Abstract
The objective of this work was to isolate a polysaccharide similar to pectin from Crataeva tapia leaves, not yet reported in the literature, and to evaluate its antioxidant, cytotoxic and immunomodulatory profile. Pectin was extracted from the leaves in three stages, organic solvent followed by acidified water and ethanol precipitation. With the pectin obtained, the physicochemical characterization of the molecule was carried out using high-performance liquid chromatography, Fourier-transform infrared spectroscopy, nuclear magnetic resonance (13C and 1H) and different thermal and elemental analysis. Furthermore, the antioxidant activities were evaluated in vitro, and using human peripheral blood mononuclear cell culture, cytotoxicity and immunostimulatory actions were investigated. Physical and chemical analyses showed characteristic signs of pectin. Antioxidant activity tests showed that pectin had moderate to low antioxidant activity. Furthermore, pectin did not affect the viability of erythrocytes and PBMC and induced an immunostimulatory state when it promoted the production of cytokines IL-6, IL-10 and TNF-α and increased the activation of CD8 + T lymphocytes. This study showed that pectin from Crataeva tapia is not cytotoxic and promoted a pro-inflammatory profile in peripheral blood mononuclear cell with application as an immunostimulating and emulsifying compound.
Keywords: Crataeva tapia, Emulsifier, Immunostimulation, Pectin, Proinflammatory
Introduction
Crataeva tapia L., Capparaceae, popularly known as “trapiá”, “tapiá” or “cabaceira”, is a forest species belonging to the Capparaceae or Capparidaceae family, native to Brazil, however, not endemic (Cornejo and Iltis 2008; Sharma et al. 2013). It is also distributed in different countries, namely: Mexico, Honduras, Guatemala, Ecuador, El Salvador, Nicaragua, Panama, Colombia, Venezuela, Peru and Argentina (Campos and Albuquerque 2021). It is a small- to medium-sized tree, between 5 and 12 m tall. It has trifoliate leaves, 8 cm leaflets, smooth, white flowers (Santos-Moura et al. 2014; Alves et al. 2017). Its fruits are rounded, 4 cm, smooth, turning yellow when ripe. Its pulp is white and involves the seeds (1 cm of brown color) (Cornejo and Iltis 2008; Sharma et al. 2013; Santos-Moura et al. 2014; Alves et al. 2017; Campos and Albuquerque 2021).
This plant has been used in afforestation and restoration of degraded areas (Pratissoli et al. 2007; Araújo et al. 2012). Its wood has great versatility and can be used in the making of canoes, musical instruments, houses, among others (Pratissoli et al. 2007). In folk medicine, its bark, flowers and fruits have been used to fight different infections (Guedes et al. 2011). In addition, its fruits can be eaten as a soft drink and a wine drink (Santos-Moura et al. 2014).
The biological potential of this plant is in its chemical composition, it has cellulose, polioses, lignin, extractives and mineral salts, which can be isolated and promote different biological activities (Araújo et al. 2012; Cabral et al. 2015; Xavier et al. 2019; Arruda et al. 2021). The extracts obtained from this species have different bioactive molecules and can be used as an antioxidant, insecticide, anti-tumor (Araújo et al. 2012), anti-inflammatory (Sharma et al. 2013), antimicrobial (Cabral et al. 2015) and herbicide (Xavier et al. 2019).
Much is known in relation to extracts; however, few works are found with the other constituents. The lignin from this plant, for example, can be used in cosmetic and pharmaceutical formulations (Arruda et al. 2021). Mineral salts and polysaccharides of C. tapia can be used as functional foods in the preparation of flour (Souza 2014). Few studies are found with the polysaccharides obtained from Crateva tapia, among which we can highlight the one carried out by Santos-Moura et al. (2014) where the authors used different extraction methods to obtain mucilage. However, due to the diversity of polysaccharides present in plants, these, when isolated, can have different technological and pharmacological applications. Among the polysaccharides, pectins have stood out for being easy to obtain and presenting nutritional and pharmacological potential (Amorim et al. 2016; Bayar et al. 2017; Kazemi et al. 2019a, b).
Pectins are polysaccharides present in plants found in the primary cell wall of plant cells and in the intercellular layers (middle lamella), contributing to the adhesion between cells, firmness and mechanical resistance of the tissue (Bayar et al. 2017; Santos et al. 2021). These polysaccharides are formed by the skeleton of α-linked galacturonic acid (1 → 4) associated with various degrees of esterified carboxyl methyl groups (Bayar et al. 2017). The main industrial process for obtaining pectin consists of slightly acidic and heated aqueous extraction (Yuliarti et al. 2015). Its application in the food industry is due to its gelling, thickening, emulsifying and stabilizing properties (Bayar et al. 2017). Pectins have also been used in the production of cosmetics and medicines (Lupi et al. 2015) and nanobiocomposites for biomedical applications (Govindaraj et al. 2018; Cortes et al. 2021; Devasvaran and Lim 2021). The benefits that this polysaccharide can promote to human health are diverse, including intestinal immunoregulation (Langhout et al. 1999), cholesterol reduction, decreased postprandial glucose concentrations (Topping and Clifton 2001), immunomodulation (Amorim et al. 2016) and antioxidant activity (Kazemi et al. 2019a).
Therefore, the isolation of pectins and pectin-like polysaccharides from leaves is an attractive alternative from the point of view of plant preservation (Santos et al. 2021; Melo et al. 2022). This is because the leaves can be easily recovered during pruning, in addition to being produced in greater quantity and faster when compared to calculus and roots (Ranieri et al. 2018; Arruda et al. 2021; Santos et al. 2021; Melo et al. 2022).
