ABSTRACT
Objectives:
Measurement of skeletal muscle using ultrasonography (US) has received considerable attention as an alternative method of muscle assessment. However, intra- and inter-rater reliability remains controversial. Furthermore, there is no consensus regarding the relationship between muscle assessment using US and muscle mass or physical assessment. We aimed to verify the validity and reliability of muscle measurements using US and its relationships with muscle strength and physical assessment.
Methods:
The 22 participants were all healthy men. Quadriceps muscle thickness was measured by US by three different raters. Intraclass correlation coefficient (ICC) was used to assess inter- and intra-rater reliability. The maximum isokinetic strength of the quadriceps and handgrip strength were used as measures of lower and upper muscle strength, respectively. Leg muscle mass was assessed using the leg skeletal muscle index (SMI), measured by body impedance analysis, and calf circumference.
Results:
The intra-rater reliability was excellent which the ICC(1,1) ranges 0.957-0.993, and ICC(1,3) ranges 0.985-0.998. For inter-rater reliability, the values of 0.904 for ICC(2,1) and 0.966 for ICC(2,3) indicated excellent reliability. Leg SMI was significantly correlated with quadriceps thickness (r=0.36). Maximum isokinetic strength and handgrip strength showed weak but statistically significant correlations with quadriceps thickness (r=0.20, r=0.30, respectively). The correlation between quadriceps thickness and calf circumference was not statistically significant.
Conclusions:
Quadriceps muscle assessment using US is a valid and reliable technique for healthy individuals. Quadriceps muscle thickness was significantly positively correlated with upper and lower muscle strength and leg SMI. Muscle thickness assessment could replace full body muscle assessment in clinical settings.
Keywords: muscle thickness, reliability, ultrasonography, validity
INTRODUCTION
Loss of muscle mass is a powerful indicator of mortality for several diseases.1,2,3) Therefore, sarcopenia, which is related to the age-associated loss of skeletal muscle mass, has attracted attention for its efficacy in assisting prognosis.4) The gold standard methods for assessing skeletal muscle mass are computed tomography (CT), magnetic resonance imaging (MRI), and dual-energy X-ray absorptiometry, which can assess skeletal muscle mass with almost complete accuracy.5) However, these assessments cannot be performed frequently because of radiation exposure and the complexity of repeated evaluations in clinical settings.5) Therefore, an alternative assessment of skeletal muscle mass is required, especially in acute care settings, such as the intensive care unit (ICU), where skeletal muscle mass may change considerably over time.
Ultrasonography (US) is a rapid, affordable, non-invasive scanning technique that is widely available. It is also a promising technique for estimating skeletal muscle mass. Among US measurements of muscle mass, assessment of the quadriceps muscle is the most frequently used because the muscle is large and is easy to evaluate. Some studies have demonstrated the validity of US as a method for measuring muscle mass in the elderly.6) However, muscle measurement using US depends on the examiner’s measurement technique, which may affect the transducer orientation during ultrasound imaging or cause compressive or shear stress on tissue through the force used during the examination.7) A recent systematic review reported that US is less reliable and less accurate than CT and MRI in determining muscle mass.8) Therefore, although the use of quantitative ultrasound for the assessment of sarcopenia has been previously proposed, this approach has not been embraced by recent guidelines because of the lack of reliability and the limited quality of studies.4)
As a result, there remains a significant need to verify the reliability and validity of US muscle thickness assessment as a tool for muscle mass assessment in daily clinical practice. Furthermore, it remains to be established whether US-derived muscle mass can predict the whole-body muscle mass or physical assessments that are determined using other measurement methods. To fill this knowledge gap, we aimed to investigate the intra- and inter-reliability of skeletal muscle mass thickness using US. Furthermore, we evaluated the relationships between skeletal muscle thickness as determined by US and muscle mass, strength, and physical assessment by measurement of calf circumference.
