Abstract
Fragile X mental retardation protein (FMRP) binds a selected set of mRNAs and proteins to guide neural circuit assembly and regulate synaptic plasticity. Loss of FMRP is responsible for Fragile X syndrome, a neuropsychiatric disorder characterized with auditory processing problems and social difficulty. FMRP actions in synaptic formation, maturation, and plasticity are site-specific among the four compartments of a synapse: presynaptic and postsynaptic neurons, astrocytes, and extracellular matrix. This review summarizes advancements in understanding FMRP localization, signals, and functional roles in axons and presynaptic terminals.
Keywords: Fragile X mental retardation protein, neuropsychiatric disorders, RNA-binding proteins, neurotransmitter release, axon projection development, axonal granules
1. Introduction
Fragile X mental retardation protein (FMRP; also called Fragile X messenger ribonucleoprotein) is an important regulator of brain development and plasticity through its ability to bind a wide range of messenger RNAs (mRNAs) and proteins. This protein is responsible for Fragile X syndrome (FXS), a leading single-gene cause of intellectual disability and social difficulties, with prominent sensory dysfunction (Hagerman et al., 2017). FMRP is also associated with a number of other neurodevelopmental and neurodegenerative diseases (Fatemi and Folsom, 2011; Salcedo-Arellano et al., 2020; Tan et al., 2020). From the time when FMRP and its gene Fmr1 were identified as the cause of FXS (Verkerk et al., 1991; Yu et al., 1991), research on FMRP function and FXS neuropathology has been at the frontier for understanding the molecular basis of brain function and for developing treatments for neuropsychiatric disorders. In the brain, FMRP is expressed in almost all neurons as well as in several glial cell types including astrocytes and oligodendrocytes (Giampetruzzi et al., 2013; Gholizadeh et al., 2015; Zorio et al., 2017). Over the last several decades, we have come to understand the vital importance of FMRP in assembling neural circuits and modulating synaptic plasticity, among other functions. In particular, postsynaptic structures on dendrites have been identified as key sites of FMRP action, often through control of activity-dependent mRNA translation via glutamate and GABA receptor signaling (Bear et al., 2004; Dölen et al., 2007; Lozano et al., 2014; Van der Aa and Kooy, 2020). More recent studies have highlighted the contribution of astrocytes and extracellular matrix, the other two components of the ‘tetrapartite synapse’ (Dityatev and Rusakov, 2011), in FMRP regulation (Cheng et al., 2012; Siller and Broadie, 2012; Wen et al., 2018). This review focuses on the least understood FMRP localization and function, which is that in axons and presynaptic elements (Akins, 2009).
We first discuss the evidence in support of a vital role of cell autonomous FMRP in axonal events, focusing on the structural establishment of axon projection and synaptic connectivity (Section 2) and the functional maturation and plasticity of neurotransmitter release from presynaptic terminals (Section 3). We then describe FMRP localization in axons as a potential mechanism underlying this role (Section 4). In so doing, we summarize the developmental profile and circuit-dependent distribution of axonal FMRP and then discuss regulatory mechanisms that localize FMRP to axons. Next, we dissect known FMRP signals and their association with FMRP-dependent developmental and plasticity events in axons (Section 5), introducing mechanisms of FMRP action by mRNA binding, protein interaction, and phase separation. We also highlight roles of FMRP-associated cell adhesion molecules (CAMs), presynaptic cytoskeletal regulators, presynaptic ion channels and receptors, and synaptic vesicle (SV) and releasing machinery. Toward the goal of understanding FMRP function in general as well as in axons in particular, studies of the auditory system have increased, along with a growing appreciation of the high prevalence of deficits in auditory-related brain activity and auditory-mediated behaviors in FXS. Here, we review recent advances of FMRP research in the auditory system with a focus on studies that provide direct evidence of FMRP localization in axons and identify FMRP-dependent axonal events (Section 6). We also discuss multifaceted FMRP mechanisms in auditory development, function, and disease. Finally, we conclude with open questions and propose directions for future investigations (Section 7).
Understanding the nomenclature of “presynaptic FMRP” as we use it here is important. Functional significance of FMRP in presynaptic terminals is implicated in studies that employed targeted approaches to selectively manipulate FMRP expression and activity in the presynaptic, but not postsynaptic, neurons within a circuit. These functions are then often labelled “presynaptic FMRP function” or “presynaptic mechanism of FMRP”. However, most of these studies identified a requirement of cell autonomous FMRP or FMRP expression in presynaptic neurons for the examined axonal properties, rather than establishing a true involvement of “presynaptically localized FMRP”. For enhancing scientific rigor, here we limit the use of “presynaptic FMRP” to the events that indeed take place in this specialized synaptic structure and distal axons.
2. Cell autonomous FMRP regulates axon growth and establishes synaptic connectivity
Axon development is a longitudinal, multi-event process, starting with neurite formation and axon polarization. This is followed by guided growth and navigation through the brain to arrive at the target area where axon branching as well as synapse formation and reorganization take place subsequently. The importance of FMRP during early events of axon development was first appreciated under cultured conditions. Fmr1 knockout (KO) or knockdown in hippocampal neurons leads to excess filopodia and reduced motility of axonal growth cones (Antar et al., 2006), reduced growth cone collapse in response to axon guidance factor semaphorin (Li et al., 2009), and increased axon length (Zhang et al., 2015). FMRP deficiency leads to random turning of post-crossing axons in commissural neurons due to the disrupted interaction between FMRP-Frizzled3 complex and Wnt5a in the growth cone (Marfull-Oromí et al., 2022). Additionally, FMRP regulates axon branching, as demonstrated by Fmr1 KO-induced neurite overgrowth and overbranching in cultured chicken hindbrain neurons (Wang et al., 2020). Consistently, FMRP overexpression in cortical neurons resulted in a decrease in axonal arbor complexity (Zimmer et al., 2017), demonstrating a dose-dependency of FMRP regulation. Together, these culture studies demonstrate a requirement of cell autonomous FMRP expression in axon growth and branching.
In accordance with this requirement, brains without FMRP display abnormal patterns of axonal projection, as shown in Fmr1 KO animals. This includes excessive axonal branches in zebrafish motor neurons (Shamay-Ramot et al., 2015), spatially diffuse axonal arbors in the mouse somatosensory cortex (Bureau et al., 2008), and ectopic mossy fiber distribution in the mouse hippocampus (Ivanco and Greenough, 2002). In the auditory brainstem, glycinergic and GABAergic afferent inputs to the medial nucleus of the trapezoid body is altered in Fmr1 KO mice, showing stronger VGAT immunostaining (Rotschafer et al., 2015) and fewer GlyT2+ or GAD67+ presynaptic structures (McCullagh et al., 2017). Similarly, using a monosynaptic tracing method, a recent study found that the afferent inputs to cortical interneurons are disrupted in Fmr1 KO mice in an area-specific manner (Pouchelon et al., 2021). However, these constitutive KO studies could not determine the site of FMRP action in these effects. This issue was partially addressed using a Fmr1 mosaic mouse model in which FMRP-expressing and FMRP-lacking neurons were intermingled due to random x-inactivation in females. Presynaptic neurons with FMRP formed more synaptic connections than presynaptic neurons lacking Fmr1 expression (Hanson and Madison, 2007). At the same time, the postsynaptic Fmr1 genotype did not influence the probability that a neuron would receive synaptic connections, emphasizing the importance of FMRP on the presynaptic site of the circuit in establishing neuronal connectivity. This regulation was eventually confirmed with in vivo studies of the auditory brainstem following cell group-specific FMRP knockdown, revealing disoriented axonal growth, delay in midline crossing, and aberrant axonal targeting (Wang et al., 2020; Curnow and Wang, 2022)(see further discussion in Section 6).
Evidence is also available in Drosophila models. dFmr1 mutants exhibited axon extension deficits; more complex axonal architecture including overgrowth, over branching, and excess synaptic boutons; and the presence of development-arrested satellite boutons in the mushroom body and at neuromuscular junction synapses (Dockendorff et al., 2002; Morales et al., 2002; Pan et al., 2004; Gatto and Broadie, 2009; Song et al., 2022). dFMRP is also required for efficient activity-dependent pruning of axon branches in the mushroom body (Tessier and Broadie, 2008). dFMRP overexpression resulted in reduced axonal complexity and aberrant axonal pathfinding (Morales et al., 2002; Michel et al., 2004; Pan et al., 2004). Importantly, restoring presynaptic dFMRP rescued these structural deficits (Pan et al., 2004), establishing the involvement of FMRP expression in presynaptic neurons. Interestingly, dFMRP regulation of axon growth is cell-type dependent, as shown in the medulla, where axonal extension from the lobula was reduced for dorsal cluster neurons but excessive for lateral neurons in dFmr1 KOs (Morales et al., 2002).
3. Cell autonomous FMRP regulates presynaptic release and plasticity
Following the establishment of synaptic connectivity, FMRP continues to function in regulating presynaptic function and plasticity for neurotransmission. Evidence is available that a global loss of FMRP can lead to defective release of several neurotransmitters from presynaptic terminals, including glutamate, GABA, dopamine, and neuropeptides.
To study whether FMRP deletion alters the quantal content of synaptic vesicles, miniature IPSCs (mIPSCs) or EPSCs (mEPSCs) were recorded and compared between wildtype and Fmr1 KO neurons. In the anterior cingulate cortex and lateral amygdala, presynaptic glutamate release was reduced in Fmr1 KO mice, showing a decreased frequency of mEPSCs (Suvrathan et al., 2010; Yang et al., 2020a). Quantal analyses also revealed decreased amplitudes of spontaneous mIPSCs and smaller postsynaptic patch sizes of GABAA receptors at inhibitory synapses in the Fmr1 KO amygdala (Vislay et al., 2013). However, effects of FMRP deletion on quantal content of synaptic vesicles appear to be neuronal type-specific and/or developmental age-dependent. Decreases in mIPSCs amplitude and GABA concentration at amygdala synapse was less distinct at P14-16 as compared to younger or older ages (Vislay et al., 2013). In 2-week-old Fmr1 KO mice, basic quantal units of synaptic transmission appear normal in synapse strength, glutamate release probability, and vesicle release in hippocampal pyramidal neurons (Klemmer et al., 2011). The amplitude and frequency of mEPSCs were wildtype-like in adult anterior piriform cortex and CA1 pyramidal cells (Braun and Segal, 2000; Deng et al., 2011; Gocel and Larson, 2012). Similarly, the amplitude and frequency of mIPSCs were not affected in the cerebellar basket cells of 1-month-old Fmr1 KO mice (Yang et al., 2020b). In primary hippocampal cultures of Fmr1 KO rats, FMRP absence resulted in increased frequency and amplitude of spontaneous, but not of miniature, synaptic events, suggesting that FMRP is more involved in regulating the turnover of vesicles than in quantal content under this condition (Subrahmanyam et al., 2021). In contrast, silencing FMRP was found to increase mEPSC frequency of cultured mouse hippocampal neurons, suggesting that FMRP inhibits, rather than promotes, glutamate release in this cell type under this condition (Zhang et al., 2015).
FMRP is also required for presynaptic short-term plasticity. For example, at excitatory CA3–CA1 hippocampal synapses, Fmr1 KO mice displayed elevated release probability and exaggerated calcium influx during repetitive activity without affecting the basal release probability or basal synaptic transmission (Deng et al., 2011; Wang et al., 2014a). In the anterior cingulate cortex, a form of presynaptic-long term potentiation following low frequency stimulation (2 Hz) was abolished in Fmr1 KO mice (Koga et al., 2015). In the cortico-hippocampal pathway, inhibitory synapses exhibited increased paired-pulse ratio (an indicator of presynaptic short-term plasticity) and decreased GABA release probability (Wahlstrom-Helgren and Klyachko, 2016). In addition to defective glutamate and GABA release, a marked reduction of presynaptic dopaminergic uptake was reported in FXS individuals (Paucar et al.,2016) In Fmr1 KO mice of 15–20 weeks of age, electrically evoked dopamine release and uptake was diminished in striatal brain slices (Fulks et al., 2010). Finally, at the Drosophila neuromuscular junction, dFMRP overexpression dramatically reduced neuropeptide intensity per presynaptic boutons and increased the number of dense-core vesicles, upon which synaptic release of neuropeptides and neurotrophins depends (Cavolo et al., 2016).
Targeted manipulations confirmed the contribution of FMRP expression in presynaptic neurons to the observed release deficits. For example, in Aplysia, selectively downregulating FMRP in individual sensory neurons enhanced the long-term synaptic depression at the sensory-to-motor neuron synapses, although it had little to no effect on long-term synaptic facilitation (Till et al., 2011). In Fmr1 mosaic mice, FMRP loss from presynaptic neurons reduced the local excitation of layers 4 and 5 fast-spiking inhibitory neurons in the neocortex, indicating that cell autonomous FMRP promotes presynaptic release probability (Patel et al., 2013). Interestingly, presynaptic FMRP loss had no effect on excitatory synapses onto excitatory neurons, implicating a target cell-specific function for presynaptic FMRP (Patel et al., 2013). Acute reintroduction of FMRP via intracellular perfusion into individual Fmr1 KO CA3 neurons confirmed a presynaptic origin of FMRP-regulated action potential (AP) duration (Deng et al., 2013). Consistently, acute neutralization of FMRP using an antibody in wildtype CA3 neurons mimicked the abnormal AP broadening observed in Fmr1 KO mice. FMRP actions on AP waveforms were axonal/presynaptic in origin, supported by the increased amplitude of compound APs recorded from the area near presynaptic terminals and direct observation of excessive calcium influx in the presynaptic terminals (Deng et al., 2013). The reduction in GABA release in the cortico-hippocampal pathway is dependent on presynaptic GABAB receptor, as revealed by selective activation of these receptors using low concentrations of baclofen (Wahlstrom-Helgren and Klyachko, 2015, 2016). Interestingly, in the amygdala, FMRP-mediated GABA production and uptake at the synapse were associated with GABAA receptor specificity, as demonstrated by conditional Fmr1 KO and rescue specific to forebrain inhibitory neurons (Vislay et al., 2013).
