Abstract
The commercial significance of accurate and simple quantification of 2-Acetyl-1-pyrroline (2-AP) cannot be overstated. Present study was carried out to standardize a method for extraction and accurate quantitation of 2-AP from rice grain using GC-MS/MS equipped with HS-SPME auto sampler. The effect of sample quantity, addition of solvent, grinding process, sample particle size, head space parameters and SPME fiber incubation parameters, were optimized in the developed method. Dehusked rice powder (2 g) prepared under liquid nitrogen, and passed through the 80-mesh sieve, incubated for 40 min at 80 °C in headspace, followed by fiber (DVB/Carbon WR/PDMS) saturation time of 15 min, could produce the maximum response. The recovery of 2-AP from fortified sample ranged between 7.02 and 9.02% at 50–200 ng g−1 fortification irrespective of the grain matrices used. Standard addition method was appropriate to overcome the matrix effect and recovery of 2-AP was more than 90% using this method. The developed method was further utilized for quantification of 2-AP in four Basmati and two non-Basmati aromatic rice samples. The content of 2-AP ranged between 57.17 and 147.10 ng g−1 of rice and varied with geographical location. This fully automated method could improve the work efficiency and reduce error during the volatile extraction and adsorption phase.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05674-7.
Keywords: HS-SPME, Aroma, Standard addition method, Rice
Introduction
Rice is one the most important staple food and quenches the calorific requirement of one-fifth of world population (FAOSTAT 2017). Among the different rice types, aromatic rice enjoys higher consumer preference for its culinary properties. Thus, the export of aromatic rice from Asian countries to Europe, United States and Middle Eastern countries has been on rise, contributing considerable amount of forex earnings to the exporting countries. During the year 2020–2021 about 46, 30, 463.14 metric tons of basmati rice (an aromatic rice) with a monetary value of 4018.71 US$ Mill (Rs. 29,849.89 Crores) has been exported from India (APEDA 2021). The export of non-basmati rice which includes short grain aromatic rice had a growth of 109% from USD 2925 million in FY 2013–2014 to USD 6115 million in FY 2021–2022 (PIB 2022).
2-Acetyl-1-pyrroline (2-AP) is the principal compound that contributes to aroma in rice. 2-AP is a chemical that emits a buttered-popcorn-like aroma at specific concentrations. The biochemical pathways associated with 2-AP synthesis has been extensively studied and it has been found that the amino acid proline serves as one of the primary precursors for 2-AP synthesis (Mo et al. 2015; Poonlaphdecha et al. 2016). Further, molecular investigations have shown that the recessive allele of Badh2 (betaine aldehyde dehydrogenase) gene is responsible for the 2-AP synthesis. With the ample information available for 2-AP synthesis, continuous efforts are being made by researchers to increase the 2-AP content in rice varieties and to combine it with other economic traits such as disease or pest resistance.
The ability to accurately measure the quantity of 2-AP is one of the important constraints in aromatic rice breeding programs intended to enhance its content in the rice grain. Numerous methods have been developed in recent years to measure the quantity of 2-AP in rice grains such as direct extraction, distillation, headspace and solid phase micro extraction (SPME) (Mahatheeranont et al. 2001; Wangcharoen et al. 2016; Sansenya et al. 2018; Lee et al. 2019; Guo et al., 2020). Among these methods, SPME is a simple, rapid, economic and sensitive method for collecting volatiles from headspace system and this method is widely used for quantitative determination of 2-AP in rice (Mathure et al. 2011; Hopfer et al. 2016). However, a number of factors namely, the sample weight, incubation time and temperature etc. influence the accurate quantitation of 2-AP and other volatile compounds (Mathure et al. 2011). These parameters need to be validated in fully automated HS-SPME system. We assumed that fully automated system will reduce the error and will improve the efficiency of the quantitation method. Automated extraction using HS-SPME followed by estimation using chromatography instruments, could increase laboratory throughput with higher precision and accuracy, reduce costs and maximize work efficiency (Kremser et al. 2016; Locatelli et al. 2019). Further, there are reports that the matrix has a strong effect on 2-AP release. The recovery of 2-AP was as low as 0.3% using HS-SPME method when compared to the solvent extraction method (Grimm et al. 2001). Large amounts of energy are required to release the analyte from the matrix in HS-SPME (Kenessov et al. 2016), which in turn can deteriorate the SPME fiber (Reyes-Garces et al. 2017). To compensate the matrix effect, standard addition methods are recommended (Ouyang and Pawliszyn 2008; Sante 2017). Interaction of 2-AP with rice matrices may vary with different varieties depending on its biochemical content. Standard addition method was compared with scented and non-scented varieties for better result.
The present study was carried out to develop a standardized method of 2-AP extraction from rice grain for its accurate quantitation. The developed method was validated using samples of six different rice cultivars grown during the same cropping season.
Materials and methods
Chemicals and reagent
2-Acetyl-1-pyrolline (2-AP, Toranto Research Chemicals, Canada) 10% W/W in toluene was supplied by Thermo Fischer Scientific, India. Different solvents namely, hexane, toluene or DMSO were procured from Merck, India and were of GC-MS grade or the highest quality grade.
