Abstract
Objective:
To determine the role of CD38, which can function as an enzyme to degrade nicotinamide adenosine dinucleotide (NAD+), in osteoarthritis (OA) development.
Methods:
Human knee cartilage from normal and OA donors were examined for CD38 expression. Approaches of “gain-of-function” through overexpression of CD38 via transient transfection and of “loss-of-function” through pharmacologic inhibition of CD38 were used to assess the impact of CD38 on intracellular levels of NAD+/NADH and catabolic activity in chondrocytes. For joint injury-induced OA, the destabilization of medial meniscus (DMM) surgery was performed on CD38 knockout (KO) and wild type (WT, C57BL/6) mice, and WT male mice with and without apigenin treatment. Cartilage degradation, synovial inflammation, subchondral bone changes and pain behavior were evaluated post-DMM surgery. Expression of CD38 and the neuropeptide CGRP was examined in the knee sections of these mice.
Results:
CD38 expression was upregulated in human knee OA cartilage and in chondrocytes stimulated with proinflammatory cytokine IL-1β. Overexpression of CD38 in chondrocytes resulted in reduced cellular NAD+/NADH levels and augmented catabolic responses to IL-1β. These effects were reversed by pharmacologic inhibition of CD38. Cartilage degradation and synovial inflammation, associated with increased CD38 expression in cartilage and synovium, osteophyte formation and subchondral bone sclerosis, and pain-like behavior linked to increased CGRP expression in the synovium were observed in WT mice after joint injury. Such effects were significantly reduced in mice deficient in CD38 either through genetic knockout or pharmacologic inhibition.
Conclusion:
CD38 deficiency exerts OA disease modifying effects. Inhibition of CD38 has a potential to be a novel therapeutic approach for OA treatment.
Keywords: CD38, NAD+, chondrocyte/cartilage, post-traumatic OA, pain
Introduction
Joint injury is a primary risk factor for development of osteoarthritis (OA), a major cause of disability and pain in older adults (1,2). OA is considered as a disorder of whole joint (1). The main pathological changes include degradation of articular cartilage, synovial inflammation, osteophyte formation and thickening of subchondral bone (1,2). Progressive degeneration of articular cartilage is a core feature of the OA. Chondrocytes, the sole cells in articular cartilage, are responsible for maintaining the homeostatic balance between extracellular matrix anabolism and catabolism (1,2). Emerging evidence suggests that metabolic alterations in articular chondrocytes, exemplified by altered bioenergy sensing, are associated with OA (3,4). Nicotinamide adenine dinucleotide (NAD+) is a key metabolite and a necessary cofactor for metabolic pathways involved in cellular energy metabolism and adaptive responses of cells to bioenergetics (5-7). Mounting evidence indicates that intracellular NAD+ levels are significantly affected by environmental stimuli (5-7), and maintenance of a proper intracellular NAD+ concentration is critical for maintaining tissue homeostasis (5-7).
Changes in intracellular NAD+ levels are associated with NAD+ biosynthesis and/or degradation. In mammalian cells, the majority of NAD+ is synthesized through the salvage pathway from nicotinamide (NAM), a by-product of NAD+ degradation (5-7). Cluster of differentiation 38 (CD38), a type II transmembrane protein, is a major consuming enzyme of NAD+, contributing substantially to cellular NAD+ degradation (6,7). Tissue NAD+ levels are 10- to 20-fold higher in CD38 knockout (KO) compared to wild type (WT) mice (8). CD38 has both NAD+ glycohydrolase and ADP-ribosyl cyclase enzyme activities catalyzing production of ADP-ribose (ADPR), cyclic ADP-ribose (cADPR), and NAM (6,8,9). CD38 also performs a base-exchange reaction, by swapping the NAM of NAD+ for nicotinic acid (NA) in low pH conditions and generating nicotinic acid adenine dinucleotide (NAAD). However, CD38 NAD+ glycohydrolase appears to be responsible for the majority of CD38 NADase activity (6,8,9). CD38 is a ubiquitous protein expressed in multiple tissues but is highly expressed in immune cells including macrophages (10). Because the promoter region of CD38 contains binding sites for several transcription factors including NF-κB and STAT (6,9), CD38 expression can be induced by inflammatory mediators (6,9,10). The pro-inflammatory state associated with aging process provides a link between increased CD38 expression and age-related tissue NAD+ decline (6,9). Recent studies demonstrated that induction of CD38 expression in macrophages by senescence-associated inflammation drives NAD+ decline in aging (6,9). The evidence for immune cells in the synovium particularly macrophages in stimulating and modulating inflammatory responses in OA (10) implicates a potential role of CD38 in contribution to OA development.
NAD+ decline could lead to reduction of activities of NAD+-dependent enzymes such as sirtuins, leading to altered cellular metabolism, gene expression and protein function (6,7). CD38 degrades NAD+ and therefore decrease accessibility of NAD+ to sirtuins (6,7). Dysregulation of sirtuins has been implicated in OA development (11). Of the seven sirtuins, SIRT1 is the most studied in terms of its critical role in cartilage homeostasis (11). Based on these previous findings, in this study, we explored if CD38 plays a role in OA pathogenesis. We examined CD38 expression in OA vs. normal human knee cartilage in situ, determined how CD38 expression levels influenced chondrocyte catabolism in vitro, and investigated if mice with CD38 deficiency through either genetic knockout or pharmacologic inhibition were protected from OA development by assessing cartilage degradation, synovial inflammation, changes in subchondral bone and evaluating associated pain a post-traumatic OA model in vivo.