4. Axonal localization of FMRP
As described above, cell autonomous FMRP expression is required for proper structural and functional establishment of presynaptic connectivity. Due to the long distance from axonal growth cones and presynaptic terminals to the soma and the time-sensitivity of axonal endings in response to local environments, it is reasonable to speculate that FMRP exerts these functions on-site in axons and axonal endings. In support of this idea, axonal localization of FMRP has been identified, and the evidence is summarized here.
4.1. FMRP is present in axon branches and endings in a circuit-dependent manner
We know that neuronal FMRP is localized in the cytoplasm, dendrites, and nucleus (Weiler et al., 1997; Dury et al., 2013; Wang et al., 2014b). The discovery of an axonal localization of FMRP came later and was first identified via electron microscope observations of the rat brain. Although less intense than somatodendritic labeling, a subset of axon terminals contained FMRP immunogold particles (Feng et al., 1997). Further evidence was provided by light microscopy studies of cultured neurons that reported FMRP puncta in the growth cones and axonal branches of mammalian neurons (Antar et al., 2006; Hengst et al., 2006; Jain and Welshhans, 2016), in Aplysia sensory neurons (Till et al., 2011), and in Drosophila neuromuscular junction structures (Gatto and Broadie, 2009). Subsequent studies of intact mammalian brains confirmed FMRP-containing granules in developing and mature axon tracts as well as in presynaptic terminals across species including humans (Christie et al., 2009; Shepard et al., 2020). These granules belong to a subpopulation of axonal puncta known as Fragile X granules (FXG), which contain FMRP and/or Fragile X-related proteins FXR1P and FXR2P. All FXGs have FXR2P and are associated with translational machinery and mRNAs, but only some contain FMRP (Christie et al., 2009; Akins et al., 2017; Chyung et al., 2018). FMRP-containing FXGs are absent in Fxr2 (gene encoding FXR2P) KO mice, indicating that FXR2P is necessary for the axonal and presynaptic localization of FMRP to these structures (Christie et al., 2009). On the other hand, Fmr1 KO mice display a higher density of FXR2P-labeled FXGs in the frontal cortex both during development (P15) and at a more mature age (P30) (Figure 1A), suggesting an inhibitory role of FMRP in FXG formation (Christie et al., 2009). In developing chicken hindbrains, both endogenous and expressed FMRP were observed in the distal axons of auditory neurons (Wang et al., 2020). Interestingly, it was reported that FXGs in the postnatal mouse brainstem do not contain FMRP (Chyung et al., 2018). Whether this difference reflects interspecies variation or stage-dependent axonal forms of FMRP (FXGs vs. other forms) is yet to be determined.
Figure 1. A. Fragile X granules (A) and known FMRP-bound mRNAs and proteins in axons and axonal endings (B).
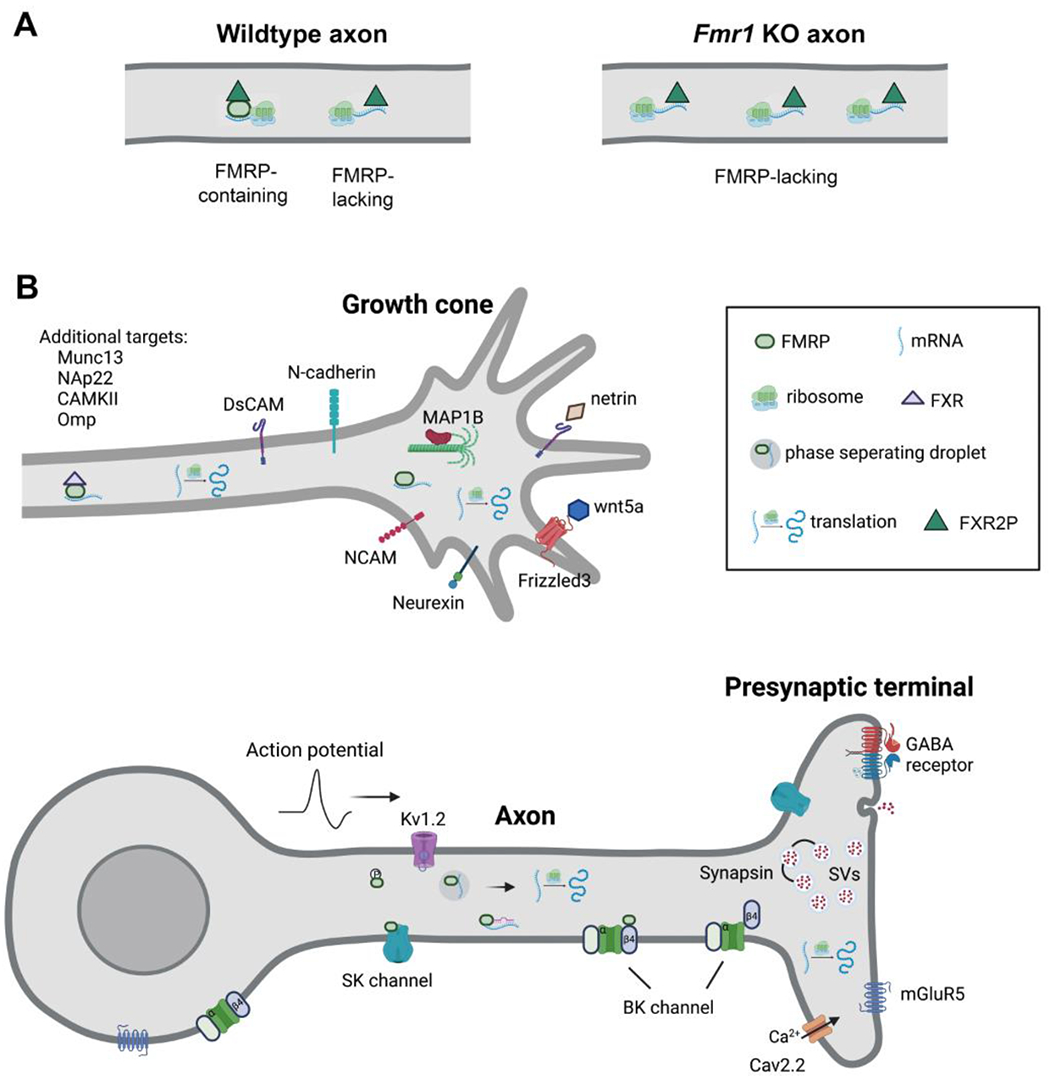
The drawing in A was built upon Akin et al., 2017. In B, The large number of SV-associated proteins and active zone members that are affected by FMRP absence are not included due to the lack of confirmation of a direct binding with FMRP. SVs: synaptic vesicles. Created with BioRender.com.
Although axonal localization of FMRP is evolutionarily conserved and spans throughout the brain, it exhibits circuit- and cell type-dependence. For example, in 2-week-old mice, a higher density of FXGs (both those containing FMRP and those lacking FMRP) was present within a restricted subset of neurons and fiber tracts including corticocortical and thalamocortical fibers, olfactory sensory neuron axons, hippocampal CA3 associational axons, and cerebellar parallel fibers than in others such as the corpus callosum and optic tract (Akins et al., 2012). Additionally, there was a particular richness of FXGs in circuits involved in sensory and motor processing (Akins et al., 2012). Strong FMRP expression and distinct axonal localization go beyond the brain into primary sensory neurons, whose somata are situated in the peripheral nervous system and extend long axons to the brain. Examples include dorsal root, trigeminal, auditory, and retinal ganglion neurons (Price et al., 2006; Frederikse et al., 2015; Guimarães-Souza et al., 2016; Wang et al., 2022). These distribution patterns may underlie prominent sensory problems in FXS (Rotschafer and Razak, 2014; McCullagh et al., 2017; Sinclair et al., 2017; Rais et al., 2018) and implicate contribution of a lost control of axonal FMRP (see Section 6 for further discussion).
4.2. Axonal localization of FMRP is most intense and regulatable in developing axons
In chicken embryos, FMRP was identified as early as embryonic day 4 (E4; chicken take 21 days to hatch) in the navigating axons (Wang et al., 2020), indicating that FMRP localizes to the axon shortly after Fmr1 gene expression and axonogenesis. This idea is consistent with the presence of expressed FMRP-GFP in newly formed neurites of PC12 cells within 1–2 hours of expression induction (De Diego Otero et al., 2002). In the mouse cortex, hippocampus, and cerebellum, the density of FMRP-containing FXGs gradually reduces starting in postnatal ages and is low in adults (Christie et al., 2009). This pattern resembles the developmental decline of the overall FMRP level across brain regions (Lu et al., 2004; Wang et al., 2004; Cook et al., 2011; Pacey et al., 2013); however, the relationship between cytoplasmic FMRP expression and its axonal localization is not clear. One exception to this pattern is the olfactory bulb in recipient of olfactory sensory neuron (OSN) axon innervation. OSNs have a high turnover rate throughout life. FMRP-containing FXGs are present in OSN axons in mice at both postnatal and adult ages, consistent with the preference of axonal FMRP in developing axons (Christie et al., 2009). In further support of this idea, neuronal regeneration following a large-scale ablation of OSNs leads to a several-fold increase in FMRP puncta density in their axons within a short 1–2 week period upon axon growth and navigation (Christie et al., 2009). Together, these observations highlight localization of FMRP in developing axons during the period of axon growth and synaptic formation/remodeling.
Mechanisms that drive and regulate the amount of FMRP in axons have not been extensively investigated, but the data so far support an involvement of other RNA-binding proteins. As described above, FXR2P is necessary for the axonal and presynaptic localization of FMRP to FXGs (Christie et al., 2009). A later study further demonstrated that FXR2P is a lipid-modified RNA-binding protein and its lipid modification via N-terminal myristoylation influences how FMRP-containing FXGs are distributed within the axonal arbor (Stackpole et al., 2014). Another RNA-binding protein that has been linked to axonal localization of FMRP is Fused in Sarcoma (FUS), mutations of which lead to the neurodegenerative disease amyotrophic lateral sclerosis (ALS). FMRP interacts with FUS in vitro and is present in FUS-containing neuronal structures (Blokhuis et al., 2016; He and Ge, 2017). In human and mouse motor neurons, an ALS-associated FUS mutation results in more FMRP puncta in axons but not in the soma (Birsa et al., 2021). A complete loss of FUS, on the other hand, does not affect the FMRP puncta density in axons, supporting the idea that a gain of function of mutant FUS enhances FMRP localization to the axon (Birsa et al., 2021).
The expression and subcellular localization of FMRP may also be regulated by afferent inputs and/or neuronal activity. Exposure to an enriched environment or enhanced light simulation increased overall FMRP levels in the visual cortex (Irwin et al., 2000, 2005; Gabel et al., 2004). FMRP upregulation was also observed in the somatosensory and motor cortex following whisker stimulation or repeated motor training (Irwin et al., 2000; Todd and Mack, 2000; Todd et al., 2003). At the cellular level, activation of metabotropic glutamate receptors enhanced FMRP level at synapses and modulated its phosphorylation (Weiler et al., 1997; Bartley et al., 2016). Phosphorylated FMRP (p-FMRP)-mediated phase separation has been considered a mechanism that underlies synaptic activity-dependent neuronal granule formation (Boeynaems et al., 2018; Tsang et al., 2019). In consistent with this possibility however, afferent deprivation caused a rapid aggregation of somatic FMRP into stress granule-like structures in auditory brainstem neurons and this aggregation was associated with the likelihood of neuronal death or survival (Yu et al., 2021). Together, these studies support the idea that neuronal activity modulates FMRP function by regulating its expression, phosphorylation, and subcellular distribution pattern. To our knowledge, how neuronal activity affects the transport and distribution of FMRP-containing granules in axons has not been examined.
5. FMRP signals in axons
There is strong support for the idea that loss of FMRP control in the axon accounts substantially for the observed deficits in FMRP loss-induced axonal and presynaptic connectivity. In particular, the requirement of FMRP in presynaptic neurons for proper axon development and synaptic plasticity supports this idea, as well as the presence of FMRP in young and mature axons and the growing evidence of axonal mRNAs and local translation (Akins, 2009). Efforts to dissect out FMRP-associated signals in the axon (Figure 1B) have moved us closer to determining axonal FMRP regulation.
5.1. FMRP binds mRNAs and proteins in axons
As a primary RNA-binding protein, FMRP binds mRNAs in all subcellular compartments including the cytoplasm, dendrites, and axons. Several high-throughput RNA sequencing studies have identified hundreds of FMRP-bound mRNAs in samples collected from primary hippocampal cultures (Miyashiro et al., 2003) and the mouse brain (Brown et al., 2001; Darnell et al., 2011), including ~200 presynaptic proteins, collectively. These proteins include CAMs, cytoskeletal elements, presynaptic ion channels and ligand-gated receptors, SV components and regulatory proteins, and the neurotransmitter releasing machinery, covering almost all major aspects of presynaptic activities.