Rice samples and its preparation
Polished rice sample of Gobindobhog, a non-basmati, short-grain aromatic rice was collected from the local market (Goodlife brand, Reliance Fresh, India) and was used for the method development of 2-AP quantification in rice. Rice samples were ground to fine powder using Ball mill grinder (MILL MIX20, DOMEL, Slovenia).
Instrumentation
Identification and quantification of 2-AP was done using GC-MS/MS (Trace 1300-TSQ 9000, Thermo Scientific, USA) equipped with HS-SPME auto sampler (TriPlus RSH, Thermo Scientific, USA) and a capillary column (TR-WAXMS; length: 30 m, i.d.: 0.25 mm, film thickness: 0.25 µm). The following oven temperature program was followed; initial hold at 50 °C for 2 min, temperature raised to 100 °C at a ramp rate of 5 °C min−1 and hold for 10 min, temperature was increased to 230 °C at the ramp rate of 15 °C min−1 and hold for 2 min. Samples were ionized by the positive electron impact (EI) mode using electron energy of − 70 eV. Helium (99.999% purity) at the flow rate of 1 mL min−1 was used as the carrier gas. Argon (99.999% purity) was used in the collision cell. Both the MS transfer line temperature and ion source temperatures were set at 230 °C. The selected reaction monitoring (SRM) mode was designated, and the following transitions were used for identification of 2-AP; m/z 111.1 > 82.1 (CE: 10 eV), m/z 111.1 > 83.1 (CE: 5 eV), m/z 83.1 > 82.1(CE: 10 eV). The SRM transition, m/z 111.1 > 83.1 (CE: 10 eV) was used for quantification.
While measuring 2-AP, rice sample was kept into a 20 mL headspace vial (Thermo Scientific, USA) and the vials were sealed with crimp cap assembled PTFE/silicone silicon septa (Thermo Scientific, USA) and robotically transposed into the TriPlus headspace system. The headspace vials were kept in an in-built agitator at a particular temperature for a particular time during which the agitator was sequentially switched on and off at every 10 s. It was concentrated by using a SPME fiber (1 cm, 50/30 μm Divinylbenzene/Carbon Wide Range/Polydimethylsiloxane, DVB/Carbon WR/PDMS, Dark Gray, Thermo Scientific, Switzerland) for a particular duration. The fibre needle speed inside the vial was 20 mm sec−1.
After the equilibrium time, the fiber was directly injected into Split–Splitless injector port for 2 min. The injector temperature was kept at 250 °C with a split flow of 5 mL min−1 and split ratio of 5. The carrier gas flow was set at 1 mL min−1. The fiber was conditioned at 250 °C for 1 min before the injection and for 15 min post injection.
Method optimization for extraction and quantification of 2-AP
The polished rice market sample of Gobindobhog was used for standardization of the method. Optimization of the method was carried out with respect to sample weight, grinding method (with or without addition of liquid nitrogen while grinding), sieving, temperature of incubation, duration of incubation, adsorption time. Absolute area count was used as a measure of thequantity during optimization. The conditions were varied to achieve maximum increase in 2-AP peak area.
Sample weight
Three different sample weights (1, 2 and 3 g) of rice grains were initially considered for the standardization process. It has been reported that the high amount of variation in peak areas were observed when 0.5 g of sample was taken for the analysis (Mathure et al. 2010). Thus, we did not go for sample weight less than 1 g. Further, peak area of 2-AP in 2 g of sample did not have significant difference as compared to 3 g of sample. Thus, we did not go beyond 3 g sample weight and 2 g of sample was used for further standardization of the method. Few reports suggested that addition of water can alter the release of 2-AP from powdered rice. The influence of water on the extraction of 2-AP was evaluated by addition of 600 µL of water into 2 g of sample.
Grinding and sieving
The effect of grinding temperature was evaluated by grinding rice grains with or without liquid nitrogen in a mill grinder (MILL MIX20, DOMEL, Slovenia). Effect of size of the rice powder was also evaluated. The powders obtained were passed through sieves of different mesh numbers (< 60, 60–80, > 80) and the passed through powders were used for the standardization.
Incubation temperature and duration in headspace
Four different incubation temperatures ranging between 60 and 90 °C (at 10 °C interval); seven different incubation time varying from 20 to 80 min (at 10 min interval) were used for method standardization. Equilibration time of the fiber was varied between 5 and 25 min (at 5 min interval) to obtain optimum adsorption time.
Effect of solvent
The impact of addition of different solvents was also evaluated by addition of 5 µL either of hexane, toluene or dimethyl sulfoxide (DMSO) to the 2 g powdered sample.
