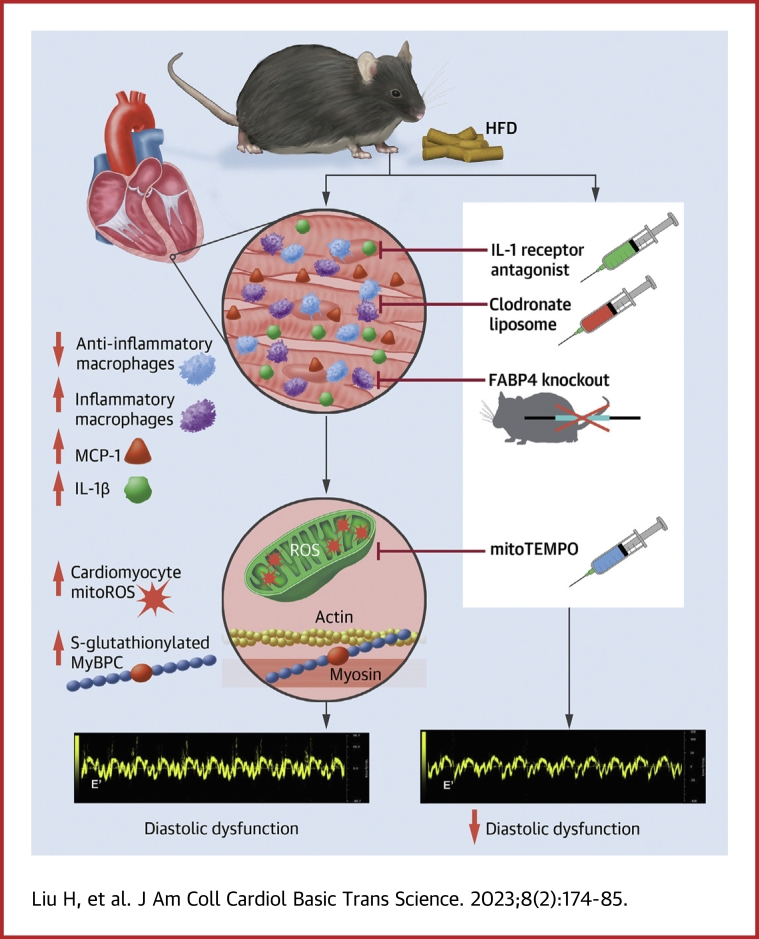
Visual Abstract
Key Words: diabetes, diastolic dysfunction, HFpEF, IL-1β, inflammation, macrophage, mitochondria
Abbreviations and Acronyms: CCR2, C-C motif chemokine receptor 2; CM, cardiomyocyte; DD, diastolic dysfunction; DM, diabetes mellitus; EF, ejection fraction; FABP4, fatty acid binding protein 4; HF, heart failure; HFD, high-fat diet; HFpEF, heart failure with preserved ejection fraction; IL, interleukin; IL1RA, interleukin 1 receptor antagonist; KO, knockout; MCP, monocyte chemoattractant protein; mitoROS, mitochondrial reactive oxygen species; MyBP-C, myosin binding protein C; TGF, transforming growth factor; TNF, tumor necrosis factor; Timd4, T cell immunoglobulin and mucin domain containing 4; WT, wild-type
Highlights
-
•
A high-fat diet caused diabetes mellitus in mice.
-
•
Inflammatory macrophages were required for development of diastolic dysfunction secondary to the high-fat diet.
-
•
Inflammatory macrophages mediated diastolic dysfunction through IL-1β and cardiomyocyte mitochondrial oxidative stress.
-
•
IL-1β receptor antagonism, mitochondrial reactive oxygen species scavenging, macrophage depletion, and macrophage phenotype modulation are potential therapeutic targets for HFpEF.
Summary
Diabetes mellitus (DM) is a main risk factor for diastolic dysfunction (DD) and heart failure with preserved ejection fraction. High-fat diet (HFD) mice presented with diabetes mellitus, DD, higher cardiac interleukin (IL)-1β levels, and proinflammatory cardiac macrophage accumulation. DD was significantly ameliorated by suppressing IL-1β signaling or depleting macrophages. Mice with macrophages unable to adopt a proinflammatory phenotype were low in cardiac IL-1β levels and were resistant to HFD-induced DD. IL-1β enhanced mitochondrial reactive oxygen species (mitoROS) in cardiomyocytes, and scavenging mitoROS improved HFD-induced DD. In conclusion, macrophage-mediated inflammation contributed to HFD-associated DD through IL-1β and mitoROS production.
Heart failure (HF) is a major and growing public health problem affecting over 6 million patients in the United States.1,2 Approximately 40% to 71% of HF cases occur in patients with a relatively normal ejection fraction (EF) and altered diastolic relaxation, known as heart failure with preserved ejection fraction (HFpEF).2 The 5-year mortality rate of HFpEF is 55% to 74%, which is similar to HF with reduced EF.2 Nevertheless, there are no pharmaceutical treatments improving diastolic dysfunction (DD) in HFpEF.
Cardiac DD is thought to be the pathological condition underlying HFpEF.2 DD is associated with a high-fat diet (HFD)/Western diet.3 HFD induces a systemic chronic low-grade inflammation by promoting inflammatory cytokine production and regulating immune cells, especially macrophages.4, 5, 6 Macrophages are a crucial component of innate immune system. In response to local environmental stimuli, macrophages can assume proinflammatory or anti-inflammatory phenotypes.7 Among the macrophage-secreted inflammatory cytokines, interleukin (IL)-1β can promote oxidative stress and reactive oxygen species production.8
In a series of publications, we have shown that cardiac oxidative stress can cause DD.9,10 In this paper, we examine the idea that activation of innate immunity mediates HFD-induced DD through IL-1β and mitochondrial reactive oxygen species (mitoROS).
