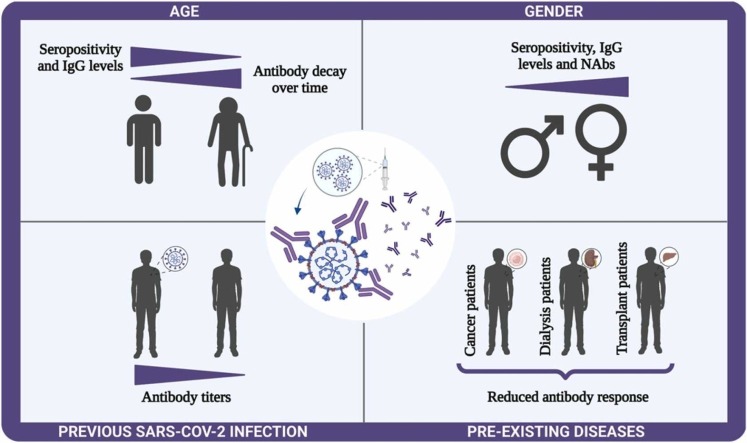
Abstract
Vaccines induce specific long-term immunological memory against pathogens, preventing the worsening of diseases. The COVID-19 health emergency has caused more than 6 million deaths and started a race for vaccine development. Antibody response to COVID-19 vaccines has been investigated primarily in healthcare workers. The heterogeneity of immune responses and the behavior of this response in particular groups were still very little explored. In this review, we discuss whether antibody responses after vaccination are influenced by age, gender, previous SARS-CoV-2 infection, or pre-existing diseases.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Antibody response
Graphical Abstract
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an RNA virus responsible for the COVID-19 pandemic that has infected hundreds of millions of people and resulted in millions of deaths worldwide (WHO, 2023). The genome of SARS-CoV-2 encodes four conserved structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N) (Kim et al., 2020). SARS-CoV-2 infects host cells through its S protein, which consists of the S1 subunit, S2 subunit, transmembrane, and cytoplasmic domains. The S1 subunit is further divided into N-terminal domain (NTD), receptor-binding domain (RBD), subdomain 1 (SD1), and subdomain 2 (SD2) (Lu et al., 2020). The RBD in the S1 subunit of SARS-CoV-2 binds to the cellular receptor angiotensin-converting enzyme 2 (ACE2) in host cells and mediates virus entry (Yesudhas et al., 2021). When the virus enters the cell, leads to the activation of the body's humoral and cellular immunity (Li et al., 2020). The humoral immune responses are crucial to fight respiratory viral infections in the current pandemic of COVID-19. During SARS-CoV-2 infection, the dynamics of antibody production are affected by the different characteristics of patients (Yaugel-Novoa et al., 2022).
The COVID-19 pandemic promoted a race for the development of vaccines (Graham, 2020, Malik et al., 2021). Until now, the world received more than 12 trillion doses of different COVID-19 vaccines (WHO, 2022). The vaccines have been an effective strategy to protect against severe cases of COVID-19 (Hu et al., 2021, Messina et al., 2019, Meyer and Zepp, 2022). Post-vaccination antibody response has been mainly described in healthcare workers (HCWs) since they were prioritized in vaccination programs (Gómez-Ochoa et al., 2021). However, the heterogeneity of immunological response and the knowledge of this response in particular groups are still limited. Also, while the immunogenicity of COVID‐19 vaccines has been characterized in several well-conducted clinical trials, real-world evidence concerning immune responses against SARS-CoV-2 raised by such vaccines is currently missing (van Praet et al., 2021).
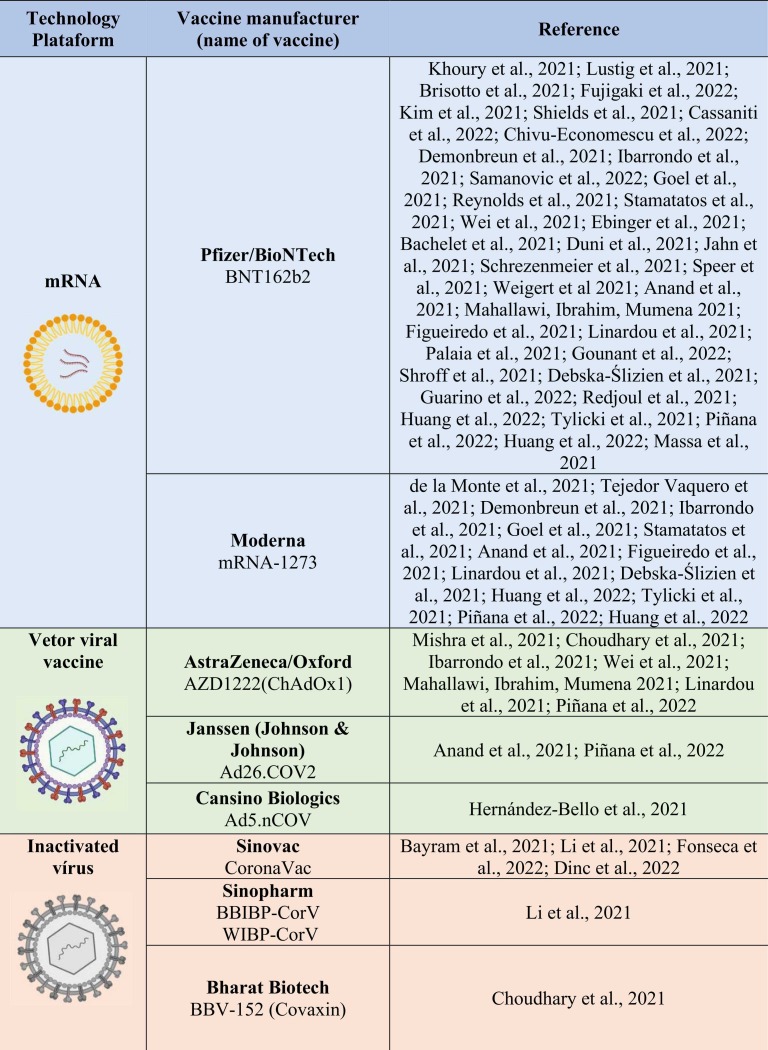
Therefore, this study aimed to review the literature on antibody response to different COVID-19 vaccine platforms ( Table 1) and determine whether this response correlates with age, gender, previous SARS-CoV-2 infection, and pre-existing diseases.
Table 1.
Vaccination platforms and the respective manuscripts evaluated in this review.
2. Antibody response
2.1. Gender and age
Gender and age differences in vaccine-induced humoral immunity have gained attention over the last years. In general, females develop higher antibody response post-vaccination compared to males, and vaccine responses are diminished in older adults due to immune senescence (Cook, 2008, Fish, 2008, Klein et al., 2010). Here, we explored whether sex and age influence the vaccine-induced SARS-CoV-2 specific antibody response.
Bayram et al. (2021) showed that seropositivity was higher among females (84.6%) than males (70.6%), after the first dose (1D) of CoronaVac. Moreover, Li et al. (2021) evaluated the effectiveness and immunogenicity of the three inactivated COVID-19 vaccines currently in use in China, namely “BBIBP-CorV”, “CoronaVac”, and “WIBP-CorV” in which female participants had significantly higher concentrations of SARS-CoV-2-specific Spike (S) IgG and neutralizing antibodies (NAbs) than male participants. In addition, Fonseca et al. (2022) reported higher seropositivity in females (90.5%) than males (79.4%) after 1D of CoronaVac. Also, antibody levels were superior in females. The median IgG level was 761.6 AU/mL in females and 626.6 AU/mL in males, after 1D, increasing to 1252 and 959.6 AU/mL, respectively, after the second dose (2D). Choudhary et al. (2021) also observed a difference between the genders in BBV-152 (COVAXIN) and Covishield vaccinated, but the difference was not statistically significant. Sex-based differences in the immune response can be explained by hormonal differences. Studies have shown that several immune cells, such as B lymphocytes, contain estrogen receptors regulated by estrogen levels (Kleina et al., 2015, Mukherjee and Pahan, 2021). In fact, estrogen has been shown to promote immunoglobins production, while testosterone can inhibit it (Ruggieri et al., 2016).