MATERIALS AND METHODS
Study Population
The 22 participants were non-disabled healthy males with a mean age of 28.3 ± 3.3 years (Table 1). None of the participants had neuromuscular or musculoskeletal disorders, and none had metal implants in the lower body. This study complied with the principles of the Declaration of Helsinki regarding investigations in humans and was approved by the Kobe University Institutional Review Board (No. 2019_820). Written informed consent was obtained from all participants.
Table 1. Participant characteristics.
| Characteristic | Mean (n=22) |
| Age, years | 28.3 ± 3.3 |
| Height, cm | 174.1 ± 6.2 |
| Weight, kg | 66.4 ± 6.86 |
| BMI, kg/m2 | 21.9 ± 1.7 |
Data are given as mean ± standard deviation.
BMI, body mass index.
Measurement of Quadriceps Muscle Thickness
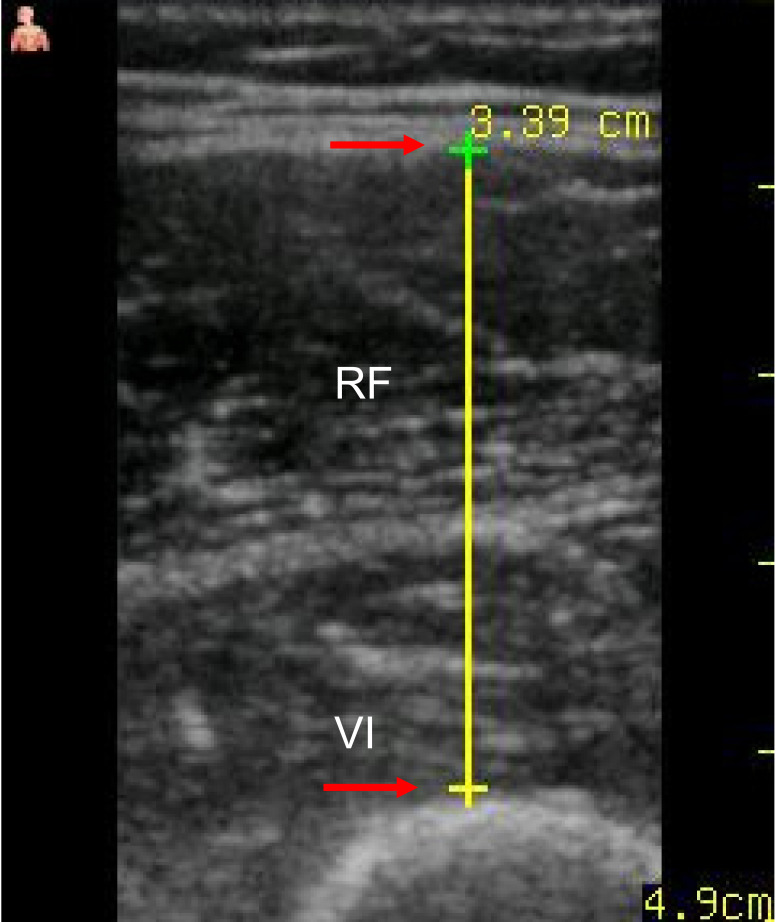
An US device probe (Vscan with Dual Probe, GE Healthcare, Tokyo, Japan) was used to obtain ultrasound images of the thigh muscles, including the rectus femoris and vastus intermedius (Fig. 1). The device was equipped with both a phased-array cardiac probe with a bandwidth of 1.7–3.8 MHz and a field of view of 70° and a linear vascular probe with a bandwidth 3.3–8.0 MHz, an aperture of 2.9 cm, and a maximum scanning depth of 8 cm.
Fig. 1.
Ultrasonography image of the quadriceps muscle. The thickness of the quadriceps muscle was defined as the combined thicknesses of the rectus femoris (RF) and the vastus intermedius (VI). The thickness was calculated by setting the cursor at the upper border of the rectus femoris (top red arrow) and the lower border of the vastus intermedius (bottom red arrow) and calculating the distance between them.
