Abstract
Behavioral interventions delivered via one-on-one telephone coaching (hereafter referred to as telehealth) for weight loss have had great population-level reach but to date limited efficacy. Acceptance and Commitment Therapy (ACT) has promise to improve behavioral weight loss treatment efficacy by addressing the fundamental challenges of weight loss and maintenance: overeating in response to internal (stress) and external (high calorie foods) cues. Here we describe the Weight Loss, Nutrition, and Exercise Study (WeLNES) randomized controlled trial that is testing the efficacy of an ACT-based telehealth coaching intervention for weight loss in comparison to a Standard Behavioral Therapy (SBT)-based telehealth coaching intervention. A total of 398 adults with overweight or obesity are being recruited and randomized to either ACT or SBT telehealth coaching. Participants in both arms are offered twenty-five telehealth coaching sessions in year one and nine booster sessions in year two. All participants receive a Bluetooth-enabled scale to self-monitor weight and a Fitbit Inspire + Fitbit app for tracking diet and physical activity. The primary aim is to determine whether a greater proportion of ACT participants will achieve a clinically significant weight loss of ≥10% compared with SBT participants at 12-months. Secondary outcomes include change in weight from baseline to 6, 12, and 24-months. Whether the effect of ACT on weight loss is mediated by ACT processes and is moderated by baseline factors will also be examined. If ACT proves efficacious, ACT telehealth coaching will offer an effective, broadly scalable weight loss treatment—thereby making a high public health impact.
Keywords: Acceptance and Commitment Therapy, Obesity, Telehealth, Telephone Coaching, Weight loss
1. INTRODUCTION
Obesity and overweight together are the second leading cause of preventable death in the United States (US),1 contributing to the development of many health conditions including diabetes, heart disease, and cancer.2-4 While some studies have argued that overweight is associated with lower risk of all-cause mortality compared to a healthy weight, other studies have found a continuous increase in the risk of death for overweight or obesity.5,6 Furthermore, there are national health and economic consequences associated with the treatment of obesity and obesity-attributable health conditions.7 The economic burden has been estimated at $48 billion in health care costs annually,8 and there are additional costs associated with obesity-attributable disability and lost work productivity.7,9 With 73.5% of US adults currently with overweight or obesity, this is a public health concern of the upmost importance.10
While numerous studies have shown that face-to-face behavioral weight loss interventions can be effective,11 these approaches require a large time commitment, have limited tailoring to individual needs, and face barriers to scalability and reach especially for those who must travel long distances to receive treatment. In comparison, telehealth coaching for weight loss offers a cost-effective alternative12 that can be effectively delivered via 20-minute telephone calls scheduled at the convenience of the individual, thereby addressing travel burden and time contraints.13,14 Another key advantage of telehealth coaching is the potential for individualized tailoring to participants’ triggers for overeating and lack of physical activity.11,15,16 Finally, telehealth coaching has greater geographical reach, its remote delivery may have health benefits in a pandemic or post-pandemic world (e.g., COVID-19), and it is estimated to remotely reach four times more people than face-to-face weight loss treatment programs.17-20
Despite the potential for great reach and population-level impact of telehealth coaching, current standard behavioral therapy (SBT) telehealth coaching interventions typically do not lead to clinically significant weight loss, whereas trials comparing in-clinic versus telephone-based interventions for weight loss have resulted in equivalent weight loss.20-24 A further challenge in all behavioral weight loss interventions is weight regain after initial weight loss and lack of evidence for long term efficacy (i.e., ≥ 12-months).25,26 Generally, SBT-based approaches to weight loss result in early rapid weight loss followed by a weight plateau and progressive regain.27,28 Needed now are novel telehealth coaching behavioral programs than can improve both initial weight loss and weight loss maintenance.
To address these needs, the current trial tests a novel behavioral weight loss intervention for telehealth coaching based on Acceptance and Commitment Therapy (ACT).29 ACT interventions focus on responding to both internal (stress) and external (high calorie foods) cues for overeating and limiting physical activity.30,31 In contrast to SBT, which emphasizes avoiding external cues to eat,32 ACT focuses on (1) increasing awareness of and willingness to experience internal cues, such as physical cravings that trigger overeating and impede physical activity while (2) making healthy choices guided by deeply held personal values. ACT-based weight loss interventions have been shown to improve both initial weight loss and weight loss maintenance relative to SBT.33-35 While ACT has been applied to many behaviors, for weight loss it has only been tested in randomized controlled trials (RCTs) as face-to-face interventions.16,36-38
Our research team developed an ACT theory-based telehealth coaching protocol39 and tested it against a SBT active control in a 2-arm pilot RCT, enrolling 105 adults recruited from 32 US states with overweight or obesity (mean body mass index, BMI=34.3, 40.7 years old, 58% female, 34% from a racial/ethnic minority group).40 Results showed that a greater proportion of ACT participants (24%) achieved ≥10% weight loss as compared to SBT (13%) at 6 months (OR = 2.45; 95% CI: 0.65, 9.23).40
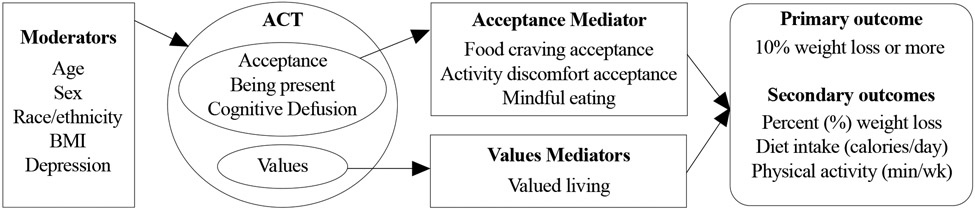
The aim of WeLNES is to compare two telehealth coaching interventions for weight loss, ACT and SBT, among 398 adults with overweight or obesity. We hypothesize that at the 12-month follow-up, a greater proportion of ACT participants will achieve a clinically significant weight loss21 of ≥10% when compared to SBT participants. Further, we hypothesize that ACT-based telehealth coaching’s efficacy on weight loss will be mediated by increasing (1) acceptance of food cravings, (2) acceptance of discomfort from physical activity, (3) mindful eating, and (4) values-guided motivation to change. We will also explore whether the 12-month weight loss outcome differs by age, sex, race/ethnicity, BMI, and depression at baseline.
