Abstract
Renal disorders are able to make patients more susceptible to infections, including those caused oral cavity infections. Today, it has been proven that during end stage renal failure, hemodialysis results in acquired immune response defects through failings of humoral and cell-mediated immune. This survey was intended to assess the molecular epidemiology and associated risk factors of Entamoeba gingivalis and Trichomonas tenax as the main oral cavity protozoa in hemodialysis patients in Lorestan Province, western Iran. The investigation was performed on 73 hemodialysis patients referring to hemodialysis centers of Lorestan Province, Iran during May 2021 to February 2022. The frequency of oral cavity protozoa was investigated using microscopic and conventional polymerase chain reaction (PCR). A provided questionnaire with a number of demographical information and related risk factors was completed for each patient. The results showed that E. gingivalis and T. tenax parasites were found in 18 (24.6%) and 20 (27.4%) of the hemodialysis participants by microscopic and PCR test, respectively. Among samples, 13 (17.8%) of the hemodialysis participants were infected with E. gingivalis; whereas 7 (9.6%) of the participants were positive for T. tenax. No significant association was reported between gender, age, education, flossing, use of mouthwash, and prevalence of oral protozoa in hemodialysis participants. However, a significant correlation was observed among between living in rural regions (OR = 13.16; 95% CI = 2.64–56.81; p = 0.002), brushing teeth (OR = 8.51; 95% CI = 1.71–42.3; p = 0.009) and prevalence of oral protozoa in hemodialysis participants. The findings of these epidemiological study clearly showed the high frequency of oral cavity parasites in hemodialysis patients in Lorestan Province, Western Iran. Awareness of the main risk factors for oral cavity parasites particularly teeth brushing is necessary in refining public and oral health policies in hemodialysis patients. Consequently, dental practitioners, nephrologist, and urologist must be alert of these risk factors to carefully identify and achieve oral health concerns in hemodialysis patients to prevent the oral diseases and infections.
Keywords: Entamoeba gingivalis, Trichomonas tenax, PCR, Renal failure, Lorestan
Introduction
With the advancement of technology and medical science, dentists are increasingly faced with systemic diseases and its specific medical complexities, and patients with these problems should be treated by a dentist (Nazir et al. 2019). Therefore, information on oral symptoms and their special considerations are very important to achieve the desired treatment. Among these various systemic diseases that affect the periodontal and oral tissues, end stage renal failure (ESRF) can be referred to as kidney disease (O’Connor and Corcoran 2012). ESRF is a progressive and chronic bilateral destruction of nephrons, the functional units of the kidney, and it occurs when more than 50% of about 2 million nephrons lose their function (Liyanage et al. 2015). At this stage of renal impairment, patients should be treated with dialysis, and this is a life-saving method that satisfactorily reduces mortality from kidney disease (Himmelfarb and Ikizler 2010). Hemodialysis patients have a weakened immune system due to abnormalities in the function of lymphocytes and a decrease in their number, disturbances in immunological responses such as phagocytosis and chemotaxis, as well as abnormalities in complement function. (Sharif et al. 2015).
The oral cavity is home to a variety of microorganisms, so that hundreds of types of microbes have been identified in the mouth (Lenka et al. 2020). Today, it has been proven that the oral cavity can even act as a primary site for the spread of microorganisms to other parts of the body and cause disease in the heart, lungs and other organs of the body (Kitamoto et al. 2020). The oral cavity as a complex ecosystem has received less attention in parasitological studies. Entamoeba gingivalis and Trichomonas tenax are oral protozoa (Deo and Deshmukh 2019). These organisms are found on the surface of teeth and gums and periodontal pockets near cervical teeth and are rarely found in tonsil crypts, and some researchers believe that they can cause tooth decay, gingivitis, gingival periodontitis and respiratory tract infections (Bonner et al. 2014; Marty et al. 2017). Where, they transmitted through the spreading of oral discharges, the habit of shared utensils, dirty hands, dental tools, and kissing (Bao et al. 2020; Eslahi et al. 2021). With respect to the prevalence of these parasites, even though several investigations have been performed in children with in people with periodontitis and gingivitis (Mehr et al. 2015), Down syndrome (Yaseen et al. 2021), patients undergoing chemotherapy (Al-Muathen et al. 2020); however, the prevalence of oral protozoa and their associated risk factors in hemodialysis (HD) patients has not been reported. This survey was intended to assess the molecular epidemiology and associated risk factors of E. gingivalis and T. tenax as the main oral cavity protozoa in hemodialysis patients in Lorestan Province, western Iran.
