Abstract
Intrauterine adhesion (IUA), resulting from pregnancy or nonpregnant uterine trauma, is one of the major causes of abnormal menstruation, infertility, or repeated pregnancy loss. Although a few methods, including hysteroscopy and hormone therapy, are routinely used for its diagnosis and treatment, they cannot restore tissue regeneration. Stem cells, which have self-renewal and tissue regeneration abilities, have been proposed as a promising therapy for patients with severe IUAs. In this review, we summarize the origin and features of endometrium-associated stem cells and their applications in the treatment of IUAs based on animal models and human clinical trials. We expect that this information will help to elucidate the underlying mechanism for tissue regeneration and to improve the design of stem cell–based therapies for IUAs.
Keywords: intrauterine adhesion, endometrial stem cells, stem cell therapy, animal model, clinical trials
Introduction
The human endometrium undergoes more than 400 cycles of menstruation during a woman’s reproductive lifetime1. It consists of the functional layer, which accounts for two-thirds of the tissue on the surface, and the basal layer, which is located underneath. During the menstrual cycle, with the change in hormone levels (mainly estrogen and progesterone)2, the functional layer is shedding periodically3, leaving the remaining basal layer, approximately 1 to 2 mm, adjacent to the myometrium. After menstruation, endometrial tissue gradually returns to normal thickness as the levels of estrogen and progesterone increase. The increase in endometrial thickness is crucial to embryo implantation and growth4 (Fig. 1).
Figure 1.
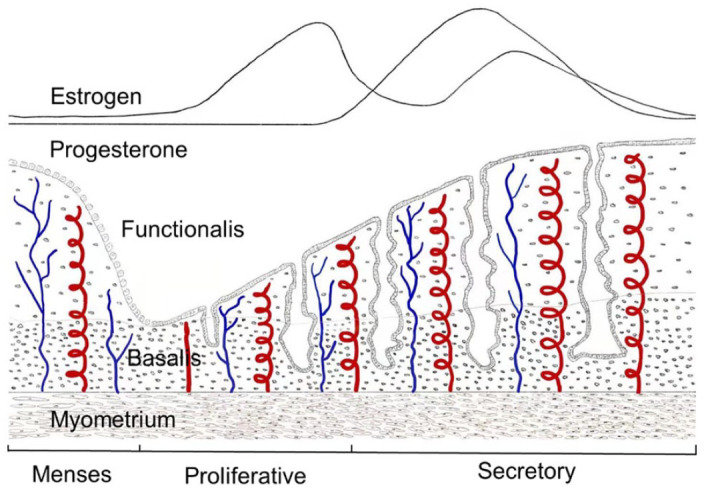
Schematic showing that the periodic shedding of human endometrial tissue with the change of estrogen and progesterone during the menstrual cycle. The menstrual cycle is divided into three main phases: menses, proliferative phase, and secretory phase. The basal layer does not change significantly with the menstrual cycle, while the function layer of the human endometrium is periodic shedding.
Intrauterine adhesions (IUAs), also called Asherman syndrome if severe uterine adhesion occurs, are mainly due to abortion or postpartum bleeding curettage5,6. This leads to partial or complete occlusion of the uterine cavity7. IUAs can cause abnormal menstruation, hypomenorrhea and amenorrhea, infertility, or repeated pregnancy loss, depending on the severity8–10. At present, the primary goal of IUAs treatment is to reconstruct the anatomical structure and restore uterine function11. For example, balloon stents and sodium hyaluronate can effectively prevent the recurrence of IUA12,13. Hysteroscopy is an effective method for diagnosis and treatment as well14,15. However, hysteroscopy also has many side effects, including bleeding, shock, and death16.
Although current IUA therapies have been proven to have clinical effectiveness, they contribute little or no to pregnancy outcomes. Studies have shown that one of the significant molecular causes of IUAs is the decrease in stem cells in the basal layer of the endometrium17,18. Stem cells capable of self-renewal and differentiation are the foundation for tissue homeostasis, repair, and regeneration. A recent study showed that endometrial stem cells extracted from IUAs patients have low angiogenic activity, clone formation, and proliferation compared with those from healthy females19. Stem cell therapy, which promotes the repair mechanism of the injured tissue by stem cells and their differentiated derivatives, is emerging as a promising strategy for IUAs.
In this review, we summarize the features of stem cells derived from the endometrial system. More importantly, we review the progress of stem cell therapy for IUAs in animal and human clinical trials.
Stem Cells in Murine Endometrium
The mouse model is crucial to our understanding of endometrial stem cells. A few studies have used label-retention methods to localize stem cells in the endometrium20. Bromodeoxyuridine (BrdU) replicate DNA molecules during cell proliferation in place of thymine (T). When BrdU is injected into the mice, all the proliferating cells are labeled, but only the quiescent are identified as label-retaining cells (LRCs)21,22.
