Abstract
Anti-Kv1.4 antibody is often detected in thymoma-associated myasthenia gravis patients with anti-acetylcholine receptor antibody. Herein, we describe 2 patients with concurrent myocarditis and myositis. In both cases, anti-Kv1.4 antibody was positive despite the absence of thymoma and anti-acetylcholine receptor antibody, and immunosuppressants eventually resolved their symptoms and cardiac function. (Level of Difficulty: Advanced.)
Key Words: anti-Kv1.4 antibody, myasthenia gravis, myocarditis, myositis
Abbreviations and Acronyms: AChR-Ab, anti-acetylcholine receptor antibody; CK, creatinine kinase; ECG, electrocardiogram; LV, left ventricular; LVEF, left ventricular ejection fraction; MG, myasthenia gravis; TTE, transthoracic echocardiography
Central Illustration
Myocarditis is an inflammatory cardiac disease with severe manifestations, including acute heart failure, fatal arrhythmia, and sudden cardiac death. The most common cause of myocarditis is a viral infection, although some autoimmune diseases, such as myasthenia gravis (MG)1 and autoimmune-related myositis,2 have been recently recognized as uncommon causes of myocarditis.
Learning Objectives
-
•
To recognize that anti-Kv1.4 antibody can be seropositive in patients with myocarditis that is suspected to be autoimmune in etiology and complicated with myositis, although thymoma, AChR-Ab, and MG are absent.
-
•
To understand that the evaluation of anti-Kv1.4 antibody in patients with suspected autoimmune myocarditis and myositis independent of the presence of thymoma, AChR-Ab, and MG may help physicians select a more appropriate treatment for myocarditis, given that anti-Kv1.4 antibody is a biomarker indicating responsiveness to immunosuppressants.
Antistriational antibodies are increasingly recognized as important biomarkers for myocarditis and/or myositis in patients with MG3 and in those receiving immune checkpoint inhibitors as biomarkers of immune-related adverse events.4 In particular, anti-Kv1.4 antibody is closely associated with autoimmune-mediated myocarditis and/or myositis, especially in thymomatous and antiacetylcholine receptor antibody (AChR-Ab)–positive MG.3,5 Few reports have described anti-Kv1.4 antibody-positive myocarditis in nonthymomatous and AChR-Ab–negative patients. Herein, we describe 2 cases of concurrent myocarditis and myositis in which anti-Kv1.4 antibody was positive, despite the absence of thymoma and AChR-Ab.
Case Presentation
Case 1
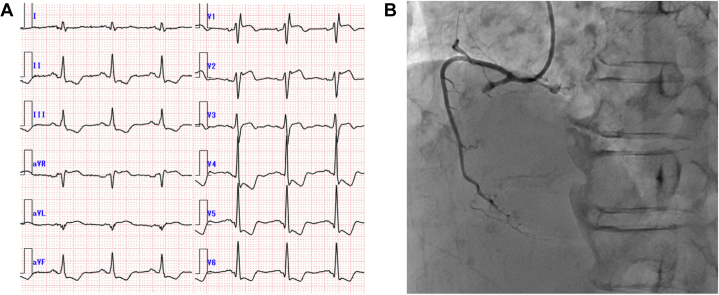
A 74-year-old woman developed diplopia and bilateral eyelid ptosis 10 days before presentation. Although she had no chest pain, a routine admission electrocardiogram (ECG) (Figure 1A) revealed complete right bundle branch block, ST-segment elevation in precordial leads V1-3, and ST-segment depression in leads V4-6 and the inferior leads. Laboratory tests revealed significantly elevated levels of creatinine kinase (CK) (6,669 IU/L), CK-MB isoenzyme (242 IU/L), high-sensitivity cardiac troponin T (1.482 ng/mL; normal range <0.014 ng/mL), brain natriuretic peptide (299 pg/mL), and C-reactive protein (1.09 mg/dL). Transthoracic echocardiography (TTE) (Video 1) demonstrated left ventricular (LV) systolic impairment (left ventricular ejection fraction [LVEF] = 40%) with akinesis of the inferior segment. Emergent coronary angiography (Figure 1B) was performed for suspected acute ST-segment elevation myocardial infarction, revealing total occlusion of the proximal lesion in the right coronary artery, which was treated using emergency percutaneous coronary intervention with deployment of 3 drug-eluting stents.
