Abstract
Background:
Young adults in the general population are at risk of experiencing loneliness, which has been associated with physical and mental health morbidities. The prevalence and consequences of loneliness in young adult survivors of childhood cancer remain unknown.
Methods:
9,664 young adult survivors of childhood cancer (median age at diagnosis 10.5 years [IQR 5-15], 27.1 years at baseline [IQR 23-32]) and 2,221 siblings enrolled in the Childhood Cancer Survivor Study completed a self-reported survey question assessing loneliness on the Brief Symptom Inventory-18 at baseline and follow-up (median follow-up=6.6 years). Multivariable models evaluated the prevalence of loneliness at baseline only, follow-up only, and baseline + follow-up, and its associations with emotional distress, health behaviors, and chronic conditions at follow-up.
Results:
Survivors were more likely than siblings to report loneliness at baseline + follow-up (prevalence ratio [PR] 2.2, 95% confidence interval [CI] 1.7-3.0) and at follow-up only (PR 1.4, 95% CI 1.1-1.7). Loneliness at baseline + follow-up was associated with elevated risk of anxiety (relative risk [RR] 9.8, 95% CI 7.5-12.7), depression (RR 17.9, 95% CI 14.1-22.7), and current smoking (odds ratio [OR] 1.7, 95% CI 1.3-2.3) at follow-up. Loneliness at follow-up only was associated with suicidal ideation (RR 1.5, 95% CI 1.1-2.1), heavy/risky alcohol consumption (RR 1.3, 95% CI 1.1-1.5), and new-onset grade 2-4 chronic conditions (RR 1.3, 95% CI 1.0-1.7).
Conclusions:
Young adult survivors of childhood cancer have elevated risk of experiencing loneliness, which is associated with future emotional distress, risky health behaviors, and new-onset chronic conditions.
Keywords: childhood cancer, survivorship, loneliness, young adult, quality of life
Precis
Young adult survivors of childhood cancer are at elevated risk of loneliness compared to siblings. Loneliness is associated with future emotional distress, risky health behaviors, new-onset chronic health conditions, and future poor quality of life.
Introduction
Over 500,000 survivors of childhood and adolescent cancer currently live in the United States,1 and these survivors have 95% probability of subsequent 15-year survival.2 The majority of childhood cancer survivors reach adulthood and many develop multiple treatment-related adverse physical and psychosocial late effects3–5 that have the potential to further impact adult development and health outcomes.
Young adulthood is a unique developmental period characterized by increasing expectations of independence and attainment of adult milestones. For childhood cancer survivors, the ability to meet these demands can be hindered by the range of physical6,7 and psychological8,9 late effects experienced during young adulthood. Indeed, survivors face challenges related to achieving expected educational attainment, acquiring employment, developing friendships, engaging in social interactions, and establishing intimate relationships including marriage.10–12 Restricted social relationships and isolation are commonly reported by young adult survivors of childhood and adolescent cancer,13 with concerns about not fitting in, feeling lonely, and being misunderstood by peers.
Loneliness, the subjective perception of social isolation,14 is increasingly recognized as an important contributor to poor health, with evidence demonstrating its unique impact on mortality15 as well as physical16,17 and psychological18 morbidity in the general population. Loneliness may result in poor health through multiple mechanisms including unhealthy behaviors, increased reactivity to stress, disrupted processes of sleep restoration and systemic inflammation.14,19 The harmful effects of loneliness through autonomic, endocrine and immune system dysregulation may take time to manifest,20 therefore longitudinal investigations of loneliness trajectories are critical. Longitudinal studies of survivors of adult-onset cancer21 and adult survivors of childhood cancer22 show that lonely survivors report a higher prevalence of pain, depression and fatigue. Despite the potential adverse effects of loneliness, the prevalence, risk factors and longitudinal consequences of loneliness on psychological and physical health remain understudied among young adult survivors of childhood cancer.
Materials and methods
Childhood Cancer Survivors Study (CCSS)
The CCSS is a retrospective cohort with longitudinal follow-up of survivors diagnosed <21 years of age, who were treated at one of 31 participating institutions in North America between 1970 and 1999, and survived ≥5 years after diagnosis of leukemia, lymphoma, central nervous system malignancy, kidney cancer, neuroblastoma, malignant bone tumor, or soft tissue sarcoma.23 A random sample of siblings was also recruited. Participants in the current study were 19–39 years of age at the time of completing a baseline survey and also completed a subsequent follow-up assessment. Participants with proxy-completed surveys were excluded. The final sample included 9,664 survivors (Figure S1; Table S1) and 2,221 siblings. IRB approval was obtained at each participating institution and all participants provided informed consent.
Primary Outcome: Loneliness
Participants completed the Brief Symptom Inventory-18 (BSI-18)24 at baseline and follow-up. The BSI is an 18-item screening tool used to assess acute symptoms of emotional distress that has been validated with adult survivors of childhood cancer.25 Loneliness was assessed using a single item of the BSI-18: “feeling lonely.” Responses were given on a 5-point Likert scale (“not at all”, “a little bit”, “moderately”, “quite a bit”, “extremely”). Participants who endorsed moderate to extreme loneliness were considered to feel lonely. Loneliness endorsed at baseline only, at follow-up only, at baseline and follow-up, and at neither time points, were considered in the current analyses.