Preparation of standard curve for quantification of 2-AP from rice matrix
2-AP stock solution was diluted appropriately in hexane and working standards were prepared. A fixed volume (5 µL) of different concentrations of 2-AP (equivalent to 1, 10, 50, 100, 200, 400 ng of 2-AP) was put into the headspace vials separately to know the linearity as well as fiber absorption/saturation capacity. 2-AP is present in all rice varieties at different concentrations. A high concentration of the volatile compound is present in the scented rice varieties including Basmati. As, we did not have any rice variety without 2-AP content, the calibration graph for 2-AP was generated by standard addition method. For this, 2 g sample of Gobindobhog rice was spiked with different concentrations of 2-AP (equivalent to 1, 10, 50, 100, 200, 400 ng of 2-AP in the vial) and was processed following the optimized conditions and peak areas were recorded. Similarly, the most popular non-aromatic rice variety Swarna (MTU 7029), grown at ICAR-NRRI, was spiked with different concentrations of 2-AP (equivalent to 1, 10, 50, 100, 200, 400 ng of 2-AP in the vial) and peak area was used to derive the standard curve.
Quantification of 2-AP in major rice varieties
The developed method was validated by further applying it for quantification of 2-AP in four Basmati varieties and two purified sort of Gobindobhog landraces. The Basmati varieties are Pusa Basmati 1121, Pusa Basmati 1609, Pusa Basmati 1718 and Pusa Basmati 1728. The Gobindobhog samples include two out of the four sorts purified at the Institute during 2015–2019 from the landrace Gobindobhog (CRRI No. 43627) collected from West Bengal. All the genotypes selected for quantification of 2-AP using the developed method were grown at the Research Farm of ICAR-National Rice Research Institute, Cuttack, Odisha, India (20.5°N, 86°E, 23.5 m above MSL) during June–December, 2020. Standard agronomic package practices were followed uniformly. At physiological maturity, grains were harvested, and sun dried to 14% moisture. Paddy samples for the four Basmati varieties were also collected from farmers’ field located at Karnal, Haryana, India (29.6857°N, 76.9905°E, 240 m above MSL), that lies in the GI (Geographical Indication) tagged area demarcated for Basmati, grown during the same cropping season of the year. The samples were used for analysis after 3 months of harvesting. Paddy samples of the respective genotypes were dehusked using Lab Husker, (Model THU 35 B, Satake make) and were ground to fine powder using Ball mill grinder (MILL MIX20, DOMEL, Slovenia). Ground samples were used for 2-AP estimation using the standard addition method. For this, each sample (2 g) was fortified with 100 ng of 2-AP standard in hexane, screw capped and mixed properly. The fortified samples were used for 2-AP estimation as per the standardized method.
Data analysis
All the experiments were carried out in completely randomized design with three replicates. Data represent mean ± standard deviation of three replicates. One-way ANOVA was done to know the differences among treatment for a particular parameter using SPSS software and post-hoc analysis using least significant difference was carried out (SPSS 2016).
Results and discussion
Method standardization
A liquid injection of 2-AP standard (5 ppm) was done in scan mode to know the mass fragments. We obtained different mass fragments and most prominent were m/z of 111.1, 83.1, and 68. The m/z of 111, 83, and 68 were often mentioned as characteristic fragments of 2-AP in the literature (Ying et al. 2011; Liu et al. 2015; Peddamma et al. 2018). Among them, the fragment ion with m/z of 83.1 was a base peak generated by α-cleavage reaction of a carbonyl group, and its fragment ion was αC5H9N. SRM was optimized to know the mass transitions. They were m/z 111.1 > 82.1 (CE: 10 eV), m/z 111.1 > 83.1 (CE: 5 eV), m/z 83.1 > 82.1 (CE: 10 eV). The SRM transition, m/z 111.1 > 83.1 (CE: 10 eV) was used for quantification. After SRM optimization, 2-AP standard solution was kept into the headspace vials to check the retention time of 2-AP obtained by using SPME fibre. It was 13.55 ± 0.05 min (Supplementary Fig. 1).
Method optimization
Quantitation of targeted compounds by HS-SPME depends on the equilibration between sample matrix, headspace of vial and fiber. Thus, variations in the extraction parameters (temperature, time, moisture content, fibre equilibration time) result in alteration in the quantified amount of 2-AP. The standardization of the parameters was done based on the response signal/area (Vas and Vekey 2004).
Impact of sample quantity
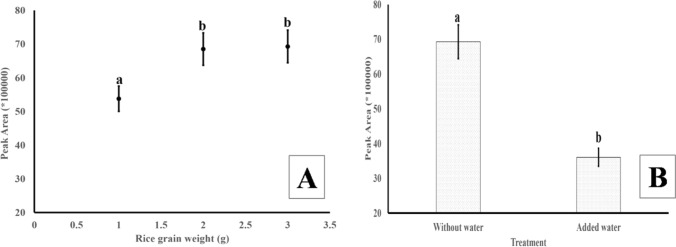
Three different quantities of rice powder i.e. 1, 2 and 3 g were used and highest peak area was observed when 3 g of sample was used. There was 27% increase in peak area when sample size was increased from 1 to 2 g and treatments were significantly different whereas, less than 2% increase in peak area was obtained from 2 to 3 g. This could be either because of the saturation of the headspace vial or the fiber itself. This suggests that 2 g of sample is appropriate for quantification of 2-AP. Thus, further standardization was carried out with 2 g of sample (Fig. 1a). In an earlier report, addition of water (300 µL g−1) in rice powder enhanced the 2-AP peak area (Mathure et al. 2010). However, a 47% decline in 2-AP peak area was observed when water (300 µL g−1) was added to the sample (Fig. 1b).
