Abstract
Soy isoflavone extracts are widely researched for their distinctive potential in contributing to various functional foods. The research work focuses on testing the toxicity of purified soy isoflavone extracts in mice models. With an agreement of the animal ethics, acute toxicity is firstly used to screen the effects of test compounds in mice for therapeutic purposes. Moreover, tests were conducted on BALB/c for estrogen in vivo and MCF7 for in vitro, screening active protection of liver cells, lipid peroxidation and scavenging free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH). Genistin and daidzin were found to be the two major compounds accounting for 47% and 35% of total purified soy isoflavones. The acute toxicity test results exhibited no effect against physiological accretion of BALB/c after 7-day administration with the given dose of 10 g/kgBW. Moreover, modified E-screen assay on MCF7 cells proved that the estrogen of isoflavone extracts induces cell proliferation by 15% compared with other non-steroid culture techniques. Therefore, this research contributes to helping researchers apply soy isoflavones in functional food, to alleviate the difficulties in menopausal symptoms for women in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05491-4.
Keywords: Soy isoflavones, Mouse acute toxicity, Hepatoprotection, Lipid peroxidation, E-screen assay
Introduction
Hormone replacement therapy (HRT) is known for its effectiveness, in most cases for alleviating menopausal symptoms; however, this therapy has fallen out of favour because it is associated with an increased risk of developing breast cancer. Therefore, to delay menopause, phytoestrogens such as soy isoflavones have been suggested as a potential alternative to HRT that can also improve the quality of life for postmenopausal women (Bennetau-Pelissero 2013).
Isoflavone composes structure close to estrogen, a female hormone that plays an important role for menopausal women, is commonly used in functional food products, pharmaceuticals, cosmetics and beverages (Bennetau-Pelissero 2013; Mazambani et al. 2018; Vitale et al. 2013). Isoflavone-enriched raw materials such as soybean, red clover, alfalfa, chickpea, and kudzu root are being intensively studied and exploited to improve the development of mankind health solutions. Isoflavone composition in plant sources depends on the climate conditions, soil and species. The most concerning isoflavone compounds are aglycones groups such as genistein, daidzein and glycitein and ß-glycosides namely genistin, daidzin and glycitin because of their high biological activity values, thermal stability and occupies a large amount in the total isoflavone components (Bennetau-Pelissero 2013). In addition, isoflavones are also of interest because of their important role in the cell membrane antioxidant activity (lipid peroxidation). This role lowers LDL against atherosclerosis or free radical scavenging ability DPPH• and FRAP, which is the basis of anti-aging, and protect cells such as liver cells and brain cells (Yoon and Park 2014).
Various extraction and purification technologies have been studied such as supercritical liquid extraction, liquid–liquid extraction and high-performance liquid chromatography preparation but are not favorable in industrial conditions (Kammerer et al. 2019). Meanwhile, the use of adsorption processes using macroporous resins presents advantages such as selective adsorption properties, low operating costs, lower solvent consumption, and has been developed as an industrial scale in the fields of separation and enrichment of bioactive compounds such as flavonoids, glycosides, saponins, carotenoids and serotonin (Zhang et al. 2007). According to Li et al. (2010), D101, which is one of the most used commercial macroporous that meets the standards for its application in food as it is relatively cheap, effective, and suitable for many adsorbents in the market, is easy to implement on an industrial scale that can gradually improve the purity of isoflavones through the usage of non-toxic solvents (i.e. distilled water and ethanol) and reused.
In this research, isoflavone extracted from soybean seeds and purified by D101 resin were firstly evaluated (1) in vitro estrogen activity through a MCF—7 cellular screening that called as E- screen (estrogen-sensitive cell line, exhibiting full biological activities of isoflavone compounds as well as ensuring the food safety (free heavy metal); (2) in vivo printing model on mouse treating VCD, approximate simulation with women's menopausal symptoms; (3) evaluation of antioxidant activity for isoflavones extract through ex vivo test against cell membranization using the hepatopathic fluid and the ability to surround free radicals to protect cells on the flex hepatitis 2. The experiments in mice were accorded to the approval letter for the research proposal, which was signed at Hanoi on 26th of June 2020.
Materials and methods
Chemicals and materials
D101 (Donghong-Chemical Co., Ltd, China), a white-colored spherical polymer of polystyrene-divinyl benzene compound, is an industrial macroporous resin used in this research. The pre-use plastic bead is treated first to remove preservatives, monomers and porogens leftover during production (Wu et al. 2015). Isoflavone standards with purity 99% were then purchased from USP-USA, including genistin, glycitin, daidzin, genistein, glycitein and daidzein. The acetonitrile solvent, methanol (Merck, Germany), and deionized water (DW) used were of HPLC grade. Soybeans acquired from five locals in Vietnam were combined and dried at 60 °C to dry matter moisture content of 9.716%. The raw sample was then powdered and kept at − 20 °C in a dark glass jar.
Purification of isoflavones by using plastic bead D101
The CET was collected from a soybean seed extraction process described by Tran et al. (2019). The isoflavone extracts were subjected to the evaporation stage, and then vacuum evaporated and sublimed in − 40 °C at 16 h to the moisture content of 1.82 ± 0.07%. Consequently, 26 g of dry extract was accurately measured and mixed in 1 L of distilled water. A plain filter paper with diameter of 8 μm (Whatman No. 1, Merck) was used to filter the suspension of the mixing. An open column chromatographic model was then prepared by first loading 100 g of macroporous D101 into the adsorption column with a 3 cm diameter with Bed-Volume (BV) of 200 mL. A volume of adsorbent at 432.3 μg/mL was flown through the column at a rate of 1.5 BV/h until the equilibrium fluid concentration of isoflavones (Ce = 10% Co). The impurities were washed with 3 BV distilled water and 2.5 BV eluted with alcohol with the determined concentration of 70% (v/v) with a speed of 1 BV/hr. Finally, the eluate was evaporated under vacuum and sublimated at − 40 °C at 16 h, reaching a moisture content of 1.32 ± 0.09%.