Materials and Methods
See details in supplemental materials
Results
Upregulation of CD38 expression in human knee OA cartilage
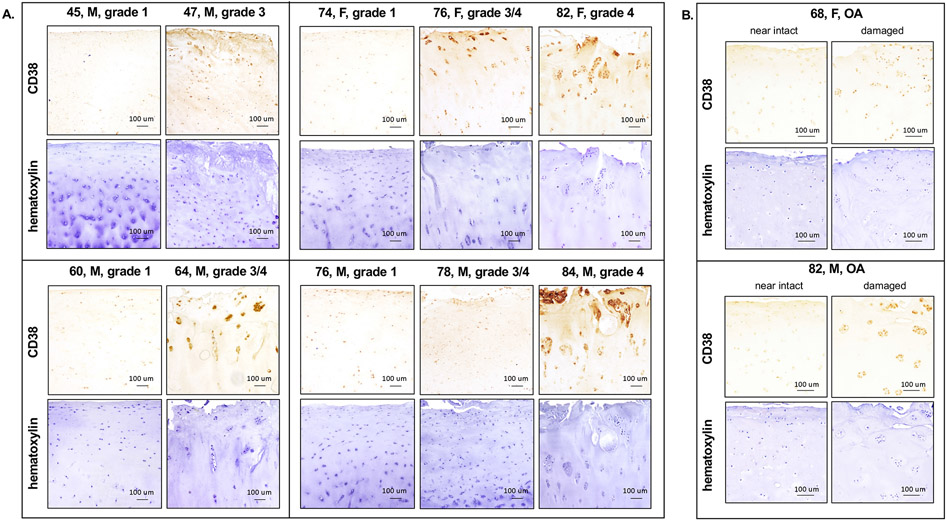
To start, we examined CD38 protein expression by IHC analysis of human knee OA cartilage (grade 3 and 4) from different age groups. Age and gender matched normal human knee cartilage (grade 1) were used as controls. Of note, the OA donors of 80s were grouped with those of 70s, because of lacking normal donors of 80s. The serial sections of the same cartilage donors were stained with hematoxylin to verify the cellularity. As seen in Figure 1A, CD38 protein expression was markedly increased in OA cartilage in all age groups, supported by average of ~80.4% cells stained positively for CD38 cross OA cartilage donors, compared to average of ~26% cells cross normal cartilage donors. Notably, in normal cartilage group, CD38 expression was increased with age, indicated by 16.6%, 22.1% and 39.3% cells stained positively for CD38 in age of 40s, 60s and 70s, respectively. Moreover, higher CD38 expression was observed in the damaged region, compared to the almost intact region, in the same OA cartilage donors. These results suggest that CD38 expression was upregulated in human knee OA cartilage and in human knee cartilage with aging. Our qRT-PCR analysis of RNA isolated directly from normal and OA human knee cartilage donors that were age and gender matched showed upregulation of CD38 mRNA expression in OA cartilage as well (Supplemental Figure 1).
Figure 1. Upregulation of CD38 protein expression in human knee OA cartilage.
Age and gender (indicated in the figure) matched human knee cartilage sections of normal and OA donors (A) and the sections of almost intact and damaged regions from the same human knee OA cartilage donors (B) were used for IHC analysis of CD38 protein expression. The serial sections of the same donors were stained for hematoxylin for the purpose of cellularity verification.
Reduced intracellular levels of NAD+/NADH and enhanced catabolic activities in chondrocytes overexpressing CD38
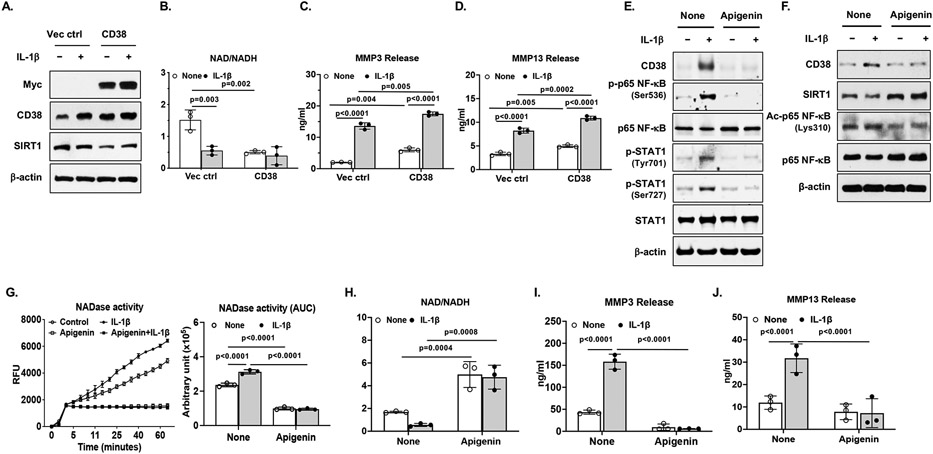
To assess functional consequence of increased CD38 expression in chondrocytes, we overexpressed CD38 via transient transfection in human chondrocytes followed by stimulation with IL-1β. CD38 expression was upregulate by IL-1β in chondrocytes transfected with the empty plasmid vector, which was further enhanced in chondrocytes transfected with the plasmid containing CD38 cDNA (Figure 2A). This was associated with markedly decreased NAD+/NADH ratio (Figure 2B). Importantly, chondrocyte catabolic capacity was augmented in chondrocytes overexpressing CD38, as IL-1β-induced MMP3 and MMP13 release was significantly enhanced (Figure 2C and 2D). These results indicate that upregulation of CD38 expression in chondrocytes caused decline of intracellular NAD+ levels and increased catabolic responses to inflammatory cytokine.
Figure 2. Modulation of intracellular levels of NAD/NADH and catabolic activity by CD38 in chondrocytes.
Human chondrocytes were transfected with pCMV3-CD38-Myc plasmid (to overexpress CD38) and the empty plasmid vector for 48 hours followed by stimulation with IL-1β (2 ng/ml) for 18 hours (A) or directly stimulated with IL-1β (2 ng/ml) in the presence or absence of apigenin for 18 hours (B). Expression of Myc, CD38, SIRT1, phosphorylated, acetylated and total p65 NF-κB, phosphorylated and total STAT1 was examined by Western blot (A, F, G). Intracellular NAD/NADH levels (B and H) were determined. NAD glycohydrolase (NADase) activity was measured and presented in kinetics and area under curve (AUC) formats (C). MMP3 and MMP13 release were measured from the conditioned media by ELISA (D, E, I, J). Data in A, F and G were representative of 3 independent experiments in chondrocytes from 3 different donors. For data in B, C and H, n=3 biological replicates. For data in D, E, I and J, n=3 biological replicates with each representing the mean of 2-3 technical replicates. Statistical analysis was performed using Two-way ANOVA with Tukey multiple comparison test (D-F). All graph data were expressed as mean±SD. p values with significance were indicated in the figures.
Inhibition of CD38 by apigenin in chondrocytes attenuated excessive catabolic activities induced by IL-1β
Stimulation of chondrocytes with apigenin, a natural flavonoid compound and a competitive CD38 inhibitor (12) prevented IL-1β-induced CD38 expression, linked to inactivation of NF-κB and STAT1, whose binding sites are in the promoter region of CD38. This was evidenced by that apigenin inhibited IL-1β-induced phosphorylation of NF-κB p65 subunit at Ser536 and STAT1 at both Tyr701 and Ser727 (Figure 2E). Notably, there was an invert correlation between CD38 and SIRT1 expression (Figure 2A and 2F). Apigenin increased SIRT1 expression and reduced acetylated p65 NF-κB (Lys310) expression at basal levels and reversed IL-1β-induced reduction of SIRT1 expression and increased acetylated p65 NF-κB expression (Figure 2F), suggesting that apigenin may also inhibit expression of NF-κB-dependent genes through deacetylation of NF-κB p65 by SIRT1. Moreover, apigenin blocked NADase activity (Figure 2G) and elevated intracellular NAD/NADH levels (Figure 2H) either at basal levels or induced by IL-1β. Furthermore, apigenin prevented IL-1β-induced loss of mRNA expression of cartilage matrix proteins aggrecan (ACAN) and type II collagen (Col2a1) and attenuated IL-1β-induced mRNA expression of cartilage degrading enzymes MMP3 (MMP3) and MMP13 (MMP13) (Supplemental Figure 2A-2D), as well as release of MMP3 and MMP13 induced by IL-1β in chondrocytes (Figure 2I and 2J) and cartilage explants (Supplemental Figure 2E and 2F). These results were further supported by that 78c, a specific CD78 inhibitor, diminished CD38 expression and attenuated MMP3 and MMP13 release induced by IL-1β in chondrocytes and cartilage explants (Supplemental Figure 3A-3D). Importantly, apigenin mitigated IL-1β-induced glycosaminoglycan (GAG) release (Supplemental Figure 2G), indicating that inhibition of CD38 could prevent cartilage matrix degradation under inflammatory condition.