Mouse endometrium stromal stem cells
Through BrdU tracing, Chan and Gargett20 found that stromal LRCs (6%) mainly reside at the endometrial–myometrial junction near blood vessels and adjacent to the luminal epithelium. Later, in 2007, Cervellóet et al. identified that the stromal LRC population expressed c-Kit (stem cell factor receptor) and octamer-binding transcription factor 4 (OCT-4, pluripotent stem cell marker), also known as POU5F1, 8 to 10 weeks after injection of BrdU. The labeled cells reside primarily in the lower part of the endometrial stroma23. A recent study found that 6 weeks after BrdU injection, LRCs primarily resided under the luminal epithelium and expressed CD44+, CD90+, CD140b+, CD146+, and Sca-1+. This study also highlights that LRC responds effectively to physiological stimuli at the onset of uterine involution and returns to the quiescent state after postpartum repair24. In a mouse menstruation model, Yin et al. confirmed that SM22α+ stromal cells, located in the stroma at an early phase, express CD34+KLF4+ markers upon estrogen induction. These cells are transferred to the epithelium during endometrial repair, and this process is called mesenchymal-to-epithelial transition (MET)25.
Mouse endometrium epithelial stem cells
Chan et al.26 concluded that epithelial LRCs were mainly located in the luminal epithelium, with few cells observed in the glandular epithelium according to the BrdU method. Huang et al.27 described a novel transition of stromal cells to epithelial cells during regeneration induced by parturition in 2012. Janzen et al. suggested that CD44+ epithelial cells were mouse epithelial progenitor cell markers, and these cells produced more glandular structures than CD44− cells in immunosuppressed mice. However, CD44+ cells were also expressed in the endometrial stroma28. In 2006, Deane et al.29 used a GFP reporter under the control of the telomerase reverse transcriptase promoter (mTert-GFP) and found that mTert-GFP was expressed by rare luminal and glandular epithelial cells. However, these cells are different from slow-cycling cells with a label-retaining phenotype.
However, BrdU has some limitations in determining stem cell populations because of the length of tracing and the interval of the initial pulse30. To solve this problem, several other techniques have been used to trace stem cells. Using a single-cell lineage tracing system, Jin et al. confirmed that in the adult mouse uterus, epithelial stem cells were located at the junction of the glandular epithelium and luminal epithelium. These epithelial stem cells are able to grow both glandular epithelial (GE) and luminal epithelial (LE) efficiently and maintain the homeostasis of the mouse endometrial epithelium31. More stringent uterine lineage tracing studies have been performed more recently. In one study, the authors found that Axin2+ cells were located at the base of the gland and were able to regenerate the entire gland32. They believed that there were two types of stem cells in the endometrium, a group of short-lived LE progenitor cells and a group of long-lived bipotent stem cells, to maintain stem cell properties and prevent cells from becoming cancerous. In another study, Seishima et al.33 confirmed that LGR5+ progenitor cells are located at the end of the endometrial glands and maintain uterine gland development after birth.
Stem Cells in Human Endometrium
It was a long time since Prianishnikov proposed that there were stem cells in the deepest basal layer of human endometrium in 1978. These stem cells have the ability to differentiate into endometrial cells, and the ratio of different cells was regulated by hormones produced by the ovaries34. Later, Chan et al.35 first isolated epithelial and stromal stem cells from human endometrium and proved that they have clonogenic activity in vitro in 2004. With the development of stem cell research over the years, stem cells in human endometrium have been identified and divided into three types: epithelial stem cells, endothelial stem cells, and endometrial mesenchymal stem cells (MSCs).
Human endometrium stromal stem cells
Endometrium stromal stem cells are similar to other MSCs derived from other human tissues. They are positive for CD105, CD73, CD44, and CD90 and negative for CD45, CD34, CD14, or CD11b36. C-kit/CD117 was first identified as an endometrial stem cell marker in 2006. Although C-kit was not detected in the fetal endometrium, it was mainly expressed in the stroma in all other lifetime endometrium samples37. Stromal cells positive for two perivascular cell markers, CD146 and platelet-derived growth factor–receptor beta (PDGFR-b), show mesenchymal stem cell (MSC) properties and are located near blood vessels in the human endometrium38. Another study identified Sushi domain containing 2+(SUSD2+) (also called W5C5) as a marker for endometrial stromal stem cells. The proliferative activity of SUSD2+ cells was three times higher than that of unsorted endometrial stromal cells and 14.7 times higher than that of W5C5− cells. These cells can also differentiate into osteocytes, adipocytes, and chondrocytes in vitro in response to specific induction cues. More importantly, compared with the combination of CD146 and PDGF-Rβ, SUSD2 can be used as a single marker for the extraction of endometrial MSCs39,40.