Secondary Outcomes
Emotional distress (anxiety, depression and somatization) was measured using the BSI-18, with T-scores ≥63 considered to represent significant symptoms.24 Because the loneliness item contributes to the depression scale, that item was treated as missing and the imputation approach from the BSI-18 manual was used to derive the depression scale score.24 Suicidal ideation was assessed through the item “thought of ending life” of the BSI-18. Because this item also contributes to the depression scale, the analyses of suicidal ideation used a depression scale score derived through a similar imputation approach described above. This is consistent with past approaches used to examine suicide ideation in CCSS.8
Health behaviors included tobacco use, alcohol use and physical activity. Self-reported smoking was used to categorize participants as never smokers (smoked <100 cigarettes in their lifetime), former smokers (smoked ≥100 cigarettes in their lifetime but do not currently smoke), and current smokers (smoked ≥100 cigarettes in their lifetime and smoke now). Alcohol use was classified according to the National Institute for Alcohol Abuse and Alcoholism guidelines as heavy drinking (binge drinking on ≥5 days in the past month) and risky drinking (>3 drinks/day or >7 drinks/week for females; >4 drinks/day or >14 drinks/week for males)26. Physical activity was classified according to CDC guidelines (30 minutes of moderate intensity physical activity on ≥5 days/week or 20 minutes of vigorous intensity physical activity on ≥3 days/week).27
Health-related quality of life was measured using the SF-36,28 with T-scores <40 indicating poor quality of life across eight domains: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional health problems, and mental health.
Chronic health conditions (CHCs) were self-reported on both baseline and follow-up questionnaires. CHCs were grouped into seven organ systems and graded in severity according to the Common Terminology Criteria for Adverse Events v.4.0 (1=mild; 2=moderate; 3=severe; 4=life-threatening).7 The highest grade for any CHC within an organ system was used to assign the grade for that organ system.
Covariates
Demographic and clinical variables included sex, race (white vs. black, other), age at diagnosis, and age at survey completion. Socioeconomic factors included educational attainment, employment in the past year, marital status, independent living, and health insurance status (categories shown in Table 1). Perceived physical health status was self-reported by participants using a single item, with responses ranging from poor to excellent. Treatment exposures included amputation, intrathecal methotrexate, cytarabine, corticosteroids , and radiation (none vs. non-cranial, cranial <20Gy and cranial ≥20Gy; cranial radiation dose was defined as sum of prescribed dose from all overlapping cranial fields).
Table 1.
Demographic and treatment characteristics of young adult survivors of childhood cancer and siblings at baseline.
| Survivors (N = 9,664) |
Siblings (N = 2,221) |
|
|---|---|---|
|
| ||
| Median (IQR) | Median (IQR) | |
|
|
||
| Age at diagnosis (y) | 10.5 (5.40-14.6) | / |
| Age at baseline (y) | 27.1 (23.3-31.5) | 28.9 (24.4,33.4) |
| Age at follow-up (y) | 33.6 (29.8-38.0) | 34.7 (30.3-39.5) |
| Time from diagnosis | 17.5 (14.2-20.8) | / |
| N (%) | N (%) | |
| Sex | ||
| Male | 4,694 (48.0) | 984 (44.3) |
| Female | 4,970 (52.0) | 1,237 (55.7) |
| Race | ||
| White | 8,331 (87.9) | 1,987 (92.5) |
| Black | 413 (4.5) | 44 (2.0) |
| Other | 702 (7.5) | 116 (5.4) |
| Educational attainment | ||
| ≤ High school | 1,899 (20.3) | 364 (16.9) |
| Some college, training | 3,507 (38.1) | 760 (35.4) |
| ≥ College graduate | 3,929 (41.5) | 1,024 (47.7) |
| Employed during the last yeara | ||
| Yes | 8,161 (85.3) | 2,029 (92.4) |
| No | 1,365 (14.7) | 167 (7.6) |
| Marital status | ||
| Single, never married | 4,618 (50.3) | 772 (35.3) |
| Married, living as married | 4,112 (42.7) | 1,251 (57.2) |
| Divorced, separated, widowed | 685 (7.0) | 165 (7.5) |
| Health insurance | ||
| Yes/Canadian resident | 8,141 (85.2) | 1,983 (90.0) |
| No | 1,382 (14.8) | 221 (10.0) |
| Perceived physical health | ||
| Poor/fair | 929 (9.7) | 95 (4.3) |
| Good/very good/excellent | 8,658 (90.3) | 2,106 (95.7) |
| Diagnosis | ||
| Leukemia | 2,691 (35.0) | / |
| CNS tumor | 1,512 (14.1) | / |
| Hodgkin lymphoma | 1,584 (14.8) | / |
| Non-Hodgkin lymphoma | 938 (8.7) | / |
| Soft tissue sarcoma | 777 (7.2) | / |
| Wilms tumors | 679 (6.3) | / |
| Neuroblastoma | 419 (3.9) | / |
| Bone tumors | 1,064 (9.9) | / |
| Amputation | ||
| Yes | 470 (4.4) | / |
| No | 9,180 (95.6) | / |
| Intrathecal methotrexate | ||
| Yes | 2,787 (38.0) | / |
| No | 6,214 (62.0) | / |
| Cytarabine | ||
| Yes | 2,178 (30.6) | / |
| No | 6,885 (69.4) | / |
| Corticosteroids | ||
| Yes | 4,328 (52.4) | / |
| No | 4,735 (47.6) | / |
| Radiation | ||
| None | 3,567 (43.8) | / |
| Non-cranial | 2,505 (25.4) | / |
| Cranial <20 Gy | 943 (11.5) | / |
| Cranial ≥20 Gy | 1,823 (19.2) | / |
Abbreviations: CNS, central nervous system; IQR, interquartile range; Gy, gray.% weighted by inverse probability of sampling.
“Yes” includes full-time employment, part-time employment, and student. “No” includes currently unemployed, unable to work, retired, seeking job, care-home.