2. METHODS
2.1. Overview
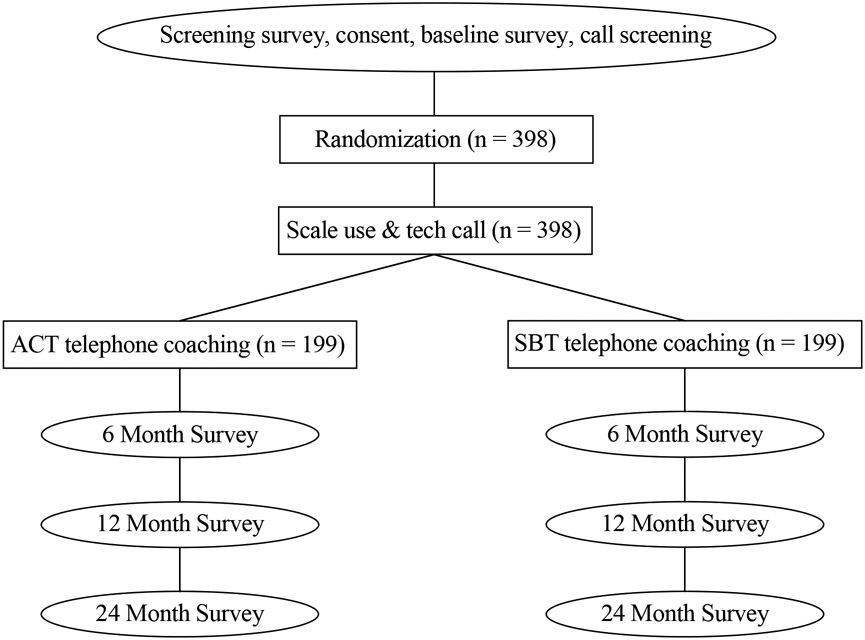
WeLNES is a 24-month, 2-arm, parallel group RCT. The aim of WeLNES is to compare the efficacy of an ACT versus SBT telehealth coaching for weight loss among 398 adults with overweight or obesity (BMI ≥27 to 45.5kg/m2) (Figure 1). The study was pre-registered on ClinicalTrials.gov (Identifier: NCT04447313; dates July 2020-June 2025). All study activities were approved by the Institutional Review Board at the Fred Hutchinson Cancer Center (Protocol number: IR-10404/RG1007177).
Figure 1.
Experimental Design Schema
2.2. Specific aims
2.2.1. Primary aim
Determine whether a greater proportion of ACT participants will achieve a clinically significant weight loss of ≥10% when compared to SBT participants at 12-months.
2.2.2. Secondary outcomes
Compare ACT versus SBT telehealth coaching on change from baseline to 6, 12, and 24-month follow-ups in (a) percent weight loss, (b) dietary intake (daily caloric intake), and (c) physical activity (minutes per week of aerobic physical activity).
2.2.3. Mediators
Determine whether ACT versus SBT telehealth coaching 12-month weight loss main outcome (i.e., ≥10% weight loss) was mediated by these ACT theory-based psychological processes: (a) acceptance of food cravings, (b) acceptance of discomfort from physical activity, (c) mindful eating, and (d) values-guided motivation to change.
2.2.4. Exploratory aim
Explore whether the 12-month weight loss main outcome for ACT versus SBT, differs by these baseline factors: (a) age, (b) sex, (c) race/ethnicity, (d) BMI categories, and (e) depression.
2.3. Participants, recruitment, and randomization
2.3.1. Eligibility
2.3.1.1. Inclusion criteria:
(1) age 18 or older; (2) overweight or obesity (BMI ≥27-45.5 kg/m2); (3) wants to lose weight in the next 30 days; (4) interest in learning skills to lose weight; (5) willing to be randomly assigned to either condition; (6) resides in the US; (7) has access to their own phone and email; (8) does not have a medical or psychiatric condition that would limit their ability to comply with the behavioral recommendations of the program or pose a risk to the participant during weight loss, including meeting criteria for binge eating disorder or severe depression, or a diagnosis of serious heart disease, diabetes, uncontrolled hypertension, or cancer without written confirmation of approval from their physician’s office; (9) not pregnant, planning to become pregnant, or breastfeeding in the next 12 months; (10) have not changed the dosage of prescription medications that can cause a significant change in weight/appetite (past 3 months), (11) have not lost more than 5% of their weight in the past 6 months; (12) able to read in English; (13) not participating in or planning to participate in other weight loss programs (i.e., in-person or telephone counseling, web-based or app-based weight loss programs); (14) has not participated in our other ACT intervention studies; (15) does not meet criteria for combined heavy plus binge drinking; (16) has access to a Bluetooth-enabled device/Wi-Fi; and (17) planning to have or has not recently had (past 12 months) bariatric surgery; (18) willing to complete follow-up surveys; and (19) provide email, phone, and mailing address.
2.3.1.2. Exclusion criteria:
(1) age <18 years old; (2) BMI <27 kg/m2; (3) does not want to lose weight in the next 30 days; (4) not interested in learning skills to lose weight; (5) not willing to be randomly assigned to either condition; (6) does not live in the US, (7) does not have access to their own phone/email; (8) has a medical or psychiatric condition that would limit their ability to comply with the behavioral recommendations; (9) pregnant, breastfeeding, or planning to become pregnant in the next 12 months; (10) changed the dosage of prescription medications that can cause a significant change in weight/appetite (past 3 months); (11) lost more than 5% of their weight in the past 6 months; (12) not able to read in English; (13) participating in or planning to participate in other weight loss programs; (14) previously participated in our other ACT intervention studies; (15) meets criteria for heavy plus binge drinking; (16) no access to a Bluetooth-enabled device/Wi-Fi; and (17) planning to have/has recently had bariatric surgery.