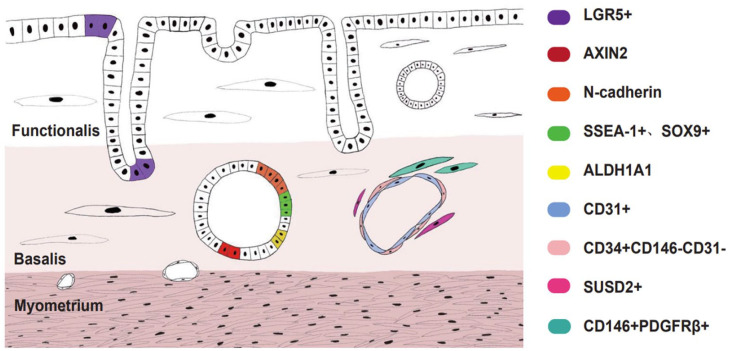
Other MSC markers, such as mesenchymal stem cell antigen-1 (MSCA-1), also known as TNAP41, and Notch142, are also found in human endometrial stem cells. The expression of TNAP in endometrial stromal cells was mainly limited to CD146(+) cells41,43. Musashi-1, which encodes an RNA-binding protein in neural stem cells44, was also indicated as an endometrial stromal stem cell marker in 2008. Researchers found that Musashi-1 was highly expressed in the proliferating endometrium, endometriosis, and endometrial carcinoma45. Recently, Musashi-1 expression was also found in neonatal endometrium at 12 weeks of gestation, and the number of positive cells decreased with increasing gestational age. When reaching reproductive age, these positive cells were mainly located in stromal cells adjacent to the myometrium46. However, no studies have yet successfully isolated Musashi-1+ cells by fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS), so its use as an endometrial stem cell marker is controversial. The schematic is listed in Fig. 2.
Figure 2.
Stem cells in human endometrium and its location. LGR5 are mainly locate in luminal and basalis epithelium. AXIN2+ epithelial cells are locate in basalis of human endometrium. N-cadherin+ epithelial cells are in deep bases of branching gland structures. SSEA-1+ SOX9+ epithelial cells locate in basal layer proximal to N-cadherin+ epithelial progenitor cells. ALDH1A1 co-localize with N-cadherin+ cells in basal glands. CD31+ cells are endothelial stem cells. CD34+ CD146− CD31− cells are perivascular in the adventitia of blood vessels. SUSD2+ eMSCs are also perivascular cells. CD140b+ CD146+ eMSCs are located mainly in both the functionalis and basalis.
Human endometrium epithelial stem cells
Endometrial epithelial stem cells are thought to reside in the base of the basal layer glands30,47. Although mouse studies indicated that parturition can promote stromal cell transfer to epithelial cells during regeneration, it has not been proven in the human system27. In humans, endometrial epithelial stem cells were first identified in 2004 as clonogenic cells marked by EpCAM. EpCAM+ cells showed self-renewal by serially cloning more than three times and proliferated greater than 30 fold during 4 months of culture35. Stage-specific embryonic antigen-1 (SSEA-1) has also been identified as a marker for glandular epithelial cells located in the basal layer of the endometrium. SSEA-1+ cells had longer telomeres and a weaker differentiated phenotype than SSEA− cells, and SSEA+ cells formed more organoids than SSEA− cells47. In addition, both SOX9 and β-catenin have been suggested as putative markers of human endometrial epithelial stem cells with SSEA-147. Another putative marker for endometrial epithelial stem cells is N-cadherin, an intercellular adhesion molecule that stabilizes connections between epithelial cells48. Immunofluorescence staining showed that N-cadherin was mainly located at the basal layer near the myometrium, and N-cadherin+ epithelial cells had stronger colony formation ability in vitro than N-cadherin− epithelial cells49.
A few other genes have been proposed as endometrial epithelial stem cell markers; however, the results are not convincing due to the lack of functional analysis. In one study, the researchers used in situ hybridization (ISH), qPCR, and a systems biology approach to study the expression of leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), a marker for the intestinal epithelium and hair follicle stem cells, in human endometrium. They found that LGR5 was more dominantly expressed in the luminal epithelium than in other epithelial compartments in the healthy human endometrium50. However, due to the poor quality of LGR5 antibodies, further functional analysis could not be conducted. In another study, Ma et al. found that aldehyde dehydrogenase 1 isoform (ALDH1A1), a cancer stem cell marker51, was highly expressed in the epithelium of basalis in the human endometrium and colocalized with N-cadherin in the glands of stratum basalis52. Another putative marker is ectonucleoside triphosphate diphosphohydrolase-2 (NTPDase2), a cell surface enzyme that can catalyze the dephosphorylation of ATP53. A recent study found that NTPDase2 is present in the surrounding basal glands but absent in functional glands. They proposed that NTPDase2 may be related to endometrial epithelial stem cell storage54. All markers and details are listed in Table 1.
Table 1.
Human Endometrial Stem Cells.