Similar to dendrites, FMRP regulates local protein translation in axons. In mammalian brains, FMRP-containing FXGs are associated with mRNAs, ribosomes, and other translational machinery. Fmr1 KO mice display reduced polyribosome association of several presynaptic FMRP mRNA targets (Brown et al., 2001; Akins et al., 2017) and increased axonal protein levels of one FMRP-associated mRNA (olfactory marker protein) (Akins et al., 2017). This effect is likely cell autonomous, as demonstrated by selective deletion of FMRP from presynaptic neurons, which impairs the overall level of activity-dependent protein synthesis in mouse mossy boutons as measured by puromycin labeling of nascent peptides (Monday et al., 2022). Similarly, translation of CAMKII mRNA, another FMRP target, is increased following FMRP reduction in Drosophila local interneurons (Sudhakaran et al., 2014). Thus, identifying mRNAs whose protein level is altered in the absence of FMRP provides additional mRNA candidates for axonal FMRP (Liao et al., 2008; Klemmer et al., 2011; Broek et al., 2016). Consistently, presynaptic ion channels, neurotransmitter transporters, CAMs, SV proteins, and presynaptic specialization were detected in this mRNA candidate pool. While the mechanisms underlying FMRP-controlled local translation in axons are not fully understood, phosphorylation in FMRP C-terminal potentially mediates liquid-liquid phase separation in axons to regulate protein synthesis (Kim et al., 2019; Tsang et al., 2019). Additionally, FMRP has been shown as a key component of RNA interference (RNAi) complex in developing axons and growth cones (Hengst et al., 2006). This complex regulates the local translation of RhoA mRNA which is required for Semaphorin-3A induced growth cone collapse (Hengst et al., 2006).
Whether FMRP regulates mRNA trafficking in axons is controversial and may be specific to certain circuits. In the mouse olfactory bulb, axonal localization of a number of mRNAs including β-catenin and omp appear normal in Fmr1 KO mice, suggesting that FMRP may not be required for axonal transport of its target mRNAs (Akins et al., 2017). However, Fmr1 KO led to increased numbers of FXR1P and FXR2P-immunoreactive FXGs, as observed in the frontal cortex (Christie et al., 2009), raising the possibility that FXR1P and FXR2P may compensate the loss of FMRP in axonal trafficking of mRNAs. In a study of cortical neuron cultures, FMRP was found interacting with another mRNA-binding protein TRF2-S. Loss of this interaction increased Aplp1 and Rab3a mRNA transport to axons and enhance axonal outgrowth and neurotransmitter release (Zhang et al., 2015). It is unknown how FXR1P, FXR2P and other potential compensation mechanisms occur under this condition. Interestingly, FMRP overexpression-induced axon overgrowth is restricted to the full-length and a subset of FMRP alternative splicing isoforms, revealing its independence of RNA binding and possible involvement of phosphorylation and nuclear export (Zimmer et al., 2017).
In addition to binding mRNAs, FMRP also forms protein-protein interactions. A list of FMRP-interacting proteins has been summarized in a snapshot (Pasciuto and Bagni, 2014). Of particular relevance to axon activities, FMRP interacts with several presynaptic ion channels as a means of modulating presynaptic calcium signal and AP properties (discussed further below). Additionally, FMRP is physically associated with other RNA-binding proteins in the axon. As an example, telomere repeat-binding factor 2 (TRF2) interacts directly with its target mRNAs to facilitate their axonal delivery. FMRP occupies the GAR domain of TRF2 to block the assembly of TRF2-mRNA complexes; FMRP silencing promotes axon entry of the mRNAs that directly bind TFR2 but not FMRP (Zhang et al., 2015).
In addition to binding individual mRNAs and proteins, another form of FMRP-mediated axonal signaling may involve phase separation. Activity-dependent translation requires the transport of mRNAs within membraneless protein assemblies known as neuronal granules (e.g., FXGs) from the cell body toward distant subcellular regions. Translation of mRNA is inhibited in these granules during transport but is quickly activated in response to neuronal stimuli at the destined location (such as presynaptic terminals). Multiple studies have demonstrated FMRP’s ability to phase separate RNA into liquid droplets via phosphorylation of its C-terminal intrinsically disordered region (Kim et al., 2019; Tsang et al., 2019; Chakraborty et al., 2021) with the involvement of additional players. For example, Fmr1 KO mice display an increase in synapsin level in the brain (Klemmer et al., 2011; Belagodu et al., 2017), a necessary protein for SV phase separation or clustering (Guarnieri et al., 2017; Milovanovic and De Camilli, 2017; Milovanovic et al., 2018). Also, RNA-binding protein FUS regulates FMRP phase separation in vitro and protein translation in cell bodies (Birsa et al., 2021). FMRP-mediated phase separation has not been directly observed in axons.
5.2. FMRP regulates cell adhesion in axons
Cell adhesion is critical for directed axon growth and targeting as well as synaptic maintenance and dynamics (Sytnyk et al., 2017; Yuzaki, 2018). mRNAs for several CAMs are associated with FMRP in axons. In Drosophila, dFMRP is co-immunoprecipitated with Down syndrome CAM (Dscam) mRNA and suppresses its protein translation (Kim et al., 2013b). Dscam protein level is dynamically associated with the size of the presynaptic arbor of D4 dendritic arborization neurons. Presynaptic terminal outgrowth in dFMRP null flies was completely abolished when Dscam was absent, demonstrating that dFMRP regulates Dscam expression to control presynaptic arbor growth in this cell type (Kim et al., 2013b). Colocalization of Dscam mRNA and FMRP has been observed in axonal growth cones of mouse hippocampal neurons in which Dscam level controls axon outgrowth and branching triggered by axonal guidance cue netrin (Jain and Welshhans, 2016). Interestingly, dFMRP also suppressed protein translation of netrin-B in Drosophila mushroom bodies, while dFMRP loss-induced β lobe fusion was mimicked by overexpression of netrin-B and rescued by knock-down of netrin-B (Kang et al., 2019). Although the cellular site of these FMRP actions is unknown, these observations suggest a potential dual action of FMRP in cell adhesion interactions by controlling both the axonal guidance cues and axonal CAMs.
FMRP may also regulate the interaction between presynaptic neurexin and postsynaptic neuroligin from either side, as suggested by altered expression of a subset of neurexin and neuroligin mRNAs in Fmr1 KO mouse brain (Lai et al., 2016). As further support, mRNAs for neurexin 1, 2, and 3 are localized in axons and associated with FXG (Akins et al., 2017). Other CAMs whose mRNAs are associated with FXG in axons include neural CAM (NCAM) and β-catenin (Akins et al., 2017). However, the functional roles of FMRP regulation of neurexin, NCAM, and β-catenin have not been determined. Finally, N-cadherin mRNAs are associated with FMRP in embryonic mouse brains and FMRP loss reduces the overall level of N-cadherin mRNA and protein and alters the distribution of N-cadherin across cortical layers (La Fata et al., 2014). Overexpressing N-cadherin normalized the early neuron activity deficits in Fmr1 KO mice (La Fata et al., 2014), demonstrating the functional significance of FMRP binding with N-cadherin mRNAs. Finally, FMRP deficiency-induced random turning of post-crossing axons in commissural neurons has been attributed to the disrupted interaction between FMRP-Frizzled3 complex and Wnt5a in the growth cone (Marfull-Oromí et al., 2022).
5.3. FMRP regulates of axonal cytoskeleton
Axonal growth and synapse formation/reorganization involve structural changes. The cytoskeleton of distal axons and axon endings is composed of actins and microtubules. Several lines of evidence have supported microtubule-associated protein 1B (MAP1B) as an FMRP target in axons. First, FMRP is associated with MAP1B mRNA, showing colocalization in axonal growth cones of hippocampal neurons (Antar et al., 2006) and co-immunoprecipitation in the intact brain (Zalfa et al., 2003; Lu et al., 2004). Next, FMRP controls MAP1B translation, as demonstrated by elevated protein levels of MAP1B in Fmr1 KO mice and inhibited MAP1B synthesis in the distal axons of Fmr1 KO hippocampal neurons in response to semaphorin-3A (Li et al., 2009). Further evidence shows that FMRP mediates axonal delivery of miR-181d which targets the MAP1B and calmodulin mRNA to negatively regulate their local translation in sensory neurons (Wang et al., 2015). Finally, Fmr1 KO cortical neurons displayed abnormally increased microtubule stability in the cell body (Lu et al., 2004), although whether this holds true in axons is unclear. With this regard, Drosophila studies provide direct evidence of FMRP modulation of presynaptic microtubule organization and the establishment of presynaptic connectivity (Bodaleo and Gonzalez-Billault, 2016). This modulation was found through regulated translation of MAP1B, but not MAP1A, a related protein with partially overlapping functions (Noiges et al., 2002).
Apart from the canonical function of MAP1B in stabilizing microtubules, FMRP regulation of MAP1B may have additional implications in dendritic and axonal events. For example, dendritic MAP1B was found to regulate synaptic transmission by modifying channel activity of several ion channels or ligand-gated receptors (such as Nav1.6, GABAC receptor, NMDA receptor, and several mGluR types), and restricting the access of AMPA receptors to dendritic spines and the postsynaptic membrane (Palenzuela et al., 2017). In axons, MAP1B binds Cav2.2 channels in hippocampal neurons, promoting their anchoring and stabilization in axons. Moreover, MAP1B interacts with proteins linked to neurodegenerative disease, apoptosis, autophagy, and mRNA- and membrane-associated proteins (Villarroel-Campos and Gonzalez-Billault, 2014). Thus, FMRP-MAP1B regulation may influence the axon both structurally and physiologically via diverse functions of MAP1B.
Dysregulation of actin cytoskeleton has also been observed in FXS, primarily from studies of dendritic spines (reviewed by (Michaelsen-Preusse et al., 2018)). Protein translation or activity of multiple actin modulators are controlled by FMRP either through FMRP-mRNA binding (e.g., for Cofilin 1, Rac1, p21-activated kinases, and armadillo protein p0071) or via FMRP-protein interactions (e.g., CYFIP1 and CYFIP2) (Bongmba et al., 2011; Dolan et al., 2013; Nolze et al., 2013; Abekhoukh and Bardoni, 2014; Feuge et al., 2019). Very few studies have examined FMRP regulation of actin organization in axons, but evidence is available in support of this regulation. In hippocampal neurons, FMRP deficiency causes reduced axonal growth cone collapse induced by Semaphorin 3A (Li et al., 2009), an event involving controlled actin assembling/disassembling. In Fmr1 KO mice, mossy fiber boutons from the hippocampal granule cells to CA3 neurons contain ribosomes and actin mRNA, and knocking out FMRP expression in granule cells increases actin synthesis (Monday et al., 2022).
5.4. FMRP regulates presynaptic ion channels and receptors
Fast neurotransmitter release is a multistaged procedure. During APs, extracellular calcium ions influx into the presynaptic terminal through calcium channels and receptors. Changes in the intracellular calcium concentration ([Ca2+]i) is sensed by calcium sensors on neurotransmitter-containing SVs. This triggers SV fusion with the presynaptic membrane and neurotransmitter release.
Multiple types of ion channels are located on the membranes of distal axons near or within presynaptic terminals, where they play distinct roles in regulating calcium influx, AP properties, and neurotransmitter release (Meir et al., 1999). A series of studies have established a high-conductance calcium- and voltage-dependent potassium (BK) channel as an underlying mechanism of FMRP-regulated neurotransmitter release. It is well known that presynaptic BK channels play critical roles in transmitter release by influencing calcium entry and AP waveforms (Griguoli et al., 2016). At the hippocampal CA3–CA1 synapses, FMRP binds to the β4 subunit of BK channels and modulates channel activity including calcium sensitivity (Deng et al., 2013). Loss of FMRP reduces BK channel activity and leads to excessive AP broadening, elevated presynaptic calcium influx, and enhanced synaptic transmission and short-term plasticity (Deng et al., 2013). Mutant FMRP that disrupts FMRP-BK channel interaction loses the ability to rescue presynaptic AP broadening in Fmr1 KO mice (Myrick et al., 2015). Genetic upregulation of BK channel activity by knocking out its negative regulator BKβ4 normalizes the deficits in AP, glutamate release, and short-term plasticity in Fmr1 KO mice (Deng and Klyachko, 2016). Finally, agonist activation of BK channels normalizes activity-dependent bulk endocytosis in Fmr1 KO hippocampal neurons (Bonnycastle et al., 2022). In neurons, BK channels are enriched in axon tracts and terminals and also localized in the somatodendritic compartment (Contet et al., 2016). The axon/presynaptic loci of FMRP actions on AP waveform suggests an on-site regulation of FMRP on BK channels in presynaptic terminals.
In addition to BK channel-mediated AP duration, FMRP may regulate AP firing (frequency) through another potassium channel, voltage-independent small conductance calcium-activated potassium (SK) currents (Deng et al., 2019). SK-mediated potassium currents are reduced in Fmr1 KO mice due to a loss of FMRP interaction with the SK channels without changes in channel expression. This deficit is responsible for decreased AP threshold, increased AP firing, and abnormal input-output function of neurotransmission at the CA3–CA1 synapses. Similar to BK channels, the FMRP-SK channel interaction is cell autonomous in origin, as validated by intracellular filling of SK channel openers or FMRP fragment. This SK channel deficit in Fmr1 KO is proposed to underlie CA3 neuronal hyperexcitability in Fmr1 KO mice (Deng et al., 2019). Additionally, FMRP directly binds the phosphorylated form of voltage-gated potassium channel Kv1.2 and regulates axonal trafficking of Kv1.2 channels in the cerebellum (Yang et al., 2020b). In Fmr1 KO mice, excessive calcium signal and GABA release from presynaptic terminals were observed in the inhibitory neurons, which attenuates the firing frequency of postsynaptic Purkinje neurons. Similarly, FMRP action on Kv1.2 is cell autonomous, as confirmed using intracellular introduction of FMRP fragment or antibody. Activation of Kv1.2 using an agonist normalizes the inhibitory function in Fmr1 KO mice (Yang et al., 2020b).