Determination of acute toxicity
The determination of acute toxicity of the extracts was performed on rats since they were given the same dose of the samples under the same stable conditions, observing the reactions occurring for 72 h. For preliminary experiment, 6 white rats per lot (50% male, 50% female) was weighted in range of 19–24 g, and fasted for at least 12 h before being delivered the samples of CET and PET with a single dose of 10 g/kgBW (volume range of 0.2 mL = 0.3 mL/10 kgBW) by oral (Al-Afifi et al. 2018; Kifayatullah et al. 2015). Their overall movements, behavioral manifestations, hair status, eating, urination and death were monitored and counted within 72 h while rats expressed no signs of abnormality or death would be continued to follow up to 7 days. There are 3 experimental plots that were established as follows: Lot 1, the control has been just dosed distilled water at the rate of 0.2‒0.3 mL per mice; Lot 2, 6 rats were dosed CET at the rate of 10 g/kgBW; Lot 3, 6 rats were dosed PET at the same rate in Lot 2.
Estrogen activity test in vitro
The in vitro E-screen was released to reveal the estrogen activity of CET and PET. The E-screen estrogen assessment tests were processed using the method of Minervini et al. (2005) with some modifications. MCF7 cells supplied by American Type Culture Collection (ATCC) were cultured in steroid-free DMEM (Sigma, USA) without red phenol, with 5% charcoal dextran stripped 10% fetal bovine serum (FBS, Sigma), 2% L-glutamine 200 mM, 2% HEPES 1 M and 1% penicillin/streptomycin of 10 mg/L. Cells were cultured in 96 well plates with a concentration of 2 × 104 cells in a well, incubated at 37 °C, 5% CO2 for 24 h. The medium is then aspirated after passing over centrifugation at 2500 rpm for 10 min and replaced with a new medium containing different concentrations of test compounds at 0.8 μg/mL, 4.0 μg/mL, 20.0 μg/mL and 100.0 μg/mL. A negative control with the solvent for dissolving samples, and a positive control with 17β-estradiol at the concentrations from 1 × 10−4 to 1 × 10−1 µM was chosen. After 5 days of incubation in an incubator of 37 °C, 5% CO2, cell proliferation was assessed using MTT method described by Minervini et al. (2005), based on the yellow transformation of MTT into purple formazan crystals through cellular metabolic activity. 20 µL MTT (5 mg/mL MTT in PBS) was added to each well and incubated at 37 °C for 4 h, the formazan crystals produced were dissolved by adding 200 µL of 100% DMSO. The spectral absorbance of formazane is measured in a spectrophotometer with a wavelength of 590 nm. The amount of proliferating cells compared with the negative control will be calculated by the Eq. (1):
| 1 |
where, , denotes the absorbance of sample test with isoflavones and DMSO at 590 nm.
Estrogen activity test in vivo
In estrogen activity test in vivo, 30 white, and thoroughbred female mice type species BALB/c at 28 days of age (Biological Laboratory, Vietnam Biotechnology Institute) were ensured the hygiene conditions, diets according to the needs of mice, and weighted before conducting experiments. Based on the method of determining estrogen actual activity in vivo through causing ovaries with 4-vinylcyclohexene dioxide (VCD) model, a substance suppresses directly phosphorylation of c-Kit receptor on the cell membrane of the oocytes. This receptor plays an important role in the life of the oocytes. Therefore, VCD works to accelerate the process of apoptosis, causing a decline in primitive and primary eggs. This phenomenon simulates natural menopause in humans because most women enter menopause due to reducing the ovarian function and retaining the remaining ovarian tissue, the weakening of the follicle is shown gradually (Mazambani et al. 2018; Shin et al. 2017). VCD model was performed as follows: Experimental mouse was injected VCD mixed a sesame oil in an abdomen continuous 15-day with a dose of 160 mg/kg/P/Day/Time excluding mice with the sesame oil used as a physiological control. Systematically, experimental mice were divided into five lots with a dose of 1000 mg/kg/day for being dosed PET (lot-01), with 2000 mg/kg/day for being dosed CET (lot-02), with only sesame oil for the physiological control (lot-03), with 17β-E2 intramuscular injection of 0.1 mg/kg for positive control (lot-04), and remaining lot for negative control (lot-05) during 14-day observation. After that, the surgery of weighed mice was performed to separate their uterus. The ovary of the mouse in plots was isolated, weighed, and fixed in Formol 10%, casting paraffin and sliced, dyed hematoxylin and eosin. The number of follicles in each type (e.g. primordial, primary, secondary and graft follicle) was determined and compared with pathological control.
Determination of estradiol content in serum
Blood samples are centrifuged, separated serum and use to determine the level of 17β-estradiol referring to the Kit of BioVision’s mouse Estradiol E2 ELISA Kit (Catalog: K3830-100). 50 μL serum (diluted) or 50 μL standard estradiol at different concentrations were included in 96 wells with mouse estradiol antibody. 50 μL buffer was included in a well as a zero well. The 96 wells were then incubated for 30 min at 37 °C. The wells were then washed 5 times with washing buffer after removing the solution. 50 μL material solution melted horseradish peroxidase (HRP) was added to each well except the Zero well and incubated for 30 min at 37 °C, which was rejected before the well was washed 5 times with buffer solution. 50 μL Chromogen A and 50 μL Chromogen B were adjusted into each well before being incubated at 37 °C in the dark in 15 min. 50 μL stop solution was added to each well. This solution has measured the adsorption with the ELISA meter at 450 nm wavelength.
