Abstract
Amblyopia is a disorder of neurodevelopment that occurs when there is discordant binocular visual experience during the first years of life. While treatments are effective in improving visual acuity, there are significant individual differences in response to treatment that cannot be attributed solely to difference in adherence. In this considerable variability in response to treatment, we argue that treatment outcomes might be optimized by utilizing deep phenotyping of amblyopic deficits to guide alternative treatment choices. In addition, an understanding of the broader knock-on effects of amblyopia on developing visually-guided skills, self-perception, and quality of life will facilitate a whole person healthcare approach to amblyopia.
Keywords: Amblyopia, Child development, Deep phenotyping, Whole person health
1. Introduction
1.1. Amblyopia
Amblyopia is a disorder of neurodevelopment that occurs when there is discordant binocular visual experience during the first years of life. It is the most common cause of monocular visual acuity impairment in children, affecting 2–4% (Birch, 2013). Clear retinal images in each eye and straight ocular alignment are necessary conditions for normal visual development. Anisometropia and strabismus are two common pediatric eye conditions with discordant visual input that can disrupt visual development, placing children at risk for amblyopia. The predominant theory is that amblyopia is a result of the mismatch between the images to each eye; information from the misaligned strabismic eye or blurred eye with higher refractive error is suppressed (Birch, 2013; Hess and Thompson, 2015).
Patching or penalization of the fellow eye with atropine or filters are mainstays of amblyopia treatment. These treatments are effective in children ≤7 years old, with diminished benefit for older children (Holmes and Levi, 2018). Binocular amblyopia treatments show similar effectiveness to patching in children ≤7 years old but, like patching and atropine, are less effective for older children, teens, and adults (Birch et al., 2020b). Even among children aged ≤7 years, complete recovery of normal visual acuity occurs in less than 50% of children regardless of treatment approach; many amblyopic children have lifelong residual amblyopia post-treatment (Awan et al., 2005; Birch et al., 2004, 2019b; Birch and Stager, 2006; Jost et al., 2020, 2022; Kelly et al., 2016b; Pediatric Eye Disease Investigator Group, 2003a, b, 2010; Stewart et al., 2004; Woodruff et al., 1994) and regression following treatment is common (Bhola et al., 2006; Birch, 2013; Holmes et al., 2004, 2007). See Table 1 for information about who responds better to amblyopia treatment. Recent research from our lab and others has revealed that amblyopia has a complex phenotype with individual variability in treatment response and a broad impact on the whole child.
Table 1.
Randomized clinical trials evaluating patching and binocular treatment for amblyopia.
| Study | Intervention | Comparison Group | VA improved | VA improved more | Primary outcome (weeks) | Mean improvement (lines) | Mean Age (y) (range) | Adherence | >7 y (%) | Prior treatment (%) | Not orthotropic (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Pediatric Eye Disease Investigator Group et al., 2006b) | patching | glasses | Yes | Yes | 5 | 1.1 | 5.4 (3–7) |
“good to excellent” | 7% | 11% | NA |
| PEDIG (2010) | patching | Bangerter filter | Yes | No | 24 | 1.9 | 6.3 (3–9) |
“good to excellent” | 36% | 17% | NA |
| (Wang and Neely, 2016) | patching | Intermittent occlusion | Yes | No | 12 | 1.5 | 5.8 (3–8) |
92% | 12% | 0% | NA |
| (Holmes et al., 2016) | dichoptic game (reduced fellow eye contrast) | patching | Yes | No | 24 | 1.1 | 8.5 (5–12) |
46% | 76% | 78% | 32% |
| Kelly et al. (2016b) | patching | Yes | Yes | 4 | 1.8 | 6.8 | 103% | 25% | 58% | 21% | |
| (Birch et al., 2020b) | (4–10) | ||||||||||
| (Gao et al, 2018) | sham game | No | No | 6 | 0.6 | 13.6 (7-adult) |
“moderate to poor” | 61% | 77% | 25% | |
| (Manh et al., 2018) | patching | No | No | 16 | 0.7 | 14.3 (13–16) |
21% | 100% | 84% | 37% | |
| (Pediatric Eye Disease Investigator Group, 2019) | glasses | Yes | No | 4 | 1.3 | 9.6 (7–12) |
65% | 100% | 96% | 34% | |
| Manny et al. (2022) | glasses | Yes | Yes | 4 | 1.1 | 5.8 (4–7) |
45% | 0% | 64% | 18% | |
| (Rajavi et al., 2021) | dichoptic VR game (fellow eye views background and fixed objects with desaturated color, blur, and reduced contrast) | patching | Yes | No | 4 | 0.7 | 7.1 (4–11) |
84% | NA | NA | 0% |
| Jost et al. (2022) | dichoptic videos (reduced fellow eye contrast or fellow eye blur) | patching | Yes | No | 2 | 0.7 | 6.0 (3–7) |
95% | 0% | 80% | NA |
| (Xiao et al., 2022) | glasses | Yes | Yes | 12 | 1.8 | 6.1 (4–7) |
88% | 0% | 76% | NA | |
| (Wygnanski-Jaffe et al., 2022) | patching | Yes | No | 16 | 2.8 | 6.8 (4–9) |
91% | NA | 52% | 8% |
All RCTs included spectacle adaptation prior to treatment start and treatment was administered at-home.
1.2. Variability in response to amblyopia treatment
Failure to recover normal visual acuity with patching treatment has often been ascribed to poor adherence. Patching is associated with skin irritation, red eyelids, and itching in 28–54% of children, including 6–8% who have a severe adverse reaction (Menon et al., 2008; Pediatric Eye Disease Investigator Group, 2002b). Atropine treatment is associated with mild reduction in visual acuity of the fellow eye and light sensitivity (Pediatric Eye Disease Investigator Group, 2002b; Scheiman et al., 2008). Most of the available dichoptic games for binocular amblyopia treatment are not engaging enough to promote good adherence for more than 2–4 weeks (Manh et al., 2018; Manny et al., 2022; Pediatric Eye Disease Investigator Group, 2019). Poor adherence to prescribed treatment may also be associated with parents’ poor understanding of amblyopia and their role in managing it, family stress associated with treatment, social stigma of treatment, and socioeconomic factors (Cole et al., 2001; Dixon-Woods et al., 2006; Holmes et al., 2008; Newsham, 2000; Wang, 2015).
When objectively monitored with a sensor under the patch, patching adherence is only 33–58% (Awan et al., 2005; Stewart et al., 2004, 2017). Moreover, adherence decreases from an initial level to 40%–60% after week 6 and 30% after week 12 (Loudon et al., 2002; Stewart et al., 2001, 2017; Wallace et al., 2013), consistent with the finding that 80% of visual acuity improvement occurs during the first 6 weeks of patching treatment (Stewart et al., 2004, 2017). Nevertheless, this is not the whole story. Excellent adherence with patching does not always ensure a good outcome. With 200 hours of patching, verified objectively with a sensor, visual acuity improvement ranges from 0.2 to 0.8 logMAR (Fig. 1A) (Stewart et al., 2004). Even with 300–800 hours of patching, some children respond with as little as 0.1 logMAR (1 line) improvement in visual acuity. It is also clear that adherence to glasses wearing is good (70%), but less than optimal among amblyopic children treated with patching and this may affect treatment response (Maconachie and Gottlob, 2015). Individual variability in the dose-response to binocular treatment has also been reported (Birch et al., 2020b) (Fig. 1B).
Fig. 1.
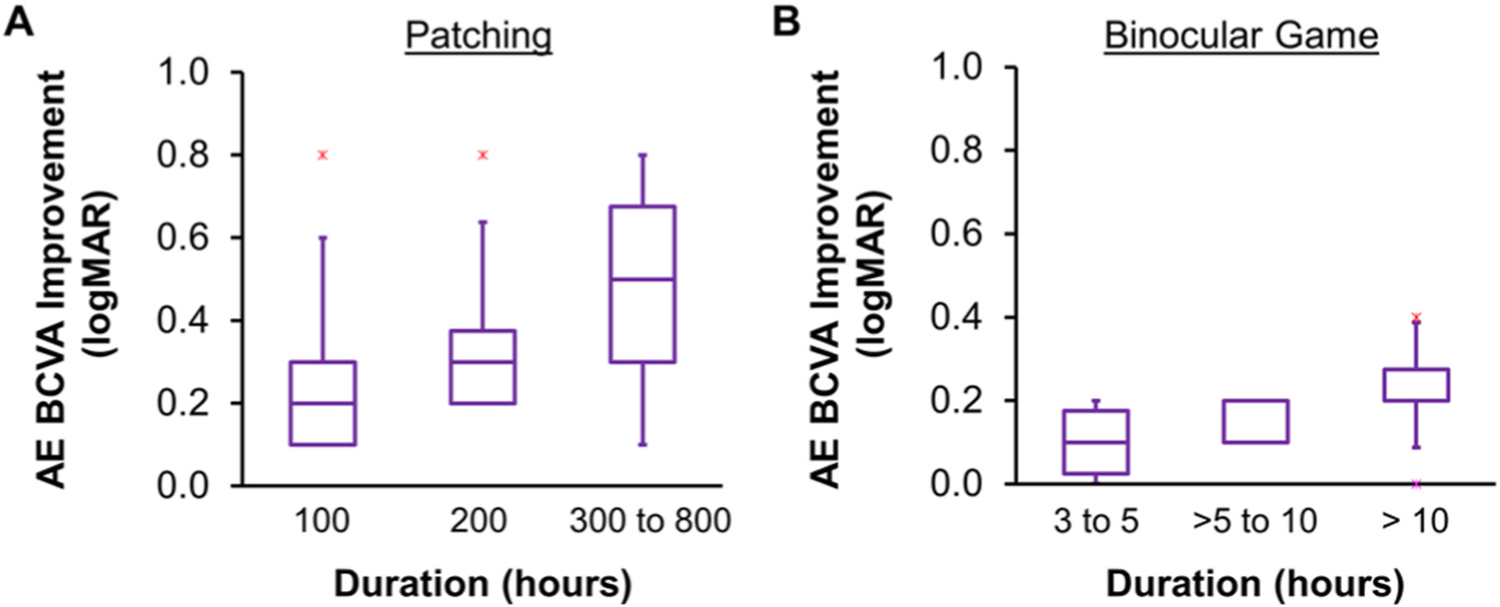
Objectively monitored dose-response for (A) patching (Stewart et al., 2004a) and (B) binocular game treatment (Birch et al., 2020b) to illustrate the dose-response relationships. Note that, even within a group of children who achieved approximately the same number of hours of a treatment, there were considerable individual differences in the amount of visual acuity improvement. The horizontal line within each box represents the median improvement in amblyopic eye best corrected visual acuity (AE BCVA, logMAR), the boxes correspond to the 25th to 75th percentiles, and the whiskers correspond to the fifth and 95th percentiles.
Clearly, poor adherence is not the only factor influencing visual acuity outcome — other unidentified factors must contribute to individual differences. This argues for a personalized approach to amblyopia treatment, choosing among a variety of effective approaches to determine what works best for each child.
1.3. Improving amblyopia treatment outcomes
Our knowledge base for amblyopia has expanded greatly over the past 25 years. Researchers have identified the broader effects of amblyopia on visual function, carefully detailing a large constellation of sensory deficits as well as knock-on effects on reading, visually-guided skills, self-perception, and quality of life. Yet, there remain significant gaps in utilizing these emerging data to guide precision medicine and whole person healthcare. In the setting of considerable variability in response to patching treatment, treatment outcomes might be optimized by utilizing deep phenotyping of amblyopic deficits to guide alternative treatment choices. In addition, an understanding of the broader effects of amblyopia on developing reading, visually-guided skills, self-perception, and quality of life will facilitate a whole person healthcare approach to amblyopia.
2. Deep phenotyping of amblyopia
2.1. Sensory and ocular motor deficits in amblyopia
While the hallmark of amblyopia is reduced visual acuity accompanied by an amblyogenic factor (typically strabismus or anisometropia), recent research has illuminated the broader scope of the effects of abnormal visual experience on the developing visual system. Initial phenotyping of amblyopia was based on the amblyogenic factor and, on that basis, sub-classified as strabismic, anisometropic, or combined mechanism amblyopia. Studies comparing these subtypes of amblyopia reached diverse conclusions about whether they differ in patterns of visual deficits. One telling example is the comparison of reports that show unlike those with anisometropic amblyopia, adults with strabismic amblyopia have disproportionately larger deficits in optotype acuity and vernier acuity than in grating acuity (Levi and Klein, 1982a; Levi and Klein, 1982b; Levi and Klein, 1985; Levi et al., 1994). We confirmed that, among children with moderate amblyopia, the ratio of vernier to grating acuity was significantly different for anisometropic versus strabismic etiology but, among children with severe amblyopia, the difference between subgroups was not evident (Birch and Swanson, 2000). We suggested that the functional distinctions between anisometropic and strabismic subgroups might not depend on which amblyogenic factor was present, but instead on factors such as age of onset and severity of amblyopia.
It remains unclear whether there are distinctive patterns of deficits in subgroups of children with amblyopia. The limited inventory of assessments that are typical of clinical evaluation may account for this uncertainty. Early discordant binocular visual experience can yield a broad range of sensory deficits in children (Table 2), including impaired amblyopic eye visual acuity (Birch and Holmes, 2010; Pediatric Eye Disease Investigator Group, 2002a), contrast sensitivity (Abrahamsson and Sjostrand, 1988; Repka et al., 2009; Rogers et al., 1987), vernier acuity (Birch and Swanson, 2000; Drover et al., 2010; Subramanian et al., 2012), spatial integration (Chandna et al., 2001, 2004; Jeffrey et al., 2004; Subramanian et al., 2012), global motion perception (Meier et al., 2016), and perception of motion-defined form (Giaschi et al., 2015; Hayward et al., 2011; Ho and Giaschi, 2009), as well as crowding (Greenwood et al., 2012; (Levi and Klein, 1985); Levi et al., 2007), deficits in fellow eye function (Birch et al., 2019c, 2019d; Giaschi et al., 1992; Ho et al., 2005), and in binocular vision (Birch and Holmes, 2010; Birch et al., 2016; Li et al., 2011; Narasimhan et al., 2012; Pediatric Eye Disease Investigator Group, 2002a; Webber et al., 2020; Webber et al., 2019). Additionally, amblyopia is associated with ocular motor deficits, including poor accommodative accuracy (Chen et al., 2018; Jost et al., 2019; Manh et al., 2015), fixation instability (Birch et al., 2013; González et al., 2012; Kelly et al., 2019; Shaikh et al., 2016; Subramanian et al., 2013), vergence instability (Kelly et al., 2019), and alterations in saccades (Kelly et al., 2022). Not all of these deficits are present in every child with amblyopia. Deep phenotyping would provide a complete description of the spectrum of visual abnormalities associated with amblyopia in individual children. This knowledge may be useful to guide treatment in the context of precision medicine; that is, it may be useful to provide the best available care for each individual, based on distinct phenotypic classification.
Table 2.
Visual and ocular motor deficits in children with amblyopia.
2.2. Deep phenotyping
An initial attempt at a phenotypic classification of amblyopia was described by McKee and colleagues in 2003 (McKee et al., 2003). They evaluated visual acuity, contrast sensitivity, and binocular function in a large cohort of adults with strabismic, anisometropic, or combined mechanism amblyopia. Factor analysis revealed two main dimensions of variation in visual performance, one related to resolution (optotype, vernier, and grating acuity) and the other related to contrast sensitivity. A third dimension, binocular vision, was identified separately. The distinctive distributions of visual loss for different clinical subgroups of amblyopic adults was consistent with the hypothesis that deficits in resolution and binocularity are the primary determinants of the pattern of visual deficit. The impact of this phenotypic classification of amblyopia based on three dimensions has been limited, however, because it primarily segregated groups along the same boundaries as the amblyogenic factors of strabismus, anisometropia, or both. Namely, combined mechanism amblyopia was associated with severe deficits in resolution and binocularity, strabismic amblyopia was associated with a moderate deficit in resolution and a severe deficit in binocularity, and anisometropic amblyopia was associated with moderate deficits in resolution, binocularity, and contrast sensitivity. Since the time data were collected for the study, many other sensory and ocular motor deficits have been described in children with amblyopia and other amblyopia treatment options have become available. An updated, more comprehensive approach to phenotyping is needed to guide which treatment approach is best for each child.
Initially, precision medicine was aimed at taking advantage of genetic markers to direct treatment choice. This narrow approach simply reduces patients to their genetic profiles and is a different way of grouping patients, not personalizing their treatment. More recently, advocates of precision medicine have emphasized the importance of carefully defining the individual phenotype beyond what is typically recorded in medical charts (Delude, 2015). Because little is known about the genes that may contribute to the development of amblyopia, the success of deep phenotyping in amblyopia will depend on careful characterization of sensory and ocular motor deficits. Use of additional sensory and ocular motor tests (Table 2) and expansion of the scope of health-related data to include variables that have been shown to affect amblyopia outcomes (Table 3) (Abbott and Shah, 2020; Birch and Holmes, 2010; Holmes et al., 2011; Holmes and Levi, 2018; Pediatric Eye Disease Investigator Group, 2002a; Repka, 2020; Repka et al., 2022; Townsend, 2009) are essential to understanding individual differences in response to the various amblyopia treatment options. Algorithms to integrate sensory and ocular motor data and personal information could then be used to guide precision medicine, and suggest measures to ameliorate the effects of multiple deprivation and health disparities.
Table 3.
Health-related data by exploring known to affect amblyopia outcomes.
| Age | (Birch and Holmes, 2010; Holmes et al., 2011; Holmes and Levi, 2018; Pediatric Eye Disease Investigator Group, 2002a; Repka et al, 2022) |
| Race/ethnicity | (Repka, 2020; Repka et al, 2022) |
| Medicaid/CHIP/private insurance | (Repka et al, 2022) |
| Multiple deprivation: overall health, diet, clothing, housing, environmental, educational, working and social conditions | Townsend (2009) |
| Health disparity: Deprived or adverse family background | Abbott and Shah (2020) |
2.3. Matching treatment options with deep phenotype
While deep phenotyping is not yet routinely used to guide amblyopia treatment, there are some hints in the literature as to how it might be applied. For binocular amblyopia treatment, we know that outcomes are better for younger children (aged 3–6 years) than for older children (Birch et al., 2015; Holmes et al., 2011; Holmes and Levi, 2018; Jost et al., 2020, 2022; Kelly et al., 2016b; Manny et al., 2022), children who have moderate amblyopia and are orthotropic (Birch et al., 2019b), and children who have had no prior response to patching treatment (Jost et al., 2022). Patching treatment is more appropriate than binocular amblyopia treatment for children with strabismus because most binocular treatments are intolerant of strabismus or tolerant only of micro-tropia. Additionally, we know that children with severe amblyopia have larger visual acuity gains with patching treatment than those with moderate amblyopia, especially those aged 3–5 years (Holmes et al., 2011), but may not be able to participate in binocular treatment because severe amblyopia is associated with severe suppression (Birch et al., 2016; Li et al., 2011). Treatment with spectacles alone is effective in achieving full recovery of visual acuity in 32% of children less than 7 years old, but only in 14% of those aged 7–12 years and 11% in children aged 13–17 years (Asper et al., 2018; Pediatric Eye Disease Investigator Group et al., 2006a(Pediatric Eye Disease Investigator Group et al., 2006b); Pediatric Eye Disease Investigator Group et al., 2012). Addressing the need to also rehabilitate fellow eye vision in some children with amblyopia, fellow eye motion perception deficits improve with binocular treatment (Webber et al., 2019) but not with patching (Giaschi et al., 2015). Finally, it is clear that children from socioeconomically-deprived homes and those with an adverse family background need additional support for them and their families to achieve the same vision outcomes as children from non-deprived stable homes (O’Colmain et al., 2020). Suggestions to minimize the effects of multiple deprivation and health disparities include developing methods to improve parental education about amblyopia and their role in its management (Alsaqr and Masmali, 2019; Holmes and Levi, 2018; Repka, 2020; Repka et al., 2022; Tjiam et al., 2012; Wang, 2015), education of children about the importance of timely treatment and adherence (Aljohani et al., 2020), increasing awareness about unconscious provider bias (Ricks et al., 2022), and providing school-based eye care (Black et al., 2019; Burnett et al., 2018; Chu et al., 2015; Collins et al., 2022; Lyons et al., 2011; Mudie et al., 2022; Repka et al., 2022). At this early stage, attempts at using amblyopia phenotyping to guide treatment are piecemeal. Nonetheless, these examples highlight the need for routinely gathering additional sensory and ocular motor data and for expanding the scope of health-related data collected.