FMRP interaction with BK, SK, and Kv1.2 channels are via direct protein-protein binding. Whether FMRP regulates presynaptic potassium channels by its mRNA binding ability is unknown. A number of mRNAs encoding potassium channels have been identified as FMRP mRNA target candidates (Darnell et al., 2011). Among them, FMRP association and translation control have been confirmed for voltage-gated potassium channels Kv3.1b and Kv4.2 mRNA in the somatodendritic compartment (Strumbos et al., 2010; Gross et al., 2011; Lee et al., 2011; Ferron, 2016), however a similar action of FMRP has not been identified in the axon and presynaptic compartment. AP deficits observed in Fmr1 KO hippocampus appeared to be translational-independent (Deng et al., 2013, 2019), implicating a neglectable role of FMRP as a protein translation regulator in presynaptic AP modulation.
N-type voltage-gated calcium channels (Cav2) provide a main route for extracellular calcium ions entering presynaptic terminals. Among the three Cav2 isoforms, FMRP interacts with the c-terminal domain of CaV2.2 (Ferron et al., 2014, 2020). FMRP knockdown leads to increased Cav2.2 current density and increased surface Cav2.2 in presynaptic terminals of dorsal root ganglion neurons, and consistently, Fmr1 KO mice display elevated Cav2.2 level in synaptosome preparations (Ferron et al., 2014). The increase in surface Cav2.2 may be partially due to enhanced Cav2.2 trafficking from the endoplasmic reticulum to the plasma membrane and partially due to reduced proteasomal degradation (Ferron et al., 2014, 2020). Functionally, Fmr1 knockdown leads to larger calcium transients and enhanced SV exocytosis, which can be corrected using Cav2.2 blocker, demonstrating that FMRP regulates presynaptic release via CaV2.2 channels in this cell type (Ferron et al., 2014, 2020).
In addition to ion channels, FMRP also regulates the activity of ligand-gated receptors such as mGluR5, GABAA and GABAB receptors, and adrenergic receptors (βAR) on the presynaptic membrane. Apart from the well-characterized mGluR5 mechanism in the postsynaptic structures (Bear et al., 2004; Stoppel et al., 2021), mGluR5 is also localized in presynaptic terminals and modulates synaptic activities (Schrader and Tasker, 1997; Kim et al., 2009; Xie et al., 2017; Colmers and Bains, 2018; Fitzgerald et al., 2019; Fernandes et al., 2021). Activation of presynaptic mGluR5 corrects FMRP loss-induced releasing and plasticity deficits in lateral amygdala neurons and restores fear learning, distinct from the observations that inhibiting mGluR5 restores postsynaptic functions (Fernandes et al., 2021). In addition, defective glutamate release is partially contributed by impaired inhibitory presynaptic function in the absence of FMRP. In Fmr1 KO mouse hippocampus, deficits in α1 subunit of GABAB receptor expression was identified in presynaptic terminals, and the ability of GABAB receptors to suppress glutamate release was compromised (Kang et al., 2017). In the auditory cortex, the developmental strengthening of basal synaptic transmission and synaptic plasticity in the L4–L2/3 connectivity was accelerated in Fmr1 KO mice and this effect was associated with reduced GABAA receptor α3 unit in presynaptic terminals (Song et al., 2021). Finally, βAR colocalizes with presynaptic proteins Munc13, vGluT1, and vGluT2 in the cerebrocortical synaptosomes and agonist activation of βARs induces glutamate release potentiation (García-Font et al., 2019). This effect is diminished in Fmr1 KO and appears to result from an impaired capability of the receptor to mobilize SVs to the active zone.
5.5. FMRP regulates synaptic vesicles
SVs within presynaptic terminals are the primary vehicle for neurotransmitter release. Specialized transports uptake their corresponding neurotransmitters into vesicles. Depending on their releasing probability and the distance from the active zone, SVs are generally divided into two pools, the readily releasable or docked vesicles and the reserved vesicles. During APs, calcium influx is sensed by the calcium sensor synaptotagmins on the vesicles, which triggers the fusion of vesicles (primarily the docked ones) with the terminal membrane at the active zone in a process called exocytosis to release the neurotransmitter into the synaptic cleft. SVs are then recycled into the terminal through endocytosis to replenish the pool for the next APs.
Without FMRP, SVs are defective in number, type, distribution, and turnover rate. Ultrastructural analyses revealed fewer vesicles overall, but a higher number of docked vesicles vs. reserved vesicles, in FXS human embryonic stem cells and the CA1 region of the Fmr1 KO hippocampus (Klemmer et al., 2011; Telias et al., 2015), while other studies demonstrated normal vesicle density in the cerebellum (Broek et al., 2016) and larger pools of both readily-releasable and reserved vesicles (Deng et al., 2011; García-Font et al., 2019). In Drosophila, dFmr1 KO neurons similarly displayed elevated presynaptic vesicle pools (Pan et al., 2008). These differences may reflect cell-type specificity and/or across-study variations. SV recycling can be examined using an FM1-43 dye that is internalized during vesicle endocytosis and released with vesicle exocytosis (Gaffield and Betz, 2006). Using this method, a reduction in vesicle unloading was identified in the hippocampus, while vesicle unloading was enhanced in the cerebellum, indicating altered turnover rate of SV recycling with cell-type specificity (Broek et al., 2016). Primary hippocampal neuron cultures from Fmr1 KO rats displayed defective activity-dependent bulk endocytosis during trains of high-frequency stimulation (Bonnycastle et al., 2022) and more spontaneous, but not evoked, SV fusion events (Subrahmanyam et al., 2021), demonstrating multi-faceted FMRP modulation of vesicle fusion, endocytosis, and exocytosis.
Many SV proteins and their regulators are potential targets of FMRP, directly or indirectly. Quantitative proteomic analyses identified increased protein levels of 17 presynaptic proteins (syntaxin, Rab3A, piccolo, synapsin-1, synaptophysin, AP-2, V-ATPase, Syt1, Syt5, syntaphilin, a-synuclein, Munc13, SV glycoprotein 2A, VAMP2, SNAP25, complexin-2, and Myc box dependent-interacting protein) from the Fmr1 KO mouse brain and neurons (Liao et al., 2008; Klemmer et al., 2011; Tang et al., 2015). Among them, elevated synapsin-1 level has been confirmed in Fmr1 KO cortex and hippocampus (Klemmer et al., 2011; Belagodu et al., 2017). However, other studies failed to detect a change in synapsin level in the cortex (Li et al., 2002). FMRP regulation of synapsin translation maybe age-dependent because synapsin level in cortical samples is increased at P15–17 but not in mice older than 4 weeks (Tang et al., 2015). Although synapsin mRNA is identified as an mRNA candidate target (Darnell et al., 2011), whether FMRP directly binds synapsin mRNA or protein is unknown and synapsin mRNA was not identified in FXGs (Akins et al., 2017). Loss of synapsin 1, 2, and 3 leads to reduced levels of many SV proteins without affecting presynaptic membrane proteins such as SNAP25 and bassoon, demonstrating a specific requirement of synapsin for vesicle integrity (Vasileva et al., 2012). Indeed, synapsin functions in liquid-liquid phase separation for SV clustering (Wang and Kaeser, 2018), which may provide a potential mechanism of FMRP-mediated phase separation by controlling synapsin level in presynaptic terminals. In the mouse auditory brainstem, FMRP absence leads to altered developmental profiles of five essential SV proteins, including neurotransmitter transporters vGluT1/2 and VGAT as well as SV calcium sensors Syt1/2, in the medial nucleus of trapezoid body (MNTB) along its tonotopic axis (Yu and Wang, 2022). This finding demonstrates that FMRP-regulated expression of SV proteins is tightly associated with functional requirements of these proteins for information processing.
Finally, FMRP has the potential to regulate other aspects of the presynaptic membrane machinery. The association of presynaptic FMRP targets such as Munc13 and NAP22 with polyribosome is reduced in the absence of FMRP (Brown et al., 2001). FMRP also regulates accumulation of active zone protein Munc18-1 via suppression for local translation in axons during synaptogenesis in mouse cortical cultures (Parvin et al., 2019). mRNAs for Bassoon and Piccolo, two large scaffolding proteins of the cytomatrix assembled at the active zone, were identified in axons and associated with FXG (Akins et al., 2017). In fact, Bassoon mRNA has the highest affinity among all mRNAs in binding with FMRP (Darnell et al., 2011). Piccolo level is reduced in Fmr1 KO mouse neurons (Klemmer et al., 2011). The functional significance of FMRP regulation of these active zone membranes remains to be investigated.
6. Axonal FMRP in auditory function and disease
Pioneer studies of axonal FMRP actions were mostly performed within cortical and hippocampal circuits. In the last decade, auditory and other sensory systems have received increased attention toward the goals of understanding FMRP mechanisms, both in general and specifically in axons (Figure 2). In part, the increased attention is the result of our growing appreciation of the high prevalence of deficits in auditory-related brain activity and auditory-mediated behaviors in FXS (Rotschafer and Razak, 2014; Sinclair et al., 2017; Rais et al., 2018; McCullagh et al., 2020a; Razak et al., 2021, p 202). Our recent review contains a summary of FMRP-related auditory problems (McCullagh et al., 2020a). New, unique approaches to study the development and function of auditory circuits at both the single-cell and network levels have further facilitated in-depth investigation of underlying molecular and cellular mechanisms of FXS as well as functional association of FMRP with auditory processing.
Figure 2. Axon-related FMRP regulation in the auditory system.
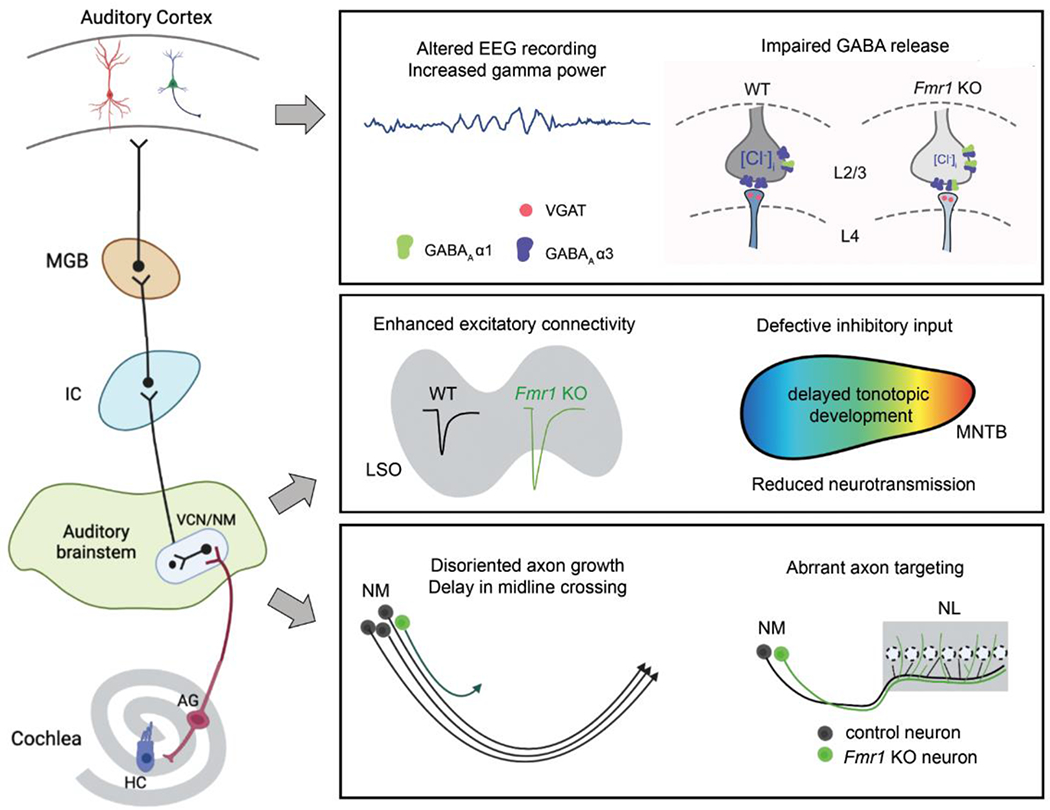
The auditory ascending pathway starts from the auditory ganglion (AG) which innervate both hair cells (HCs) and ventral cochlear nucleus (or nucleus magnocellularis or NM in birds) in the brainstem. VCN and NM neurons project to other cell groups within the brainstem. Acoustic signal is then conveyed from the brainstem to the cortex via inferior colliculus (IC) and medial geniculate body (MGV). Auditory neurons at all levels of this pathway express FMRP at high levels, particularly in AG neurons. Within the cortex, both pyramidal neurons (PNs) and interneurons neurons (INs) require FMRP for their normal function. GABAA receptor α3 unit in presynaptic terminals of INs is reduced in Fmr1 KO, underlying accelerated synaptic plasticity of PNs. Neural network activity is enhanced showing increased gamma power of cortical EEG recording. At the brainstem level, Fmr1 KO leads to enhanced excitatory connectivity to the lateral superior olive (LSO) and defective organization of inhibitory inputs in the medial nucleus of the trapezoid body (MNTB). The latter includes delayed tonotopic development and reductions of the presynaptic machinery as well as weakened neurotransmission. In the avian brain, selective FMRP knockout or knockdown in the NM results in altered axon navigation and projection to its target in the nucleus laminaris (NL). Created with BioRender.com.
