Abstract
The Elongator complex associated with elongating RNA polymerase II in Saccharomyces cerevisiae was originally reported to have three subunits, Elp1, Elp2, and Elp3. Using the tandem affinity purification (TAP) procedure, we have purified a six-subunit yeast Holo-Elongator complex containing three additional polypeptides, which we have named Elp4, Elp5, and Elp6. TAP tapping and subsequent purification of any one of the six subunits result in the isolation of all six components. Purification of Elongator in higher salt concentrations served to demonstrate that the complex could be separated into two subcomplexes: one consisted of Elp1, -2, and -3, and the other consisted of Elp4, -5, and -6. Deletions of the individual genes encoding the new Elongator subunits showed that only the ELP5 gene is essential for growth. Disruption of the two nonessential new Elongator-encoding genes, ELP4 and ELP6, caused the same phenotypes observed with knockouts of the original Elongator-encoding genes. Results of microarray analyses demonstrated that the gene expression profiles of strains containing deletions of genes encoding subunits of either Elongator subcomplex, in which we detected significantly altered mRNA expression levels for 96 genes, are very similar, implying that all the Elongator subunits likely function together to regulate a group of S. cerevisiae genes in vivo.
The transition from transcriptional initiation to elongation is associated with a change in the factors that are associated with RNA polymerase II (RNAPII). Whereas general transcription factors are required for promoter-directed transcription initiation and the multisubunit Srb/mediator complex is associated with the RNAPII holoenzyme that binds to promoters in the yeast Saccharomyces cerevisiae, RNAPII can elongate the transcript unaided. Nevertheless, several factors have been discovered that stimulate elongation on nonchromatin, naked DNA templates (e.g., TFIIS, elongin, ELLs, and TFIIF) (3, 20, 23, 24, 30, 33). Recently, other factors have been identified that are thought to affect RNAPII transcript elongation via effects on chromatin. These factors include the S. cerevisiae factors Spt4 and Spt5 (corresponding to human DSIF) (31), Spt6 (10), Spt16/Pob3 (corresponding to human FACT) (18), and Elongator (19).
Elongator was originally found stoichiometrically associated with the elongating form of RNA polymerase II, and it was shown to preferentially bind to the hyperphosphorylated form of RNAPII in vitro (19). As a result, it has been hypothesized that Elongator replaces mediator during the switch from transcription initiation to elongation. It was originally reported that Elongator could be extracted and purified from chromatin and is comprised of three subunits of 150, 90, and 60 kDa, the products of ELP1/IKI3, ELP2, and ELP3 genes, none of which is essential for S. cerevisiae growth (7, 19, 34). Elp1/Iki3 appears to have no significant homology to proteins of known function but may be the S. cerevisiae homolog of human IKAP (5). The IKI1 and IKI3 genes were originally identified in a genetic screen for resistance to the Kluyveromyces lactis toxin (35). Elp2 has eight WD-40 repeats, which are thought to mediate protein-protein interactions (7, 27). The 60-kDa subunit of Elongator, Elp3, is a highly conserved histone acetyltransferase (HAT) and is capable of acetylating histones in vitro (34). Whereas nucleosome arrays have been shown to inhibit transcription elongation (11, 32), histone acetylation can partly overcome this inhibition (29). It has, therefore, been suggested that the HAT activity of Elp3 may assist RNAPII during transcription elongation on a chromatin template.
Here we describe the purification of Elongator using the tandem affinity purification (TAP) procedure (21). Each of the three previously characterized subunits of Elongator was TAP tagged and purified. In each case, the tagged subunits copurified with three additional uncharacterized polypeptides, which we have named Elp4, Elp5, and Elp6. Furthermore, tagging of the three new subunits also resulted in the isolation of the entire six-subunit complex. Purification of the complexes at higher salt concentrations served to demonstrate that Elongator could be separated into two subcomplexes. One subcomplex consisted of Elp1, Elp2, and Elp3. The other subcomplex consisted of the three new polypeptides, one of which, Elp5, was found to be the product of an essential gene. Deletion of the two nonessential new genes ELP4 and ELP6 caused the same set of phenotypes attributed to knockouts of the three original subunits. Also, microarray analyses were used to demonstrate that the gene expression profiles of strains containing deletions of individual genes encoding subunits of either subcomplex are almost identical, suggesting that all six proteins are likely to function together in vivo.
MATERIALS AND METHODS
Protein purification and identification.
Tagged Elongator complexes were purified essentially as described previously (21) on immunoglobulin G (IgG) and calmodulin columns from extracts of S. cerevisiae cells grown in YPD (yeast extract-peptone-dextrose) medium to an optical density at 600 nm (OD600) of 1.0 to 1.5. The cell pellets were frozen in liquid nitrogen and lysed with dry ice in a Krups coffee grinder (model 203-70). An equal volume of yeast extraction buffer (250 mM KCl, 100 mM HEPES-KOH, 1 mM EDTA, 2.5 mM dithiothreitol) was added, and following centrifugation, the extract was dialyzed with either IPP125 or IPP200 buffer (IPP buffer consists of 10 mM Tris-Cl [pH 7.9], 0.1% Triton X-100; the number indicates the millimolar NaCl concentration). The remainder of the purification was done as described previously (21). The purified complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were visualized by silver staining. The protein bands were reduced, alkylated, and subjected to in-gel tryptic digestion, and the peptides were then purified and identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry with a PerSeptive DE STR instrument (16).