Statistical Analysis
Descriptive statistics were calculated for all outcomes, exposures, and covariates. Inverse probability weights were used to account for purposeful under-sampling of leukemia survivors in the expansion cohort of CCSS. Comparison of loneliness prevalence in survivors and siblings was conducted through multinomial logistic regression, accounting for intra-family correlations (21% of the survivors had matched sibling). Multinomial logistic regressions examined associations of loneliness with primary diagnoses and treatment exposures (in separate models to avoid problems due to collinearity of childhood cancer diagnosis and treatments), adjusting for age at follow-up, sex, race, and age at diagnosis. Among survivors, multinomial logistic regression models or modified Poisson regression models29 examined the associations between loneliness and emotional distress, health behaviors and health-related quality of life, at follow-up, as appropriate. All models were adjusted for age at follow-up, sex, race, grade 3-4 CHCs before follow-up, and socioeconomic factors at follow-up (marital status, educational attainment, health insurance status, and employment). Each model was also adjusted for the same construct at baseline (e.g., the model for depression at follow-up was further adjusted for depression at baseline) as appropriate. The model for physical activity was adjusted for perceived health status at baseline. The models for health behaviors were also adjusted for the BSI depressive symptoms without the loneliness item. The model for suicidal ideation was also adjusted for the BSI raw score of depression without loneliness and suicidal ideation items. In sensitivity analyses, models were adjusted for independent living (as an additional indicator of social support) in place of marital status, due to significant overlap between the two variables that precluded inclusions of both in the main models. New-onset CHCs were defined as grade 2-4 CHCs at follow-up among survivors who had grade 0-1 CHCs at baseline (n=4,263; median follow-up=6.6 years). Chi-square tests assessed the distribution of new-onset CHCs by organ system across the loneliness categories. Multivariable modified Poisson regression models examined the associations between loneliness and any new-onset grade 2-4 (versus grade 0-1) CHCs, adjusted for age at follow-up, sex, and race. Further exploratory models were conducted for the organ systems showing unequal distribution of loneliness. Prevalence ratios (PRs), relative risks (RRs) and odds ratio (ORs) with 95% confidence intervals (CIs) are reported. All analyses were conducted using Statistical Analysis System (SAS 9.4, Cary NC).
Results
Characteristics of survivors
Table 1 shows the baseline characteristics of study participants. Survivors were a median of 27.1 years of age at baseline (interquartile range: 23.3-31.5) and 17.5 years from diagnosis (interquartile range: 14.2-20.8). The most prevalent diagnoses included leukemia (35%), CNS tumors (14%), Hodgkin lymphoma (15%), and bone tumors (10%). Fifty-six percent of survivors were treated with radiation therapy (25% non-cranial; 31% cranial).
Prevalence of loneliness
Fourteen percent of survivors reported moderate to extreme loneliness at either baseline or follow-up compared to 9% of siblings (P’s<0.001). Five percent of survivors reported loneliness at both time points compared to 3% of siblings (PR: 2.21: 95% CI, 1.66-2.96; Table 2). Prevalence estimates using ≥a little bit are reported in Table S2. Survivors of leukemia (OR: 2.52, 95% CI, 1.86-3.40), CNS tumors (OR: 2.59, 95% CI, 1.84-3.66), Hodgkin lymphoma (OR: 1.69, 95% CI, 1.17-2.45), soft tissue sarcoma (OR: 1.78, 95% CI, 1.13-2.80), neuroblastoma (OR: 2.32, 95% CI, 1.38-3.89), and bone tumors (OR: 2.12, 95% CI, 1.43-3.14) were more likely to report loneliness at both baseline and follow-up than siblings (Table 3). Table S3 shows the odds of loneliness among survivors only.
Table 2.
Prevalence of moderate to extreme loneliness in young adult childhood cancer survivors and siblings.
| Survivors (N = 9,664) |
Siblings (N = 2,221) |
||||
|---|---|---|---|---|---|
|
|
|||||
| n | % | n | % | PR (95% CI) | |
| Loneliness at either time point* | |||||
| Baseline | |||||
| none/a little bit | 8,190 | 86.1 | 1,985 | 90.4 | 1.00 |
| moderate to extreme | 1,333 | 13.9 | 211 | 9.6 | 1.04 (1.03 - 1.05) |
| Follow-up | |||||
| none/a little bit | 7,565 | 85.9 | 2,004 | 90.6 | 1.00 |
| moderate to extreme | 1,221 | 14.1 | 208 | 9.4 | 1.04 (1.03 - 1.06) |
| Loneliness at two time points | |||||
| Neither baseline nor follow-up | 6,720 | 77.6 | 1,830 | 83.6 | 1.00 |
| Baseline only | 748 | 8.3 | 152 | 6.9 | 1.29 (1.06 - 1.56) |
| Follow-up only | 754 | 8.6 | 148 | 6.8 | 1.37 (1.13 - 1.67) |
| Baseline and follow-up | 448 | 5.4 | 58 | 2.7 | 2.21 (1.66 - 2.96) |
Abbreviations: CI, confidence interval; PR, prevalence ratio.
% among non-missing and weighted by inverse probability of sampling. Loneliness at either time point refers to loneliness status at either baseline or follow-up, independent of loneliness status as at the other time point (e.g., “baseline” indicates loneliness at baseline irrespective of loneliness status at follow-up). Loneliness at two time points refers to loneliness status based on both baseline and follow-up (e.g., “baseline only” indicates loneliness at baseline and not follow-up). Bold font indicates significant results. Missing data for 141 survivors and 25 siblings at baseline, and 878 survivors and 9 siblings at follow-up.
Table 3.