6.1. Axonal FMRP in auditory neurons
In the mouse brain, FXGs are rich in sensory and motor processing circuits (Akins et al., 2012). Further evidence of axonal localization of FMRP was provided in studies of the auditory brainstem (Wang et al., 2020; Curnow and Wang, 2022). The nucleus magnocellularis (NM) in birds and its mammalian analog, the ventral cochlear nucleus (VCN), are primary recipients of the auditory ganglion (also called spiral ganglion in mammals) situated in the inner ear. NM/VCN neurons in turn innervate other brainstem and midbrain cell groups along the ascending auditory pathway, thus serving as the primary gate through which acoustic signals enter the brain.
During development, NM neurons are derived from Atoh1-expressing precursors in the embryonic hindbrain (Kohl et al., 2012; Hirsch et al., 2021). Combining FMRP immunostaining and genetic labeling of NM precursors, the presence of FMRP was detected in the cell bodies and growing axons of NM precursors as early as embryonic day 4 (E4) (Wang et al., 2020). Since these neurons are born at E2–2.5 (Rubel et al., 1976), this finding indicates that FMRP localizes to the axon shortly after Fmr1 gene expression and axonogenesis, consistent with observations from other cell types (see Section 4.2). This early-presence highlights the necessity of FMRP for axon growth. To demonstrate this, targeted FMRP downregulation in NM precursors in live embryos was achieved using in ovo electroporation of CRISPR-mediated KO or shRNA-mediated knockdown constructs (Wang et al., 2018; Fan et al., 2022). Such manipulation resulted in disoriented axonal growth as well as delay in axon midline crossing, confirming a role of FMRP at early stages of axon navigation (Wang et al., 2020). During the period of circuit assembly, axons of FMRP-deficient NM neurons arrive at their target, the nucleus laminaris (NL), but form aberrant axonal termination there. Neurons in the NL are bipolar, morphologically and functionally similar to the medial superior olive in mammals. Each of their two sets of dendrites receive inputs separately (from one ear) through the NM, forming the fundamental substrate for computing interaural time difference (ITD). Normally, single axons of NM neurons bifurcate and innervate the dorsal dendrites of the ipsilateral NL and the ventral dendrites of the contralateral NL. When FMRP expression was reduced, NM axons terminated on both dendritic sets of the same NL neurons (Wang et al., 2020), which likely interrupts ITD coding. Additionally, these observations demonstrate that FMRP regulates multiple developmental events in axons during discrete developmental periods, a concept consistent with the wide range of deficits in neural and synaptic development across cell types.
In addition to axon navigation and targeting, the absence of FMRP also impairs the development and maturation of presynaptic terminals in auditory circuits. In the lateral superior olive (LSO), the number of cochlear nucleus fibers converging onto individual postsynaptic neurons was increased in Fmr1 KO, leading to enhanced excitation (Garcia-Pino et al., 2017). Without FMRP, LSO neurons showed increased firing rate, broadened frequency tunning, and impaired ability of binaural processing. In the MNTB, glycinergic and GABAergic afferent inputs was altered in Fmr1 KO mice, showing stronger VGAT immunostaining (Rotschafer et al., 2015) and fewer GlyT2+ or GAD67+ presynaptic structures (McCullagh et al., 2017). Whether these changes reflect alterations in connectivity or protein levels of specific presynaptic markers is unclear. Regardless, presynaptic release of GABA was impaired in the MNTB (Curry et al., 2018). A more recent study demonstrated distinct developmental patterns across protein markers in excitatory presynaptic terminals in MNTB (Yu and Wang, 2022). Importantly, this study identified a developmental delay in the tonotopic organization of several presynaptic proteins in Fmr1 KO MNTB (Yu and Wang, 2022), demonstrating a requirement of FMRP in the timely maturation of presynaptic protein machinery.
Given the importance of cell autonomous FMRP in axon developmental events of auditory neurons, we wonder whether the long auditory axon fibers between the auditory ganglion and brain are disorganized when FMRP is absent and how this may affect the development and mature function of the central auditory system. Indeed, a strong expression of FMRP in auditory ganglion neurons has been confirmed in several avian and rodent species (Wang et al., 2022). Intriguingly, auditory ganglion neurons maintain a high level of FMRP throughout development and into adulthood, distinct from the developmental trajectory of FMRP in the brain where it declines dramatically after critical developmental periods. This feature highlights the potential importance of FMRP to both the development and mature functions of the auditory ganglion, and strongly implicates that the inner ear is a key pathological site for the generation of auditory problems in FXS. The idea that FMRP is important to both development and mature functions is supported by studies of other sensory systems: The dorsal root, trigeminal, and retinal ganglion neurons all express FMRP (Price et al., 2006; Frederikse et al., 2015; Guimarães-Souza et al., 2016). In non-FXS mouse models of autism spectrum disorders, peripheral somatosensory neurons are functionally impaired and this impairment underlies tactile and behavior deficits in these mice (Orefice et al., 2016, 2019).
6.2. Additional implications of axonal FMRP in normal and disordered auditory processing
In addition to potentially far-reaching influence from an impaired auditory periphery and defective axon projection and synaptic connectivity in the brain, FMRP may also regulate auditory function at specific aspects of neural activity. Here we highlight FMRP regulation of AP duration, and neuronal correlation as well as auditory plasticity and critical period, with a discussion of how axonal mechanisms may be involved. We also discuss how loss of these FMRP actions may contribute to acoustic hypersensitivity and central auditory processing problems in FXS and non-syndromic hearing disorders.
FMRP regulation of presynaptic ion channels and AP waveforms may have profound implications in auditory processing. Auditory neurons are characterized with narrow APs in coping with the need of fast spiking. It is also well appreciated that distinct subtypes of potassium channels play key roles in shaping presynaptic AP waveforms of auditory neurons (Brown and Kaczmarek, 2011; Tong et al., 2013; Hong et al., 2018; Choudhury et al., 2020). Additionally, Cav1 channels shape AP waveforms and modulate sound-level coding in the inferior colliculus (IC) (Grimsley et al., 2016). AP duration, measured as half the width of the AP, is significantly decreased with development and varies across functionally distinct cell types within the same cell groups (Wang et al., 2017b; Curry and Lu, 2021; Feng et al., 2022). At given neurons, AP duration is dynamic, showing a gradual broadening in response to trains of current pulses, as recorded from cultured mouse and gerbil spiral ganglion neurons (Lin, 1997; Lin and Hant, 2001). Computer simulation approaches further demonstrated that this AP broadening enhances neurotransmitter release and amplifies responses of postsynaptic receptors (Lin and Hant, 2001). Together, these studies demonstrate AP duration as a coding factor in auditory information processing. FMRP regulates the channel activity, tonotopic organization, and sound exposure-induced dynamics of potassium channels (Kv3 and Slack) (Brown et al., 2010; Strumbos et al., 2010), although there is no direct evidence that axons are a site of action. It is possible that FMRP modulates AP kinetics of auditory neurons under both physiological and pathological conditions. This regulation may help maintain the excitation/inhibition balance, loss of which could potentially contribute to auditory processing problems and hyperexcitability in FXS. In support of this idea, AP broadening has been considered an underlying mechanism for tinnitus: A tinnitus-inducing drug, quinine, blocks potassium channels and prolongs AP duration (Lin et al., 1998). More recently, decreased AP broadening was reported at the calyx synapses in the MNTB of a mouse model of Angelman syndrome (Wang et al., 2017a), another neurodevelopmental disorder.
A distinct phenotype of FXS is altered electroencephalography (EEG) recording from the auditory cortex. The specific aspects of EEG that are altered in Fmr1 KO were summarized recently and their association with acoustic hyperactivity was discussed (Razak et al., 2021). One underlying mechanism has been attributed to abnormal development of parvalbumin-expressing inhibitory interneurons, enhanced matrix-metalloproteinase-9 (MMP-9) activity, and reduced formation of extracellular matrix structures (Razak et al., 2021). Based on this knowledge, efforts towards normalizing EEG recordings have targeted GABAB agonist racemic baclofen, CB1 receptor agonist 2-arachidonoyl-sn-glycerol, and MMP-9 inhibitor minocycline (Lovelace et al., 2020; Pirbhoy et al., 2021; Jonak et al., 2022), among others. Additionally, the 1C has been identified as another site that contributes to the generation of cortical EEG alterations as well as more frequent auditory seizures. In Fmr1 KO mice, developing neurons in the 1C display enhanced responsiveness to tone bursts and amplitude-modulated tones and broader frequency tuning curves (Nguyen et al., 2020). Selective FMRP deletion in brainstem neurons (including 1C) resulted in several aspects (phase-locking) of EEG alterations similar to those observed in Fmr1 KO (Holley et al., 2022). Interestingly, treating Fmr1 KO mice with serotonin-1A receptor agonist weakened IC activity, reduced seizure, and increased mouse survival (Tao et al., 2022).
Several lines of evidence support an involvement of FMRP in auditory plasticity and critical periods. For example, the overall level of Kv3.1b was elevated in auditory brainstem neurons in the MNTB and VCN of wildtype, but not Fmr1 KO, mice (Strumbos et al., 2010). Somatic FMRP immunoreactivity aggregated into large granular structures in a subset of afferent-deprived NM neurons that died later (Yu et al., 2021), though it is not known whether the distribution of FMRP-containing granules in NM axons is affected by activity deprivation. Presynaptic actions of FMRP-regulated synaptic plasticity were recently identified in the auditory cortex. The developmental strengthening of basal synaptic transmission and synaptic plasticity in the L4–L2/3 connectivity was accelerated in Fmr1 KO mice and this effect was associated with reduced GABAA receptor α3 unit in presynaptic terminals (Song et al., 2021). Additionally, sound frequency-specific stimulation-induced reorganization of tonotopic representation was diminished in Fmr1 KO mice, demonstrating reduced dynamics of the auditory cortex (Kim et al., 2013a).
Supported by FMRP involvement in the development, information processing, plasticity, and pathology of auditory neurons and circuits, it is reasonable to speculate that FMRP may underlie other types of hearing problems in addition to FXS. For example, a strong association of FMRP downregulation and phosphorylation has been established with the progress of Alzheimer’s disease (Malter et al., 2010; Sokol et al., 2011; Murakami and Ono, 2022; Wu et al., 2022), in which the auditory system is a key pathological site (Blackwood et al., 1988; Martorell et al., 2019). Auditory brainstem responses in individuals with FXS and Fmr1 KO mice display altered threshold and/or peak latencies (Garcia-Pino et al., 2017; McCullagh et al., 2020b; Chawla and McCullagh, 2021), suggesting a potential involvement of FMRP in developmental and age-related hearing loss. Individuals with FXS are characterized with language learning disabilities and speech sound processing is compromised in a rat model when FMRP is absent (Engineer et al., 2014; Hagerman et al., 2017), giving rise to the possibility that FMRP is required in auditory processing for speech recognition.
7. Open questions
The journey toward understanding FMRP actions at the presynaptic site of synaptic connectivity assembly and neurotransmission has advanced significantly in the last decade. In particular, we have observed axonal localization of FMRP, determined cell autonomous and stage-specific roles for FMRP in axon development and synaptic functions, and identified several groups of FMRP signals in axon endings. Several questions remain.
It appears that FMRP functions similarly in axons and dendrites with respect to the general mechanisms of mRNA binding and protein interaction. FMRP also employs some signals common to both structures, including mGluR5 signaling, GABA receptors, ion channels, and cytoskeleton proteins. However, we understand little about how FMRP regulates the large number of presynaptic-specialized proteins such as presynaptic CAMs, SV-associated elements, and active zone members, although the evidence supporting the presence of such regulation is strong. It is interesting to note that FMRP absence consistently leads to upregulation, but not downregulation, of SV and active zone proteins identified so far, suggesting that FMRP acts as a translational repressor for the SV and active zone machinery. Mechanisms underlying the specificity of FMRP to bind different mRNAs at different locations and stages remain to be determined.
The regulatory mechanisms underlying the developmental profile and circuit-specificity of FMRP localization in axons warrant further exploration. The relationship among FXG, phase-separated droplets, and FMRP-associated neuronal granules remains blurry, as only a selected subpopulation of FMRP mRNA targets localizes to the axonal FXGs. Understanding this relationship would provide fundamental knowledge needed for restoring FMRP control of axonal events by targeting to specific age groups and/or symptoms.
The relative contribution of the peripheral and central nerve systems to the emergence of FXS neuropathology during development is obscure. Sensory ganglion neurons extend long axons to the brain, providing an experimentally advantageous and clinically relevant model for investigating FMRP actions in the axons in vivo and for determining the contribution of periphery nerve system-central nervous system interface to psychiatric disorders.
The auditory system has been established as a clinically relevant site for further investigation of FMRP functions including those specialized in axons and presynaptic terminals. Elaborating the timing and molecular basis of FMRP-mediated signals in regulating auditory circuit assembly and plasticity would advance our understanding of both neural development and auditory dynamics. Given the wide-ranging impact of auditory problems on social and cognitive functions (Kral et al., 2016), correcting auditory problems is expected to reduce the severity of other symptoms in FXS. Additionally, it is important to determine the degree of FMRP deficits in aging and non-syndromic hearing disorders such as hearing loss and central auditory processing disorders, which is of potentially broader clinical impact.