| Surface markers | Cell type | Location | Function | Reference |
|---|---|---|---|---|
| TERT | Epithelial | Glandular | Correlated with epithelial proliferation | 55 |
| CD326 (EPCAM) | Epithelial | Basalis and functionalis | Endometrial EpCAM(+) epithelial cells own great proliferation abilities | 56 |
| SOX9 | Epithelial | Basalis (46.2–52.3%) functionalis (8–12.1%) | Epithelial repair and glandular regeneration | 47 |
| SSEA1 | Epithelial | Basalis | Epithelial repair and glandular regeneration | 47 |
| CTNNB1 (β-catenin) | Epithelial | Basalis | Could have stem cell properties | 47 |
| Notch | Epithelial | Basalis and functionalis | Maintain cells in an undifferentiated state57 | 57 |
| CHD2 (N-cadherin) | Epithelial | Basalis | Glandular regeneration and self-renew | 49 |
| ALDH1AI | Epithelial | Deep glands of basalis | Endometrium regeneration, involved in the pathology of endometriosis | 52 |
| LRG5 | Epithelial | Luminal epithelium and stratum basalis | Correlate with epithelial proliferation | 50 |
| MSCA-1 (TNAP) | Stromal | Pericyte | High levels in the luminal space of glandular epithelia | 41 |
| W5C5 (SUSD2) | Stromal | Perivascular, basalis and functionalis | Reconstituting endometrial stromal tissues | 39 |
| CD146 PDGF-Rb (CD140b) | Stromal | Perivascular location in the basalis (mainly) | Colony-forming ability and multipotency | 38 |
| NTPDase2 | Stromal | Basalis | NTPDase2 was expressed by the SUSD2+ endometrial mesenchymal stem cells | 54 |
| c-KIT/CD117 | Epithelial/stromal | Mainly in the stroma of the basalis | Persistent from the fetal to the postmenopausal period | 37 |
| Musashi-1 | Epithelial/stromal | Basalis and functionalis | Progenitor cells in proliferating endometrium | 45 |
Endometrial-Derived Stem Cells for IUA Treatment
Animal models for IUA
A suitable animal model that recapitulates the pathology of IUA is crucial for testing novel regenerative therapies in preclinical phases. Till now, mice, rats, rabbits, and nonhuman primates have been used to model IUA. Owing to the size of the animals and the cost, rats are most often used for research and preclinical studies. Uterine histology, morphology, and pregnancy outcome are usually assessed to evaluate whether the models mimic the physiology and pathology of the disease58,59.
Endometrial injury was induced by physical damage, chemical damage, or a combination of both60. Physical injury by surgical blades is often performed on rats to mimic the process of human endometrial curettage IUAs61. This process is always accompanied by side effects, such as abdominal adhesion. In addition, the process is laborious and depends highly on the surgical technique, which makes consistency a major issue in interpreting endpoint results. Chemical damage by ethanol is another well-used method to mimic IUAs62,63. However, the retention time and the dosage of ethanol are difficult to determine to avoid excessive or insufficient endometrial damage64. Trichloroacetic acid (TCA), a chemical agent that causes chemical burns to tissue once in contact, is also used for IUAs models. TCA is used clinically to treat patients with dysfunctional uterine bleeding (DUB) through endometrial ablation65. However, TCA is reported to be carcinogenic according to the World Health Organization’s International Agency66. A comprehensive comparison of various rat IUAs models was conducted by one group, and they concluded that compared with heat stripping, mechanical injury, and the combination of mechanical injury and infection (dual-injury), ethanol injection was the best based on histological and immunohistochemical analysis67. More recently, a study established an IUAs model by surgical abortion and curettage in pregnant rats. They claim that this process mimics abortion curettage or postpartum curettage in humans and that the pathology is more comparable to human IUAs68.
Despite numerous improvements in animal models, there is wide variation in procedures, timing, endpoints, and assessment criteria, resulting in inconsistent results between studies and inaccurate conclusions for efficacy tests. To avoid future failure in human clinical trials, appropriate assessment plans with validated methods, statistically prejustified sample sizes, timing, endpoints, and evaluation methods should be developed for preclinical animal studies of stem cell therapy.
Menstrual blood–derived stem cells for IUA treatment
Menstrual blood–derived stem cells (MenSCs) were first identified in 2007 and found to express CD9, CD29, CD41a, CD44, CD59, CD73, CD90, and CD105, which are also expressed by MSCs from other tissues. Compared to MSCs, MenSCs have several advantages over MSCs derived from other tissues for stem cell therapy69. First, MenSCs can be easily obtained with noninvasive operation and no ethical concerns70,71. Second, MenSCs are able to proliferate rapidly and remain karyotype stable after 68 generations72. Finally, MenSCs express high level of matrix metalloproteases, which is beneficial for tissue repair69. So far, animal experiments have shown that MenSCs have therapeutic effects on a variety of diseases, including type 1 diabetes73, cardiac diseases74, cartilage damage75, osteochondral defects76, premature ovarian failure77, and liver disease78,79.
MenSCs can differentiate into endometrium cells with growth factor-β (TGF-β), epidermal growth factor (EGF), platelet-derived growth factor BB (PDGF-BB) in vitro. Thus, they are supposed to contribute to endometrium growth and recovery of fertility72. Animal experiments in rats confirmed that MenSCs was indeed beneficial to the recovery of IUA, and the effect is better when culture MenSCs with FGF-280. Transplantation of MenSCs with platelet-rich plasma (PRP), a platelet concentrate product with rich growth factors and proteins, improves uterine proliferation and endometrium damage repair in IUA rats81. Based on these promising results, MenSCs-based treatment are proposed as a personalized strategy for reproductive therapy.