Each participant was scanned in a relaxed supine position. The examiner placed the probe on the anterior aspect of the thigh, perpendicular to its long axis at a point midway between the anterior superior iliac spine and the proximal end of the patella according to a previous study.9) The examiner identified the subcutaneous adipose tissue, rectus femoris, vastus intermedius, and the femur. Excess gel was applied to the skin to minimize distortion. Three examiners performed image acquisition to investigate inter- and intra-rater reliabilities on the dominant limb. Among the three examiners, two were physicians and one was a physiotherapist. The examiners were specialists who had conducted evaluations using US in a clinical setting for at least 3 years. Furthermore, each examiner had received training from an experienced musculoskeletal sonographer (R.H.). All trials by the three examiners in the present study were conducted independently within 2 h of the first examination to avoid fluctuations in the measurement and analysis of muscle parameters. On the US device screen, the cursor was used to mark the top border of the rectus femoris and the bottom border of the vastus intermedius. This allowed the instrument to calculate the muscle thickness as the sum of the muscle thickness of the rectus femoris and vastus intermedius. Each examiner performed three measurements to allow assessment of intra-rater reliability. After each investigation, the participant was returned to the initial position and the skin was cleaned to remove any gel or markings. This ensured that each image and dataset were acquired independently with reduced risk of measurement bias, such as anchoring.
Assessment of Muscle Strength
The maximum isokinetic strength of the quadriceps was assessed using a dynamometer (MYORET RZ-450; Kawasaki Heavy Industries, Kobe, Japan). Prior to the muscle strength test, participants warmed up using a stationary cycling ergometer for 5 min at low resistance. The participant sat on the seat of the dynamometer and was stabilized using straps. The test was first performed with the dominant leg. Each participant performed two practice contractions, followed by five maximal effort contractions at 60°/s. The test was repeated on the non-dominant leg. The peak extension torque was recorded as raw data in Newton meters (Nm) and was normalized according to body weight (Nm/kg).
Handgrip strength was measured using a grip strength dynamometer (TKK 5401; Takei Scientific Instruments, Niigata, Japan). To perform the test, the participant was seated in a chair with the shoulders neutral, elbows at 90° flexion, and forearms neutral in supination/pronation. The participant was given verbal encouragement to squeeze the dynamometer as tightly as possible for 2 or 3 s. Two trials were performed for each measurement, and the higher value was used. The order of measurement between the right and left hands was randomized for each participant.10)
Assessment of Body Impedance Analysis and Calf Circumference
A portable multifrequency bio-impedance device (InBody S10; In Body, Tokyo, Japan) was used for non-invasive body impedance analysis (BIA). Measurements were conducted while participants rested in the supine position, and electrodes were placed on the bilateral thumbs, third fingers, and ankles. Appendicular skeletal muscle mass was converted to skeletal muscle index (SMI) by standardizing according to height squared (kg/m2). Subsequently, we calculated leg SMI according to a previous study11) as follows:
Leg SMI (kg/m2) = Total skeletal muscle mass of bilateral lower limbs (kg)/Height (m)2.
The calf circumference was measured to the nearest 0.1 cm using a non-elastic tape measure with the knee joint flexed to 90° in the supine position. The tape measure was placed around the calf without compressing the subcutaneous tissue, and the point of greatest circumference was recorded.