Fig. 1.
Recovery of 2-AP from different sample quantities (A). Effect of addition of water on the recovery of 2-AP (B). Different letters above the bars indicate significant difference (p < 0.05)
Impact of grinding temperature and sieve size
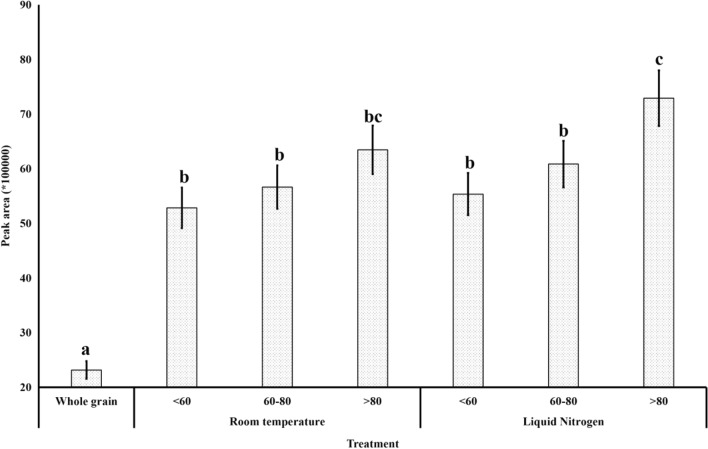
As 2-AP is volatile in nature, the heat produced during grinding might incur the loss of 2-AP. It was found that the 2-AP area was highest in rice grain powder prepared under liquid nitrogen and passed through the sieve with mesh size of > 80. The 2-AP peak area went on decreasing with increase in mesh size (Fig. 2). Samples ground in liquid nitrogen had higher peak area as compared to those ground without it (Fig. 2). The recovery of 2-AP was found to be 4–14% higher when the samples were ground in liquid nitrogen as compared to the samples ground under ambient condition. The recovery of 2-AP was 20–30% higher in rice powder passed through sieve with 80 mesh as compared to the sample which did not pass through the sieve of 60 mesh, and they differed significantly. Powdered samples with > 80 mesh sizes were chosen for further study. Generation of heat during grinding may lead to loss of the volatile compounds like 2-AP. Further, finer the powder size, higher is the release of 2-AP. Perhaps, this the first study in reporting the effect of grinding temperature and particle size on the extraction of 2-AP.
Fig. 2.
Effect of sample particle sizes and grinding temperature on the recovery of 2-AP from rice sample. Different letters above the bars indicate significant difference (p < 0.05)
Head space incubation temperature and time
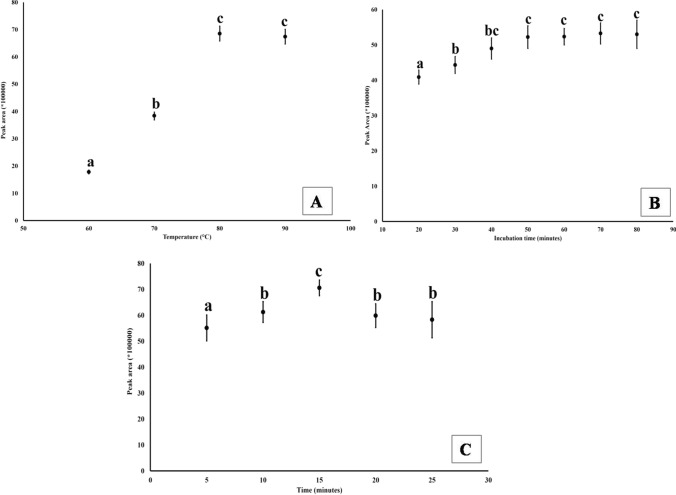
The headspace incubation temperature was varied between 60 °C and 90 °C and it was observed that peak area was increased with increase in temperature till 80 °C and effect of temperature had significant on 2-AP recovery (Fig. 3a). There was 115 and 78.4% increase in peak area when headspace incubation temperature was increased to 60 to 70 °C and 70 to 80 °C, respectively. Whereas, less than 2% variation was observed when headspace incubation temperature increased from 80 to 90 °C. Thus, the incubation temperature of 80 °C was chosen. The incubation time was increased from 20 to 80 min (Fig. 3b). The recovered quantity of 2-AP increased with time. There was increase in 7–10% of peak area with increase in 10 min’ incubation till 40 min. However, it did not increase significantly after 40 min. Thereafter, there was only 2% increase in peak area till 80 min.
Fig. 3.