2.3.2. Recruitment and enrollment
We are using recruitment strategies that have yielded high geographical reach in our previous interventions.41 First, we developed and tailored Facebook ads with ongoing adjustment for recruitment yield. Recruitment methods are designed to achieve a broad representation of adults with overweight or obesity (40% male; 40% minority race/ethnicity). Facebook ads are tailored for racial/ethnic minority groups as well as men since these groups are historically underrepresented in US weight loss trials.42,43 Second, we use our registration website to provide information about the study, FAQs, a brief video describing the study, and information about the study team and academic institution. All interested individuals are then directed to complete a web-based screening survey. Those who screen eligible are sent an email inviting them to complete an online survey to provide informed consent and complete the baseline assessment. The baseline survey collects data on (1) socio-demographics, (2) readiness/motivation to lose weight, (3) depression, (4) acceptance of food cravings, (5) discomfort from physical activity, (6) mindful eating, and (7) values-guided motivation to change.
To deter fraud, we use (1) CAPTCHA authentication, (2) IP addresses that were previously used will cause ineligibility, and (3) research staff conduct manual review of participants’ information if survey response times or preferred communication method appear suspicious. Those completing the online enrollment process are further reviewed for eligibility by a phone call from a research staff member. Participants are withdrawn if they no longer are willing or able to comply with the behavioral recommendations of the program or if doing so would pose a health risk to the participant (e.g., becoming pregnant). Everyone not confirmed eligible for any reason is sent a link to the Centers for Disease Control and Prevention Weight Loss and Management resource list and Nutrition.gov weight loss resource.
2.3.3. Randomization and double blinding
We are using permuted block randomization, stratifying on two factors with well-documented disparities in weight loss: (a) sex and (b) race/ethnicity.44,45 Random assignments are concealed from participants throughout the entire trial.46,47 To ensure that participants are blinded, each intervention is branded as “WeLNES”. Research staff have no access to upcoming randomized assignments and are blind to random assignment throughout the duration of the trial. Treatment allocation will remain concealed from investigators until data collection is completed.
2.4. Interventions
2.4.1. Health coaches
The interventions are delivered by trained health coaches with a bachelor’s or master’s degree in social work, psychology, or previous counseling experiences in either ACT or SBT. All health coaches are trained in delivering nutrition/physical activity coaching and supervised by a PhD-level psychologist with experience delivering behavioral interventions or counseling.
2.4.2. Treatment plan and intervention delivery
The treatments in this study are telehealth coaching behavioral interventions. To support weight loss, participants are being mailed an intervention packet containing (1) a resource guide tailored to each treatment arm, (2) a Bluetooth-enabled Fitbit Aria Air scale (Model FB203, Fitbit Inc.©, San Francisco, USA) to self-monitor weight, (3) a Fitbit Inspire (Model FB412BKBK, Fitbit Inc.©, San Francisco, USA) linked to the Fitbit app to self-monitor diet and physical activity; and (3) a food scale, (4) measuring cups, and (5) measuring spoons for tracking caloric intake. The call numbering and scheduling is the same in both arms: 25 one-on-one calls being delivered during 12 months after randomization, including weekly calls 1-16, followed by biweekly calls 17-23, and monthly calls 24-25. Consistent with standard telehealth coaching interventions for weight loss,18,20,32 the length of each call is 25-30 minutes for the first call and 15-20 minutes for all subsequent calls. All participants also receive a technology support call, to help troubleshoot any issues or challenges they might experience setting up the Fitbit Inspire, scale or using the Fitbit app to log food. In the second year, participants receive nine 10-minute booster calls to review progress, goals, and skills learned in year 1 and to review an action plan for tracking calories and physical activity: monthly calls 1-6 and bimonthly calls 7-9.
2.4.3. Shared and distinct components of SBT and ACT telehealth coaching
Health coaches are providing weight loss guidance and individualized tailoring to participants’ triggers for overeating and lack of physical activity equally in both arms (Table 1). Shared components of SBT and ACT telehealth coaching include: (1) nutrition education and calorie intake goals based on initial weight and sex (i.e., 1200-1800 kcal/day), (2) physical activity education with gradual increases to ≥150 minutes/week of physical activity, (3) intention formation, (4) self-monitoring, (5) feedback on progress, (6) stimulus control, (7) relapse prevention, and (8) social support. Weight data is securely viewed by coaches, for discussion of progress during coaching calls. Participants are encouraged to keep daily logs of caloric intake and physical activity via the Fitbit app. These components are very similar to those used in Look AHEAD and the Diabetes Prevention Program protocols.48,49
Table 1.