Based on the Estradiol standard curve, the estradiol content was determined by calculating the percentage of estradiol from the VCD injected mouse and also taking the pattern with the VCD content of normal physiological mice.
Active testing of liver cells
HepG2 cells (Long Island Univesity, US) were cultured in DMEM culture medium with the attached component including 2 mM L-glutamine, 10 mM Hepes, and 1.0 mM sodium pyruvate, additional 10% Fetal Bovine Serum-FBS (Gibco), incubated at 37 °C, 5% CO2 conditions. HepG2 cells were raised in 96 wells with a concentration of 3 × 104 cells.well−1 and incubated overnight at 37 °C, 5% CO2. Quercetin control and test samples were included in cultured wells at different concentrations with the presence of 40 mM CCl4 before being incubated for 2 h.
The ability to survive cells under the impact of CCl4 was determined through the MTT test. After removing the culture media, an additional 50 μL of 1 mg/mL MTT was added to each well and incubated at 37 °C temperature in 4 h. The color of Formazan was formed since it was dissolved with DMSO and representative an absorbance at the wavelength of 540 nm. The cell viability was defined as the ratio of the absorbance of treated cells to that of untreated control cells and is expressed as a percentage, physiological control samples were considered as 100% (González et al. 2017). Based on the surviving cell, the cytoprotective effect (% HE) was calculated by the Eq. (2) (Abdel-Monem et al. 2013):
| 2 |
where, , , refers to the absorbance of the sample treated by isoflavones with CCl4, the absorbance of the sample treated by CCl4, (pathological control sample) and absorbance of sample no treatment, (physiological control sample).
Chemical properties of extracts
The following method is on HPLC analytical sample set up: Soybean extracts were modified based on the method (AOAC 2008.03) for determining 6 isoflavone compounds and total soy isoflavones in the dried extract. This was a rationale for comparing extracts through the content of isoflavones as described in the previous study by Tran et al. (2019). Dried soy extract of 0.02 to 0.05 mg was added into a 10 mL screw-capped tube, 8.5 mL of acetonitrile along with 5 mL of water was added and vortex at room for 15 min. 6.5 mL of DW was then added, the tube was then vortex and centrifuged at 5000 rpm for 5 min. Before injection into the HPLC analysis, the supernatant was filtered using a filter membrane (PTFE, 0.2 µm, Sartorius, code 17,575 K). Agilent 1200 System (Agilent Technologies, CA, USA) equipped with a Lichrosphere 100 RP column (C18, 5 μm × 4.6 mm × 250 mm, USA) were used to conduct HPLC analysis of isoflavones. In this study, mobile phase A consists of 0.05% phosphoric acid in water and mobile phase B was acetonitrile. The wavelength for the diode array UV detector was set up at 260 nm and the flow rate was at 1.5 mL/min. The sample was eluted by gradient elution as follows: 100% of A in 5 min initial, change from 10 to 30% of B (linear) over a time frame of 60 min, 5 min wash 90% of B, 10 min equilibration at 10% B. Each isoflavone was quantified using an external standard method that involves integrating chromatographic peak areas. The content of each isoflavone compound (Xi (µg/g)) and the total of isoflavones in the dry material was calculated according to Tran et al. (2019).
Dried extracts were determined by standard oven drying method at an air temperature of 100 ± 2 °C for 72 h and subsequently cooling in desiccators for one hour before taking a final weight. The determination of total sugar content and soluble protein content was performed according to Dubois method and Bradford method (Bradford, 1976; Dubois et al. 1956).
Inhibition of lipid peroxidation
The antioxidant method through inhibition of membrane lipid peroxidation (MDA test) was performed according to the method of Ruberto et al. (2000) with modifications to suit laboratory conditions. In this study, malonyl dialdehyde (MDA) content, a product of lipid peroxidation of cell membranes was measured to determine the ability to inhibit lipid peroxidation of the sample. MDA can form trimethin complex when it reacts with thiobarbituric acid at a maximum absorption peak (λ = 532 nm).
The target samples were mixed into different concentrations (10,000 µg/mL, 2000 µg/mL, 400 μg/mL, 80 µg/mL and 16 µg/mL). The liver homogenate was prepared by separating the rat liver and grinding it in phosphate buffer (pH = 7.4) at a ratio of 1:10 at 0‒4 °C. Then, 0.1 mL of the target sample was added at the tested concentrations react with 1 mL of rat liver homogenate with adding 0.8 mL of phosphate buffer, along with 0.1 mL of Fenton reagent (FeSO4 0.1 mM: H2O2 in a ratio of 1:1) to the final volume of 2 mL, where sample concentrations in the test tube were reduced 20 times to 500.0 µg/mL,100.0 µg/mL, 20.0 µg/mL, 4.0 µg/mL, 0.8 µg/mL, respectively. The mixture was incubated at 37 °C for 15 min. To stop the reaction, 1 mL of 10% trichloroacetic acid was added before centrifuging at 12,000 rpm for 5 min to pick up the suspension, which was reacted with 1 mL of 0.8% thiobarbituric acid (in the ratio 2:1). This mixture was incubated at 100 °C for 15 min, then cooled and measured at 532 nm. Trolox was used as a positive control. The Inhibition of oxidation percentage was calculated using Eq. (3) below:
| 3 |
where, , denotes the absorbance of the control and absorbance of the target sample.