3. Whole person health and amblyopia
3.1. Amblyopia and development
While the initial development of visual system circuitry is guided by a sequence of patterned gene expression that interacts with intrinsically-driven activity, postnatal concordant binocular visual experience is needed to optimize function. If discordant binocular visual experience occurs, due to strabismus or anisometropia, the vulnerability of the visual system during this critical period can misguide its development, posing a risk for amblyopia. Strabismus significantly reduces the number of binocular neurons in visual cortex, yielding a visual system with neurons that mostly connect to one or the other eye, but not to both (Kiorpes et al., 1998). Anisometropia can result in a diminished neural representation of the eye with chronic blur and a reduction in binocularly-driven neurons (Kiorpes et al., 1998). In primary visual cortex, the changes in neural circuitry are clearly associated with deficits in visual acuity and contrast sensitivity for the amblyopic eye (Kiorpes et al., 1998). Higher visual cortical areas are also affected by discordant binocular visual experience. In visual area V2, amblyopia is associated with neurons that exhibit noisy spiking (Wang et al., 2017), and receptive fields that are spatially disordered (Tao et al., 2014). In the middle temporal (MT) motion processing area, neurons that normally favor binocular stimulation are altered to favor stimulation by the non-amblyopic eye, with deficits in motion sensitivity (El-Shamayleh et al., 2010). Misguided development of the visual cortical areas may feed forward, disrupting other sensorimotor systems that underlie the child’s competence in performing everyday tasks that rely on vision, such as eye-hand coordination, navigating the world, and reading. Coordination of the eyes with the hands and body, along with normal ocular motor function, are crucial for planning and guiding reaching, grasping, aiming, and catching movements, as well as postural stability (balance) and navigating the environment. The development of fine and gross motor skills is dependent upon intact binocular vision during infancy and childhood, and thus can be impacted by early discordant visual experience from amblyopia. The vision and ocular motor deficits typical of amblyopia have the potential to also interfere with reading, a complex skill, relying on an interplay between cognitive, sensory, and ocular motor competences. Early reading ability predicts reading performance in later grades (Butler et al., 1985; Cunningham and Stanovich, 1997), and thus students with reading difficulties may be at risk of lower academic achievement. Impaired visuomotor ability during development can potentially lead to delayed acquisition of motor and cognitive milestones, as well as limitatations on academic achievement and life-long problems. Slow reading in childhood has the potential to persist throughout one’s lifetime, which can negatively influence virtually all aspects of life, including how leisure time is spent, job performance, and activities of daily living. These functional consequences, in turn, can cause issues of self-perception and quality of life, especially if they persist throughout childhood and into adulthood. Understanding the impact of amblyopia on visuomotor development, reading, self-perception, and quality of life is key to a whole person health approach to treatment.
3.2. Fine motor impairments in amblyopia
Amblyopic adults and children are impaired on tasks such as placing pegs into holes, threading beads, putting coins into a slot, grasping common objects, and catching (Grant and Moseley, 2011; O’Connor et al., 2010a; O’Connor et al., 2010b; Webber et al., 2008a). Most investigations of fine motor skills explored the role of strabismus or abnormal binocularity in motor performance (Alramis et al., 2016; Caputo et al., 2007; Hemptinne et al., 2020; Hrisos et al., 2006; Mazyn et al., 2004; O’Connor et al., 2010a; O’Connor et al., 2010b; Watt et al., 2003). We assessed motor ability during binocular viewing in children with strabismic or anisometropic amblyopia using the Movement Assessment Battery for Children-2 (ABC-2) to determine clinical and sensory factors related to poor fine motor skills. The Movement ABC-2 is a standardized test used to identify delayed or impaired motor development in children, and administered in three age bands (3–6, 7–10, and 11–16 years. Fine motor skills assessed including unimanual dexterity (e.g., posting coins with one hand), bimanual dexterity (e.g. lacing a thread through a board using two hands), drawing trail (stay inside the lines with a pen), catching (a bean bag or ball), and aiming (hit a target with a beanbag or ball). Amblyopic children scored lower than controls on almost all fine motor tasks, except aiming at a target (Fig. 2). Factors associated with poor performance in amblyopic children are shown in Table 4. In general, lower scores were associated with amblyopia, regardless of etiology or severity of amblyopia, with the following exceptions; 1) anisometropia or mild amblyopia for unimanual dexterity, and 2) anisometropia and stereoacuity present for catching. The lack of associations found within the amblyopic group does not rule out the role of these factors; it is difficult to tease apart the separate effects of amblyopia severity, stereoacuity, and suppression when they co-occur. Instead, our findings indicate that the early experience with binocular discordant input shapes the development of motor ability in a different manner than children who experience balanced binocular input during the critical period.
Fig. 2.
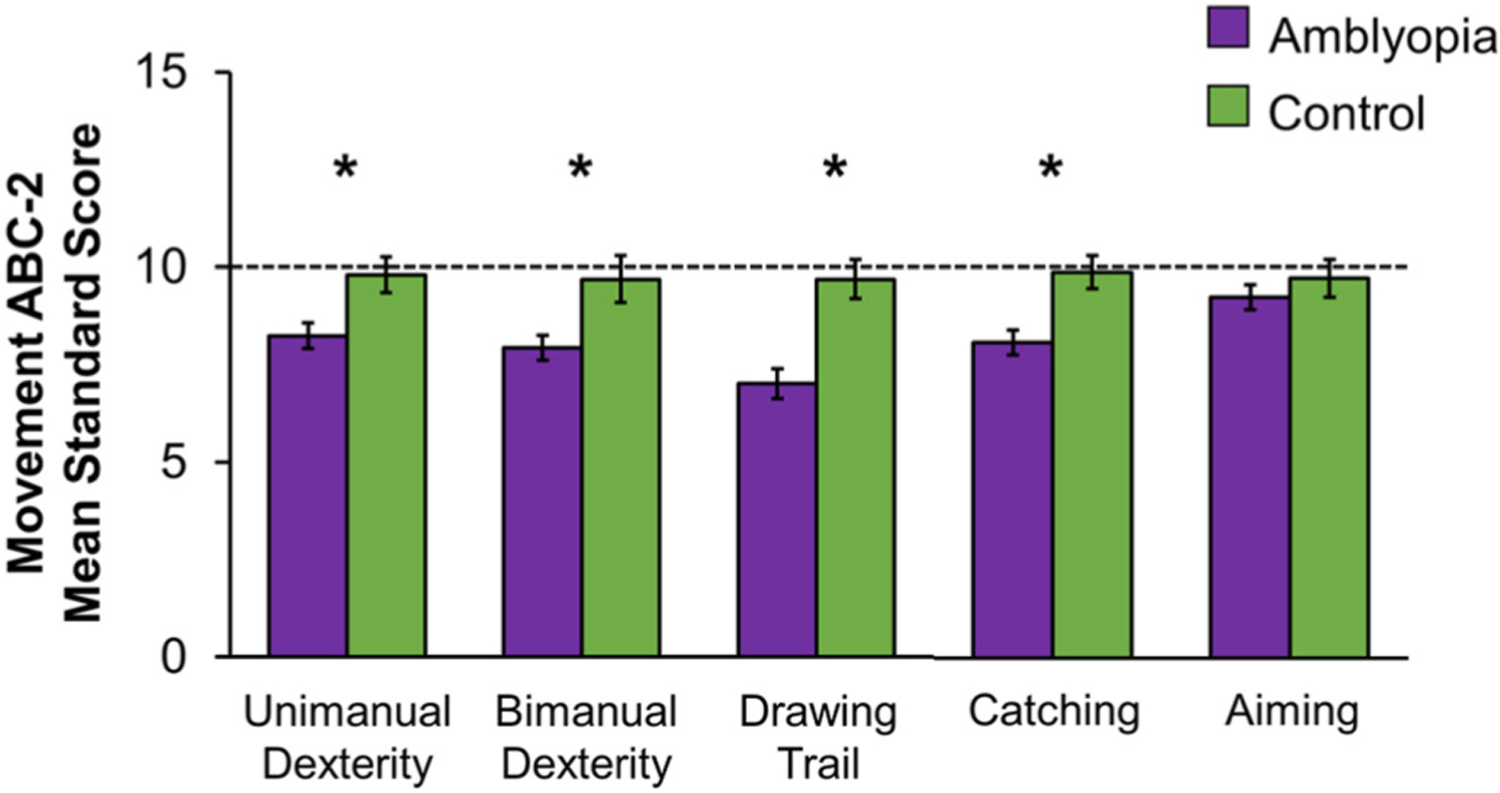
Bar graphs showing mean standard scores for fine motor tasks of the Movement ABC-2 among children ages 3–13 years. Amblyopic children (purple, n = 98) had lower standard scores (i.e., performed worse) compared to control children (green, n = 38) for unimanual dexterity, bimanual dexterity, drawing trail, and catching, but not for aiming. Based on data from (Kelly et al., 2020). Error bars represent ± SEM. Dashed lines represent 50th percentile (standard score of 10). *Significantly different from controls.
Table 4.
Factors affecting fine motor performance in amblyopic children.
| Factor | Unimanual Dexterity | Bimanual Dexterity | Drawing Trail | Catching | Aiming |
|---|---|---|---|---|---|
| Etiology | |||||
| Strabismus | ⨯ | ⨯ | ⨯ | ⨯ | ✓ |
| Anisometropia | ✓ | ⨯ | ⨯ | ✓ | ✓ |
| Severity of Amblyopia | |||||
| Milda | ✓ | ⨯ | ⨯ | ⨯ | ✓ |
| Moderate/Severeb | ⨯ | ⨯ | ⨯ | ⨯ | ✓ |
| Stereoacuity | |||||
| Present | ⨯ | ⨯ | ⨯ | ✓ | ✓ |
| Not Present | ⨯ | ⨯ | ⨯ | ⨯ | ✓ |
× Scored lower than controls ✓Not different from controls.
Mild, amblyopic eye visual acuity of 0.2 logMAR.
Moderate/Severe, amblyopic eye visual acuity of ≥0.3 logMAR.
It is evident from our research and others that fine motor skills are impaired in amblyopic children, but it is unclear what is happening when they move that results in these impairments. Recently, we investigated the role of eye-hand coordination during visually-guided reaching by tracking the hand (Leap Motion Controller) and eyes (EyeLink 1000 binocular eye tracker) as strabismic children age 7–12 years reached out to touch a small dot on a screen during binocular viewing (Kelly et al., 2021). Reaching is typically completed in two phases that reflect initial feedforward control of motor planning (acceleration phase) and online feedback control (deceleration phase) (Desmurget and Grafton, 2000). Amblyopic children (n = 19) were slower to reach than controls, especially in the final approach (i.e., deceleration phase) and were less accurate at touching the dot (Fig. 3A). In addition to impaired reach kinematics, children with strabismic amblyopia (n = 15) also had reduced saccade precision (i.e., more variability in the landing of the saccade) and produced more reach-related corrective saccades than controls (Fig. 3B) (Kelly et al., 2022). Slower reach in the final approach, lower touch accuracy, and increased reach-related saccades points to inefficient use of visual feedback during visually-guided reaching. Our findings are consistent with reports of younger amblyopic children (age 4–8 years) who have prolonged reach in the final approach during grasping (Grant et al., 2014; Suttle et al., 2011). However, our findings are in contrast to strabismic adults who have increased secondary corrective saccades before reach initiation (Niechwiej-Szwedo et al., 2012), and spend more time in the initial approach (acceleration phase), signifying more reliance on the visuomotor plan than visual feedback. Children with strabismic amblyopia may not have yet formed an efficient compensatory strategy for visually-guided reaching, and compensatory strategies may yet develop with age; however, it is unknown at what age this change occurs.
Fig. 3.
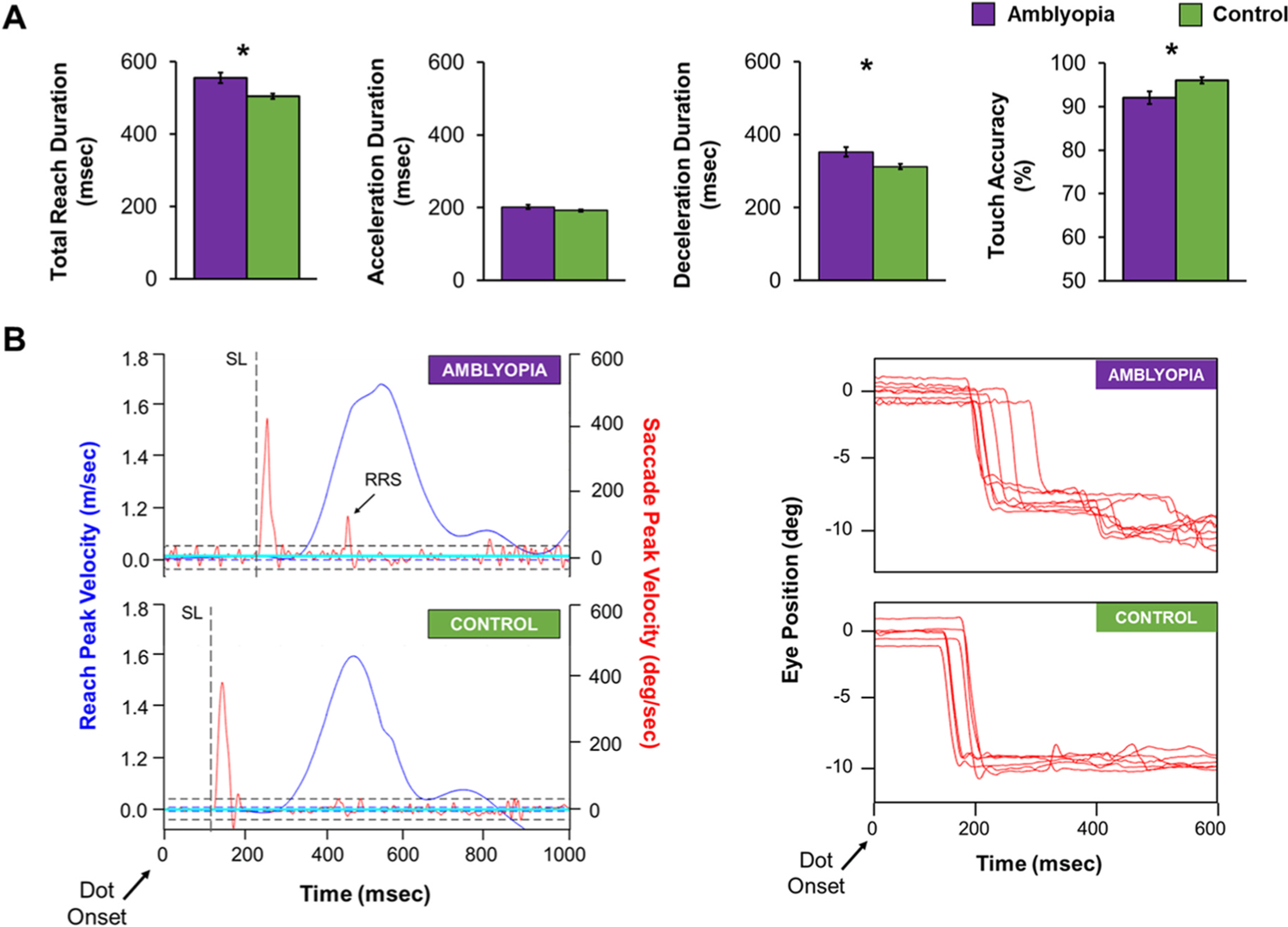
Eye-hand coordination during visually-guided reaching in strabismic children with amblyopia (purple, n = 19) and controls (green, n = 35) aged 7–12 years while completing a point to touch task. (A) Bar graphs showing mean total reach duration, acceleration duration, deceleration duration, and touch accuracy for the amblyopic group (purple) and control group (green). Amblyopic children took longer to touch the dot, due to longer deceleration duration, and had poorer touch accuracy than controls. Errors bars represent ± SEM. *significantly different from controls. (B) Examples of a typical visually-guided reaching trial for an amblyopic child (top) and a control child (bottom). Children were asked to reach out and touch a dot on a screen 35 cm away while their eye and index finger movements were being simultaneously recorded. The right panel shows velocity for the reach to the dot (right y-axis, blue) and for the saccade to the dot (left y-axis, red). The dotted line indicates primary saccade latency (SL). For the amblyopic child, saccade latency was prolonged and a reach-related saccade (RRS) was present. The left panel shows reduced saccade precision (i.e., more variability in saccade landing position) in the amblyopic child compared to the control child. Based on data from (Kelly et al., 2021 and Kelly et al., 2022).
Whether eye-hand coordination deficits can be ameliorated with amblyopia treatment has been paid little attention. Severity of amblyopia is not directly associated with severity of fine motor impairment based on standardized motor testing (Webber et al., 2008a). Thus, amblyopia treatments targeting visual acuity may not be of benefit to fine motor skills. On the other hand, fine motor impairments are closely associated with impaired stereoacuity and suppression (Grant and Moseley, 2011; Kelly et al., 2020; O’Connor et al., 2010a; O’Connor et al., 2010b), therefore, binocular amblyopia treatments that target reducing interocular suppression may be of more benefit. There is evidence that binocular amblyopia treatment can lessen visuomotor deficits (Birch et al., 2023; Webber et al., 2016). Yet, despite these improvements, fine motor deficits persisted compared to controls.
3.3. Gross motor impairments in amblyopia
Strabismus, a major amblyogenic factor, is related to postural instability in children and adults, with binocular dysfunction (poor stereoacuity and suppression) playing a large role in this instability (Lions et al., 2013a; Matsuo et al., 2010; Odenrick et al., 1984; Zipori et al., 2018). Nonamblyopic, strabismic children rely more on proprioception (body’s ability to perceive its own position in space) than controls to help control their postural stability (Lions et al., 2014), possibly because their visual input is not efficient. While several studies have focused on balance in strabismus, few studies have focused on balance in amblyopic children. Of those that have, impaired balance was found on standardized tests of motor ability such as the Bruininks-Osteretsky Test of Motor Proficiency and the Movement ABC (Engel-Yeger, 2008; Zipori et al., 2018). We previously reported that amblyopic children scored lower than controls on standardized balance tasks from the Movement ABC-2 (Kelly et al., 2020), including static balance (e.g. balance on one foot), walking a straight line, and jumping on mats (Fig. 4). This was due to moderate and severe amblyopia (>0.2 logMAR), no measurable stereoacuity, and no fusion at distance (Fig. 5). Further, parents of amblyopic children completed the Movement ABC-2 checklist, a questionnaire that reflects the parent’s evaluation of their child’s visuomotor abilities in static predictable environments (e.g., avoiding desks while navigating a classroom) or dynamic unpredictable environments (e.g., avoiding other children or moving balls while playing sports). Parents scored their child’s abilities significantly higher if their child had some level of measurable stereoacuity compared with parents of children with no stereoacuity present. These data point to impaired gross motor development as a result of discordant binocular input during the critical period.
Fig. 4.