Figure 1.
Electrocardiographic and Coronary Angiographic Findings Pertaining to Case 1
(A) Electrocardiogram on admission showing a complete right bundle branch block, ST-segment elevation in leads V1-3, and ST-segment depression in leads V4-6 and the inferior leads. (B) Right coronary angiography before percutaneous coronary intervention. The proximal right coronary artery was obstructed.
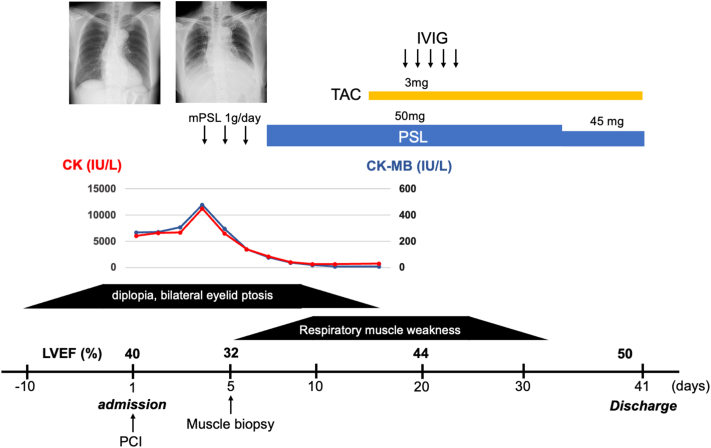
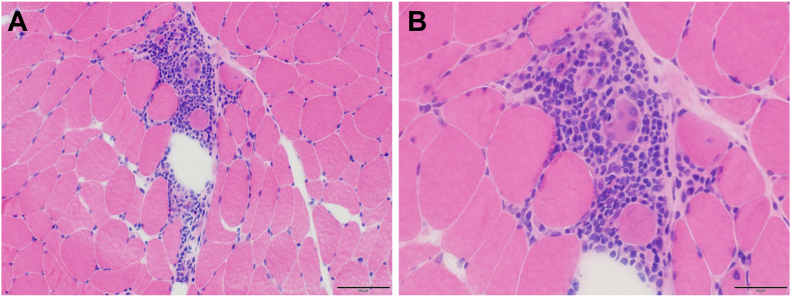
Despite successful coronary revascularization, the ST-segment changes on ECG and elevated serum CK level persisted, and LVEF progressively decreased to 32%. The patient went on to develop symptoms of heart failure during hospitalization, including dyspnea; chest X-ray revealed increased pleural effusion compared with that observed at admission. The patient’s clinical course is shown in Figure 2. A left biceps brachii muscle biopsy specimen (Figures 3A and 3B) showed the infiltration of inflammatory cells along with necrotic myofibrils, confirming a diagnosis of inflammatory myositis. The progressive decrease in the cardiac function after revascularization and the concurrent myositis raised a possibility of myocarditis; however, cardiac biopsy and magnetic resonance imaging were not feasible due to the deteriorated respiratory status. Steroid pulse therapy was administered and resulted in the resolution of diplopia, bilateral eyelid ptosis, and the serum CK concentration. However, owing to respiratory muscle failure, noninvasive positive-pressure ventilation was administered. After treatment with tacrolimus and intravenous immunoglobulin therapy, her respiratory muscle function gradually improved. Her LVEF improved to 50%, and she was discharged on day 41 after rehabilitation.
Figure 2.