S. cerevisiae strain construction.
All of the strains used in this study (Table 1) are congenic with W303-1A (28). Deletions were constructed by PCR-mediated gene targeting with the KanMX marker (2) and confirmed by PCR after selection on YPD medium containing Geneticin at a concentration of 150 μg/ml. Tags were fused to genes with a similar PCR-based one-step in vivo tagging strategy using the TAP tag vector pBS1479 (21). Transformed cells (9) were plated onto synthetic medium lacking tryptophan. Integration of the tag-encoding DNA was confirmed by PCR and then Western blotting with rabbit IgG to preferentially recognize the protein A component of the TAP tag. Strains harboring the correct insertions were sporulated, and tetrads were dissected on YPD agar and replica plated to appropriate selective media. Sensitivity to 6-AU was tested by plating strains harboring pRS316 (26) onto plates lacking uracil and containing 25 μg of 6-AU per ml.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| W303-1A | MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 can1-100 | 26 |
| YCK245 | As W303-1A but trp1Δ | C. Koth, unpublished data |
| YNJK1 | As W303-1A but trp1Δ elp1-TAP::TRP1 | This study |
| YNJK2 | As W303-1A but trp1Δ elp3-TAP::TRP1 | This study |
| YNJK36 | As W303-1A but elp1Δ::Km | This study |
| YNJK37 | As W303-1A but elp2Δ::Km | This study |
| YNJK39 | As W303-1A but elp4Δ::Km | This study |
| YNJK40 | As W303-1A but elp6Δ::Km | This study |
| YNJK44 | As W303-1A but trp1Δ elp2-TAP::TRP1 | This study |
| YNJK45 | As W303-1A but trp1Δ elp4-TAP::TRP1 | This study |
| YNJK46 | As W303-1A but trp1Δ elp5-TAP::TRP1 | This study |
| YNJK127 | As W303-1A but elp2Δ::Km ppr2Δ::URA3 | This study |
| YNJK128 | As W303-1A but elp4Δ::Km ppr2Δ::URA3 | This study |
| YNJK129 | As W303-1A but elp6Δ::Km ppr2Δ::URA3 | This study |
| YF2223 | W303-1B ppr2::hisG-URA3-hisG | D. B. Jansma, unpublished data |
RNA isolation.
S. cerevisiae cells were grown in YPD medium at 30°C and harvested at OD600 of 0.4 to 0.6. Cells were washed once with water and resuspended in LETS buffer (100 mM LiCl, 10 mM EDTA, 10 mM Tris-HCl [pH 7.4], 0.2% SDS). After the addition of 0.4 volume of acid-washed glass beads (diameter, 425 to 600 μm; Sigma) and an equal volume of LETS buffer-equilibrated phenol, efficient lysis of the cells was obtained through vortexing. The supernatant was extracted three times with LETS buffer-equilibrated phenol-chloroform, and the RNA was ethanol precipitated and quantified by measuring the OD260. For each DNA microarray, 75 μg of total RNA was reverse transcribed using 400 U of SuperScript II (Gibco, Life Technologies). The reverse transcription (RT) step was primed using an AncT primer (T20VN; Gibco, Life Technologies) and was performed with dATP, dGTP, dTTP (Pharmacia) (final concentration, 168 μM), dCTP (Pharmacia) (final concentration, 50 μM), and Cy3-dCTP or Cy5-dCTP (Amersham) (final concentration, 50 μM). The reaction mixture was heated at 65°C for 5 min and then at 42°C for 5 min, and following the addition of the enzyme, the mixture was incubated at 42°C for an additional 3 h. RT was stopped by the addition of EDTA (final concentration, 6.25 mM), and the RNA was hydrolyzed by adding 10 N NaOH to a final concentration of 0.5 N and then incubating the mixture at 65°C for 30 min. The reaction mixture was neutralized by adding 5 M acetic acid to a final concentration of 0.5 M, and the cDNA was precipitated with isopropanol. The labeled cDNA was resuspended in 5 μl of diethyl pyrocarbonate-treated water.
Hybridization.
For each DNA microarray, 5 μl of Cy5-labeled cDNA and 5 μl of Cy3-labeled cDNA were added to 60 μl of DIG Easy hybridization buffer (Boehringer Mannheim). Following the addition of 2 μl of yeast tRNA (Sigma) (10 mg/ml) and 2 μl of single-stranded salmon sperm DNA (Sigma) (10 mg/ml), the mixture was heated at 65°C for 2 min. The solution was applied to a custom-made yeast whole-genome microarray (Microarray Facility, Ontario Cancer Institute) and incubated in a hybridization chamber at 37°C for 10 to 12 h. The slides were washed three times (15 min each time at 50°C) in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS and rinsed three times (5 min each time at room temperature) in 1× SSC. After the slides were dried by centrifugation, the arrays were read on a laser scanner (GenePix 4000A Integrated Microarray Scanner; Axon Instruments, Inc.), and the images generated were analyzed with Quantarray Data Handler 3.0 (GSI Lumonics) and Array File Maker 4.0 (AFM) (4).
RESULTS
Purification of a Holo-Elongator complex.