Determination of free radical reduction using the DPPH assay
The antioxidant activity of target samples was determined through a free radical scavenging reaction (DPPH) based on the method of Brand-Williams et al. (1995), on the principle of 1,1-diphenyl-2-picrylhydrazyl (DPPH) that is capable of generating stable free radicals in methanol (Zimmer et al. 2012).
The target sample was prepared with a stock isoflavone concentration of about 10,000 µg/mL, where the diluent stock solution was aspirated different volumes in turn and added methanol to a volume of 1000 µL, 4 mL of DPPH solution with a concentration of 0.1 mM was adjusted in the mixture, well-shaken and let stable in the dark for 30 min, then measured UV–Vis spectrophotometer at 515 nm, from which the percentage of free radical scavenging was calculated due to the DPPH of the test sample according to the Eq. (4) (Zimmer et al. 2012):
| 4 |
where, , , refers to the absorbance of DPPH solution in methanol and the absorbance of DPPH solution with the addition of the target sample.
An equation expressing the correlation between IC % and the concentration of the sample used was established, from which the IC50 value was calculated with Vitamin C as reference. Results are also expressed as AEAC (Ascorbic Acid equivalent antioxidant capacity) in grams and by Eq. (5):
| 5 |
where, , , refers to the IC50 value for ascorbic acid and sample.
Statistical analysis
The experiments were conducted with three replications per treatment (n ≥ 30). The results of the experiments are expressed as mean ± SD. Data were analyzed for variance (ANOVA) and tested for significant difference (p < 0.05) using GraphPad Prism 4.0 Software, San Diego, CA, USA. The IC50 and EC50 values of the tests were determined using Tablecurve2Dv4 software.
Results and discussions
Extraction and purification of soy isoflavones
The results of the HPLC chromatogram of isoflavones purified from D101 fully expressed from 6 isoflavone compounds (see Online Resource 1). CET and PET were concentrated and freeze-dried to yellow–brown color. As a result of purification, the composition of PET contained 18.63 ± 0.64% of total sugar, 5.42 ± 0.26% soluble protein content, that was more than 3 times reduction of those in crude one (56.82 ± 1.04% total sugar, 16.38 ± 0.51% total protein). Hence, it was easy to store, and not hygroscopic.
The content of isoflavones in PET achieved 144.66 ± 1.21 mg/g and was 9 times higher than CET. Due to the interaction force between the components isoflavone and D101 macroporous the proportion of pure isoflavone and crude isoflavone between them is different, ranging from 7.1 to 9.5. The desorption of isoflavones on this open column achieved a desorption rate above 78%, and the recovery rate was over 77%. Wang et al. (2018) reported the podophyllotoxin enrichment from Sinopodophyllum hexandrum by using plastic bead D101 with the results showing that purified podophyllotoxin increased 7.4 times compared with the original extract, achieving a recovery efficiency of 74.6%. Pan et al. (2017) also reported that the enrichment of chelidonine from Chelidonium majus with a recovery efficiency was 80.8%, increasing the concentration of chelidonine from 2.7 to 37.8%. If the requirement in the concentration of isoflavones increased (isoflavones up to 58%) by using XAD-4 (Wu et al. 2015), genistein was up to approximately 90% by using AB-8 (Li et al. 2012), it is necessary to purify deeply before passing the column, resulting in the low recovery efficiency. Therefore, with an attempt on increasing the isoflavone content to add to food products or as oral tablets, the method using D101 met this goal to purify isoflavones using distilled water and ethanol. Specially, these solvents were used to be safe, non-toxic, and easy to recover as well as achieve high recovery efficiency. On the other hand, the results illustrated the content of heavy metals including lead (Pb), cadmium (Cd), arsenic (As), mercury (Hg) in two samples of extract were examined under the quantitative limitation of the analytical method.
Acute toxicity of soy isoflavone extract
The mice were dosed extract samples at the rate of 10 g/kgBW. The results in acute toxicity after 72 h and rat's weight are recorded (see Supplementary material). The results showed that both extract samples did not cause any death of experimental mice by oral administration. Thus, extracts do not cause acute toxicity in white mice BALB/c orally with the highest test dose of 10 g/kgBW.
The average mass tracking of the mouse during the 7-day tests showed there was no difference in the average mass of the mouse as compared to the control group before drinking the extracts (P > 0.05). Likewise, within the 1st, 4th and 7th-day tests, the experimental mice in the control group and the test group did not raise the difference (P > 0.05). This result was consistent with those previous studies, that purified genistein and daidzein were tested according to the above method with doses of 2000 mg/kg (McClain et al. 2006) and 5000 mg/kgBW, respectively, which also did not affect the normal growth of experimental mice (Laddha et al. 2020). Our result in this section was almost accorded to previous studies, for instance, the acute toxicity test for the ethanolic extract from leaves of Pericampylus glaucus (Lam.) Merr. in BALB/c mice was performed with the oral dose of 4 g/kg with no visualization in symptom or mortality after oral administration.
Estrogen activity test in vitro
MCF7 cells are cells with estrogen receptors and dependent estrogen. Therefore, MCF7 cells need to be nourished in estrogen media. Based on this feature, MCF7 cells are included in the E-Screen assay and are raised in the DMEM with free steroids. Thus, MCF7 cells will not be able to proliferate. Therefore, using E-Screen assay, the proliferation of MCF7 cells under the impact of the sample enables the estrogen activity of the research sample to be evaluated. The results indicated that the activity of estrogen in extracts have proliferated cells of 7.4%, 14.9%, respectively as compared to negative controls that cells are only cultured in a non-steroid media, while positive 17β-estradiol was stable in experiments (Fig. 1).