CD38 deficiency exhibited chondroprotective effect in mice
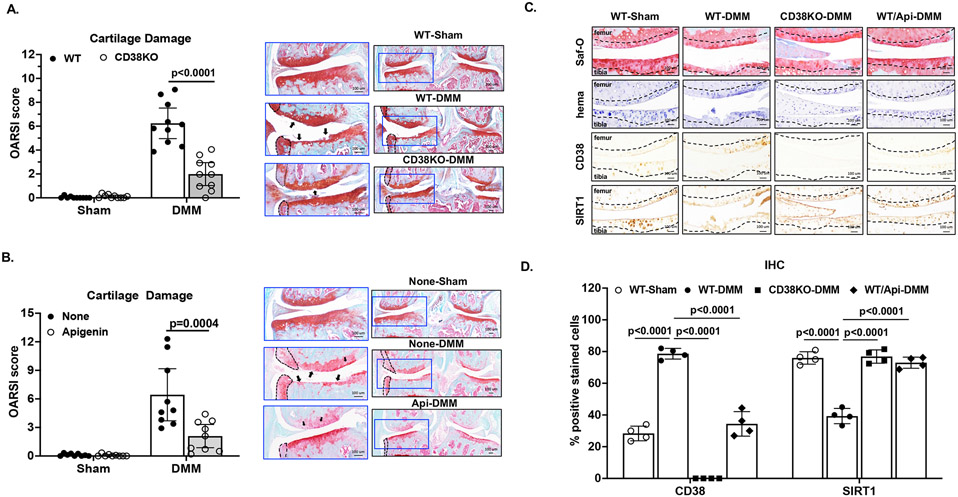
We performed DMM surgery on both CD38KO and WT mice. Cartilage degeneration was evaluated at 10 weeks post-DMM surgery. As expected, no cartilage damage was observed in sham control knees (Figure 3A). WT DMM knees exhibited prominent cartilage degradation (black arrows, Figure 3A) with a mean OARSI score of 6.24 (95% confidence interval (CI) 4.96 to 7.52). In comparison, cartilage degradation was suppressed in CD38KO DMM knees with a significantly lower mean OARSI score (1.99, 95% CI 1.03 to 2.95) (Figure 3A). Similar results were observed in apigenin-treated WT mice, indicated by considerable differences in mean OARSI scores, which were 2.09 (95% CI 0.87 to 3.31) for apigenin-treated and 6.44 (95% CI 3.7 to 9.17) for non-treated groups (Figure 3B). IHC analysis showed increased CD38 expression and decceased SIRT1 expression in WT-DMM compared to WT-sham knee cartilage sections, which were reversed in apigenin-treated WT-DMM and CD38KO-DMM knee cartilage sections (Figure 3C and 3D). These data suggest CD38 deficiency in mice achieved through genetic knockout or pharmacologic inhibition had chondroprotective effect after joint injury, which may be in part mediated through SIRT1.
Figure 3. Chondroprotective effect of CD38 deficiency in mice.
DMM surgery was performed on WT (C57BL/6) and CD38KO (A, n=10/group), as well as WT mice with and without apigenin treatment (n=9/group). Sham surgeries performed on separate groups of mice were used as controls. All mice were sacrificed at 10 weeks post-DMM surgery, and knee sections of these mice were subjected to histological and IHC analyses. The OARSI scores and the representative images of safranin-O staining of the DMM knees with medial compartment enlarged were shown in A and B. Representative IHC images of expression of CD38 and SIRT1 in articular cartilage and percentage of cells with positive staining for CD38 and SIRT1 relative to all cells present in the non-calcified regions of femoral and tibial cartilage indicated by hematoxylin staining were shown in C and D (n=4/group). Statistical analysis was performed using unpaired Student t-test in A and B (WT vs. CD38KO and non-treated vs. apigenin-treated), one-way ANOVA with Tukey multiple comparison test in D. The data were expressed as mean±95% CI (A, B) or mean±SD (D). p values with significance were indicated in the figures.