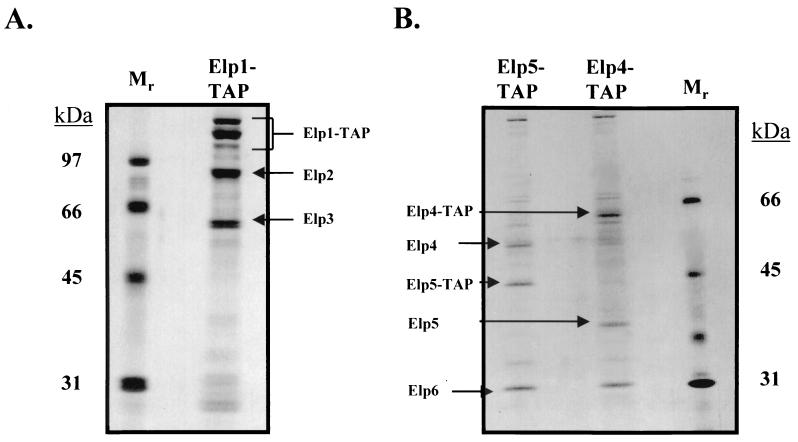
In order to characterize Elongator as it exists in vivo, we used single-step transformation with PCR products and a TRP1 selective marker to place TAP tags containing a calmodulin-binding peptide and Staphylococcus aureus protein A, separated by a tobacco etch virus protease cleavage site, at the carboxy termini of the Elp1, Elp2, and Elp3 subunits of Elongator (21). Subsequent purification on IgG and calmodulin columns of the tagged Elp proteins from an extract prepared from each of the tagged strains then resulted in the purification of a six-protein Holo-Elongator complex (Fig. 1A). The additional proteins that copurified with Elongator had apparent molecular masses of 51, 38, and 31 kDa. Although staining with silver, as shown in Fig. 1, is not quantitative, we have also stained SDS-polyacrylamide gels containing purified Elongator with Coomassie blue (data not shown) and have concluded that there are approximately equimolar amounts of the six Holo-Elongator subunits. Three electrophoretically distinct forms of Elp1 were consistently observed, and it remains to be seen if they are due to degradation or posttranslational modification.
FIG. 1.
Isolation of the six-protein Holo-Elongator complex. (A) TAP of Elongator from strains containing either no tagged protein or TAP-tagged versions of Elp1, Elp2, and Elp3 in buffers containing 125 mM NaCl was performed. The purified preparations were then analyzed by SDS-PAGE and silver staining. In each case, the tagged proteins copurified stoichiometrically with five additional proteins, including three uncharacterized polypeptides with apparent molecular masses of 50, 38, and 31 kDa. We have named the new subunits Elp4, Elp5, and Elp6. Coomassie blue staining also revealed that all six proteins were seemingly stoichiometric (data not shown). The three different electrophoretic forms of Elp1 may be a consequence of degradation or posttranslational modifications. Each purified polypeptide was identified by trypsin digestion and MALDI-TOF mass spectrometry. (B) Purification of Holo-Elongator using TAP-tagged Elp4 and Elp5. The presence of the additional subunits in Elongator was confirmed by TAP tapping the fourth and fifth largest components of the complex. Following purification in buffers containing 125 mM NaCl, all six subunits were isolated in each case.
Peptide mass fingerprinting using MALDI-TOF mass spectrometry was used to identify the additional subunits of Elongator as the products of the S. cerevisiae genes YPL101w, YHR187w/IKI1, and YMR312w, which we have named ELP4, ELP5, and ELP6, respectively. TAP tapping and purification of the newly discovered Elongator subunits resulted in the isolation of the identical six-protein Holo-Elongator complex, confirming that all six subunits are bona fide components of this elongation factor (Fig. 1B and data not shown). Consistent with this conclusion, the new subunit Yhr187w/Iki1 and the original subunit Elp1/Iki3 were originally identified in a genetic screen for resistance to the Kluyveromyces lactis toxin (35). Of the newly identified subunits, only Elp4 seems to have closely related homologues in Schizosaccharomyces pombe, Drosophila, and humans. Interestingly, Elp4 and Elp5 have also been identified in another HAT complex, NuA3 (14).
Elongator was originally isolated from the chromatin fraction of an S. cerevisiae cell extract and found to be stoichiometrically associated with the elongating form of RNAPII (19). Our purification of Elongator from the soluble portion of an S. cerevisiae cell extract using the TAP-tagged strains did not lead to the copurification of RNAPII. Furthermore, utilizing a strain harboring a TAP-tagged version of the third largest subunit of RNAPII, Rbp3, to purify RNAPII from a soluble fraction did not result in the copurification of Elongator (data not shown).
Purification of Elongator subcomplexes.
The Elongator purifications shown in Fig. 1 were performed using buffers containing 125 mM NaCl. Using the strain containing the TAP-tagged version of Elp1 in a purification with a higher concentration of salt (200 mM NaCl) in the buffer, the original three-subunit Elongator complex, consisting of Elp1, Elp2, and Elp3, was isolated (Fig. 2A). Similarly, when purification was performed on extracts from strains harboring tagged versions of the fourth and fifth largest subunits, a second subcomplex, comprising Elp4, Elp5, and Elp6, was purified (Fig. 2B). These results imply that Elongator consists of two three-subunit subcomplexes which can be separated by higher concentrations of salt.
FIG. 2.
Separation of Elongator into two subcomplexes. Elongator was purified in buffers containing 200 mM NaCl using strains containing either no tag or TAP-tagged versions of Elp1 (A) and Elp4 or Elp5 (B). These purifications served to demonstrate that Holo-Elongator could be separated into two subcomplexes, one containing Elp1, Elp2, and Elp3 and one containing Elp4, Elp5, and Elp6. Each purified polypeptide was identified by trypsin digestion and MALDI-TOF mass spectrometry after SDS-PAGE and silver staining. Mr lanes, molecular mass standards.