Clinical Course of Case 1
Clinical course of Case 1. Revascularization of the right coronary artery failed to resolve elevation of the CK level, whereas steroid pulse therapy resulted in resolution of the elevated CK level and extraocular muscle paralysis. Although respiratory muscle failure worsened thereafter, the addition of TAC and IVIg eventually improved respiratory muscle failure. Cardiac function was also improved using immunosuppressive therapy. CK = creatinine kinase; CK-MB = MB isoenzyme of creatinine kinase; IVIg = intravenous immunoglobulin; mPSL = methylprednisolone; PCI = percutaneous coronary intervention; PSL = prednisolone; TAC = tacrolimus.
Figure 3.
Histologic Assessment of the Skeletal Muscle in Case 1
(A and B) Histologic examination of skeletal muscle biopsy specimens from the left biceps brachii muscle. Hematoxylin and eosin staining (A) and its enlarged image (B) showed extensive inflammatory cell infiltration into the endomysium along with necrotic myofibrils.
Serologic tests demonstrated that anti-Kv1.4 antibody and antititin antibody were positive, whereas AChR-Ab and anti–muscle-specific kinase antibody were negative. Computed tomography revealed no thymoma, and ice pack and repetitive nerve stimulation tests were negative. Thus, the diagnostic criteria for MG were not met; however, MG could not be excluded, considering that extraocular and respiratory muscle paralysis are common in MG.
Case 2
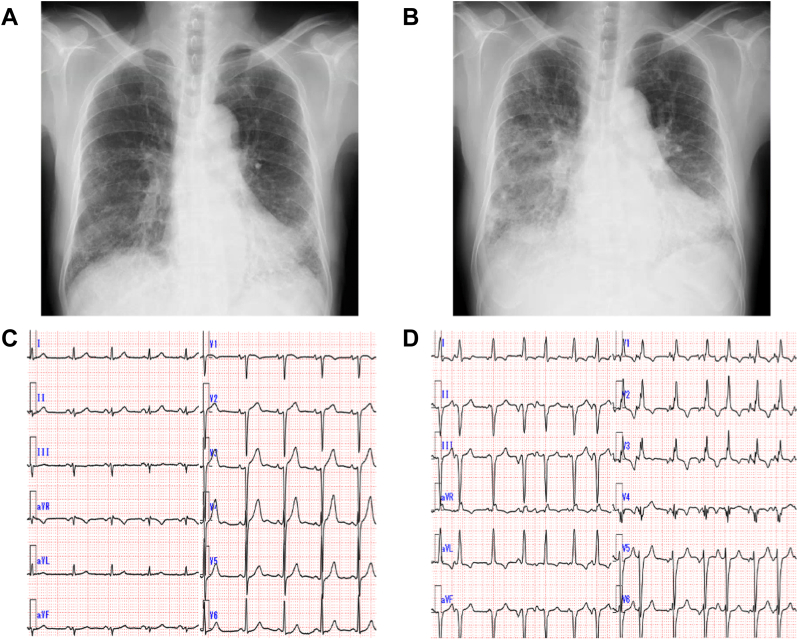
A 70-year-old man with a history of interstitial lung disease diagnosed based on reticular shadows on chest X-ray (Figure 4A) presented with orthopnea. Six months previously, he was diagnosed with polymyositis and commenced tacrolimus and prednisolone therapy. On admission, oxygen saturation was 92% with oxygen administered at 3 L/min. Chest X-ray (Figure 4B) showed pulmonary congestion and markedly exacerbated reticular shadows in the lower lung fields. Although TTE and ECG findings (Figure 4C) at the time of polymyositis diagnosis were normal, repeat TTE demonstrated severe global hypokinesis with LVEF of 22% (Video 2). ECG on arrival demonstrated frequent premature atrial complexes with complete right bundle branch block and left anterior fascicular blocks (Figure 4D). Laboratory tests revealed elevated levels of brain natriuretic peptide (976.1 pg/mL), high-sensitivity cardiac troponin T (0.197 ng/mL), and C-reactive protein (1.27 mg/dL), whereas the CK level was normal (110 IU/L). KL-6 is a serum biomarker that is used to help diagnose and assess disease activity for different causes of interstitial lung disease. In addition to acute decompensated heart failure, this patient was also suspected of having acute exacerbation of interstitial lung disease on the basis of an elevated KL-6 level of 1,763 U/mL. The patient was diagnosed with congestive heart failure and acute exacerbation of interstitial lung disease associated with polymyositis. Steroid pulse therapy was administered on hospital day 3, followed by 60 mg/day of oral prednisolone. Steroid therapy and heart failure treatments gradually improved his heart failure condition and reticular shadows in the lower lung fields.