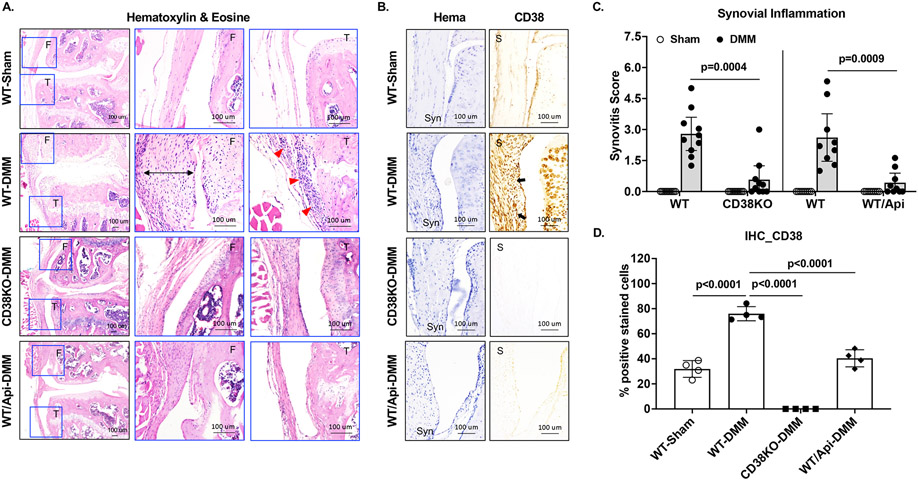
Mice with CD38 deficiency displayed reduced synovial inflammation after joint injury
We examined synovial inflammation by histological analysis of all mice at 10 weeks after DMM surgery. As depicted in Figure 4A, WT-DMM compared to WT-sham knees exhibited thickening of synovium (black arrow bar) and increased infiltration of cells in the sublining of synovium (red arrows), which were much less in CD38KO-DMM and apigenin-treated WT-DMM (WT/Api-DMM) mice. The mean synovitis scores of the DMM knees were 2.79 (95% CI 1.99 to 3.6) and 0.58 (0 to 1.25) for WT and CD38KO mice respectively, and 2.61 (95% CI 1.46 to 3.76) and 0.43 (0 to 0.89) for non-treated (WT) and apigenin-treated (WT/Api) mice respectively (Figure 4C). These results indicate that CD38 deficiency alleviated synovial inflammation in mice after joint injury. IHC analysis showed significantly higher CD38 expression in the synovium of WT-DMM than that in the synovium of WT-sham and WT/Api-DMM mice (Figure 4B and 4D), suggesting an association of increased CD38 expression with synovial inflammation. Since synovial inflammation occurs in both early and late stage of OA, we also examined CD38 expression in the synovium of these mice at 2 weeks post-DMM surgery. Given the involvement of macrophages in modulating synovial inflammation, we performed IHC using the fluorescence labeled CD38 (red) and F4/80 (green), the mouse macrophage marker. As seen in the Supplemental Figure 4, H&E staining showed that synovial inflammation was evident in WT-DMM mice but mild in CD38KO and WT/Api-DMM mice at 2 weeks post-DMM surgery. Both CD38 and F4/80 expression were observed in a number of cells (white arrows) in WT-DMM synovium. Remarkably, about 86% of F4/80 positive cells were colocalized with CD38 positive cells (white arrows), implicating that CD38 expression were mainly upregulated in macrophages in the synovium 2 weeks after joint injury. In comparison, CD38 expression was not seen in the synovium of CD38KO mice as expected and was much less in the synovium of apigenin-treated WT mice, supported by fewer cells with colocalization of CD38 and F4/80. Immunofluorescence IHC analysis of synovial expression of CD38 at 10 weeks post-DMM surgery revealed similar result that increased expression of CD38 in the synovium, which was also mostly in macrophages, in WT-DMM mice (Supplemental Figure 5). Again, synovial CD38 expression was not seen in CD38KO-DMM mice and was very limited in WT/Api-DMM mice (Supplemental Figure 5). Taken together, these results suggest that restrained CD38 expression in macrophages may be responsible for reduced synovial inflammation in mice deficient in CD38.
Figure 4. Reduced synovial inflammation in mice with CD38 deficiency post-DMM surgery.
Synovitis was evaluated semi-quantitatively in DMM knees of WT, CD38KO, non-treated and apigenin-treated mice at 10 weeks after DMM surgery. Representative images of H&E staining of the medial compartment of DMM knees (lower magnification) with femoral and tibial regions enlarged (higher magnification) were shown in A, and synovitis scores were presented in C (n=10 each for WT and CD38KO, n=9 each for WT and WT+apigenin). Representative images of IHC analysis of CD38 expression and percentage of CD38 positively stained cells were shown in B and D (n=4/group). Statistical analysis was performed using unpaired Student t-test in C (WT vs CD38KO and WT vs WT+Apigenin) and one-way ANOVA with Tukey multiple comparison test in D. The data in C and D were expressed as mean±95% CI and mean±SD, respectively. p values with significance were indicated in the figures.
Mice with CD38 deficiency exhibited less subchondral bone alterations after joint injury
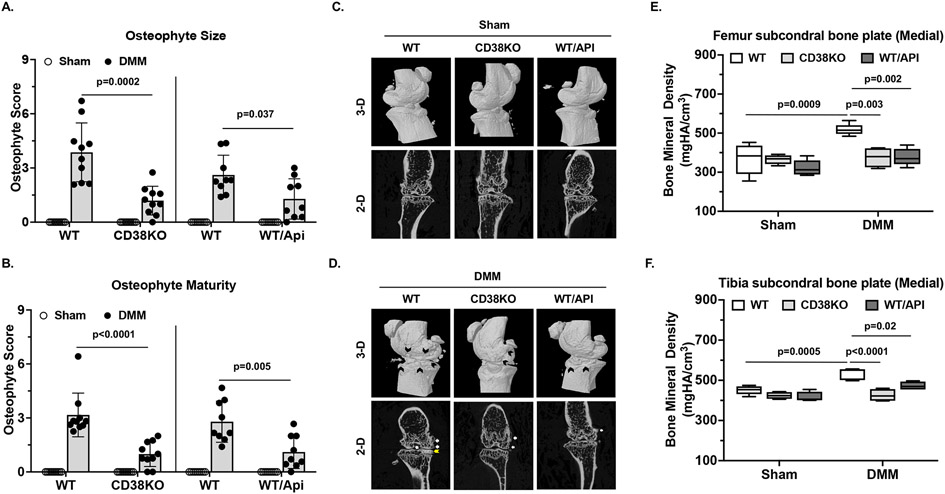
Histological analysis revealed no osteophyte formation was observed in WT sham control mice as expected. However, WT-DMM knees presented prominent osteophyte formation (black dot-lined area in Figure 3A) both in size and maturity with mean scores of 3.86 (95% CI 2.7 to 5.03) and 3.16 (95% CI 2.3 to 4.03, respectively) (Figure 5A and 5B). Despite that osteophyte formation was still evident in CD38KO DMM knees (black dot-lined area in Figure 3A), the size and maturity of osteophytes were reduced, supported by significantly lower mean osteophyte scores, which were 1.19 (95% CI 0.62 to 1.76) and 0.99 (95% CI 0.49 to 1.48) for size and maturity, respectively (Figure 5A and 5B). Similar results were seen in apigenin-treated (WT/Api) compared to non-treated (WT) mice (Figure 5A and 5B).
Figure 5. Reduced subchondral bone alterations in mice with CD38 deficiency post-DMM surgery.
Osteophyte formation including both size (A) and maturity (B) was evaluated semi-quantitatively in sham and DMM knees of WT, CD38KO, and WT treated with apigenin mice at 10 weeks after DMM surgery (n=10 each for WT and CD38KO, n=9 each for WT and WT+apigenin). Representative 3-D and 2-D reconstruction images generated from micro-CT scan (n=5/group) were shown in C (sham) and D (DMM). Subchondral bone plate BMDs in both femur and tibia in medial compartment of DMM knees were quantified (E and F). Statistical analysis was performed using non-parametric Mann Whitney test in A and B (WT vs. CD38KO and WT vs WT+Apigenin), and Two-way ANOVA with Tukey multiple comparison test in E and F. The data were expressed as mean±95% CI in A and B and in box plot (Min to Max) for E and F. p values with significance were indicated in the figures.