Highlights.
Fragile X mental retardation protein (FMRP) binds a selected set of mRNAs and proteins to guide neural circuit assembly and regulate synaptic plasticity.
Loss of FMRP is responsible for Fragile X syndrome, a neuropsychiatric disorder characterized with auditory processing problems and social difficulty.
FMRP actions in synaptic formation, maturation, and plasticity are site-specific among the four compartments of a synapse: presynaptic and postsynaptic neurons, astrocytes, and extracellular matrix.
This review summarizes advancements in understanding FMRP localization, signals, and functional roles in axons and presynaptic terminals, and discussed the axonal FMRP in auditory function and disease.
Acknowledgements
This study was supported by the National Institute of Mental Health (R01MH126176), United States – Israel Binational Science Foundation (2015087 to Y.W. and D.S.-D.), Israel Science Foundation (No. 1341/21), National Natural Science Foundation of China grant (No. 32000697, 82271196), Guangdong Natural Science Foundation (2021A1515010619), Science and Technology Program of Guangzhou (202102080139). We thank Dr. Terra Bradley for the careful editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Interest
The authors declare no conflict of interest.
References
- Abekhoukh S, Bardoni B. 2014. CYFIP family proteins between autism and intellectual disability: links with Fragile X syndrome. Front Cell Neurosci 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins M 2009. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits [Internet] 3. Available from: 10.3389/neuro.04.017.2009/abstract [DOI] [PMC free article] [PubMed]
- Akins MR, Berk-Rauch HE, Kwan KY, Mitchell ME, Shepard KA, Korsak LIT, Stackpole EE, Warner-Schmidt JL, Sestan N, Cameron HA, Fallon JR. 2017. Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Hum Mol Genet:ddw 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins MR, LeBlanc HF, Stackpole EE, Chyung E, Fallon JR. 2012. Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol 520:3687–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. 2006. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Molecular and Cellular Neuroscience 32:37–48. [DOI] [PubMed] [Google Scholar]
- Bartley CM, O’Keefe RA, Blice-Baum A, Mihailescu M-R, Gong X, Miyares L, Karaca E, Bordey A. 2016. Mammalian FMRP S499 Is Phosphorylated by CK2 and Promotes Secondary Phosphorylation of FMRP. eNeuro 3:ENEURO.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. 2004. The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377. [DOI] [PubMed] [Google Scholar]
- Belagodu AP, Zendeli L, Slater BJ, Galvez R. 2017. Blocking elevated VEGF-A attenuates non-vasculature Fragile X syndrome abnormalities. Dev Neurobiol 77:14–25. [DOI] [PubMed] [Google Scholar]
- Birsa N, Ule AM, Garone MG, Tsang B, Mattedi F, Chong PA, Humphrey J, Jarvis S, Pisiren M, Wilkins OG, Nosella ML, Devoy A, Bodo C, de la Fuente RF, Fisher EMC, Rosa A, Viero G, Forman-Kay JD, Schiavo G, Fratta P. 2021. FUS-ALS mutants alter FMRP phase separation equilibrium and impair protein translation. Sci Adv 7:eabf8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, St Clair DM, Muir WJ, Oliver CJ, Dickens P. 1988. The development of Alzheimer’s disease in Down’s syndrome assessed by auditory event-related potentials. J Ment Defic Res 32 ( Pt 6):439–453. [DOI] [PubMed] [Google Scholar]
- Blokhuis AM, Koppers M, Groen EJN, van den Heuvel DMA, Dini Modigliani S, Anink JJ, Fumoto K, van Diggelen F, Snelting A, Sodaar P, Verheijen BM, Demmers JAA, Veldink JH, Aronica E, Bozzoni I, den Hertog J, van den Berg LH, Pasterkamp RJ. 2016. Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways. Acta Neuropathol 132:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaleo FJ, Gonzalez-Billault C. 2016. The Presynaptic Microtubule Cytoskeleton in Physiological and Pathological Conditions: Lessons from Drosophila Fragile X Syndrome and Hereditary Spastic Paraplegias. Front Mol Neurosci 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P, Fuxreiter M. 2018. Protein Phase Separation: A New Phase in Cell Biology. Trends in Cell Biology 28:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongmba OYN, Martinez LA, Elhardt ME, Butler K, Tejada-Simon MV. 2011. Modulation of dendritic spines and synaptic function by Rac1: a possible link to Fragile X syndrome pathology. Brain Res 1399:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnycastle K, Kind PC, Cousin MA. 2022. FMRP Sustains Presynaptic Function via Control of Activity-Dependent Bulk Endocytosis. J Neurosci 42:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Segal M. 2000. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex 10:1045–1052. [DOI] [PubMed] [Google Scholar]
- Broek JAC, Lin Z, de Gruiter HM, van ‘t Spijker H, Haasdijk ED, Cox D, Ozcan S, van Cappellen GWA, Houtsmuller AB, Willemsen R, de Zeeuw Cl, Bahn S. 2016. Synaptic vesicle dynamic changes in a model of fragile X. Molecular Autism 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kaczmarek LK. 2011. Potassium channel modulation and auditory processing. Hear Res 279:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula V-R, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. 2010. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci 13:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. 2001. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107:477–487. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GMG, Svoboda K. 2008. Circuit and Plasticity Defects in the Developing Somatosensory Cortex of Fmr1 Knock-Out Mice. Journal of Neuroscience 28:5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolo SL, Bulgari D, Deitcher DL, Levitan ES. 2016. Activity Induces Fmr1-Sensitive Synaptic Capture of Anterograde Circulating Neuropeptide Vesicles. J Neurosci 36:11781–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Dutta A, Dettori LG, Li J, Gonzalez L, Xue X, Hehnly H, Sung P, Bah A, Feng W. 2021. FMRP DIRECTLY INTERACTS WITH R-LOOP AND SHOWS COMPLEX INTERPLAY WITH THE DHX9 HELICASE. Biochemistry. Available from: http://biorxiv.org/lookup/doi/10.1101/2021.04.21.440759 [Google Scholar]
- Chawla A, McCullagh EA. 2021. Auditory Brain Stem Responses in the C57BL/6J Fragile X Syndrome-Knockout Mouse Model. Front Integr Neurosci 15:803483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Sourial M, Doering LC. 2012. Astrocytes and developmental plasticity in fragile X. Neural Plast 2012:197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N, Linley D, Richardson A, Anderson M, Robinson SW, Marra V, Ciampani V, Walter SM, Kopp-Scheinpflug C, Steinert JR, Forsythe ID. 2020. Kv3.1 and Kv3.3 subunits differentially contribute to Kv3 channels and action potential repolarization in principal neurons of the auditory brainstem. J Physiol 598:2199–2222. [DOI] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. 2009. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci 29:1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyung E, LeBlanc HF, Fallon JR, Akins MR. 2018. Fragile X granules are a family of axonal ribonucleoprotein particles with circuit-dependent protein composition and mRNA cargos. J Comp Neurol 526:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers PLW, Bains JS. 2018. Presynaptic mGluRs Control the Duration of Endocannabinoid-Mediated DSI. J Neurosci 38:10444–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Goulding SP, Kuljis DA, Barth AL. 2016. Chapter Eight - BK Channels in the Central Nervous System. In: Contet C, editor. International Review of Neurobiology. Vol. 128. Big on Bk. Academic Press, p 281–342. Available from: https://www.sciencedirect.com/science/article/pii/S0074774216300678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Sanchez-Carbente M del R, Lachance C, Radzioch D, Tremblay S, Khandjian EW, DesGroseillers L, Murai KK. 2011. Fragile X related protein 1 clusters with ribosomes and messenger RNAs at a subset of dendritic spines in the mouse hippocampus. PLoS ONE 6:e26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnow E, Wang Y. 2022. New Animal Models for Understanding FMRP Functions and FXS Pathology. Cells 11:1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry RJ, Lu Y. 2021. Intrinsic properties of avian interaural level difference sound localizing neurons. Brain Res 1752:147258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry RJ, Peng K, Lu Y. 2018. Neurotransmitter- and Release-Mode-Specific Modulation of Inhibitory Transmission by Group I Metabotropic Glutamate Receptors in Central Auditory Neurons of the Mouse. J Neurosci 38:8187–8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. 2011. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell 146:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego Otero Y, Severijnen L-A, van Cappellen G, Schrier M, Oostra B, Willemsen R. 2002. Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol Cell Biol 22:8332–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Carlin D, Oh YM, Myrick LK, Warren ST, Cavalli V, Klyachko VA. 2019. Voltage-Independent SK-Channel Dysfunction Causes Neuronal Hyperexcitability in the Hippocampus of Fmr1 Knock-Out Mice. J Neurosci 39:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Klyachko VA. 2016. Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J Physiol 594:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. 2013. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Sojka D, Klyachko VA. 2011. Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci 31:10971–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Rusakov DA. 2011. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff TC, Su HS, McBride SMJ, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. 2002. Drosophila lacking dFmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34:973–984. [DOI] [PubMed] [Google Scholar]
- Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko H-Y, Lin GG, Govindarajan A, Choi S-Y, Tonegawa S. 2013. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci U S A 110:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF. 2007. Correction of Fragile X Syndrome in Mice. Neuron 56:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dury AY, El Fatimy R, Tremblay S, Rose TM, Côté J, De Koninck P, Khandjian EW. 2013. Nuclear Fragile X Mental Retardation Protein is localized to Cajal bodies. PLoS Genet 9:e1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Rahebi KC, Buell EP, Kilgard MP. 2014. Degraded speech sound processing in a rat model of fragile X syndrome. Brain Res 1564:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Zhang X, Wang Y, Wang X. 2022. Dissecting Cell-Autonomous Function of Fragile X Mental Retardation Protein in an Auditory Circuit by In Ovo Electroporation. JoVE (Journal of Visualized Experiments):e64187. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. 2011. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology 60:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Gutekunst C-A, Eberhart DE, Yi H, Warren ST, Hersch SM. 1997. Fragile X Mental Retardation Protein: Nucleocytoplasmic Shuttling and Association with Somatodendritic Ribosomes. J Neurosci 17:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhang Q, Lu X, Li X, Liu W. 2022. Development of neuronal excitability of the bushy cells in anteroventral cochlear nucleus of rats. Dev Neurosci. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Mishra PK, Nawaz MS, Donlin-Asp PG, Rahman MM, Hazra A, Kedia S, Kayenaat A, Songara D, Wyllie DJA, Schuman EM, Kind PC, Chattarji S. 2021. Correction of amygdalar dysfunction in a rat model of fragile X syndrome. Cell Rep 37:109805. [DOI] [PubMed] [Google Scholar]
- Ferron L 2016. Fragile X mental retardation protein controls ion channel expression and activity. J Physiol 594:5861–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. 2014. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat Commun 5:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L, Novazzi CG, Pilch KS, Moreno C, Ramgoolam K, Dolphin AC. 2020. FMRP regulates presynaptic localization of neuronal voltage gated calcium channels. Neurobiology of Disease 138:104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuge J, Scharkowski F, Michaelsen-Preusse K, Korte M. 2019. FMRP Modulates Activity-Dependent Spine Plasticity by Binding Cofilin1 mRNA and Regulating Localization and Local Translation. Cereb Cortex 29:5204–5216. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Mackie K, Pickel VM. 2019. Ultrastructural localization of cannabinoid CB1 and mGluR5 receptors in the prefrontal cortex and amygdala. Journal of Comparative Neurology 527:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikse PH, Nandanoor A, Kasinathan C. 2015. Fragile X Syndrome FMRP Co-localizes with Regulatory Targets PSD-95, GABA Receptors, CaMKIIα, and mGluR5 at Fiber Cell Membranes in the Eye Lens. Neurochem Res 40:2167–2176. [DOI] [PubMed] [Google Scholar]
- Fulks JL, O’Bryhim BE, Wenzel SK, Fowler SC, Vorontsova E, Pinkston JW, Ortiz AN, Johnson MA. 2010. Dopamine Release and Uptake Impairments and Behavioral Alterations Observed in Mice that Model Fragile X Mental Retardation Syndrome. ACS Chem Neurosci 1:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel LA, Won S, Kawai H, McKinney M, Tartakoff AM, Fallon JR. 2004. Visual experience regulates transient expression and dendritic localization of fragile X mental retardation protein. J Neurosci 24:10579–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Betz WJ. 2006. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat Protoc 1:2916–2921. [DOI] [PubMed] [Google Scholar]
- García-Font N, Martín R, Torres M, Oset-Gasque MJ, Sánchez-Prieto J. 2019. The loss of β adrenergic receptor mediated release potentiation in a mouse model of fragile X syndrome. Neurobiol Dis 130:104482. [DOI] [PubMed] [Google Scholar]
- Garcia-Pino E, Gessele N, Koch U. 2017. Enhanced Excitatory Connectivity and Disturbed Sound Processing in the Auditory Brainstem of Fragile X Mice. J Neurosci 37:7403–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. 2009. Temporal requirements of the fragile x mental retardation protein in modulating circadian clock circuit synaptic architecture. Front Neural Circuits 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh S, Haider SK, Hampson DR. 2015. Expression of fragile X mental retardation protein in neurons and glia of the developing and adult mouse brain. Brain Research 1596:22–30. [DOI] [PubMed] [Google Scholar]