Figure 4.
Radiographic and Electrocardiographic Findings Pertaining to Case 2
(A) Chest X-ray pertaining to Case 2 obtained at the time of diagnosis of interstitial lung disease 9 months before the current admission, which showed a reticular shadow in the lower lung fields. (B) Chest X-ray obtained at admission showing pulmonary congestion and an exacerbated reticular shadow in the lower lung fields. (C) ECG at polymyositis diagnosis. No significant abnormalities were observed. (D) ECG on admission demonstrating frequent premature atrial complexes with complete right bundle branch and left anterior fascicular blocks. ECG = electrocardiogram.
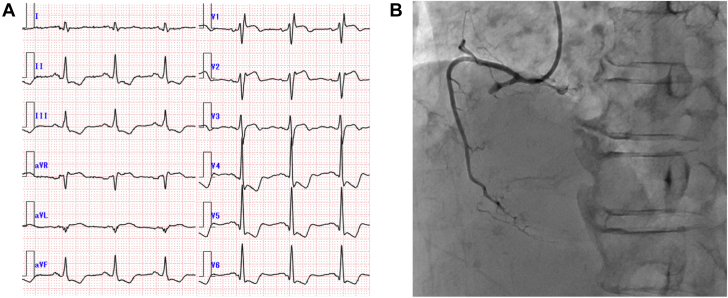
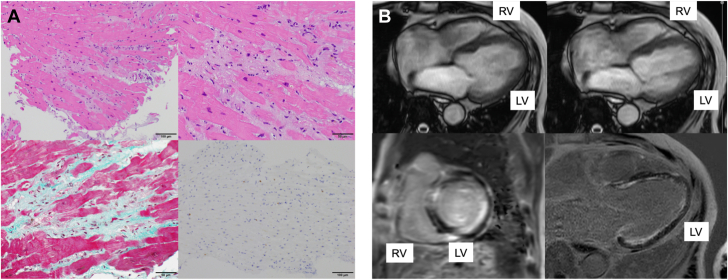
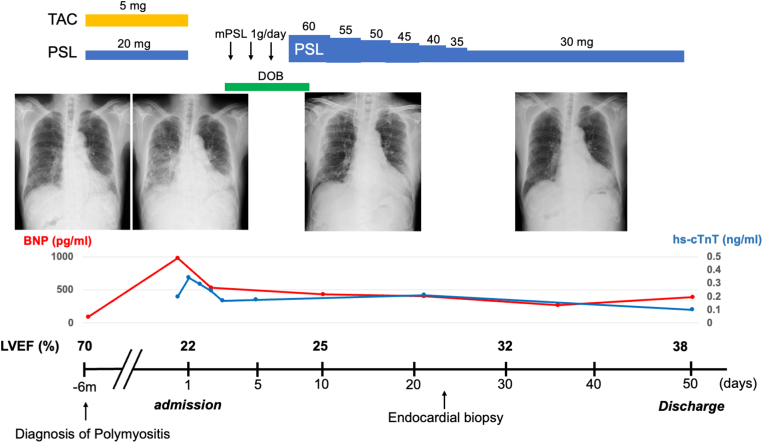
Even after stabilization of heart failure, the LV function remained impaired (LVEF 32%). Therefore, coronary angiography was performed and showed no significant coronary artery stenosis. Endomyocardial biopsy from the right ventricle showed the mononuclear cell infiltration with cardiomyocyte necrosis and replacement fibrosis, whereas granulomas, eosinophils, and giant cells were absent (Figure 5A), indicating lymphocytic myocarditis. Cardiac magnetic resonance imaging (Figure 5B) revealed severely impaired LV function (LVEF 20%). Late gadolinium enhancement images showed diffuse endocardial late gadolinium enhancement involving the left ventricle. The patient was discharged 50 days after rehabilitation. A summary of his clinical course is shown in Figure 6.