The 3-dimensional (3-D) and 2-D reconstructive images from micro-CT scan revealed no subchondral bone alterations in mice received sham surgery (Figure 5C). However, protruding osteophytes were observed in WT-DMM knees, evidenced by appearance of outgrowing bone surface (Figure 5C, black arrows in 3-D, white arrows in 2-D). In comparison, formation of osteophytes was significantly limited in CD38KO-DMM or WT/Api-DMM knees (Figure 5C), which agreed with the findings from histological assessment. The subchondral bone density changes were noticed in the knees of WT-DMM mice, especially in the tibial subchondral bone of medial compartment in 2-D images (Figure 5D, yellow arrow). These were not observed in the either CD38KO-DMM or WT/Api-DMM knees (Figure 5D). Both femoral and tibial subchondral bone plate bone mineral density (BMD) in the medial compartment was significantly increased in WT-DMM but not CD38KO-DMM or WT/Api-DMM knees (Figure 5E and 5F). No subchondral bone plate BMD changes were seen in the lateral compartment of all knees studied (data not shown). Taken together, these data suggest the beneficial effect of CD38 deficiency in limiting abnormal subchondral changes after joint injury.
Mice with CD38 deficiency exhibited less pain associated with OA
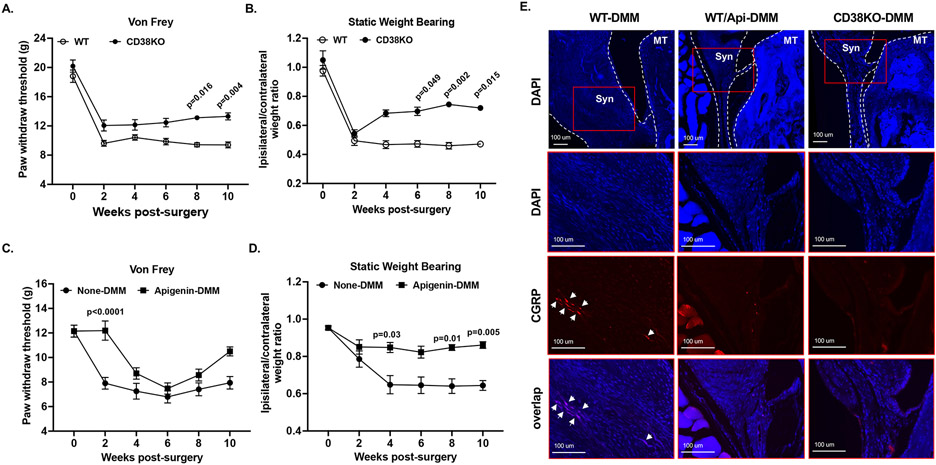
We performed von Frey and static weight bearing tests bi-weekly to assess and compare mechanical stimulus and non-stimulus evoked nociception respectively. The von Frey test revealed that paw withdraw response threshold was markedly decreased in WT mice (Figure 6A) or non-treated mice (Figure 6C) at 2 weeks post-DMM surgery. This was sustained in the rest of study period. The paw withdraw response threshold was also notably reduced but to a less extent in CD38KO mice with a slow trend of increasing in later time points, especially at 8- and 10-weeks post-DMM surgery (Figure 6A). The apigenin-treated mice also exhibited reduction of paw withdraw response threshold post-DMM surgery but with a delay of 2 weeks (Figure 6C). Similarly, the extent of reduction of paw withdraw response threshold was less in apigenin-treated compared to non-treated mice (Figure 6C). Area under the curve (AUC) value of paw withdrawal response threshold for the entire time course was significantly higher in CD38KO mice (Supplemental Figure 6A) and apigenin-treated mice (Supplemental Figure 6C). The static weight bearing test showed that both WT and CD38KO mice displayed the similar extent of weight bearing deficit at 2 weeks post-DMM surgery (Figure 6B). This deficit was sustained in WT mice for the rest of study period, but was considerably improved in CD38KO mice, particularly at 6-, 8- and 10-weeks post-DMM surgery (Figure 6B). The non-treated mice also showed weight bearing deficit that reached to the lowest point at 4 weeks post-DMM surgery, and this was maintained at similar levels during the rest of time course (Figure 6D). In comparison, the apigenin-treated mice exhibited only mild weight bearing deficit at all time points, evidenced by significantly higher ipsilateral to contralateral weight bearing ratio at 4-, 8- and 10-weeks post-surgery (Figure 6D). Both CD38KO mice and apigenin-treated mice had significant higher AUC value of ipsilateral to contralateral weight bearing ratio for the entire time course (Supplemental Figure 6B and 6D). These data indicate that mice deficient in CD38 experienced less pain after joint injury.
Figure 6. Mice with CD38 deficiency exhibited less pain associated with OA post-DMM surgery.
Von Frey and static weight bearing tests were performed to evaluate stimulus-evoked (A, C) and non-stimulus evoked pain (B, D), respectively, in WT vs. CD38KO (A, B, n=10/group) and non-treated vs. apigenin-treated (C, D, n=9/group) mice biweekly after DMM surgery. Representative fluorescence IHC images of CGRP (red), DAPI (blue) and their overlap (violet) in the synovium were shown in E (n=3/group). Statistical analysis was conducted using Kruskal-Wallis test with Dunn’s multiple comparisons (A-D). The data in A-D were expressed as mean±SEM. p values with significance were indicated in the figures.
Next, we performed immunofluorescence IHC analysis of expression calcitonin gene-related peptide (CGRP), a neuropeptide involved in peripheral pain sensitization. As shown in Figure 6E, CGRP signal was detected with a strong intensity in the synovium of WT-DMM mice, indicating occurrence of innervation. In comparison, much weaker or little CGRP signal was observed in WT/Api-DMM and CD38KO-DMM mice, suggesting protective effect of CD38 deficiency on peripheral pain sensitization.
Discussion
Our data revealed that CD38 expression was upregulated in human knee OA cartilage and in human chondrocytes challenged with pro-inflammatory cytokine IL-1β. In addition, chondrocytes with increased expression of CD38 had significantly decreased intracellular NAD+/NADH ratio and augmented catabolic capacity in response to IL-1β. Moreover, IL-1β-induced CD38 expression was abrogated by either apigenin or 78c in chondrocytes. This was likely mediated through inhibition of activation of NF-κB and STAT1, as both of which bind to CD38 promoter. Notably, apigenin also reversed IL-1β-induced downregulation of SIRT1 expression and increased acetylation of p65 NF-κB (Lys310). Since SIRT1 activation can suppress inflammatory responses through promotion of deacetylation of p65 NF-κB and inhibition of NF-κB activity (13), these data suggest that inhibition of CD38 can restrict expression of NF-κB-dependent genes through SIRT1 in chondrocytes under inflammatory conditions. Indeed, apigenin attenuated IL-1β-induced expression of catabolic genes MMP3 and MMP13, which are NF-κB dependent. Apigenin also reversed IL-1β-induced reduction of expression of anabolic genes aggrecan and col2a1, indicating that inhibition of CD38 may help maintain cartilage extracellular matrix homeostasis. The ability of apigenin to mitigate IL-1β-induced release of MMP3, MMP13 and GAG release suggests that inhibition of CD38 could prevent cartilage extracellular matrix degradation.