Associations between diagnosis and treatment exposures and moderate to extreme loneliness.
| Baseline only (n = 748) |
Follow-up only (n = 754) |
Baseline and follow-up (n = 448) |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Model 1: Diagnosisa | ||||||
| Siblings | 1.00 | - | 1.00 | - | 1.00 | - |
| Leukemia | 1.21 | 0.97-1.49 | 1.21 | 0.97-1.50 | 2.52 | 1.86-3.40 |
| CNS tumor | 1.24 | 0.95-1.61 | 1.60 | 1.25-2.05 | 2.59 | 1.84-3.66 |
| Hodgkin lymphoma | 1.22 | 0.94-1.59 | 1.19 | 0.91-1.54 | 1.69 | 1.17-2.45 |
| Non-Hodgkin lymphoma | 1.35 | 1.00-1.82 | 1.16 | 0.85-1.59 | 1.33 | 0.83-2.12 |
| Soft tissue sarcoma | 1.47 | 1.08-2.01 | 1.39 | 1.02-1.92 | 1.78 | 1.13-2.80 |
| Wilms tumors | 1.25 | 0.89-1.74 | 1.13 | 0.80-1.61 | 1.27 | 0.76-2.14 |
| Neuroblastoma | 1.23 | 0.81-1.85 | 1.51 | 1.02-2.23 | 2.32 | 1.38-3.89 |
| Bone tumors | 1.31 | 0.98-1.77 | 1.53 | 1.16-2.03 | 2.12 | 1.43-3.14 |
| Model 2: Treatment exposureb | ||||||
| Amputation | 1.67 | 1.14-2.44 | 1.21 | 0.81-1.83 | 1.82 | 1.14-2.91 |
| Intrathecal methotrexate | 1.39 | 1.06-1.81 | 1.10 | 0.84-1.45 | 0.92 | 0.67-1.26 |
| Cytarabine | 0.67 | 0.53-0.86 | 0.97 | 0.76-1.24 | 1.17 | 0.88-1.55 |
| Corticosteroids | 0.97 | 0.78-1.20 | 0.83 | 0.67-1.03 | 1.31 | 1.02-1.70 |
| Non-cranial radiation | 1.29 | 1.04-1.60 | 1.12 | 0.90-1.39 | 1.10 | 0.83-1.44 |
| Cranial radiation < 20 Gy | 1.07 | 0.81-1.41 | 0.98 | 0.74-1.29 | 1.32 | 0.98-1.78 |
| Cranial radiation ≥ 20 Gy | 1.61 | 1.30-1.99 | 1.73 | 1.41-2.12 | 1.56 | 1.21-2.02 |
Abbreviations: CI, confidence interval; CNS, central nervous system; OR, odds ratio. Survivors with loneliness at neither baseline nor follow-up as reference group. Bold font indicates significant results.
Multivariable models adjusted for age at follow-up, sex, and race.
Multivariable models among survivors only adjusted for age at diagnosis, age at follow-up, sex, and race
Treatment exposures associated with loneliness
Survivors treated with intrathecal methotrexate (OR: 1.39, 95% CI, 1.06-1.81), amputation (OR: 1.67, 95% CI, 1.14-2.44), non-cranial radiation (OR: 1.29, 95% CI, 1.04-1.60), and ≥20Gy cranial radiation (OR: 1.61, 95% CI, 1.30-1.99) were more likely to report loneliness at baseline only compared to no loneliness at both time points. Survivors treated with amputation (OR: 1.82, 95% CI, 1.14-2.91), corticosteroids (OR: 1.31, 95% CI, 1.02-1.70), and ≥20Gy cranial radiation (OR: 1.56, 95% CI, 1.21-2.02) were more likely to report loneliness at baseline and follow-up compared to no loneliness at both time points (Table 3).
Loneliness and emotional distress
Loneliness at any time point was significantly associated with increased risk of elevated symptoms of somatization, anxiety, and depression at follow-up (Table 4). For example, loneliness at both time points was associated with increased risk of somatization (RR: 3.22, 95% CI, 2.71-3.84), anxiety (RR: 9.75, 95% CI, 7.47-12.72), and depression (RR: 17.86, 95% CI, 14.09-22.65) at follow-up. Loneliness at baseline only (RR: 1.74, 95% CI, 1.27-2.39) or follow-up only (RR: 1.52, 95% CI, 1.13-2.06) was associated with increased risk of suicidal ideation at follow-up.
Table 4.
Associations between moderate to extreme loneliness at baseline and follow-up and emotional distress and health behaviors at follow-up.