Fig. 1.
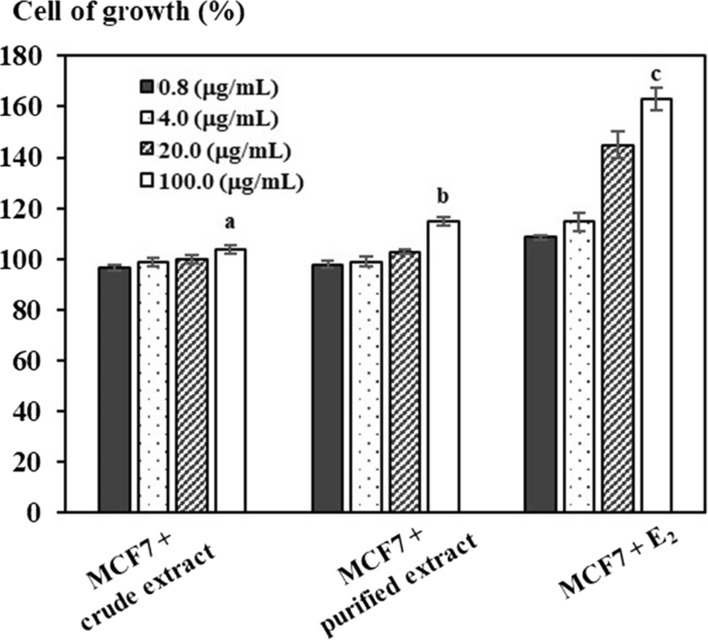
Growth of cell under an impact of experimental estrogen extracts
The results showed that at a concentration of 100 μg dry matter(DM)/mL, extracts exhibited cell proliferation, 7.4% and 14.9%, respectively, compared with a negative control where cells were cultured only in a steroid-free medium. While 17β-estradiol was consistently positive in the experiment, there was an EC50 value of 12.86 ± 2.19 nM. Thus, at the same concentration of 100 μgDM/mL, total isoflavones in PET were 37.23 μM greater approximately 9 times than CET. Meanwhile, the cytostimulation rate of PET was 3.83 times higher than that of CET. Consequently, there is a positive correlation between the proliferation rate and isoflavones concentration in target samples. However, if the concentration in the proliferative samples was increased, it has an inhibitory effect on MCF-7 cell growth. Uifălean et al. (2016) evaluated the estrogenic activity of isoflavones in the MCF-7 cell line, reported that the 20% cytostimulation rate was 22.59 µg extract/mL and the 20% cell inhibition rate was 166.34 µg extract/mL, but total isoflavones of the sample were not indicated. Our study firstly illustrated the estrogenic activity of target samples, but they were subjected to experimental analysis of this activity in rats for further evaluation.
Estrogen activity test in vivo
Furthermore, estrogen activity in vivo tests with 5 experimental lots established above was investigated at its impact on the weight of experimental mice, which was determined before and after dosing extracts. The results in Table 1 showed that the mass of the mouse has caused the ovarian disorder after using extracts without differences compared to pathological control (P > 0.05). However, the 17β-estradiol (E2) injected in the mouse has caused an increase in the weight of the mouse compared to pathological controls and pre-uptaking dose of extracts.
Table 1.
Effect of estrogen activity on the uterine weight of mice
| Experimental lot | Body weight (g) | Uterine weight after treatment (mg/10 g BW) | ||
|---|---|---|---|---|
| Before treatment | After treatment | |||
| Physiological control | 33.00 ± 2.36 | 36.10 ± 0.38 | 29.8a ± 1.1 | |
| VCD + Crude extract | 30.88 ± 1.64 | 31.18 ± 1.58 | 27.3b ± 1.4 | |
| VCD + purified extract | 31.50 ± 1.43 | 31.08 ± 1.45 | 26.6b ± 1.4 | |
| VCD | 31.00 ± 2.30 | 30.32 ± 2.75 | 20.7c ± 1.3 | |
| VCD + E2 | 30.68 ± 1.96 | 33.00 ± 2.44 | 28.2ab ± 1.7 | |
The letter a, b, c showed significant differences at P < 0.05
Results in Table 1 indicated that the uterine mass per the weight of 6 mice from pathological control lot was lower than physiological lot without injecting VCD at a statistically significant level (P < 0.05). On the other hand, the mouse was treated with extracts form, this index increased and was higher than the pathological control. However, the difference has not shown statistical significance (P > 0.05). Similarly, the experiments were realized to express the impact of isoflavones on the uterus of the mouse, whose mass was recorded the fluctuation during the 7th-day treatment shown in Fig. 2.
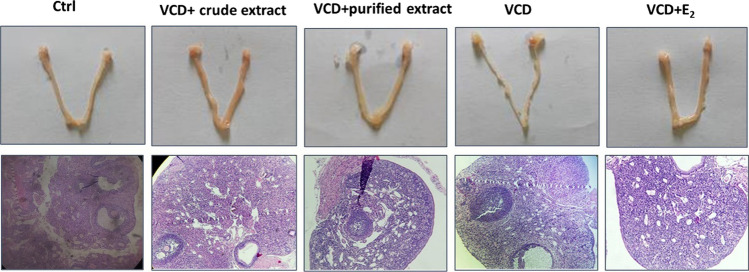
Fig. 2.