Methods
Detailed methods are described in the Supplemental Appendix. Briefly, male C57BL/6J mice were fed a HFD to induce DD. Age- and sex-matched mice with a normal diet were used as control mice. C57BL/6J mice with HFD were randomized into 3 treatment groups: mitoTEMPO, IL-1 receptor antagonist (IL1RA), and clodronate liposomes to deplete macrophages. USP sterile water or plain liposomes were used as placebo. Macrophage infiltration and phenotypes were measured and characterized by flow cytometry. Cardiac diastolic function was evaluated with echocardiography and invasive hemodynamic tests. Monocyte chemoattractant protein (MCP)-1 and several macrophage-secreted cytokines, including IL-1β, IL-6, IL-10, transforming growth factor (TGF)-β, and tumor necrosis factor (TNF)-α, were measured in heart tissue by immunoblotting and enzyme-linked immunosorbent assay. Isolated cardiomyocytes (CMs) were treated with IL-1β, and the myocyte mitoROS was assessed by confocal microscope using mitoSOX stain. To confirm the role of proinflammatory macrophages in the pathogenesis of HFD-induced DD, fatty acid binding protein 4 (FABP4) knockout (KO) mice and a macrophage cell line were employed in the study. FABP4 KO macrophages were analyzed with microarray for phenotype characterization. FABP4 KO and wild-type (WT) mice were fed a HFD, and the cardiac IL-1β level and diastolic function were compared with the FABP4 WT mice on a normal diet.
Animal care and interventions were provided in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals, and all animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Statistics
Continuous data are presented as mean ± SEM and checked for normality using D’Agostino and Pearson Omnibus or Shapiro-Wilks test for small group size. For the dot plots, the lines indicate the mean values, and the error bars indicate SEM. Data were analyzed using 2-tailed Student’s t-test or 1-way analysis of variance with Bonferroni’s post hoc test for multiple pairwise comparisons. All statistical analyses were performed with GraphPad Prism software version 5.0 (GraphPad Software). A P value of <0.05 was considered statistically significant.
Results
HFD caused diabetes mellitus and HFpEF
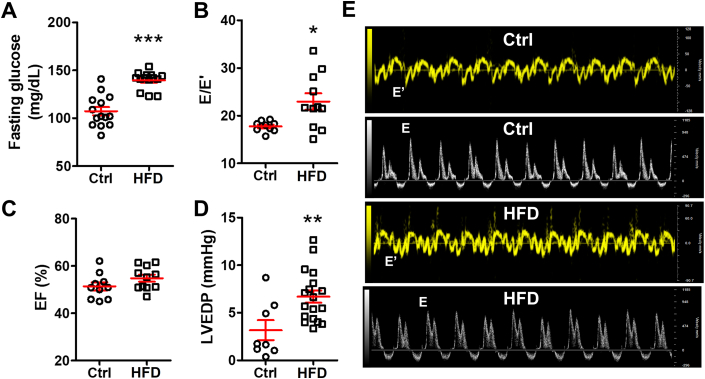
As we demonstrated previously,9 HFD induced type II diabetes mellitus (DM) and HFpEF in mice. In this study, we confirmed that HFD significantly raised the fasting glucose level (107.3 ± 4.4 mg/dL in control mice vs 139.6 ± 2.5 mg/dL in HFD mice; P = 0.0001) (Figure 1A). Further, the E/E′ ratio, an echocardiographic indicator of cardiac diastolic function, increased in the HFD mice (23.0 ± 1.7) compared with the control mice (17.8 ± 0.4; P = 0.015) (Figure 1B and 1E) despite comparable cardiac systolic EF (52.0% ± 1.8% in control mice vs 54.8% ± 1.5% in HFD mice; P = 0.14) (Figure 1C). Invasive hemodynamic study detected higher left ventricular end-diastolic pressure (2.4 ± 0.8 mm Hg in control mice vs 6.7 ± 0.6 mm Hg in HFD mice; P = 0.001) (Figure 1D). These results demonstrated that HFD caused DM and that HFD mice developed HFpEF with impaired cardiac diastolic function.
Figure 1.
HFD Causes Cardiac Diastolic Heart Failure in Mice
High-fat diet (HFD)-induced (A) hyperglycemia (n = 14 mice per group), (B) an increased ratio of transmitral Doppler early filing velocity to tissue Doppler early diastolic mitral annular velocity (E/E′) (n = 9 to 11 per group), and (D) left ventricular end-diastolic pressure (LVEDP) elevation by hemodynamic test (n = 7 to 18 mice per group) with (C) preserved ejection fraction (EF) value (n = 9 to 11 mice per group). (E) Representative echocardiographic images of tissue Doppler and pulsed wave Doppler from control (Ctrl) and HFD mice. Bars are mean ± SEM. Unpaired t-test was used. ∗P <0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs Ctrl.
IL-1β mediated HFD-induced DD
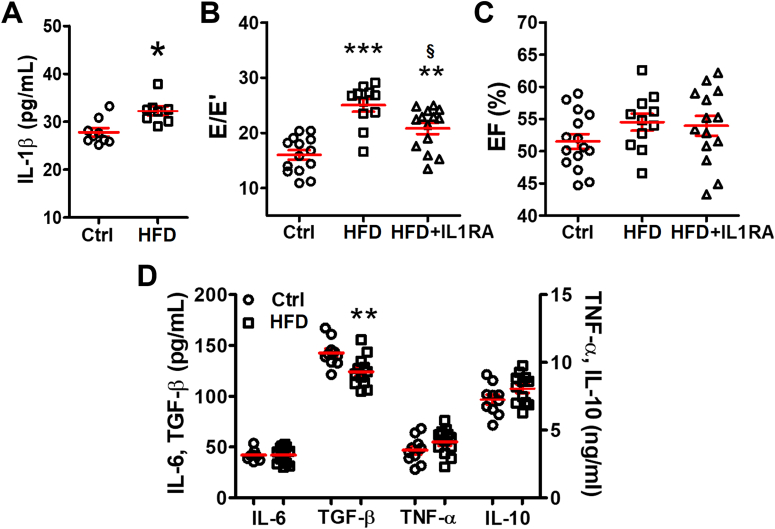
IL-1β is a key proinflammatory cytokine in the immune response to infection and injury.11 Previously, we and others have found that a high IL-1β level is associated with cardiac electrical abnormalities in HFD mice and DM mice leading to arrhythmia.8,12 In this study, we confirmed that the cardiac IL-1β level was significantly higher in HFD mice compared with the control mice (27.8 ± 0.9 pg/mL in control mice vs 32.2 ± 0.9 pg/mL in HFD mice; P = 0.033) (Figure 2A). Inhibiting IL-1β with IL1RA for 2 weeks caused substantial improvement of E/E′ (20.8 ± 1.0 in HFD+IL1RA vs 25.0 ± 1.1 in HFD mice; P = 0.047) (Figure 2B) without affecting the EF (Figure 2C). As seen previously, IL1RA did not affect insulin resistance.8
Figure 2.