| Emotional distressa | Suicide ideationb | Alcohol usec | Smoking statusd | Physical activitye | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Somatization | Anxiety | Depression | Yes | Heavy/risky drinking | Former smoker | Current smoker | Inactive | |
|
|
||||||||
| RR (95% CI) |
RR (95% CI) |
RR (95% CI) |
RR (95% CI) |
RR (95% CI) |
OR (95% CI) |
OR (95% CI) |
RR (95% CI) |
|
|
|
||||||||
| Loneliness | ||||||||
| Neither baseline nor follow-up | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Baseline only | 1.76 (1.44-2.14) | 2.29 (1.66-3.16) | 3.26 (2.35-4.53) | 1.74 (1.27-2.39) | 0.98 (0.83-1.16) | 1.58 (1.28-1.94) | 1.70 (1.35-2.14) | 0.99 (0.97-1.02) |
| Follow-up only | 3.28 (2.80-3.85) | 9.33 (7.50-11.60) | 19.37 (15.94-23.53) | 1.52 (1.13-2.06) | 1.27 (1.05-1.54) | 1.09 (0.84-1.41) | 1.78 (1.39-2.27) | 0.97 (0.94-1.00) |
| Baseline and follow-up | 3.22 (2.71-3.84) | 9.75 (7.47-12.72) | 17.86 (14.09-22.65) | 1.26 (0.87-1.81) | 1.16 (0.91-1.48) | 1.34 (0.99-1.83) | 1.70 (1.26-2.30) | 0.97 (0.93-1.01) |
| Educational attainment | ||||||||
| > College graduate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Some college, training | 1.50 (1.31-1.73) | 1.09 (0.90-1.31) | 1.05 (0.92-1.20) | 1.08 (0.91-1.29) | 1.06 (0.95-1.19) | 1.88 (1.63-2.15) | 3.61 (3.08-4.23) | 1.02 (1.00-1.04) |
| ≤ High school | 1.82 (1.56-2.13) | 1.27 (1.04-1.56) | 1.03 (0.87-1.23) | 0.91 (0.72-1.16) | 1.06 (0.90-1.25) | 2.08 (1.72-2.51) | 4.71 (3.88-5.73) | 1.06 (1.03-1.09) |
| Employment in the past year | ||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.51 (1.32-1.73) | 1.33 (1.12-1.60) | 1.37 (1.20-1.56) | 0.90 (0.74-1.09) | 0.93 (0.80-1.07) | 1.02 (0.87-1.21) | 1.21 (1.02-1.44) | 1.02 (0.99-1.04) |
| Marital status | ||||||||
| Married, living as married | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Single, never married | 0.93 (0.81-1.07) | 0.80 (0.67-0.96) | 0.87 (0.76-0.99) | 1.36 (1.13-1.64) | 1.04 (0.92-1.16) | 0.77 (0.66-0.89) | 1.10 (0.94-1.30) | 1.03 (1.01-1.05) |
| Divorced, separated, widowed | 1.13 (0.95-1.35) | 1.04 (0.82-1.32) | 1.01 (0.84-1.21) | 1.01 (0.77-1.31) | 1.24 (1.04-1.49) | 1.16 (0.92-1.47) | 2.60 (2.10-3.22) | 1.00 (0.97-1.03) |
| Health Insurance | ||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 1.23 (1.04-1.46) | 1.02 (0.83-1.26) | 1.17 (1.01-1.36) | 1.19 (0.95-1.49) | 1.08 (0.90-1.30) | 1.37 (1.11-1.70) | 2.13 (1.75-2.59) | 0.99 (0.96-1.02) |
Abbreviations: CI, confidence interval; OR, odds ratio; RR, relative risk. All models were adjusted for age at follow-up, sex, race, socioeconomic factors at follow-up (educational attainment, employment in the past year, marital status and health insurance), and grade 3-4 chronic health conditions before follow-up. Bold font indicates significant results.
Model adjusted for emotional distress symptoms at baseline.
Model adjusted for suicide ideation at baseline and raw score of depression (without loneliness and suicidal ideation) at baseline.
Model adjusted for alcohol use at baseline and depression (without loneliness) at follow-up.
Model adjusted for depression (without loneliness) at follow-up.
Model adjusted for physical health status at baseline and depression (without loneliness) at follow-up.
Loneliness and health behaviors
Survivors who reported loneliness at follow-up only (RR: 1.27, 95% CI,1.05-1.54) had an increased risk of heavy/risky alcohol consumption at follow-up compared to survivors who reported no loneliness at both time points (Table 4). For smoking status, loneliness at both time points was associated with increased odds of smoking at follow-up (current smoker: OR: 1.70, 95% CI, 1.26-2.30) compared to loneliness at neither time points (Table 4). Loneliness was not significantly associated with physical activity at follow-up (Table 4). The sensitivity analysis adjusted for independent living showed similar results (Table S4).
Loneliness and health-related quality of life
Loneliness at any time point was associated with increased risk of impaired quality of life at follow-up (Figure 1; Table S5). For example, loneliness at both time points was associated with increased risk of impaired general health (RR: 2.56, 95% CI, 2.26-2.90), impaired social function (RR: 4.95, 95% CI, 4.26-5.74), and impaired mental health (RR: 6.94, 95% CI, 6.14-7.85) at follow-up. The sensitivity analysis adjusted for independent living showed similar results (Table S6).
Figure 1.