We have constructed some haploid strains in which one gene encoding a nonessential Elongator subunit is deleted and a different subunit is TAP tagged. Purification of the tagged subunit was then done in an effort to understand the assembly of Elongator. Using an elp2Δ strain with a TAP tag on the Elp1 subunit, it was found that a roughly stoichiometric amount of Elp3 copurified with Elp1, but substoichiometric amounts of Elp4, Elp5, and Elp6 were also isolated (data not shown). This suggests that Elp2 has a role in binding of the smaller subcomplex to Elp1 but is not necessary for effective association of the HAT activity-associated component, Elp3, to the largest subunit of Elongator. Similar strains have been constructed in order to understand further the assembly of the complex (N. J. Krogan and J. F. Greenblatt, unpublished results).
Phenotypes of elp4Δ, elp5Δ, and elp6Δ strains.
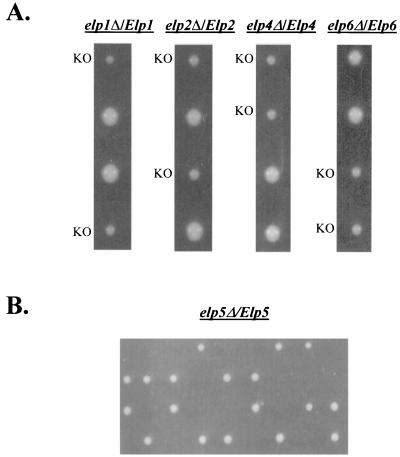
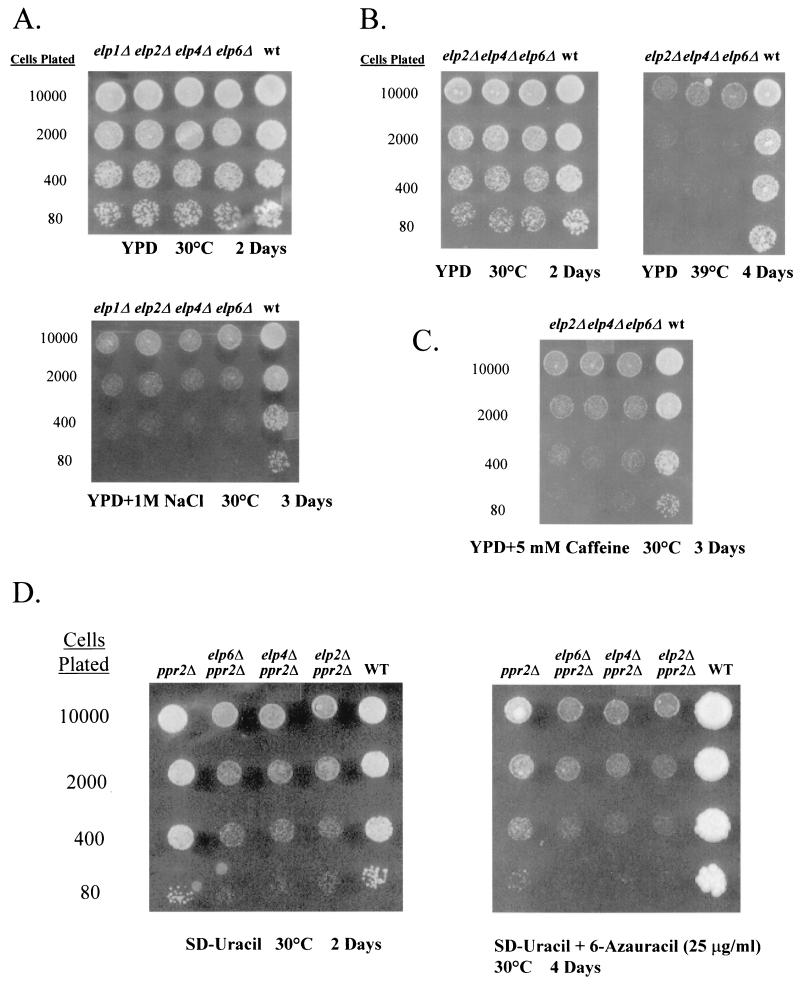
To investigate the function of the three additional subunits of Elongator, each of the corresponding open reading frames (ORFs) was deleted, along with ELP1 and ELP2, in a W303 diploid strain, and the resulting strains were induced to sporulate. Following tetrad dissection, it was discovered that the fifth largest subunit of Elongator was essential for the growth of S. cerevisiae (Fig. 3B). A recent study reported that ELP5/IKI was nonessential (8); however, the differences seen here could be due to strain and/or marker variation. The ORFs corresponding to ELP4 and ELP6 were found to be nonessential, but spores harboring elp4Δ and elp6Δ mutations displayed the slow-start phenotype originally described by Otero et al. (19) for strains with deletions of ELP1, ELP2, or ELP3 (Fig. 3A). Similarly, elp4Δ and elp6Δ strains are slow to adapt to a change in carbon source from glucose to galactose (data not shown), as previously described for elp1Δ, elp2Δ, and elp3Δ strains (19). Also, deletion of the two new nonessential Elongator subunits resulted in sensitivity to salt, caffeine, and temperature, all phenotypes that are also associated with deletions of the three largest subunits of the complex (Fig. 4A to C).
FIG. 3.
Growth of elp deletion strains. (A) Slow-start phenotypes of elp deletion strains. elp4Δ and elp6Δ display the same slow start as elp1Δ and elp2Δ strains. Tetrad dissections of elp1Δ/ELP1, elp2Δ/ELP2, elp4Δ/ELP4, and elp6Δ/ELP6 diploids following sporulation are shown after 3 days of incubation on YPD medium at 30°C. Haploid progeny in which an ELP gene has been deleted (knockout [KO]) are indicated. Smaller colony size is evidence of the slow start (19). (B) The ELP5 gene is essential for growth. Dissection of elp5Δ/ELP5 tetrads revealed 2:2 segregation of viable and inviable spores, indicating that the fifth largest subunit of Elongator is essential for cell growth.