Regarding the influence of age, Bayram et al. (2021) reported seropositivity of 85.4%, 68.2%, and 37.5% among HCWs between 18-34 years, 35-59 years, and ≥ 60 years, respectively, following 1D of CoronaVac. According to Li et al. (2021), no obvious correlations between age and the concentrations of antibodies were observed after 2D. In addition, Fonseca et al. (2022) described seropositivity of 92.5% in the 18–30 age group, 88.1% in the 31–50 age group, and 80.4% in the ≥ 51 years group after 1D of CoronaVac. The median of IgG levels was 755.3 in the age group 18–30 years, 742.3 in the age group 31–50 years, and 459.9 AU/mL in the age group ≥ 51 years, following 1D, increasing to 1197, 1213, and 1191, respectively, after 2D. They highlighted that the participants ≥ 51 years exhibited the lowest IgG anti-S titers, mainly following 1D. However, the antibodies reached a level close to the others age group, after 2D. Therefore, the results indicated the benefit of 2D for older people.
Closely, age and sex-based immunological differences contribute to variations in the immune response to mRNA vaccines. Khoury et al. (2021) noticed that participants ≥ 50 years vaccinated with BNT162b2 had lower antibody titers than patients < 30 years: mean titer 14786 ± 15471 AU/mL and 33660 ± 20771 AU/mL, respectively. Moreover, in the participants older, higher titers were seen in females than in males: mean titer 24013 ± 16684 and 8909 ± 7674 AU/mL, respectively. Additionally, Lustig et al. (2021) reported IgG concentrations 1.34–1.57 times higher in HCWs younger than 46 years vaccinated with BNT162b2 when compared with older participants. Farther, the NAbs titers were 2.23–3.92 times higher in the younger group. Moreover, Brisotto et al. (2021) reported that IgG concentration negatively correlated with age, with median values of 716.1 AU/mL for subjects < 30 years and 450.3 AU/mL for individuals ≥ 60 years, but no difference in the antibody titer between men and women was found. Finally, Fujigaki et al. (2022) showed that the IgG titer of females (263.6 ± 158.0 U/mL) was significantly higher than that of males (209.9 ± 137.6 U/mL), after 2D of BNT162b2. Also, they reported that in males, age correlated negatively with IgG (r = − 0.410) responses to SARS-CoV-2 receptor-binding domain (RBD) although antibody titer did not show a correlation with age for females. According to Kim et al. (2022), no significant difference in antibody levels between men and women was observed after BNT162b2 vaccination, although the mean antibody level showed a trend of rapid decrease in the older group over time, reinforcing the importance of booster doses in this group. Regarding the Moderna mRNA-1273 vaccine, de la Monte et al. (2021) observed no significant association between IgG responses and sex or age.
Sex and age also can impact immune responses to viral vector-based vaccines. Mishra et al. (2021) described that women have a 2.19-fold rise in titer between 1D and 2D of AZD1222 vaccine compared to a 1.03-fold rise for men. However, six months after 2D, women exhibited a faster decay in antibody levels at 53% compared to 12% for men. Additionally, persons < 45 years had higher mean titer than people ≥ 45 years at all time points evaluated. Further, Hernández-Bello et al. (2021) associated age with lower levels of NAbs in those vaccinated with the viral vector vaccine Ad5.nCoV. In opposition, Choudhary et al. (2021) observed no statistically significant difference in post-vaccination antibody production and its persistence across gender and age in AZD1222 recipients.
2.2. Previous SARS-CoV-2 infection
Most studies showed the benefit of vaccination in previously infected people although the number of doses required and how this could be modified by vaccine type is still unclear (Karachaliou et al., 2022).
Previously infected HCWs had higher antibody levels after 1D of CoronaVac when compared with HCWs not previously infected. Fonseca et al. (2022) reported that antibody titers were found five times higher in those who had COVID-19 (1512 AU/mL) than those who did not (303.6 AU/mL). Accordingly, Dinc et al. (2022) showed a median antibody titer of 1331.2 AU/mL in participants with prior history of COVID-19 and 48.4 AU/mL in the infection-naive group. Similarly, Bayram et al. (2021) reported that antibody titers were found 3–4 times higher in those who had COVID-19 than those who did not.
Interestingly, the 2D of CoronaVac did not increase the antibody response in HCWs previously infected. According to Fonseca et al. (2022), in the HCWs without previous COVID-19, IgG levels increased 3.5 times after the 2D in relation to 1D. However, in the group of HCWs with previous COVID-19, an increase of anti-S IgG was not observed after 2D. Likewise, Dinc et al. (2022) reported that the median antibody titer was 48.4 AU/mL after 1D in the infection-naive group, which increased to 707.1 AU/mL after 2D. While in participants with prior history of COVID-19, the median antibody titer was 1331.2 and 1090.0 AU/mL, respectively. Thus, HCWs with a history of COVID-19 seem to reach a peak of antibodies already in the 1D (Fonseca et al., 2022). Their previous infections served as a dose of antigen and the 1D of vaccine as a booster dose (Purushotham et al., 2021).
Similarly, SARS-CoV-2 previously infected individuals developed higher antibody titers to the mRNA vaccines than non-pre-exposed individuals. Shields et al. (2021) observed that antibody responses were faster and of greater magnitude in HCWs previously exposed to the SARS-CoV-2 after vaccination with a dose of BNT162b2 compared with non-pre-exposed HCWs. Moreover, Cassaniti et al. (2022) reported seropositivity in 100% of SARS-CoV-2 experienced subjects and in 96.1% of SARS-CoV-2 naive subjects, after 1D with BNT162b2. Also, they related that all the SARS-CoV-2 naive subjects developed a positive IgG anti-RBD response after 2D, although at significantly lower levels. Additionally, Brisotto et al. (2021), detailed that among vaccinated with two doses of BNT162b2, HCWs with previous SARS-CoV-2 infection showed antibody median title of 1072.65 AU/mL, while HCWs without previous infection showed antibody median title of 532.55 AU/mL, one month after 2D. Among those vaccinated with two doses of mRNA-1273, HCWs with and without previous SARS-CoV-2 infection showed antibody median titles 1813.4 and 736.95 AU/mL, respectively. Further, Chivu-Economescu et al. (2022) described that the highest levels of anti‐S after BNT162b2 vaccination were observed in persons who had prior infection.
Unlike naïve individuals, COVID-19-recovered subjects did not mount a recall antibody response upon the second vaccine dose of mRNA. Tejedor Vaquero et al. (2021) showed that previously SARS-CoV-2 infected individuals had a vigorous humoral response after 1D of mRNA-1273. However, 2D did not produce an increase in antibody titers. Likewise, Demonbreun et al. (2021), Ibarrondo et al. (2021), and Samanovic et al. (2022) observed an increased viral neutralization after 1D of BNT162b2 or mRNA-1273 in previously SARS-CoV-2 infected participants while previously uninfected individuals showed a strong NAbs response only after two doses. Closely, Goel et al. (2021), Reynolds et al. (2021), and Stamatatos et al. (2021) showed that a single dose of BNT162b2 or mRNA-1273 in previously infected persons induced a strong antibody response (Ibarrondo et al., 2021). Additionally, Samanovic et al. (2022) pointed out those convalescent individuals showed continuously higher levels of NAbs after 2D of BNT162b2, while uninfected individuals showed no significant difference when compared to levels after the first immunization. According to Torres-Estrella et al. (2022), the strong antibody response after 1D in persons with a history of SARS-CoV-2 infection was a combination of stimulation from natural infection and vaccination. Although, Ibarrondo et al. (2021) highlighted that a single dose would not be sufficient to generate a reliably effective immunological memory against the virus, regardless of the history of infection before vaccination. Therefore, the importance of completing the vaccination cycle for both groups is emphasized.