Shared and distinct components of SBT and ACT telehealth coaching
| Shared Components | 1. Nutritional education, 1200-1800 calorie goal (depending on weight and preferences); 2. Physical activity education, gradual increases up to 150 minutes per week of aerobic activity; 3. Intention formation, including setting specific, actionable and time limited goals for eating and physical activity; 4. Self-monitoring of diet, exercise, & weight. (e.g., weighing & measuring all foods); 5. Provide feedback on progress; 6. Stimulus control (e.g., removal of problematic foods from home and work; portion control); 7. Relapse prevention (e.g., identifying triggers for overeating/lack of physical activity); 8. Social support | |
| Included only in SBT | Included only in ACT | |
|---|---|---|
| General approach for all triggers | Avoidance: Actively trying not to experience urges, emotions, & thoughts with the intent that they change (e.g., desire for urge to reduce). (e.g., Asking: “How can you avoid or control your urges to eat?”) | Acceptance: Openness to experience urges, emotions, and thoughts and without any intent that they change (e.g., no desire that urge reduces). (e.g., Asking: “How willing are you to have, and not try to change, your urges to eat?” |
| Specific approach for cravings or emotions | Avoidant Urge/Emotion Coping Skills: A broad set of strategies designed to manage or control urges and emotions that cue eating/inactivity (e.g., engaging in a distracting activity.) | Being Present: Being fully aware of the present moment with openness and curiosity. Observation and non-judgmental description of experiences in the present moment (e.g., noticing all 5 senses while eating or exercising) |
| Specific approach for thoughts | Cognitive Restructuring: Changing the content of one’s unrealistic/irrational beliefs and/or replacing them with realistic/rational beliefs (e.g., “Eating sweets makes me feel better” is replaced with: “Sweets increase my weight in the long run; I have other ways to cope with feelings.”) | Cognitive Defusion: Stepping back from the process of thinking. Recognizing thoughts, self-judgments, and memories as just words and pictures. Allowing them to come and go without trying to control or avoid them (e.g., imagining thoughts like leaves floating down a stream.) |
| Specific approach for motivation | Expectancies: Beliefs about what actions will produce goal. (e.g., Listing expected outcomes of losing weight vs not losing) | Values: Chosen life directions that guide goals and actions (e.g., creating a personal weight loss vision statement that guides goals and specific actions for diet and activity) |
2.4.4. Active Treatment Control Arm: Standard Behavioral Therapy (SBT)
The overall approach of SBT for addressing weight loss is to “control what you eat and how much you exercise.” SBT encourages individuals to actively try not to experience urges that triggers overeating and impede physical activity with the overall intent of changing the content of thoughts and by directly modifying their food and physical activity environments. For example, a coach would say: “There are some things you can’t control, like seeing an ad for a burger. Some things you can control are making choices about what you eat and how much you exercise. To change your home environment, remove high calorie foods from your home. Stock up your home with low-calorie foods like fruits and vegetables.” In SBT, the approach for addressing cravings that cue overeating and impede physical activity may include distracting strategies, as a form of avoidance, designed to control urges that cue these behaviors. In SBT, participants are motivated to reduce caloric intake/engage in physical activity via expectancies.
2.4.5. Experimental Arm: Acceptance and Commitment Therapy (ACT)
The overall approach of ACT-based intervention for weight loss is a “control what you can and accept what you can’t” framework.33,34 This acceptance-based approach teaches participants to distinguish between aspects of their experience that can be modified (i.e., their food and physical activity environments) versus aspects of their experience that are not under voluntary control (e.g., cravings). ACT focuses on increasing willingness to experience cravings, emotions, and thoughts that trigger overeating and impede physical activity. To illustrate the concept of willingness, a coach would introduce the “Car Journey” metaphor: you are the driver and in the backseat are your triggers for craving high calorie foods. You can try removing these passengers, but they keep coming back. Instead, focus on the road ahead while making room for your passengers”. An experimental exercise in being willing to have “passengers” would be to pause when having an urge, noticing your body sensations, with the goal of not acting on the urge but instead just letting it be as it is. In ACT, participants are motivated to reduce caloric intake and engage in physical activity via encouragement to making healthy choices guided by deeply held values. For example, a coach will ask “What is inspiring you to lose weight?”.
2.5. Measures
2.5.1. Treatment fidelity
An evaluation of the coach’s competence is being rated by two trained independent raters by assessing a 20% random sample of audiotaped calls on adherence to and competence in delivering shared, SBT-only, and ACT-only components of the intervention. Calls are evaluated with a comprehensive rating system that is based on our prior experience with telephone and in-person delivered ACT interventions.16,34,40,50-52 The rating system assesses fidelity to following specific intervention processes: triggers for eating, values, acceptance, committed action, defusion, goal setting, tracking, stimulus control, and social support. Calls are then rated for adherence to treatment and treatment implementation skill. Two raters code each randomly selected call to assess inter-rater reliability. A third rater who is a PhD-level expert in ACT and SBT for weight loss then provides a "gold standard" rating for a 20% random sample subset of all ratings. Discrepancies in ratings are handled through discussion.
2.5.2. Weight outcome measures
Weight is being measured objectively via the Bluetooth-enabled Fitbit Aria scale that securely transmits participant data to the study database, using a standardized protocol that has been previously validated against in-clinic visits.53,54 All participants are instructed in mailed study materials that they should be the only person to use their scale and to follow the scale manual instructions for measurements (e.g., weigh yourself at the same time each day and similarly clothed on a hard, flat surface). After stepping on the scale, users must save weights in the Fitbit app for measurements to be logged. We believe this step, in combination with the robust outlier detection described in Section 2.6.3., will greatly minimize cases of non-study participant weights being recorded. Baseline weight is the first scale weight recorded after randomization and before the start of coaching calls. During the 6, 12, and 24-month follow-ups, participants weigh themselves to assess weight loss for the primary outcome. The scale weight that is timestamped closest to the time of follow-up survey completion is used.
2.5.3. Diet and physical activity outcome measures
Fitbits are mailed to participants at randomization. We ask participants to set up Fitbit accounts and authorize our access to them as part of enrollment. Access is then available within a week of randomization and before they start of coaching calls. Participants can log their food throughout the day to track their estimated calories eaten versus calories burned. Physical activity outcomes are being measured with the Fitbit Inspire via the Fitbit app. These include daily minutes of moderate-to-vigorous physical activity (MVPA) and total step count. Fitbit devices calculate MVPA through metabolic equivalents, which factors in participant weight to estimate intensity. Days with a minimum of 1000 steps recorded will be considered valid wear days.55,56 Measurements at baseline and follow-ups will be averaged over seven days of wear.55,57
2.5.4. Potential mediators
2.5.4.1. Acceptance of food cravings
is being measured using the 10-item Food Craving Acceptance and Action Questionnaire developed to measure the acceptance of cravings to eat or the extent to which individuals might try to control or change food related cravings, which has good reliability (α=0.93) and validity.58 A sample item is: “Despite my cravings for unhealthy foods, I continue to eat healthy”.
2.5.4.2. Acceptance of discomfort from physical activity
is being measured using the 10-item Physical Activity Acceptance Questionnaire developed to measure the extent to which individuals are willing and able to accept discomfort related to engaging in physical activity, which has good reliability (α=0.89) and validity.59 A sample item is “It is okay to experience discomfort while I am exercising”.