Statistical Analysis
Demographic data are presented as mean ± standard deviation. Intra- and inter-rater reliabilities were evaluated using the intraclass correlation coefficient (ICC) with 95% confidence interval (CI). Intra-rater reliability was determined using ICC(1,1) and ICC(1,3) methods.12) Subsequently, inter-rater reliability was determined using the ICC(2,1) and ICC(2,3) methods.12) ICC values were interpreted according to the following: <0.5, poor reliability; 0.5–0.75, moderate reliability; 0.75–0.90, good reliability; >0.90, excellent reliability.13)
The concurrent validity of the quadriceps muscle thickness was examined by calculating Spearman’s correlation coefficient for comparisons of quadriceps muscle thickness with muscle strength, leg SMI assessed by BIA, and calf circumference. In addition, because the quadriceps muscle thickness is represented by a one-dimensional unit (length), we also compared quadriceps muscle thickness squared with muscle strength to match the unit dimensions. We also investigated the relationship between leg SMI and calf circumference by calculating Spearman’s correlation coefficient. The sample size was calculated based on a previous study and unpublished data. We determined a minimum acceptable reliability level of 0.70 and an expected minimum acceptable reliability level of 0.90 for the three different raters. A type I error rate of 0.05 and 90% power were assumed (n=20). Statistical significance was set at P<0.05. Statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We evaluated 22 male participants; their clinical characteristics are shown in Table 1.
Intra-rater Reliabilities
For the three raters, the ICC(1,1) values for intra-rater reliability were 0.966, 0.957, and 0.993. The ICC(1,3) values for intra-rater reliability were 0.988, 0.985, and 0.998, respectively (Table 2). All ICCs were greater than 0.90, indicating excellent reliability.
Table 2. Intra-rater reliability based on ICC values for measurement of quadriceps thickness by US.
| Rater | ICC(1,1) | 95% CI | ICC(1,3) | 95% CI |
| 1 | 0.966 | 0.951–0.981 | 0.988 | 0.981–0.993 |
| 2 | 0.957 | 0.931–0.975 | 0.985 | 0.976–0.991 |
| 3 | 0.993 | 0.988–0.997 | 0.998 | 0.996–0.999 |
Inter-rater Reliabilities
Table 3 shows the inter-rater reliability results. The mean muscle thicknesses of the three raters were 4.143 ± 0.523, 4.289 ± 0.512, and 4.247 ± 0.492 cm, respectively. ICC(2,1) was 0.904 (0.837–0.940) and ICC(2,3) was 0.966 (0.939–0.979), indicating excellent reliability.
Table 3. Inter-rater reliability based on ICC values for measurement of quadriceps thickness by US.
| Quadriceps thickness, cm | ICC(2,1) | 95% CI | ICC(2,3) | 95% CI | ||
| Rater 1 | Rater 2 | Rater 3 | ||||
| 4.143 ± 0.523 | 4.289 ± 0.512 | 4.247 ± 0.492 | 0.904 | 0.837–0.940 | 0.966 | 0.939–0.979 |
Data for quadriceps thickness given as mean ± standard deviation.
Comparison with Other Parameters
The mean leg muscle mass as assessed using BIA analysis was 5.74 ± 0.73 kg/m2. Leg SMI was weakly but significantly correlated with quadriceps thickness (r=0.36, P=0.02; Table 4). The mean of maximum isokinetic strength normalized for body weight was 1.41 ± 0.24 Nm/kg, which showed weak correlations with quadriceps thickness with statistical significance (r=0.20; P=0.04). This correlation remained unchanged for quadriceps thickness squared (r=0.28; P=0.04). Similarly, there was weak correlation between grip strength and quadriceps thickness with statistical significance (r=0.30; P=0.04). Calf circumference (36.01 ± 2.88 cm) did not show statistically significant correlation with quadriceps thickness (r=0.26; P=0.09), but was positively correlated with leg SMI (r=0.42; P=0.03).
Table 4. Concurrent validity between quadriceps muscle thickness and muscle mass, strength, and calf circumference.
| Variable | Spearman’s correlation coefficient | P value |
| Leg SMI | 0.36 | 0.02 |
| Maximum isokinetic strength | 0.20 | 0.04 |
| Grip strength | 0.30 | 0.04 |
| Calf circumference | 0.26 | 0.09 |
DISCUSSION
This study investigated the intra- and inter-rater reliability of US for the assessment of quadriceps muscle thickness. All ICC values for both intra-rater and inter-rater reliability were above 0.80, indicating excellent reliability. This is the first study to demonstrate the relationship between quadriceps muscle thickness and leg muscle mass, strength, and calf circumference. Furthermore, there were significant positive correlations between quadriceps muscle thickness and lower muscle mass, upper muscle strength (measured by handgrip strength), and lower muscle strength (maximum isokinetic strength of the quadriceps), respectively.