IL-1β Mediates HFD-Induced Diastolic Dysfunction
(A) Cardiac interleukin (IL)-1β level tested by enzyme-linked immunosorbent assay was increased in the mice with HFD; n = 8 to 9 mice per group. (B) IL-1β antagonist improved E/E′ in HFD mice; n = 11 to 14 mice per group. (C) The EF remained unchanged after IL1RA treatment; n = 11 to 14 mice per group. (D) Other cytokines in hearts were tested by enzyme-linked immunosorbent assay; n = 10 to 12 mice per group. Bars are mean ± SEM. Unpaired t-test (A and D) or 1-way analysis of variance with Bonferroni post hoc tests (B and C) were used. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs Ctrl; §P < 0.05 vs HFD. IL1RA = interleukin 1 receptor antagonist; TGF = transforming growth factor; TNF = tumor necrosis factor; other abbreviations as in Figure 1.
Other proinflammatory cytokines, IL-6, and TNF-α, were comparable between the control and the HFD groups (Figure 2D). The anti-inflammatory cytokine, TGF-β, was significantly reduced (142.6 ± 4.2 pg/mL in control hearts vs 124.3 ± 4.3 pg/mL in HFD hearts; P = 0.007) (Figure 2D), whereas IL-10 was unchanged (Figure 2D), supporting the idea that IL-1β–mediated inflammation was involved in HFD-induced DD.
Cardiac macrophages were activated in HFD mice
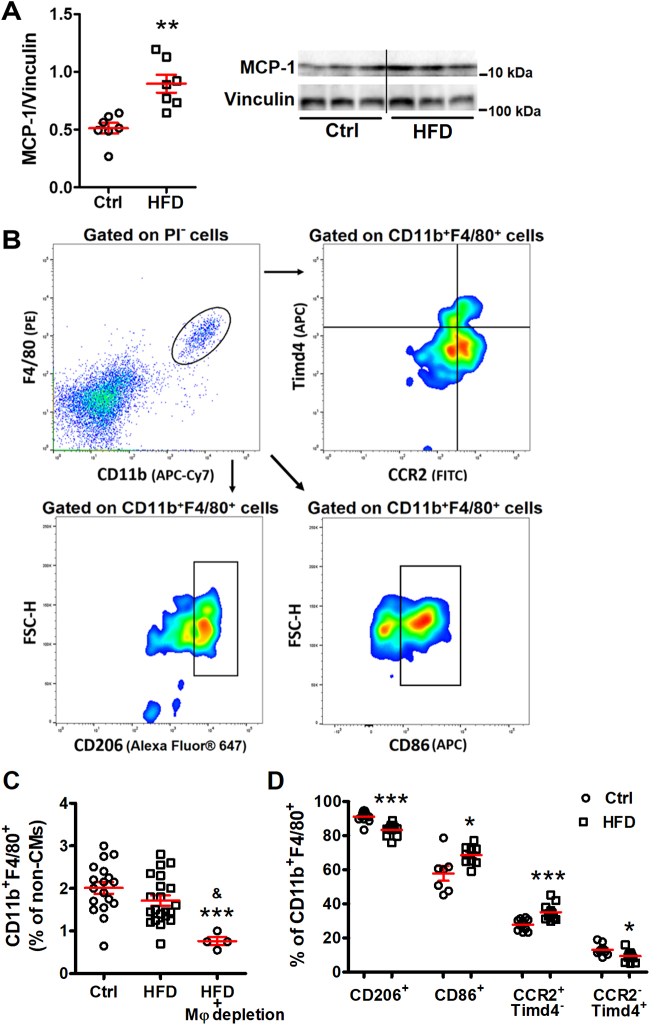
Macrophages are an important source of IL-1β.11 In response to inflammation and tissue injury, monocytes are recruited into the tissue where they become macrophages that are central to the initiation and resolution of inflammation. MCP-1 is the main chemokine that regulates monocyte recruitment.13 As shown in Figure 3A, MCP-1 was significantly up-regulated in the hearts of HFD mice (0.51 ± 0.05 in control mice vs 0.90 ± 0.08 in HFD mice; P = 0.001) (Figure 3A), indicating promoted monocyte recruitment to hearts by HFD.
Figure 3.
Cardiac Macrophages Are Activated in HFD Hearts
(A) Monocyte chemoattractant protein (MCP)-1 level was significantly higher in HFD hearts by Western blot; n = 7 mice per group. (B) Representative flow cytometry images showing the gating strategy and analysis to identify macrophages subsets. Isolated noncardiomyocyte cells were pregated on CD11b+F4/80+ as cardiac macrophages and were further divided into subsets based on their expression of CCR2, T cell immunoglobulin and mucin domain containing 4 (Timd4), CD206, or CD86. (C) Percentage of cardiac macrophages (CD11b+F4/80+) among noncardiomyocyte interstitial cells; n = 4 to 21 mice per group. (D) Percentage change of each macrophage subset; n = 8 to 12 mice per group. Bars are mean ± SEM. Unpaired t-test (A and D) or 1-way analysis of variance with Bonferroni post hoc tests (C) were used. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs Ctrl; and &P < 0.01 vs HFD. CCR2 = C-C motif chemokine receptor 2; PI = propidium iodide; other abbreviations as in Figure 1.