2.5.4.3. Mindful eating
is being measured using the 28-item Mindful Eating Questionnaire developed to describe a nonjudgmental awareness of sensations associated with eating, which has good reliability (α=0.84) and predicts lower weight.60 A sample item is: “I recognize when food advertisements make me want to eat”.
2.5.4.4. Valued living
or values guided motivation to change behavior are being measured with the 10-item Valuing Questionnaire developed to measure the enactment of personal values that guide behavior change, which has good reliability (α=0.87) and also predicts weight loss.61 A sample item is: “I worked toward my goals even if I didn’t feel motivated to”.
2.5.5. Potential moderators
ACT-based intervention research suggests that key baseline factors might moderate the impact of ACT on weight loss, including age,62 sex, 63 race/ethnicity,37 BMI categories,16,36 and depression.64,65 These factors define major subgroups of adults who might benefit more from an ACT-based intervention that focuses on responding effectively to internal cues. For example, participants with higher baseline BMI might respond more favorably to an ACT-based approach to weight loss because those with higher BMI may have more difficulty with coping with distress.16,36 Regarding depression, individuals with depression have responded more favorably to an ACT-based approach due its focus on coping with internal distress.64,65
2.5.5.1. Age, sex, and race/ethnicity
are being measured via the baseline survey.
2.5.5.2. BMI
is calculated as weight in kg/m2 using objectively measured weight via scale and self-reported height via the baseline survey.
2.5.5.3. Depression
is being measured using the 10-item CESD scale (cutoff≥10).66
2.5.6. Methods for outcome data retention
An established protocol that we have successfully implemented and that has yielded high outcome data retention rates (85-90%) in our previous trials is being implemented.41 Participants receive $20 at each 6, 12, and 24-month assessment when they provide the scale-measured weight and outcome survey within 15 days, with an extra $20 bonus when completed within 72 hours. Each assessment timepoint has a 90-day completion window from when the follow-up sequence begins: from Day 0, with the first email invitation, and then for 74 days after the final call attempt is made on Day 16, to allow for delayed responses. Participants also get a letter notifying them of the upcoming survey, and a $2 pre-incentive, mailed 2 weeks before the survey window opens. On Day 90, the collection window is closed. Any assessments returned after this point are not included in the analysis. To further promote data retention, emailed newsletters are being sent to participants every 3 months.
2.5.7. Treatment adherence and satisfaction
Treatment adherence is being measured as the number of coaching calls completed. Satisfaction with the intervention and the coaches is being assessed via study questionnaires at each follow-up (e.g., overall satisfaction with the intervention, would recommend coaching program, helpfulness of coaching for losing weight).
2.5.8. Follow-ap data collection
The follow-up period lasts for 24 months and consists of three surveys. The 6, 12 and 24-month survey primarily collects data on ACT-based processes and treatment satisfaction.
2.6. Statistical Analysis Plan
2.6.1. Primary hypothesis
At the 12-month follow-up, participants assigned to ACT telehealth coaching will have a higher proportion of individuals who have achieved ≥10% weight loss than those assigned to SBT telehealth coaching.
2.6.2. Sample size
The sample size was aimed at 80% power using these parameters: (1) participants randomized to one of the two telehealth intervention arms; and (2) two-sided test with α=.05. Sample sizes were calculated using the proportion with ≥10% weight loss at 6-months in our pilot trial40 with penalized imputation to be conservative (i.e., missing values coded as <10% weight loss). Estimates account for a decrease from the observed ≥10% weight loss outcome o f 24% in the ACT arm at 6-months to an estimated 20% at 12-months. The 12-month ≥10% weight loss outcome for SBT counseling was estimated as 10%. Obtaining 80% power to detect these estimated weight loss outcomes requires 398 randomized participants (199 in each arm).
2.6.3. Approach
Baseline demographic and clinical characteristics will be summarized by treatment group. Differences between treatment arms will be assessed using t-tests for continuous variables and Fisher’s exact test for categorical variables to determine the need for covariate adjustment.67 Variables with baseline differences between arms that are predictive of outcome, as well as variables used in the stratified randomization will be included as covariates in analyses.67
Prior to analysis of study outcomes, data will be evaluated for completeness and distributional properties to ensure the appropriateness of analytic models. We will use a multi-step weight measurement cleaning algorithm that examines the standard deviations of consecutively measured weights. 68,69 Clinically implausible measurements occurring on the same day or weights that substantially deviate from plausible trends over time will be flagged as outliers and removed. We will compare the two telehealth interventions on the primary outcome of ≥10% weight loss, with missing 12-month outcome data coded as <10% weight loss. To account for potential within-coach correlations, random effects logistic regression with fixed intervention effect and random coach effects will be fit using R package ‘lme4’.70 The main outcome analysis will be repeated for sensitivity using two robust imputation methods. First, we will use a linear model for missing weights, assuming that dropouts steadily regain weight at a rate of 0.3kg per month.71 Second, we will use multiple imputation of missing weights. Chained equations will be conducted using the R package ‘mice’72 to generate 10 imputed complete data sets which will be pooled for analysis using Rubin’s rules.73 We will also conduct a subgroup analysis of those using other weight loss interventions after randomization. All analyses will exclude any participants who are flagged post-randomization as ineligible (e.g., fraudulent, duplicates). Participants will be coded as fraudulent or duplicate if they fail CAPTCHA authentication, their IP address is duplicated, survey response times are suspiciously short, or review of contact information indicates enrollment in our previous trials.
3. DISCUSSION
Overweight and obesity are a major public health problem that continues to increase.10,74 Telehealth coaching for weight loss with potential for high reach is a population level approach addressing this problem.17-20 However, a critical barrier to progress in the field is that traditional SBT approaches in telehealth coaching interventions have had small effect sizes.18,20-24 ACT approaches may be useful to improve the efficacy of existing weight loss programs by addressing the fundamental challenges of weight loss: overeating in response to cues both internal (stress) and external ( high-calorie foods).