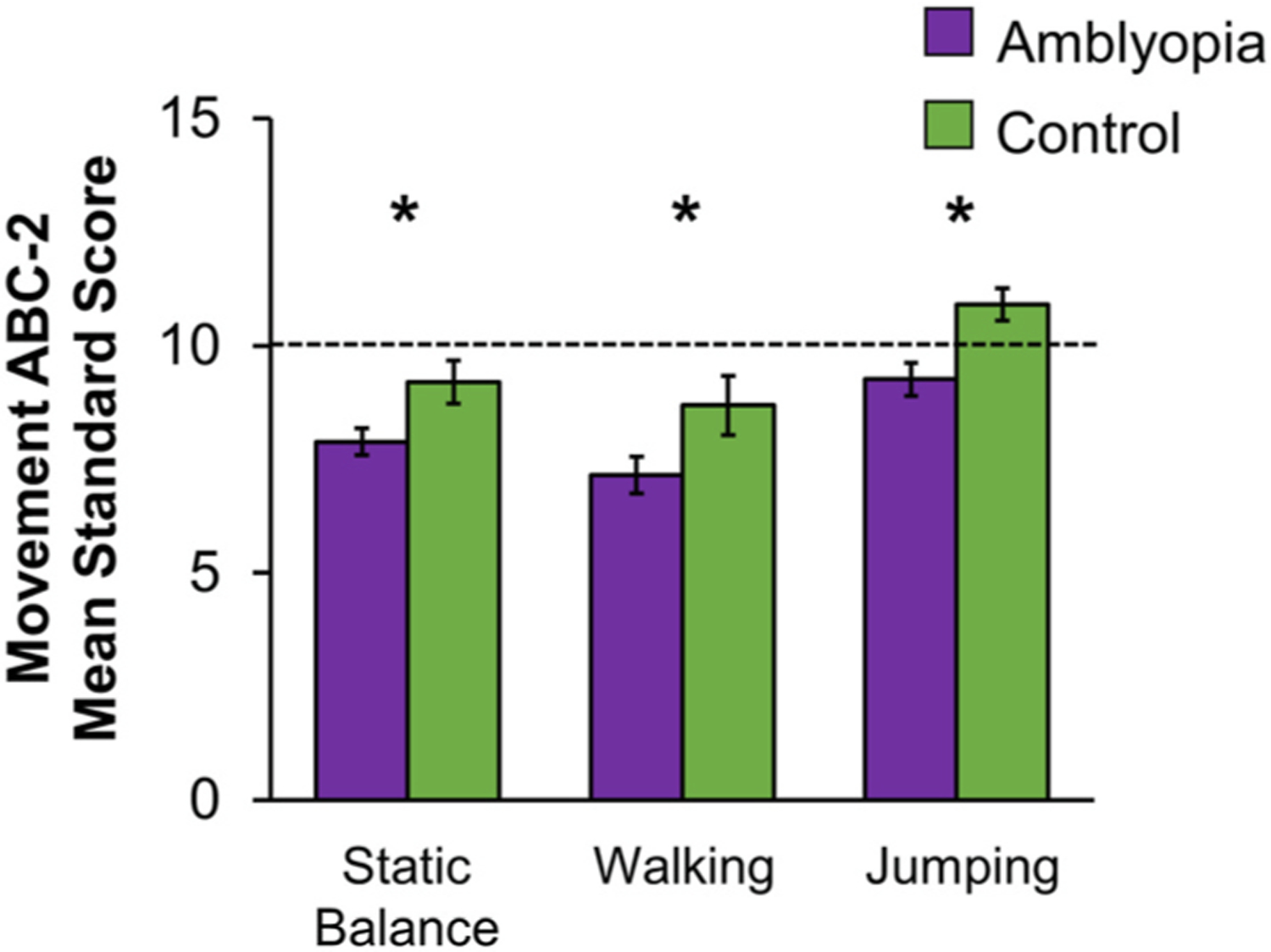
Bar graphs showing mean standard scores for gross motor subtasks of the Movement ABC-2 in children ages 3–13 years. Amblyopic children (purple, n = 98) had lower mean standard scores (i.e., performed worse) for static balance, walking, and jumping compared to controls (green, n = 38). Based on data from (Kelly et al., 2020). Error bars represent ± SEM. Dashed lines represent 50th percentile (standard score of 10). *Significantly different from controls.
Fig. 5.
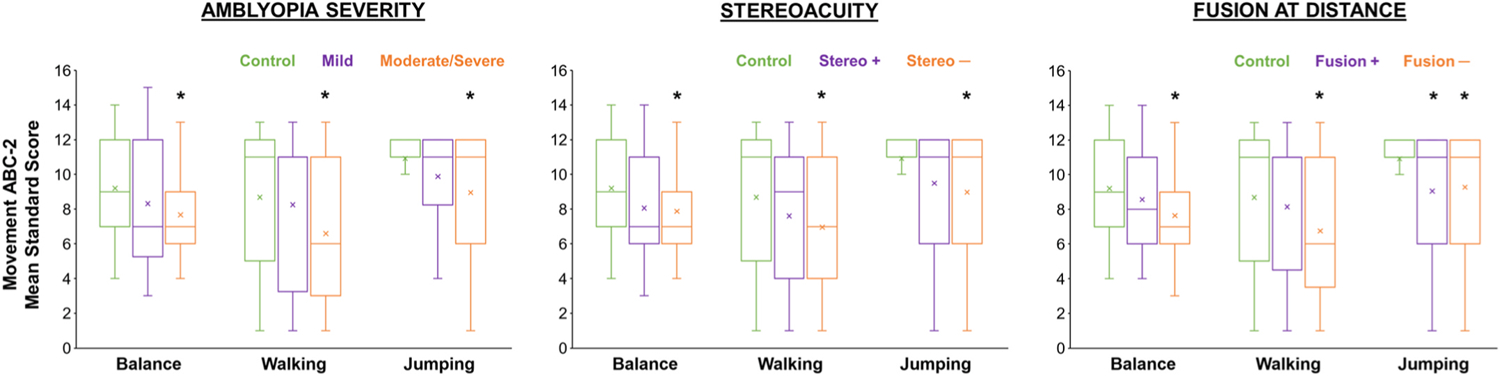
Factors associated with gross motor skills from the Movement ABC-2 in amblyopic children, including amblyopia severity (mild 0.2 logMAR; moderate/severe >0.2 logMAR), stereoacuity present (yes, stereo +; no, stereo −), and fusion at distance (3 m) evaluated with the Worth 4-dot test (yes, fusion +; no, fusion −). Based on data from (Kelly et al., 2020). The horizontal line within each box represents the median amblyopic eye (AE) visual acuity, the boxes correspond to the 25th to 75th percentiles, and the whiskers correspond to the fifth and 95th percentiles. *Significantly different from controls (green).
Although it is clear from our research and others that gross motor skills are deficient in amblyopic children, the reason for the impairment is unclear. Both the Bruininks-Osteretsky Test of Motor Proficiency and the Movement ABC-2 measure balance based on how long the child can maintain balance rather than assessing body kinematics such as anterior-posterior sway, center of pressure, gait, or the coordination between the eyes and the body during balance. As the dominant sense for movement planning and guidance, the visual system provides pertinent near and far information almost simultaneously, for regulation of movement locally (postural balance and step-by-step) and globally for route planning. Thus, coordination of the eyes and body is essential for balance, navigating the environment, and avoiding falls or collisions. Vergence movements reduce postural sway in nonamblyopic children with strabismus, and realigning the eyes with surgery improves postural stability, possibly due to the change in vergence angle (Legrand et al., 2011, 2012). These data suggest that ocular motor signals send feedback into mechanisms controlling postural stability, and that the vergence angle particularly contributes. We used the Standing Balance Task from the Motor Domain of the NIH Toolbox® to measure postural stability during static balance in amblyopic children. This test uses the accelerometer built-in to an iPod to measure anterior-posterior sway. Preliminary data show that amblyopic children have poorer static balance than controls during binocular viewing (Fig. 6). The ocular motor dysfunction present in amblyopia (fixation instability, abnormal saccades, reduced vergence) may play a role in poor balance (Kelly et al., 2016a, 2019; Perdziak et al., 2014, 2016).
Fig. 6.
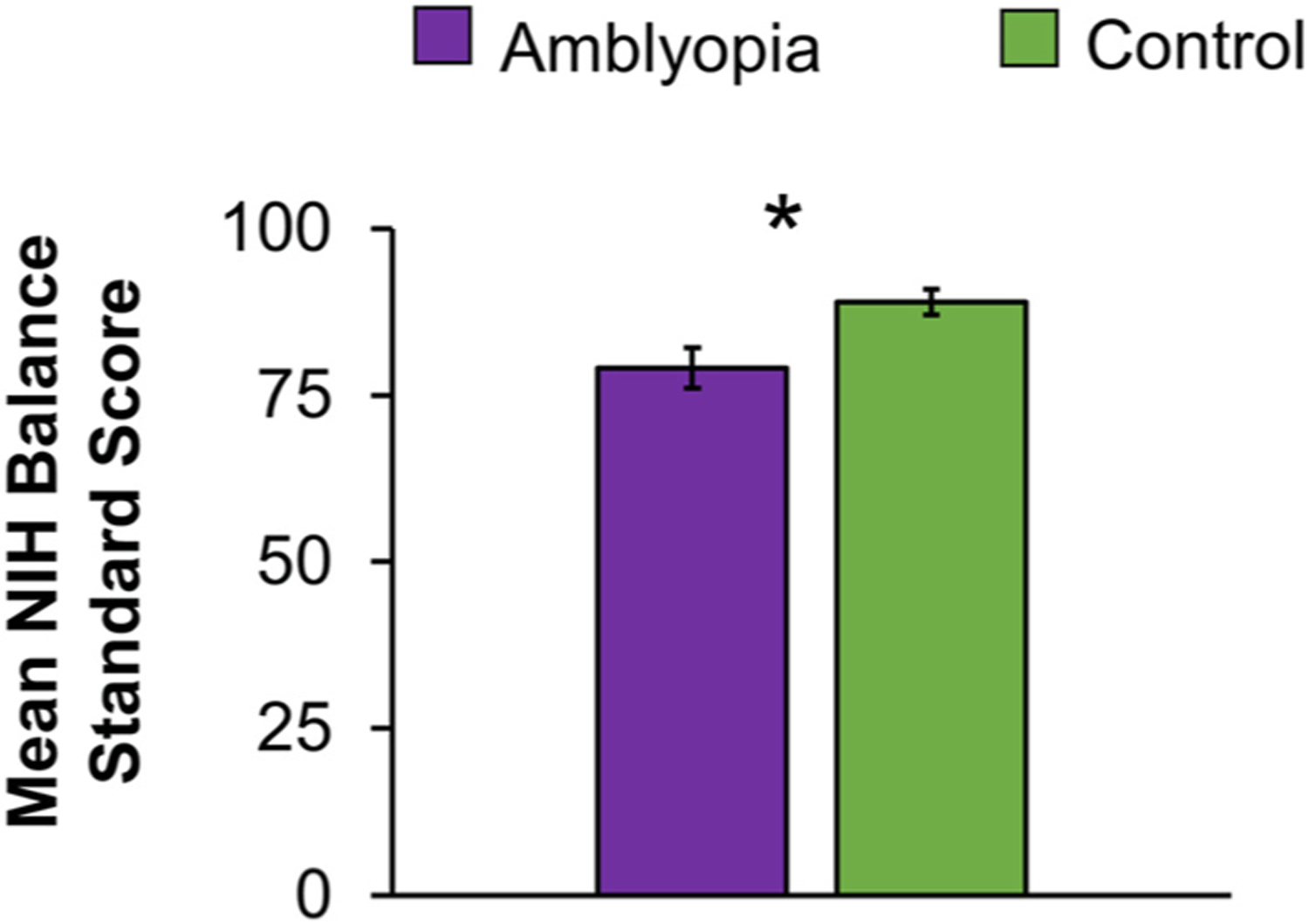
Preliminary data showing postural stability measured by the Standing Balance Task from the Motor Domain of the NIH ToolboxR. Children age 7–11 years were asked to hold their balance for 50 s while completing 5 different balance positions; 1) eyes open, both feet on the floor, 2) eyes closed, both feet on the floor, 3) eyes open, both feet on a foam mat, 4) eyes closed, both feet on a foam mat, and 5) eyes open, feet tandem (heel touching toe) on the floor. An iPod attached to the child’s waist measured anterior-posterior sway using the built-in accelerometer. An age corrected standard score is calculated combining the sway data from all 5 balance positions. Amblyopic children (purple, n = 13) had lower mean age-corrected standard scores (i.e., poorer balance) than control children (green, n = 13). Error bars represent ± SEM. *Significantly different from controls.
Binocularity is also important for providing information used to locate footholds during walking, with stereodeficient adults biasing their gaze closer to footholds in rougher terrain (Bonnen et al., 2021; Hayhoe et al., 2009). During dynamic balance (i.e. walking), stereodeficient adults and strabismic children are more cautious than controls when navigating the environment. They take shorter steps, reduce their walking velocity, and increase their toe clearance when navigating higher obstacles, suggesting a deficit in using visual input to plan the approach and increased uncertainty about the obstacle’s characteristics (i.e., height, location) (Buckley et al., 2010; Odenrick et al., 1984). In stereodeficient adults, occluding one eye affects gait during walking, suggesting that both eyes contribute and that field of view is important during walking (Buckley et al., 2010). These studies point to a higher risk of falling or tripping during natural walking when binocularity is disrupted. There is a need for research to quantify the role of ocular motor dysfunction in eye-body coordination in amblyopic children during static balance and dynamic balance.
Visuomotor impairments have the potential to negatively impact a child’s academic success. In the early grades, learning vocabulary and counting is contingent on fine motor skills for object manipulation. Amblyopic children in later grades take 28% longer than their peers to transfer answers to a multiple-choice form (Kelly et al., 2018), which can impact performance on time standardized tests that are used for school admissions, to assess academic achievement, and determine eligibility for academic tracks. Impaired visuomotor ability may also impact a child’s physical competence in sports and navigating their environment, which increases the risk of injuries and falling (Pineles et al., 2016, 2021), and inhibit a child’s decision to take part in physical activities (Engel-Yeger, 2008; Zipori et al., 2018). Amblyopic children spend less time participating in nonschool-related physical activity, and are 6 times more likely to report engaging in no physical activity than their peers (Harrington et al., 2022). Motor deficits in amblyopia increase the risk of obesity in adolescence (Drews-Botsch et al., 2022), and persist into adulthood, increasing the risk of falls (Pineles et al., 2016, 2021).
Binocular amblyopia treatment may be effective in improving gross motor deficits because of the relationship between gross motor deficits and impaired binocularity. In another vein, treatments that target the visual acuity deficit may also be of benefit given the relationship between amblyopic eye severity and static and dynamic balance (Kelly et al., 2020). Indirect evidence supports this hypothesis; children with successfully treated amblyopia were 5 times more likely to engage in regular physical activity than children whose amblyopia had not yet resolved, suggesting an advantage of amblyopic treatment on visuomotor ability (Harrington et al., 2022).
3.4. Amblyopia and slow reading
Earlier reports showed slow reading in amblyopic children and adults compared to controls (; Kanonidou et al., 2010; Kugathasan et al., 2019; Levi et al., 2007; Lions et al., 2013b; Stifter et al., 2005a; Stifter et al., 2005b). Some of these studies focused solely on strabismic amblyopia or used different reading conditions; i.e., monocular viewing, one line sentences, limited eye movements, oral reading, or far reading distances, and none assessed factors associated with slow reading. To determine the impact of amblyopia on silent, binocular reading in children, we conducted an initial study using the Readalyzer eye movement recording system (Compevo AB, Stockholm, Sweden), which provides information such as how fast a child can read and how their eyes are moving through the text (Kelly et al., 2015). Reading speed was 27% slower in children with strabismic and anisometropic amblyopia compared to controls (148 words per minute versus 204 words per minute). However, children with strabismus but no amblyopia read at a similar rate as controls (198 words per minute), pointing to a key role of amblyopia rather than strabismus in slow reading. Yet, it is unclear why amblyopic children are slow at reading. Based on our finding of slow reading in amblyopic children, we have conducted a series of studies to try and pinpoint the cause of the reading impairment. Children diagnosed with reading disorders or dyslexia and those with poor reading comprehension secondary to learning disabilities were excluded to ensure any deficits in reading speed were not due to other issues.
In examining clinical and sensory factors that may be related to slow binocular reading in amblyopic children, we have failed to find a relationship with the severity of amblyopia. Children with mild (0.2 logMAR), moderate (0.3–0.6 logMAR), and severe (≥0.7 logMAR) do not differ in reading rate, and all three were slower than controls (Fig. 7A). Binocularity status (stereoacuity, interocular suppression; Fig. 7B) and diagnosis (strabismic, anisometropic) also yielded no relationships with slow reading in our amblyopic group suggesting amblyopia alone is sufficient to impair reading (Kelly et al., 2015, 2017).
Fig. 7.
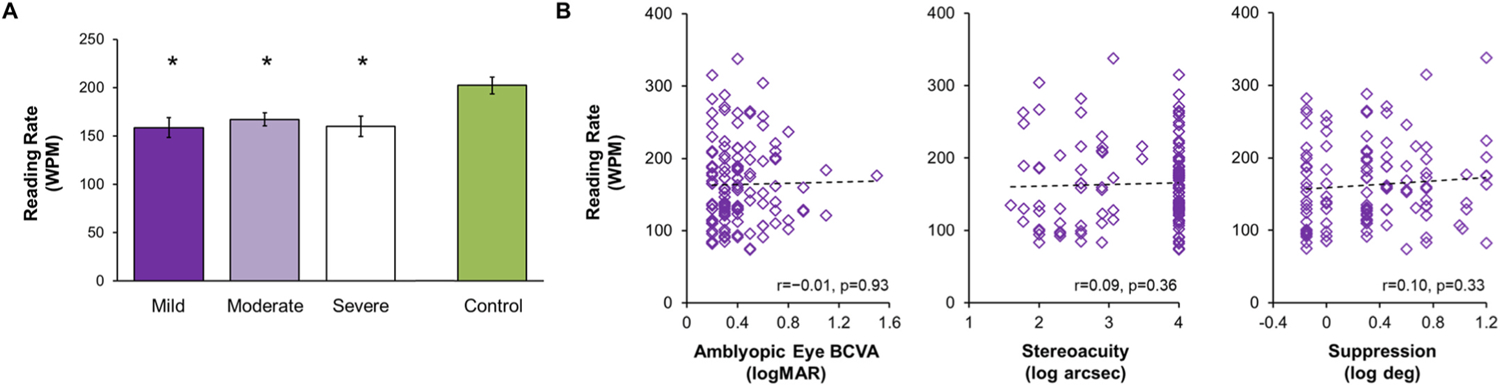
A) Bar graph for amblyopic children who completed grades 1–6 (ages 7–12 years) showing no difference in the Readalzyer mean reading rate (words per minute, WPM) based on amblyopic eye best corrected visual acuity (BCVA; mild, 0.2 logMAR, n = 29, dark purple bar; moderate, 0.3–0.6 logMAR, n = 97, light purple bar; severe, ≥0.7 logMAR, n = 20, white bar). All three amblyopic categories read significantly slower (i.e. lower reading rate) than controls (n = 72, green bar). Error bars represent SEM. *Significantly different from controls. B) Scatterplots showing no relationship of reading rate (words per minute, WPM) with amblyopic eye BCVA, stereoacuity, or suppression in children with amblyopia. Based on data from (Kelly et al., 2015; Kelly et al., 2017).
It has been proposed that slow reading may be due to binocular inhibition, i.e., the amblyopic eye is interfering with the fellow eye during binocular reading. If so, fellow eye reading performance with the amblyopic eye occluded would be better than binocular reading in amblyopic children. Yet, this is not the case (Kelly et al., In Press). While amblyopic children were slower than controls in both binocular and fellow eye reading, there was no difference in reading rate between the two viewing conditions (Fig. 8).
Fig. 8.