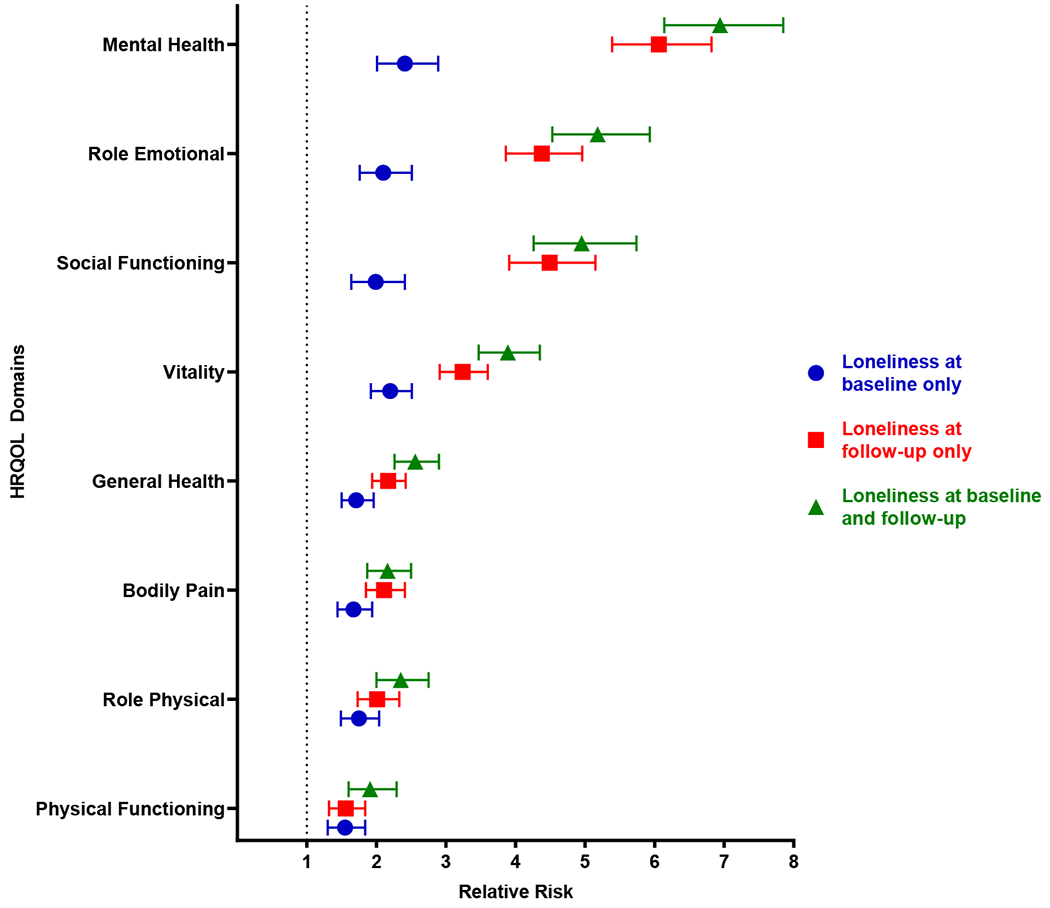
Loneliness and relative risk (RR) of impaired quality of life. RRs and 95% confidence intervals are shown for loneliness at baseline only (blue circles), at follow-up only (red squares), and at baseline and follow-up (green triangles) compared to no loneliness at both time points. The black dotted line represents the null reference association (RR = 1, loneliness at neither time point).
Loneliness and new-onset chronic health conditions
Table S7 shows the distribution of organ-specific new-onset CHCs by loneliness status. Loneliness at follow-up only was associated with any new-onset grade 2-4 (RR: 1.29, 95% CI, 1.01-1.65) and neurologic (RR: 4.37, 95% CI, 2.14-8.91) CHCs (Table 5). The risk of new-onset endocrine CHCs was also elevated (RR: 1.58, 95% CI, 0.99-2.52), but did not reach statistical significance.
Table 5.
Association between moderate to extreme loneliness at baseline and follow-up and grade 2-4 new-onset chronic health conditions at follow-up.
| Any | Neurologic | Endocrine | |
|---|---|---|---|
|
|
|||
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Loneliness | |||
| Neither baseline nor follow-up | 1.00 | 1.00 | 1.00 |
| Baseline only | 1.18 (0.93-1.51) | 2.31 (0.94-5.68) | 1.34 (0.87-2.06) |
| Follow-up only | 1.29 (1.01-1.65) | 4.37 (2.14-8.91) | 1.58 (0.99-2.52) |
| Baseline and follow-up | 0.94 (0.63-1.39) | 2.88 (0.88-9.45) | 1.36 (0.73-2.52) |
Abbreviations: CI, confidence interval; RR, relative risk.
Models were adjusted for age at follow-up, sex, and race. Bold font indicates significant results.
Discussion
In a large cohort of young adult survivors of childhood cancer, we observed an elevated prevalence of loneliness in survivors compared to sibling controls and found that loneliness was associated with emotional, behavioral, and physical health morbidities. Young adult survivors of childhood cancer navigate a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health. Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.
In our sample, 14% of survivors reported moderate to extreme loneliness at baseline, a prevalence significantly greater than observed in siblings. Recently, a small study of German adult survivors of childhood cancer that was not restricted to young adults reported the prevalence of mild, moderate and severe loneliness as 18%.22 If mild loneliness is considered in our sample, the prevalence of loneliness increases to 35%. This heightened prevalence of loneliness in young adulthood is consistent with data from the general population indicating a U-shaped distribution of loneliness, with peaks observed for younger and older adults.30 Importantly, our comparison with siblings without a cancer history suggests that survivors of childhood cancer may be particularly vulnerable to experiencing loneliness during the critical developmental period of young adulthood.
We identified several cancer-related risk factors for loneliness. Survivors of all diagnostic groups except non-Hodgkin lymphoma and Wilms tumor were more likely than siblings to report feeling lonely, suggesting a generalized risk of loneliness in childhood cancer survivors. However, among survivors, those diagnosed with leukemia, CNS tumors and bone tumors were more likely to report loneliness, and treatment exposures common to these diagnoses, including amputation, corticosteroids, and ≥20Gy CRT also were significantly associated with risk of loneliness. Cranial radiation is a well-established risk factor for neurocognitive deficits31 and emotional distress9 which may lead to social isolation and feelings of disconnectedness in subgroups of survivors,32 while amputation and corticosteroids are associated with activity limitations,33 secondary to osteonecrosis34 and reduced bone mineral density.35
Loneliness was strongly associated with risk of future emotional distress symptoms including anxiety, depression, and suicide ideation, consistent with the aforementioned German study.22 Loneliness can adversely impact mental health by triggering psychological processes that increase anger, negative affect, hypervigilance to threats, fear of negative evaluation, and reduce self-esteem, optimism, perceived social competence and social support.36 Moreover, loneliness may lead to suicidal ideation by contributing to the inability to develop a sense of belonging,37 or by acting as a motivational factor where a person perceives no prospect of escape38. We also observed that loneliness was associated with future risky/heaving drinking and smoking. This pattern has been observed in non-cancer populations,39 but is particularly concerning among childhood cancer survivors for whom excessive alcohol consumption and smoking may contribute to their vulnerable health status. We adjusted our analyses for distal (structural) socioeconomic correlates of loneliness,40 which also contributed to the outcomes. However, the strong associations that remain for loneliness highlight the importance of the subjective perception of connectedness for mental health and healthy behaviors in young adult survivors. Moreover, the results of the sensitivity analysis underscore the importance of the subjective experience of feeling lonely, as even after adjusting for living arrangement which may be considered a more objective indicator of social isolation, the impact of loneliness remained significant. Nonetheless, the current study does not allow us to disentangle potential effects of loneliness from different yet related constructs of social health including emotional and tangible social support.