FIG. 4.
Phenotypic characterization of elp deletion strains. (A) Salt-sensitive phenotype of elp deletion strains. Cells containing the indicated elp deletions were plated in a dilution series onto YPD plates either lacking or containing 1 M NaCl as indicated and were grown for 3 days at 30°C. (B) Temperature sensitivity of elp deletion strains. Wild-type (wt) cells and strains containing deletions of ELP2, ELP4, or ELP6 were plated in a dilution series and grown on YPD medium at 30°C for 2 days or at 39°C for 4 days. (C) Caffeine sensitivity of elp deletion strains. Wild-type cells and the elp2Δ, elp4Δ, and elp6Δ strains were plated in a dilution series on YPD medium containing 5 mM caffeine and were grown for 3 days at 30°C. (D) Genetic interaction of ELP genes with PPR2. Strains containing the double deletions elp2Δ ppr2Δ, elp4Δ ppr2Δ, or elp6Δ ppr2Δ were plated on synthetic defined medium without uracil (SD-uracil) with or without the drug 6-AU (25 μg/ml) and were grown at 30°C for 4 days. WT, wild type.
In contrast to previous observations (7, 19, 34), replacement of ELP1, ELP2, ELP4, or ELP6 with a kanamycin resistance marker in our strain background did not result in any detectable sensitivity to 6-AU (data not shown). The drug 6-AU results in the reduction of UTP and GTP concentrations in S. cerevisiae cells, leads to the induction of the PUR5 gene (22), and has been shown to affect the elongation efficiency of RNAPII (6). Therefore, sensitivity to this compound has been used as an indicator for involvement in transcriptional elongation. Studies using 6-AU have helped to show that genes like PPR2/TFIIS encode factors having a role in transcriptional elongation (1). When a ppr2Δ mutation, which itself causes sensitivity to 6-AU, was combined with a deletion of ELP2, ELP4, or ELP6, the resulting strains grew more slowly on media containing 25 μg of 6-AU per ml than a ppr2Δ strain alone. However, strains in which two of these genes were deleted also grew more slowly than a ppr2Δ strain on media lacking 6-AU (Fig. 4D).
Microarray analyses.
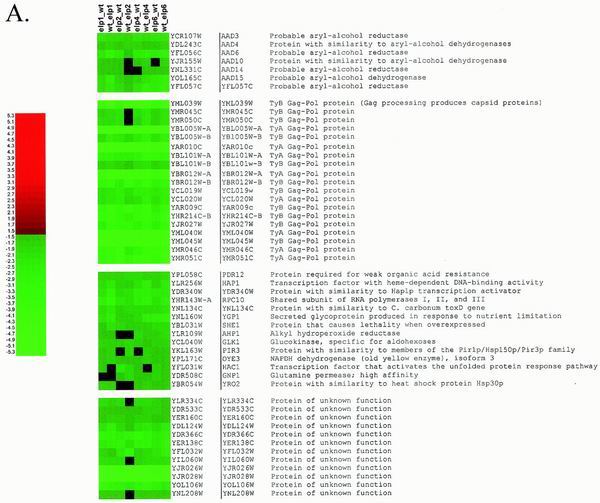
To examine whether Elongator is important for gene expression in vivo and further compare the roles of the original and new subunits of Elongator, microarray analyses were performed on strains with deletions of the Elongator genes ELP1, ELP2, ELP4, and ELP6. Fluorescently labeled cDNAs were generated from total mRNA derived from Elongator deletion and wild-type strains, mixed together, and hybridized to DNA microarrays representing the S. cerevisiae genome. Differential effects on the transcript levels measured for individual genes were normalized so that the total fluorescent signals were equal for the wild-type and mutant mRNAs. A subset of 52 genes had expression levels reduced at least 1.5-fold when Elongator deletion mutants were compared to the wild type (Fig. 5 and 6A) (complete data are available upon request and will be displayed on a laboratory website currently under construction). More importantly, the effects of the elp4Δ and elp6Δ mutations correlated almost perfectly with the effects of elp1Δ (Fig. 5 and 6A) and elp2Δ mutations (Fig. 6A and data not shown), strongly indicating that the two subcomplexes of Elongator have similar effects on gene expression. It is not evident what is in common among the genes that are down-regulated in Elongator deletion mutants. They are longer than average, but this may be because many are Ty elements with long transcripts.
FIG. 5.
Effects of elp4 (A) and elp6 (B) deletions on gene expression are strongly correlated with the effects of an elp1 deletion. The mRNA level for each gene in an elp deletion strain was calculated, after normalization, relative to that of the same gene in a wild-type (wt) strain. Using scatter plots in which each point represents one gene, these data were then used to graphically compare the effect of an elp1 deletion with the effects of elp4 and elp6 deletions.
FIG. 6.
List of genes whose expression is most affected on our arrays by the deletion of Elongator-encoding genes. (A) Genes whose mRNA levels are down-regulated at least 1.5-fold in at least three of four Elongator deletion strains. (B) Genes whose mRNA levels are up-regulated at least 1.5-fold in at least three of four Elongator deletion strains. In each case (e.g., elp1Δ_wt), the first name listed corresponds to cDNA labeled with Cy5-dCTP and the second represents cDNA that has been labeled with Cy3-dCTP. Equal amounts of the two types of cDNA were mixed and hybridized to the same array. After normalization, the results are always expressed as the amount of elpΔ relative to that for the wild type (wt). A black box indicates that the effect of the elp deletion on gene expression is less than 1.5-fold. C. carbonum, Cochliobolus carbonum; l-aspartate 4-P-transferase, l-aspartate 4-phosphate-transferase.