Human clinical trials
As stem cell therapy becomes more standardized and promising, an increasing number of clinical trials of stem cell treatments for IUAs are being conducted worldwide. For example, researchers have used autologous MenSCs to treat IUAs, in which endometrial thickness increased in all patients, and three of seven eventually became pregnant82. In another study, the researchers adopted UC-MSC+ collagen scaffolds to treat IUAs. The endometrial thickness of all patients who accepted the treatment was increased, and 10 of 26 achieved successful pregnancy83. Similar clinical trials to improve the thickness of IUAs patients included amnion84 and b-FGF85, and both obtained promising results. To date, a great number of clinical trials have been conducted to treat infertility in women caused by endometrial factors, and details are listed in Table 2.
Table 2.
The List of Some Clinical Trials Using Mesenchymal Stem Cells to Treat Intrauterine Adhesions (or Thin Endometrium, Asherman’s Syndrome). All Data Come From: Home - ClinicalTrials.gov.
| Pathological condition | Cell therapy | Estimated enrollment | Intervention/treatment | Cliniclatrials.gov identifier | Outcome measures |
|---|---|---|---|---|---|
| Infertile women with severe intrauterine adhesions or endometrial dysplasia | Collagen scaffold loaded with autologous BMSCs | 30 participants | Autologous bone marrow stem cells | NCT02204358 | Evaluation of the reduction of intrauterine scar area, the change of intrauterine adhesion, endometrial thickness, and the menstrual blood volume |
| Women with intrauterine adhesion | UC-MSCs loaded in collagen scaffold | 18 participants | UCMSC combined with collagen scaffold | NCT03724617 | Evaluation of the change of endometrial thickness, pregnancy rate, live birth rate, abortion rate |
| Women with scarring and adhesions | AMSCs and a biodegradable carrier | 10 participants | Autologous mesenchymal stem cells | NCT04432467 | Evaluation of the number of cured patients, and patients with treatment-related adverse events |
| Women infertility with severe refractory Asherman’s syndrome | hAESCs | 50 participants | hAESCs-based therapy | NCT03223454 | Evaluation of menstrual blood volume, Endometrial thickness, Uterine volume, Ongoing pregnancy rate |
| Women with severe refractory Asherman’s syndrome | hAESCs | 20 participants | hAESCs-based therapy | NCT03381807 | Evaluation of the changes of Endometrial thickness, Menstrual blood volume, pregnancy rate |
| Infertility women with Intrauterine adhesions | UCMSCs | 26 participants | UCMSC-based therapy | NCT02313415 | Evaluation of live birth rate, endometrial thickness, reduction of intrauterine adhesion, change of menstrual blood volume |
| Patients with Asherman’s Syndrome | CD133+ autologous BMSCs | 16 participants | Bone marrow CD133+ stem cell transplantation | NCT02144987 | Evaluation of Live-birth rate, Ongoing pregnancy rate, Implantation Rate, Endometrial thickness prior to the treatment |
| Asherman Syndrome Endometrium Recurrent Implantation Failure |
Autologous BMSCs | 30 participants | BMSCs-based therapy | NCT05343572 | Evaluation of endometrial thickness and implantation rates |
AMSCs: adipose tissue derived mesenchymal stem cells; BMSCs: bone marrow stem cells; hAESCs: Human amniotic epithelial stem cells; UCMSC: umbilical cord mesenchymal stem cells.
All clinical trials of MSC therapy are interventional trials. Most patients recruited were women of normal reproductive age with a history of uterine adhesions or thin endometrium and no history of human chorionic gonadotrophin (HCG) use. Various sources of cells, different injection methods, and combinations with different materials and hormonal supports have been applied in clinical trials. MSCs come from a wide range of sources, such as umbilical cord, adipose, amniotic and bone marrow. The effects of MSC therapy can be measured in many ways, such as endometrial thickness, changes in menstrual volume, reduction of intrauterine scar area, implantation rate, and pregnancy rate before and after treatment. In all clinical trials that we counted, half of the trials were over and had good results. In addition, in all of the clinical trials, only autologous BMSC transplantation was in phase 4, and the remaining trials were in phase 1 and phase 2. These results indicate that autologous BMSCs have great advantages in the treatment of IUAs, and autologous stem cell transplantation will be the first choice of stem cell therapy in the future.
Conclusion
Great progress has been made in stem cell therapy with the rapid development of stem cell research. Since the first in vitro fertilization (IVF) baby was born in 1978 in the United Kingdom86, IVF has made tremendous progress worldwide in the past 30 years. However, female infertility includes not only the problem of seeds (embryo) but also the problems of soil (human endometrium). Stem cells are essential for the maintenance of normal endometrial function and morphology. Therefore, transplantation of stem cells is considered a promising therapeutic strategy to promote endometrial repair or regeneration in IUAs patients. Although many preclinical and clinical studies have shown the potential therapeutic effect of MSCs derived from either endometrial or nonendometrial tissues on IUAs, improvements should be made on several issues before we develop a novel stem cell therapy with high efficacy and safety: (1) a thorough understanding of adult stem cells and their regulatory mechanisms in the human endometrium; (2) reliable animal models that mimic human IUAs with good predictability of clinical outcomes; and (3) Good manufacturing practices (GMP) regulations on the isolation, expansion, and transplantation of stem cells for clinical application. We believe that in the near future, stem cell therapy will become one of the most effective therapies for a variety of diseases, including IUAs.