Like inactivated virus and mRNA vaccines, Hernández-Bello et al. (2021) reported that 100% of individuals with prior COVID-19 developed NAbs after a single dose of Ad5-nCoV vaccine, a non-replicating viral vector vaccine, against 7.4% of individuals without prior COVID-19. Also, Mishra et al. (2021) showed that 2D of AZD1222 (ChAdOx1) did not boost the antibody levels of HCWs with a history of COVID-19. They pointed out that the lack of increase in IgG titer is due to the robust antibody response after a single dose of AZD1222 vaccine in people previously infected (Ebinger et al., 2021, Wei et al., 2021).
2.3. Pre-existing diseases
Some people are immunocompromised because of a medical condition or a treatment for a disease. For this reason, this population has reduced seroconversion to routine vaccines, compared to healthy controls (Broeders et al., 2011, Buti et al., 1992, Peces et al., 1997, Soni et al., 2013). Since immunocompromised patients were excluded from vaccine registration trials, it is still unknown whether they would elaborate a protective antibody response after vaccination. In the literature, the main studies with population immunocompromised involved cancer, dialysis, and transplant patients, therefore, these groups were approached in this section.
2.3.1. Cancer patients
Reduced humoral response to SARS-CoV-2 vaccines was reported in cancer patients. According to Figueiredo et al. (2021), 22.6% of patients did not seroconvert after 2D of the immunizers BNT162b2 or mRNA-1273. Also, antibody levels were lower in patients with cancer (solid tumors: median = 8581 AU/mL; hematologic cancer: median= 1128 AU/mL) compared to healthy controls (median = 11794 AU/mL). Additionally, only 24% of patients with solid tumor and 17% of patients with hematologic malignancy maintained high antibody levels 4–6 months following 2D. Accordingly, Linardou et al. (2021) demonstrated a seropositivity rate of 90.5% in solid tumor cancer patients after vaccination with BNT162b2, mRNA-1273, or AZD1222 and 98% in the healthy group. In addition, the median of antibody titers was significantly higher in controls than in cancer patients (2050 vs. 523 BAU/mL). On the other hand, Palaia et al. (2021) reported no differences between gynecologic cancer patients and healthy controls in terms of antibody titers, one month after the vaccination with BNT162b2. Nonetheless, there was a more rapid trend of reduction over time among cancer patients compared with healthy women, as their titers were significantly lower after 3 months from vaccination. Gounant et al. (2022) evaluated the humoral responses to the SARS-CoV-2 vaccine in patients with thoracic cancer that mostly received BNT162b2. The median serum anti-S IgG titers in the healthy control group were significantly higher than in the cancer group (10594 AU/mL versus 4725 AU/mL, respectively), 6.3% of patients did not seroconvert (<50 AU/mL) and 11% had low antibody titers (< 300 AU/mL) 14 days following 2D. Thereby, patients with low or no antibody titers received a 3D and as a result, 92% of these patients showed substantial increases in IgG anti-S antibodies. Additionally, Shroff et al. (2021) described a threefold increase in Nabs in patients with solid tumors following 3D of the BNT162b2 vaccine. Thereby, 3D administration could contribute to adequate seroprotect in patients who did not seroconvert after 2D.
Age, gender, and smoking habits were associated with a low or negative response to COVID-19 vaccines in cancer patients. Gounant et al. (2022) identified lowest immunization rate in octogenarian cancer patients, after BNT162b2 vaccination. Similarly, Linardou et al. (2021) observed that antibody titers were higher in patients < 50 years (median value <50 years old = 1060 versus ≥50 = 491.5 BAU/mL), after vaccination with BNT162b2, mRNA-1273 or the AZD1222. Additionally, female patients presented higher antibody titers compared to males (median value 665 versus 456 BAU/mL). Moreover, non-smoker cancer patients showed higher antibody titers than smokers (value 632 versus 409.5 BAU/mL). In contrast, natural SARS-CoV-2 infection was a stronger stimulus for the immune response to COVID-19 vaccines in cancer patients. Gounant et al. (2022) observed that previously COVID-19 infected patients showed the highest antibody titers after the 2D of BNT162b2.
Cancer type also influenced the immune responses of patients to COVID-19 vaccination. In accordance with Linardou et al. (2021), patients with small-cell lung cancer (SCLC), colorectal and pancreatic cancer had considerably lower antibody titers as opposed to patients with breast or ovarian cancer, after vaccination with BNT162b2, mRNA-1273 or the AZD1222. Also, the comparison of IgG antibodies levels in the subgroup of patients with breast, non-small cell lung cancer, SCLC, pancreatic, colorectal, and ovarian cancer showed a significant difference among them, with ovarian cancer patients achieving the highest median number of antibodies among all, and those with pancreatic cancer the lowest (p = 0.041). Furthermore, Figueiredo et al. (2021) described those patients with solid tumors achieved and sustained higher levels of humoral response, following two doses of the immunizers BNT162b2 or mRNA-1273, compared to those with hematological malignancies (median = 8581 vs 1128 AU/mL, respectively). In the hematologic malignancies group, multiple myeloma patients had significantly lower antibody levels (median = 794 AU/mL). One explanation is that multiple myeloma is known to be one of the most defective immune conditions among cancers (Pimpinelli et al., 2020) since the dysfunctional tumor plasma cells and bone marrow microenvironment generate an immunosuppressive context characterized by loss of effective antigen presentation, dysregulation of effector cells, and expansion of immunosuppressive cells (Leone et al., 2020).
Cancer treatment can decrease humoral response post-COVID-19 vaccination. Gounant et (2022) associated long-term corticosteroids use with the absence or low antibody levels in patients with thoracic cancer after two doses of BNT162b2 vaccine. According to them, it can probably be explained by either lower total T-cell and CD4 + T-lymphocyte counts or T-cell–specific responses to S protein. Likewise, Figueiredo et al. (2021) showed that in patients with solid tumors, those on immune checkpoint inhibitors (ICI) therapy with or without chemotherapy had lower antibody levels and decayed more rapidly compared to those treated with chemotherapy alone or other therapies. The authors observed that treatment with ICIs before to vaccine eliminates vaccine-induced T-cell responses due to apoptosis of overactivated T cells (Verma et al., 2019). Another relevant point is that although the targeting of PD-1 in cancer by ICIs focuses primarily on its role in T lymphocytes, PD-1 is also expressed in B lymphocytes and PD-1/PD-L1 signaling is a critical part of the immune response that can influence antibody production (Good-Jacobson et al., 2010). Further, Figueiredo et al. (2021) reported that patients with hematologic malignancies who received B-cell therapies had lower IgG anti-S antibody titers compared to those who did not receive this treatment (Figueiredo et al., 2021). Therapies targeting B cells may adversely affect antibody production in response to SARS-CoV-2 vaccination due to the depletion of these cells and/or disruption of their receptor signaling pathway (Ghione et al., 2021). Figueiredo et al. (2021) reported still that the time between vaccine administration and cancer treatments influenced the antibody response following vaccination. Vaccination after four weeks from any type of B-cell therapy generated higher antibody titers compared with earlier vaccination. Also, a drop in antibody levels after 4–6 months of vaccination was more pronounced among patients who were vaccinated after starting ICI compared with before treatment (median = 278 vs. median = 1327 AU/mL respectively). Perhaps this happens due to the different pharmacokinetic of these two agents and the specific types of immune cells targeted in the treatment. This information may help some medical decisions about when to administer vaccines or boosters to cancer patients.