Effect of head space incubation temperature (A), time (B) and SPME absorption time (C) on the recovery of 2-AP from rice sample. Different letters above the bars indicate significant difference (p < 0.05)
Incubation temperature and time are two critical parameters which need to be standardized. It has been observed that high pretreatment temperature during sample preparation and headspace incubation may alter the 2-AP recovery due to volatilization as well as new molecule formation (Yoshihashi 2006; Maraval et al. 2010; Hopfer et al. 2016). But synthesis of new 2-AP in rice sample during high incubation temperature is yet to be unanimously accepted. The incubation temperatures varied from 50 to 120 °C in different studies (Hu et al. 2014; Hopfer et al. 2016; Peddamma et al. 2018; Sansenya et al. 2018). Based on our results, we propose the optimum head space temperature of 80 °C. High temperatures (> 85 °C) along with longer period of incubation may result in the deformation of crimp cap assembled PTFE/silicone silicon septa of the sampling vials and may lead to loss of aroma (Grimm et al. 2001). High energy may release more analyte from the matrix in HS-SPME (Kenessov et al. 2016), but it can deteriorate the SPME fiber (Reyes-Garces et al. 2017).
Impact of adsorption time
SPME fibre was kept for 2-AP adsorption in the head space vial for different duration from 5 to 25 min with the increment of 5 min each. The highest peak area was observed when 15 min of incubation was done. There was decrease in peak area after 15 min of adsorption (Fig. 3c).
Impact of solvent addition
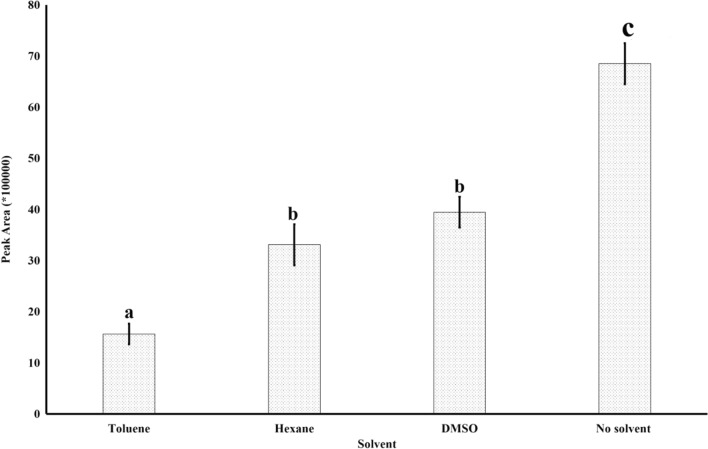
Few literatures suggested addition of solvents can improve the extraction efficiency of 2-AP (Grimm et al. 2001). However, it was observed that the peak area decreased considerably when solvents (hexane, toluene and DMSO) were added as compared to the rice blank samples. The area of 2-AP was lowest in case of toluene followed by hexane and DMSO (Fig. 4). This indicates that addition of other compounds can suppress the extraction of the targeted compound. This may be due to the fiber saturation or adsorption of the solvent molecules. The sorption material of the fiber is DVB/Carbon WR/PDMS which has both polar and non-polar surfaces. Upon heating, solvent vapors attracted over the fiber keeping limited number of spaces for 2-AP sorption. DMSO has the lowest effect, may be due to its high boiling point.
Fig. 4.
Effect of solvent addition on the recovery of 2-AP from rice sample. Different letters above the bars indicate significant difference (p < 0.05)
Preparation of standard curve for quantification of 2-AP
The standard curve for quantification of 2-AP was prepared at different concentration levels. Solvent matched standard curve was prepared from 1 to 200 µg L−1 concentration with regression coefficient value of 0.999 (Table 1). Beyond 200 µg L−1, there was 2-AP saturation in the SPME fibre. The standard curve with the scented rice (Gobindobhog) and non-scented rice (Swarna) was prepared with 1–400 µg kg−1 concentrations with r2 value of 0.993 and 0.995, respectively. The equations were y = 33125x + 3,000,000, y = 35394x + 802,491 for the scented rice (Gobindobhog) and non-scented rice (Swarna), respectively. The recovery of 2-AP from Gobindobhog and Swarna as 7.9–29.2% and 7.02–24.58% in 1–200 ppb fortification, respectively. The higher recovery was obtained at 1 and 10 ppb level of 2-AP fortification in both the varieties. Whereas, the recovery of 2-AP was 7.9–9.2 and 7.02–7.55% within 50–200 ppb level of fortification. In a previous literature, recovery of 2-AP was as low as 0.3% using HS-SPME method when compared to the solvent extraction method (Grimm et al. 2001). Generally, the matrix effect caused by starch adsorption of 2-AP. Being highly lipophilic in nature, 2-AP could be attached with lipid molecules in the starch matrix (Yoshihashi et al. 2005). It has been recommended that matrix-matched standard curve should be used for 2-AP estimation to avoid matrix effects (Jung et al. 2019; Lee et al. 2019).
Table 1.