Macrophages have been traditionally categorized into 2 subsets, namely classically activated macrophages that secrete proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and alternatively activated macrophages that can produce anti-inflammatory cytokines such as TGF-β and IL-10.14 Nevertheless, macrophages are remarkably plastic cells that change their function in response to environmental cues. Because the origin and residence are the major determinants of cellular identity, macrophages are now categorized as either self-renewing resident macrophages or monocyte-derived macrophages.15 In the heart, Dick et al16 showed that C-C motif chemokine receptor 2 (CCR2) and T cell immunoglobulin and mucin domain containing 4 (Timd4) are durable markers of recruited and resident macrophages, respectively. Notably, Timd4+ resident macrophages have been shown to be proresolving17, 18, 19 as opposed to monocyte-derived macrophages.16 Additional markers such as the mannose receptor CD206 have been associated with an anti-inflammatory and reparative macrophage phenotype.20, 21, 22 On the other hand, the CCR2+ monocyte-derived or CD86+ macrophages appear to promote inflammation,16,21,23 although a small population of CCR2+ resident macrophages with an intermediate phenotype has been recently characterized.24
In the present study, cardiac interstitial cells (non-CMs) were isolated from mouse hearts and characterized based on their cell surface markers using flow cytometry (Figure 3B). Although the number of cardiac macrophages (CD11b+F4/80+) was not changed (2.0% ± 0.1% in control mice vs 1.7% ± 0.1% in HFD mice; P = 0.30) (Figure 3C), the percentage of proinflammatory macrophages (CD11b+F4/80+CD86+) was increased in DD hearts (57.8% ± 4.3% in control hearts vs 68.5% ± 1.9% in HFD hearts; P = 0.027) (Figure 3D), whereas anti-inflammatory macrophages (CD11b+F4/80+CD206+) decreased (91.1% ± 1.0% in control hearts vs 83.5% ± 1.0% in HFD hearts; P < 0.0001) (Figure 3D). Recruited proinflammatory macrophages (CCR2+Timd4−) increased (27.9% ± 1.0% in control hearts vs 35.1% ± 1.3% in HFD hearts; P =0.0003) (Figure 3D), whereas resident proresolving macrophages (CCR2-Timd4+) decreased (13.1% ± 1.0% in control hearts vs 9.4% ± 0.9% in HFD hearts; P = 0.011) (Figure 3D). The results indicated that cardiac macrophages assumed a proinflammatory phenotype in HFD-induced DD.
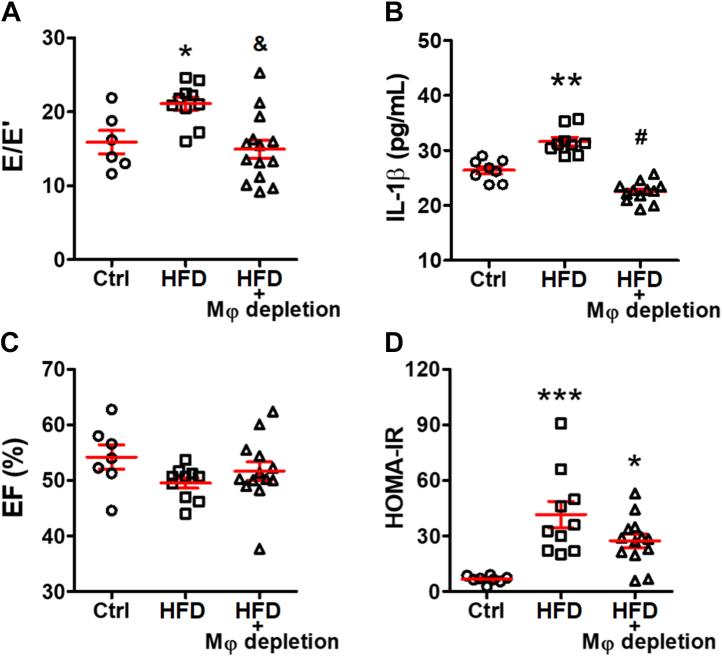
Macrophage depletion mitigated HFD-induced DD
To test whether macrophages directly promote the development of DD, macrophages were depleted by clodronate liposomes in HFD mice.25 Within 2 weeks, clodronate liposomes significantly reduced cardiac macrophages (CD11b+F4/80+) by over 50% (1.7% ± 0.1% in HFD vs 0.8% ± 0.1% in HFD+depletion; P = 0.009) (Figure 3C). Depleting macrophages reversed the E/E′ elevation (15.4 ± 1.3 in HFD+depletion vs 21.1 ± 0.9 in HFD; P = 0.005; vs 15.9 ± 1.6 in control mice; P > 0.99) (Figure 4A). Depletion significantly normalized cardiac IL-1β level (22.5 ± 0.5 pg/mL in HFD+depletion vs 31.6 ± 0.8 pg/mL in HFD; P < 0.0001; vs 26.4 ± 0.7 pg/mL in control mice; P = 0.055) (Figure 4B). The insulin resistance status and the systolic function remained comparable between the HFD groups with or without liposome treatment (Figures 4C and 4D). These data support the hypothesis that macrophages contribute to HFD-induced DD through IL-1β.
Figure 4.
Cardiac Macrophages Are Required for HFD-Induced Diastolic Dysfunction
(A) Macrophage (Mφ) depletion improved E/E′ ratio (n = 6 to 13 mice per group) and decreased (B) cardiac IL-1β level tested by enzyme linked immunosorbent assay (n = 8 to 12 mice per group) without altering (C) EF (n = 7 to 10 mice per group) or (D) homeostatic model assessment for insulin resistance (HOMA-IR), an indicator of insulin resistance (n = 8 to 13 mice per group). Bars are mean ± SEM. One-way analysis of variance with Bonferroni post hoc tests were used. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs Ctrl; and &P < 0.01; #P < 0.001 vs HFD. Abbreviations as in Figures 1 and 2.