Loneliness at follow-up only was associated with risk of new-onset CHCs, specifically neurological conditions. This is consistent with evidence from the general population identifying loneliness as a unique contributor to metabolic syndrome, coronary artery disease, and stroke.16,17 However, our results suggest a cross-sectional rather than longitudinal associations between loneliness and new-onset CHCs, and causal associations between the two cannot be established. The low frequency of incident CHCs may contribute to these null findings, but reverse directionality whereby CHCs lead to loneliness is also possible. Nevertheless, these results indicated that loneliness has implications for the health of long-term survivors of childhood cancer who are already at risk of developing treatment related CHCs. Lastly, we observed associations between loneliness, especially persistent loneliness, and future reduced quality of life, underscoring the broad negative impact of feeling lonely on the physical and mental health of young adult childhood cancer survivors even decades after their diagnosis and treatment.
Some limitations should be considered when interpreting our findings. Loneliness was assessed using a single item, which is less reliable than multi-item measures.41 Using a single item as a direct measure of acute feelings of loneliness over the past 7 days may have led to either under- or over-estimation of the prevalence of loneliness in our sample. For example, because females are more likely than males to report feeling lonely on direct compared to indirect assessments,42 the larger proportion of males lost to follow-up and the slightly larger proportion of females in the current sample may have led to an overestimation of loneliness in our sample; however, the prevalence of moderate to extreme loneliness at baseline was similar in evaluable participants and those lost to follow-up. Our sample primarily consisted of white survivors and future studies should examine loneliness among more diverse samples given evidence of higher risk of loneliness in underrepresented groups.40 In addition, loss to follow-up in the CCSS cohort may limit the external validity of the current findings though weighting by inverse probability of sampling was used to minimize potential for bias. Compared to survivors who were lost at follow-up, participants in the current analysis had more favourable characteristics on socioeconomic structural correlates of loneliness40 (i.e., marriage, education and employment) and this may have resulted in an underestimation of loneliness and an attenuation of the associations with late outcomes. Because it is possible that the experience of having a sibling with childhood cancer may impact long-term psychological health, use of siblings may result in a biased comparison (i.e., underestimation) of loneliness symptoms in survivors. However, consistent with past CCSS reports,43 siblings in our study reported psychological distress symptoms at or below levels expected in the general population and thus likely reflect an adequate comparison group. Further, the evaluation of CHCs based on self-report without external validation may have limited our ability to detect more robust associations with loneliness, and the temporal associations between loneliness and CHCs cannot be established. Future research should elucidate potential biological mechanism (e.g., DNA methylation) linking loneliness with emotional and physical health in childhood cancer survivors.
Despite these limitations, the results of this study have important implications. The current Children’s Oncology Group Long-term Follow-up Guidelines44 recommend yearly screening for depression, a potential serious antecedent as well as consequence of loneliness.45 A brief assessment of depression that includes assessment of loneliness (e.g., the Center for Epidemiologic Studies Depression scale46) may be particularly important for young adults as more than one-third of survivors in our study reported at least mild loneliness. Attention should also be paid to other psychological risk factors for loneliness, such as social anxiety or poor social network connectedness,13 that could emerge from systematic mental health screenings. There are several psychological interventions aimed at reducing loneliness that may confer benefit to survivors’ mental and physical health, including mindfulness47 and Internet-based cognitive-behavioral48 programs. These interventions have been studies in non-cancer populations and future studies should evaluate their feasibility and efficacy in survivors of childhood cancer.
Our study addresses an important public health concern given the steady increase in loneliness among young adults observed over the past several decades,49 which may be further exacerbated by the recent COVID-19 pandemic.50 While our study focused on young adulthood, future studies should characterize loneliness in the growing aging population of childhood cancer survivors.