Method validation parameters
| Concentration (ng/g) | Recovery | Matrix effect | Regression equations | ||||
|---|---|---|---|---|---|---|---|
| Gobindobhog | Swarna | Gobindobhog | Swarna | Equation | Regression coefficient | ||
| 1 | 18.35 | 21.67 | 81.65 | 78.33 | Solvent matched | y = 414390x | 0.999 |
| 10 | 29.34 | 24.59 | 70.66 | 75.41 | Matrix matched (Gobindobhog) | y = 33125x + 3,000,000 | 0.993 |
| 50 | 8.12 | 7.37 | 91.88 | 92.63 | Matrix matched (Swarna) | y = 35394x + 802,491 | 0.995 |
| 100 | 7.99 | 7.03 | 92.01 | 92.97 | |||
| 200 | 9.22 | 7.56 | 90.78 | 92.44 | |||
Quantification of 2-AP in different rice samples
The 2-AP content in different rice varieties grown is presented in Table 2. It was found that GB Type 1, a pureline derived from the short grain aromatic landrace, Gobindobhog had the highest 2-AP content (93.85 ng g−1) among the different cultivars grown in Cuttack. The 2-AP content in the Basmati varieties grown in Cuttack ranged between 57.16 and 60.97 ng g−1, the highest being observed in Pusa Basmati 1121. The 2-AP content ranged between 81.22 and 147.10 ng g−1 in Basmati varieties grown in Karnal. The 2-AP content was 1.37–2.5 times higher in rice varieties grown in the geographical region, Karnal as compared to Cuttack. Cuttack region has always had higher temperature during flowing and grain filling of rice varieties as compared to Karnal region where traditionally Basmati varieties are grown.
Table 2.
2-AP content in different rice varieties
| Rice cultivars | 2-AP content (ng/g) |
|---|---|
| Grown at Cuttack, India | |
| GB Type 1 | 93.86 ± 5.86 |
| GB Type 2 | 68.04 ± 6.64 |
| PB 1728 | 58.84 ± 7.45 |
| PB 1121 | 60.98 ± 3.56 |
| PB 1609 | 57.17 ± 6.52 |
| PB1718 | 59.29 ± 4.42 |
| Grown at Karnal, India | |
| PB 1728 | 147.10 ± 5.18 |
| PB 1121 | 87.20 ± 4.58 |
| PB 1609 | 105.19 ± 6.68 |
| PB1718 | 81.22 ± 5.56 |
Variety, rice cultivation practices, environmental conditions during production, postharvest processing, cooking conditions have all been linked to a wide range of variations in 2-AP levels in aromatic rice varieties (Wakte et al. 2016; Mahmud et al. 2018; Routray and Rayaguru 2017). Due to high matrix effect, source of variations in 2-AP content in particular variety could be linked to analysis protocols. For example, dynamic headspace sampling of 2-AP in cooked rice resulted in only 0.2–4.5 ng g−1 of 2-AP (Yang et al. 2008), whereas continuous extraction methods employing steam distillation have yielded 40–300 ng g−1 of 2-AP in cooked rice (Buttery et al. 1983). Our findings are consistent with those of scented non-basmati rice varieties collected from various locations of India, which had 2-AP concentration ranging from 0.038 to 0.920 µg g−1 and one basmati variety, Basmati-370, which had 0.4–0.45 µg g−1 of 2-AP (Mathure et al. 2014 and Hingeet al. 2016). Our findings are also similar to the SPME-based detection of 2-AP in fragrance rice (Hopfer et al. 2016). It appears that the new approach is sensitive enough to detect extremely low amounts of 2-AP in various rice cultivars. Basmati cultivars grown in geographical indication (GI) region had higher 2-AP content as compared to the non-GI region. This is due to high temperature during post flowering in eastern part of India as compared to western part of India. Higher temperature led to higher volatilization loss of 2-AP in eastern part of India.
Conclusion
Parameters to quantify the 2-AP using fully automated HS-SPME system has been standardized in the present study. The present study standardized important parameters such as sample particle size, temperature during grinding process which could impact 2-AP quantitation. Standard addition method could recover more than 90% of the 2-AP from rice matrices and the method is validated using major aromatic rice varieties to quantify the 2-AP content. As the extraction step is fully automated, the method could improve the work efficiency and reduce error during 2-AP estimation. The method could effectively be used for the screening of aroma (2-AP) content in different rice varieties. The established method further could help in promoting rice varieties in export market.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors sincerely thank Director, ICAR-National Rice Research Institute, Cuttack for providing technical and financial support. Authors also duly acknowledge the fund received under ICAR-NRRI/EAP-272. The authors declare that they have no competing interests.
Abbreviations
- 2-AP
2-Acetyl-1-pyrroline
- HS-SPME
Head space solid phase microextraction
- GC-MS/MS
Triple quadrupole gas chromatography mass spectrometry
- DVB/Carbon WR/PDMS
Divinylbenzene/carbon wide range/polydimethylsiloxane
- DMSO
Dimethyl sulfoxide
- PTFE
Polytetrafluoroethylene
Author contributions
Conceptualization: TA, SS, AM; Experiment: AM, NB, GA; Instrumentation: AM, SS, PS, TBB; Data analysis and presentation: TA, AM, MC; Draft: AM, TA, SS, GA; Editing: MC, NB, TBB, PS, TA; Project administration: SS.
Funding
Authors also duly acknowledge the fund received under ICAR-NRRI/EAP-272.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Totan Adak and Arabinda Mahanty have contributed equally.
