ABSTRACT
Background:
This case report describes the successful management of rehabilitation therapy for a hematological malignancy patient who was receiving chemotherapy and had coronavirus disease 2019 (COVID-19).
Case:
A 76-year-old man receiving chemotherapy for relapsed refractory multiple myeloma (MM) presented to our hospital with fever and dyspnea and was hospitalized with a diagnosis of COVID-19. Physical therapy (20 min/day, 5 days/week) was started on day 6 of hospitalization while the patient was receiving oxygen therapy. Conditioning exercises and movement exercises were performed in an isolation room, and blood counts, fracture susceptibility, and respiratory status were monitored. The patient was severely immunocompromised and required 34 days of isolation due to persistent severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) infection. Physical function was assessed by manual muscle testing of the lower extremities and by the extent of lower extremity fatigue and dyspnea on exertion, as assessed using the Borg scale. Motor capacity was assessed using the de Morton Mobility Index (DEMMI) score and the Barthel Index (BI). Muscle weakness and severe dyspnea developed 4 days after physical therapy was started. However, physical therapy led to improvements in DEMMI score and BI. The patient was discharged home on day 43 with home medical care.
Discussion:
Careful management of MM and COVID-19 facilitated safe treatment with physical therapy. The patient’s physical function improved with a carefully planned physical therapy program. Moreover, the patient required prolonged isolation due to persistent viral shedding; however, as a result of the treatment, which was coordinated between physicians and nurses, the patient could be discharged home.
Keywords: cancer, COVID-19, long-term isolation, risk management
INTRODUCTION
Cancer patients are at significantly increased risk for coronavirus disease 2019 (COVID-19),1) and at high risk of severe disease.1,2) In particular, patients with hematological malignancies are at high risk of infection, severe disease, and death from COVID-19.3) Patients with hematological malignancies, especially lymphoid malignancies, or patients undergoing chemotherapy, have impaired immune responses to the virus and its vaccine.4,5) Therefore, treatment of COVID-19 for such patients tends to be prolonged and is conducted in an isolated environment.
Patients with hematological malignancies have poor physical function and poor levels of physical activity.6,7,8) Those with moderate-to-severe COVID-19 experience respiratory failure and decreased physical activity, which result in functional decline. Rehabilitation therapy is important for patients with hematological malignancies or COVID-19 to improve and maintain physical function and activities of daily living (ADL). However, the management and impact of rehabilitation therapy on patients with hematological malignancies who also have COVID-19 have rarely been reported and remain unclear. Herein, we report how we provided physical therapy to improve ADL in a multiple myeloma patient with COVID-19 while maintaining infection control measures and continuing cancer care. Written informed consent was obtained from the patient and his family for publication of this case report.
CASE
The patient was a 76-year-old man receiving chemoimmunotherapy for multiple myeloma (MM) at our hospital. He was diagnosed with symptomatic MM about 8 months previously in addition to renal failure, anemia, a clavicle fracture, and intraspinal tumors at the level of the thoracic spine (Th). Chemotherapy was initiated with high-dose dexamethasone (HD-DEX), and radiation therapy was applied to the Th4 and Th11 segments, which were involved in spinal compression. The patient was then treated with bortezomib and dexamethasone, followed by daratumumab, bortezomib, melphalan, and prednisolone (DVMP). After three cycles of DVMP, it was determined that the disease was progressive and an additional compression fracture at L1 was noted. The patient was treated with vincristine, doxorubicin, and dexamethasone, but the disease continued to progress. We once again administered HD-DEX, followed by pomalidomide, cyclophosphamide, and dexamethasone. On day 12 during the third cycle, the patient developed a fever and was tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The polymerase chain reaction (PCR) test resulted in a diagnosis of severe COVID-19, even though the patient had received two doses of COVID-19 vaccine. Before admission, the patient was able to move around at home unassisted. He used a walker to ensure safe movement outside the house because of his stooped posture, which was a result of compression fractures.