Materials and methods
Ethical statement
This epidemiological survey was permitted by ethical committee of Lorestan University of Medical Sciences, Iran (IR.LUMS.REC.1401.062). However, a transcribed informed consent form was got from all participant.
Participants
The cross-sectional descriptive survey was performed on 73 hemodialysis patients referring to hemodialysis centers of Lorestan Province, Iran during August 2021 to February 2022. Participants who have received systemic antibiotics in the last 90 days and also immunocompromised patients were discarded.
Questionnaire
For each patient, before sampling, a provided questionnaire with a number of demographical information and related risk factors, e.g. age, gender, residence, smoking, education, toothbrush, and mouthwash was completed for each patient.
Sample collection
Two specimens were obtained from each patient by means of sterile swabs from saliva and dental plaques for microscopic examinations. In addition, the third sample was put into a tube with sterile physiological saline and preserved at − 20 °C for molecular tests.
Polymerase chain reaction (PCR) assay
To extract the DNA samples, Qiagen kits was were used based on the protocol of producer. The extracted DNA was applied to amplify of the SrRNA gene for E. gingivalis suing the primers of forward (5’ CGCGCATTCGAACAGGAATGTAGA-3’) and reverse (5’- CGAACGCCTTTTCAATAGTATCTTCATTCA- 3’ as well as 18S ribosomal RNA gene for T. tenax using the primers of forward (5’- CATGACCAG-TTGCATCGATGGCATTC -3’) and reverse (5’- CGTCCAAAGATTCTGCCACTAACAAG -3') according the previous study (13). The thermal condition was 6 min at 93 °C for early denaturation, 35 cycles of 30 s at 93 °C, 30 s at 57 °C, 60 s at 70 °C, and a last extension phase of 10 min at 71 °C. Then the obtained amplicons using agarose gel (1%) were electrophoresed. Positive and negative controls were DNA of standard strains and distilled water, respectively (Gupta and Yassin 2013).
Microscopic examination
The smears were prepared from saliva and dental plaque samples on a glass slide, stained by Giemsa stain and were studied using a light microscope.
Statistical analysis
We analyzed the collected data by SPSS software version 25.0. Chi-square-test, Fisher exact test, and logistic regression are applied to check the association among the variables and the prevalence of oral cavity protozoan parasites. P < 0.05 will be considered as a significant level.
Results
Participants
The mean age of the hemodialysis participants was 47.6 ± 13.2 years; whereas the majority of participants were male (52, 71.2%). The most of participants were lived in urban regions (53, 76.7%) and the rest lived in rural region. By education, 58 (79.4%) participants had higher education than diploma, while 15 (10.6%) participants had lower education than diploma. Most participants (42, 57.5%) were 30–60 years old. By brushing teeth, 43 (58.9%) participants brushed their teeth daily. Floss and mouthwash were used by 29 (39.7%) and 9 (12.3%) of participants, respectively (Table 1).
Table 1.