Figure 5.
Cardiac Histologic and Magnetic Resonance Imaging Assessments Pertaining to Case 2
(A) Histopathologic examinations of the endomyocardial biopsy specimens from the RV. Hematoxylin and eosin staining (upper left) and its enlarged image (upper right) showed cell infiltration of mononuclear cells with cardiomyocyte necrosis. Masson-trichrome staining (lower left) showed replacement fibrosis, and CD3 staining (lower right) demonstrated infiltration of CD3-positive cells into the myocardium. (B) Cardiac magnetic resonance imaging. Cine imaging in the end-diastolic phase (upper left) and end-systolic phase (upper right) revealed impaired left ventricular function (LVEF 20%), and late gadolinium enhancement imaging (short-axis, lower left; long-axis, lower right) showed diffuse endocardial late gadolinium enhancement involvement in the LV. LV = left ventricle; LVEF = left ventricular ejection fraction; RV = right ventricle.
Figure 6.
Clinical Course of Case 2
Clinical course of case 2. The pulmonary congestion and marked reticular shadow in the lower lung fields as well as the elevated levels of BNP and hs-cTnT were present on admission; these features were improved using immunosuppressive therapy and heart failure treatment. Oral prednisolone was gradually tapered as shown in this figure. BNP = brain natriuretic peptide; DOB = dobutamine; hs-cTnT = high-sensitivity cardiac troponin T; other abbreviations as in Figures 1 and 5.
Serologic tests for autoimmune markers showed anti-Kv1.4 antibody positivity, whereas antititin antibody, AChR-Ab, and anti–muscle-specific kinase antibody were negative. Throughout the entire clinical course of this case, there were no symptoms or manifestations indicating the presence of MG, and thymoma was not detected on computed tomography.
Discussion
In this case series, we described 2 cases of anti-Kv1.4 antibody-positive myocarditis with concurrent myositis. Case 1 exhibited concomitant myositis and suspected myocarditis. Although myocarditis was not histologically confirmed, it was suggested by a clinical course inconsistent with acute coronary syndrome and a recovery of cardiac function with immunosuppression therapy. Case 2 was admitted to our hospital because of acute congestive heart failure caused by myocarditis 6 months after the diagnosis of polymyositis. In both cases, anti-Kv1.4 antibody was seropositive, and immunosuppressive therapy improved the patients’ clinical conditions.
Kv1.4 is a subunit of the voltage-gated potassium channel located in the brain and skeletal and heart muscles. Anti-Kv1.4 antibody was first reported in 20055 and is closely associated with thymoma-associated MG5, myocarditis and/or myositis,1,3 and ventricular arrhythmia,6 given that Kv1.4 is expressed in the majority of left ventricular cardiomyocytes.7 Generally, anti-Kv1.4 antibody has been reported to be detected in thymomatous and AChR-Ab–positive MG.3,5 However, unlike previously reported cases, ours were both anti-Kv1.4 antibody–positive despite the absence of thymoma and AChR-Ab. Furthermore, in Case 2, all manifestations indicative of MG were absent. There are few previous reports on anti-Kv1.4 antibody–positive cases with myocarditis in nonthymomatous, AChR-Ab–negative, or even non-MG patients. To our best knowledge, this is the first report describing anti-Kv1.4 antibody–positive cases with concurrent myocarditis and myositis, independent of the presence of thymoma, AChR-Ab, and MG, which may highlight the importance of evaluating anti-Kv1.4 antibody in patients with myocarditis (especially those with myositis), although MG-related manifestations or findings, including thymoma and AChR-Ab, are absent.