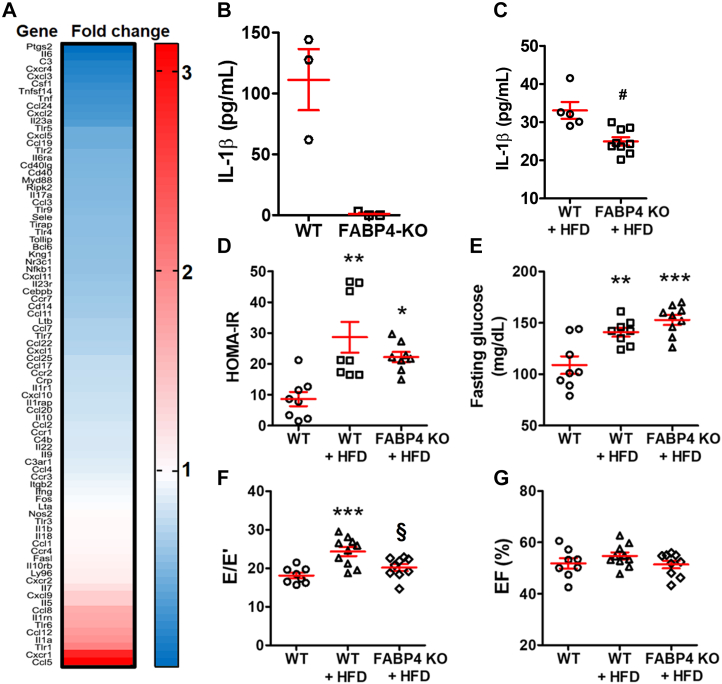
Mice without proinflammatory macrophages were resistant to HFD-induced DD
To investigate the role of proinflammatory macrophages in HFD-induced DD, we employed a mouse strain with a constitutive FABP4 KO. Under inflammatory stimulation, FABP4 KO macrophages do not express IL-1β, IL-6, and TNF-α, suggesting a reduced inflammatory capacity.26 To verify the phenotype of the FABP4 KO macrophages, a total of 84 key inflammatory genes were examined by microarray. Over 70 inflammatory genes were down-regulated in FABP4 KO macrophages compared with the WT macrophages (Figure 5A). At the protein level, FABP4 KO macrophages failed to generate IL-1β, even after lipopolysaccharide stimulation (Figure 5B). The results suggested that the FABP4 KO macrophages could not assume an inflammatory phenotype.
Figure 5.
Suppressing Proinflammatory Macrophages Prevented HFD-Induced Diastolic Dysfunction
(A) Inflammatory gene expression of fatty acid binding protein 4 (FABP4) knockout (KO) macrophage cell line by microarray. (B) FABP4 KO macrophages cannot secrete IL-1β under lipopolysaccharide stimulation; n = 3 independent experiments per group. (C) With HFD, FABP4 KO mice had lower cardiac IL-1β level; n = 5 to 9 mice per group. (D) Insulin resistance as indicated by HOMA-IR (n = 8 to 9 mice per group) and (E) hyperglycemia (n = 8 to 9 mice per group) were unchanged between wild-type (WT)+HFD and KO+HFD groups. (F) FABP4 KO mice were resistant to HFD-induced diastolic dysfunction; n = 8 to 9 mice per group. (G) EF was unaffected by FABP4 KO; n = 8 to 10 mice per group. Bars are mean ± SEM. Unpaired t-test (C) or 1-way analysis of variance with Bonferroni post hoc tests (D to G) were used. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs WT; §P < 0.05; #P < 0.01 vs WT+HFD. Abbreviations as in Figures 1, 2, and 4.
Consistent with these results, the cardiac IL-1β level was lower in FABP4 KO mice (KO+HFD, 25.0 ± 1.1 pg/mL) than WT (WT+HFD, 33.1 ± 2.2 pg/mL; P = 0.003) (Figure 5C). In FABP4 KO mice, HFD still caused DM and insulin resistance (Figures 5D and 5E), but HFD-induced DD was prevented (E/E′, 20.2 ± 0.9 in KO+HFD vs 24.4 ± 1.2 in WT+HFD; P = 0.017; vs 18.1 ± 0.7 in WT; P = 0.46) (Figure 5F). No change to cardiac systolic function was detected (Figure 5G). Combined, these results suggested that proinflammatory macrophages led to HFD-induced DD.
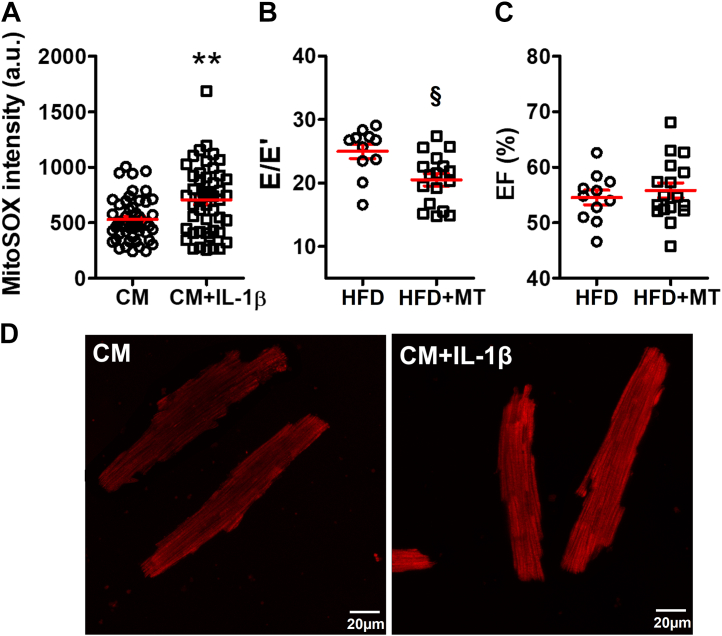
Evidence that IL-1β acted through mitoROS
Previously, we have demonstrated that IL-1β causes arrhythmia in HFD mice through modulating mitoROS generation.8 In the present study, we confirmed a shared pathogenic cascade. IL-1β escalated mitoROS levels in cultured CMs (mitoSOX, 530.7 ± 28.7 arbitrary units in CMs vs 706.8 ± 44.5 arbitrary units in CMs+IL-1β; P = 0.001) (Figure 6A and 6D). Treating HFD mice with a mitochondrial specific antioxidant, mitoTEMPO, significantly improved DD (E/E′, 20.5 ± 1.0 in HFD+MT vs 25.0 ± 1.1 in HFD; P = 0.019) (Figure 6B) in the absence of changes of the EF (Figure 6C) or insulin resistance as we have seen previously.8
Figure 6.