2.3.2. Dialysis patients
The studies showed that most Dialysis patients (DP) were able to produce an immune response after 2D of BNT162b2 vaccine. Bachelet et al. (2021) reported that 94,3% of patients became seropositive for SARS-CoV-2 anti-S IgG, 30 days after 2D. In addition, they demonstrated that the adjunction of 3D increased the seropositivity to 98% (Bachelet et al., 2021). Moreover, Duni et al. (2021) announced that 91.8% of DP and 29.6% of kidney transplant recipients became seropositive, 2 weeks after 2D. Likewise, Jahn et al. (2021) pointed out those two weeks after 2D, 93% of DP tested positive for SARS-CoV-2 IgG. Similarly, Schrezenmeier et al. (2021) described that 88.9% of DP developed SARS-CoV-2 IgG after complete vaccination. Further, Speer et al. (2021) reported that 95% of peritoneal dialysis patients, 88% of hemodialysis patients, and 100% of healthy controls had detectable anti-S IgG, 12 weeks following two doses of vaccine. However, they detailed that the anti-S level was significantly lower in hemodialysis patients compared with peritoneal dialysis patients or healthy controls with a median of 7.0, 21.8 and 134.9 AU/mL, respectively (Speer et al., 2021). Finally, Weigert et al. (2021) described 91% and 95% of seropositivity in hemodialysis patients and controls, respectively, 21 days post-2D.
The antibody response to SARS-CoV-2 vaccination waned rapidly in DP. Schrezenmeier et al. (2021) reported that the proportion of DP with detectable IgG antibodies decreased from 88.9% to 84.37%, 10 weeks after 2D of BNT162b2. Similarly, Weigert et al. (2021) observed a reduction in anti-S levels only four months after 2D of BNT162b2 vaccine. They highlighted that IgG levels decreased even more approximately 5 months following 2D, increasing seronegativity from 9% to > 30% (Weigert et al., 2021). Likewise, Anand et al. (2022) reported that among vaccinated patients with mRNA-1273, BNT162b2 e Ad26. COV2, an undetectable IgG response increased from 6.6% (range 14–30 days) after vaccination to 20.2% at 5–6 months after vaccination. In addition, Speer et al. (2021) detailed those twelve weeks after 2D the levels of Nabs significantly decreased from 87% to 79% in hemodialysis patients and from 93% to 85% in peritoneal dialysis patients, respectively. The fast decay of antibodies in DP can explain the number of breakthrough infections higher in this group when compared to healthy cohorts, as reported by Anand et al. (2022). Together, these data reinforce the importance of conducting longitudinal studies on this DP after COVID-19 vaccination.
Factors associated with a high or low response to COVID-19 immunization in DP have been described. Bachelet et al. (2021) showed that the immunocompromised status was associated with the absence of seroconversion in DP vaccinated with BNT162b2. They also identified age, nutritional status, chronic inflammation, and low lymphocyte count with a low response to vaccine (IgG<500 AU/mL) (Bachelet et al., 2021). Accordingly, Weigert et al. (2021) detailed lower antibody response in participants ≤ 70 years with immunosuppressive therapies compared to controls, after 1D of BTN162b2. Moreover, Speer et al. (2021) reported that older age negatively affected anti-S IgG concentration to BNT162b2. On the other hand, previous SARS-COV-2 infection was associated with high response (IgG>7000 AU/mL) to vaccination (Bachelet et al., 2021).
Several studies have compared the antibody response across different vaccines. In the work of Mahallawi Ibrahim Mumena (2021) it was observed that the BNT162b2 vaccine was superior to ChAdOx1 (AstraZeneca) in maintaining higher anti-S IgG levels (0.41 ± 0.94 vs 0.03 ± 0.30, respectively) in DP. On the other hand, Anand et al. (2022) analyzed the humoral response after complete vaccination in a cohort of DP vaccinated with mRNA-1273, BNT162b2, and Ad26. COV2. This study showed that those vaccinated with mRNA-1273 had higher mean IgG levels and a lower proportion of subjects without an IgG response than those receiving BNT162b2 due to the higher mRNA dose in the mRNA1273 formulation (Anand et al., 2022). Regarding the Ad26. COV2. S vaccine, Anand et al. (2022) noticed that a single dose did not produce a detectable antibody response in more than half of the patients, but the number of patients who received this vaccine was small. Therefore, caution is suggested in the interpretations of response comparisons with this vaccine (Anand et al., 2022).
2.3.3. Transplant patients
Reduced humoral response to SARS-CoV-2 was reported in solid organ recipients vaccinated with mRNA COVID-19 vaccines. Dębska-ślizień et al. (2021)analyzed the antibody response in kidney transplant recipients vaccinated with BNT162b2 or mRNA-1273 compared to volunteers without kidney disease, both without evidence of previous COVID-19 infection. Seroconversion was seen in 51.41% of transplant recipients and in 100% of healthy volunteers, after the 2D. Also, the median antibody titers of kidney transplant recipients were eight times lower than healthy volunteers (Dębska-ślizień et al., 2021). Similarly, Guarino et al. (2022) evaluated the immunogenicity of the BNT162b2 vaccine in liver transplant recipients and healthy controls, measuring IgG anti-S titers. After three months of the 2D, the seroconversion rate was 75% in transplanted patients and 97.72% in the control group. In addition, liver transplant recipients had statistically lower titers than the control group.
Several factors were associated with a negative or low response to mRNA COVID-19 vaccines in transplant patients. Debska-Ślizien et al. (2021) reported that the seroconversion rate in kidney transplant recipients was higher among the younger patients aged (<54 years), with the longest duration of transplantation (>8 years), treated with no more than two immunosuppressants, without corticosteroids or mycophenolate mofetil/Na (MMF/MPS) treatment, and vaccinated with mRNA-1273 vaccine. . Similarly, Guarino et al. (2022) pointed out that older age (>40 years), shorter time from liver transplantation (<5 years), and immunosuppressive regimens with multiple drugs or with antimetabolites were significantly associated with non-response to vaccination (Guarino et al., 2022). Moreover, Piñana et al. (2022) showed that among the recipients of allogeneic hematopoietic cell transplantation (allo-HCT), vaccination within one year after transplantation, the development of graft-versus-host disease (GVHD), and lymphopenia (< 1 × 109/mL) were factors related to low humoral detection. Lymphopenia was also observed in another study as a risk factor for an inadequate response after 2D of BNT162b2 vaccine for this same group of patients (Redjoul et al., 2021). Furthermore, among the recipients of autologous hematopoietic stem cells, patients diagnosed with non-Hodgkin's lymphoma and those on corticosteroid therapy showed low antibody detections (Piñana et al., 2022). Similarly, Huang et al. (2022) found a difference regarding the time interval between transplantation and vaccination. Patients vaccinated with BNT162b2 or mRNA-1273 whose transplantation occurred 3–6 months and 6–12 months prior to vaccination produced significantly lower antibody titers than 12 months post-transplant patients and the healthy controls group. In stratified analysis, allogeneic recipients older than 65 years, on immunosuppression for prevention or treatment of acute GVHD and those with relapsed disease also had low humoral immune responses to the vaccine. In contrast, the intensity of the conditioning regimen, underlying disease (myeloid, lymphoid, or other leukemia), and the presence of chronic GVHD treatment had no impact on antibody levels. Together, the studies pointed out age, the interval between the time of transplantation and vaccination, and the use of corticoids as the main factors that influenced the immune response post-COVID-19 vaccination in transplant patients. As discussed earlier, aging is associated with declines in adaptive and innate immunity (Crooke et al., 2019). The negative correlation between shorter time from transplantation and non-response to vaccination can be explained because of the higher level of immunosuppression immediately post-transplant that is softened over time, allowing the reconstitution of immunological capacity (Debska-Slizien et al., 2022, Guarino et al., 2022) and corticoids are immunosuppressive agents.