Uterine and light visualization of mouse follicle in experimental lots
According to Shin et al. (2017), the uterine weight with an injection of VCD usually decreases because VCD causes a reduction in estrogen, reducing the effect of estrogen on uterus. Uptaking extracts has improved this index and figured out a positive impact of the test samples on the recovery of uterus after causing a genital impairment due to VCD injection. Furthermore, the influence of experimental extracts was investigated at the growth of mouse follicles with VCD (Figs. 2 and 3). The results figured out the injection of VCD caused a reduction in primitive, preliminary and secondary follicles compared to physiological control (P < 0.05). However, since uptaking extracts, both follicles were higher than the negative control. In particular, the number of preliminary follicles with mice in lot-02 was higher than that in lot-01 (P > 0.05).
Fig. 3.
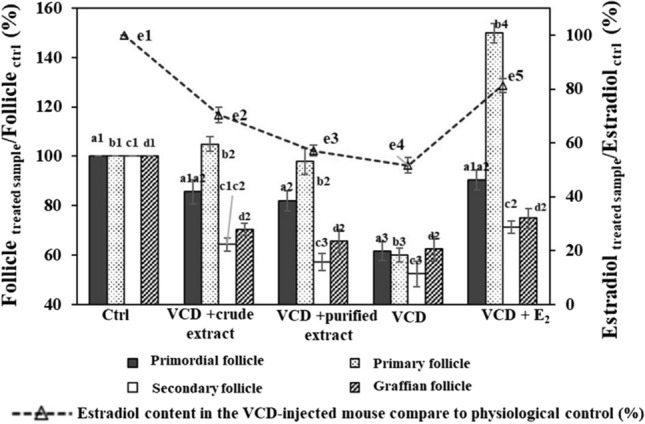
Growth rate of follicles and estrogen in experimental lots compared to the physiological control. Ctrl: Physiological control lot; VCD + M1: Lot treated with VCD and dosed CET; VCD + M2: Lot treated with VCD and untaken PET; VCD + E2: Lot treated with VCD and dosed E2. The letter a, b, c showed significant differences at P < 0.05
The estradiol was well-performed in this experimental model, in which the percentage of secondary follicles increased up to 1.5 times compared with the physiological control (P < 0.05). Thus, the analyzed samples had positive effects on the recovery and development of oocytes in mice with ovarian atrophy. Mazambani et al. (2018) performed tests on daidzin-rich isoflavones from extract with a VCD-injected rat model. Their result showed that this composition increased the menstrual cycle of rats compared with the negative control. This has been a common phenomenon in premenopausal women due to a lack of estrogen. Ma et al. (2021) also reported an increase in the menstrual cycle in experimental mice treated to cause polycystic ovary syndrome (Table 2).
Table 2.
Inhibition of lipid peroxidation ex vivo of isoflavone extracts using liver homogenates as media
| Concentration (µg/mL) | % Inhibition | ||
|---|---|---|---|
| Crude extract | Purified extract | Trolox | |
| 500.0 | 34.92 ± 1.79 | 58.24 ± 1.17 | ‒ |
| 100.0 | 10.02 ± 1.64 | 29.41 ± 1.03 | 76.79 ± 0.36 |
| 20.0 | 1.27 ± 0.18 | 20.83 ± 0.64 | 66.83 ± 0.72 |
| 4.0 | ‒ | 15.00 ± 1.62 | 45.23 ± 0.90 |
| 0.8 | ‒ | ‒ | 23.26 ± 1.93 |
| IC50 | > 500 | 360.65 ± 17.49 | 6.97 ± 0.67 |
Besides VCD impairs ovarian function and the number of follicles, it significantly alters the production of steroid hormones by the ovaries including decreasing the hormone of 17β-estradiol. As known, this action causes most of the disorders associated with menopause. The results in Fig. 3 showed that the estradiol content in the group of mice injected with VCD as a negative control decreased sharply compared to the physiological control at a statistically significant level (P < 0.05). In contrast, the estradiol content in positive control increased compared to the control at a statistically significant level (P < 0.05). Meanwhile, the estradiol content in the mice with PET was lower than that with CET, but this difference did not show statistical significance (P > 0.05).
Thus, supplementing isoflavones with soybean extract did not affect the BW of mice, increased the volume of the uterus of mice compared to the physiological control with ovarian insufficiency. On the other hand, isoflavone extracts increased follicular growth, and the serum estradiol content compared to the negative control. Isoflavone extracts have somewhat demonstrated the similar function of estrogen, improving the physiology of rats with impaired ovarian function.
Active test for the protection of liver cells
In order to evaluate liver protection in vitro, research samples were rated on the model of HepG2 cell culture along with the presence of CCl4, which can be toxic that directly or indirectly impacted through the creation of free radical, leading to a process of peroxys in lipid molecules of cell and seriously damaging to cells. Besides, HepG2 cells are considered a suitable in vitro model for this test, because they still maintain a lot of physiological and morphological characteristics similar to benign liver cells. Therefore, this model can be used to research the damage in liver cells and screening natural compounds against hepatotoxicity (González et al. 2017; Pareek et al. 2013). Research samples and quercetin have surveyed the impact of HepG2 cell protection from toxic effects of CCl4 and results were shown in Fig. 4.
Fig. 4.