MitoROS Scavenging Reduced HFD-Induced Diastolic Dysfunction
(A) IL-1β incubation up-regulated the cardiomyocyte (CM) mitochondrial reactive oxygen species (mitoROS) level. The cardiomyocytes were isolated from 5 mice, and 48 cardiomyocytes were tested for each group. (B) Mitochondrial antioxidant, mitoTEMPO (MT), improved E/E′ in HFD mice without affecting (C) the EF; n = 11 to 17 mice per group. The HFD group was the same as in Figures 2B and 2C. (D) Representative confocal microscopy images showing mitoROS in cardiomyocytes by mitoSOX red staining; scale bar represents 20 μm. Bars are mean ± SEM. Unpaired t-test were used. ∗∗P < 0.01 vs CM; §P < 0.05 vs HFD. a.u. = arbitrary units; other abbreviations as in Figures 1 and 2.
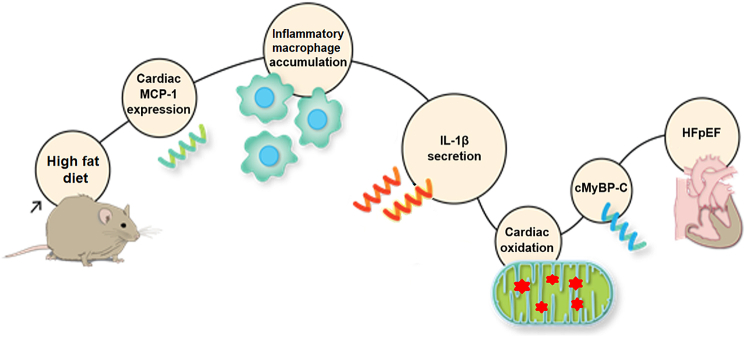
Discussion
As summarized in Figure 7, in the present study, we found that HFD-induced DD was accompanied by increased cardiac MCP-1 and IL-1β, an increase in proinflammatory and decrease in anti-inflammatory macrophages, and elevated CM mitoROS. Inhibiting IL-1β or mitoROS was sufficient to ameliorate DD, as was macrophage depletion or anti-inflammatory macrophage phenotypic modulation. Collectively, these findings indicated that HFD-induced DD was mediated by inflammatory macrophages secreting IL-1β to cause cardiomyocyte mitoROS. IL-1β receptor antagonism had beneficial effects on cardiac mitochondrial oxidative stress. Nevertheless, we cannot rule out IL-1β receptor antagonism had extracardiac mechanisms that contributed to the improvement of DD. On the other hand, we have shown that mitoROS is sufficient to cause DD associated with oxidative modification of the contractile protein, cardiac myosin binding protein C (MyBP-C), and that DD can be relieved at the CM level with a mitochondrial targeted antioxidant.9
Figure 7.
Innate Immunity Mediates High-Fat Diet–Induced HFpEF Through IL-1β Secretion and Mitochondrial Reactive Oxygen Species Modulation
cMyBP-C = cardiac myosin binding protein C; HFpEF = heart failure with preserved ejection fraction; other abbreviations as in Figures 2 and 3.
Unlike HF with reduced EF where there is increasing consensus from human and animal research that recruited monocytes or CCR2+ macrophage infiltration is enhanced causing persistent inflammation, which worsens systolic function,24,27, 28, 29, 30 the role of macrophages in HFpEF is less understood. Glezeva et al31 reported increased monocytosis and monocyte differentiation to M2 macrophages in HFpEF and in asymptomatic DD patients. In hypertensive mice, CCR2-dependent monocyte recruitment is responsible for the macrophage expansion and the associated DD.32 In the present study, we used the HFD-induced DD model and revealed that proinflammatory macrophages expanded (Figure 3D), and depleting macrophages with clodronate liposomes (Figure 4A) or suppressing the proinflammatory macrophage phenotype (Figure 5F) both improved DD. A macrophage-dependent inflammatory milieu was further suggested by increased expression of IL-1β and decreased expression of TGF-β (Figure 2D).
Macrophages represent a continuum of highly plastic cells with a spectrum of diverse phenotype states.33 Traditionally, macrophages are classified into proinflammatory and anti-inflammatory phenotypes. In heart, the macrophage population can be divided into resident and monocyte-recruited populations. Our results point to an inflammatory macrophage subtype causing HFD-induced DD. Although not conclusive, cell surface marker characterization suggested that CD86+ and CCR2+Timd4− macrophages might be responsible for DD in our model. By contrast, an enhancement of CD86+ as well as CCR2+ macrophages is consistent with an inflammatory phenotype causing DD in our model. This is supported by the fact that FABP4 KO mouse macrophages do not adopt an inflammatory phenotype, and these mice were resistant to HFD-induced HEpEF. An expansion of CCR2+Timd4− macrophage population suggests that recruited macrophages may play a role in HFD-induced DD. This is consistent with elevated expression of cardiac MCP-1. Although macrophages are involved, our data do not exclude a role for other immune cell types in the pathogenesis of DD. Despite that, macrophage depletion alone completely reversed DD, suggesting macrophages were a main contributor to HFD-induced DD. In summary, our data strongly support that inflammatory macrophages are responsible for HFD-induced DD.