Acknowledgments
Not applicable.
Footnotes
Author Contributions: Kai Chen: Drafted and edited the manuscript.
Shengxia Zheng: Concept of manuscript and provided advice about the content.
Fang Fang: Concept and design of manuscript, edited the manuscript, and final approval of the manuscript.
Availability of Data and Materials: Not applicable.
Ethical Approval: Not applicable.
Statement of Human and Animal Rights: This study did not involve any human or animal subjects.
Statement of Informed Consent: Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the National Natural Science Foundation of China (grant nos. 32070830 and 81971339) and the research funds from the University of Science and Technology of China (grant nos. WK9110000141 and YD9100002007).
ORCID iD: Kai Chen  https://orcid.org/0000-0002-2125-6887
https://orcid.org/0000-0002-2125-6887
References
- 1. Henriet P, Gaide Chevronnay HP, Marbaix E. The endocrine and paracrine control of menstruation. Mol Cell Endocrinol. 2012;358(2):197–207. [DOI] [PubMed] [Google Scholar]
- 2. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27(1):17–46. [DOI] [PubMed] [Google Scholar]
- 3. Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–51. [DOI] [PubMed] [Google Scholar]
- 4. Tomic V, Kasum M, Vucic K. Impact of embryo quality and endometrial thickness on implantation in natural cycle IVF. Arch Gynecol Obstet. 2020;301(5):1325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santamaria X, Isaacson K, Simón C. Asherman’s Syndrome: it may not be all our fault. Hum Reprod. 2018;33(8):1374–80. [DOI] [PubMed] [Google Scholar]
- 6. Friedler S, Margalioth EJ, Kafka I, Yaffe H. Incidence of post—abortion intra—uterine adhesions evaluated by hysteroscopy —a prospective study. Hum Reprod. 1993;8(3):442–44. [DOI] [PubMed] [Google Scholar]
- 7. March CM. Asherman’s syndrome. Semin Reprod Med. 2011;29(2):83–94. [DOI] [PubMed] [Google Scholar]
- 8. Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome —one century later. Fertil Steril. 2008;89(4):759–79. [DOI] [PubMed] [Google Scholar]
- 10. Myers EM, Hurst BS. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril. 2012;97(1):160–64. [DOI] [PubMed] [Google Scholar]
- 11. Hooker AB, de Leeuw RA, Twisk JWR, Brölmann HAM, Huirne JAF. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan Z, Goldberg JM. Hysteroscopic management of Asherman’s syndrome. J Minim Invasive Gynecol. 2018;25(2):218–28. [DOI] [PubMed] [Google Scholar]
- 13. Yang X, Liu Y, Li TC, Xia E, Xiao Y, Zhou F, Song D, Zhou Q. Durations of intrauterine balloon therapy and adhesion reformation after hysteroscopic adhesiolysis: a randomized controlled trial. Reprod Biomed Online. 2020;40(4):539–46. [DOI] [PubMed] [Google Scholar]
- 14. Hanstede MM, van der Meij E, Goedemans L, Emanuel MH. Results of centralized Asherman surgery, 2003-2013. Fertil Steril. 2015;104(6):1561–68. [DOI] [PubMed] [Google Scholar]
- 15. March CM. Management of Asherman’s syndrome. Reprod Biomed Online. 2011;23(1):63–76. [DOI] [PubMed] [Google Scholar]
- 16. Cholkeri -Singh A, Sasaki KJ. Hysteroscopy safety. Curr Opin Obstet Gynecol. 2016;28(4):250–54. [DOI] [PubMed] [Google Scholar]
- 17. Verdi J, Tan A, Shoae-Hassani A, Seifalian AM. Endometrial stem cells in regenerative medicine. J Biol Eng. 2014;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. Plos One. 2014;9(5): e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Min J, Lu N, Huang S, Chai X, Wang S, Peng L, Wang J. Phenotype and biological characteristics of endometrial mesenchymal stem/stromal cells: a comparison between intrauterine adhesion patients and healthy women. Am J Reprod Immunol. 2021;85(6): e13379. [DOI] [PubMed] [Google Scholar]
- 20. Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24(6):1529–38. [DOI] [PubMed] [Google Scholar]
- 21. Humphreys BD. Cutting to the chase: taking the pulse of label-retaining cells in kidney. Am J Physiol Renal Physiol. 2015;308(1): F29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Webster AF, Williams A, Recio L, Yauk CL. Bromodeoxyuridine (BrdU) treatment to measure hepatocellular proliferation does not mask furan-induced gene expression changes in mouse liver. Toxicology. 2014;323:26–31. [DOI] [PubMed] [Google Scholar]
- 23. Cervelló I, Martínez-Conejero JA, Horcajadas JA, Pellicer A, Simón C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22(1):45–51. [DOI] [PubMed] [Google Scholar]
- 24. Cao M, Chan RW, Yeung WS. Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev. 2015;24(6):768–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin M, Zhou HJ, Lin C, Long L, Yang X, Zhang H, Taylor H, Min W. CD34(+)KLF4(+) stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 2019;27(9):2709–27243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan RW, Kaitu’u-Lino T, Gargett CE. Role of label-retaining cells in estrogen-induced endometrial regeneration. Reprod Sci. 2012;19(1):102–14. [DOI] [PubMed] [Google Scholar]