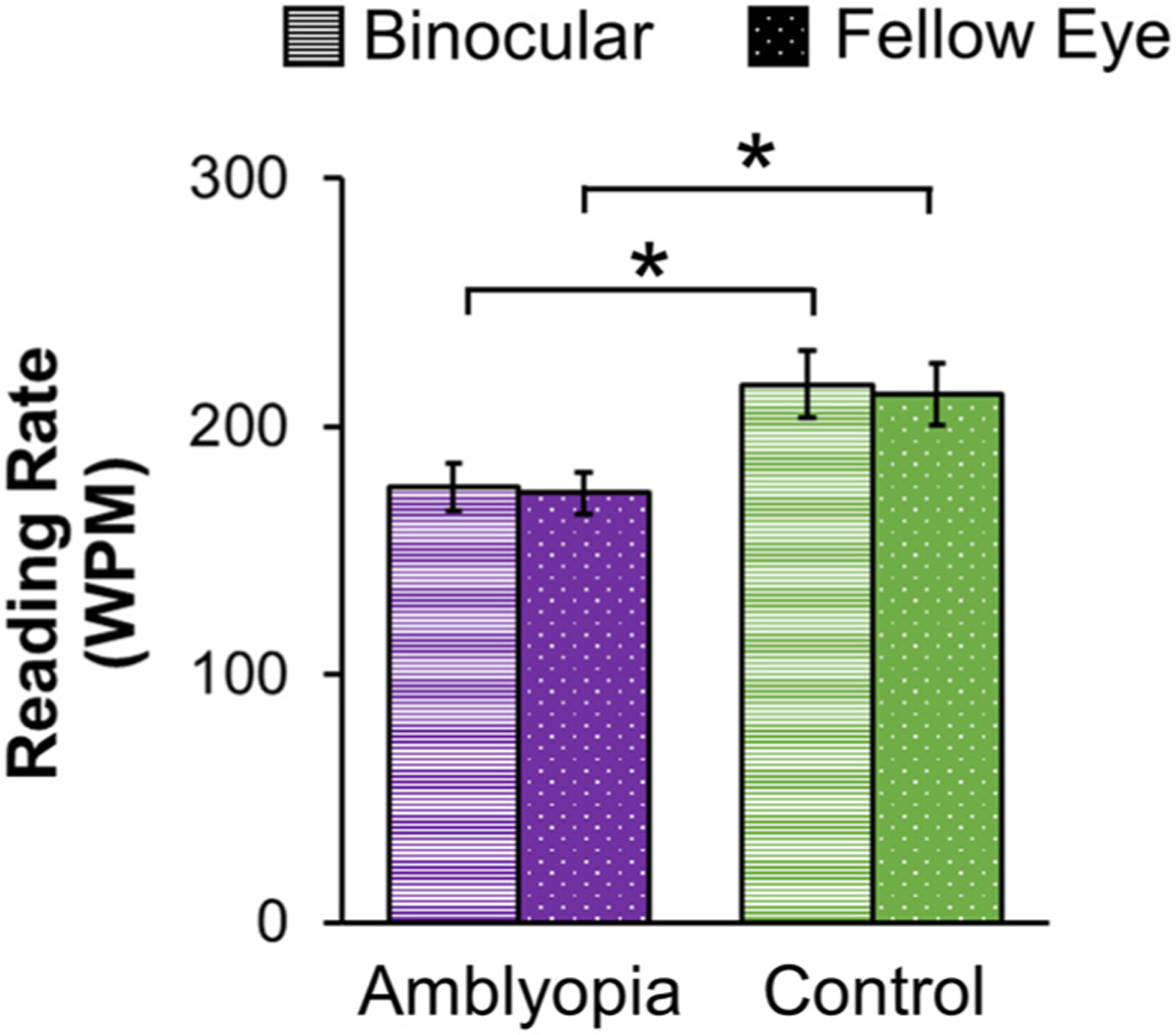
Bar graphs for children who completed grades 1–6 (ages 7–12 years) showing no difference in Readalyzer mean reading rate (words per minute, WPM) between binocular reading (striped bars) and fellow eye reading (dotted bars) in amblyopic children (purple, n = 38), but slower binocular and fellow eye reading (i.e. lower reading rate) than controls (green, n = 36). Based on data from (Kelly et al. 2023). Error bars represent ± SEM. *Significantly different from controls.
The more likely culprit for slow reading is ocular motor dysfunction. During reading, saccades allow the eyes to move forward and regress backward through lines of text, while fixations (pauses) allow decoding of phonemes to occur. We and others have reported the role of eye movements during binocular reading in amblyopic participants (Bhutada et al., 2022; Kanonidou et al., 2010; Kelly et al., 2015; Kelly et al., 2017; Lions et al., 2013b). In our studies of amblyopic children, we found that slow reading was related to more forward saccades (Fig. 9; Fig. 10A and B) and to fixation instability (Fig. 10C) (Kelly et al., 2015, 2017). In our investigations (Kelly et al., 2015, 2017; Kelly et al., In Press), slow reading was not related to regressive saccades, fixation duration, or comprehension, measures that are hallmarks of dyslexia (Handler and Fierson, 2011). The lack of increased regressions and longer fixation duration supports an ocular motor deficit rather than a disorder of language processing.
Fig. 9.
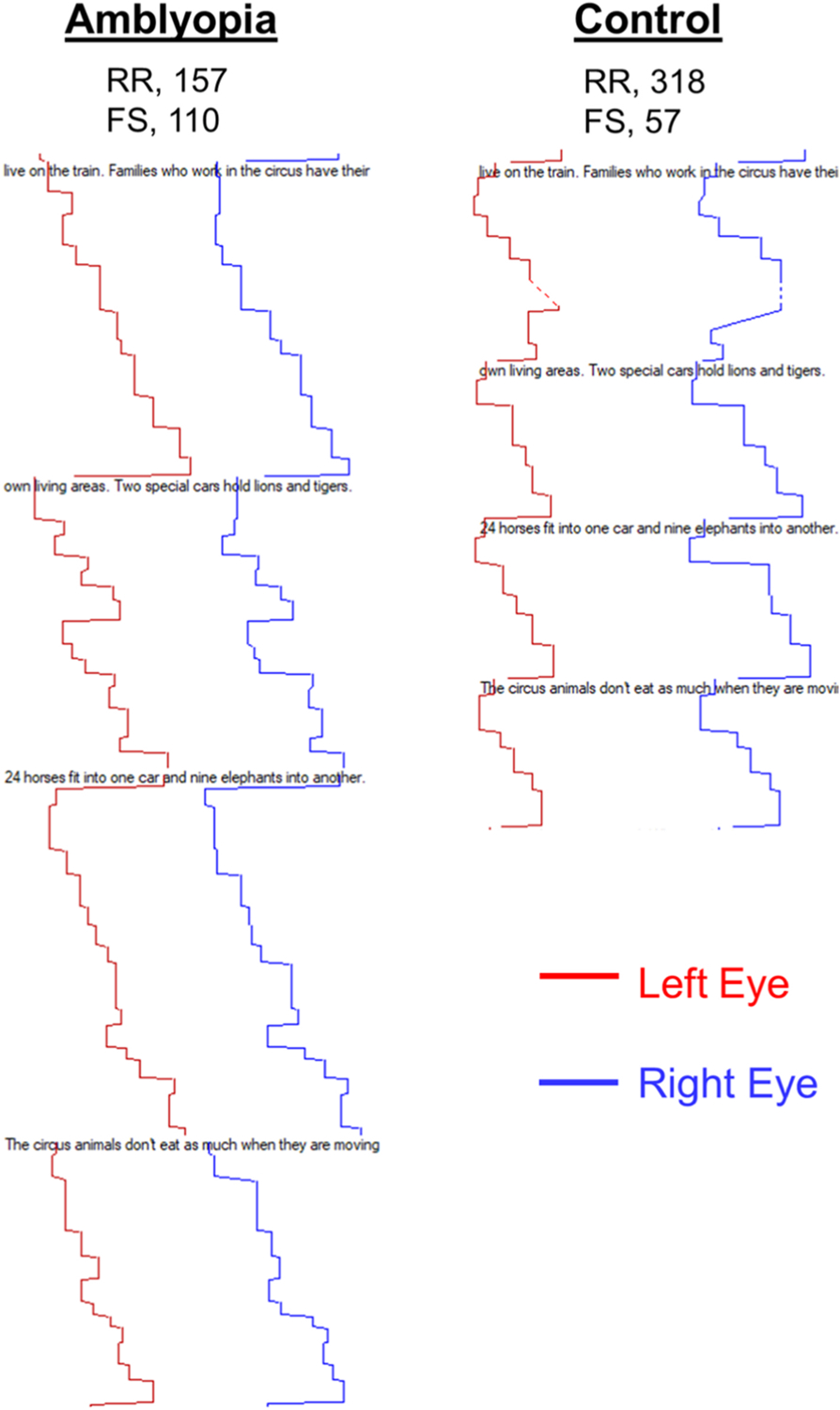
Left eye (red line) and right eye (blue line) horizontal eye movements as an amblyopic child and a control child read through a grade 6 paragraph. The amblyopic child reads slower (157 vs 318 words per minute) and makes more forward saccades (110 vs 57). RR, reading rate; FS, forward saccades.
Fig. 10.
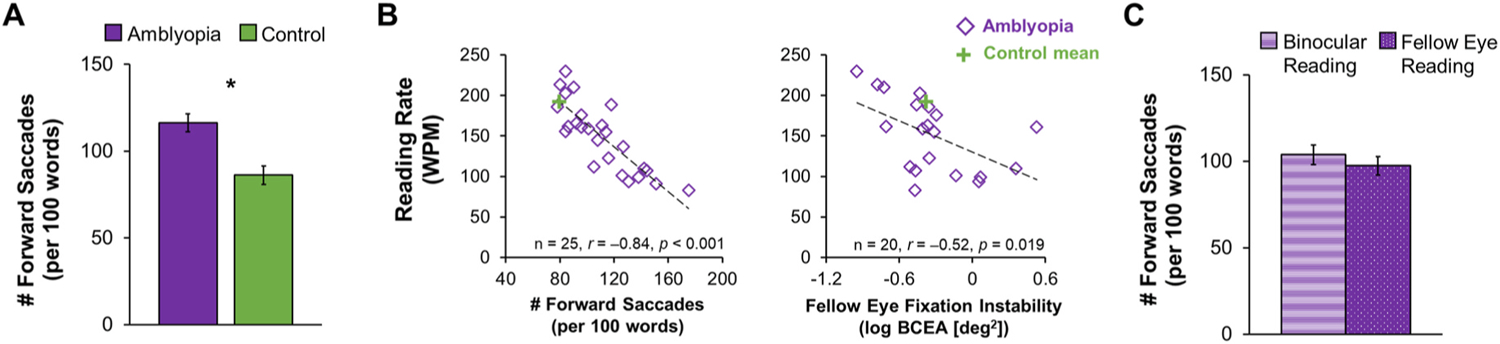
The relationship between slow reading and eye movements in children with amblyopia who completed grades 1–6 (ages 7–12 years). (A) Bar graphs showing an increase in the number of forward saccades (per 100 words) during binocular reading for amblyopic children (purple, n = 25) compared to controls (green, n = 25). Adapted from (Kelly et al., 2015). (B) Scatterplots showing significant correlations of binocular reading rate (words per minute, WPM) with the number of forward saccades (per 100 words) produced during binocular reading and with fellow eye fixation instability for children with anisometropic amblyopia. Adapted from Kelly et al., 2017). (C) Bar graphs showing no difference in the number of forward saccades (per 100 words) during binocular reading (purple stripes) and fellow eye reading (purple dots) in amblyopic children (n = 38). *Significantly different from controls. Adapted from (correction for current Kelly citation with no date). Error bars represent ± SEM.
To further investigate the role of ocular motor dysfunction in slow reading, we minimized the need for inter-word saccades using a rapid serial visual presentation (RSVP) task (Mir Norouzi et al., 2022). In our RSVP task, grade-appropriate sentences were shown as one word at a time in the center of the screen in quick succession. (Fig. 11A). ‘Yes’ or ‘No’ comprehension questions were asked and sentences were presented faster or slower depending on their answer so that maximum speed at which the child could read with comprehension was determined. Our hypothesis was that if inter-word saccades were the driving force behind slow reading, removing the requirement for saccades would allow children with amblyopia to read as quickly as controls. In this preliminary study of 18 amblyopic children and 15 age-similar controls, amblyopic children were still slower at binocular RSVP reading than controls, even though there was no need for inter-word saccades (Fig. 11B). Nonetheless, slow RSVP reading does not rule out a role for other types of ocular motor dysfunction such as fixation instability in slow reading. An amblyopic child’s eyes might be moving involuntarily when attempting to fixate on a word, or their fixation location may not optimize their visual span.
Fig. 11.
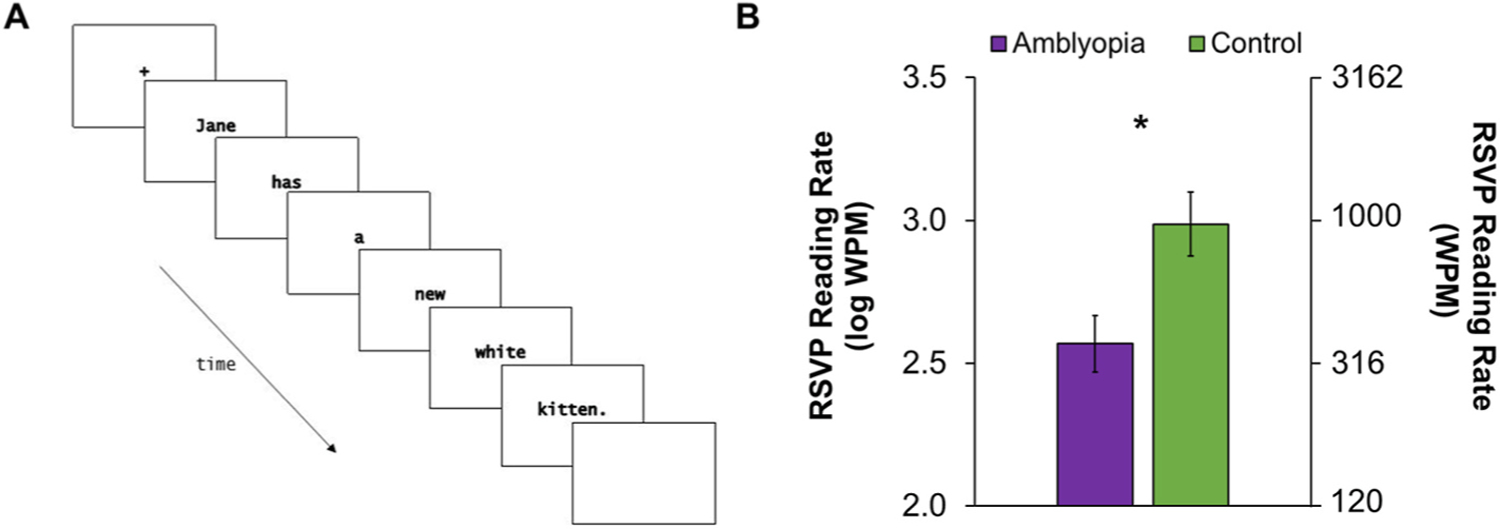
A) Time course of one trial of rapid serial visual presentation (RSVP) reading. Each word of one sentence is presented centrally one at a time for a fixed exposure time, which varies during the experiment to determine the fastest exposure time (i.e., threshold) that the child can read. B) Log mean RSVP reading rate (words per minute, WPM) for children with amblyopia who completed grades 1–6 (ages 7–12 years; purple, n = 18) was slower than the control group (green, n = 15) (Adapted from Mir Norouzi et al., 2022 Vision Sciences Society Meeting). Error bars represent ±SEM. *Significantly different from controls.
It could be hypothesized that slow reading is a consequence of a more general issue associated with amblyopia. Prior to entering kindergarten and learning to read, children must acquire the skills, knowledge, and attitudes toward reading and writing that serve as the cornerstone for the development of reading and writing proficiency, i.e. early literacy skills (Lonigan et al., 2000; Whitehurst and Longian, 1998)). Because early literacy performance can predict reading success in later grades (Whitehurst and Longian, 1998), it could be the case that slow reading in amblyopic children is due to impaired early literacy skills. However, based on a reanalysis of all binocular reading data from our published and ongoing studies, slow reading in amblyopia is not apparent until grade 3 (Fig. 12). Thus, early literacy skills that are acquired prior to reading are unlikely to have been adversely impacted by amblyopia.
Fig. 12.
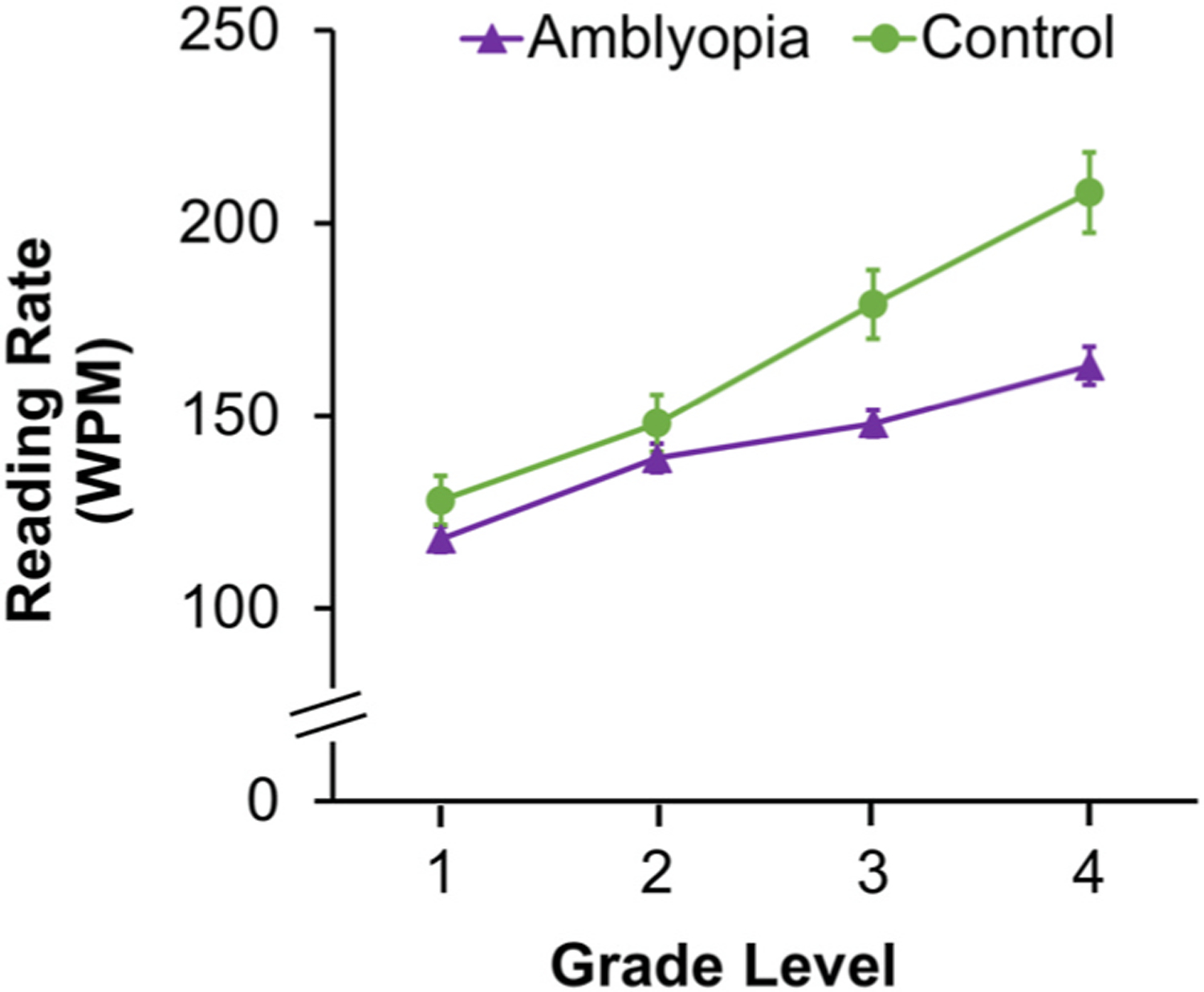
Line graph showing significantly slower reading rate (words per minute, WPM) for amblyopic children (purple line, triangle, n = 140) compared with controls (green line, circle, n = 67) who have completed grades 3 and 4 but not for children who have completed grades 1 or 2. Approximate age equivalents for children who have completed grade 1 (7.7 ± 0.5 years), grade 2 (8.7 ± 0.4 years), grade 3 (age 9.6 ± 0.4 years, and grade 4 (10.6 ± 0.4 years). Error bars represent ±SEM. *Significantly different from controls.
Slow reading in children with amblyopia may hinder academic success. Parents, educators, and doctors of children with amblyopia who read slowly may not know that their eye condition is impacting their reading speed because the child has 20/20 vision in the fellow eye. Yet, amblyopic children who read slowly may not qualify for academic accommodations in the context of strictly timed academic achievement tests and entrance exams because the federal definition of visual impairment is 20/40 or worse in the better-seeing eye (US Center for Disease Control; https://www.cdc.gov/visionhealth/vehss/data/studies/vision-impairment-and-blindness.html). There are lackluster findings on the impact of amblyopia on education (Chua and Mitchell, 2004; Hill et al., 2019; Rahi et al., 2006; Wilson and Welch, 2013). In children, one study used the Bracken School Readiness Assessment and concluded that while amblyopia was not significantly related to negative cognitive performance between 3 and 7 years of age, children 3 years of age with strabismic or mixed amblyopia were at a greater risk than controls of having impaired school readiness (Gitsels et al., 2020). However, it is unclear whether these children were impaired in reading once they began to read. In amblyopic adults, a few studies found no impact on occupation, education level, and income, while one study found that they were less likely to have completed university degrees (control, 7.2% vs amblyopia, 2.5%) (Chua and Mitchell, 2004). However, shortcomings of these studies include reliance on parental report of amblyopia history (Hill et al., 2019) and use of data from adults born in the 1920’s to 1970’s when societal and academic environments were different from today’s (Chua and Mitchell, 2004; Rahi et al., 2006; Wilson and Welch, 2013). Currently, there are no studies assessing directly the impact of amblyopia on school performance in children.