The presence of anti-Kv1.4 antibody in patients with MG has predicted an improved therapeutic response to the use of a calcineurin inhibitor.8 Therefore, assessment of anti-Kv1.4 antibody in patients with myocarditis may help physicians identify responders to calcineurin inhibitors. Indeed, in Case 1, steroid therapy was ineffective in resolving the patient’s clinical condition; however, the addition of a calcineurin inhibitor resulted in the recovery of cardiac function and resolution of myositis-related symptoms. Still, given the paucity of evidence regarding the clinical significance of anti-Kv1.4 antibody positivity, particularly in nonthymomatous MG and non-MG patients, further large-scale investigations are strongly warranted.
Conclusions
We have described 2 anti-Kv1.4 antibody–positive and AChR-Ab–negative cases with concomitant myocarditis and myositis, despite the absence of thymoma or diagnosis of MG. Evaluation of anti-Kv1.4 antibody in patients with myocarditis and myositis independent of the presence of MG may help physicians select a more appropriate treatment for myocarditis.
Funding Support and Author Disclosures
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 21K08131). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank these 2 patients for giving consent to publish.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Parasternal Short-Axis View of Transthoracic Echocardiography in Case 1 on Admission
Left ventricular systolic impairment (LVEF 40%) with akinesis of the inferior segment was observed. LVEF = left ventricular ejection fraction.
Apical 4-Chamber View of Transthoracic Echocardiography in Case 2 on Admission
The left ventricular function was severely and diffusely impaired (LVEF 22%). Abbreviation as in Video 1.
References
- 1.Suzuki S., Baba A., Kaida K., et al. Cardiac involvements in myasthenia gravis associated with anti-Kv1.4 antibodies. Eur J Neurol. 2014;21:223–230. doi: 10.1111/ene.12234. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg I.E. Cardiac involvement in autoimmune myositis and mixed connective tissue disease. Lupus. 2005;14:708–712. doi: 10.1191/0961203305lu2205oa. [DOI] [PubMed] [Google Scholar]
- 3.Kufukihara K., Watanabe Y., Inagaki T., et al. Cytometric cell-based assays for anti-striational antibodies in myasthenia gravis with myositis and/or myocarditis. Sci Rep. 2019;9:5284. doi: 10.1038/s41598-019-41730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono R., Iwai Y., Yamazaki T., et al. Nivolumab-induced myositis and myocarditis with positive anti-titin antibody and anti-voltage-gated potassium channel Kv1.4 antibody: a case report. Intern Med. 2022;61:2973–2979. doi: 10.2169/internalmedicine.8772-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki S., Satoh T., Yasuoka H., et al. Novel autoantibodies to a voltage-gated potassium channel Kv1.4 in a severe form of myasthenia gravis. J Neuroimmunol. 2005;170:141–149. doi: 10.1016/j.jneuroim.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Grune J., Yamazoe M., Nahrendorf M. Electroimmunology and cardiac arrhythmia. Nat Rev Cardiol. 2021;18:547–564. doi: 10.1038/s41569-021-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmajothi M.V., Campbell D.L., Rasmusson R.L., et al. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagane Y., Suzuki S., Suzuki N., Utsugisawa K. Factors associated with response to calcineurin inhibitors in myasthenia gravis. Muscle Nerve. 2010;41:212–218. doi: 10.1002/mus.21462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal Short-Axis View of Transthoracic Echocardiography in Case 1 on Admission
Left ventricular systolic impairment (LVEF 40%) with akinesis of the inferior segment was observed. LVEF = left ventricular ejection fraction.
Apical 4-Chamber View of Transthoracic Echocardiography in Case 2 on Admission
The left ventricular function was severely and diffusely impaired (LVEF 22%). Abbreviation as in Video 1.