WeLNES is a 24-month, 2-arm RCT designed to test the efficacy of ACT telehealth coaching for weight loss against SBT among adults with overweight and obesity. Results from this trial will demonstrate the efficacy of a newer behavioral approach for weight loss that focuses on helping individuals increase their willingness to experience cravings that trigger overeating and impede physical activity, against a traditional SBT approach that focuses on using reason and logic to control urges to overeat and impede physical activity. Given our recent success with ACT for weight loss in a pilot telehealth coaching intervention,40 telehealth ACT has the potential to boost success over and above SBT telehealth coaching.
3.1. Strengths
The strengths of this trial include: (1) randomized controlled study design, (2) active control comparison, (3) double blinding random assignments, (4) long-term follow-up and maintenance phase to prevent weight regain, (4) clinically significant ≥10% weight loss primary outcome, (5) objectively measured weight outcome, (6) fully-powered sample size to detect weight loss effects, identify mediators, and explore moderators, (7) nationwide recruitment, (8) validated assessments, and (9) coaching implementation rated for treatment fidelity.
3.2. Challenges & potential limitations
Challenges and potential limitations of this trial include (1) high attrition, (2) high dropout, and (3) risk of weight regain after 12-months. To help minimize the limitations associated with attrition and dropout, we are employing retention strategies that yielded high retention rates in previous trials.41 Additionally, robust methods to impute missing data will be used. To help minimize weight regain, the trial is administering nine booster calls in Year 2.
3.3. Conclusions
The project will help shift the behavioral paradigm of telehealth coaching interventions for weight loss. If successful, ACT telehealth coaching will offer a more effective, broadly scalable weight loss treatment—thereby making a high public health impact.
Figure 2.
Conceptual model of telehealth coaching ACT for weight loss
Table 2.
Measurements by timepoint
| Measure | Screen | Baseline | 6-month | 12-month | 24-month | Purpose |
|---|---|---|---|---|---|---|
| Eligibility criteria | X | X | Eligibility | |||
| Demographics | X | Eligibility & moderation | ||||
| Weight (Fitbit Aria Air scale) | X | X | X | X | Primary/secondary aims | |
| Physical activity outcomes (Fitbit Inspire) | X | X | X | X | Secondary aim | |
| Diet outcomes (Fitbit app: food log) | X | X | X | X | Secondary aim | |
| Food Cravings Acceptance Action (FAAQ) | X | X | X | Mediation aim | ||
| Physical Activity Acceptance (PAAQ) | X | X | X | Mediation aim | ||
| Mindful Eating | X | X | X | Mediation aim | ||
| Valuing Questionnaire | X | X | X | Mediation aim | ||
| Depression Scale (CESD-20) | X | Moderation exploratory aim |
CESD, Center for Epidemiological Studies Depression Scale
Acknowledgements:
We appreciate the tireless contributions of the entire study staff, most notably Sara Fey-Hinckley, Jessica C. Harris, Vanessa Imus, Julie S. Packard, Victoria Sanborn, and Christeine Terry. We are very appreciative of the study participants.
Funding:
This study is supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grant R01 DK124114 awarded to Dr. Bricker. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ACT
Acceptance and Commitment Therapy
- BMI
Body Mass Index
- SBT
Standard Behavioral Therapy
- RCT
Randomized Controlled Trial
- US
United States
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Office of the Surgeon General (US). The Surgeon General's Call To Action To Prevent and Decrease Overweight and Obesity. Rockville (MD): Office of Disease Prevention and Health Promotion (US); Centers for Disease Control and Prevention (US); National Institutes of Health (US);2001. [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a Disease: The Obesity Society 2018 Position Statement. Obesity (Silver Spring). 2019;27(1):7–9. [DOI] [PubMed] [Google Scholar]
- 4.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregg EW, Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377(1):80–81. [DOI] [PubMed] [Google Scholar]
- 7.Waters H, Graf M. America’s Obesity Crisis. The Health and Economic Consequences of Excess Weight. Milken Institute; 2018. [Google Scholar]
- 8.Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health. 2017;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. [DOI] [PubMed] [Google Scholar]
- 10.Hales CM, Carroll DM, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief no 360. Hyattsville, MD: National Center for Health Statistics;2022. [Google Scholar]
- 11.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172–1191. [DOI] [PubMed] [Google Scholar]
- 12.Radcliff TA, Bobroff LB, Lutes LD, Durning PE, Daniels MJ, Limacher MC, Janicke DM, Martin AD, Perri MG. Comparing Costs of Telephone vs Face-to-Face Extended-Care Programs for the Management of Obesity in Rural Settings. J Acad Nutr Diet. 2012;112(9):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, Dale MS, Daniels MJ, Radcliff TA, Martin AD. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perri MG, Shankar MN, Daniels MJ, Durning PE, Ross KM, Limacher MC, Janicke DM, Martin AD, Dhara K, Bobroff LB, Radcliff TA, Befort CA. Effect of Telehealth Extended Care for Maintenance of Weight Loss in Rural US Communities: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(6):e206764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob A, Moullec G, Lavoie KL, Laurin C, Cowan T, Tisshaw C, Kazazian C, Raddatz C, Bacon SL. Impact of cognitive-behavioral interventions on weight loss and psychological outcomes: A meta-analysis. Health Psychol. 2018;37(5):417–432. [DOI] [PubMed] [Google Scholar]
- 16.Forman EM, Butryn ML, Manasse SM, Crosby RD, Goldstein SP, Wyckoff EP, Thomas JG. Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity (Silver Spring). 2016;24(10):2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association AT. About telemedicine http://www.americantelemed.org/main/about/telehealth-faqs-. Accessed October 9, 2018.