A subset of 44 genes had mRNA levels that were increased at least 1.5-fold when an Elongator gene was deleted (Fig. 5 and 6B). Many of these induced genes are involved in amino acid metabolism, and a similar subset of genes has previously been shown to be induced when S. cerevisiae cells were grown in the presence of carcinogenic alkylating agents, oxidizing agents, and ionizing radiation (12). Also, deletion of the Gcn4 transcriptional activator results in a similar induction of genes involved in amino acid metabolism (17). Therefore, it is likely that the up-regulation that occurs for a number of genes when an Elongator gene is deleted is an indirect consequence of the reduced expression of one or more genes that also occurs when Elongator is missing.
DISCUSSION
We have used the TAP procedure to purify a new form of Elongator from the soluble fraction of an S. cerevisiae cell extract. The Elongator protein complex was originally found associated with the elongating form of RNAPII. This new form of Elongator has three new subunits (50, 38, and 31 kDa), which we have named Elp4, Elp5, and Elp6, respectively. Interestingly, Elongator in the soluble fraction did not copurify with RNAPII. Similarly, when we purify RNAPII from the soluble fraction using a strain containing a TAP-tagged subunit of the enzyme, we do not find Elongator associated with RNAPII. Using the monoclonal antibody 8WG16 directed against Rbp1 (8WG16), Western blotting analyses revealed no detectable RNAPII when Elongator was purified from strains with TAP-tagged Elp1 or Elp5, even when approximately 1 μg of Elongator was present (data not shown). However, when strains harboring TAP-tagged versions of subunits of other known transcriptional elongation factors (TFIIF, TFIIS, and Spt5) were purified, RNAPII was shown by Western blotting and MALDI-TOF analysis to copurify with these proteins (Krogan and Greenblatt, unpublished). These results suggest that Elongator may bind only to actively transcribing, chromatin-bound RNAPII and that our purification procedure does not successfully extract transcribing RNAPII.
The issue of whether all six subunits of Elongator are bound to RNAPII during chain elongation in vivo remains to be addressed. The similar phenotypes that result when ELP1, ELP2, ELP4, or ELP6 is deleted, as well as the virtually identical transcription patterns in these strains, imply that the old and new subunits of Elongator are equally involved in its function. One possibility is that Elp4, -5, and -6 are associated with RNAPII during elongation. Alternatively, these three new subunits may be involved only in the assembly of the larger subcomplex onto RNAPII during the transition from transcription initiation to elongation.
Since the initial isolation of Elongator (19) involved purification steps in which the salt concentration was high, it is conceivable that the new subunits we have identified were lost during the original purification procedure. We have provided evidence that this may, indeed, be the case. Purification of tagged Elongator in buffer containing 200 mM NaCl, rather than 125 mM NaCl, results in the isolation of two distinct subcomplexes, one containing Elp1, Elp2, and Elp3 and the other consisting of Elp4, Elp5, and Elp6. Conventional purifications usually require high salt concentrations to reduce nonspecific protein binding to the column matrix, but high salt concentrations could disrupt weaker interactions that exist in vivo. The TAP method we have used here (21) resulted in a low background even when we used lower salt concentrations that may be closer to the conditions that exist in the cell. Based on these and other experiments (Krogan and Greenblatt, unpublished), we believe that complexes containing proteins that are weakly associated in vivo have a greater chance of remaining associated when the purification is carried out using near-physiological ionic strengths.
Using a haploid strain harboring elp2Δ and a TAP-tagged version of Elp1, Elongator was purified, and equal yields of Elp1 and Elp3 were obtained (data not shown). However, substoichiometric amounts of the three smallest subunits were also present, indicating a role for Elp2 in the association of Elp4, Elp5, and Elp6 with Elp1. Since Elp2 has eight WD-40 repeats and WD-40 repeats are thought to have a role in protein-protein interactions, it seems reasonable that Elp2 could have a major role in the assembly of the two subcomplexes. However, the loss of Elp2 has no effect on the binding of Elp3 HAT to Elp1.
Deletion of each of the genes encoding the three new subunits of Elongator showed that the only essential component of the complex is Elp5. This result implies either that Elp5 is the only subunit essential for the activity of Elongator or that Elp5 has an additional function. Recently, Elp4 and Elp5 were found to be associated with another HAT, Sas3, in the chromatin-remodelling complex, NuA3 (14). Since Elp4 and Elp5 are found in two distinct HAT complexes, they could have a role in regulating the acetyltransferase activities of Elp3 and Sas3. Since our purification of TAP-tagged versions of Elp4 and Elp5 led only to the isolation of Elongator and not NuA3, the NuA3 complex may be much less abundant than Elongator or may not exist in the soluble fraction of an S. cerevisiae cell extract. Still another possibility is that tags on Elp4 and Elp5 both interfere with the assembly of NuA3, but not Elongator. Recently, Elp5 (YHR187w) and Kti12 were shown to coimmunoprecipitate with the three largest subunits of Elongator (8). The genes that encode both these proteins were originally discovered in a screen to isolate zymocin-resistant mutants from S. cerevisiae. While we find that Yhr187w/Elp5 is a component of Elongator, TAP tapping and purification of Kti12 resulted in the isolation of a different and seemingly unrelated protein complex (data not shown). Perhaps different purification conditions are needed to successfully detect these interactions.