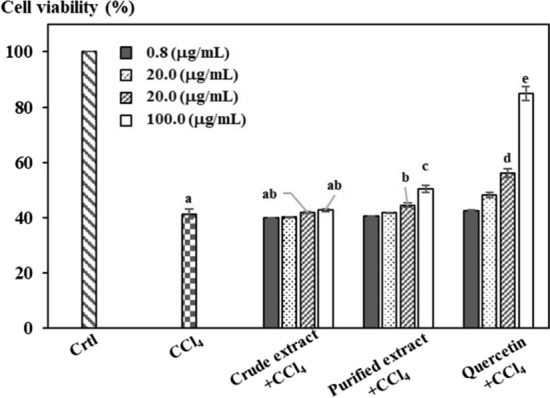
Cell viability of target samples at different concentrations compared to the control
Under the impact of CCl4, extracts have shown the capacity of liver cell protection at a 100 μg/mL concentration of extracts, reaching 2.3 ± 0.05% and 15.4 ± 1.2% respectively. In addition, the protection activity of the research samples has not reached 50%, so it does not identify the EC50 value, and quecertin operates stably in the experiment at this value of 57.45 ± 2.9%.
Inhibition of lipid peroxidation in liver homogenate
The determination of antioxidant activity through inhibition of membrane lipid peroxidation (MDA test) resulted in Online Resource 2, illustrating that extract samples were capable of inhibiting the lipid peroxidation, in which IC50 of latter was 360.65 ± 17.49 µg/mL, was lower than that of former, and 51.74 times compared to that of Trolox. In an in vitro experiment, Rüfer and Kulling (2006) reported that the antioxidant activities of six naturally inducing isoflavones were more effective than the positive controls quercetin and ascorbic acid. Likewise, daidzein was indicated the moderate inhibitory effect among TPA-induced H2O2 formation in research of Wei et al. (1995). Furthermore, their study clearly indicated the effects of flavones/isoflavones on H2O2 production by 12–0-tetradecanoylphorboi-13-acetate (TPA) but genistein poorly inhibited a potent protein kinase C activity with an apparent IC50 > 100 µg/mL. It was seen in Online Resource 1 that genistein compounds have just accounted for 5% of total isoflavones while genistin and daidzin were dominant compositions in soybean. These compounds were in majority of aglycones that were well-documented in the antioxidant activity of soy isoflavones (Liu et al. 2005; Rajaei et al. 2019). With this result, it was possible to resist lipid peroxide, protect consumers' health of soy isoflavones purified by D101 macroporous.
DPPH scavenging activity
The linear equation correlation between the ability to scan the free radicals of the samples and IC50 of the isoflavones-inducing extracts were built as IC50-CET of 2474.2 µg/mL and IC50-PET of 335.1 µg/mL (see Online Resource 3). Consequently, the survey in the efficiency of isoflavone freedom radicals DPPH was proportional to the amount of Isoflavones in extract, this is likely to increase the scavenging of free radicals in PET to 7.4 times. Compared to IC50-Ascorbic of 12.4 µg/mL, the antioxidant capacity in grams of CET and PET was 0.005 (gAEAC) and 0.037 (gAEAC), respectively.
Conclusion
With incalculable dangers from functional foods that women mostly used, soy isoflavones are considered to be the safest alternative to purified estrogen. The process of soy isoflavones extraction requires merely simple operations from Vietnamese soybean and purified by plastic bead D101. The toxicity test on crude and PET showed that there was no acute toxicity found in mice. Likewise, the evaluation of extract for estrogen activity in vivo, in vitro, and ex vivo indicated the remarkable recovery of uterine mice after using the VCD model for uterus depletion. Therefore, the analysis of this study can be applied for further advancement on the application of soy isoflavones. Finally, the novelty of this current work proves that PET from this purification model was capable of good antioxidant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Domestic Master/Ph.D. Scholarship Programme of Vingroup Innovation Foundation, under grant number VINIF.2019.TS.63.
Abbreviations
- BALB/c
Bagg albino/c mice
- BW
Body weight
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- E2
17β-Estradiol
- E-Screen
Estrogenicity screen
- FBS
Fetal bovine serum
- FRAP
Ferric reducing antioxidant power
- HRT
Hormone replacement therapy
- LDL
Low density lipoprotein
- MCF-7
Michigan Cancer Foundation-7
- MDA
Malonyl dialdehyde
- MTT
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- VCD
4-Vinylcyclohexen diepoxide
- CET
Crude extract
- PET
Purified extract
Author contribution
TNTT Writing—original draft; Methodology, Validation, Formal analysis: Visualization, TMHT Validation, Formal analysis, TDPN Conceptualization; Supervision, Funding acquisition, Project administration, VXB Writing—review and editing, DTT Writing—review and editing, LT-v Writing—review and editing, KSK Writing—review and editing, Project administration, KWC Writing—review and editing, PLS Supervision, Project administration.
Funding
This research work was supported under the Domestic Master/Ph.D. Scholarship Programme of Vingroup Innovation Foundation, under grant number VINIF.2019.TS.63.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not Applicable.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Not Applicable.
Consent to participate
All authors declare their consent to participate in this research work.
Consent for publication
The authors declare that the work described has not been published nor under consideration for publication elsewhere and that if accepted, it will not be published elsewhere in the same form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thi Ngoc Thu Tran, Email: ttnthu@ute.udn.vn.
Thi Minh Hanh Truong, Email: ttmhanh@dut.udn.vn.
Pau Loke Show, Email: PauLoke.Show@nottinham.edu.my, Email: showpauloke@gmail.com.