- 18.Kozak AT, Buscemi J, Hawkins MA, Wang ML, Breland JY, Ross KM, Kommu A. Technology-based interventions for weight management: current randomized controlled trial evidence and future directions. J Behav Med. 2017;40(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev Med. 2017;100:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang JW, Lin YY, Wu NY. The effectiveness of telemedicine on body mass index: A systematic review and meta-analysis. Journal of telemedicine and telecare. 2018:1357633x18775564. [DOI] [PubMed] [Google Scholar]
- 21.U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr., Grossman DC, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Behavioral Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(11):1163–1171. [DOI] [PubMed] [Google Scholar]
- 22.Befort CA, VanWormer JJ, Desouza C, Ellerbeck EF, Gajewski B, Kimminau KS, Greiner KA, Perri MG, Brown AR, Pathak RD, Huang TT, Eiland L, Drincic A. Effect of Behavioral Therapy With In-Clinic or Telephone Group Visits vs In-Clinic Individual Visits on Weight Loss Among Patients With Obesity in Rural Clinical Practice: A Randomized Clinical Trial. JAMA. 2021;325(4):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly JE, Goetz J, Gibson C, Sullivan DK, Lee R, Smith BK, Lambourne K, Mayo MS, Hunt S, Lee JH, Honas JJ, Washburn RA. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity (Silver Spring). 2013;21(10):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, Gibson C, Sullivan DK. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes (Lond). 2007;31(8):1270–1276. [DOI] [PubMed] [Google Scholar]
- 25.Hall KD, Kahan S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am. 2018;102(1):183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordmo M, Danielsen YS, Nordmo M. The challenge of keeping it off, a descriptive systematic review of high-quality, follow-up studies of obesity treatments. Obes Rev. 2020;21(1):e12949. [DOI] [PubMed] [Google Scholar]
- 27.Flore G, Preti A, Carta MG, Deledda A, Fosci M, Nardi AE, Loviselli A, Velluzzi F. Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients. 2022;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paixao C, Dias CM, Jorge R, Carraca EV, Yannakoulia M, de Zwaan M, Soini S, Hill JO, Teixeira PJ, Santos I. Successful weight loss maintenance: A systematic review of weight control registries. Obes Rev. 2020;21(5):e13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1–25. [DOI] [PubMed] [Google Scholar]
- 30.Forman EM, Butryn ML. A new look at the science of weight control: how acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman EM, Butryn ML, Manasse SM, Bradley LE. Acceptance-based behavioral treatment for weight control: a review and future directions. Curr Opin Psychol. 2015;2:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podina IR, Fodor LA. Critical review and meta-analysis of multicomponent behavioral e-health interventions for weight loss. Health Psychol. 2018;37(6):501–515. [DOI] [PubMed] [Google Scholar]
- 33.Forman EM, Butryn ML. Effective Weight Loss: An Acceptance-based Behavioral Approach. New York: Oxford University Press; 2016. [Google Scholar]
- 34.Forman EM, Manasse SM, Butryn ML, Crosby RD, Dallal DH, Crochiere RJ. Long-Term Follow-up of the Mind Your Health Project: Acceptance-Based versus Standard Behavioral Treatment for Obesity. Obesity (Silver Spring). 2019;27(4):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillis J, Dunsiger S, Thomas JG, Ross KM, Wing RR. Novel behavioral interventions to improve long-term weight loss: A randomized trial of acceptance and commitment therapy or self-regulation for weight loss maintenance. J Behav Med. 2021;44(4):527–540. [DOI] [PubMed] [Google Scholar]
- 36.Lillis J, Niemeier HM, Thomas JG, Unick J, Ross KM, Leahey TM, Kendra KE, Dorfman L, Wing RR. A randomized trial of an acceptance-based behavioral intervention for weight loss in people with high internal disinhibition. Obesity (Silver Spring). 2016;24(12):2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butryn ML, Forman EM, Lowe MR, Gorin AA, Zhang F, Schaumberg K. Efficacy of environmental and acceptance-based enhancements to behavioral weight loss treatment: The ENACT trial. Obesity (Silver Spring). 2017;25(5):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattivelli R, Guerrini Usubini A, Manzoni GM, Vailati Riboni F, Pietrabissa G, Musetti A, Franceschini C, Varallo G, Spatola CAM, Giusti E, Castelnuovo G, Molinari E. ACTonFood. Acceptance and Commitment Therapy-Based Group Treatment Compared to Cognitive Behavioral Therapy-Based Group Treatment for Weight Loss Maintenance: An Individually Randomized Group Treatment Trial. Int J Environ Res Public Health. 2021;18(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman EM, Butryn ML. Effective weight loss: An acceptance-based behavioral approach, clinician guide. New York: Oxford University Press; 2016. [Google Scholar]
- 40.Bricker JB, Mull KE, Sullivan BM, Forman EM. Efficacy of telehealth acceptance and commitment therapy for weight loss: a pilot randomized clinical trial. Transl Behav Med. 2021;11(8):1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson NL, Mull KE, Heffner JL, McClure JB, Bricker JB. Participant Recruitment and Retention in Remote eHealth Intervention Trials: Methods and Lessons Learned From a Large Randomized Controlled Trial of Two Web-Based Smoking Interventions. J Med Internet Res. 2018;20(8):e10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haughton CF, Silfee VJ, Wang ML, Lopez-Cepero AC, Estabrook DP, Frisard C, Rosal MC, Pagoto SL, Lemon SC. Racial/ethnic representation in lifestyle weight loss intervention studies in the United States: A systematic review. Prev Med Rep. 2018;9:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring). 2012;20(6):1234–1239. [DOI] [PubMed] [Google Scholar]