References
- APEDA (2021) Basmati Rice. http://apeda.gov.in/apedawebsite/SubHead_Products/Basmati_Rice.htm. Accessed on 10 June 2022
- Buttery RG, Ling LC, Juliano BO, Turnbaugh JC. Cooked rice aroma and 2-acetyl-1-pyrroline. J Agric Food Chem. 1983;31:823–826. doi: 10.1021/jf00118a036. [DOI] [Google Scholar]
- BM Corp. Released (2016). IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. SPSS Statistics for Windows, version 16 (SPSS Inc., Chicago, Ill., USA)
- FAOSTAT (2017) Crops/regions/world list/production quantity (pick lists), rice (paddy), 2018. UN Food and Agriculture Organization, Corporate Statistical Database 2020. Archived from the original on 11 May 2017
- Grimm CC, Bergman C, Delgado JT, Bryant R. Screening for 2-acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. J Agric Food Chem. 2001;49:245–249. doi: 10.1021/jf0008902. [DOI] [PubMed] [Google Scholar]
- Guo Z, Huang S, Chen M, Ni Y, Hu X, Sun N. Identification and quantitative determination of 2-acetyl-1-pyrroline using GC-TOF MS combined with HS and HS-SPME pretreatment. J Cereal Sci. 2020;93:102975. doi: 10.1016/j.jcs.2020.102975. [DOI] [Google Scholar]
- Hinge VR, Patil HB, Nadaf AB. Aroma volatile analyses and 2AP characterization at various developmental stages in basmati and non-basmati scented rice (Oryza sativa L.) cultivars. Rice. 2016;9:38. doi: 10.1186/s12284-016-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer H, Jodari F, Negre-Zakharov F, Wylie PL, Ebeler SE. HS-SPME-GC-MS/MS method for the rapid and sensitive quantitation of 2-acetyl-1-pyrroline in single rice kernels. J Agric Food Chem. 2016;64(20):4114–4120. doi: 10.1021/acs.jafc.6b00703. [DOI] [PubMed] [Google Scholar]
- Hu CY, Zhu YL, Ye YX, Qiao ZY, Cheng HY. Preconcentration and determination of 2-acetyl pyrrolidine in rice based on nafion and PDDAC coated solid-phase microextraction. J Suzhou Uni Sci Technol Nat Sci. 2014;31(4):42–45. [Google Scholar]
- Jung D, Kreher JD, Kratz HU, Michalik U. A new matrix-matched calibration strategy for static headspace gas chromatography to enable high throughputs in pharmaceutical quality control laboratories. Anal Methods. 2019;11:4242–4248. doi: 10.1039/C9AY00400A. [DOI] [Google Scholar]
- Kenessov B, Koziel JA, Bakaikina NV, Orazbayeva D. Perspectives and challenges of on-site quantification of organic pollutants in soils using solid phase microextraction. TrAC Trends Anal Chem. 2016;85:111–122. doi: 10.1016/j.trac.2016.04.007. [DOI] [Google Scholar]
- Kremser A, Jochmann MA, Schmidt TC. PAL SPME arrow—evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal Bioanal Chem. 2016;408:943–952. doi: 10.1007/s00216-015-9187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Oh Y, Kim TH, Cho YH. Quantitation of 2-acetyl-1-pyrroline in aseptic-packaged cooked fragrant rice by HS-SPME/GC-MS. Food Sci Nutr. 2019;7(1):266–272. doi: 10.1002/fsn3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Rao D, Ren Y, Qiu Y, Chen X, Xu Z. A method on determination the 2-acetyl-1-pyrroline of aromatic rice. J Hunan Agric Univ. 2015;41(3):234–238. [Google Scholar]
- Locatelli M, Tartaglia A, Piccolantonio S, et al. Innovative configurations of sample preparation techniques applied in bioanalytical chemistry: a review. Curr Anal Chem. 2019;15(7):731–744. doi: 10.2174/1573411015666190301145042. [DOI] [Google Scholar]
- Mahatheeranont S, Keawsa-ard S, Dumri K. Quantification of the rice aroma compound, 2-acetyl-1-pyrroline, in uncooked khao dawk mali 105 brown rice. J Agric Food Chem. 2001;49(2):773–779. doi: 10.1021/jf000885y. [DOI] [PubMed] [Google Scholar]
- Mahmud MMC, Oh Y, Kim TH, Cho YH, Lee YS. Effects of milling on aromatics, lipophilic phytonutrients, and fatty acids in unprocessed white rice of scented rice ‘Cheonjihyang-1-se’. Food Sci Biotechnol. 2018;27:383–392. doi: 10.1007/s10068-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraval I, Sen K, Agrebi A, Menut C, Morere A, Boulanger R, Gay F, Mestres C, Gunata Z. Quantification of 2-acetyl-1-pyrroline in rice by stable isotope dilution assay through headspace solid-phase microextraction coupled to gas chromatography-tandem mass spectrometry. Anal Chim Acta. 2010;675(2):148–155. doi: 10.1016/j.aca.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Mathure S, Jawali N, Nadaf A. Diversity analysis in selected non-basmati scented rice collection. Rice Sci. 2010;17(1):35–42. doi: 10.1016/S1672-6308(08)60102-X. [DOI] [Google Scholar]