- Giampetruzzi A, Carson JH, Barbarese E. 2013. FMRP and myelin protein expression in oligodendrocytes. Mol Cell Neurosci 56:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocel J, Larson J. 2012. Synaptic NMDA receptor-mediated currents in anterior piriform cortex are reduced in the adult fragile X mouse. Neuroscience 221:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griguoli M, Sgritta M, Cherubini E. 2016. Presynaptic BK channels control transmitter release: physiological relevance and potential therapeutic implications. J Physiol (Lond) 594:3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Green DB, Sivaramakrishnan S. 2016. L-type calcium channels refine the neural population code of sound level. J Neurophysiol 116:2550–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. 2011. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci 31:5693–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri FC, Pozzi D, Raimondi A, Fesce R, Valente MM, Delvecchio VS, Van Esch H, Matteoli M, Benfenati F, D’Adamo P, Valtorta F. 2017. A novel SYN1 missense mutation in nonsyndromic X-linked intellectual disability affects synaptic vesicle life cycle, clustering and mobility. Human Molecular Genetics 26:4699–4714. [DOI] [PubMed] [Google Scholar]
- Guimarães-Souza EM, Perche O, Morgans CW, Duvoisin RM, Calaza KC. 2016. Fragile X Mental Retardation Protein expression in the retina is regulated by light. Experimental Eye Research 146:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, Hagerman PJ. 2017. Fragile X syndrome. Nat Rev Dis Primers 3:17065. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. 2007. Presynaptic Fmr1 Genotype Influences the Degree of Synaptic Connectivity in a Mosaic Mouse Model of Fragile X Syndrome. Journal of Neuroscience 27:4014–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Ge W. 2017. The tandem Agenet domain of fragile X mental retardation protein interacts with FUS. Sci Rep 7:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. 2006. Functional and selective RNA interference in developing axons and growth cones. J Neurosci 26:5727–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch D, Kohl A, Wang Y, Sela-Donenfeld D. 2021. Axonal Projection Patterns of the Dorsal Interneuron Populations in the Embryonic Hindbrain. Front Neuroanat 15:793161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley AJ, Shedd A, Boggs A, Lovelace J, Erickson C, Gross C, Jankovic M, Razak K, Huber K, Gibson JR. 2022. A sound-driven cortical phase-locking change in the Fmr1 KO mouse requires Fmr1 deletion in a subpopulation of brainstem neurons. Neurobiol Dis 170:105767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Wang X, Lu T, Zorio DAR, Wang Y, Sanchez JT. 2018. Diverse Intrinsic Properties Shape Functional Phenotype of Low-Frequency Neurons in the Auditory Brainstem. Front Cell Neurosci 12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Christmon CA, Grossman AW, Galvez R, Kim SH, DeGrush BJ, Weiler IJ, Greenough WT. 2005. Fragile X mental retardation protein levels increase following complex environment exposure in rat brain regions undergoing active synaptogenesis. Neurobiol Learn Mem 83:180–187. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Swain RA, Christmon CA, Chakravarti A, Weiler IJ, Greenough WT. 2000. Evidence for altered Fragile-X mental retardation protein expression in response to behavioral stimulation. Neurobiol Learn Mem 73:87–93. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. 2002. Altered mossy fiber distributions in adultFmr1 (FVB) knockout mice. Hippocampus 12:47–54. [DOI] [PubMed] [Google Scholar]
- Jain S, Welshhans K. 2016. Netrin-1 induces local translation of down syndrome cell adhesion molecule in axonal growth cones: Local Translation of Dscam. Devel Neurobio 76:799–816. [DOI] [PubMed] [Google Scholar]
- Jonak CR, Pedapati EV, Schmitt LM, Assad SA, Sandhu MS, DeStefano L, Ethridge L, Razak KA, Sweeney JA, Binder DK, Erickson CA. 2022. Baclofen-associated neurophysiologic target engagement across species in fragile X syndrome. J Neurodev Disord 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Zhao J, Jiang X, Li G, Huang W, Cheng H, Duan R. 2019. Drosophila Netrin-B controls mushroom body axon extension and regulates courtship-associated learning and memory of a Drosophila fragile X syndrome model. Mol Brain 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-Y, Chadchankar J, Vien TN, Mighdoll MI, Hyde TM, Mather RJ, Deeb TZ, Pangalos MN, Brandon NJ, Dunlop J, Moss SJ. 2017. Deficits in the activity of presynaptic γ-aminobutyric acid type B receptors contribute to altered neuronal excitability in fragile X syndrome. J Biol Chem 292:6621–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Gibboni R, Kirkhart C, Bao S. 2013a. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J Neurosci 33:15686–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Wang X, Coolon R, Ye B. 2013b. Dscam expression levels determine presynaptic arbor sizes in Drosophila sensory neurons. Neuron 78:827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Tsang B, Vernon RM, Sonenberg N, Kay LE, Forman-Kay JD. 2019. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 365:825–829. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park C-K, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. 2009. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci 29:10000–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmer P, Meredith RM, Holmgren CD, Klychnikov OI, Stahl-Zeng J, Loos M, van der Schors RC, Wortel J, de Wit H, Spijker S, Rotaru DC, Mansvelder HD, Smit AB, Li KW. 2011. Proteomics, ultrastructure, and physiology of hippocampal synapses in a fragile X syndrome mouse model reveal presynaptic phenotype. J Biol Chem 286:25495–25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Liu M-G, Qiu S, Song Q, O’Den G, Chen T, Zhuo M. 2015. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. J Neurosci 35:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl A, Hadas Y, Klar A, Sela-Donenfeld D. 2012. Axonal Patterns and Targets of dA1 Interneurons in the Chick Hindbrain. Journal of Neuroscience 32:5757–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Kronenberger WG, Pisoni DB, O’Donoghue GM. 2016. Neurocognitive factors in sensory restoration of early deafness: a connectome model. Lancet Neurol 15:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fata G, Gärtner A, Domínguez-Iturza N, Dresselaers T, Dawitz J, Poorthuis RB, Averna M, Himmelreich U, Meredith RM, Achsel T, Dotti CG, Bagni C. 2014. FMRP regulates multipolar to bipolar transition affecting neuronal migration and cortical circuitry. Nat Neurosci 17:1693–1700. [DOI] [PubMed] [Google Scholar]
- Lai JKY, Doering LC, Foster JA. 2016. Developmental expression of the neuroligins and neurexins in fragile X mice: Neuroligins and neurexins in fragile X mice. J Comp Neurol 524:807–828. [DOI] [PubMed] [Google Scholar]
- Lee HY, Ge W-P, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. 2011. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72:630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bassell G, Sasaki Y. 2009. Fragile X Mental Retardation Protein is involved in protein synthesis-dependent collapse of growth cones induced by Semaphorin-3A. Front Neural Circuits [Internet] 3. Available from: http://journal.frontiersin.org/article/10.3389/neuro.04.011.2009/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez J-L, Carlen PL. 2002. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci 19:138–151. [DOI] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P, Yates JR. 2008. Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. PNAS 105:15281–15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X 1997. Action potentials and underlying voltage-dependent currents studied in cultured spiral ganglion neurons of the postnatal gerbil. Hear Res 108:157–179. [DOI] [PubMed] [Google Scholar]
- Lin X, Chen S, Tee D. 1998. Effects of quinine on the excitability and voltage-dependent currents of isolated spiral ganglion neurons in culture. J Neurophysiol 79:2503–2512. [DOI] [PubMed] [Google Scholar]
- Lin X, Hant J. 2001. Computer-simulation studies on roles of potassium currents in neurotransmission of the auditory nerve. Hear Res 152:90–99. [DOI] [PubMed] [Google Scholar]
- Lovelace JW, Ethell IM, Binder DK, Razak KA. 2020. Minocycline Treatment Reverses Sound Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome. Front Neurosci 14:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Hare EB, Hagerman RJ. 2014. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat 10:1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’donnell WT, Li W, Warren ST, Feng Y. 2004. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA 101:15201–15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter JS, Ray BC, Westmark PR, Westmark CJ. 2010. Fragile X Syndrome and Alzheimer’s Disease: Another story about APP and beta-amyloid. Curr Alzheimer Res 7:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfull-Oromí P, Onishi K, Zou Y. 2022. The Fragile X Syndrome Protein Participates in Axon Guidance Mediated by the Wnt/Planar Cell Polarity Pathway. Neuroscience:S0306-4522(22)00488–2. [DOI] [PubMed] [Google Scholar]
- Martorell AJ, Paulson AL, Suk H-J, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim DN-W, Kritskiy O, Barker SJ, Mangena V, Prince SM, Brown EN, Chung K, Boyden ES, Singer AC, Tsai L-H. 2019. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 177:256–271.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh EA, Rotschafer SE, Auerbach BD, Klug A, Kaczmarek LK, Cramer KS, Kulesza RJ, Razak KA, Lovelace JW, Lu Y, Koch U, Wang Y. 2020a. Mechanisms underlying auditory processing deficits in Fragile X syndrome. FASEB J 34:3501–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh EA, Rotschafer SE, Auerbach BD, Klug A, Kaczmarek LK, Cramer KS, Kulesza RJ, Razak KA, Lovelace JW, Lu Y, Koch U, Wang Y. 2020b. Mechanisms underlying auditory processing deficits in Fragile X syndrome. FASEB J 34:3501–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh EA, Salcedo E, Huntsman MM, Klug A. 2017. Tonotopic alterations in inhibitory input to the medial nucleus of the trapezoid body in a mouse model of Fragile X syndrome. J Comp Neurol 525:3543–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir A, Ginsburg S, Butkevich A, Kachalsky SG, Kaiserman I, Ahdut R, Demirgoren S, Rahamimoff R. 1999. Ion Channels in Presynaptic Nerve Terminals and Control of Transmitter Release. Physiological Reviews 79:1019–1088. [DOI] [PubMed] [Google Scholar]
- Michaelsen-Preusse K, Feuge J, Korte M. 2018. Imbalance of synaptic actin dynamics as a key to fragile X syndrome?: Synaptic actin and fragile X syndrome. J Physiol 596:2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CI, Kraft R, Restifo LL. 2004. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci 24:5798–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, De Camilli P. 2017. Synaptic Vesicle Clusters at Synapses: A Distinct Liquid Phase? Neuron 93:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Wu Y, Bian X, De Camilli P. 2018. A liquid phase of synapsin and lipid vesicles. Science 361:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. 2003. RNA Cargoes Associating with FMRP Reveal Deficits in Cellular Functioning in Fmr1 Null Mice. Neuron 37:417–431. [DOI] [PubMed] [Google Scholar]
- Monday HR, Kharod SC, Yoon YJ, Singer RH, Castillo PE. 2022. Presynaptic FMRP and local protein synthesis support structural and functional plasticity of glutamatergic axon terminals. Neuron 110:2588–2606.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. 2002. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34:961–972. [DOI] [PubMed] [Google Scholar]
- Murakami K, Ono K. 2022. Interactions of amyloid coaggregates with biomolecules and its relevance to neurodegeneration. FASEB J 36:e22493. [DOI] [PubMed] [Google Scholar]
- Myrick LK, Deng P-Y, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, Cheng X, Warren ST, Klyachko VA. 2015. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AO, Binder DK, Ethell IM, Razak KA. 2020. Abnormal development of auditory responses in the inferior colliculus of a mouse model of Fragile X Syndrome. J Neurophysiol 123:2101–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, Propst F. 2002. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci 22:2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolze A, Schneider J, Keil R, Lederer M, Hüttelmaier S, Kessels MM, Qualmann B, Hatzfeld M. 2013. FMRP regulates actin filament organization via the armadillo protein p0071. RNA 19:1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, Ye M, Chirila AM, Emanuel AJ, Rankin G, Fame RM, Lehtinen MK, Feng G, Ginty DD. 2019. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell 178:867–886.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. 2016. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 166:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LKK, Xuan ICY, Guan S, Sussman D, Henkelman RM, Chen Y, Thomsen C, Hampson DR. 2013. Delayed myelination in a mouse model of fragile X syndrome. Human Molecular Genetics 22:3920–3930. [DOI] [PubMed] [Google Scholar]
- Palenzuela R, Gutiérrez Y, Draffin JE, Lario A, Benoist M, Esteban JA. 2017. MAP1B Light Chain Modulates Synaptic Transmission via AMPA Receptor Intracellular Trapping. J Neurosci 37:9945–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Woodruff E III, Liang P, Broadie K. 2008. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Molecular and Cellular Neuroscience 37:747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Zhang YQ, Woodruff E, Broadie K. 2004. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol 14:1863–1870. [DOI] [PubMed] [Google Scholar]
- Parvin S, Takeda R, Sugiura Y, Neyazaki M, Nogi T, Sasaki Y. 2019. Fragile X mental retardation protein regulates accumulation of the active zone protein Munc18-1 in presynapses via local translation in axons during synaptogenesis. Neuroscience Research 146:36–47. [DOI] [PubMed] [Google Scholar]
- Pasciuto E, Bagni C. 2014. Snapshot: FMRP interacting proteins. Cell 159:218–218.e1. [DOI] [PubMed] [Google Scholar]