In vivo studies using a post-traumatic OA model through the DMM surgery demonstrated that CD38KO and apigenin-treated WT mice, compared to WT mice, exhibited less cartilage damage after joint injury. This was associated with preservation of SIRT1 expression and restriction of CD38 expression in CD38KO-DMM and WT/Api-DMM knee cartilage, indicating chondroprotective effect of CD38 deficiency mediating in part through SIRT1. It is known that SIRT1 plays a critical role in cartilage homeostasis (10). Chondrocyte-specific SIRT1 KO mice had accelerated cartilage degradation in a post-traumatic OA model (14). In contrast, chondrocyte-specific SIRT1 knock-in (KI) mice, in which SIRT1 was overexpressed specifically in chondrocytes, exhibited less cartilage damage (15). In addition, mice received treatment with SIRT1 specific activators systemically display significantly decreased cartilage degradation (16,17). Since inhibition of CD38 can mitigate NADase activity which would prevent NAD+ degradation, it would be interesting to determine if other NAD+-dependent SIRTs contribute to chondroprotective effect of CD38 efficiency.
Both CD38KO and apigenin-treated WT mice, which had no or less CD38 expression in the synovium, exhibited significantly lower synovitis and osteophyte scores compared to WT mice at 10 weeks post-DMM surgery. Synovial inflammation is implicated to associate with more rapid progression of cartilage loss in human OA joints (18). Synovial macrophages can contribute to OA progression directly or indirectly through the induction of inflammatory mediators, growth factors and proteinases, leading to enhanced cartilage degeneration and osteophyte formation (10,19,20). We noticed that significant number of cells in the synovium of WT mice at 2 weeks post-DMM surgery had already expressed CD38, and most of them appeared to be macrophages, evidenced by co-localization of CD38 with F4/80 in these cells. This was not observed in CD38KO mice and was much less in apigenin-treated WT mice. Similar result was also seen in these mice at 10 weeks post-DMM surgery. These data indicate that upregulation of CD38 expression in macrophages may contribute to synovial inflammation at both early and late stage of OA. Accumulation of pro-inflammatory M1 macrophages is observed in both human and mouse OA synovial tissues (20). CD38 is found to be highly expressed in pro-inflammatory macrophages (5, 21). Given that inhibition of CD38 can reduce M1 macrophage polarization (21,22), the effect of CD38 deficiency in limiting synovial inflammation and osteophyte formation after joint injury may be in part resulted from preventing synovial macrophage polarization to pro-inflammatory phenotype. WT mice but not CD38KO or apigenin-treated mice were notably to have a significant increase in the medial subchondral bone plate BMD at 10 weeks post-DMM surgery, implicating involvement of CD38 in dysregulation of bone metabolism in injury-induced OA. Although the mechanism of which remains to be determined, CD38 deficiency appear to protect mice from abnormal subchondral bone changes after joint injury.
The pain-like behavior study revealed that both CD38KO and apigenin-treated mice, compared to WT mice, experienced less pain post-DMM surgery. This was associated with little or low CGRP expression in the synovium, suggesting these mice were protected from peripheral innervation after joint injury. CGRP is a key contributor to pain sensitization in OA (23). Thus, CD38 deficiency may exert its pain-relieving effect by reducing pain sensitivity. Since synovitis is seen to associate with neuropathy-like pain sensitization in knee OA patients (24), and histological evidence of synovitis is shown to associate with persistent mechanical allodynia in mouse DMM model (25), the pain-relieving effect of CD38 deficiency may mediate through inhibition of synovial inflammation after joint injury. Remarkably, paw withdraw threshold was not decreased and static weight bearing was mildly reduced in apigenin-treated mice but not CD38KO mice at 2 weeks post-DMM surgery, indicating the ability of apigenin in relieving post-surgery pain in a CD38-independent manner.
This study has some limitations. First, because apigenin was given to mice orally and CD38KO mice have global CD38 deficiency, we cannot rule out potential benefit of systemic CD38 deficiency contributing to the chondroprotective effect. Second, apigenin is a competitive but not specific CD38 antagonist. It has been shown to have other cellular targets besides CD38 (26). Although we showed that apigenin can inhibit CD38 expression and NADase activity in chondrocytes in vitro, the protective effect of apigenin observed in our in vivo studies may not only be attributed to CD38 inhibition. For example, apigenin can activate AMP-activated protein kinase (AMPK) in several different cell types and disease models in mice (27-32). We recently showed that metformin and berberine, which are AMPK activators, can limit OA development and relieve OA pain in an AMPK-dependent manner in mice after DMM surgery (33,34). AMPK is emerged as a novel pain target (35,36). The pain-relieving effect of apigenin observed in this study may be partly due to AMPK activation. A recent study demonstrated that apigenin is a positive allosteric modulator of α7 nicotinic acetylcholine receptors (α7-nAChRs) (37). Activation of α7-nAChRs can alleviate monosodium iodoacetate (MIA)-induced joint degradation and OA pain (38,39). These implicate that apigenin may also activate α7-nAChRs to exert its pain-relieving effect in our study. Third, both ADPR and cADPR, the by-products of NAD+ degradation by CD38, can act as second messengers controlling cell functions through calcium (Ca2+) mobilization (6,7). Alterations in Ca2+ signaling may lead to the defective synthesis of extracellular matrix and aggravated catabolic responses in OA(40), as well as involve in pain modulation (41). Whether ADPR and cADPR play a role in OA development by disturbing cartilage matrix homeostasis and OA-associated pain remain to be determined.
In summary, CD38 expression was upregulated, associated with decreased intracellular NAD+/NADH levels, in human knee OA cartilage and in chondrocytes challenged with pro-inflammatory cytokine IL-1β. Inhibition of CD38 prevented intracellular NAD+/NADH reduction and attenuated chondrocyte/cartilage catabolic activities in responses to IL-1β. CD38KO mice or mice received apigenin treatment exhibited significantly less joint structural changes (e.g., cartilage degradation, synovial inflammation, osteophyte formation and subchondral bone sclerosis) and pain-like behavior in a post-traumatic OA model. Aging is a major risk factor for OA development (1). It appeared that CD38 expression in human knee cartilage was also increased with aging. Since CD38 substantially contributes to age-related NAD+ decline (5,6,9), it may play an important role in pathogenesis of age-related spontaneous OA, which is currently under our investigation. In conclusion, CD38 deficiency has OA disease modifying effects in experimental mouse OA. Inhibition of CD38 has a potential to be a novel therapeutic approach for OA treatment.