Regarding the molecular effector of proinflammatory macrophages, our data suggest that IL-1β signaling is crucial. This is consistent with the data that macrophage-dependent IL-1β production mediates arrhythmia in HFD mice.8 It is unclear though why cardiac IL-1β was enhanced, whereas the other 2 main inflammatory cytokines (IL-6 and TNF-α) were not changed by HFD (Figure 2D). Inhibiting IL-1β significantly improved HFD-induced DD, indicating IL-1β–mediated inflammation plays an important role in the pathogenesis of DD. This result is consistent with the observations that renormalizing cardiac IL-1β level by macrophage depletion or by FABP4 KO reversed/prevented DD in HFD mice, which also suggested that macrophages were a major source of pathogenic IL-1β. Nevertheless, we did not rule out amplification of this macrophage-dependent signal by other cell sources of IL-1β. We also saw a decrease of TGF-β in HFD heart. TGF-β is a powerful anti-inflammatory factor and can antagonize the inflammatory effect of IL-1β.34,35 Thus, it is plausible that TGF-β down-regulation may synergize with IL-1β enhancement, leading to HFD-induced DD. It cannot be excluded that changes in other cytokines not measured contributed to the results, however.
In clinical trials, IL-1β inhibition has shown a significantly reduced cardiovascular events in the CANTOS study (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), although this trial was not designed to test an effect on HFpEF.36 The DHART (Diastolic Heart Failure Anakinra Response Trial) showed that 2 weeks of IL-1 β inhibition with anakinra improved peak oxygen consumption and C-reactive protein (CRP) levels.37 DHART2 showed that 12 weeks of anakinra improved quality-of-life metrics, CRP, and brain natriuretic protein, but did not change peak oxygen consumption in patients with HFpEF.38 Therefore, the value of IL-1β inhibition in human HFpEF remains to be determined. Any role for macrophage modulation in human HFpEF remains to be explored.
Major risk factors for human HFpEF include age, DM, and hypertension.39,40 It is possible that the 3 different risk factors promote HFpEF by different pathogenic mechanisms. Hulsmans et al32 implicated macrophage-derived, IL-10–stimulated cardiac fibrosis contributing to hypertension-induced DD. Consistently, we have found fibrosis is also a factor in age-associated HFpEF.41 On the other hand, we have reported previously that hypertension and HFD can cause DD at the myocyte level through oxidative stress without significant cardiac fibrosis.9,10 Further, cardiac IL-10 was unaltered in HFD mice in the present study. A possible cause for the discrepancy between our results and those of Hulsmans et al42 is that they used an aldosterone-induced hypertension model that is known to induce cardiac fibrosis. A HFD/Western diet is strongly associated with increased risk of type II DM43 as evidenced in our HFD mice. DD is observed in over 40% of DM patients.44,45 Nevertheless, correcting hyperglycemia failed to improve HFD-induced DD as we reported previously.9 Furthermore, our data in this study indicated that inhibiting macrophage-secreting IL-1β, despite reversed HFD-induced DD, had no effects on blood glucose level and insulin resistance. A possible explanation is that whereas DD may be initiated by DM, it is sustained by macrophage-mediated inflammation.
Previously, we have demonstrated that mitoROS contributes to HFD-induced DD.9 In the present study, we recapitulated the findings that IL-1β up-regulated the mitoROS level in cardiomyocytes and showed that a mitoROS scavenger (mitoTEMPO) generated similar effects on DD as the IL-1 receptor antagonist. Given that we have shown previously that the IL-1β effects through mitoROS in HFD mice,8 it seems likely that the same pathogenic cascade is in play here. If this is the case, it may explain the epidemiological association of HFpEF and arrhythmias.46,47 Nevertheless, it is possible that there are intermediate signals between IL-1β and mitochondrial oxidative stress, such as IL-18.48
Study limitations
A limitation to our study is that we used an IL-1β receptor antagonist, thus we cannot rule out a potential effect of other cytokines acting on the IL-1 receptor, such as IL-1α. Nevertheless, IL-1β concentration is 4 times higher than IL-1α in DM patients.49 Therefore, it is reasonable to conclude that IL-1β is the main effector. Further investigation with specific IL-1β neutralizing antibody is needed to confirm our findings. In addition, clodronate liposomes nonspecifically deplete all phagocytes including dendritic cells.50 Nevertheless, dendritic cells are a remarkably minor population compared with macrophages in cardiac interstitial cells.51 Last, because we used a full-body KO of FABP4, and FABP4 is mainly expressed in macrophages and adipocytes,52 we cannot rule out some component of the prevention of DD occurred because of reduction in adipocyte or systemic inflammatory influence.53 On the other hand, a similar effect on DD with clodronate would suggest that macrophages were most important. Finally, female mice were excluded because they are less susceptible to HFD-induced metabolic disturbances and inflammation.54 Therefore, the results must be extrapolated with caution to the female sex.
Conclusions
HFD results in activation of a cardiac innate immune response associated with impaired diastolic function, which could be inhibited by depleting macrophages, modulating macrophage phenotype, antagonizing IL-1β, and diminishing mitoROS. Each of these approaches represents a possible new therapy for HFD-induced DD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HFD causes activation of proinflammatory macrophages that secrete IL-1β leading to DD.
TRANSLATIONAL OUTLOOK: HFD-induced DD can be inhibited by IL-1β antagonism, mitochondrial reactive oxygen species scavenging, macrophage depletion, and macrophage phenotype modulation, suggesting macrophage-mediated inflammation as a potential therapeutic targets for HFpEF.
Funding Support and Author Disclosures
This project was supported by National Institutes of Health grants R01 HL104025 (Dr Dudley) and R01 HL106592 (Dr Dudley). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank D. Dicky for the assistance on FABP4 mice breeding and maintenance.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section, please see the online version of this paper.