Frequency of E. gingivalis and T. tenax in hemodialysis participants in Lorestan Province, western Iran according to the demographic features and related risk factors by univariate regression analysis
| Group | Totally | Oral cavity parasites | Crude OR | 95%CI | P value | |
|---|---|---|---|---|---|---|
| No. (%) | Positive No. (%) | Negative No. (%) | ||||
| Age | ||||||
| < 30 yrs | 11 (15.1) | 4 (36.4) | 7 (63.6) | 1 | 1 | – |
| 30–60 yrs | 42 (57.5) | 13 (31.0) | 29 (69.0) | 2.54 | 0.63–10.2 | 0.189 |
| 60 < | 20 (27.4) | 3 (15.0) | 17 (85.0) | |||
| Education | ||||||
| < Diploma | 15 (20.5) | 4 (26.7) | 77 (73.3) | 1 | 1 | – |
| ≥ Diploma | 58 (79.5) | 16 (27.6) | 42 (72.4) | 1.04 | 0.29–3.77 | 0.943 |
| Residence | ||||||
| Rural | 17 (23.2) | 12 (70.6) | 5 (19.4) | 1 | 1 | – |
| Urban | 56 (76.8) | 8 (14.3) | 48 (85.7) | 0.069 | 0.019–0.251 | < 0.001* |
| Gender | ||||||
| Female | 21 (28.8) | 3 (14.3) | 18 (85.7) | 0.291 | 0.75–11.27 | 0.121 |
| Male | 52 (71.2) | 17 (32.7) | 35 (67.3) | 1 | 1 | – |
| Brushing | ||||||
| Yes | 43 (58.9) | 5 (11.6) | 38 (88.4) | 0.132 | 0.04–0.426 | 0.001* |
| No | 30 (41.1) | 15 (50) | 15 (50) | 1 | 1 | – |
| Flossing | ||||||
| Yes | 29 (29.7) | 9 (31.0) | 20 (69) | 1 | 1 | – |
| No | 44 (60.3) | 11 (25.0) | 33 (75.0) | 0.135 | 0.476–3.82 | 0.572 |
| Mouthwash | ||||||
| Yes | 9 (12.3) | 3 (33.3) | 6 (66.6) | 1.38 | 0.311–6.15 | 0.671 |
| No | 64 (87.7) | 20 (27.4) | 53 (72.6) | 1 | 1 | – |
Frequency of oral cavity protozoan parasites
The results showed that E. gingivalis and T. tenax parasites were reported in 18 (24.6%) of the hemodialysis participants by microscopic examination. Among samples, 12 (66.7%) of the hemodialysis participants were infected with E. gingivalis; whereas 6 (33.3%) of the participants were positive for T. tenax. By PCR, E. gingivalis and T. tenax parasites were observed in 20 (27.4%) of the hemodialysis participants, respectively (Fig. 1). Among samples, 13 (65.0%) of the hemodialysis participants were infected with E. gingivalis; whereas 7 (35.0%) of the participants were positive for T. tenax.
Fig. 1.
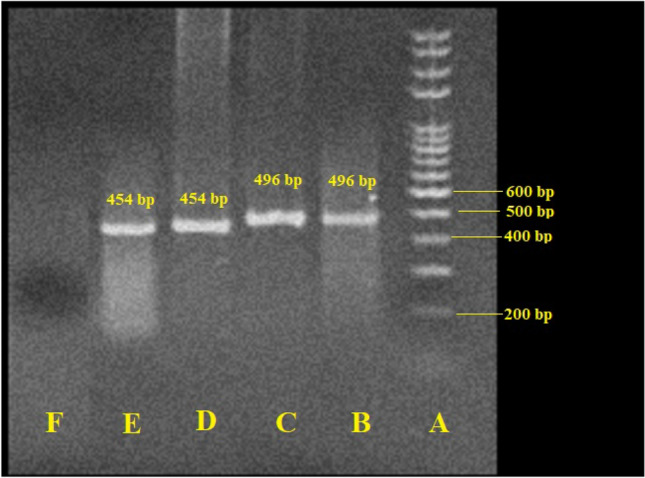
Agarose gel electrophoresis of SrRNA and 18 s rRNA genes for Entamoeba gingivalis and Trichomonas tenax, respectively. A: Ladder (size marker), 100 bp; B: positive control (T. tenax, 496 bp); C: positive sample of T. tenax; D: positive control (E. gingivalis, 454 bp); E: positive sample of E. gingivalis; E: negative control (distilled water)
Risk factors
No significant association was found among gender (p = 0.151), age (p = 0.324), education (p = 0.998), flossing (p = 0.601), use of mouthwash (p = 0.698) and prevalence of oral protozoa in hemodialysis participants. However, a significant correlation was observed among between living in rural regions (p < 0.001), brushing teeth (p < 0.001) and prevalence of oral protozoa in hemodialysis participants. As indicated in Table 2, in the multivariate model, living in rural regions (p = 0.002) and use of mouthwash (p < 0.001) were significantly associated with the frequency of these parasites.