Supplementary Material
Suppl Figure 1. Upregulation of CD38 mRNA expression in human knee OA cartilage. Total RNA directly isolated from age and gender matched human knee cartilage of normal and OA donors (n=6 pair) were subjected to qRT-PCR analysis of CD38 mRNA expression (A). Information about the donors’ age and gender was shown in B. Statistical analysis was performed using Student t-test. Data in A were expressed as mean±SD. p value with significance was indicated in the figure.
Suppl Figure 2. Attenuation of chondrocyte catabolic activities induced by IL-1β by apigenin. Human chondrocytes and human knee cartilage explants were treated with IL-1β (2 ng/ml) in the presence or absence of apigenin (25 μM) for 6 and 24 hours, respectively. The chondrocyte samples were used for evaluating mRNA expression of ACAN, Col2a1, MMP3 and MMP13 by qRT-PCR analysis (A-D), and the cartilage explant samples were used for measuring release of MMP3, MMP13 and glycosaminoglycan (GAG) from the conditioned media by ELISA and the Dimethylmethlyene Blue (DMB) dye methods (E-G). For data in A-D, n=3 biological replicates with each representing the mean of 3 technical replicates. For data in E-G, n=12 cartilage explants collected from 4 different donors (3 explants from each donor). Statistical analysis was performed using Two-way ANOVA with Tukey multiple comparison test. All data were expressed as mean±SD. p values with significance were indicated in the figures.
Suppl Figure 3. The CD38-specific inhibitor 78c had the same effects as apigenin on attenuation of IL-βinduced CD38 expression and catabolic activity in chondrocytes. Human chondrocytes were stimulated with IL-1β (2 ng/ml) in the presence or absence of apigenin (25 μM) or 78c (25 μM) for 18 hours. CD38 protein expression was examined by Western blot analysis (A). The conditioned media was used for ELISA analysis of MMP3 and MMP13 release in chondrocytes (B and C) or MMP3 release in cartilage explants (D). Data in A were representative of 3 independent experiments in chondrocytes from 3 different donors. For data in B and C, n=3 biological replicates with each representing the mean of 3 technical replicates. For data in D, n=6 cartilage explants collected from 3 different donors and 2 explants from each donor. Statistical analysis was performed using Two-way ANOVA with Tukey multiple comparison test. Data were expressed as mean±SD. p values with significance were indicated in the figures.
Suppl Figure 4. Increased CD38 expression in the synovial macrophages in WT mice but CD38KO mice and apigenin-treated WT mice at 2 weeks post-DMM surgery. Knee sections of WT-sham, WT-DMM, CD38KO-DMM, WT/Api-DMM mice at 2 weeks after DMM surgery were subjected to H&E staining and fluorescence IHC analysis for expression of CD38 (red) and F4/80 (green). DAPI staining (nuclear stain) was included (blue). Representative H&E staining images of the synovium in the medial femur (MF) and medial tibia (MT) compartment and immunostaining images of the synovium in the MT compartment were shown (n=4/group).
Suppl Figure 5. Increased CD38 expression in the synovial macrophages in WT mice but CD38KO mice and apigenin-treated WT mice at 10 weeks post-DMM surgery. WT-DMM, CD38KO-DMM, and WT/Api-DMM mice at 10 weeks after DMM surgery were subjected to fluorescence IHC analysis for expression of CD38 (red) and F4/80 (green). DAPI staining was included (blue). Representative immunostaining images of the synovium in the MT compartment were shown (n=4/group).
Suppl Figure 6. Mice with CD38 deficiency exhibited less pain associated with OA post-DMM surgery. The time course data of Von Frey and weight bearing tests in Figure 6 were presented in the format of Area under curve (AUC) in box plots (Min to Max). For data in A and B, n=10/group. For data in C and D, n=9/group. Statistical analysis was conducted using unpaired Student t-test. p values with significance were indicated in the figures.
Acknowledgements
We thank Dr. Martin Lotz (The Scripps Research Institute, La Jolla, CA) for providing us with total RNA and slides of human normal and OA knee cartilage sections. We are grateful to Drs Robert Sah and Albert Chen at UCSD Bioengineering/ Orthopaedic MicroCT facility for their help with MicroCT scanning mouse knees.
This work was supported by the Rheumatology Research Foundation Innovative Research Award (RL-B) and the Department of Veterans Affairs Merit Review grant 1I01BX002234 (RL-B).
Footnotes
Competing interests: None.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/art.42351
Data availability statement
The data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author upon reasonable request.
References:
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol 2007;213:626–34. [DOI] [PubMed] [Google Scholar]
- 3.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum F, Griffin TM, Liu-Bryan R. Review: Metabolic Regulation of Inflammation in Osteoarthritis Arthritis Rheumatol. 2017;69:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chini CCS, Zeidler JD, Kashyap S, Warner G, Chini EN. Evolving concepts in NAD+ metabolism. Cell Metab. 2021;33:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–92. [DOI] [PubMed] [Google Scholar]
- 9.Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab. 2020;2:1284–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodell-May JE, Sommerfeld SD. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J Orthop Res. 2020;38:253–257. [DOI] [PubMed] [Google Scholar]
- 11.Dvir-Ginzberg M, Mobasheri A, Kumar A. The Role of Sirtuins in Cartilage Homeostasis and Osteoarthritis. Curr Rheumatol Rep. 2016;18:43. [DOI] [PubMed] [Google Scholar]
- 12.Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, et al. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 2012;7:e46364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki T, Matsushita T, Takayama K, Matsumoto T, Nishida K, Kuroda R, et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann Rheum Dis. 2014;73:1397–404. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Miyaji N, Kataoka K, Nishida K, Nagai K, Kanzaki N, et al. Knee Osteoarthritis Progression Is Delayed in Silent Information Regulator 2 Ortholog 1 Knock-in Mice. Int J Mol Sci. 2021;22:10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyaji N, Nishida K, Tanaka T, Araki D, Kanzaki N, Hoshino Y, et al. Inhibition of Knee Osteoarthritis Progression in Mice by Administering SRT2014, an Activator of Silent Information Regulator 2 Ortholog 1 Knock-in Mice. Int J Mol Sci. 2021;22:10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida K, Matsushita T, Takayama K, Tanaka T, Miyaji N, Ibaraki K, et al. Intraperitoneal injection of the SIRT1 activator SRT1720 attenuates the progression of experimental osteoarthritis in mice. Bone Joint Res. 2018;7:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555–561. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, et al. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS One. 2015;10:e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amici SA, Young NA, Narvaez-Miranda J, Jablonski KA, Arcos J, Rosas L, et al. CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions. Front Immunol. 2018;9:1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu B, Feng Y, Gui Y, Lu Q, Wei W, Xue X, et al. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-κB signaling suppression. Cell Signal. 2018;42:249–258. [DOI] [PubMed] [Google Scholar]