If the presence of amblyopia is the key factor in slow reading and not the severity of the visual acuity deficit, it should stand to reason that successful treatment of amblyopia (i.e., achieving 20/20 vision) should eliminate the deficit. In contrast to this hypothesis, several studies report persistent reading deficits following successful amblyopia treatment. Yet, these studies focused on monocular testing of the amblyopic eye (Repka et al., 2008; Stifter et al., 2005a; Zurcher, 1980), a scenario which may exacerbate fixation instability. There is one report on 10 children with a history of successfully treated strabismic amblyopia who had binocular oral reading speed comparable to controls (Fernandes and Ferraz, 2022). Using silent, natural reading conditions, we reported no binocular reading deficit in nonamblyopic strabismic children (Kelly et al., 2015). More recently, we have observed the benefit of successful amblyopia treatment in ameliorating reading difficulties (Fig. 13). Prospective studies assessing reading rate before and after amblyopia treatment should be conducted to further ascertain whether slow reading can be improved with successful amblyopia treatment.
Fig. 13.
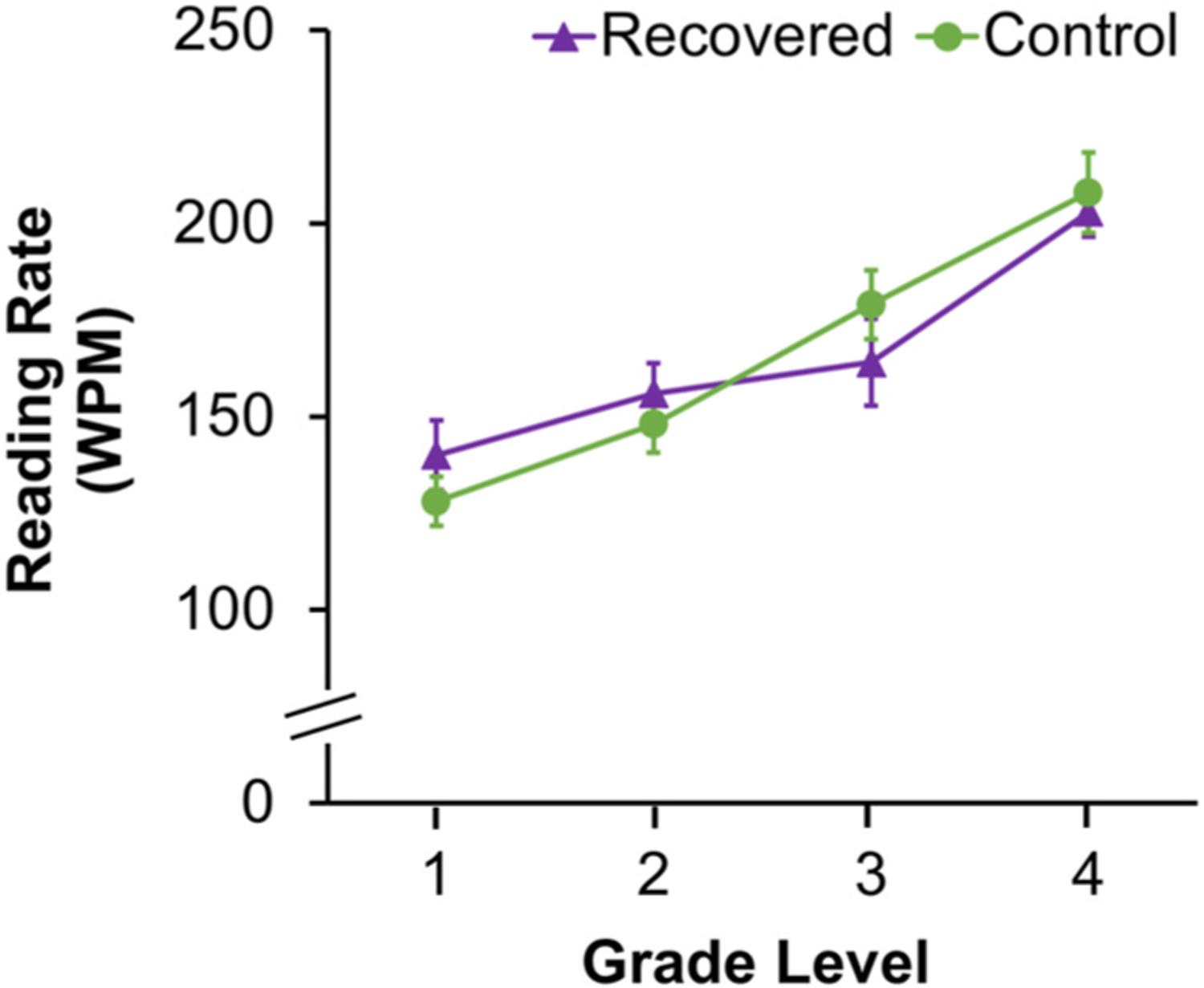
Line graph showing no difference in reading rate (words per minute, WPM) between amblyopic children who have recovered normal visual acuity (purple line, triangle, n = 31) and controls (green line, circle, n = 67). Approximate age equivalents for children who have completed grade 1 (7.7 ± 0.5 years), grade 2 (8.7 ± 0.4 years), grade 3 (age 9.6 ± 0.4 years, and grade 4 (10.6 ± 0.4 years). Error bars represent ± SEM.
Up to 50% of children treated for amblyopia will not recover normal visual acuity (Birch, 2013; Buckle et al., 2019; Pediatric Eye Disease Investigator Group, 2003a; 2004, 2010; Repka et al., 2014; Repka et al., 2005; Stewart et al., 2004; (Pediatric Eye Disease Investigator Group et al., 2006b); Woodruff et al., 1994). For these children, alternative interventions may need to be explored. Eye movement training has improved reading speed in adults with age-related macular degeneration (Nguyen et al., 2011; Seiple et al., 2005). Whether reading interventions for children with amblyopia should target eye movements during reading remain to be determined.
3.5. Self-perception
The bulk of research on well-being of children with amblyopia has focused on the effects of patching treatment on self-esteem (Felius et al., 2010; Hrisos et al., 2004; Koklanis et al., 2006; Loudon et al., 2009; Packwood et al., 1999; Webber et al., 2008b; Williams et al., 2006). However, many of these studies were conducted during an era in which amblyopic children were patching 6 hours or more per day or with older school-age children. As a result, treatment often required that the children wear their patches to school and while engaging in other activities outside the home. These early studies include reports of experiencing stigma, bullying, low social acceptance, poor self-image, interference with performance in school and sports, and family stress. The current standard-care is initial treatment with spectacles alone, with any residual amblyopia treated with 2 hours per day patching, increasing the number of hours only if residual amblyopia is unresponsive to 2 hours per day (https://www.aao.org/preferred-practice-pattern/amblyopia-ppp-2017). This more modest treatment schedule of 2 h per day allows young children aged ≤ 7years to complete their treatment mostly during hours when they are at home and avoids the potential social stigma and bullying that can be experienced in other settings. Within this younger age group with more limited treatment hours, we were able to largely avoid the social stigma associated with patching, and obtain a clearer picture of how amblyopia itself affects children’s self-perception.
Children aged 3 to 7 years. Self-perception begins developing during the preschool years (Harter, 2006, 2012). During these early years, children begin to make judgments about their competence, including cognitive capability and physical skills, and evaluate their social acceptance in terms of whether or not they receive support from friends, parents, and teachers (Harter, 2012; Harter and Pike, 1984). Using the Pictorial Scale of Perceived Competence and Social Acceptance for Young Children, we evaluated four components of self-perception in children aged 3–7 years: cognitive competence, physical competence, peer acceptance and maternal acceptance (Fig. 14) (Birch et al., 2019a). Children with amblyopia had significantly impaired self-perception of physical competence and peer acceptance compared with controls (Fig. 15). These factors may be inter-related because strong peer bonds are often formed through team sports and other group physical activities. The validity of the reduced self-perception scores for physical competence were supported by the finding of poorer performance on aiming and catching tasks of the Movement ABC-2 compared with the performance of controls. Lower physical competence scores also were correlated with more severely impaired stereoacuity, but not with the severity of visual acuity impairment.
Fig. 14.
Sample items from the Preschool-Kindergarten Pictorial Scale of Perceived Competence and Social Acceptance for Young Children (Harter and Pike, 1984). These items were chosen from the version designed for use with girls in preschool.
Fig. 15.
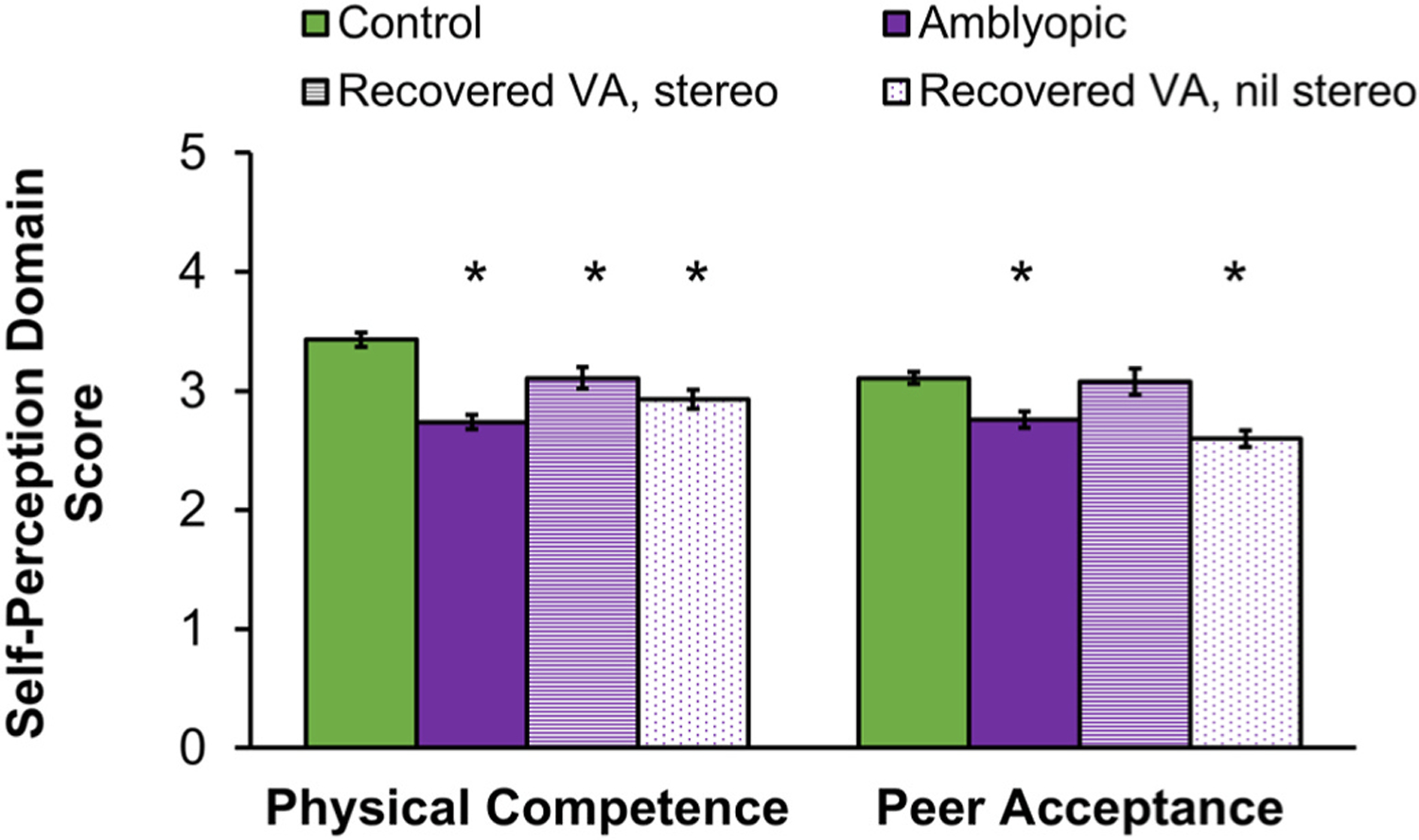
Self-perception domain scores from the Preschool-Kindergarten Pictorial Scale of Perceived Competence and Social Acceptance for Young Children (Harter and Pike, 1984) for amblyopic children aged 3–7 years at baseline (purple, n = 60; reported in (Birch et al., 2019a) and following recovery of normal visual acuity with treatment (n = 82). Data from age-similar controls (green, n = 20) are shown for comparison. Self-perception was lower (i.e., poorer) for amblyopic children than controls for physical competence and peer acceptance. Despite recovery of normal visual acuity with treatment, physical competence domain scores for children remained significantly lower than controls, even among those who also had improvement of stereoacuity (striped purple, n = 40). Peer acceptance domain scores improved for amblyopic children who recovered visual acuity and had improved stereoacuity while those who failed to make gains in stereoacuity (dotted, n = 42) remained significantly lower than controls. Error bars represent ±SEM. *Significantly different from controls.
We explored whether rehabilitation of visual acuity and stereoacuity with amblyopia treatment resulted in improved self-perception of peer acceptance and physical competence testing a separate group of 82 children who were tested following successful amblyopia treatment. All children had recovered normal visual acuity and had ≤0.1 logMAR interocular difference in visual acuity; about half (n = 42) also had improved stereoacuity. The children’s self-perception of peer acceptance did improve, and no longer differed from controls when both visual acuity and stereoacuity improved with amblyopia treatment but not when stereoacuity failed to improve (Fig. 15). This result suggests that treatments that promote recovery of stereoacuity as well as visual acuity may provide additional benefit to the child with amblyopia. On the other hand, recovery of normal visual acuity, with or without improved stereoacuity did not result in improved self-perception of physical competence (Fig. 15). Early intervention, before age 3–7 years, may be important for the development of physical competence when visuomotor skills are being learned. Alternatively, the persistence of lower self-perception of physical competence despite improved vision suggests that remediation of amblyopia may be needed prior to entry into formal schooling when peer comparisons begin to emerge. The foundations of self-perception are already established by age 5 years (Cvencek et al., 2016; Harter, 2006) and it is unclear whether later treatment, even if it is effective in rehabilitating vision, will be able to alter some or all aspects of self-perception.
Children aged 8 to 13 years. The reading speed and eye-hand coordination deficits present in school-age children with amblyopia (Kelly et al., 2015, 2017, 2020) may impede their ability to demonstrate their academic knowledge in standardized tests, compete in physical activities, and interact socially with their peers. Beginning in middle childhood, children are able to make domain specific evaluations of their competence or adequacy (Harter, 2012). Using the Self-Perception Profile for Children Survey (Harter, 1982), we evaluated five components of self-perception: scholastic competence, social competence, athletic competence, physical appearance, and behavioral conduct, and global self-worth (Birch et al., 2018). Children with amblyopia had significantly impaired self-perception of scholastic, social, and athletic competence (Fig. 16). Scholastic competence scores were positively associated with reading speed during natural binocular silent reading of grade-appropriate printed passages assessed with the Readalyzer. Social and athletic competence scores were not found to be associated with amblyopic eye visual acuity or stereoacuity, but were positively associated with performance on aiming and catching tasks of the Movement ABC-2. Children without amblyopia who had been treated for an amblyogenic factor (strabismus or anisometropia) also had impaired self-perception of social and athletic competence but not impaired scholastic competence. This result is consistent with our finding that amblyopia, not the associated etiologic factors of strabismus or anisometropia, is the key factor in slow reading in school age children with amblyopia (Kelly et al., 2015, 2017). Our finding that children with amblyopia and children who had been treated for strabismus or anisometropia but never developed amblyopia have comparably impaired self-perception of social and athletic competence suggests that discordant binocular visual experience, and not amblyopia, is influencing self-perception in these domains (Birch et al., 2018).
Fig. 16.
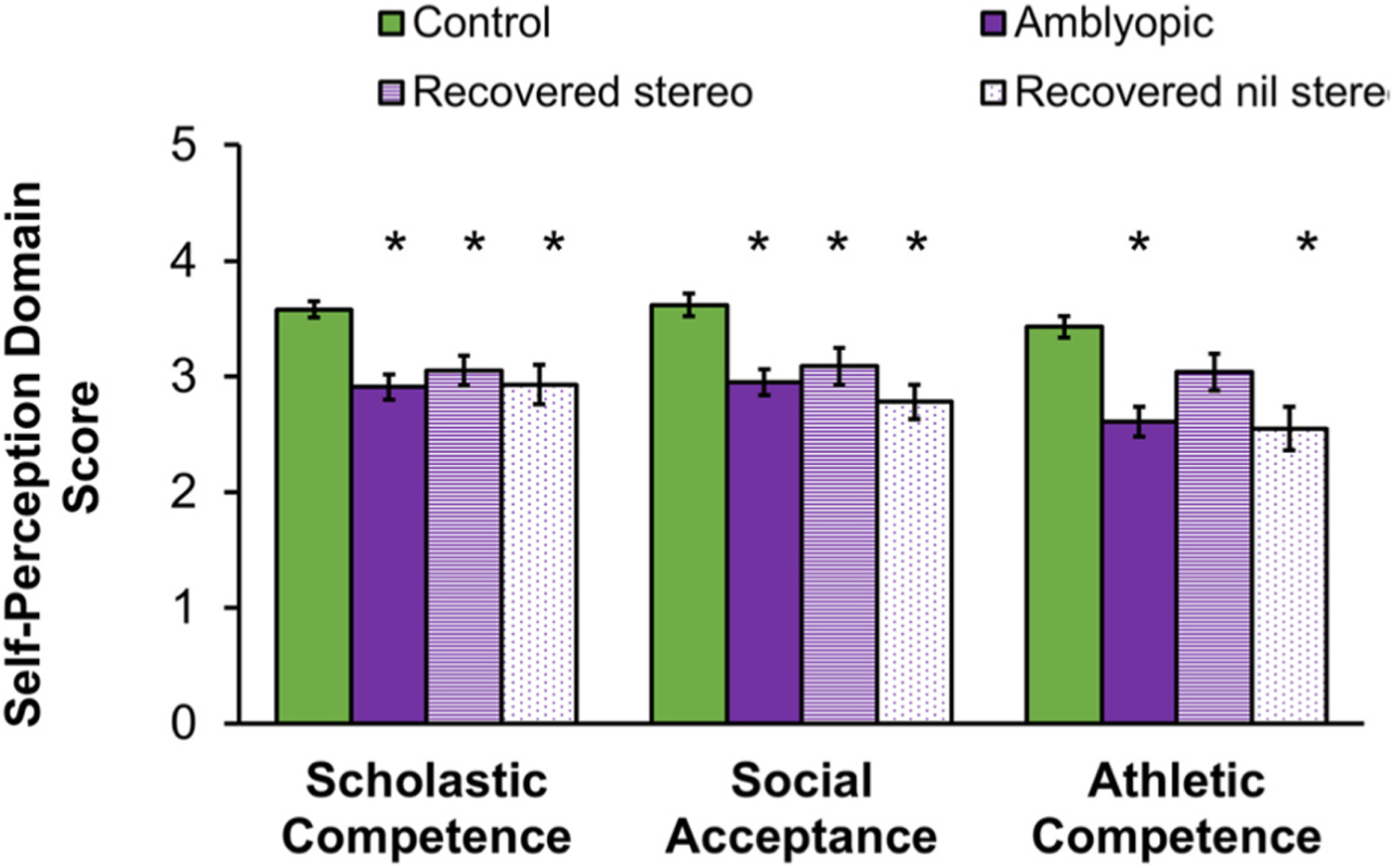
Self-perception domain scores from the Self-Perception Profile for Children Survey (Harter, 1982) for amblyopic children in aged 8–13 years at baseline (purple, n = 50, reported in (Birch et al., 2018) and following recovery of normal visual acuity with treatment (n = 38). Data from age-similar controls (green, n = 18) are shown for comparison. Self-perception was lower (i.e., poorer) for amblyopic children than controls for scholastic competence, social acceptance, and athletic competence. Despite recovery of normal visual acuity with treatment, scholastic competence and social acceptance domain scores for children remained significantly lower than control, even among those who also had improvement of stereoacuity (striped purple, n = 17). Athletic competence domain scores improved for amblyopic children who recovered visual acuity and had improved stereoacuity while those who failed to make gains in stereoacuity (dotted, n = 21) remained significantly lower than control. Error bars represent ±SEM. *Significantly different from controls.