Treatment of COVID-19
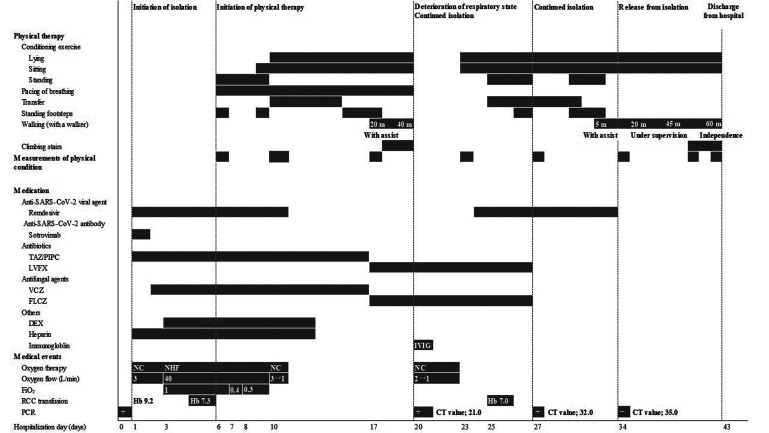
Chemotherapy for MM was temporarily suspended and treatment for COVID-19 was initiated. The course of treatment and general condition during hospitalization are shown in Fig. 1 On admission, the patient had a fever and respiratory failure. Initially, he was administered 3 L/min of oxygen with a nasal cannula; however, the respiratory status deteriorated on day 3 of hospitalization, and nasal high flow (NHF) oxygen therapy was introduced. The respiratory condition gradually improved after treatment, which included an antiviral drug (remdesivir), an antibody drug (sotrovimab), dexamethasone, an antithrombotic agent (heparin), antibiotics, and an antifungal agent. The patient was weaned from NHF on day 10 and no longer required supplemental oxygen on day 12. However, on day 20, his respiratory condition worsened again, and computed tomography revealed bilateral infiltrations and pleural effusion. A SARS-CoV-2 antigen test was highly positive, and a PCR test was positive with a cycle threshold (CT) value at 21.0, revealing that viral elimination was insufficient and the patient was still infectious. He was kept isolated, and COVID-19 treatment was continued, including an antiviral drug and γ globulin, as his globulin levels were extremely low. On day 34, an antigen test was negative and a SARS-CoV-2 PCR test showed a CT value of 35.0; therefore, isolation was discontinued.
Fig. 1.
Course of treatment and general condition of the patient. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TAZ/PIPC, tazobactam/piperacillin; LVFX, levofloxacin; VCZ, voriconazole; FLCZ, fluconazole; DEX, dexamethasone; FiO2, fraction of inspiratory oxygen; RCC, red cell concentrate; PCR, polymerase chain reaction, IVIG, intravenous immunoglobulin; NC, nasal cannula; NHF, nasal high flow; Hb, hemoglobin; CT value, cycle threshold value.