COVID-19 mRNA vaccination after natural SARS-CoV-2 infection was a stronger stimulus for the immune response in transplant patients. Tylicki et al. (2021) showed a seroconversion rate of 100% in vaccinated kidney transplant recipients previously infected with SARS-CoV-2 compared to 45.78% in recipients never infected, after 2D of BNT162b2 or mRNA-1273. However, they detailed that decay of the antibodies post-2D was faster in the previously infected group compared to the non-infected group.
Antibody titers were higher in transplant recipients who received mRNA-1273 when compared to BNT162b2 recipients. Debska-Ślizien et al. (2021) revealed that the seroconversion rates and the magnitude of the antibody titer were significantly higher after 2D of the mRNA-1273 vaccine compared to BNT162b2. Similar findings were shown by Tylicki et al. (2021) where after 3D, 66% of patients vaccinated with BNT162b2 and 82.6% of patients vaccinated with mRNA-1273 seroconverted with a median anti-S titer of 384.5 and 1620 BAU/mL, respectively. As mentioned before, one explanation for the higher mRNA-1273 vaccine immunogenicity may be the three-times-higher dose of mRNA. Thus, vaccines with a higher dose of mRNA can be the better choice in immunocompromised individuals.
Administration of the third dose boosted the immune response to SARS-CoV-2 in transplant recipients. Massa et al. (2021) and Tylicki et al. (2021) evaluated the immunogenicity in kidney transplant recipients that received three doses of the BNT162b2 vaccine. According to Massa et al. (2021) the seroconversion rate increased of 44.3% after 2D to 62.3% after 3D. Also, the antibody levels boosted about 5–4 times, and a better serum-neutralizing activity was observed following 3D. Likewise, Tylicki et al. (2021) pointed out that fewer than 50% of transplant patients showed any antibody-mediate response following the primary vaccination. However, with 3D, seroconversion was reached in almost half of the patients in whom the primary vaccination failed. Further, antibody titers were 30% higher than that after 2D. It is important to highlight that 37.7% of patients in the study by Massa et al. (2021) and 21.42% of the study by Tylicki et al. (2021) remained seronegative, even after 3D. This finding revealed the need for alternative strategies to improve COVID-19 immunization in this population.
3. Conclusion
The studies showed a negative correlation between age and immune response after immunization. In most studies, females showed a more robust immune response to COVID-19 vaccination than males. Prior SARS-CoV-2 infection influenced positively the humoral response induced by COVID-19 vaccines. Prior infected healthy individuals already exhibited high antibody levels after the first dose. The second dose of vaccines did not increase the antibody response on them. The studies with immunosuppressed patients showed a reduced antibody response to COVID-19 vaccination and the influence of additional factors in this response as therapy and timing of treatment relative to vaccination. The benefits of the third dose in this population were highlighted.
CRediT authorship contribution statement
M.C.R.F, G.S.V., A.C.L.M., T.C.M., L.F.C and M.H.G.F conceived, design of the study and performed the analysis and interpretation of data. M.H.G.F and F.M.C.A. reviewed critically the intellectual content. M.C.R.F, G.S.V., A.C.L.M., T.C.M., L.F.C, M.H.G.F and F.M.C.A approved the final version to be submitted.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The project is funded by Fiocruz and Ministério da Saúde, Brazil.
The authors would like to thank Manoela Rebouças and Maria Claudia dos Santos Luciano for helping review the design of the graphical abstract presented in the manuscript.
Data Availability
Data will be made available on request.
References
- Anand S., Montez-Rath M.E., Han J., Garcia P., Cadden L., Hunsader P., Morgan C., Kerschmann R., Beyer P., Dittrich M., Block G.A., Chertow G.M., Parsonnet J. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann. Intern. Med. 2022;175:371–378. doi: 10.7326/M21-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelet T., Bourdenx J.P., Martinez C., Mucha S., Martin-Dupont P., Perier V., Pommereau A. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS One. 2021:16. doi: 10.1371/JOURNAL.PONE.0257646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram A., Demirbakan H., Günel Karadeniz P., Erdoğan M., Koçer I. Quantitation of antibodies against SARS‐CoV‐2 spike protein after two doses of CoronaVac in healthcare workers. J. Med. Virol. 2021;93:5560–5567. doi: 10.1002/JMV.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisotto G., Muraro E., Montico M., Corso C., Evangelista C., Casarotto M., Caffau C., Vettori R., Cozzi M.R., Zanussi S., Turetta M., Ronchese F., Steffan A. IgG antibodies against SARS-CoV-2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin. Chim. Acta. 2021;523:476. doi: 10.1016/J.CCA.2021.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeders N.E., Hombrouck A., Lemy A., Wissing K.M., Racapé J., Gastaldello K., Massart A., van Gucht S., Weichselbaum L., de Mul A., Brochier B., Thomas I., Abramowicz D. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: a cohort study. Clin. J. Am. Soc. Nephrol. 2011;6:2573–2578. doi: 10.2215/CJN.04670511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buti Maria, Viladomiu L., Jardi R., Olmos A., Rodriguez J.A., Bartolome J., Esteban R., Guardia J., Buti M. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am. J. Nephrol. 1992;12:144–147. doi: 10.1159/000168436. [DOI] [PubMed] [Google Scholar]
- Cassaniti I., Bergami F., Percivalle E., Gabanti E., Sammartino J.C., Ferrari A., Adzasehoun K.M.G., Zavaglio F., Zelini P., Comolli G., Sarasini A., Piralla A., Ricciardi A., Zuccaro V., Maggi F., Novazzi F., Simonelli L., Varani L., Lilleri D., Baldanti F. Humoral and cell-mediated response against SARS-CoV-2 variants elicited by mRNA vaccine BNT162b2 in healthcare workers: a longitudinal observational study. Clin. Microbiol. Infect. 2022;28(301) doi: 10.1016/J.CMI.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivu-Economescu M., Bleotu C., Grancea C., Chiriac D., Botezatu A., Iancu I. v, Pitica I., Necula L.G., Neagu A., Matei L., Dragu D., Sultana C., Radu E.L., Nastasie A., Voicu O., Ataman M., Nedeianu S., Mambet C., Diaconu C.C., Ruta S.M. Kinetics and persistence of cellular and humoral immune responses to SARS‐CoV‐2 vaccine in healthcare workers with or without prior COVID‐19. J. Cell. Mol. Med. 2022;26:1293. doi: 10.1111/JCMM.17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary H.R., Parai D., Chandra Dash G., Kshatri J.S., Mishra N., Choudhary P.K., Pattnaik D., Panigrahi K., Behera S., Ranjan Sahoo N., Podder S., Mishra A., Raghav S.K., Mishra S.K., Pradhan S.K., Sahoo S.K., Pattnaik M., Rout U.K., Nanda R.R., Mondal N., Kanungo S., Palo S.K., Bhattacharya D., Pati S. Persistence of antibodies against spike glycoprotein of SARS-CoV-2 in healthcare workers post double dose of BBV-152 and AZD1222 vaccines. Front. Med. 2021;8 doi: 10.3389/FMED.2021.778129/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/J.VACCINE.2008.04.054. [DOI] [PubMed] [Google Scholar]
- Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing. 2019;2019 16(1 16):1–16. doi: 10.1186/S12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S.M., Long C., Szczepanski N., Griffin C., Fitzgerald A., Chapin K. Heterogeneous longitudinal antibody responses to Covid-19 mRNA vaccination. Clin. Pathol. 2021:14. doi: 10.1177/2632010X211049255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debska-Slizien A., Muchlado M., Slizien Z., Kubanek A., Piotrowska M., Dabrowska M., Bzoma B., Konopa J., Renke M., Biedunkiewicz B., Tylicki L. Significant humoral response to mRNA COVID-19 vaccine in kidney transplant recipients with prior exposure to SARS-CoV-2: the COViNEPH project. Pol. Arch. Intern. Med. 2022:132. doi: 10.20452/PAMW.16142. [DOI] [PubMed] [Google Scholar]
- Dębska-ślizień A., Ślizień Z., Muchlado M., Kubanek A., Piotrowska M., Dabrowska˛ M., Tarasewicz A., Chamienia A., Biedunkiewicz B., Renke M., Tylicki L. Predictors of humoral response to mRNA COVID19 vaccines in kidney transplant recipients: a longitudinal study—the COViNEPH project. Vaccines. 2021;Vol. 9:1165. doi: 10.3390/VACCINES9101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun A.R., Sancilio A., Velez M.P., Ryan D.T., Saber R., Vaught L.A., Reiser N.L., Hsieh R.R., D’Aquila R.T., Mustanski B., McNally E.M., McDade T.W. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021:38. doi: 10.1016/j.eclinm.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinc H.O., Saltoglu N., Can G., Balkan I.I., Budak B., Ozbey D., Caglar B., Karaali R., Mete B., Tuyji Tok Y., Ersoy Y., Ahmet Kuskucu M., Midilli K., Ergin S., Kocazeybek B.S. Inactive SARS-CoV-2 vaccine generates high antibody responses in healthcare workers with and without prior infection. Vaccine. 2022;40:52. doi: 10.1016/J.VACCINE.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duni A., Markopoulos G.S., Mallioras I., Pappas H., Pappas E., Koutlas V., Tzalavra E., Baxevanos G., Priska S., Gartzonika K., Mitsis M., Dounousi E. The humoral immune response to BNT162b2 vaccine is associated with circulating CD19+ B lymphocytes and the Naïve CD45RA to memory CD45RO CD4+ T helper cells ratio in hemodialysis patients and kidney transplant recipients. Front. Immunol. 2021:12. doi: 10.3389/FIMMU.2021.760249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., van Eyk J.E., Braun J.G., Cheng S., Sobhani K. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981. doi: 10.1038/S41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo J.C., Merin N.M., Hamid O., Choi S.Y., Lemos T., Cozen W., Nguyen N., Finster L.J., Foley J., Darrah J., Gong J., Paquette R., Mita A.C., Vescio R., Mehmi I., Basho R., Tourtellotte W.G., Huynh C.A., Melmed G.Y., Braun J., McGovern D.P.B., Mengesha E., Botwin G., Prostko J.C., Frias E.C., Stewart J.L., Joung S., van Eyk J., Ebinger J.E., Cheng S., Sobhani K., Reckamp K.L., Merchant A. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Cancer Res. 2021;81:6273–6280. doi: 10.1158/0008-5472.CAN-21-3554/670658/AM/LONGITUDINAL-SARS-COV-2-MRNA-VACCINE-INDUCED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/NRI2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M.H.G., de Souza T., de F.G., de Carvalho Araújo F.M., de Andrade L.O.M. Dynamics of antibody response to CoronaVac vaccine. J. Med. Virol. 2022;94:2139–2148. doi: 10.1002/JMV.27604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigaki H., Yamamoto Y., Koseki T., Banno S., Ando T., Ito H., Fujita T., Naruse H., Hata T., Moriyama S., Takahashi Y., Suzuki T., Murakami T., Yoshida Y., Yagura Y., Oyamada T., Takemura M., Kondo M., Iwata M., Saito K. Antibody responses to BNT162b2 vaccination in Japan: monitoring vaccine efficacy by measuring IgG antibodies against the receptor-binding domain of SARS-CoV-2. Microbiol. Spectr. 2022:10. doi: 10.1128/SPECTRUM.01181-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghione P., Gu J.J., Attwood K., Torka P., Goel S., Sundaram S., Mavis C., Johnson M., Thomas R., McWhite K., Darrall A., DeMarco J., Kostrewa J., Mohr A., Rivas L., Neiders M., Suresh L., Segal B.H., Griffiths E.A., Ramsperger V., Shen L., Hernandez-Ilizaliturri F.J. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138:811–814. doi: 10.1182/BLOOD.2021012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., Dysinger S., Lundgreen K.A., Kuri-Cervantes L., Adamski S., Hicks A., Korte S., Oldridge D.A., Baxter A.E., Giles J.R., Weirick M.E., McAllister C.M., Dougherty J., Long S., D’Andrea K., Hamilton J.T., Betts M.R., Luning Prak E.T., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:1–19. doi: 10.1126/SCIIMMUNOL.ABI6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ochoa S.A., Franco O.H., Rojas L.Z., Raguindin P.F., Roa-Díaz Z.M., Wyssmann B.M., Guevara S.L.R., Echeverría L.E., Glisic M., Muka T. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am. J. Epidemiol. 2021;190:161–175. doi: 10.1093/AJE/KWAA191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson K.L., Szumilas C.G., Chen L., Sharpe A.H., Tomayko M.M., Shlomchik M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010;2010 11(6 11):535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounant V., Ferré V.M., Soussi G., Charpentier C., Flament H., Fidouh N., Collin G., Namour C., Assoun S., Bizot A., Brouk Z., Vicaut E., Teixeira L., Descamps D., Zalcman G. Efficacy of severe acute respiratory syndrome coronavirus-2 vaccine in patients with thoracic cancer: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J. Thorac. Oncol. 2022;17:239–251. doi: 10.1016/J.JTHO.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/SCIENCE.ABB8923/ASSET/94AE5ABC-0C84-401A-815D-AB7FAA82F4FE/ASSETS/GRAPHIC/368_945_F2.JPEG. [DOI] [PubMed] [Google Scholar]
- Guarino, M., Esposito, I., Portella, G., Cossiga, V., Loperto, I., Tortora, R., Cennamo, M., Capasso, M., Terracciano, D., Lanza, A.G., Somma, S. di, Picciotto, F.P., Morisco, F., 2022. Humoral Response to 2-dose BNT162b2 mRNA COVID-19 Vaccination in Liver Transplant Recipients. https://doi.org/10.1016/j.cgh.2022.01.012. [DOI] [PMC free article] [PubMed]
- Hernández-Bello J., Morales-Núñez J.J., Machado-Sulbarán A.C., Díaz-Pérez S.A., Torres-Hernández P.C., Balcázar-Félix P., Gutiérrez-Brito J.A., Lomelí-Nieto J.A., Muñoz-Valle J.F. Neutralizing antibodies against sars-cov-2, anti-ad5 antibodies, and reactogenicity in response to ad5-ncov (Cansino biologics) vaccine in individuals with and without prior sars-cov-2. Vaccines. 2021:9. doi: 10.3390/vaccines9091047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021 doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., Cicin-Sain C., Pasin C., Epp S., Audigé A., Müller N.J., Nilsson J., Bankova A., Wolfensberger N., Vilinovszki O., Nair G., Hockl P., Schanz U., Kouyos R.D., Hasse B., Zinkernagel A.S., Trkola A., Manz M.G., Abela I.A., Müller A.M.S. Antibody response to SARS-CoV-2 vaccination in patients following allogeneic hematopoietic cell transplantation. Transpl. Cell Ther. 2022;28(214) doi: 10.1016/J.JTCT.2022.01.019. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo F.J., Hofmann C., Fulcher J.A., Goodman-Meza D., Mu W., Hausner M.A., Ali A., Balamurugan A., Taus E., Elliott J., Krogstad P., Tobin N.H., Ferbas K.G., Kitchen S.G., Aldrovandi G.M., Rimoin A.W., Yang O.O. Primary, recall, and decay kinetics of SARS-CoV-2 vaccine antibody responses. ACS Nano. 2021;15:11180–11191. doi: 10.1021/acsnano.1c03972. [DOI] [PubMed] [Google Scholar]