Several studies have evaluated the validity of muscle thickness in ICU patients,14) patients with diabetes mellitus,15) and healthy individuals.16) The results of the present study demonstrate similar ICC values to those of previous studies. Our results show that with adequate positioning and proper protocols, assessment of muscle thickness using US is sufficiently robust for use in clinical evaluation. However, there remains some potential for measurement error because of variations in measurement position and probe placement according to the methods of the examiners. Regarding the relationship between muscle thickness and muscle mass, some studies have reported a positive correlation between thickness measurements of the quadriceps muscle and quadriceps muscle strength.17,18,19) However, different evaluation methods were used for muscle strength measurements in each study, and reliable assessments are lacking. We used an isokinetic dynamometer, which has been used as a reliable and objective measuring apparatus for recording lower-extremity muscle strength. In pulmonary disease patients, Seymour et al.20) stated that the cross-sectional area of the rectus femoris muscle was significantly correlated with isometric quadriceps strength. However, we demonstrated that quadriceps muscle thickness showed a statistically significant positive correlation with lower-extremity muscle strength in healthy participants. Ideally, it would be dimensionally correct to compare muscle strength with muscle thickness squared. In clinical settings, it is not feasible to estimate muscle strength from squaring the muscle thickness. Moreover, there was no significant change in the results when muscle thickness was squared. Therefore, our results imply that the measurement of muscular thickness by US could be an alternative to the measurement of muscle strength in situations where maximal muscle strength cannot be achieved, as exemplified in critically ill patients.
There was also significant correlation between the quadriceps muscle thickness and grip strength. Although few studies have examined muscle strength and quadriceps thickness, it is generally recognized that grip strength is related to whole-body muscle strength.21) The findings of the present study suggest that the assessment of quadriceps muscle thickness, which does not require patient effort, can be used as a surrogate assessment of muscle strength in the upper and lower limbs. However, the correlation between quadriceps muscle thickness and leg SMI, while significant, was not strong. This result could be explained by compromised muscle quality, following the report by Fukumoto et al. that demonstrated that skeletal muscle quality was associated with muscle strength.22) Although our study using a portable US device did not assess muscle quality, it is possible that more detailed muscle assessment could be achieved by monitoring the echo intensity.
Muscle thickness measured using US was also compared with calf circumference, but only a weak correlation (r=0.26) was observed that was not statistically significant (P=0.09). Calf circumference measurement has been suggested as an alternative method of assessing muscle mass and is recommended in the criteria for defining sarcopenia.4) In the present study, calf circumference showed a significant relationship with leg SMI. However, no significant relationship was observed between muscle thickness and calf circumference. This result may have been caused by a lack of assessment of muscle quality or the sample size. Furthermore, given that the effects of fat mass and edema are generally problematic when assessing leg circumference,23) the factors of age and sex should be taken into consideration. Despite these concessions, our results suggest that muscle mass assessment using US could be performed when muscle mass cannot be measured.
Muscle mass assessment can be utilized for non-invasive daily clinical assessment of total body muscle mass and strength, particularly in acute care or ICU settings where muscle mass fluctuates dynamically. In situations where patients are unable to actively use their muscles, the US evaluation of muscle thickness can replace a full body muscle assessment. A previous study demonstrated that muscle mass decreases significantly over the first 2–3 weeks of ICU stay, reaching approximately half of what it was when the patient was hospitalized.24) Therefore, muscle thickness assessment can provide insight into the patient’s condition over time and can be used to assess the efficacy of nutritional and rehabilitation therapies. Furthermore, it is preferable to evaluate large muscle groups, such as the quadriceps, as demonstrated in the present study, because US examination of very small muscles has been shown to have poor inter-rater reliability.25)
The portable, battery-powered, and inexpensive US device used in this study allows routine bedside examination in clinical settings and is recommended as an alternative assessment of muscle mass and strength. Toledo et al.1) reported that a quadriceps muscle thickness of 1.64 cm can be used as a threshold value that predicts worse outcomes, making it a useful prognostic predictor. The evidence presented here suggests that the assessment of muscle thickness using US could be used to improve patient management. Given the simplicity and the potential benefits of the technique, its omission from routine clinical practice for many rehabilitation patients may be hard to justify.