- Mathure SV, Wakte KV, Jawali N, Nadaf AB. Quantification of 2-acetyl-1-pyrroline and other rice aroma volatiles among Indian scented rice cultivars by HS-SPME/GC-FID. Food Anal Methods. 2011;4:326–333. doi: 10.1007/s12161-010-9171-3. [DOI] [Google Scholar]
- Mathure SV, Jawali N, Thengane RJ, Nadaf AB. Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food Chem. 2014;142:383–391. doi: 10.1016/j.foodchem.2013.07.066. [DOI] [PubMed] [Google Scholar]
- Mo Z, Li W, Pan S, Fitzgerald TL, Xiao F, Tang Y, Wang Y, Duan W, Tian H, Tnag X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice. 2015;8:1–10. doi: 10.1186/s12284-015-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang G, Pawliszyn J. A critical review in calibration methods for solid-phase microextraction. Anal Chim Acta. 2008;627(2):184–197. doi: 10.1016/j.aca.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Peddamma SK, Ragichedu PK, Maddala S, Rao DS, Lella VSR, Konne K, Sripada P, Krishnan GS, Singh AK, Maganti SM. Insight of aroma in brown rice through chemical assessment of 2-acetyl-1-pyrroline (2-AP) in aromatic germplasm of India. Cereal Chem. 2018;95:679–688. doi: 10.1002/cche.10081. [DOI] [Google Scholar]
- PIB (2022) https://pib.gov.in/PressReleasePage.aspx?PRID=1818313 Accessed on 8 Dec 2022
- Poonlaphdecha J, Gantet P, Maraval I, Sauvage FX, Menut C, Morere A, Boulanger R, Wust M, Gunata Z. Biosynthesis of 2-acetyl-1- pyrroline in rice calli cultures: demonstration of 1-pyrroline as a limiting substrate. Food Chem. 2016;197:965–971. doi: 10.1016/j.foodchem.2015.11.060. [DOI] [PubMed] [Google Scholar]
- Reyes-Garces N, Gionfriddo E, Gómez-Ríos GA, Alam MN, Boyacı E, Bojko B, Singh V, Grandy J, Pawliszyn J. Advances in solid phase microextraction and perspective on future directions. Anal Chem. 2017;90(1):302–360. doi: 10.1021/acs.analchem.7b04502. [DOI] [PubMed] [Google Scholar]
- Routray W, Rayaguru K. 2-acetyl-1-pyrroline: a key aroma component of aromatic rice and other food products. Food Rev Int. 2017 doi: 10.1080/87559129.2017.1347672. [DOI] [Google Scholar]
- Sansenya S, Hua Y, Chumanee S. The Correlation between 2-acetyl-1-pyrroline content, biological compounds and molecular characterization to the aroma intensities of thai local rice. J Oleo Sci. 2018;67(7):893–904. doi: 10.5650/jos.ess17238. [DOI] [PubMed] [Google Scholar]
- SANTE (2017) Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed Document SANTE/11813/2017
- Vas G, Vékey K. Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J Mass Spectrom. 2004;39(3):233–254. doi: 10.1002/jms.606. [DOI] [PubMed] [Google Scholar]
- Wakte K, Zanan R, Hinge V, Khandagale K, Nadaf A, Henry R. Thirty three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): a status review. J Sci Food Agric. 2016;97(2):384–395. doi: 10.1002/jsfa.7875. [DOI] [PubMed] [Google Scholar]
- Wangcharoen W, Phanchaisri C, Daengpok W, Phuttawong R, Hangsoongnern T, Phanchaisri B. Consumer acceptance test and some related properties of selected KDML 105 rice mutants. J Food Sci Technol. 2016;53:3550–3556. doi: 10.1007/s13197-022-05567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Shewfelt RL, Lee KS, Kays SJ. Comparison of odor-active compounds from six distinctly different rice flavor types. J Agric Food Chem. 2008;56:2780–2787. doi: 10.1021/jf072685. [DOI] [PubMed] [Google Scholar]
- Ying X, Xu X, Ouyang Y, Zhu Z, Chen M, Han S, Min J. Analysis of characteristic compound in aroma rice by gas chromatography/mass spectrometry with solid-phase microextraction. J Anal Sci. 2011;27(1):69–71. [Google Scholar]
- Yoshihashi T. Quantitative analysis on 2-acetyl-1-pyrroline of an aromatic rice by stable isotope dilution method and model studies on its formation during cooking. J Food Sci. 2006;67(2):619–622. doi: 10.1111/j.1365-2621.2002.tb10648.x. [DOI] [Google Scholar]
- Yoshihashi T, Huong NTT, Surojanametakul V, Tungtrakul P, Varanyanond W. Effect of storage conditions on 2-acetyl-1 pyrroline content in aromatic rice variety, Khaodawk mali 105. J Food Sci. 2005;70:S34–S37. doi: 10.1111/j.1365-2621.2005.tb09061.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.