To examine whether rehabilitation of visual acuity and stereoacuity with amblyopia treatment resulted in improved self-perception we tested a separate group of 38 children who had recovered normal visual acuity and had ≤0.1 logMAR interocular difference in visual acuity; about half (n = 17) also had improved stereoacuity. Children’s self-perception of athletic competence did improve, and no longer differed from controls when both visual acuity and stereoacuity improved with amblyopia treatment but not when stereoacuity failed to improve (Fig. 16). Improved self-perception of athletic competence when there is some recovery of stereoacuity that accompanies resolution of visual acuity suggests that binocularity may be the driving force behind lower self-perception for this domain. This result parallels our finding in the 3-to 7-year-old group and supports a potential additional benefit of amblyopia treatments that promote recovery of stereoacuity as well as visual acuity. Recovery of normal visual acuity, with or without improved stereoacuity did not result in improved self-perception of scholastic competence or social acceptance (Fig. 16). Residual effects of amblyopia on these aspects of self-perception of school-age children, even when visual acuity has resolved, suggests that early intervention may be needed for prevention or remediation.
3.6. Quality of life
Recent years have seen substantial growth in research concerned with developing, evaluating, and applying quality of life (QOL) measures within health-related research. The goals are to inform those tasked with health rationing, to inform decision making by agencies such as the Food and Drug Administration, and to provide an important outcome measure in clinical trials comparing therapies. In the context of randomized clinical trials, QOL outcome can be an important contributor to cost-benefit analysis for economic decisions about access to experimental therapies that may provide only a minimal increase to quality of life. Development of a comprehensive instrument that evaluates QOL across a group of related health conditions has been strongly recommended by national agencies as preferable to multiple different instruments specific to individual diseases (Medical Research Council, 2009; Mokkink et al., 2010; U.S. Department of Health and Human Services et al., 2009; Varma et al., 2010). Such instruments allow for assessment of the relative impact of a range of related health conditions, providing consistency and comparability across clinical trials and in healthcare decision making. Interpretation and publication of QOL data can help identify needs for health policies and legislation, guide allocation of resources, and be used to monitor the effectiveness of community-wide interventions.
Pediatric patient-reported outcomes (PROs) are primarily generic surveys designed to compare the quality of life of young children and infants with chronic medical problems in the context of other pediatric diseases (Payot and Barrington, 2011). As a result, these surveys typically lack sensitivity to changes in children’s quality of life associated with amblyopia (Hatt et al., 2020; Steel et al., 2019; Wen et al., 2011). To overcome this limitation, a number of pediatric PROs for amblyopia have been developed to evaluate the effects of amblyopia and its treatment on children’s quality of life, performance daily activities, and symptoms (Bokhary et al., 2013; Carlton, 2019; Choong et al., 2004; Cole et al., 2001; Hrisos et al., 2004; Sabri et al., 2006). Overall, most of the concerns expressed were related to patching treatment rather than the amblyopia per se, including distress to the family related to the patching treatment, dislike of the feeling of the patch, adherence difficulties, and worry about whether patching would be effective, as well as some issues related to the social stigma of patching, teasing, and bullying. Despite these concerns, parents typically reported that their children were happy, generally cooperative, good-humored, and had no behavioral problems. These surveys had one or more shortcomings as a measure of QOL in amblyopia (Table 5): 1) not derived from interviews with children and their families, 2) designed to be administered to only parents as proxies for their children rather than the children themselves, 3) did not utilize item response theory, 4) were limited to a small subset of ages, or 5) focused on performance of activities, symptoms, treatments, or self-esteem rather than QOL. Moreover, none of these instruments allow for assessment of the relative impact of a range of pediatric eye conditions to allow for consistency across clinical trials in pediatric ophthalmology and in healthcare decision making for children with pediatric eye conditions.
Table 5.
Pediatric patient-reported outcomes for children with amblyopia.
| Child Interviews | Child Questionnaire | Rasch Analysis | Limited Age Range (y) | Strong Focus on Performance of Activities, Symptoms, Treatments, or Self-Esteem | |
|---|---|---|---|---|---|
| ATI a | Noh | Noh | Noh | 3–6h | Yesh |
| CAT-QoL b | Yes | Yes | Yes | 4–7h | Yesh |
| CVLS c | Yes | Yes | Yes | 5–12 | Yesh |
| EIAQ d | Noh | Noh | Noh | 4–5h | No |
| PIQ e | Yes | Yes | Noh | 16–18h | No |
| PPQ f | Noh | Noh | Noh | 3–6h | No |
| PedEyeQ g | Yes | Yes | Yes | 0–17 | No |
Amblyopia Treatment Index (Cole et al., 2001).
Child Amblyopia Treatment Questionnaire (Carlton, 2019).
Children’s Vision for Living Scale (Bokhary et al., 2013).
Emotional Impact of Amblyopia Questionnaire (Hrisos et al., 2004).
Psychological Impact Questionnaire (Sabri et al., 2006).
Perceived Psychosocial Questionnaire (Choong et al., 2004).
PedEyeQ (Hatt et al., 2019)
Shortcoming in questionnaire design
It was in this context that we undertook development of the PedEyeQ to evaluate eye condition-related quality of life (ERQOL) across a wide spectrum of pediatric eye conditions in children aged 0–17 years. Children (n = 180) experiencing a range of pediatric eye conditions and their parents (n = 328) participated in semi-structured interviews to identify specific concerns. Coded concerns were reviewed to formulate questions to address specific child concerns and were grouped into bins of like questions (Hatt et al., 2018). Factor analysis was performed to identify unidimensional domains, and Rasch analysis was used to reduce the number of items based on response ordering, local dependence, infit, outfit, differential item functioning, and targeting (Hatt et al., 2019). The PedEyeQ is a suite of questionnaires with queries about functional vision and ERQOL, with separate versions for children (5–11 and 12–17 years versions), parents as proxies for their children (0–4, 5–11, and 12–17 years versions), and parents themselves. The questionnaires in English and Spanish, along with instructions for administration and scoring with the provided Rasch Look-up Tables can be found at: https://public.jaeb.org/pedig/view/reference#pedeyeq.
Using the PedEyeQ to assess children with residual amblyopia aged 8–11 years, both the children themselves and parents completing the proxy survey reported significantly lower (worse) PedEyeQ scores compared to controls for Functional Vision, Bothered by Eyes/Vision, Social, and Frustration/Worry domains (Hatt et al., 2020). On the Parent PedEyeQ, parents reported that their child’s amblyopia had significant Impact on Parent and Family and they had significant Worry About Child’s Eye Condition, Worry About Child’s Self-Perception and Interactions, and Worry About Child’s Functional Vision (Hatt et al., 2020). A strength of this study is that the children had residual amblyopia and were no longer being treated for amblyopia, so patching treatment effects on ERQOL were not in play. However, most of the concerns expressed by children with amblyopia and their parents were also expressed by visually normal controls wearing glasses (Hatt et al., 2020). Nearly all amblyopic children wear glasses for hyperopia or anisometropia, so this finding highlights the difficulty in unambiguously attributing the lower ERQOL scores to amblyopia.
For 189 children aged 5–11 years with strabismus or anisometropia and amblyopia, the PedEyeQ revealed significantly lower Functional Vision and Bothered by Eyes/Vision domain scores for children with moderate amblyopia (0.3–0.6 logMAR), compared with mild amblyopia (≤0.2 logMAR), and significantly lower Functional Vision and Bothered by Eyes/Vision domain scores for children with severe amblyopia (≤0.7 logMAR), compared with mild and moderate amblyopia (Table 6). In addition, Social domain scores were significantly lower for children with severe amblyopia than for children with mild amblyopia.
Table 6.
Mean (standard error) PedEyeQ domain scores for 184 children with amblyopia.
| Amblyopic Eye Visual Acuity, logMAR | Functional Vision, mean (se) | Bothered by Eyes/Vision, mean (se) | Social, mean (se) | Frustration/Worry, mean (se) |
|---|---|---|---|---|
| Mild (0.1–0.2) | 82.0 (2.0) | 86.6 (1.9) | 87.4 (1.9) | 80.9 (3.0) |
| Moderate (0.3–0.6) | 75.0 (1.7)* | 79.2 (1.9)* | 82.0 (1.7) | 75.2 (1.9) |
| Severe (≥0.7) | 66.2 (4.2)* ** | 69.3 (4.6)* ** | 74.6 (3.6)* | 70.8 (4.2) |
Unpublished data from the Retina Foundation of the Southwest.
significantly worse score than mild amblyopia.
significantly worse score than moderate amblyopia.
The applicability of the PedEyeQ to a spectrum of pediatric eye conditions allows us to compare the ERQOL of children with amblyopia to the ERQOL of children with other eye conditions. The scores of children with amblyopia for the PedEyeQ Functional Vision, Bothered by Eyes/Vision, and Social domains were comparable to those of children with cataract, nystagmus, or strabismus (Leske et al., 2021). For the Frustration/Worry domain, children with amblyopia had scores similar to those of children with glaucoma, nystagmus, retinal conditions, strabismus, or uveitis (Leske et al., 2021).
In children with strabismus or anisometropia with and without amblyopia, lower PedEyeQ Functional Vision, Bothered by Eyes/Vision, Social, and Frustration/Worry domain scores were related to lower self-perception of physical competence (Birch et al., 2020a). Additionally, lower Social and Frustration/Worry domain scores were related to lower self-perception of peer acceptance (Birch et al., 2020a). For children with strabismus or anisometropia, PedEyeQ domain scores were not correlated with better eye or worse eye acuity, but note that all had normal visual acuity in at least one eye and about half of the cohort studied had normal visual acuity in both eyes so there may not have been sufficient power to evaluate a weak or moderate correlation (Birch et al., 2020a). Given the strong and moderate correlations with self-perception, ERQOL may be more related to self-perception than vision-related variables.
Self-efficacy has been identified as a contributor to children’s long-term QOL but has barely been explored in the context of amblyopia. Self-efficacy is an individual’s belief in their capacity to act in the ways necessary to reach specific goals and is a pivotal factor in career choice and development (Bandura, 1977; Bandura et al., 2001). Children’s perceived academic, social, and self-regulatory efficacy influence both the types of occupations that they judge to be appropriate for themselves and the direction of their academic course toward those occupations. Stronger perceived self-efficacy is associated with higher goal aspirations and stronger commitment to the goal (Locke and Latham, 1990). Children’s perceived efficacy rather than their actual academic achievement is a key determinant of their perceived occupational self-efficacy and preferred choice of career (Bandura et al., 2001). To date, there is only one published assessment of self-efficacy and general QOL in children with amblyopia aged 11–17 years (Mazlominezhad and Moghadam, 2022). These adolescents with amblyopia scored low (45 out of 100) on the World Health Organization’s Quality of Life questionnaire (WHOQOL) and scored moderate for self-efficacy. There was a significant positive correlation between self-efficacy scores and WHOQOL scores.
Self-efficacy also is known to be a predictor of successful health treatment outcomes and it has been suggested that enhancement of self-efficacy may be a valuable clinical intervention to promote treatment success (Rounds-Bryant et al., 1997). Self-efficacy was found to be a strong predictor of coping behavior and perseverance in performing behaviors necessary to produce a desired health outcome (Bandura and Locke, 2003). Despite promising research with adults who have substance abuse disorders, this approach has yet to be evaluated for children.
4. Future directions
4.1. Deep phenotyping
The last two decades of amblyopia research have seen an emphasis on single-center and multi-center randomized clinical trials (RCTs). RCTs are the cornerstone of evidence-based medicine, and are essential to establishing the effectiveness of treatments with rigorous methodology that avoids biases of confounding factors, selection, and interpretation. This approach has resulted in a strong evidence-base to guide amblyopia treatment (Repka, 2020). Yet, while RCT methodology has many strengths, it is designed to evaluate treatment effectiveness for a group defined by a few common traits (e.g., moderate strabismic or anisometropic amblyopia in a limited age range) and provide information about how effective the treatment is for the group as a whole. In most cases, RCTs enroll an amalgam of good and poor responders, with differing constellations of amblyopic eye, fellow eye, and binocular sensory and ocular motor deficits. As a result, there are considerable individual differences in response to treatment reported in RCTs (Manny et al., 2022; Pediatric Eye Disease Investigator Group, 2002b, 2003a, b, 2010; Stewart et al., 2004, 2017). Socioeconomic factors, health disparities, and adverse family background can limit families’ participation in randomized clinical trials. As a result, the effect of these factors on treatment outcomes has not been addressed in the context of RCTs even though there is evidence that they affect amblyopia treatment outcomes (Abbott and Shah, 2020; Repka, 2020; (Repka et al., 2022) Townsend, 2009).
Now that thousands of children have participated in RCTs of amblyopia treatment, we need to progress to include not only statistical analyses of large randomized cohorts of amblyopic children but also begin to explore a more individualized approach guided by the child’s deep phenotype. Rather than using a few characteristics to identify children eligible for randomization (e.g., amblyogenic factor, severity of visual acuity deficit, age), deep phenotyping emphasizes more precise characterization. To achieve this goal in amblyopia, we will need a consensus on collecting an expanded core set of sensory, ocular motor, and health-related data. In addition, temporal factors (progression or onset of new symptoms), as well as socioeconomic and family environment determinants of health and access to care should be addressed. Taken together with the development of analytic algorithms, such an approach would allow for quantitative characterization of amblyopia phenotypes, help to identify phenotypic features with high diagnostic value, facilitate recognition of amblyopia variants, and support progress towards precision medicine for amblyopia.
4.2. Whole person health
A plethora of research has been conducted on the sensory and ocular motor consequences of amblyopia, with little attention paid to the functional consequences of amblyopia. More recently, evidence highlighting multiple areas of concern have emerged; fine and gross motor deficits, slow reading, and negative impacts on a child’s self-perception and quality of life. Fine motor and gross motor deficits are clearly present in amblyopic children (Kelly et al., 2020), yet the causes of these impairments remain unclear. The role of ocular motor dysfunction, and clinical and sensory factors, and eye-hand/body coordination must be further examined. Additional research on the impact of amblyopia treatment on visuomotor deficits should include direct comparisons before and after treatment. Or, if not effective in resolving the deficits, other forms of interventions should be explored to prevent or ameliorate visuomotor impairments in amblyopic children. Given the damaging consequences that slow reading and visuomotor deficits can impart upon a child as they move through the school system, and into the real world as adults, their causes need to be further explored. Future research on slow reading in amblyopia should use more sensitive eye tracking technology to further characterize ocular motor abnormalities during reading that could be targeted for intervention. Participation in physical activity both inside and outside of the school setting should be investigated as a possible way of cultivating a child’s self-perception and quality of life. Athletic competence is a major contributor to social well-being, self-efficacy, and quality of life (Shapiro and Martin, 2014; Te Velde et al., 2018). Lastly, the progression of these knock-on effects of amblyopia and whether they snowball into more difficulties with age in education, job prospects, physical activity, and family life are unknown. Or, perhaps children develop compensatory strategies to circumvent problems that arise due to their amblyopia.
Currently, the emphasis of treating amblyopia is on visual acuity, and sometimes secondary binocular vision outcomes; but not the whole person. With the more recent focus of the role of binocularity in amblyopia, and the clear knock-on effects, healthcare for amblyopic children should be more holistic, taking into account more than just improving their visual acuity deficit. There needs to be a focus on determining multiple competencies, including reading, self-perception, quality of life, as well as the burden of treatment. Thus, research should explore these intersecting areas to address how best to treat the effects of amblyopia on everyday life functioning, how to design personalized approach to treatments, and how to take into account other determinants of health. The much-needed continuation of research investigating causes of knock-on effects of amblyopia will lead to clinicians, educators, and scientists using this information to develop programs and interventions to prevent or ameliorate slow reading, visuomotor impairment, and low self-perception and quality of life in amblyopic children. This approach can transform vision care into whole person healthcare (See Box 1 for a summary of our suggested future directions).
Box 1. Future directions in amblyopia research.
| AREA OF RESEARCH | FUTURE DIRECTIONS |
|---|---|
| Deep Phenotyping |
|
| Whole Person Health |
|
5. Summary
Amblyopia occurs during a critical period of development, resulting in a visual acuity deficit in the affected eye, many additional sensory deficits in the amblyopic and fellow eyes, binocular dysfunction, impairments in performance of activities of daily life, lower self-perception, and lower quality of life. Yet, many treatments for amblyopia focus solely on rehabilitation of monocular visual acuity, which resolves in only about half of children. More recent treatments may also target interocular suppression. Significant individual differences in response to treatment are not solely due to differences in treatment adherence. Deep phenotyping would offer a complete description of the spectrum of visual abnormalities associated with amblyopia in individual children that might be used to guide treatment in the context of precision medicine. In addition, a whole person healthcare approach to amblyopia should be considered to address the broad effects of amblyopia on child development. Parents, educators, and clinicians should be aware of the potential problems facing children with amblyopia, and guide the family through navigating obstacles to proficient reading, visuomotor ability, and lower self-perception and quality of life. Findings from this evolving field of research has important implications for the development of novel clinical programs and interventions to surmount obstacles to proficient reading and visuomotor skills, and promote physical, social, and academic success, and provide evidence for more in-depth whole health evaluations of children with amblyopia.
Funding
This work was supported by the National Institutes of Health [EY022313, EY028224; EY024333]; Knights Templar Eye Foundation [16-015-CS]; Fight for Sight [PD15002]; Moody Foundation; John and Bonnie Strauss Foundation; Harry Bass Foundation.
Footnotes
CRediT author statement
Eileen E Birch: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition, Krista R Kelly: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None of the authors has a financial conflict of interest with the material presented in this manuscript.
Data availability
Data will be made available on request.