- 27. Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. Plos One. 2012;7(8): e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janzen DM, Cheng D, Schafenacker AM, Paik DY, Goldstein AS, Witte ON, Jaroszewicz A, Pellegrini M, Memarzadeh S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells. 2013;31(4):808–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deane JA, Ong YR, Cain JE, Jayasekara WS, Tiwari A, Carlone DL, Watkins DN, Breault DT, Gargett CE. The mouse endometrium contains epithelial, endothelial and leucocyte populations expressing the stem cell marker telomerase reverse transcriptase. Mol Hum Reprod. 2016;22(4):272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci USA. 2019;116(14):6848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, Whan RM, Alterman D, Tanwar PS. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26(1):64–80.e13. [DOI] [PubMed] [Google Scholar]
- 33. Seishima R, Leung C, Yada S, Murad KBA, Tan LT, Hajamohideen A, Tan SH, Itoh H, Murakami K, Ishida Y, Nakamizo S, et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 2019;10(1):5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18(3):213–23. [DOI] [PubMed] [Google Scholar]
- 35. Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–50. [DOI] [PubMed] [Google Scholar]
- 36. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. [DOI] [PubMed] [Google Scholar]
- 37. Cho NH, Park YK, Kim YT, Yang H, Kim SK. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81(2):403–407. [DOI] [PubMed] [Google Scholar]
- 38. Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. [DOI] [PubMed] [Google Scholar]
- 39. Masuda H, Anwar SS, Bühring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21(10):2201–14. [DOI] [PubMed] [Google Scholar]
- 40. Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–43. [DOI] [PubMed] [Google Scholar]
- 41. Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Orgel M, Treml S, Cerabona F, de Zwart P, Ochs U, Müller CA, Gargett CE, et al. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 2010;19(5):669–77. [DOI] [PubMed] [Google Scholar]
- 42. Schüring AN, Schulte N, Kelsch R, Röpke A, Kiesel L, Götte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95(1):423–26. [DOI] [PubMed] [Google Scholar]
- 43. Tempest N, Maclean A, Hapangama DK. Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci. 2018;19(10):3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kudinov AE, Karanicolas J, Golemis EA, Boumber Y. Musashi RNA-binding proteins as cancer drivers and novel therapeutic targets. Clin Cancer Res. 2017;23(9):2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Götte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schüring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215(3):317–29. [DOI] [PubMed] [Google Scholar]
- 46. Lu X, Lin F, Fang H, Yang X, Qin L. Expression of a putative stem cell marker Musashi-1 in endometrium. Histol Histopathol. 2011;26(9):1127–33. [DOI] [PubMed] [Google Scholar]
- 47. Valentijn AJ, Palial K, Al-Lamee H, Tempest N, Drury J, Von Zglinicki T, Saretzki G, Murray P, Gargett CE, Hapangama DK. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28(10):2695–2708. [DOI] [PubMed] [Google Scholar]
- 48. Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–52. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen HPT, Xiao L, Deane JA, Tan KS, Cousins FL, Masuda H, Sprung CN, Rosamilia A, Gargett CE. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32(11):2254–68. [DOI] [PubMed] [Google Scholar]
- 50. Tempest N, Baker AM, Wright NA, Hapangama DK. Does human endometrial LGR5 gene expression suggest the existence of another hormonally regulated epithelial stem cell niche? Hum Reprod. 2018;33(6):1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7(10):11018–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma S, Hirata T, Arakawa T, Sun H, Neriishi K, Fukuda S, Nakazawa A, Wang Y, Harada M, Hirota Y, Koga K, et al. Expression of ALDH1A Isozymes in human endometrium with and without endometriosis and in ovarian endometrioma. Reprod Sci. 2020;27(1):443–52. [DOI] [PubMed] [Google Scholar]
- 53. Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49(6):473–97. [DOI] [PubMed] [Google Scholar]
- 54. Trapero C, Vidal A, Rodríguez-Martínez A, Sévigny J, Ponce J, Coroleu B, Matias-Guiu X, Martín-Satué M. The ectonucleoside triphosphate diphosphohydrolase-2 (NTPDase2) in human endometrium: a novel marker of basal stroma and mesenchymal stem cells. Purinergic Signal. 2019;15(2):225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanaka M, Kyo S, Takakura M, Kanaya T, Sagawa T, Yamashita K, Okada Y, Hiyama E, Inoue M. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am J Pathol. 1998;153(6):1985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qi S, Zhao X, Li M, Zhang X, Lu Z, Yang C, Zhang C, Zhang H, Zhang N. Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod Biol Endocrinol. 2015;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fan Y, Sun J, Zhang Q, Lai D. Transplantation of human amniotic epithelial cells promotes morphological and functional regeneration in a rat uterine scar model. Stem Cell Res Ther. 2021;12(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tal R, Shaikh S, Pallavi P, Tal A, López-Giráldez F, Lyu F, Fang YY, Chinchanikar S, Liu Y, Kliman HJ, Alderman M, 3rd, et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17(9): e3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Polishuk WZ. Endometrial regeneration and adhesion formation. S Afr Med J. 1975;49(12):440–42. [PubMed] [Google Scholar]