Appendix
References
- 1.Hunt S.A., Abraham W.T., Chin M.H., et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Plitt G.D., Spring J.T., Moulton M.J., Agrawal D.K. Mechanisms, diagnosis, and treatment of heart failure with preserved ejection fraction and diastolic dysfunction. Expert Rev Cardiovasc Ther. 2018;16:579–589. doi: 10.1080/14779072.2018.1497485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone S., Canada J.M., Buckley L.F., et al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Trans Science. 2017;2:513–525. [Google Scholar]
- 4.Duan Y., Zeng L., Zheng C., et al. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9:2649. doi: 10.3389/fimmu.2018.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H., Barnes G.T., Yang Q., et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiran S., Rakib A., Kodidela S., Kumar S., Singh U.P. High-fat diet-induced dysregulation of immune cells correlates with macrophage phenotypes and chronic inflammation in adipose tissue. Cells. 2022;11(8):1327. doi: 10.3390/cells11081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atri C., Guerfali F.Z., Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19(6):1801. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H., Zhao Y., Xie A., et al. Interleukin-1β, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. J Am Coll Cardiol Basic Trans Science. 2021;6:42–52. doi: 10.1016/j.jacbts.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong E.M., Chung J., Liu H., et al. Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc. 2016;5(5) doi: 10.1161/JAHA.115.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silberman G.A., Fan T.H., Liu H., et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Castejon G., Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monnerat G., Alarcón M.L., Vasconcellos L.R., et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7 doi: 10.1038/ncomms13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshmane S.L., Kremer S., Amini S., Sawai B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y., Surachai S., Nurkesh A., Zharkinbekov Z., Saparov A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int J Mol Sci. 2021;22(5):2715. doi: 10.3390/ijms22052715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blériot C., Chakarov S., Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020;52:957–970. doi: 10.1016/j.immuni.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Dick S.A., Macklin J.A., Nejat S., et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20:29–39. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalli J., Chiang N., Serhan C.N. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci U S A. 2014;111:E4753–E4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stables M.J., Shah S., Camon E.B., et al. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Maeyer R.P.H., van de Merwe R.C., Louie R., et al. Publisher correction: blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21:696. doi: 10.1038/s41590-020-0686-5. [DOI] [PubMed] [Google Scholar]
- 20.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y., Mouton A., Lindsey M. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambara K., Ohashi W., Tomita K., et al. In vivo depletion of CD206+ M2 macrophages exaggerates lung injury in endotoxemic mice. Am J Pathol. 2015;185:162–171. doi: 10.1016/j.ajpath.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Koelwyn G.J., Moore K.J. Defining macrophages in the heart one cell at a time. Trends Immunol. 2019;40:179–181. doi: 10.1016/j.it.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajpai G., Bredemeyer A., Li W., et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124:263–278. doi: 10.1161/CIRCRESAHA.118.314028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Amerongen M.J., Harmsen M.C., van Rooijen N., Petersen A.H., van Luyn M.J. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H., Hertel A.V., Steen K.A., Bernlohr D.A. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Mol Endocrinol. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBerge M., Shah S.J., Wilsbacher L., Thorp E.B. Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol Med. 2019;25:328–340. doi: 10.1016/j.molmed.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel B., Bansal S.S., Ismahil M.A., et al. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. J Am Coll Cardiol Basic Trans Science. 2018;3:230–244. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajpai G., Schneider C., Wong N., et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24:1234–1245. doi: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sager H.B., Hulsmans M., Lavine K.J., et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glezeva N., Voon V., Watson C., et al. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail. 2015;21:167–177. doi: 10.1016/j.cardfail.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Hulsmans M., Sager H.B., Roh J.D., et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parisi L., Gini E., Baci D., et al. Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res. 2018;2018 doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebel K., Rudolph M., Kosai B., Chang H.D., Butzmann J., Brunner-Weinzierl M.C. IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J Immunol. 2011;187:5627–5635. doi: 10.4049/jimmunol.1003998. [DOI] [PubMed] [Google Scholar]
- 35.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 37.Van Tassell B.W., Arena R., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Tassell B.W., Trankle C.R., Canada J.M., et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 40.Gong F., Jelinek M., Castro J., et al. Risk factors for incident heart failure with preserved or reduced ejection fraction, and valvular heart failure, in a community-based cohort. Open Heart. 2018;5 doi: 10.1136/openhrt-2018-000782. e000782corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed A.L., Tanaka A., Sorescu D., et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301:H824–H831. doi: 10.1152/ajpheart.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lijnen P., Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 43.Marshall J.A., Essenes D.H. Dietary fat and the development of type 2 diabetes. Diabetes. Care. 2002;25:620–622. doi: 10.2337/diacare.25.3.620. [DOI] [PubMed] [Google Scholar]
- 44.Owain T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson M.J., Zadourian A., Taub P.R. Heart failure and diabetes mellitus: defining the problem and exploring the interrelationship. Am J Cardiol. 2019;124(suppl 1):S3–S11. doi: 10.1016/j.amjcard.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Walker A.M., Cubbon R.M. Sudden cardiac death in patients with diabetes mellitus and chronic heart failure. Diab Vasc Dis Res. 2015;12:228–233. doi: 10.1177/1479164115573225. [DOI] [PubMed] [Google Scholar]
- 47.Veglio M., Chinaglia A., Cavallo-Perin P. QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest. 2004;27:175–181. doi: 10.1007/BF03346265. [DOI] [PubMed] [Google Scholar]
- 48.Toldo S., Mezzaroma E., O'Brien L., et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisser S.B., van Rooijen N., Sly L.M. Depletion and reconstitution of macrophages in mice. J Vis Exp. 2012;66:4105. doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forte E., Perkins B., Sintou A., et al. Cross-priming dendritic cells exacerbate immunopathology after ischemic tissue damage in the heart. Circulation. 2021;143:821–836. doi: 10.1161/CIRCULATIONAHA.120.044581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makowski L., Boord J.B., Maeda K., et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Jeinsen B., Ritzen L., Vietheer J., et al. The adipokine fatty-acid binding protein 4 and cardiac remodeling. Cardiovasc Diabetol. 2020;19:117. doi: 10.1186/s12933-020-01080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettersson U.S., Wilden T.B., Carlsson P.O., Jansson L., Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.