References
- Abbott J, Shah P, 2020. Amblyopia, deprivation and health disparities research: challenges in 2020. Eye 34, 1491–1493. 10.1038/s41433-020-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson M, Sjostrand J, 1988. Contrast sensitivity and acuity relationship in strabismic and anisometropic amblyopia. Br. J. Ophthalmol 72, 44–49. 10.1136/bjo.72.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljohani S, Bailey J, Perno L, et al. , 2020. The feasibility of an educational cartoon video to improve compliance with patching in amblyopic children. Invest. Ophthalmol. Vis. Sci 61, 506. [Google Scholar]
- Alramis F, Roy E, Christian L, Niechwiej-Szwedo E, 2016. Contribution of binocular vision to the performance of complex manipulation tasks in 5–13 years old visually-normal children. Hum. Mov. Sci 46, 52–62. 10.1016/j.humov.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Alsaqr AM, Masmali AM, 2019. The awareness of amblyopia among parents in Saudi Arabia. Ther Adv Ophthalmol 11, 2515841419868103. 10.1177/2515841419868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asper L, Watt K, Khuu S, 2018. Optical treatment of amblyopia: a systematic review and meta-analysis. Clin. Exp. Optom 101, 431–442. 10.1111/cxo.12657. [DOI] [PubMed] [Google Scholar]
- Awan M, Proudlock FA, Gottlob I, 2005. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Invest Ophalmol Vis Sci 46, 1435–1439. 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- Bandura A, 1977. Self-efficacy: toward a unifying theory of behavioral change. Psychol. Rev 84, 191–215. 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A, Locke EA, 2003. Negative self-efficacy and goal effects revisited. J. Appl. Psychol 88, 87–99. 10.1037/0021-9010.88.1.87. [DOI] [PubMed] [Google Scholar]
- Bandura A, Barbaranelli C, Caprara GV, Pastorelli C, 2001. Self-efficacy beliefs as shapers of children’s aspirations and career trajectories. Child Dev. 72, 187–206. 10.1111/1467-8624.00273. [DOI] [PubMed] [Google Scholar]
- Bhola R, Keech RV, Kutschke P, Pfeifer W, Scott WE, 2006. Recurrence of amblyopia after occlusion therapy. Ophthalmology 113, 2097–2100. 10.1016/j.ophtha.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Bhutada I, Skelly P, Jacobs J, et al. , 2022. Reading difficulties in amblyopia: consequence of visual sensory and oculomotor dysfunction. J. Neurol. Sci 442, 120438 10.1016/j.jns.2022.120438. [DOI] [PubMed] [Google Scholar]
- Birch EE, 2013. Amblyopia and binocular vision. Prog. Retin. Eye Res 33, 67–84. 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Holmes JM, 2010. The clinical profile of amblyopia in children younger than 3 years of age. J AAPOS 14, 494–497. 10.1016/j.jaapos.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR, 2006. Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS 10, 409–413. 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Birch EE, Swanson WH, 2000. Hyperacuity deficits in anisometropic and strabismic amblyopes with known ages of onset. Vis. Res 40, 1035–1040. 10.1016/s0042-6989(00)00011-0. [DOI] [PubMed] [Google Scholar]
- Sr Birch EE, Stager DR, Berry P, Leffler J, 2004. Stereopsis and long-term stability of alignment in esotropia. J AAPOS 8, 146–150. 10.1016/S1091853103003124. [DOI] [PubMed] [Google Scholar]
- Birch EE, Subramanian V, Weakley DR, 2013. Fixation instability in anisometropic children with reduced stereopsis. J AAPOS 17, 287–290. 10.1016/j.jaapos.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Li SL, Jost RM, et al. , 2015. Binocular iPad treatment for amblyopia in preschool children. J AAPOS 19, 6–11. 10.1016/j.jaapos.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Morale SE, Jost RM, et al. , 2016. Assessing suppression in amblyopic children with a dichoptic eye chart. Invest. Ophthalmol. Vis. Sci 57, 5649–5654. 10.1167/iovs.16-19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Castaneda YS, Cheng-Patel CS, et al. , 2018. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol 137, 167–174. 10.1001/jamaophthalmol.2018.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Castaneda YS, Cheng-Patel CS, et al. , 2019a. Self-perception in children aged 3 to 7 years with amblyopia and its association with deficits in vision and fine motor skills. JAMA Ophthalmol 137, 499–506. 10.1001/jamaophthalmol.2018.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Jost RM, De La Cruz A, et al. , 2019b. Binocular amblyopia treatment with contrast-rebalanced movies. J AAPOS 23, 160. 10.1016/j.jaapos.2019.02.007 e161–160 e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Jost RM, Wang YZ, Kelly KR, Giaschi DE, 2019c. Impaired fellow eye motion perception and abnormal binocular function. Invest. Ophthalmol. Vis. Sci 60, 3374–3380. 10.1167/iovs.19-26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Kelly KR, Giaschi DE, 2019d. Fellow eye deficits in amblyopia. J. Binocul. Vis. Ocul. Motil 69, 116–125. 10.1080/2576117X.2019.1624440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Castaneda YS, Cheng-Patel CS, et al. , 2020a. Associations of eye-related quality of life with vision, visuomotor function, and self-perception in children with strabismus and anisometropia. Invest. Ophthalmol. Vis. Sci 61, 22. 10.1167/iovs.61.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Jost RM, Kelly KR, et al. , 2020b. Baseline and clinical factors associated with response to amblyopia treatment in a randomized clinical trial. Optom. Vis. Sci 97, 316–323. 10.1097/OPX.0000000000001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Morale SE, Jost RM, Cheng-Patel C, Kelly KR, 2023. Binocular amblyopia treatment improves manual dexterity. J AAPOS S1091–8531 (22). 10.1016/j.jaapos.2022.10.006, 00798–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SA, McConnell EL, McKerr L, et al. , 2019. In-school eyecare in special education settings has measurable benefits for children’s vision and behaviour. PLoS One 14, e0220480. 10.1371/journal.pone.0220480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhary KA, Suttle C, Alotaibi AG, Stapleton F, Boon MY, 2013. Development and validation of the 21-item children’s vision for living scale (CVLS) by Rasch analysis. Clin. Exp. Optom 96, 566–576. 10.1111/cxo.12055. [DOI] [PubMed] [Google Scholar]
- Bonnen K, Matthis JS, Gibaldi A, et al. , 2021. Binocular vision and the control of foot placement during walking in natural terrain. Sci. Rep 11, 20881 10.1038/s41598-021-99846-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle M, Billington C, Shah P, Ferris JD, 2019. Treatment outcomes for amblyopia using PEDIG amblyopia protocols: a retrospective study of 877 cases. J AAPOS 23, 98. 10.1016/j.jaapos.2018.12.007 e91–98 e94. [DOI] [PubMed] [Google Scholar]
- Buckley JG, Panesar GK, MacLellan MJ, Pacey IE, Barrett BT, 2010. Changes to control of adaptive gait in individuals with long-standing reduced stereoacuity. Invest. Ophthalmol. Vis. Sci 51, 2487–2495. 10.1167/iovs.09-3858. [DOI] [PubMed] [Google Scholar]
- Burnett AM, Yashadhana A, Lee L, et al. , 2018. Interventions to improve school-based eye-care services in low- and middle-income countries: a systematic review. Bull. World Health Organ 96, 682–694D. 10.2471/BLT.18.212332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SR, Marsh HW, Sheppard MJ, Sheppard JL, 1985. Seven-year longitudinal study of the early prediction of reading achievement. J. Educ. Psychol 77, 349–361. 10.1037/0022-0663.77.3.349. [DOI] [Google Scholar]
- Caputo R, Tinelli F, Bancale A, et al. , 2007. Motor coordination in children with congenital strabismus: effects of late surgery. Eur. J. Paediatr. Neurol 11, 285–291. 10.1016/j.ejpn.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Carlton J, 2019. Refinement of the child amblyopia treatment questionnaire (CAT-QoL) using Rasch analysis. Strabismus 27, 66–77. 10.1080/09273972.2019.1601743. [DOI] [PubMed] [Google Scholar]
- Chandna A, Pennefather PM, Kovacs I, Norcia AM, 2001. Contour integration deficits in anisometropic amblyopia. Invest. Ophthalmol. Vis. Sci 42, 875–878. [PubMed] [Google Scholar]
- Chandna A, Gonzalez-Martin JA, Norcia AM, 2004. Recovery of contour integration in relation to logMAR visual acuity during treatment of amblyopia in children. Invest. Ophthalmol. Vis. Sci 45, 4016–4022. 10.1167/iovs.03-0795. [DOI] [PubMed] [Google Scholar]
- Chen AM, Manh V, Candy TR, 2018. Longitudinal evaluation of accommodation during treatment for unilateral amblyopia. Invest. Ophthalmol. Vis. Sci 59, 2187–2196. 10.1167/iovs.17-22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong YF, Lukman H, Martin S, Laws DE, 2004. Childhood amblyopia treatment: psychosocial implications for patients and primary carers. Eye 18, 369–375. 10.1038/sj.eye.6700647. [DOI] [PubMed] [Google Scholar]
- Chu R, Huang K, Barnhardt C, Chen A, 2015. The effect of an on-site vision examination on adherence to vision screening recommendations. J. Sch. Nurs 31, 84–90. 10.1177/1059840514524599. [DOI] [PubMed] [Google Scholar]
- Chua B, Mitchell P, 2004. Consequences of amblyopia on education, occupation, and long term vision loss. Br. J. Ophthalmol 88, 1119–1121. 10.1136/bjo.2004.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Beck RW, Moke PS, et al. , 2001. The amblyopia treatment index. J AAPOS 5, 250–254. 10.1067/mpa.2001.117097. [DOI] [PubMed] [Google Scholar]
- Collins ME, Guo X, Repka MX, Neitzel AJ, Friedman DS, 2022. Lessons learned from school-based delivery of vision care in Baltimore, Maryland. Asia Pac. J. Ophthalmol. (Phila) 11, 6–11. 10.1097/APO.0000000000000488. [DOI] [PubMed] [Google Scholar]
- Cunningham AE, Stanovich KE, 1997. Early reading acquisition and its relation to reading experienceand ability 10 years later. Dev. Psychol 33, 934–945. 10.1037//0012-1649.33.6.934. [DOI] [PubMed] [Google Scholar]
- Cvencek D, Greenwald A, Meltzoff A, 2016. Implicit measures for preschool children confirm self-esteem’s role in maintaining a balanced identity. J. Exp. Soc. Psychol 62, 50–57. 10.1016/j.jesp.2015.09.015. [DOI] [Google Scholar]
- Delude CM, 2015. Deep phenotyping: the details of disease. Nature 527, S14–S15. 10.1038/527S14a. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S, 2000. Forward modeling allows feedback control for fast reaching movements. Trends Cognit. Sci 4, 423–431. 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Dixon-Woods M, Awan M, Gottlob I, 2006. Why is compliance with occlusion therapy for amblyopia so hard? A qualitative study. Arch. Dis. Child 91, 491–494. 10.1136/adc.2005.090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews-Botsch C, Machicado K, Lambert SR, Weinstein A, 2022. Prevalence of obesity in adolescents with amblyopia. Invest. Ophthalmol. Vis. Sci 63, 4212. [Google Scholar]
- Drover JR, Morale SE, Wang YZ, Stager DR, Birch EE, 2010. Vernier acuity cards: examination of development and screening validity. Optom. Vis. Sci 87, E806–E812. 10.1097/OPX.0b013e3181f6fb5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shamayleh Y, Kiorpes L, Kohn A, Movshon JA, 2010. Visual motion processing by neurons in area MT of macaque monkeys with experimental amblyopia. J. Neurosci 30, 12198–12209. 10.1523/JNEUROSCI.3055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel-Yeger B, 2008. Evaluation of gross motor abilities and self perception in children with amblyopia. Disabil. Rehabil 30, 243–248. 10.1080/09638280701257221. [DOI] [PubMed] [Google Scholar]
- Felius J, Chandler DL, Holmes JM, Chu RH, Cole SR, Hill M, Huang K, Kulp MT, Lazar EL, Matta NS, Melia M, Wallace DK, 2010. Evaluating the burden of amblyopia treatment from the parent and child’s perspective. J AAPOS 14, 389–395. 10.1016/j.jaapos.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AG, Ferraz NN, 2022. The effects of amblyopia on children’s reading performance after patching treatment. Eur. J. Ophthalmol 32, 575–579. 10.1177/1120672121998248. [DOI] [PubMed] [Google Scholar]
- Giaschi DE, Regan D, Kraft SP, Hong XH, 1992. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Invest. Ophthalmol. Vis. Sci 33, 2483–2489. [PubMed] [Google Scholar]
- Gao TY, Guo CX, et al. , BRAVO Study Team, 2018. Effectiveness of a binocular video game vs placebo video game for improving visual functions in older children, teenagers, and adults with amblyopia: A randomized clinical trial. JAMA Ophthalmol 136, 172–181. 10.1001/jamaophthalmol.2017.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaschi D, Chapman C, Meier K, Narasimhan S, Regan D, 2015. The effect of occlusion therapy on motion perception deficits in amblyopia. Vis. Res 114, 122–134. 10.1016/j.visres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Gitsels LA, Cortina-Borja M, Rahi JS, 2020. Is amblyopia associated with school readiness and cognitive performance during early schooling? Findings from the Millennium Cohort Study. PLoS One 15. 10.1371/journal.pone.0234414 e0234414–e0234414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EG, Wong AMF, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ, 2012. Eye position stability in amblyopia and in normal binocular vision. Invest. Ophthalmol. Vis. Sci 53, 5386–5394. 10.1167/iovs.12-9941. [DOI] [PubMed] [Google Scholar]
- Grant S, Moseley MJ, 2011. Amblyopia and real-world visuomotor tasks. Strabismus 19, 119–128. 10.3109/09273972.2011.600423. [DOI] [PubMed] [Google Scholar]
- Grant S, Suttle C, Melmoth DR, Conway ML, Sloper JJ, 2014. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest. Opthalmol. Vis. Sci 55, 5687–5700. 10.1167/iovs.14-14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Tailor VK, Sloper JJ, et al. , 2012. Visual acuity, crowding, and stereo-vision are linked in children with and without amblyopia. Invest. Ophthalmol. Vis. Sci 53, 7655–7665. 10.1167/iovs.12-10313. [DOI] [PubMed] [Google Scholar]
- Handler SM, Fierson WM, 2011. Learning disabilities, dyslexia, and vision. Pediatrics 127, e818–e856. 10.1542/peds.2010-3670. [DOI] [PubMed] [Google Scholar]
- Harrington S, Kearney J, O’Dwyer V, 2022. Visual factors associated with physical activity in schoolchildren. Clin. Exp. Optom 1–11. 10.1080/08164622.2022.2106780. [DOI] [PubMed] [Google Scholar]
- Harter S, 1982. The perceived competence scale for children. Child Dev. 87–97 10.2307/1129640. [DOI] [PubMed] [Google Scholar]
- Harter S, 2006. The development of self-representations. In: Fisher C, Lerner R (Eds.), Applied Developmental Science: an Encyclopedia of Research, Policies, and Programs. Sage Publications, Thousand Oaks, CA. [Google Scholar]
- Harter S, 2012. The Construction of the Self: Developmental and Sociocultural Foundations. Guillford Press, New York, NY. [Google Scholar]
- Harter S, Pike R, 1984. The pictorial scale of perceived competence and social acceptance for young children. Child Dev. 55, 1969–1982. 10.2307/1129772. [DOI] [PubMed] [Google Scholar]
- Hatt SR, Leske DA, Castaneda YS, Wernimont SM, Liebermann L, Cheng-Patel CS, Birch EE, Holmes JM, 2018. Patient-derived questionnaire items for patient-reported outcome measures in pediatric eye conditions. J AAPOS 22, 445–448. 10.1016/j.jaapos.2018.05.020 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt SR, Leske DA, Castaneda YS, Wernimont SM, Liebermann L, Cheng-Patel CS, Birch EE, Holmes JM, 2019. Development of pediatric eye questionnaires for children with eye conditions. Am. J. Ophthalmol 200, 201–217. 10.1016/j.ajo.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt SR, Leske DA, Castaneda YS, Wernimont SM, Liebermann L, Cheng-Patel CS, Birch EE, Holmes JM, 2020. Understanding the impact of residual amblyopia on functional vision and eye-related quality of life using the PedEyeQ. Am. J. Ophthalmol 218, 173–181. 10.1016/j.ajo.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe M, Gillam B, Chajka K, Vecellio E, 2009. The role of binocular vision in walking. Vis. Neurosci 26, 73–80. 10.1017/S0952523808080838 (The). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J, Truong G, Partanen M, Giaschi D, 2011. Effects of speed, age, and amblyopia on the perception of motion-defined form. Vis. Res 51, 2216–2223. 10.1016/j.visres.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Hemptinne C, Aerts F, Pellissier T, Vanderveken C, 2020. Motor skills in children with strabismus. J AAPOS 24, e76. 10.1016/j.jaapos.2020.01.005 e76. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, 2015. Amblyopia and the binocular approach to its therapy. Vis. Res 114, 4–16. 10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Hill M, Hall A, Williams C, Emond AM, 2019. Impact of co-occurring hearing and visual difficulties in childhood on educational outcomes: a longitudinal cohort study. BMJ Paediatr. Open 3, e000389. 10.1136/bmjpo-2018-000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, 2009. Low- and high-level motion perception deficits in anisometropic and strabismic amblyopia: evidence from fMRI. Vis. Res 49, 2891–2901. 10.1016/j.visres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, Boden C, et al. , 2005. Deficient motion perception in the fellow eye of amblyopic children. Vis. Res 45, 1615–1627. 10.1016/j.visres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Levi DM, 2018. Treatment of amblyopia as a function of age. Vis. Neurosci 35, E015 10.1017/S0952523817000220. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Kraker RT, et al. , 2004. Risk of amblyopia recurrence after cessation of treatment. J AAPOS 8, 420–428. 10.1016/S1091853104001612. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Manh VM, Pediatric Eye Disease Investigator, Group, et al. , 2016. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: A randomized clinical trial. JAMA 134, 1391–1400. 10.1001/jamaophthalmol.2016.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Melia M, Bradfield YS, Cruz OA, Forbes B, 2007. Factors associated with recurrence of amblyopia on cessation of patching. Ophthalmology 114, 1427–1432. 10.1016/j.ophtha.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Strauber S, Quinn GE, et al. , 2008. Further validation of the amblyopia treatment index parental questionnaire. J AAPOS 12, 581–584. 10.1016/j.jaapos.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Lazar EL, Melia BM, et al. , 2011. Effect of age on response to amblyopia treatment in children. Arch. Ophthalmol 129, 1451–1457. 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrisos S, Clarke MP, Wright CM, 2004. The emotional impact of amblyopia treatment in preschool children: randomized controlled trial. Ophthalmology 111, 1550–1556. 10.1016/j.ophtha.2003.12.059. [DOI] [PubMed] [Google Scholar]
- Hrisos S, Clarke MP, Kelly T, Henderson J, Wright CM, 2006. Unilateral visual impairment and neurodevelopmental performance in preschool children. Br. J. Ophthalmol 90, 836–838. 10.1136/bjo.2006.090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey BG, Wang YZ, Birch EE, 2004. Altered global shape discrimination in deprivation amblyopia. Vis. Res 44, 167–177. 10.1016/j.visres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Jost RM, Hunter JS, Dao L, et al. , 2019. Amblyopic eye accommodative response in children with binocular treatment. J AAPOS 21, e30–e31. [Google Scholar]
- Jost RM, Kelly KR, Hunter JS, et al. , 2020. A randomized clinical trial of contrast increment protocols for binocular amblyopia treatment. J AAPOS 24, 282 e281–e282. 10.1016/j.jaapos.2020.06.009 e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost RM, Hudgins LA, Dao LM, Stager DR Jr., Luu B, Beauchamp CL, Hunter JS, Giridhar P, Wang YZ, Birch EE, 2022. Randomized clinical trial of streaming dichoptic movies versus patching for treatment of amblyopia in children aged 3 to 7 years. Sci. Rep 12, 4157. 10.1038/s41598-022-08010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanonidou E, Proudlock F, Gottlob I, 2010. Reading strategies in mild to moderate strabismic amblyopia: an eye movement investigation. Invest. Ophthalmol. Vis. Sci 51, 3502–3508. 10.1167/iovs.09-4236. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, De La Cruz A, Birch EE, 2015. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 19, 515–520. 10.1016/j.jaapos.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Felius J, Ramachandran S, et al. , 2016a. Congenitally impaired disparity vergence in children with infantile esotropia. Invest. Ophthalmol. Vis. Sci 57, 2545–2550. 10.1167/iovs.15-18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Dao L, et al. , 2016b. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol 134, 1402–1408. 10.1001/jamaophthalmol.2016.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, De La Cruz A, et al. , 2017. Slow reading in children with anisometropic amblyopia is associated with fixation instability and increased saccades. J AAPOS 21, 447–451. 10.1016/j.jaapos.2017.10.001e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, De La Cruz A, Birch EE, 2018. Multiple-choice answer form completion time in children with amblyopia and strabismus. JAMA Ophthalmol 136, 938–941. 10.1001/jamaophthalmol.2018.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Cheng-Patel CS, Jost RM, Wang YZ, Birch EE, 2019. Fixation instability during binocular viewing in anisometropic and strabismic children. Exp. Eye Res 183, 29–37. 10.1016/j.exer.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Morale SE, Beauchamp CL, et al. , 2020. Factors associated with impaired motor skills in strabismic and anisometropic children. Invest. Ophthalmol. Vis. Sci 61, 43. 10.1167/iovs.61.10.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Hunter J Jr., Norouzi DM, et al. , 2021. Reach kinematics during binocular viewing in 7- to 12-year-old children with strabismus. Invest. Ophthalmol. Vis. Sci 62, 21. 10.1167/iovs.62.15.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Hudgins LA, et al. , 2023. Slow binocular reading in amblyopic children is a fellow eye deficit Optom Vis Sci. In Press. [DOI] [PMC free article] [PubMed]
- Kelly KR, Mir Norouzi D, Nouredanesh M, et al. , 2022. Temporal eye-hand coordination during visually-guided reaching in 7 to 12 year old children with strabismus. Invest. Ophthalmol. Vis. Sci 63, 10. 10.1167/iovs.63.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA, 1998. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J. Neurosci 18, 6411–6424. 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koklanis K, Abel LA, Aroni R, 2006. Psychosocial impact of amblyopia and its treatment: a multidisciplinary study. Clin. Exp. Ophthalmol 34, 743–750. 10.1111/j.1442-9071.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- Kugathasan L, Partanen M, Chu V, Lyons C, Giaschi D, 2019. Reading ability of children treated for amblyopia. Vis. Res 156, 28–38. 10.1016/j.visres.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Legrand A, Quoc EB, Vacher SW, et al. , 2011. Postural control in children with strabismus: effect of eye surgery. Neurosci. Lett 501, 96–101. 10.1016/j.neulet.2011.06.056. [DOI] [PubMed] [Google Scholar]
- Legrand A, Bui-Quoc E, Bucci MP, 2012. Re-alignment of the eyes, with prisms and with eye surgery, affects postural stability differently in children with strabismus. Graefes Arch. Clin. Exp. Ophthalmol 250, 849–855. 10.1007/s00417-011-1845-z. [DOI] [PubMed] [Google Scholar]
- Leske DA, Hatt SR, Wernimont SM, et al. , 2021. Association of visual acuity with eye-related quality of life and functional vision across childhood eye conditions. Am. J. Ophthalmol 223, 220–228. 10.1016/j.ajo.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D, Klein S, 1982a. Differences in vernier discrimination for gratings between strabismic and anisometropic amblyopes. Invest. Ophthalmol. Vis. Sci 23, 398. [PubMed] [Google Scholar]
- Levi DM, Klein S, 1982b. Hyperacuity and amblyopia. Nature 298, 268–270. 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, 1985. Vernier acuity, crowding and amblyopia. Vis. Res 25, 979–991. 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Wang H, 1994. Amblyopic and peripheral vernier acuity: a test-pedestal approach. Vis. Res 34, 3265–3292. 10.1016/0042-6989(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Levi DM, Song S, Pelli DG, 2007. Amblyopic reading is crowded. J. Vis 7, 1–17. 10.1167/7.2.21.Introduction. [DOI] [PubMed] [Google Scholar]
- Li J, Thompson B, Lam CS, et al. , 2011. The role of suppression in amblyopia. Invest. Ophthalmol. Vis. Sci 52, 4169–4176. 10.1167/iovs.11-7233. [DOI] [PubMed] [Google Scholar]
- Lions C, Bui-Quoc E, Bucci MP, 2013a. Postural control in strabismic children versus non strabismic age-matched children. Graefes Arch. Clin. Exp. Ophthalmol 251, 2219–2225. 10.1007/s00417-013-2372-x. [DOI] [PubMed] [Google Scholar]
- Lions C, Bui-Quoc E, Seassau M, Bucci MP, 2013b. Binocular coordination of saccades during reading in strabismic children. Invest. Ophthalmol. Vis. Sci 54, 620–628. 10.1167/iovs.12-10526. [DOI] [PubMed] [Google Scholar]
- Lions C, Quoc EB, Wiener-vacher S, Maria P, 2014. Postural control in strabismic children: importance of proprioceptive information. Front. Physiol 5, 1–7. 10.3389/fphys.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke EA, Latham GP, 1990. A Theory of Goal Setting & Task Performance. Prentice Hall, Englewood Cliffs, N.J. [Google Scholar]
- Loudon SE, Polling JR, Simonsz HJ, 2002. A preliminary report about the relation between visual acuity increase and compliance in patching therapy for amblyopia. Strabismus 10, 79–82. 10.1076/stra.10.2.79.8143. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Burgess SR, Anthony JL, 2000. Development of emergent literacy and early reading skills in preschool children: evidence from a latent-variable longitudinal study. Dev Psychol 36, 596–613. 10.1037/0012-1649.36.5.596. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Passchier J, Chaker L, et al. , 2009. Psychological causes of non-compliance with electronically monitored occlusion therapy for amblyopia. Br. J. Ophthalmol 93, 1499–1503. 10.1136/bjo.2008.149815. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Johnson C, Majzoub K, 2011. School based vision centers: striving to optimize learning. Work 39, 15–19. 10.3233/WOR-2011-1146. [DOI] [PubMed] [Google Scholar]
- Maconachie GD, Gottlob I, 2015. The challenges of amblyopia treatment. Biomed. J 38, 510–516. 10.1016/j.bj.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manh V, Chen AM, Tarczy-Hornoch K, Cotter SA, Candy TR, 2015. Accommodative performance of children with unilateral amblyopia. Invest. Ophthalmol. Vis. Sci 56, 1193–1207. 10.1167/iovs.14-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manh VM, Holmes JM, Pediatric Eye Disease Investigator Group, et al. , 2018. A randomized trial of a binocular iPad game versus part-time patching in children aged 13 to 16 years with amblyopia. Am J Ophthalmol 186, 104–115. 10.1016/j.ajo.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manny RE, Holmes JM, Kraker RT, et al. , 2022. A randomized trial of binocular Dig Rush game treatment for amblyopia in children aged 4 to 6 years. Optom. Vis. Sci 99, 213–227. 10.1097/OPX.0000000000001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Yabuki A, Hasebe K, et al. , 2010. Postural stability changes during the prism adaptation test in patients with intermittent and constant exotropia. Invest. Ophthalmol. Vis. Sci 51, 6341–6347. 10.1167/iovs.10-5840. [DOI] [PubMed] [Google Scholar]
- Mazlominezhad A, Moghadam FA, 2022. Evaluation of quality of life and self-efficacy in adolescents with amblyopia. J. Med. Life 15, 499–503. 10.25122/jml-2020-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazyn LIN, Lenoir M, Montagne G, Savelsbergh GJP, 2004. The contribution of stereo vision to one-handed catching. Exp. Brain Res 157, 383–390. 10.1007/s00221-004-1926-x. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA, 2003. The pattern of visual deficits in amblyopia. J. Vis 3, 380–405. 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Medical Research Council, 2009. Patient-reported Outcome Measures (PROMs): Identifying UK Research Priorities. https://webarchive.nationalarchives.gov.uk/ukgwa/20090410114333/http://www.diabetes.nhs.uk/news-folder/guidance-on-the-routine-collection-of-patient-reported-outcome-measures-proms/.(Accessed 10 September 2022).