Table 2.
Comparison of E. gingivalis and T. tenax in hemodialysis participants in Lorestan Province, western Iran based on the associated risk factors by multivariate regression analysis
| Group | Crude OR | 95%CI | P value |
|---|---|---|---|
| Age | 0.144 | 0.15–13.75 | 0.749 |
| Gender | 0.570 | 0.09–3.31 | 0.532 |
| Education | 0.766 | 0.115–5.10 | 0.783 |
| Residence | 12.16 | 2.64–56.81 | 0.002* |
| Brushing | 8.51 | 1.71–42.3 | 0.009* |
| Flossing | 0.430 | 0.10–1.82 | 0.253 |
| Mouthwash | 0.202 | 0.212–19.24 | 0.541 |
*p < 0.05, difference was statistically significant
Discussion
Renal disorders are able to make patients more susceptible to infections, including those caused oral cavity infections (Liyanage et al. 2015). Today, it has been proven that during ESRF, hemodialysis results in acquired immune response defects through failings of humoral and cell-mediated immune (Girndt et al. 1999). Moreover, previous study revealed that hemodialysis not only persuades T cells apoptosis, and declined phagocytic ability of immune cells (e.g. monocytes and neutrophils), but also disrupt pro-inflammatory cytokines production through direct interaction with artificial membranes in HD machines (Eleftheriadis et al. 2007; Muniz-Junqueira et al. 2005; Tetta et al. 1990). Today, it has been proven that the oral cavity can even act as a primary site for the spread of microorganisms to the body and cause disease in the heart, lungs and other organs of the body (Lenka et al. 2020). Hence, this survey was intended to assess the molecular epidemiology and related risk factors of E. gingivalis and T. tenax in hemodialysis patients in Lorestan Province, western Iran.
The results showed that E. gingivalis and T. tenax parasites were found in 18 (24.6%) of the hemodialysis participants by microscopic examination. Among samples, 12 (66.7%) of the hemodialysis participants were infected with E. gingivalis; whereas 6 (33.3%) of the participants were positive for T. tenax. By PCR, E. gingivalis and T. tenax parasites were reported in 20 (27.4%) of the hemodialysis participants, respectively. Among samples, 13 (65.0%) of the hemodialysis participants were infected with E. gingivalis; whereas 7 (35.0%) of the participants were positive for T. tenax. Considering the prevalence of oral cavity parasites in Iran, Mehr et al. (2015) showed that the frequency of T. tenax in 52 Down syndrome patients with periodontal disease referred to Dental Clinics in Tabriz, Iran was 18.8% by PCR assay. Gharavi et al. (2006) have showed that between 240 patients in Tehran, 41.7% and 9.2% of the patients were positive for E. gingivalis and T. tenax, respectively. Maraghi et al. (2012) also have revealed that the frequency of E. gingivalis and T. tenax was 0.5% and 0% by microscopic examinations in periodontitis patients in Khozestan Province, Iran, respectively. Another study conducted by Sharifi et al. (2020) on 315 adolescents in Kerman province by culturing and PCR, the findings exhibited that by culture media and PCR the oral cavity parasites was 9.2% and 11.4%, respectively; whereas, 11.7% and 2.2% of the adolescents were positive E. gingivalis and T. tenax. By the prevalence of these parasites in the present studied region, Derikvand (2018) have showed that the frequency rate of E. gingivalis and T. tenax in 76 periodontitis patients was 17.1% and 14.5%, respectively. In the study conducted, Mahmoudvand et al. (2018) exhibited that from 140 patients referred to Khorramabad Dental clinics, 15.4% and 10.7% were positive by microscopic examinations for E. gingivalis and T. tenax, respectively. Nevertheless, it should be mentioned that the variance among the results of the current study and previous surveys may related to various factors, e.g. target group, sample size, used diagnostic methods, and lifestyles of studies regions (Özçelık et al. 2010).