- 61. Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, Ma L, Gao C, Zhang S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160–71. [DOI] [PubMed] [Google Scholar]
- 62. Zhang S, Sun Y, Jiang D, Chen T, Liu R, Li X, Lu Y, Qiao L, Pan Y, Liu Y, Lin J. Construction and optimization of an endometrial injury model in mice by transcervical ethanol perfusion. Reprod Sci. 2021;28(3):693–702. [DOI] [PubMed] [Google Scholar]
- 63. Xu QX, Zhang WQ, Liu XZ, Yan WK, Lu L, Song SS, Wei SW, Liu YN, Kang JW, Su RW. Notch1 signaling enhances collagen expression and fibrosis in mouse uterus. Biofactors. 2021;47:852–864. [DOI] [PubMed] [Google Scholar]
- 64. Kim YY, Choi BB, Lim JW, Kim YJ, Kim SY, Ku SY. Efficient production of murine uterine damage model. Tissue Eng Regen Med. 2019;16(2):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kucukozkan T, Kadioglu BG, Uygur D, Moroy P, Mollamahmutoglu L, Besli M. Chemical ablation of endometrium with trichloroacetic acid. Int J Gynaecol Obstet. 2004;84(1):41–46. [DOI] [PubMed] [Google Scholar]
- 66. Peng X, Yu S, Lin H, Wu F, Yang J, Zhou C, Zhang L, Yang J, Zhang W. Time-concentration-dependent profile of histone modifications on human hepatocytes treated by trichloroacetic acid. Int J Environ Health Res. 2022;32:2376–2384. [DOI] [PubMed] [Google Scholar]
- 67. Sun L, Zhang S, Chang Q, Tan J. Establishment and comparison of different intrauterine adhesion modelling procedures in rats. Reprod Fertil Dev. Epub 2019 Apr 9. [DOI] [PubMed] [Google Scholar]
- 68. Feng Q, Gao B, Zhao X, Huang H, Yi S, Zou L, Liu X, Xue M, Xu D. Establishment of an animal model of intrauterine adhesions after surgical abortion and curettage in pregnant rats. Ann Transl Med. 2020;8(4):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lv H, Hu Y, Cui Z, Jia H. Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell Res Ther. 2018;9(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang S, Chang Q, Li P, Tong X, Feng Y, Hao X, Zhang X, Yuan Z, Tan J. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale. 2021;13(15):7334–47. [DOI] [PubMed] [Google Scholar]
- 72. Zheng SX, Wang J, Wang XL, Ali A, Wu LM, Liu YS. Feasibility analysis of treating severe intrauterine adhesions by transplanting menstrual blood-derived stem cells. Int J Mol Med. 2018;41(4):2201–12. [DOI] [PubMed] [Google Scholar]
- 73. Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23(11):1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu Y, Niu R, Li W, Lin J, Stamm C, Steinhoff G, Ma N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol Life Sci. 2019;76(9):1681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Uzieliene I, Urbonaite G, Tachtamisevaite Z, Mobasheri A, Bernotiene E. The potential of menstrual blood-derived mesenchymal stem cells for cartilage repair and regeneration: novel aspects. Stem Cells Int. 2018;2018:5748126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khanmohammadi M, Golshahi H, Saffarian Z, Montazeri S, Khorasani S, Kazemnejad S. Repair of osteochondral defects in rabbit knee using menstrual blood stem cells encapsulated in fibrin glue: a good stem cell candidate for the treatment of osteochondral defects. Tissue Eng Regen Med. 2019;16(3):311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Feng P, Li P, Tan J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev Rep. 2019;15(2):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. 2017;6(1):272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen L, Guo L, Chen F, Xie Y, Zhang H, Quan P, Sui L. Transplantation of menstrual blood-derived mesenchymal stem cells (MbMSCs) promotes the regeneration of mechanical injuried endometrium. Am J Transl Res. 2020;12(9):4941–54. [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–29. [DOI] [PubMed] [Google Scholar]
- 83. Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L, Zhou Q, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li C, Cai A, Sun C, Wu B, Chen X, Mao Y, Zhang Y, Gou Y, Yu J, Wang Y, Yu H, et al. The study on the safety and efficacy of amnion graft for preventing the recurrence of moderate to severe intrauterine adhesions. Genes Dis. 2020;7(2):266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jiang P, Tang X, Wang H, Dai C, Su J, Zhu H, Song M, Liu J, Nan Z, Ru T, Li Y, et al. Collagen-binding basic fibroblast growth factor improves functional remodeling of scarred endometrium in uterine infertile women: a pilot study. Sci China Life Sci. 2019;62(12):1617–29. [DOI] [PubMed] [Google Scholar]
- 86. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. [DOI] [PubMed] [Google Scholar]