- Meier K, Sum B, Giaschi D, 2016. Global motion perception in children with amblyopia as a function of spatial and temporal stimulus parameters. Vis. Res 127, 18–27. 10.1016/j.visres.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Menon V, Shailesh G, Sharma P, Saxena R, 2008. Clinical trial of patching versus atropine penalization for the treatment of anisometropic amblyopia in older children. J AAPOS 12, 493–497. 10.1016/j.jaapos.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Mir Norouzi D, Dao LM, Beauchamp CL, et al. , 2022. Assessing the contribution of eye movements to slow binocular reading in children with amblyopia. J. Vis 22, 3106. 10.1167/jov.22.14.3106. [DOI] [Google Scholar]
- Mokkink LB, Terwee CB, Patrick DL, et al. , 2010. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual. Life Res 19, 539–549. 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudie LI, Guo X, Slavin RE, Madden N, Wolf R, Owoeye J, Friedman DS, Repka MX, Collins ME, 2022. Baltimore Reading and Eye Disease Study: vision outcomes of school-based eye care. Can. J. Ophthalmol 57, 36–40. 10.1016/j.jcjo.2021.02.013. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Harrison ER, Giaschi DE, 2012. Quantitative measurement of interocular suppression in children with amblyopia. Vis. Res 66, 1–10. 10.1016/j.visres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Newsham D, 2000. Parental non-concordance with occlusion therapy. Br. J. Ophthalmol 84, 957–962. 10.1136/bjo.84.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NX, Stockum A, Hahn GA, Trauzettel-Klosinski S, 2011. Training to improve reading speed in patients with juvenile macular dystrophy: a randomized study comparing two training methods. Acta Ophthalmol. 89, e82–e88. 10.1111/j.1755-3768.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chandrakumar M, Goltz HC, Wong AMF, 2012. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: saccadic eye movements. Invest. Ophthalmol. Vis. Sci 53, 7458–7468. 10.1167/iovs.12-10550. [DOI] [PubMed] [Google Scholar]
- O’Colmain U, Neo YN, Gilmour C, MacEwen CJ, 2020. Long-term visual and treatment outcomes of whole-population pre-school visual screening (PSVS) in children: a longitudinal, retrospective, population-based cohort study. Eye 34, 2315–2321. 10.1038/s41433-020-0821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H, 2010a. Relationship between binocular vision, visual acuity, and fine motor skills. Optom. Vis. Sci 87, 942–947. 10.1097/OPX.0b013e3181fd132e. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H, FSOS Research Group, 2010b. The functional significance of stereopsis. Invest. Ophthalmol. Vis. Sci 51, 2019–2023. 10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- Odenrick P, Sandstedt P, Lennerstrand L, 1984. Postural sway and gait of children with convergent strabismus. Dev. Med. Child Neurol 26, 495–499. 10.1111/j.1469-8749.1984.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Packwood E, Cruz O, Rychwalski P, Keech R, 1999. The psychosocial effects of amblyopia study. J AAPOS 3, 15–17. 10.1016/s1091-8531(99)70089-3. [DOI] [PubMed] [Google Scholar]
- Payot A, Barrington KJ, 2011. The quality of life of young children and infants with chronic medical problems: review of the literature. Curr. Probl. Pediatr. Adolesc. Health Care 41, 91–101. 10.1016/j.cppeds.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2002a. The clinical profile of moderate amblyopia in children younger than 7 years. Arch. Ophthalmol 120, 281–287. [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2002b. A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children. Arch. Ophthalmol 120, 268–278. 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2003a. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch. Ophthalmol 121, 603–611. 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2003b. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 110, 2075–2087. 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2004. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology 111, 2076–2085. 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2010. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology 117, 998–1004. 10.1016/j.ophtha.2009.10.014 e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2012. Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology 119, 150–158. 10.1016/j.ophtha.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2006a. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology 113, 895–903. 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2006b. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology 113, 904–912. 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdziak M, Witkowska D, Gryncewicz W, Przekoracka-Krawczyk A, Ober J, 2014. The amblyopic eye in subjects with anisometropia show increased saccadic latency in the delayed saccade task. Front. Integr. Neurosci 8 10.3389/fnint.2014.00077, 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group, 2019. A randomized trial of binocular Dig Rush game treatment for amblyopia in children aged 7 to 12 years. Ophthalmology 126, 456–466. 10.1016/j.ophtha.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdziak M, Witkowska DK, Gryncewicz W, Ober JK, 2016. Not only amblyopic but also dominant eye in subjects with strabismus show increased saccadic latency. J. Vis 16, 1–11. 10.1167/16.10.12. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Repka MX, Yu F, Lum F, 2016. Risk of musculoskeletal injuries, fractures, and falls in medicare beneficiaries with disorders of binocular vision. JAMA Ophthalmol. 133, 60–65. 10.1001/jamaophthalmol.2014.3941.Risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Repka MX, Yu F, et al. , 2021. Risk of physical injuries in children and teens with ophthalmic diagnoses in the OptumLabs Data Warehouse. J AAPOS 25, 346 e341–e346 e347. 10.1016/j.jaapos.2021.07.007. [DOI] [PubMed] [Google Scholar]
- Rahi JS, Cumberland PM, Peckham CS, 2006. Does amblyopia affect educational, health, and social outcomes? Findings from 1958 British birth cohort. BMJ 332, 820–825. 10.1136/bmj.38751.597963 (AE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavi Z, Soltani A, Vakili A, et al. , 2021. Virtual reality game playing in amblyopia therapy: A randomized clinical trial. J Pediatr Ophthalmol Strabismus 58, 154–160. 10.3928/01913913-20210108-02. [DOI] [PubMed] [Google Scholar]
- Repka MX, 2020. Amblyopia outcomes through clinical trials and practice measurement: room for improvement: the LXXVII Edward Jackson Memorial lecture. Am. J. Ophthalmol 219, A1–A26. 10.1016/j.ajo.2020.07.053. [DOI] [PubMed] [Google Scholar]
- Repka MX, Wallace DK, Beck RW, et al. , 2005. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch. Ophthalmol 123, 149–157. 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Beck RW, et al. , 2008. Monocular oral reading performance following amblyopia treatment in children. Am. J. Ophthalmol 146, 942–947. 10.1016/j.ajo.2008.06.022.Monocular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Beck RW, et al. , 2009. Contrast sensitivity following amblyopia treatment in children. Arch. Ophthalmol 127, 1225–1227. 10.1001/archophthalmol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Holmes JM, et al. , 2014. Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol 132, 799–805. 10.1001/jamaophthalmol.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Li C, Lum F, 2022. Multivariable analyses of amblyopia treatment outcomes from a clinical data registry. Ophthalmology 130, 164–166. 10.1016/j.ophtha.2022.09.005, 00692–00693. [DOI] [PubMed] [Google Scholar]
- Ricks TN, Abbyad C, Polinard E, 2022. Undoing racism and mitigating bias among healthcare professionals: lessons learned during a systematic review. J. Racial. Ethn. Health Disparit 9, 1990–2000. 10.1007/s40615-021-01137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G, Bremer D, Leguire L, 1987. The contrast sensitivity function and childhood amblyopia. Am. J. Ophthalmol 104, 64–68. 10.1016/0002-9394(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Rounds-Bryant JL, Flynn PM, Craighead LW, 1997. Relationship between self-efficacy perceptions and in-treatment drug use among regular cocaine users. Am. J. Drug Alcohol Abuse 23, 383–395. 10.3109/00952999709016884. [DOI] [PubMed] [Google Scholar]
- Sabri K, Knapp CM, Thompson JR, Gottlob I, 2006. The VF-14 and psychological impact of amblyopia and strabismus. Invest. Ophthalmol. Vis. Sci 47, 4386–4392. 10.1167/iovs.05-1365. [DOI] [PubMed] [Google Scholar]
- Scheiman MM, Hertle RW, Kraker RT, et al. , 2008. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Arch. Ophthalmol 126, 1634–1642. 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiple W, Szlyk JP, McMahon T, Pulido J, Fishman GA, 2005. Eye-movement training for reading in patients with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 46, 2886–2896. 10.1167/iovs.04-1296. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Otero-Millan J, Kumar P, Ghasia FF, 2016. Abnormal fixational eye movements in amblyopia. PLoS One 11, e0149953. 10.1371/journal.pone.0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DR, Martin JJ, 2014. The relationships among sport self-perceptions and social well-being in athletes with physical disabilities. Disabil. Health J 7, 42–48. 10.1016/j.dhjo.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Steel DA, Codina CJ, Arblaster GE, 2019. Amblyopia treatment and quality of life: the child’s perspective on atropine versus patching. Strabismus 27, 156–164. 10.1080/09273972.2019.1643894. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Fielder AR, Moseley MJ, Stephens DA, 2001. Is it all over in 6 weeks? Intrerim analysis of the monitored occlusion treatment for amblyopia study (MOTAS). Invest. Ophthalmol. Vis. Sci 42, S399. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA, Fielder AR, 2004. Treatment dose-response in amblyopia therapy: the monitored occlusion treatment of amblyopia study (MOTAS). Invest. Ophthalmol. Vis. Sci 45, 3048–3054. 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Georgiou P, Fielder AR, 2017. Occlusion dose monitoring in amblyopia therapy: status, insights, and future directions. J AAPOS 21, 402–406. 10.1016/j.jaapos.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Stifter E, Burggasser G, Hirmann E, Thaler A, Radner W, 2005a. Evaluating reading acuity and speed in children with microstrabismic amblyopia using a standardized reading chart system. Graefes Arch. Clin. Exp. Ophthalmol 243, 1228–1235. 10.1007/s00417-005-1187-9. [DOI] [PubMed] [Google Scholar]
- Stifter E, Burggasser G, Hirmann E, Thaler a., Radner W, 2005b. Monocular and binocular reading performance in children with microstrabismic amblyopia. Br. J. Ophthalmol 89, 1324–1329. 10.1136/bjo.2005.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Morale SE, Wang YZ, Birch EE, 2012. Abnormal radial deformation hyperacuity in children with strabismic amblyopia. Invest. Ophthalmol. Vis. Sci 53, 3303–3308. 10.1167/iovs.11-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Jost RM, Birch EE, 2013. A quantitative study of fixation stability in amblyopia. Invest. Ophthalmol. Vis. Sci 54, 1998–2003. 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S, 2011. Eye-hand coordination skills in children with and without amblyopia. Invest. Ophthalmol. Vis. Sci 52, 1851–1864. 10.1167/iovs.10-6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Zhang B, Shen G, et al. , 2014. Early monocular defocus disrupts the normal development of receptive-field structure in V2 neurons of macaque monkeys. J. Neurosci 34, 13840–13854. 10.1523/JNEUROSCI.1992-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velde SJ, Lankhorst K, Zwinkels M, et al. , 2018. Associations of sport participation with self-perception, exercise self-efficacy and quality of life among children and adolescents with a physical disability or chronic disease-a cross-sectional study. Sports Med. Open 4, 38. 10.1186/s40798-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjiam AM, Holtslag G, Vukovic E, et al. , 2012. An educational cartoon accelerates amblyopia therapy and improves compliance, especially among children of immigrants. Ophthalmology 119, 2393–2401. 10.1016/j.ophtha.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Townsend P, 2009. Deprivation. J. Soc. Pol 16, 125–146. 10.1017/S0047279400020341. [DOI] [Google Scholar]
- U.S. Department of Health and Human Services, 2009. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. https://www.fda.gov/media/77832/download. (Accessed 6 September 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Richman EA, Ferris FL 3rd, Bressler NM, 2010. Use of patient-reported outcomes in medical product development: a report from the 2009 NEI/FDA Clinical Trial Endpoints Symposium. Invest. Ophthalmol. Vis. Sci 51, 6095–6103. 10.1167/iovs.10-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MP, Stewart CE, Moseley MJ, et al. , 2013. Compliance with occlusion therapy for childhood amblyopia. Invest. Ophthalmol. Vis. Sci 54, 6158–6166. 10.1167/iovs.13-11861. [DOI] [PubMed] [Google Scholar]
- Wang J, 2015. Compliance and patching and atropine amblyopia treatments. Vis. Res 114, 31–40. 10.1016/j.visres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Neely DE, et al. , 2016. A pilot randomized clinical trial of intermittent occlusion therapy liquid crystal glasses versus traditional patching for treatment of moderate unilateral amblyopia. J AAPOS (20), 326–331.doi. 10.1016/j.jaapos.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang B, Tao X, et al. , 2017. Noisy spiking in visual area V2 of amblyopic monkeys. J. Neurosci 37, 922–935. 10.1523/JNEUROSCI.3178-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SJ, Bradshaw MF, Clarke TJ, Elliot KM, 2003. Binocular vision and prehension in middle childhood. Neuropsychologia 41, 415–420. 10.1016/S0028-3932(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B, 2008a. The effect of amblyopia on fine motor skills in children. Invest. Ophthalmol. Vis. Sci 49, 594–603. 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B, 2008b. Effect of amblyopia on self-esteem in children. Optom. Vis. Sci 85, 1074–1081. 10.1097/OPX.0b013e31818b9911. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Thompson B, 2016. Fine motor skills of children with amblyopia improve following binocular treatment. Invest. Ophthalmol. Vis. Sci 57, 4713–4720. 10.1167/iovs.16-19797. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Thompson B, Birch EE, 2019. From suppression to stereoacuity: a composite binocular function score for clinical research. Ophthalmic Physiol. Opt 39, 53–62. 10.1111/opo.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Mandall TR, Molloy DT, Lister LJ, Birch EE, 2020. Worth 4 dot app for determining size and depth of suppression. Transl. Vis. Sci. Technol 9, 9. 10.1167/tvst.9.4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G, McKean-Cowdin R, Varma R, et al. , 2011. General health-related quality of life in preschool children with strabismus or amblyopia. Ophthalmology 118, 574–580. 10.1016/j.ophtha.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehurst GJ, Longian CJ, 1998. Child development and emergent literacy. Child Dev 69, 848–872. [PubMed] [Google Scholar]
- Williams C, Horwood J, Northstone K, et al. , 2006. The timing of patching treatment and a child’s wellbeing. Br. J. Ophthalmol 90, 670–671. 10.1136/bjo.2006.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GA, Welch D, 2013. Does amblyopia have a functional impact? Findings from the dunedin multidisciplinary health and development study. Clin. Exp. Ophthalmol 41, 127–134. 10.1111/j.1442-9071.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, Smith LK, 1994. Factors affecting the outcome of children treated for amblyopia. Eye 8, 627–631. 10.1038/eye.1994.157. [DOI] [PubMed] [Google Scholar]
- Wygnanski-Jaffe T, Kushner BJ, Moshkovitz A, Belkin M, Yehezkel O, CureSight Pivotal Trial Group, 2022. An eye-tracking-based dichoptic home treatment for amblyopia: A multicenter randomized clinical trial. Ophthalmology. 10.1016/j.ophtha.2022.10.020, 2022, In Press. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Xiao S, Angjeli E, Wu HC, Gaier ED, et al. , Luminopia Pivotal Trial Group, 2022. Randomized controlled trial of a dichoptic digital therapeutic for amblyopia. Ophthalmology 129, 77–85. 10.1016/j.ophtha.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Zipori AB, Colpa L, Wong AMF, Cushing SL, Gordon KA, 2018. Postural stability and visual impairment: assessing balance in children with strabismus and amblyopia. PLoS One 13, 1–18. 10.1371/journal.pone.0205857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher BLJ, 1980. Reading capacity in cases of cured strabismic amblyopia. Trans. Ophthalmol. Soc. U. K 100, 501–503. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