We reported no significant association between gender, age, education, flossing (p = 0.601), use of mouthwash and prevalence of oral protozoa in hemodialysis participants. However, a significant correlation was observed among between living in rural regions (p < 0.001), brushing teeth (p < 0.001) and prevalence of oral protozoa in hemodialysis participants. In consistent with our results, in studies performed by Derikvand (2018) and Mahmoudvand et al. (2018), a significant correlation between teeth brushing and the frequency of these oral cavity parasites. Previous studies have reported people living in rural regions poor due to lack of attention to oral health, more frequency of tooth loss and gum diseases was observed (Chen 2018; Zander et al. 2013). Likewise, our results exhibited that there was a significant correlation between living in rural regions and the frequency of these parasites.
Conclusion
The findings of these epidemiological study clearly showed the high frequency of oral cavity parasites in hemodialysis patients in Lorestan Province, Western Iran. Awareness of the main risk factors for oral cavity parasites particularly teeth brushing is necessary in refining public and oral health policies in hemodialysis patients. Consequently, dental practitioners, nephrologist, and urologist must be alert of these risk factors to carefully identify and achieve oral health concerns in hemodialysis patients to prevent the oral diseases and infections.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Muathen DM, Yasir LA, Sachit HG. Incidence of Entamoeba gingivalis and Trichomonas tenax in the oral cavity of periodontal and patients under chemotherapy, confirmed with in vivo study. Indian J Forensic Med Toxicol. 2020;14(3):2450–2455. [Google Scholar]
- Bao X, Wiehe R, Dommisch H, Schaefer AS. Entamoeba gingivalis causes oral inflammation and tissue destruction. J Dent Res. 2020;99(5):561–567. doi: 10.1177/0022034520901738. [DOI] [PubMed] [Google Scholar]
- Bonner M, Amard V, Bar-Pinatel C, Charpentier F, Chatard JM, Desmuyck Y, et al. Detection of the amoeba Entamoeba gingivalis in periodontal pockets. Parasite. 2014;21:30. doi: 10.1051/parasite/2014029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY. Misperception of oral health among adults in rural areas: a fundamental but neglected issue in primary healthcare. Int J Environ Res Public Health. 2018;15(10):2187. doi: 10.3390/ijerph15102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derikvand N. Frequency and associated risk factors of Entamoeba gingivalis and Trichomonas tenax among patients with periodontitis in Western Iran. J Rese Med Dental Sci. 2018;6(5):99–103. [Google Scholar]
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20(5):440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Eslahi AV, Olfatifar M, Abdoli A, Houshmand E, Johkool MG, Zarabadipour M, Abadi PA, Ghorbani A, Mirzadeh M, Badri M. The neglected role of Trichomonas tenax in oral diseases: a systematic review and meta-analysis. Acta Parasitol. 2021;66(3):715–732. doi: 10.1007/s11686-021-00340-4. [DOI] [PubMed] [Google Scholar]
- Gharavi MJ, Hekmat S, Ebrahimi A, Jahani MR. Buccal cavity protozoa in patients referred to the faculty of dentistry in Tehran, Iran Iranian. J Parasitol. 2006;1(1):43–46. [Google Scholar]
- Girndt M, Sester U, Sester M, Kaul H, Kohler H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transpl. 1999;14(12):2807–2810. doi: 10.1093/ndt/14.12.2807. [DOI] [PubMed] [Google Scholar]
- Gupta V, Yassin MH. Infection and hemodialysis access: an updated review. Infect Disord Drug Targets. 2013;13(3):196–205. doi: 10.2174/1871526511313030008. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Ikizler TA. Hemodialysis. New England J Med. 2010;363(19):1833–1845. doi: 10.1056/NEJMra0902710. [DOI] [PubMed] [Google Scholar]