References
- Abdel-Monem NM, Abdel-Azeem AM, El-Ashry E-SH, Ghareeb DA, Nabil-Adam A. Pretreatment hepatoprotective effect of the marine fungus derived from sponge on hepatic toxicity induced by heavy metals in rats. Biomed Res Int. 2013;2013:1–15. doi: 10.1155/2013/510879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Afifi NA, Alabsi AM, Bakri MM, Ramanathan A. Acute and sub-acute oral toxicity of Dracaena cinnabari resin methanol extract in rats. BMC Complement Altern Med. 2018;18:1–14. doi: 10.1186/s12906-018-2110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetau-Pelissero C (2013) Isoflavonoids and phytoestrogenic activity 77
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PtSmith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- González LT, Minsky NW, Espinosa LEM, Aranda RS, Meseguer JP, Pérez PC. In vitro assessment of hepatoprotective agents against damage induced by acetaminophen and CCl4. BMC Complement Altern Med. 2017;17:1–10. doi: 10.1186/s12906-016-1506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer DR, Kammerer J, Carle R (2019) Adsorption and ion exchange for the recovery and fractionation of polyphenols: principles and applications. In: Polyphenols in plants. Elsevier, pp 327–339
- Kifayatullah M, Mustafa MS, Sengupta P, Sarker MMR, Das A, Das SK. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J Acute Dis. 2015;4:309–315. doi: 10.1016/j.joad.2015.06.010. [DOI] [Google Scholar]
- Laddha AP, Murugesan S, Kulkarni YA (2020) In-vivo and in-silico toxicity studies of daidzein: an isoflavone from soy. Drug Chem Toxicol 1–9 [DOI] [PubMed]
- Li J, Chase HA. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat Prod Rep. 2010;27:1493–1510. doi: 10.1039/c0np00015a. [DOI] [PubMed] [Google Scholar]
- Li H, Liu J, Li D, Wang H. Study on separation and purification of genistein in the soybean residue using macroporous resin adsorption. Ind Eng Chem Res. 2012;51:44–49. doi: 10.1021/ie200057e. [DOI] [Google Scholar]
- Liu J, Chang SK, Wiesenborn D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J Agric Food Chem. 2005;53:2333–2340. doi: 10.1021/jf048552e. [DOI] [PubMed] [Google Scholar]
- Ma X, Li X, Ma L, Chen Y, He S. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF-κB signaling pathway. Bioengineered. 2021;12:7215–7223. doi: 10.1080/21655979.2021.1979864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazambani S, Johnson K, Vemuri S, Alshafi S, Cheriyath V. Daidzin-rich soy isoflavone extracts promote estrous cycling in VCD-induced menopause mouse model. Nutr Food Sci Int J. 2018;4:96–99. [Google Scholar]
- McClain RM, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Minervini F, Giannoccaro A, Cavallini A, Visconti A. Investigations on cellular proliferation induced by zearalenone and its derivatives in relation to the estrogenic parameters. Toxicol Lett. 2005;159:272–283. doi: 10.1016/j.toxlet.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Pan J, Yang Y, Zhang R, Yao H, Ge K, Zhang MMa L Enrichment of chelidonine from Chelidonium majus L. using macroporous resin and its antifungal activity. J Chromatogr B. 2017;1070:7–14. doi: 10.1016/j.jchromb.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150:973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]
- Rajaei S, Alihemmati A, Abedelahi A. Antioxidant effect of genistein on ovarian tissue morphology, oxidant and antioxidant activity in rats with induced polycystic ovary syndrome. Int J Reprod Biomed. 2019;17:11. doi: 10.18502/ijrm.v17i1.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberto G, Baratta MT, Deans SG, Dorman HD. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- Rüfer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54:2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- Shin D, Ha J, Hong SB, Kang G-H, Hwang D-S, Bae H. Schisandrae Fructus reduces symptoms of 4-vinylcyclohexene diepoxide-induced ovarian failure in mice. Evid Based Complement Altern Med. 2017;2017:1–9. doi: 10.1155/2017/2564787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TNT, Chew KW, Bui XV, Nguyen TDP, Le TTA, Truong TMH, Show PL. Optimization of isoflavones extraction from soybeans using full factorial design. J Food Process Preserv. 2019;43:e14078. doi: 10.1111/jfpp.14078. [DOI] [Google Scholar]
- Uifălean A, Schneider S, Gierok P, Ionescu C, Iuga CA, Lalk M. The impact of soy isoflavones on MCF-7 and MDA-MB-231 breast cancer cells using a global metabolomic approach. Int J Mol Sci. 2016;17:1443. doi: 10.3390/ijms17091443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang G, Chi X, Chen S. Green and efficient extraction of podophyllotoxin from Sinopodophyllum hexandrum by optimized subcritical water extraction combined with macroporous resin enrichment. Ind Crops Prod. 2018;121:267–276. doi: 10.1016/j.indcrop.2018.05.024. [DOI] [Google Scholar]
- Wei H, Bowen R, Cai Q, Barnes S, Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 1995;208:124–130. doi: 10.3181/00379727-208-43844. [DOI] [PubMed] [Google Scholar]
- Wu S, Wang Y, Gong G, Li F, Ren H, Liu Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.) Food Bioprod Process. 2015;93:148–155. doi: 10.1016/j.fbp.2013.12.006. [DOI] [Google Scholar]
- Yoon G-A, Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr Res Pract. 2014;8:618–624. doi: 10.4162/nrp.2014.8.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EJ, Ng KM, Luo KQ. Extraction and purification of isoflavones from soybeans and characterization of their estrogenic activities. J Agric Food Chem. 2007;55:6940–6950. doi: 10.1021/jf0708903. [DOI] [PubMed] [Google Scholar]
- Zimmer AR, Leonardi B, Miron D, Schapoval E, de Oliveira JR, Gosmann G. Antioxidant and anti-inflammatory properties of Capsicum baccatum: from traditional use to scientific approach. J Ethnopharmacol. 2012;139:228–233. doi: 10.1016/j.jep.2011.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not Applicable.