Physical Therapy
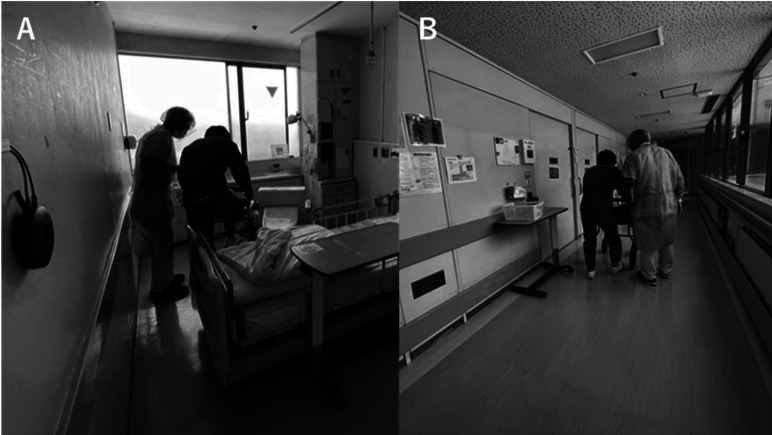
The course of physical therapy is shown in Fig. 1 Therapy began on day 6 of hospitalization and was conducted in the patient’s private isolation room by therapists wearing personal protective equipment (Fig. 2). Hemoglobin and platelet levels were monitored to assess the risk of anemia and bleeding, and bone fracture susceptibility due to MM was considered when determining the appropriate exercise load. Anemia can lead to inadequate oxygen supply, which can lead to falls and decreased performance. Therefore, the discontinuation criterion was set at a hemoglobin level of 7.0 g/dL. Moreover, if the platelet count fell below 10,000/μl, the patient was to avoid active exercise and reduce his ADL as much as possible. The patient had previously suffered fractures of the spine and clavicle, and bone fragility due to multiple myeloma was noted. New fractures would likely result in a further decline in ADL. Therefore, the patient was treated with great care to avoid falls and movements that put stress on the spine and ribs. Physical therapy sessions lasting 20 min were performed 5 days/week. Items needed for physical therapy treatment that could be disinfected or disposed of (tools for climbing up and down stairs and rubber bands that can be disposed of after use) were brought into the isolation room. On days when physical therapy was not provided, exercises were carried out under the supervision of nurses based on leaflets prepared by the physiotherapist. The leaflets were additionally used to encourage self-exercise.
Fig. 2.
(A) Physical therapy in the isolation room; (B) physical therapy after the patient was released from isolation.
Measurements
Physical measurements reflecting the outcome of physical therapy are shown in Table 1. Physical function was assessed using manual muscle testing (MMT) of the proximal and distal lower extremity muscles. Dyspnea and lower extremity fatigue on exertion were assessed using the Borg scale. The de Morton Mobility Index (DEMMI) score was used to assess movement ability. ADL was assessed using the Barthel Index (BI).
Table 1. Measurements of the patient’s physical condition.
| Before admission |
Hospitalization day | ||||||||
| Day 6 | Day 10 | Day 17 | Day 23 | Day 27 | Day 34 | Day 41 | Day 43 | ||
| MMT of lower extremity, 0–5 grade | |||||||||
| Proximal muscles | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Distal muscles | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Borg scale (on exertion), 6–20 points | |||||||||
| Dyspnea | 14 | 16 | 15 | 16 | 14 | 14 | 14 | 14 | |
| Leg fatigue | 15 | 16 | 15 | 15 | 15 | 14 | 13 | 13 | |
| DEMMI score, 0–100 points | 100 | 33 | 27 | 33 | 24 | 36 | 36 | 39 | 39 |
| BI, 0–100 points | Walking without assistance | 30 | 20 | 50 | 25 | 35 | 60 | 75 | 75 |
MMT, manual muscle testing; DEMMI score, de Morton Mobility Index score; BI, Barthel Index.
Progress of Physical Therapy
The progress of physical therapy is shown in Fig. 1. At the beginning of the treatment, the patient was under NHF oxygen therapy but did not complain of dyspnea; his vital signs were stable. The patient was able to perform transfers under observation. Therefore, pacing instruction regarding how to breath comfortably during exertion was provided as part of the respiratory rehabilitation therapy, and conditioning exercises in the standing position were initiated to prevent decline in physical function. On day 10, the patient was weaned from NHF therapy. Although the patient had recovered from respiratory failure and was able to breathe independently after treatment, he had difficulty standing up without assistance. There was no decrease in oxygen saturation on exertion; however, the patient had an increased respiratory rate, complained of severe fatigue and dyspnea, required assistance when transferring to a portable toilet, and had difficulty with the stepping exercise. Severe fatigue in the lower limb muscles and dyspnea persisted during exertion, and a decline in ADL was observed. Therefore, the level of physical therapy treatment was reduced, and conditioning exercises were performed with the patient in the supine or seated position, according to the respiratory status. For respiratory rehabilitation therapy, we encouraged the patient to relax his respiratory muscles, and gave pacing instructions to avoid stretching of the thoracic cage; this was done because the patient had a stooped posture and generalized bone weakness due to MM. Furthermore, self-exercise was encouraged by the therapists and medical staff by providing instructions for independent training because the patient tended to remain in bed in his isolated environment. Although the patient’s dyspnea and fatigue persisted, muscle strength gradually improved. By day 20, he was able to stand up with minimal assistance and walk around the room with the use of a walker. However, on day 20, respiratory status again deteriorated, and because of his positive SARS-CoV-2 PCR status, physical therapy treatment continued to be conducted in the isolation room. By day 25, the patient’s general condition and respiratory status had again improved and he was able to undergo the same treatment that was initially given. At this point, the patient was able to sit independently, stand up with minimal assistance, and perform some standing lower extremity exercises. The standing exercises were gradually increased in frequency to improve muscle strength.