- Kitamoto S, Nagao-Kitamoto H, Hein R, Schmidt TM, Kamada N. The bacterial connection between the oral cavity and the gut diseases. J Dental Res. 2020;99(9):1021–1029. doi: 10.1177/0022034520924633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka S, Swain SK, Bhuyan R, Sahu MC. Fungal infection in the oral cavity: a review. Int J Cur Res Rev. 2020;12(18):149–153. doi: 10.31782/IJCRR.2020.121821. [DOI] [Google Scholar]
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Sepahvand A, Niazi M, Momeninejad N, Sepahvand SM, Behzadian M. Prevalence and risk factors of oral cavity protozoa (Entamoeba gingivalis and Trichomonas tenax) among patients with dental cavity caries. J Res Med Dent Sci. 2018;6(5):42–46. [Google Scholar]
- Maraghi S, Azizi A, Rahdar M, Vazirianzadeh B. A study on the frequency of buccal cavity protozoa in patients with periodontitis and gingivitis in Ahvaz, southwest of Iran in 2009. Jundishapur J Health Sci. 2012;4(4):85–89. [Google Scholar]
- Marty M, Lemaitre M, Kemoun P, Morrier JJ, Monsarrat P. Trichomonas Tenax and periodontal diseases: a concise review. Parasitology. 2017;144:1417–1425. doi: 10.1017/S0031182017000701. [DOI] [PubMed] [Google Scholar]
- Mehr AK, Zarandi A, Anush K. Prevalence of oral Trichomonas tenax in periodontal lesions of down syndrome in Tabriz, Iran. J Clin Diagn Res. 2015;9(7):ZC88–90. doi: 10.7860/JCDR/2015/14725.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz-Junqueira MI, Braga Lopes C, Magalhaes CA, Schleicher CC, Veiga JP. Acute and chronic influence of hemodialysis according to the membrane used on phagocytic function of neutrophils and monocytes and pro-inflammatory cytokines production in chronic renal failure patients. Life Sci. 2005;77(25):3141–3155. doi: 10.1016/j.lfs.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Nazir MA, Izhar F, Akhtar K, Almas K. Dentists' awareness about the link between oral and systemic health. J Fam Commun Med. 2019;26(3):206. doi: 10.4103/jfcm.JFCM_55_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. American family physician. 2012;85(7):705–710. [PubMed] [Google Scholar]
- Özçelık SE, Gedık T, Gedık R, Malatyali E. Investigation of the relationship between oral and dental health and presence of Entamoeba gingivalis and Trichomonas tenax. Turkiye Parazitolojii Dergisi. 2010;34(4):155–159. doi: 10.5152/tpd.2010.03. [DOI] [PubMed] [Google Scholar]
- Sharif MR, Chitsazian Z, Moosavian M, Raygan F, Nikoueinejad H, Sharif AR, Einollahi B. Immune disorders in hemodialysis patients. Iran J Kidney Dis. 2015;9(2):84–96. [PubMed] [Google Scholar]
- Sharifi M, Jahanimoghadam F, Babaei Z, Mohammadi MA, Sharifi F, Hatami N, Danesh M, Poureslami P, Poureslami H. Prevalence and associated-factors for Entamoeba gingivalis in adolescents in Southeastern Iran by culture and PCR, 2017. Iran J Public Health. 2020;49(2):351. [PMC free article] [PubMed] [Google Scholar]
- Tetta C, Camussi G, Turello E, Salomone M, Aimo G, Priolo G, et al. Production of cytokines in hemodialysis. Blood Purif. 1990;8(6):337–346. doi: 10.1159/000169988. [DOI] [PubMed] [Google Scholar]
- Yaseen A, Mahafzah A, Dababseh D, Taim D, Hamdan AA, Al-Fraihat E, Hassona Y, Şahin GÖ, Santi-Rocca J, Sallam M. Oral Colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-based study in health, gingivitis, and periodontitis. Front Cell Infect Microbiol. 2021;11:782805. doi: 10.3389/fcimb.2021.782805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander A, Sivaneswaran S, Skinner J, Byun R, Jalaludin B. Risk factors for dental caries in small rural and regional Australian communities. Rural Remote Health. 2013;13(3):2492. [PubMed] [Google Scholar]