- Jahn M., Korth J., Dorsch O., Anastasiou O.E., Sorge-Hädicke B., Tyczynski B., Gäckler A., Witzke O., Dittmer U., Dolff S., Wilde B., Kribben A. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines. 2021:9. doi: 10.3390/VACCINES9040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachaliou M., Moncunill G., Espinosa A., Castaño-Vinyals G., Rubio R., Vidal M., Jiménez A., Prados E., Carreras A., Cortés B., Blay N., Bañuls M., Pleguezuelos V., Melero N.R., Serra P., Parras D., Izquierdo L., Santamaría P., Carolis C., Papantoniou K., Goldberg X., Aguilar R., Garcia-Aymerich J., de Cid R., Kogevinas M., Dobaño C. SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: a cohort study in Catalonia. BMC Med. 2022:20. doi: 10.1186/S12916-022-02547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury J., Najjar-Debbiny R., Hanna A., Jabbour A., Abu Ahmad Y., Saffuri A., Abu-Sinni M., Shkeiri R., Elemy A., Hakim F. COVID-19 vaccine – long term immune decline and breakthrough infections. Vaccine. 2021;39:6984. doi: 10.1016/J.VACCINE.2021.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914. doi: 10.1016/J.CELL.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Yun H.J., Kim J., Kym S., Choi Q. Antibody response to second dose of the BNT162b2 mRNA vaccine in the first 12 weeks in South Korea: a prospective longitudinal study. Vaccine. 2022;40:437. doi: 10.1016/J.VACCINE.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleina S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med Hyg. 2015;109:9–15. doi: 10.1093/TRSTMH/TRU167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone P., Solimando A.G., Malerba E., Fasano R., Buonavoglia A., Pappagallo F., de Re V., Argentiero A., Silvestris N., Vacca A., Racanelli V. Actors on the scene: Immune cells in the myeloma niche. Front. Oncol. 2020;10:1–15. doi: 10.3389/FONC.2020.599098/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/JMV.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xiang T., Liang B., Deng H., Wang H., Feng X., Quan X., Wang X., Li S., Lu S., Yang X., Wang B., Zelinskyy G., Trilling M., Sutter K., Lu M., Dittmer U., Yang D., Zheng X., Liu J. Characterization of SARS-CoV-2-specific humoral and cellular immune responses induced by inactivated COVID-19 vaccines in a real-world setting. Front Immunol. 2021:12. doi: 10.3389/fimmu.2021.802858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardou H., Spanakis N., Koliou G.-A., Christopoulou A., Karageorgopoulou S., Alevra N., Vagionas A., Tsoukalas N., Sgourou S., Fountzilas E., Sgouros J., Razis E., Chatzokou D., Lampaki S., Atanackovic D. Responses to SARS-CoV-2 vaccination in patients with cancer (ReCOVer Study): a prospective cohort study of the hellenic cooperative oncology group. Zacharenia Saridaki. 2021;11:4621. doi: 10.3390/cancers13184621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., Indenbaum V., Mandelboim M., Doolman R., Amit S., Mendelson E., Ziv A., Huppert A., Rubin C., Freedman L., Kreiss Y. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021;9:999. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W.H., Ibrahim N.A., Mumena W.A. Effectiveness of COVID-19 vaccines in patients under maintenance hemodialysis. Risk Manag. Health Policy. 2021;14:5081–5088. doi: 10.2147/RMHP.S345686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J.A., Mulla A.H., Farooqi T., Pottoo F.H., Anwar S., Rengasamy K.R.R. Targets and strategies for vaccine development against SARS-CoV-2. Biomed. Pharm. 2021:137. doi: 10.1016/J.BIOPHA.2021.111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F., Cremoni M., Gérard A., Grabsi H., Rogier L., Blois M., Couzin C., Hassen N., ben, Rouleau M., Barbosa S., Martinuzzi E., Fayada J., Bernard G., Favre G., Hofman P., Esnault V.L.M., Czerkinsky C., Seitz-Polski B., Glaichenhaus N., Sicard A. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73 doi: 10.1016/J.EBIOM.2021.103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina N.L., Zimmermann P., Curtis N. The impact of vaccines on heterologous adaptive immunity. Clin. Microbiol. Infect. 2019;25:1484–1493. doi: 10.1016/J.CMI.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Meyer C.U., Zepp F. Methods in Molecular Biology. Humana,; New York, NY: 2022. Principles in immunology for the design and development of vaccines. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Pradhan S.K., Pati S., Sahu S., Nanda R.K. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: a prospective longitudinal study. Cureus. 2021:13. doi: 10.7759/CUREUS.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Pahan K. Is COVID-19 gender-sensitive? J. Neuroimmune Pharmacol. 2021;16:38–47. doi: 10.1007/S11481-020-09974-Z/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaia, I., Caruso, G., Donato, V. di, Vestri, A., Napoli, A., Perniola, G., Casinelli, M., Fegatelli, D.A., Campagna, R., Tomao, F., D’aniello, D., Antonelli, G., Muzii, L., 2021. Pfizer-BioNTech COVID-19 Vaccine in Gynecologic Oncology Patients: A Prospective Cohort Study. https://doi.org/10.3390/vaccines10010012. [DOI] [PMC free article] [PubMed]
- Peces R., de La Torre M., Alcázar R., Urra J.M. Prospective analysis of the factors influencing the antibody response to hepatitis B vaccine in hemodialysis patients. Am. J. Kidney Dis. 1997;29:239–245. doi: 10.1016/S0272-6386(97)90036-6. [DOI] [PubMed] [Google Scholar]
- Pimpinelli F., Marchesi F., Piaggio G., Giannarelli D., Papa E., Falcucci P., Pontone M., Martino S., di, Laquintana V., Malfa A., la, Domenico G., di, di Bella O., Falzone G., Ensoli F., Vujovic B., Morrone A., Ciliberto G., Mengarelli A. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J. Hematol. Oncol. 2020;14:81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñana J.L., López-Corral L., Martino R., Montoro J., Vazquez L., Pérez A., Martin-Martin G., Facal-Malvar A., Ferrer E., Pascual M.J., Sanz-Linares G., Gago B., Sanchez-Salinas A., Villalon L., Conesa-Garcia V., Olave M.T., López-Jimenez J., Marcos-Corrales S., García-Blázquez M., Garcia-Gutiérrez V., Hernández-Rivas J.Á., Saus A., Espigado I., Alonso C., Hernani R., Solano C., Ferrer-Lores B., Guerreiro M., Ruiz-García M., Muñoz-Bellido J.L., Navarro D., Cedillo A., Sureda A. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: prospective survey from the Spanish hematopoietic stem cell transplantation and cell therapy group. Am. J. Hematol. 2022;97:30–42. doi: 10.1002/AJH.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham J.N., van Doremalen N., Munster V.J. SARS-CoV-2 vaccines: anamnestic response in previously infected recipients. Cell Res. 2021;31:827. doi: 10.1038/S41422-021-00516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redjoul R., le Bouter A., Beckerich F., Fourati S., Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398:298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., Butler D.K., Otter A.D., Menacho K., Fontana M., Smit A., Sackville-West J.E., Cutino-Moguel T., Maini M.K., Chain B., Noursadeghi M., Brooks T., Semper A., Manisty C., Treibel T.A., Moon J.C., Valdes A.M., McKnight Á., Altmann D.M., Boyton R. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/SCIENCE.ABH1282/SUPPL_FILE/ABH1282_TABLES5.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A., Anticoli S., D’ambrosio A., Giordani L., Viora M. Monographic section the influence of sex and gender on immunity, infection and vaccination. Ann. Ist. Super. Sanità. 2016;52:198–204. doi: 10.4415/ANN_16_02_11. [DOI] [PubMed] [Google Scholar]