The present study has several limitations. First, although the sample size was calculated prior to the start of the study, the sample size was small and included only male participants. Therefore, sex-related differences were not observed. Second, we only evaluated muscle thickness to assess muscle mass, and muscle quality was not evaluated. In future studies, US should be used to assess muscle quality, as recently reported Akazawa et al., who used US to measure muscle echo intensity to assess muscle quality.26) Muscle quality should be evaluated together with muscle thickness and their impact on physical function and functional prognosis should be investigated in the future. Third, this study was conducted using a portable US device and cannot be generalized to all US devices. Despite these limitations, our results are novel and provide a basis for further studies on the routine assessment of muscle mass, clinical outcomes, and interventions for rehabilitation.
CONCLUSION
We investigated the intra- and inter-reliability of quadriceps muscle thickness measured using US. Both intra- and inter-rater reliabilities were excellent in healthy individuals. The quadriceps muscle thickness showed significant positive correlations with upper and lower muscle strength. Muscle thickness by US was also correlated with leg SMI. The present study suggests that muscle thickness assessment, which does not require patient effort, can be used as a valid surrogate assessment of muscle strength in the upper and lower limbs.
ACKNOWLEDGMENTS
The authors thank all participants in the study.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Toledo DO,Freitas BJ,Dib R,Pfeilsticker FJ,Santos DM,Gomes BC,Silva-Jr JM: Peripheral muscular ultrasound as outcome assessment tool in critically ill patients on mechanical ventilation: an observational cohort study. Clin Nutr ESPEN 2021;43:408–414. 10.1016/j.clnesp.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Rier HN,Jager A,Sleijfer S,Maier AB,Levin MD: The prevalence and prognostic value of low muscle mass in cancer patients: a review of the literature. Oncologist 2016;21:1396–1409. 10.1634/theoncologist.2016-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haehling S,Garfias Macedo T,Valentova M,Anker MS,Ebner N,Bekfani T,Haarmann H,Schefold JC,Lainscak M,Cleland JG,Doehner W,Hasenfuss G,Anker SD: Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle 2020;11:1242–1249. 10.1002/jcsm.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LK,Woo J,Assantachai P,Auyeung TW,Chou MY,Iijima K,Jang HC,Kang L,Kim M,Kim S,Kojima T,Kuzuya M,Lee JS,Lee SY,Lee WJ,Lee Y,Liang CK,Lim JY,Lim WS,Peng LN,Sugimoto K,Tanaka T,Won CW,Yamada M,Zhang T,Akishita M,Arai H: Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307. 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Mitsiopoulos N,Baumgartner RN,Heymsfield SB,Lyons W,Gallagher D,Ross R: Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115–122. 10.1152/jappl.1998.85.1.115 [DOI] [PubMed] [Google Scholar]
- 6.Nijholt W,Scafoglieri A,Jager-Wittenaar H,Hobbelen JS,van der Schans CP: The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–712. 10.1002/jcsm.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris-Love MO,Monfaredi R,Ismail C,Blackman MR,Cleary K: Quantitative ultrasound: measurement considerations for the assessment of muscular dystrophy and sarcopenia. Front Aging Neurosci 2014;6:172. 10.3389/fnagi.2014.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceniccola GD,Castro MG,Piovacari SM,Horie LM,Corrêa FG,Barrere AP,Toledo DO: Current technologies in body composition assessment: advantages and disadvantages. Nutrition 2019;62:25–31. 10.1016/j.nut.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 9.Young HJ,Jenkins NT,Zhao Q,Mccully KK: Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 2015;52:963–971. 10.1002/mus.24656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaubert KL,Bohannon RW: Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res 2005;19:717–720. [DOI] [PubMed] [Google Scholar]