Outcome
The patient was discharged from hospital on day 43 and was able to walk with the help of a walker. Outdoors, he was wheelchair-bound. On discharge, MMT was 3 for the proximal leg muscles and 3 for the distal leg muscles; these scores were lower than those at the start of physical therapy treatment for the proximal leg muscles. The lowest DEMMI score during the hospital stay was 24 points, and this had improved to 39 points at discharge. The lowest BI was 20 points, but this had improved to 75 points at discharge.
DISCUSSION
The patient was receiving chemoimmunotherapy for MM as an outpatient when he contracted SARS-CoV-2. Due to MM, long-term treatment for COVID-19 was necessary, and the patient was isolated for a long period of time, resulting in decreased physical activity and physical function, as well as an eventual decline in ADL. Early physical therapy treatment, including exercise therapy and movement practice, was successfully performed while infection control measures were taken against COVID-19 and cancer care was managed; this regimen resulted in an improvement in ADL.
At the beginning of physical therapy, the patient was able to rise from bed, stand up, maintain a standing position, and walk under supervision; however, within a few days, the patient showed marked progressive muscle weakness and increased dyspnea on exertion. Despite physical therapy, in the following few days, physical activity and ADL decreased to levels that required assistance. Both dyspnea and lower extremity fatigue worsened during this period, according to the Borg scale. The cause of this decline in ADL was the increased breathing workload after the patient was weaned off NHF therapy, resulting in increased dyspnea. Furthermore, we believe that the decreased supply of stable oxygenation also led to a decrease in muscle strength on exertion. Respiratory pacing and movement exercises during exertion improved ADL, but muscle weakness and dyspnea during exertion were prolonged. Various factors were considered to be responsible for this, including decreased muscle function due to COVID-19, myopathy due to steroid use, and decreased physical activity and physical function due to isolation. It has been reported that COVID-19 induces hypercatabolism, resulting in decreased muscle function and sarcopenia.9,10) Steroid myopathy is also associated with muscle weakness with proximal muscle predominance, resulting in limitation of activities such as standing up.11) Furthermore, disuse syndrome due to inactivity causes generalized muscle weakness, and ICU-acquired weakness reportedly causes rapid muscle weakness.12,13) Our patient had all of these conditions; however, as laboratory examination could not be performed because of the patient’s isolation, we were unable to determine the exact cause of the muscle weakness. Another major complaint from the patient was respiratory distress, for which there were multiple possible causes. Respiratory distress can arise from systemic muscle dysfunction. Both skeletal and respiratory muscles can be affected by steroid myopathy.11,14) The patient had a history of spinal compression fracture and rib fracture, which might have contributed to both dyspnea and poor compliance of the thorax. However, it was feared that stretching the thorax and excessive spinal extension might cause new fractures and were therefore avoided.