Strains containing elp4 and elp6 deletions have phenotypes previously attributed to the other Elongator deletions, including a slow-start phenotype, sensitivity to salt and caffeine, and temperature sensitivity at 39°C. Strains with Elongator subunit deletions were previously also found to be sensitive to 6-AU, which usually implies a role in transcriptional elongation. However, all strains with Elongator deletions that we tested were not significantly sensitive to 6-AU. Perhaps sensitivity to this pyrimidine analogue is dependent on the strain and/or marker. We did find that deletion of the PPR2 gene, which encodes the elongation factor TFIIS, causes sensitivity to 6-AU in our strain background. Moreover, ppr2Δ combined with elp2Δ, elp4Δ, or elp6Δ does result in the strain being more sensitive to 6-AU than is a ppr2Δ strain alone. However, strains containing these double deletions also grow more slowly on synthetic complete media lacking uracil than does a ppr2Δ strain, suggesting that Elongator mutations do not really cause enhanced 6-AU sensitivity in our strain background. Recently, an elp1 deletion has been shown to be synthetically lethal with a deletion of the gene encoding the protein kinase Ctk1 (15). However, elp gene deletions have also been found to genetically interact with the seemingly unrelated cell polarity gene bni1 as well as with other genes involved in cytoskeleton synthesis and DNA damage repair (A. Tong and C. Boone, personal communication). It seems that many mutations not necessarily compromising transcription may weaken the already compromised elpΔ strains. This suggests that the genetic interaction between the subunits of Elongator and PPR2 should not necessarily be viewed as conclusive evidence that the Elongator complex is involved in transcriptional elongation.
The activity of Elongator purified as described here was tested using a tailed-template transcription assay (25) containing RNAPII and naked DNA as a template (N. J. Krogan, J. F. Greenblatt, and A. Shilatifard, unpublished data). The RNAPII used in the assay was isolated with an Rbp3 TAP-tagged strain, which proved to be an excellent source of transcriptionally active, partially phosphorylated RNAPII. However, the rate of transcription did not seem to increase in the presence of Elongator. S. cerevisiae TFIIF was added as a positive control, and transcriptional elongation was considerably stimulated in this case. Since the Elp3 subunit of Elongator possesses HAT activity (34), it is possible that stimulation of transcription by Elongator would be detected only on a chromatin template.
Microarray analyses demonstrated that the gene expression profiles generated by strains harboring deletions of genes encoding subunits of the initial and the newly identified subcomplexes of Elongator were very similar (Fig. 5 and 6). This suggests that the two subcomplexes function together in vivo. In particular, when the gene expression profiles of elp4Δ and elp6Δ strains were compared with those of elp1Δ and elp2Δ strains in a graph, they were shown to be almost identical (Fig. 5 and data not shown). About 52 genes having no clear relationship consistently had reduced mRNA levels when an Elongator-encoding gene was deleted. Conversely, 44 genes were up-regulated when an Elongator-encoding gene was deleted, and many of these are involved in amino acid metabolism. A similar subset of genes has recently been reported to be coinduced under unfavorable conditions, such as growth in the presence of the agent alkylating methyl methanesulfonate (13) or deletion of the gene encoding the Gcn4 transcriptional activator (17). Perhaps because Elongator is important for the expression of certain genes, Elongator gene deletions seem to cause similar problems for the cell, resulting in the up-regulation of this particular group of stress-induced genes.
In summary, we have used the TAP system (21) to isolate a novel six-subunit Holo-Elongator complex. High salt concentrations induce Holo-Elongator to split into two subcomplexes, one containing the previously characterized subunits and the other comprised of three novel polypeptides. The results of genetic and microarray analyses have shown that the new nonessential subunits of Elongator have roles in vivo that are similar to those of the original subunits. Further analysis will be required to uncover functional differences between the three-subunit Elongator and the six-protein Holo-Elongator complexes.
ACKNOWLEDGMENTS
We thank M. S. Kobor and D. B. Jansma for critical reading of the manuscript and G. Zhong for excellent technical assistance. We also thank B. J. Breitkreutz and P. Jorgensen for help with the microarray analyses.
This research was supported in part by grants to J.F.G. from the Medical Research Council of Canada and from the National Cancer Institute of Canada with funds from the Canadian Cancer Society. J.F.G. is an International Research Scholar of the Howard Hughes Medical Institute. N.J.K. was financially supported by a PGS-B Scholarship Award from the Natural Sciences and Engineering Research Council of Canada (NSERC).