Supplementary Material
Funding support
This study was supported by grant U24 CA055727 (Armstrong) from the National Cancer Institute. Additional support was provided to St. Jude Children’s Research Hospital by the Cancer Center Support (CORE) grant CA21765 and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest disclosures
The authors have no relevant disclosures.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nature Reviews Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 4.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 8.Recklitis CJ, Diller LR, Li X, Najita J, Robison LL, Zeltzer L. Suicide ideation in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(4):655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer AG, Verbeek JH, van DFJ. Adult survivors of childhood cancer and unemployment: A metaanalysis. Cancer. 2006;107(1):1–11. [DOI] [PubMed] [Google Scholar]
- 12.Foster RH, Stern M. Peer and romantic relationships among adolescent and young adult survivors of childhood hematological cancer: a review of challenges and positive outcomes. Acta Haematol. 2014;132(3-4):375–382. [DOI] [PubMed] [Google Scholar]
- 13.Poudel PG, Bauer HE, Srivastava DK, et al. Online Platform to Assess Complex Social Relationships and Patient-Reported Outcomes Among Adolescent and Young Adult Cancer Survivors. JCO Clinical Cancer Informatics. 2021;5(5):859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkley LC, Cacioppo JT. Loneliness Matters: A Theoretical and Empirical Review of Consequences and Mechanisms. Annals of Behavioral Medicine. 2010;40(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rico-Uribe LA, Caballero FF, Martín-María N, Cabello M, Ayuso-Mateos JL, Miret M. Association of loneliness with all-cause mortality: A meta-analysis. PLOS ONE. 2018;13(1):e0190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriksen RE, Nilsen RM, Strandberg RB. Loneliness as a risk factor for metabolic syndrome: results from the HUNT study. J Epidemiol Community Health. 2019;73(10):941–946. [DOI] [PubMed] [Google Scholar]
- 17.Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland H, Evans JJ, Nowland R, Ferguson E, O’Connor RC. Loneliness as a predictor of suicidal ideation and behaviour: a systematic review and meta-analysis of prospective studies. Journal of Affective Disorders. 2020;274:880–896. [DOI] [PubMed] [Google Scholar]
- 19.Smith KJ, Gavey S, Riddell NE, Kontari P, Victor C. The association between loneliness, social isolation and inflammation: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2020;112:519–541. [DOI] [PubMed] [Google Scholar]
- 20.Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain, behavior, and immunity. 2003;17 Suppl 1:S98–105. [DOI] [PubMed] [Google Scholar]
- 21.Jaremka LM, Andridge RR, Fagundes CP, et al. Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychology. 2014;33(9):948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst M, Brähler E, Wild PS, Faber J, Merzenich H, Beutel ME. Loneliness predicts suicidal ideation and anxiety symptoms in long-term childhood cancer survivors. International Journal of Clinical and Health Psychology. 2021;21(1):100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute–Supported Resource for Outcome and Intervention Research. Journal of Clinical Oncology. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derogatis LR. BSI18, Brief Symptom Inventory 18: Administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson; 2001. [Google Scholar]
- 25.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory--18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18(1):22–32. [DOI] [PubMed] [Google Scholar]
- 26.National Institute on Alcohol Abuse and Alcoholism. The Physician’s Guide to Helping Patients with Alcohol Problems. National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health; 1995. NIH Pub. No. 95–3769. [Google Scholar]
- 27.U.S. Department ofHealth and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, D.C.: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 28.Ware JE Jr, . SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–3139. [DOI] [PubMed] [Google Scholar]
- 29.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 30.Lasgaard M, Friis K, Shevlin M. “Where are all the lonely people?” A population-based study of high-risk groups across the life span. Social Psychiatry and Psychiatric Epidemiology. 2016;51(10):1373–1384. [DOI] [PubMed] [Google Scholar]
- 31.van der Plas E, Nieman BJ, Butcher DT, et al. Neurocognitive Late Effects of Chemotherapy in Survivors of Acute Lymphoblastic Leukemia: Focus on Methotrexate. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2015;24(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- 32.Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I’ll show them: the social construction of (in)competence in survivors of childhood brain tumors. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2008;25(3):164–174. [DOI] [PubMed] [Google Scholar]
- 33.Marina N, Hudson MM, Jones KE, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: a report from the childhood cancer survivor study. Arch Phys Med Rehabil. 2013;94(6):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in Adult Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2008;26(18):3038–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–713. [DOI] [PubMed] [Google Scholar]
- 36.Cacioppo JT, Hawkley LC, Ernst JM, et al. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40(6):1054–1085. [Google Scholar]
- 37.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE. The interpersonal theory of suicide. Psychological Review. 2010;117(2):575–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor RC, Kirtley OJ. The integrated motivational–volitional model of suicidal behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018;373(1754):20170268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyal SR, Valente TW. A Systematic Review of Loneliness and Smoking: Small Effects, Big Implications. Substance Use & Misuse. 2015;50(13):1697–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkley LC, Hughes ME, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. From Social Structural Factors to Perceptions of Relationship Quality and Loneliness: The Chicago Health, Aging, and Social Relations Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2008;63(6):S375–S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolaisen M, Thorsen K. Who are Lonely? Loneliness in Different Age Groups (18–81 Years Old), Using Two Measures of Loneliness. The International Journal of Aging and Human Development. 2014;78(3):229–257. [DOI] [PubMed] [Google Scholar]
- 42.Shiovitz-Ezra S, Ayalon L. Use of Direct Versus Indirect Approaches to Measure Loneliness in Later Life. Research on Aging. 2012;34(5):572–591. [Google Scholar]
- 43.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):435–446. [DOI] [PubMed] [Google Scholar]
- 44.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers v5.0. October 2018 October 2018. [Google Scholar]
- 45.Ren L, Mo B, Liu J, Li D. A cross-lagged regression analysis of loneliness and depression: A two-year trace. European Journal of Developmental Psychology 2020;19(2):198–212. [Google Scholar]
- 46.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research In the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 47.Lindsay EK, Young S, Brown KW, Smyth JM, Creswell JD. Mindfulness training reduces loneliness and increases social contact in a randomized controlled trial. Proceedings of the National Academy of Sciences. 2019;116(9):3488–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Käll A, Jägholm S, Hesser H, et al. Internet-Based Cognitive Behavior Therapy for Loneliness: A Pilot Randomized Controlled Trial. Behavior Therapy. 2020;51(1):54–68. [DOI] [PubMed] [Google Scholar]
- 49.Buecker S, Mund M, Chwastek S, Sostmann M, Luhmann M. Is loneliness in emerging adults increasing over time? A preregistered cross-temporal meta-analysis and systematic review. Psychol Bull. 2021;147(8):787–805. [DOI] [PubMed] [Google Scholar]
- 50.Yan A, Howden K, Mahar AL, et al. Experiences of adolescent and young adult cancer survivors during the COVID-19 pandemic. Journal of Cancer Survivorship. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