Physical therapy is useful in improving physical function and ADL in these medical conditions.15,16,17) The efficacy of exercise therapy is well known in the rehabilitation therapy of cancer patients undergoing chemotherapy; even low-intensity exercise therapy is effective.18) A rehabilitation program adapted to the general condition and respiratory status has been recommended for COVID-19 patients.19) The present case report concerned an elderly cancer patient undergoing chemoimmunotherapy in whom COVID-19 might have caused progressive hypercatabolism. Therefore, low-intensity exercise was started on the bed in accordance with the patient’s decreased physical function and respiratory status. Blood data were carefully monitored for anemia so that the exercise load could be adjusted if necessary to avoid overloading the patient. To prevent new fractures, respiratory rehabilitation consisted mainly of respiratory pacing during exertion. By gradually increasing the amount and load of exercise according to the patient’s respiratory condition and general condition, and by integrating programs for respiratory rehabilitation and muscle function, it is possible to improve physical function as assessed by DEMMI and BI. Patients with hematological malignancies are immunodeficient because of the primary disease and chemoimmunotherapy, which impairs antibody production. Therefore, when such patients are infected with SARS-CoV-2, viral elimination takes longer.20) The current patient had to be isolated for 33 days because his SARS-CoV-2 viral load remained high. A self-exercise instruction leaflet was prepared, and exercise was encouraged by the therapist and medical staff to further improve physical function and movement ability of the patient. Moreover, with careful management of MM treatment, the patient could be discharged without secondary disabilities such as falls or bone fractures, although he still had dyspnea and decreased levels of ADL compared to those before hospitalization.
In cases of COVID-19 in hematological malignancy patients undergoing chemotherapy, a longer isolation period may be necessary, and therapists should organize a physical therapy program taking into consideration the isolation period due to the risk of COVID-19, the pathophysiology of the cancer, and the risks posed by chemoimmunotherapy. In the present case, the patient was discharged from hospital after undergoing physical therapy; special attention was paid to his physical condition, and treatments to increase activity in cooperation with various medical professionals were carried out while he was in isolation. For cancer patients with COVID-19, early and prolonged treatment with physical therapy is important. Careful management of cancer and infection control measures against COVID-19 can enable physical therapy to be conducted safely.
ACKNOWLEDGMENTS
The authors are grateful to the patient, to the physical therapists in the Department of Rehabilitation, and to the physicians in the Department of General Medicine and Hematology at Matsushita Memorial Hospital.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Wang Q,Berger NA,Xu R: Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–227. 10.1001/jamaoncol.2020.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M,Liu D,Liu M,Zhou F,Li G,Chen Z,Zhang Z,You H,Wu M,Zheng Q,Xiong Y,Xiong H,Wang C,Chen C,Xiong F,Zhang Y,Peng Y,Ge S,Zhen B,Yu T,Wang L,Wang H,Liu Y,Chen Y,Mei J,Gao X,Li Z,Gan L,He C,Li Z,Shi Y,Qi Y,Yang J,Tenen DG,Chai L,Mucci LA,Santillana M,Cai H: Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–791. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharafeldin N,Bates B,Song Q,Madhira V,Yan Y,Dong S,Lee E,Kuhrt N,Shao YR,Liu F,Bergquist T,Guinney J,Su J,Topaloglu U: Outcomes of COVID-19 in patients with cancer: Report from the National COVID Cohort Collaborative (N3C). J Clin Oncol 2021;39:2232–2246. 10.1200/JCO.21.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog Tzarfati K,Gutwein O,Apel A,Rahimi-Levene N,Sadovnik M,Harel L,Benveniste-Levkovitz P,Bar Chaim A,Koren-Michowitz M: BNT162B2 COVID‐19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol 2021;96:1195–1203. 