REFERENCES
- 1.Archambault J, Lacroute F, Ruet A, Friesen J D. Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol Cell Biol. 1992;12:4142–4152. doi: 10.1128/mcb.12.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bähler J, Wu J, Longtine M S, Shah N G, McKenzie III A, Steever A B, Wach A, Philippsen P, Pringle J R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Bradsher J N, Jackson K W, Conaway R C, Conaway J W. An RNA polymerase II transcription factor SIII. Identification, purification, and properties. J Biol Chem. 1996;268:25587–25593. [PubMed] [Google Scholar]
- 4.Breitkreutz, B. J., P. Jorgensen, A. Breitkreutz, and M. Tyers. 2001. AFM 4.0: a toolbox for DNA microarray analysis. Genome Biol. 2:software0001.1–software0001.3. [Online.] http://www.genomebiology.com. [DOI] [PMC free article] [PubMed]
- 5.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:225–226. [Google Scholar]
- 6.Exinger F, Lacroute F. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr Genet. 1992;22:9–11. doi: 10.1007/BF00351735. [DOI] [PubMed] [Google Scholar]
- 7.Fellows J, Erdjument-Bromage H, Tempst P, Svejstrup J Q. The Elp2 subunit of elongator and elongating RNA polymerase II holoenzyme is a WD40 repeat protein. J Biol Chem. 2000;275:12896–12899. doi: 10.1074/jbc.275.17.12896. [DOI] [PubMed] [Google Scholar]
- 8.Frohloff F, Fichtner L, Jablonowski D, Breunig K D, Schaffrath R. Saccharomyces cerevisiae mutations confer resistance to the Kluyveromyces lactic zymocin. EMBO J. 2001;20:1993–2003. doi: 10.1093/emboj/20.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gietz R D, Schiestl R H. Transforming yeast with DNA. Methods Mol Cell Biol. 1995;5:255–269. [Google Scholar]
- 10.Hartzog G A, Wada T, Handa H, Winston F. Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev. 1998;12:357–369. doi: 10.1101/gad.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izban M G, Luse D S. Transcription on nucleosomal templates by RNA polymerase II in vitro: inhibition of elongation with enhancement of sequence-specific pausing. Genes Dev. 1991;5:683–696. doi: 10.1101/gad.5.4.683. [DOI] [PubMed] [Google Scholar]
- 12.Jelinsky S A, Estep P, Church G M, Samson L D. Regulatory networks revealed by transcriptional profiling of damaged Saccharomyces cerevisiae cells: Rpn4 links base excision repair with proteasomes. Mol Cell Biol. 2000;20:8157–8167. doi: 10.1128/mcb.20.21.8157-8167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jelinsky S A, Samson L D. Global response of Saccharomyces cerevisiae to an alkylating agent. Proc Natl Acad Sci USA. 1999;96:1486–1491. doi: 10.1073/pnas.96.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John S, Howe L, Tafrov S T, Grant P A, Sternglanz R, Workman J L. The something about silencing protein, Sas3, is the catalytic subunit of NuA3, a yTAFII30-containing HAT complex that interacts with the Spt16 subunit of the yeast CP (Cdc68/Pob3)-FACT complex. Gene Dev. 2000;14:1196–1208. [PMC free article] [PubMed] [Google Scholar]
- 15.Jona G, Wittschieben B, Svejstrup J Q, Gileadi O. Involvement of yeast carboxy-terminal domain kinase I (CTDK-I) in transcription elongation in vivo. Gene. 2001;267:31–36. doi: 10.1016/s0378-1119(01)00389-4. [DOI] [PubMed] [Google Scholar]
- 16.Mann M, Hojrup P, Roepstorff P. Use of mass spectrometry molecular weight information to identify proteins in sequence databases. Biol Mass Spectrom. 1993;22:338–345. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan K, Meyer M R, Jackson B M, Slade D, Roberts C, Hinnebusch A G, Marton M J. Transcriptional profiling shows that Gcn4 is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21:4347–4368. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orphanides G, LeRoy G, Chang C H, Luse D S, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 19.Otero G, Fellows J, Li Y, de Bizemont T, Dirac A M G, Gustafsson C M, Erdjument-Bromage H, Tempst P, Svejstrup J Q. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 20.Price D H, Sluder A E, Greenleaf A L. Dynamic interaction between a Drosophila transcription factor and RNA polymerase II. Mol Cell Biol. 1989;9:1465–1475. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Séraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotech. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 22.Shaw R J, Reines D. Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol Cell Biol. 2000;20:7427–7437. doi: 10.1128/mcb.20.20.7427-7437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shilatifard A. Identification and purification of the Holo-ELL complex. J Biol Chem. 1998;273:11212–11217. doi: 10.1074/jbc.273.18.11212. [DOI] [PubMed] [Google Scholar]
- 24.Shilatifard A, Haque D, Conaway R C, Conaway J W. Structure and function of an RNA polymerase II elongation factor ELL. Identification of two overlapping ELL functional domains that govern its interaction with polymerase and the ternary elongation complex. J Biol Chem. 1997;272:22355–22363. doi: 10.1074/jbc.272.35.22355. [DOI] [PubMed] [Google Scholar]
- 25.Shilatifard A, Lane W S, Jackson K W, Conaway R C, Conaway J W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 26.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith T F, Gaitatzes C, Saxena K, Neer E J. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 28.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 29.Tse C, Sera T, Wolfe A P, Hansen J C. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol Cell Biol. 1998;18:4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uptain S M, Kane C M, Chamberlin M J. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 31.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog G A, Winston F, Buratowski S, Handa H. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasylyk B, Chambon P. Transcription by eukaryotic RNA polymerase A and B of chromatin assembled in vitro. Eur J Biochem. 1979;98:317–327. doi: 10.1111/j.1432-1033.1979.tb13191.x. [DOI] [PubMed] [Google Scholar]
- 33.Wind M, Reines D. Transcription elongation factor SII. BioEssays. 2000;22:327–336. doi: 10.1002/(SICI)1521-1878(200004)22:4<327::AID-BIES3>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittschieben B, Otero G, de Bizemont T, Fellows J, Erdjument-Bromage H, Ohba R, Li Y, Allis C D, Tempst P, Svejstrup J Q. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 35.Yajima H, Tokunaga M, Nakayama-Murayama A, Hishinuma F. Characterization of IK11 and IK13 genes conferring pGKL killer sensitivity on Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 1997;61:704–709. doi: 10.1271/bbb.61.704. [DOI] [PubMed] [Google Scholar]