- 44.Christensen P, Meinert Larsen T, Westerterp-Plantenga M, Macdonald I, Martinez JA, Handjiev S, Poppitt S, Hansen S, Ritz C, Astrup A, Pastor-Sanz L, Sando-Pedersen F, Pietilainen KH, Sundvall J, Drummen M, Taylor MA, Navas-Carretero S, Handjieva-Darlenska T, Brodie S, Silvestre MP, Huttunen-Lenz M, Brand-Miller J, Fogelholm M, Raben A. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes, obesity & metabolism. 2018;20(12):2840–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis KK, Tate DF, Lang W, Neiberg RH, Polzien K, Rickman AD, Erickson K, Jakicic JM. Racial Differences in Weight Loss Among Adults in a Behavioral Weight Loss Intervention: Role of Diet and Physical Activity. Journal of physical activity & health. 2015;12(12):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juul S, Gluud C, Simonsen S, Frandsen FW, Kirsch I, Jakobsen JC. Blinding in randomised clinical trials of psychological interventions: a retrospective study of published trial reports. BMJ Evid Based Med. 2021;26(3):109. [DOI] [PubMed] [Google Scholar]
- 47.Lang TA, Stroup DF. Who knew? The misleading specificity of "double-blind" and what to do about it. Trials. 2020;21(1):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. [DOI] [PubMed] [Google Scholar]
- 49.Simkin-Silverman LR, Conroy MB, Bhargava T, McTigue KM. Development of an online diabetes prevention lifestyle intervention coaching protocol for use in primary care practice. The Diabetes educator. 2011;37(2):263–268. [DOI] [PubMed] [Google Scholar]
- 50.Bricker JB, Sullivan BM, Mull KE, Torres AJ, Carpenter KM. Full-scale randomized trial comparing Acceptance and Commitment Therapy (ACT) telephone-delivered coaching with standard telephone-delivered coaching among Medicare/uninsured quitline callers. Nicotine Tob Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilardaga R, Heffner JL, Mercer LD, Bricker JB. Do counselor techniques predict quitting during smoking cessation treatment? A component analysis of telephone-delivered Acceptance and Commitment Therapy. Behav Res Ther. 2014;61:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob Res. 2014;16(11):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pebley K, Klesges RC, Talcott GW, Kocak M, Krukowski RA. Measurement Equivalence of E-Scale and In-Person Clinic Weights. Obesity (Silver Spring). 2019;27(7):1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krukowski RA, Ross KM. Measuring Weight with Electronic Scales in Clinical and Research Settings During the Coronavirus Disease 2019 Pandemic. Obesity (Silver Spring). 2020;28(7):1182–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darvall JN, Wang A, Nazeem MN, Harrison CL, Clarke L, Mendoza C, Parker A, Harrap B, Teale G, Story D, Hessian E. A Pedometer-Guided Physical Activity Intervention for Obese Pregnant Women (the Fit MUM Study): Randomized Feasibility Study. JMIR Mhealth Uhealth. 2020;8(5):e15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardcastle SJ, Jimenez-Castuera R, Maxwell-Smith C, Bulsara MK, Hince D. Fitbit wear-time and patterns of activity in cancer survivors throughout a physical activity intervention and follow-up: Exploratory analysis from a randomised controlled trial. PLoS One. 2020;15(10):e0240967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostlind E, Sant'Anna A, Eek F, Stigmar K, Ekvall Hansson E. Physical activity patterns, adherence to using a wearable activity tracker during a 12-week period and correlation between self-reported function and physical activity in working age individuals with hip and/or knee osteoarthritis. BMC Musculoskelet Disord. 2021;22(1):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juarascio A, Forman E, Timko CA, Butryn M, Goodwin C. The development and validation of the food craving acceptance and action questionnaire (FAAQ). Eating behaviors. 2011;12(3):182–187. [DOI] [PubMed] [Google Scholar]
- 59.Butryn ML, Arigo D, Raggio GA, Kaufman AI, Kerrigan SG, Forman EM. Measuring the Ability to Tolerate Activity-Related Discomfort: Initial Validation of the Physical Activity Acceptance Questionnaire (PAAQ). Journal of physical activity & health. 2015;12(5):717–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Framson C, Kristal AR, Schenk JM, Littman AJ, Zeliadt S, Benitez D. Development and validation of the mindful eating questionnaire. Journal of the American Dietetic Association. 2009;109(8):1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smout M, Davies M, Burns N, Christie A. Development of the Valuing Questionnaire (VQ). J Contextual Behav Sci. 2014;3:164–172. [Google Scholar]
- 62.Giluk TL. Mindfulness, Big Five personality, and affect: A meta-analysis. Personality and Individual Differences. 2009;47(8):805–811. [Google Scholar]
- 63.Barnes RD, Tantleff-Dunn S. A preliminary investigation of sex differences and the mediational role of food thought suppression in the relationship between stress and weight cycling. Eat Weight Disord. 2010;15(4):e265–269. [DOI] [PubMed] [Google Scholar]
- 64.Arch JJ, Ayers CR. Which treatment worked better for whom? Moderators of group cognitive behavioral therapy versus adapted mindfulness based stress reduction for anxiety disorders. Behav Res Ther. 2013;51(8):434–442. [DOI] [PubMed] [Google Scholar]
- 65.Gilpin HR, Keyes A, Stahl DR, Greig R, McCracken LM. Predictors of Treatment Outcome in Contextual Cognitive and Behavioral Therapies for Chronic Pain: A Systematic Review. J Pain. 2017;18(10):1153–1164. [DOI] [PubMed] [Google Scholar]
- 66.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 67.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064–1069. [DOI] [PubMed] [Google Scholar]
- 68.Batch BC, Goldstein K, Yancy WS Jr., Sanders LL, Danus S, Grambow SC, Bosworth HB. Outcome by Gender in the Veterans Health Administration Motivating Overweight/Obese Veterans Everywhere Weight Management Program. J Womens Health (Larchmt). 2018;27(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr., Weidenbacher HJ, Livingston EH, Olsen MK. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016;151(11):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bates D, Mächler M, Bolker B, Fitting SW Linear Mixed-Effects Models Using lme4 Journal of Statistical Software. 2015;67(1)(1):1–48. [Google Scholar]
- 71.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. [DOI] [PubMed] [Google Scholar]
- 72.mice: multivariate imputation by chained equations in R. J Stat Softw. 2011. [Google Scholar]
- 73.R DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 74.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. The New England journal of medicine. 2019;381(25):2440–2450. [DOI] [PubMed] [Google Scholar]