- Patel AB, Hays SA, Bureau I, Huber KM, Gibson JR. 2013. A target cell-specific role for presynaptic Fmr1 in regulating glutamate release onto neocortical fast-spiking inhibitory neurons. J Neurosci 33:2593–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucar M, Beniaminov S, Paslawski W, Svenningsson P. 2016. PSP-CBS with Dopamine Deficiency in a Female with a FMR1 Premutation. Cerebellum 15:636–640. [DOI] [PubMed] [Google Scholar]
- Pirbhoy PS, Jonak CR, Syed R, Argueta DA, Perez PA, Wiley MB, Hessamian K, Lovelace JW, Razak KA, DiPatrizio NV, Ethell IM, Binder DK. 2021. Increased 2-arachidonoyl-sn-glycerol levels normalize cortical responses to sound and improve behaviors in Fmr1 KO mice. J Neurodev Disord 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchelon G, Dwivedi D, Bollmann Y, Agba CK, Xu Q, Mirow AMC, Kim S, Qiu Y, Sevier E, Ritola KD, Cossart R, Fishell G. 2021. The organization and development of cortical interneuron presynaptic circuits are area specific. Cell Rep 37:109993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F, Hargreaves KM. 2006. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience 141:2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais M, Binder DK, Razak KA, Ethell IM. 2018. Sensory Processing Phenotypes in Fragile X Syndrome. ASN Neuro 10:1759091418801092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Binder DK, Ethell IM. 2021. Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome. Front Psychiatry 12:720752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Marshak S, Cramer KS. 2015. Deletion of Fmr1 Alters Function and Synaptic Inputs in the Auditory Brainstem. PLoS ONE 10:e0117266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Razak KA. 2014. Auditory processing in fragile x syndrome. Front Cell Neurosci 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Smith DJ, Miller LC. 1976. Organization and development of brain stem auditory nuclei of the chicken: ontogeny of n. magnocellularis and n. laminaris. J Comp Neurol 166:469–489. [DOI] [PubMed] [Google Scholar]
- Salcedo-Arellano MJ, Dufour B, McLennan Y, Martinez-Cerdeno V, Hagerman R. 2020. Fragile X syndrome and associated disorders: Clinical aspects and pathology. Neurobiology of Disease 136:104740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Tasker JG. 1997. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol 77:527–536. [DOI] [PubMed] [Google Scholar]
- Shamay-Ramot A, Khermesh K, Porath HT, Barak M, Pinto Y, Wachtel C, Zilberberg A, Lerer-Goldshtein T, Efroni S, Levanon EY, Appelbaum L. 2015. Fmrp Interacts with Adar and Regulates RNA Editing, Synaptic Density and Locomotor Activity in Zebrafish. PLOS Genetics 11:e1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard KA, Korsak LIT, DeBartolo D, Akins MR. 2020. Axonal localization of the fragile X family of RNA binding proteins is conserved across mammals. J Comp Neurol 528:502–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller SS, Broadie K. 2012. Matrix metalloproteinases and minocycline: therapeutic avenues for fragile X syndrome. Neural Plast 2012:124548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Oranje B, Razak KA, Siegel SJ, Schmid S. 2017. Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models. Neurosci Biobehav Rev 76:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Long JM, Ray B, Lahiri DK. 2011. Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 76:1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leahy SN, Rushton EM, Broadie K. 2022. RNA-binding FMRP and Staufen sequentially regulate the Coracle scaffold to control synaptic glutamate receptor and bouton development. Development 149:dev200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YJ, Xing B, Barbour AJ, Zhou C, Jensen FE. 2021. Dysregulation of GABAA Receptor-Mediated Neurotransmission during the Auditory Cortex Critical Period in the Fragile X Syndrome Mouse Model. Cereb Cortex 32:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole EE, Akins MR, Fallon JR. 2014. N-myristoylation regulates the axonal distribution of the Fragile X-related protein FXR2P. Molecular and Cellular Neuroscience 62:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel DC, McCamphill PK, Senter RK, Heynen AJ, Bear MF. 2021. mGluR5 Negative Modulators for Fragile X: Treatment Resistance and Persistence. Front Psychiatry 12:718953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. 2010. Fragile X Mental Retardation Protein Is Required for Rapid Experience-Dependent Regulation of the Potassium Channel Kv3.1b. Journal of Neuroscience 30:10263–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam R, Dwivedi D, Rashid Z, Bonnycastle K, Cousin MA, Chattarji S. 2021. Reciprocal regulation of spontaneous synaptic vesicle fusion by Fragile X mental retardation protein and group I metabotropic glutamate receptors. J Neurochem 158:1094–1109. [DOI] [PubMed] [Google Scholar]
- Sudhakaran IP, Hillebrand J, Dervan A, Das S, Holohan EE, Hülsmeier J, Sarov M, Parker R, VijayRaghavan K, Ramaswami M. 2014. FMRP and Ataxin-2 function together in longterm olfactory habituation and neuronal translational control. Proc Natl Acad Sci USA 111:E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. 2010. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA 107:11591–11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns’ka I, Schachner M. 2017. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends in Neurosciences 40:295–308. [DOI] [PubMed] [Google Scholar]
- Tan Y, Sgobio C, Arzberger T, Machleid F, Tang Q, Findeis E, Tost J, Chakroun T, Gao P, Höllerhage M, Bötzel K, Herms J, Höglinger G, Koeglsperger T. 2020. Loss of fragile X mental retardation protein precedes Lewy pathology in Parkinson’s disease. Acta Neuropathol 139:319–345. [DOI] [PubMed] [Google Scholar]
- Tang B, Wang T, Wan H, Han L, Qin X, Zhang Y, Wang J, Yu C, Berton F, Francesconi W, Yates JR, Vanderklish PW, Liao L. 2015. Fmr1 deficiency promotes age-dependent alterations in the cortical synaptic proteome. Proc Natl Acad Sci USA 112:E4697–E4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Newman-Tancredi A, Varney MA, Razak KA. 2022. Acute and Repeated Administration of NLX-101, a Selective Serotonin-1A Receptor Biased Agonist, Reduces Audiogenic Seizures in Developing Fmr1 Knockout Mice. Neuroscience 509:113–124. [DOI] [PubMed] [Google Scholar]
- Telias M, Kuznitsov-Yanovsky L, Segal M, Ben-Yosef D. 2015. Functional Deficiencies in Fragile X Neurons Derived from Human Embryonic Stem Cells. Journal of Neuroscience 35:15295–15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. 2008. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development 135:1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till SM, Li H-L, Miniaci MC, Kandel ER, Choi Y-B. 2011. A presynaptic role for FMRP during protein synthesis-dependent long-term plasticity in Aplysia. Learn Mem 18:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ. 2000. Sensory stimulation increases cortical expression of the fragile X mental retardation protein in vivo. Brain Res Mol Brain Res 80:17–25. [DOI] [PubMed] [Google Scholar]
- Todd PK, Malter JS, Mack KJ. 2003. Whisker stimulation-dependent translation of FMRP in the barrel cortex requires activation of type I metabotropic glutamate receptors. Molecular Brain Research 110:267–278. [DOI] [PubMed] [Google Scholar]
- Tong H, Kopp-Scheinpflug C, Pilati N, Robinson SW, Sinclair JL, Steinert JR, Barnes-Davies M, Allfree R, Grubb BD, Young SM, Forsythe ID. 2013. Protection from noise-induced hearing loss by Kv2.2 potassium currents in the central medial olivocochlear system. J Neurosci 33:9113–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang B, Arsenault J, Vernon RM, Lin H, Sonenberg N, Wang L-Y, Bah A, Forman-Kay JD. 2019. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc Natl Acad Sci USA 116:4218–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Aa N, Kooy RF. 2020. GABAergic abnormalities in the fragile X syndrome. Eur J Paediatr Neurol 24:100–104. [DOI] [PubMed] [Google Scholar]
- Vasileva M, Horstmann H, Geumann C, Gitler D, Kuner T. 2012. Synapsin-dependent reserveo pool of synaptic vesicles supports replenishment of the readily releasable pool under intense synaptic transmission: Synapsin-dependent vesicles and synaptic transmission. European Journal of Neuroscience 36:3005–3020. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP. 1991. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914. [DOI] [PubMed] [Google Scholar]
- Villarroel-Campos D, Gonzalez-Billault C. 2014. The MAP1B case: an old MAP that is new again. Dev Neurobiol 74:953–971. [DOI] [PubMed] [Google Scholar]
- Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. 2013. Homeostatic Responses Fail to Correct Defective Amygdala Inhibitory Circuit Maturation in Fragile X Syndrome. Journal of Neuroscience 33:7548–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom-Helgren S, Klyachko VA. 2015. GABAB receptor-mediated feed-forward circuit dysfunction in the mouse model of fragile X syndrome. J Physiol 593:5009–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom-Helgren S, Klyachko VA. 2016. Dynamic balance of excitation and inhibition rapidly modulates spike probability and precision in feed-forward hippocampal circuits. J Neurophysiol 116:2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Pan L, Wei M, Wang Q, Liu W-W, Wang N, Jiang X-Y, Zhang X, Bao L. 2015. FMRP-Mediated Axonal Delivery of miR-181d Regulates Axon Elongation by Locally Targeting Map1b and Calm1. Cell Rep 13:2794–2807. [DOI] [PubMed] [Google Scholar]
- Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y. 2004. Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet 13:79–89. [DOI] [PubMed] [Google Scholar]
- Wang SSH, Kaeser PS. 2018. A Presynaptic Liquid Phase Unlocks the Vesicle Cluster. Trends Neurosci 41:772–774. [DOI] [PubMed] [Google Scholar]
- Wang T, van Woerden GM, Elgersma Y, Borst JGG. 2017a. Enhanced Transmission at the Calyx of Held Synapse in a Mouse Model for Angelman Syndrome. Front Cell Neurosci 11:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fan Q, Yu X, Wang Y. 2022. Cellular distribution of the Fragile X mental retardation protein in the inner ear: a developmental and comparative study in the mouse, rat, gerbil, and chicken. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hong H, Brown DH, Sanchez JT, Wang Y. 2017b. Distinct Neural Properties in the Low-Frequency Region of the Chicken Cochlear Nucleus Magnocellularis. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kohl A, Yu X, Zorio DAR, Klar A, Sela-Donenfeld D, Wang Y. 2020. Temporal-specific roles of fragile X mental retardation protein in the development of the hindbrain auditory circuit. Development 147:dev188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zorio DAR, Schecterson L, Lu Y, Wang Y. 2018. Postsynaptic FMRP Regulates Synaptogenesis In Vivo in the Developing Cochlear Nucleus. J Neurosci 38:6445–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-S, Peng C-Z, Cai W-J, Xia J, Jin D, Dai Y, Luo X-G, Klyachko VA, Deng P-Y. 2014a. Activity-dependent regulation of release probability at excitatory hippocampal synapses: a crucial role of fragile X mental retardation protein in neurotransmission. Eur J Neurosci 39:1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sakano H, Beebe K, Brown MR, de Laat R, Bothwell M, Kulesza RJ, Rubel EW. 2014b. Intense and specialized dendritic localization of the fragile X mental retardation protein in binaural brainstem neurons: a comparative study in the alligator, chicken, gerbil, and human. J Comp Neurol 522:2107–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. 1997. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 94:5395–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, Razak KA, Ethell IM. 2018. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb Cortex 28:3951–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xu J, Naguib M, Bie B. 2022. Blockade of Type 2A Protein Phosphatase Signaling Attenuates Complement C1q-Mediated Microglial Phagocytosis of Glutamatergic Synapses Induced by Amyloid Fibrils. Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J-D, Chen S-R, Pan H-L. 2017. Presynaptic mGluR5 receptor controls glutamatergic input through protein kinase C-NMDA receptors in paclitaxel-induced neuropathic pain. J Biol Chem 292:20644–20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L-K, Lu L, Feng B, Wang X-S, Yue J, Li X-B, Zhuo M, Liu S-B. 2020a. FMRP acts as a key messenger for visceral pain modulation. Mol Pain 16:1744806920972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y-M, Arsenault J, Bah A, Krzeminski M, Fekete A, Chao OY, Pacey LK, Wang A, Forman-Kay J, Hampson DR, Wang L-Y. 2020b. Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain. Mol Psychiatry 25:2017–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D. 1991. Fragile X genotype characterized by an unstable region of DNA. Science 252:1179–1181. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang X, Sakano H, Zorio DAR, Wang Y. 2021. Dynamics of the fragile X mental retardation protein correlates with cellular and synaptic properties in primary auditory neurons following afferent deprivation. J Comp Neurol 529:481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang Y. 2022. Tonotopic differentiation of presynaptic neurotransmitter-releasing machinery in the auditory brainstem during the prehearing period and its selective deficits in Fmr1 knockout mice. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M 2018. Two Classes of Secreted Synaptic Organizers in the Central Nervous System. Annu Rev Physiol 80:243–262. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. 2003. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112:317–327. [DOI] [PubMed] [Google Scholar]
- Zhang P, Abdelmohsen K, Liu Y, Tominaga-Yamanaka K, Yoon J-H, Ioannis G, Martindale JL, Zhang Y, Becker KG, Yang IH, Gorospe M, Mattson MP. 2015. Novel RNA- and FMRP-binding protein TRF2-S regulates axonal mRNA transport and presynaptic plasticity. Nat Commun 6:8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer SE, Doll SG, Garcia ADR, Akins MR. 2017. Splice form-dependent regulation of axonal arbor complexity by FMRP. Dev Neurobiol 77:738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorio DAR, Jackson CM, Liu Y, Rubel EW, Wang Y. 2017. Cellular distribution of the fragile X mental retardation protein in the mouse brain. J Comp Neurol 525:818–849. [DOI] [PMC free article] [PubMed] [Google Scholar]