- 23.Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol. 2015;80:965–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu CC, Zaki S, Ravi V, Schiavinato A, Smith MM, Little CB. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: differential effect of stem cell and hyaluronan treatment. Arthritis Res Ther. 2020;22:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, van Schooten W. The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci. 2018;39:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijani S, Dizaji R, Sharafi A, Hosseini MJ. Neuroprotective Effect of Apigenin on Depressive-Like Behavior: Mechanistic Approach. Neurochem Res. 2021Oct 27. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Bu H, Jiang Y, Sun G, Jiang R, Huang X, et al. The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol Med Rep. 2019;20:2867–2874. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Meng Z, Cheng B, Liu M, Tao S, Guan S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp Ther Med. 2019;18:2965–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi WH, Son HJ, Jang YJ, Ahn J, Jung CH, Ha TY. Apigenin Ameliorates the Obesity-Induced Skeletal Muscle Atrophy by Attenuating Mitochondrial Dysfunction in the Muscle of Obese Mice. Mol Nutr Food Res. 2017;61(12). [DOI] [PubMed] [Google Scholar]
- 31.Tong X, Smith KA, Pelling JC. Apigenin, a chemopreventive bioflavonoid, induces AMP-activated protein kinase activation in human keratinocytes. Mol Carcinog. 2012;51:268–79. [DOI] [PubMed] [Google Scholar]
- 32.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–91. [DOI] [PubMed] [Google Scholar]
- 33.Asiedu MN, Dussor G, Price TJ. Targeting AMPK for the alleviation of pathological pain. Exp Suppl 2016;107:257–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price TJ, Das V, Dussor G. Adenosine monophosphate-activated protein kinase (AMPK) activators for the prevention, treatment and potential reversal of pathological pain. Curr Drug Targets 2016;17:908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Zhang B, Liu WX, Lu K, Pan H, Wang T et al. Metformin limits osteoarthritis development and progression through activation of AMPK signaling. Ann Rheum Dis. 2020;79:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Wang Y, Chen D, Liu-Bryan R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthritis Cartilage. 2022;30:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shabbir W, Yang KS, Sadek B, Oz M. Apigenin and Structurally Related Flavonoids Allosterically Potentiate the Function of Human α7-Nicotinic Acetylcholine Receptors Expressed in SH-EP1 Cells. Cells. 2021;10:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Xu S, Zhang H, Qian K, Huang J, Gu X et al. Stimulation of α7-nAChRs coordinates autophagy and apoptosis signaling in experimental knee osteoarthritis. Cell Death Dis. 2021;12:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Wu D, Song F, Zhu C, Hui Y, Zhu Q et al. Activation of α7 nicotinic acetylcholine receptors prevents monosodium iodoacetate-induced osteoarthritis in rats. Cell Physiol Biochem. 2015;35:627–38. [DOI] [PubMed] [Google Scholar]
- 40.Mobasheri A, Matta C, Uzielienè I, Budd E, Martín-Vasallo P, Bemotiene E. The chondrocyte channelome: A narrative review. Joint Bone Spine. 2019;86:29–35. [DOI] [PubMed] [Google Scholar]
- 41.Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94:81–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl Figure 1. Upregulation of CD38 mRNA expression in human knee OA cartilage. Total RNA directly isolated from age and gender matched human knee cartilage of normal and OA donors (n=6 pair) were subjected to qRT-PCR analysis of CD38 mRNA expression (A). Information about the donors’ age and gender was shown in B. Statistical analysis was performed using Student t-test. Data in A were expressed as mean±SD. p value with significance was indicated in the figure.
Suppl Figure 2. Attenuation of chondrocyte catabolic activities induced by IL-1β by apigenin. Human chondrocytes and human knee cartilage explants were treated with IL-1β (2 ng/ml) in the presence or absence of apigenin (25 μM) for 6 and 24 hours, respectively. The chondrocyte samples were used for evaluating mRNA expression of ACAN, Col2a1, MMP3 and MMP13 by qRT-PCR analysis (A-D), and the cartilage explant samples were used for measuring release of MMP3, MMP13 and glycosaminoglycan (GAG) from the conditioned media by ELISA and the Dimethylmethlyene Blue (DMB) dye methods (E-G). For data in A-D, n=3 biological replicates with each representing the mean of 3 technical replicates. For data in E-G, n=12 cartilage explants collected from 4 different donors (3 explants from each donor). Statistical analysis was performed using Two-way ANOVA with Tukey multiple comparison test. All data were expressed as mean±SD. p values with significance were indicated in the figures.
Suppl Figure 3. The CD38-specific inhibitor 78c had the same effects as apigenin on attenuation of IL-βinduced CD38 expression and catabolic activity in chondrocytes. Human chondrocytes were stimulated with IL-1β (2 ng/ml) in the presence or absence of apigenin (25 μM) or 78c (25 μM) for 18 hours. CD38 protein expression was examined by Western blot analysis (A). The conditioned media was used for ELISA analysis of MMP3 and MMP13 release in chondrocytes (B and C) or MMP3 release in cartilage explants (D). Data in A were representative of 3 independent experiments in chondrocytes from 3 different donors. For data in B and C, n=3 biological replicates with each representing the mean of 3 technical replicates. For data in D, n=6 cartilage explants collected from 3 different donors and 2 explants from each donor. Statistical analysis was performed using Two-way ANOVA with Tukey multiple comparison test. Data were expressed as mean±SD. p values with significance were indicated in the figures.
Suppl Figure 4. Increased CD38 expression in the synovial macrophages in WT mice but CD38KO mice and apigenin-treated WT mice at 2 weeks post-DMM surgery. Knee sections of WT-sham, WT-DMM, CD38KO-DMM, WT/Api-DMM mice at 2 weeks after DMM surgery were subjected to H&E staining and fluorescence IHC analysis for expression of CD38 (red) and F4/80 (green). DAPI staining (nuclear stain) was included (blue). Representative H&E staining images of the synovium in the medial femur (MF) and medial tibia (MT) compartment and immunostaining images of the synovium in the MT compartment were shown (n=4/group).
Suppl Figure 5. Increased CD38 expression in the synovial macrophages in WT mice but CD38KO mice and apigenin-treated WT mice at 10 weeks post-DMM surgery. WT-DMM, CD38KO-DMM, and WT/Api-DMM mice at 10 weeks after DMM surgery were subjected to fluorescence IHC analysis for expression of CD38 (red) and F4/80 (green). DAPI staining was included (blue). Representative immunostaining images of the synovium in the MT compartment were shown (n=4/group).
Suppl Figure 6. Mice with CD38 deficiency exhibited less pain associated with OA post-DMM surgery. The time course data of Von Frey and weight bearing tests in Figure 6 were presented in the format of Area under curve (AUC) in box plots (Min to Max). For data in A and B, n=10/group. For data in C and D, n=9/group. Statistical analysis was conducted using unpaired Student t-test. p values with significance were indicated in the figures.
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author upon reasonable request.