- 11.Shimono Y,Enomoto H,Kishino K,Moriwaki EI,Nishikawa H,Nishimura T,Iwata Y,Iijima H,Nishiguchi S: Arm skeletal muscle mass is associated with the prognosis of patients with cirrhosis. In Vivo 2020;34:1165–1171. 10.21873/invivo.11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrout PE,Fleiss JL: Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–428. 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- 13.Koo TK,Li MY: A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardo E,El Behi H,Boizeau P,Verdonk F,Alberti C,Lescot T: Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol 2018;18:205. 10.1186/s12871-018-0647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva CR,Costa AS,Rocha T,Lima DA,Nascimento T,Moraes SR: Quadriceps muscle architecture ultrasonography of individuals with type 2 diabetes: reliability and applicability. PLoS One 2018;13:e0205724. 10.1371/journal.pone.0205724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi Y,Fujino Y,Miura K,Toida A,Matsuda T,Makita S: Intra- and inter-rater reliability of rectus femoris muscle thickness measured using ultrasonography in healthy individuals. Ultrasound J 2021;13:21. 10.1186/s13089-021-00224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser EM,Draskovits T,Praschak M,Quittan M,Graf A: Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013;35:2377–2388. 10.1007/s11357-013-9517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi-Fishman G,Hicks JE,Cintas HM,Sonies BC,Gerber LH: Ultrasound imaging distinguishes between normal and weak muscle. Arch Phys Med Rehabil 2004;85:980–986. 10.1016/j.apmr.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Freilich RJ,Kirsner RL,Byrne E: Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul Disord 1995;5:415–422. 10.1016/0960-8966(94)00078-N [DOI] [PubMed] [Google Scholar]
- 20.Seymour JM,Ward K,Sidhu PS,Puthucheary Z,Steier J,Jolley CJ,Rafferty G,Polkey MI,Moxham J: Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 2009;64:418–423. 10.1136/thx.2008.103986 [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW: Grip strength: an indispensable biomarker for older adults. Clin Interv Aging 2019;14:1681–1691. 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumoto Y,Ikezoe T,Yamada Y,Tsukagoshi R,Nakamura M,Mori N,Kimura M,Ichihashi N: Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 2012;112:1519–1525. 10.1007/s00421-011-2099-5 [DOI] [PubMed] [Google Scholar]
- 23.Nindl BC,Scoville CR,Sheehan KM,Leone CD,Mello RP: Gender differences in regional body composition and somatotrophic influences of IGF-I and leptin. J Appl Physiol 2002;92:1611–1618. 10.1152/japplphysiol.00892.2001 [DOI] [PubMed] [Google Scholar]
- 24.Gruther W,Benesch T,Zorn C,Paternostro-Sluga T,Quittan M,Fialka-Moser V,Spiss C,Kainberger F,Crevenna R: Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. Acta Derm Venereol 2008;40:185–189. 10.2340/16501977-0139 [DOI] [PubMed] [Google Scholar]
- 25.English CK,Thoirs KA,Fisher L,McLennan H,Bernhardt J: Ultrasound is a reliable measure of muscle thickness in acute stroke patients, for some, but not all anatomical sites: a study of the intra-rater reliability of muscle thickness measures in acute stroke patients. Ultrasound Med Biol 2012;38:368–376. 10.1016/j.ultrasmedbio.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 26.Akazawa N,Kishi M,Hino T,Tsuji R,Tamura K,Hioka A,Moriyama H: Longitudinal relationship between intramuscular adipose tissue of the quadriceps and activities of daily living in older inpatients. J Cachexia Sarcopenia Muscle 2021;12:2231–2237. 10.1002/jcsm.12842 [DOI] [PMC free article] [PubMed] [Google Scholar]