- Samanovic M.I., Cornelius A.R., Gray-Gaillard S.L., Richard Allen J., Karmacharya T., Wilson J.P., Wesley Hyman S., Tuen M., Koralov S.B., Mulligan M.J., Sedaghat Herati R. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. Sci. Transl. Med. 2022 doi: 10.1126/scitranslmed.abi8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeier E., Bergfeld L., Hillus D., Lippert J.D., Weber U., Tober-Lau P., Landgraf I., Schwarz T., Kappert K., Stefanski A.L., Sattler A., Kotsch K., Dörner T., Sander L.E., Budde K., Halleck F., Kurth F., Corman V.M., Choi M. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front. Immunol. 2021:12. doi: 10.3389/FIMMU.2021.690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A.M., Faustini S.E., Kristunas C.A., Cook A.M., Backhouse C., Dunbar L., Ebanks D., Emmanuel B., Crouch E., Kröger A., Hirschfeld J., Sharma P., Jaffery R., Nowak S., Gee S., Drayson M.T., Richter A.G., Dietrich T., Chapple I.L.C. COVID-19: seroprevalence and vaccine responses in UK dental care professionals. J. Dent. Res. 2021;100:1220. doi: 10.1177/00220345211020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M. v, Peyton K.L., Uhrlaub J.L., Ripperger T.J., Jergović M., Dalgai S., Wolf A., Whitmer R., Hammad H., Carrier A., Scott A.J., Nikolich-Žugich J., Worobey M., Sprissler R., Dake M., LaFleur B.J., Bhattacharya D. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021;2021 27(11 27):2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R., Horowitz B., Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin. Dial. 2013;26:416–426. doi: 10.1111/SDI.12101. [DOI] [PubMed] [Google Scholar]
- Speer C., Schaier M., Nusshag C., Töllner M., Buylaert M., Kälble F., Reichel P., Grenz J., Süsal C., Zeier M., Schnitzler P., Morath C., Klein K., Benning L. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines. 2021:9. doi: 10.3390/VACCINES9101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., Feng J., Mize G., de Rosa S.C., Finzi A., Lemos M.P., Cohen K.W., Moodie Z., McElrath M.J., McGuire A.T. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. doi: 10.1126/SCIENCE.ABG9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor Vaquero S., de Campos-Mata L., Ramada J.M., Díaz P., Navarro-Barriuso J., Ribas-Llaurado C., Rodrigo Melero N., Carolis C., Cerutti A., Gimeno R., Magri G. The mRNA-1273 vaccine induces cross-variant antibody responses to SARS-CoV-2 with distinct profiles in individuals with or without pre-existing immunity. Front. Immunol. 2021:12. doi: 10.3389/fimmu.2021.737083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Estrella C.U., Reyes-Montes M.D.R., Duarte-Escalante E., Martínez M.S., Frías-De-león M.G., Acosta-Altamirano G. Vaccines against COVID-19: a review. Vaccines. 2022:10. doi: 10.3390/VACCINES10030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylicki L., Dębska-ślizień A., Muchlado M., Ślizień Z., Gołębiewska J., Dąbrowska M., Biedunkiewicz B. Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines. 2021;Vol. 10:56. doi: 10.3390/VACCINES10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V., Shrimali R.K., Ahmad S., Dai W., Wang H., Lu S., Nandre R., Gaur P., Lopez J., Sade-Feldman M., Yizhak K., Bjorgaard S.L., Flaherty K.T., Wargo J.A., Boland G.M., Sullivan R.J., Getz G., Hammond S.A., Tan M., Qi J., Wong P., Merghoub T., Wolchok J., Hacohen N., Janik J.E., Mkrtichyan M., Gupta S., Khleif S.N. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 2019;2019 20(9 20):1231–1243. doi: 10.1038/s41590-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praet J.T., Vandecasteele S., de Roo A., Vynck M., de Vriese A.S., Reynders M. Dynamics of the cellular and humoral immune response after BNT162b2 messenger ribonucleic acid coronavirus disease 2019 (COVID-19) vaccination in COVID-19-naive nursing home residents. J. Infect. Dis. 2021;224:1690–1693. doi: 10.1093/INFDIS/JIAB458. [DOI] [PubMed] [Google Scholar]
- Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., Bell J.I., Newton J.N., Farrar J., Diamond I., Rourke E., Howarth A., Marsden B.D., Hoosdally S., Jones E.Y., Stuart D.I., Crook D.W., Peto T.E.A., Pouwels K.B., Eyre D.W., Walker A.S., Lambert A., Thomas T., Black R., Felton A., Crees M., Jones J., Lloyd L., Sutherland E., Pritchard E., Vihta K.D., Doherty G., Kavanagh J., Chau K.K., Hatch S.B., Ebner D., Ferreira L.M., Christott T., Dejnirattisai W., Mongkolsapaya J., Cameron S., Tamblin-Hopper P., Wolna M., Brown R., Cornall R., Screaton G., Lythgoe K., Bonsall D., Golubchik T., Fryer H., Cox S., Paddon K., James T., House T., Robotham J., Birrell P., Jordan H., Sheppard T., Athey G., Moody D., Curry L., Brereton P., Jarvis I., Godsmark A., Morris G., Mallick B., Eeles P., Hay J., VanSteenhouse H., Lee J. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021;6:1140. doi: 10.1038/S41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A., Bergman M.L., Gonçalves L.A., Godinho I., Duarte N., Abrantes R., Borges P., Brennand A., Malheiro V., Matoso P., Akpogheneta O., Kosack L., Cruz P., Nogueira E., Pereira M., Ferreira A., Marques M., Nunes T., Faro-Viana J., Demengeot J., Penha-Gonçalves C. Longitudinal analysis of antibody responses to the mRNA BNT162b2 vaccine in patients undergoing maintenance hemodialysis: a 6-month follow-up. Front. Med. 2021:8. doi: 10.3389/FMED.2021.796676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHOCoronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data [WWW Document], n.d. URL https://covid19.who.int/ (accessed 1.22.23).
- WHO, 2022. WHO Coronavirus (COVID-19) Dashboard [WWW Document]. URL https://covid19.who.int/ (accessed 6.8.22).
- Yaugel-Novoa M., Bourlet T., Paul S. Role of the humoral immune response during COVID-19: guilty or not guilty? Mucosal Immunol. 2022;15:1170–1180. doi: 10.1038/S41385-022-00569-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesudhas D., Srivastava A., Gromiha M.M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49:199–213. doi: 10.1007/S15010-020-01516-2/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.