10.1002/ajh.26284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E,Gavriatopoulou M,Ntanasis-Stathopoulos I,Briasoulis A,Gumeni S,Malandrakis P,Fotiou D,Papanagnou ED,Migkou M,Theodorakakou F,Roussou M,Eleutherakis-Papaiakovou E,Kanellias N,Trougakos IP,Kastritis E,Dimopoulos MA: The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J 2021;11:138. 10.1038/s41408-021-00530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen RF,Jarden M,Minet LR,Frølund UC,Möller S,Abildgaard N: Physical function in patients newly diagnosed with multiple myeloma; a Danish cohort study. BMC Cancer 2020;20:169. 10.1186/s12885-020-6637-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol JL,Woodrow C,Burton NW,Mollee P,Nicol AJ,Hill MM,Skinner TL: Physical activity in people with multiple myeloma: associated factors and exercise program preferences. J Clin Med 2020;9:3277. 10.3390/jcm9103277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermaete N,Wolter P,Verhoef G,Gosselink R: Physical activity, physical fitness and the effect of exercise training interventions in lymphoma patients: a systematic review. Ann Hematol 2013;92:1007–1021. 10.1007/s00277-013-1689-1 [DOI] [PubMed] [Google Scholar]
- 9.Menozzi R,Valoriani F,Prampolini F,Banchelli F,Boldrini E,Martelli F,Galetti S,Fari’ R,Gabriele S,Palumbo P,Forni D,Pantaleoni M,D’Amico R,Pecchi AR: Impact of sarcopenia in SARS-CoV-2 patients during two different epidemic waves. Clin Nutr ESPEN 2022;47:252–259. 10.1016/j.clnesp.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piotrowicz K,Gąsowski J,Michel JP,Veronese N: Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res 2021;33:2887–2898. 10.1007/s40520-021-01942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haran M,Schattner A,Kozak N,Mate A,Berrebi A,Shvidel L: Acute steroid myopathy: a highly overlooked entity. QJM 2018;111:307–311. 10.1093/qjmed/hcy031 [DOI] [PubMed] [Google Scholar]
- 12.Wall BT,Dirks ML,van Loon LJ: Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 2013;12:898–906. 10.1016/j.arr.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 13.Vanhorebeek I,Latronico N,Van den Berghe G: ICU-acquired weakness. Intensive Care Med 2020;46:637–653. 10.1007/s00134-020-05944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Balkom RH,van der Heijden HF,van Herwaarden CL,Dekhuijzen PN: Corticosteroid-induced myopathy of the respiratory muscles. Neth J Med 1994;45:114–122. [PubMed] [Google Scholar]
- 15.Nagashima M,Takahashi D,Mizushima T,Yamauchi K: Effects of exercise in patients with connective tissue disease receiving high-dose glucocorticoids: a pilot prospective cohort study. Eur J Appl Physiol 2021;121:2253–2263. 10.1007/s00421-021-04697-2 [DOI] [PubMed] [Google Scholar]
- 16.Fuke R,Hifumi T,Kondo Y,Hatakeyama J,Takei T,Yamakawa K,Inoue S,Nishida O: Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open 2018;8:e019998. 10.1136/bmjopen-2017-019998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang K,Chen B,Wang M,Chen D,Hui L,Guo S,Ji T,Shang F: The effect of early mobilization in critically ill patients: a meta‐analysis. Nurs Crit Care 2020;25:360–367. 10.1111/nicc.12455 [DOI] [PubMed] [Google Scholar]
- 18.Fukushima T,Nakano J,Ishii S,Natsuzako A,Sakamoto J,Okita M: Low-intensity exercise therapy with high frequency improves physical function and mental and physical symptoms in patients with haematological malignancies undergoing chemotherapy. Eur J Cancer Care (Engl) 2018;27:e12922. 10.1111/ecc.12922 [DOI] [PubMed] [Google Scholar]
- 19.Felten-Barentsz KM,van Oorsouw R,Klooster E,Koenders N,Driehuis F,Hulzebos EH,van der Schaaf M,Hoogeboom TJ,van der Wees PJ: Recommendations for hospital-based physical therapists managing patients with COVID-19. Phys Ther 2020;100:1444–1457. 10.1093/ptj/pzaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helleberg M,Niemann CU,Moestrup KS,Kirk O,Lebech AM,Lane C,Lundgren J: Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020;222:1103–1107. 10.1093/infdis/jiaa446 [DOI] [PMC free article] [PubMed] [Google Scholar]