Different studies have demonstrated the importance of polysaccharides obtained from different leaves, among which we can mention the work carried out by Mzoughi et al., (2018) extracting pectin-like polysaccharide from the fructose leaves of Suaeda found that it was able to promote antioxidant, anti-inflammatory and analgesic activities. Tahmouzi and Nejat (2020) found that the pectin-like polysaccharide obtained from Althaea officinalis leaves was able to promote antioxidant and antimicrobial activity. Xia et al. (2020) evaluated a pectin-like polysaccharide obtained from leaves of Acanthopanax senticosus and observed that it could be used as an antioxidant agent.
Santos et al. (2021) isolated a pectin-like polysaccharide extracted from the leaves of Conocarpus erectus Linnaeus that was able to promote antioxidant activity, growth of probiotic Lactobacillus microorganisms (L. paracasei and L. rhamnosus), stimulate immune cells through the mechanism of oxidation stress, stimulating the proliferation and activation of TCD4 + lymphocytes, reduction in the activation of TCD8 + lymphocytes, in addition to stimulating effectors through the production of cytokines and nitric oxide, mainly producing a pro-inflammatory response of the immune system, without causing cell damage.
Melo et al. (2022) evaluating a pectin-like polysaccharide obtained from the leaves of Caesalpinia pulcherrima found that the pectin obtained did not promote a cytotoxic effect (viability > 90%), increased a subpopulation of CD8 + T lymphocytes and monocytes. Furthermore, it promoted a mostly pro-inflammatory response confirmed by the production of cytokines IL-2, -4, -6, IFN-γ and TNF-α. These studies show the potential of polysaccharides obtained from leaves.
Due to the relevance of the described biological activities, pectins have been used in emulsions as drug carriers for therapeutic applications, to increase the bioavailability of poorly water-soluble drugs in oral administration (Burapapadh et al. 2010; Sriamornsak 2011; Deshmukh 2020).
Therefore, we performed the extraction and chemical characterization of a polysaccharide similar to pectin from the leaves (which we call pectin) of Crataeva tapia, in addition to evaluating its antioxidant capacity, immunostimulating potential in human peripheral blood mononuclear cells and its properties as an emulsifying agent.
Materials and methods
Reagents
5-hydroxymethylfurfural (Merck, CAS 67-47-0), ABTS (Merck, CAS 28752-68-3), acetonitrile (Merck, CAS 75-05-8), acetic acid (Merck, CAS: 64-19-7), ascorbic acid (Cewin), phosphoric acid (Merck, CAS 7664-38-2), galacturonic acid (Merck, CAS, 91510-62-2), gallic acid (Meta Química, CAS 149-91-7), sulfuric acid (Merck, CAS 7664-93-9), trichloroacetic acid (Merck, CAS 76-03-9), anti-CD4-PerCP-Cy5.5 and anti-CD8-FITC monoclonal antibodies (BD®), arabinose (Merck,CAS 5328-37-0), sodium carbonate (Merck, CAS 497-19-8), deuterium oxide (Merck, CA S7789-20-0), DMSO (Sigma-aldrich CAS 67-68-5), DPPH (Merck, CAS 1898-66-4), ethanol (Merck, CAS 64-17-5), Folin and Ciocalteu′s phenol reagent (Merck,), dibasic sodium phosphate (Metaquimica, CAS: 10028-24-7), furfural (Merck, CAS 98-01-1), glucose (Merck, CAS 50-99-7), sodium hydroxide (Merck, CAS1310-73-2), butylated hydroxytoluene (Merck, CAS128-37-0), propidium iodide (BD Biosciences), Cytometric Bead Array kit (CBA) Human Th1/Th2 Cytokine (BD Biosciences-USA®), RPMI 1640 culture medium (Thermo Fisher Scientific), methanol (Merk, CAS 67-56-1), ammonium molybdate (Sigma-Aldrich, CAS 12054-85-2), sodium nitrite (Merck, CAS 7632-00-0), soy oil (Lyza), potassium persulfate (Dinâmica Formula), Griess reagent (Merck,), rhamnoses (Merck, CAS 10030-85-0), toluene (Merck, CAS 108-88-3), xylose (Merck, CAS 58-86-6), fetal bovine serum (Thermo Fisher Scientific), trypan blue (Merck, CAS 72-57-1), Cytofix (BD Biosciences), sodium chloride (Merck, CAS 7647-14-5), and calcium chloride (Merck, CAS 10043-52-4).
Plant
The leaves of Crataeva tapia (300 g) were collected in the garden of the Center for Biosciences of the Federal University of Pernambuco, Recife-PE, Brazil (8º 2′ 50.312 ʺ S, 34º 56′ 58.364ʺ W). A voucher specimen was identified by Marlene Barbosa and deposited at the Herbarium Geraldo Mariz of the Federal University of Pernambuco, Recife-PE, Brazil under number 87511. For the study, green and healthy leaves were selected, that is, visually intact, free of pests, diseases or color changes. The plant material was dried in an oven (Tecnal, TE-393/1) at a temperature of 60 ± 2.0 °C for 48 h. Then, it was ground in a knife mill (Fritsch-Pulverisette 14) and sieved to 100 mesh granules.
Extraction of pectin from Crataeva tapia leaves
The extraction process was carried out according to the methodology proposed by Santos et al. (2021) and Melo et al. (2022) with few modifications. Thus, the pectin extraction took place in three stages. In step I, the 100 mesh dry sheets were subjected to extraction in a Soxhlet apparatus using the toluene/ethanol system 32:68 v/v for a period of 6 h in order to remove compounds that interfere with the chemical structure of pectin (Habibi et al. 2005). Leaves free of extractives were dried in an oven at 60 ± 2.0 °C, until obtaining a constant mass. In step II, the solid fraction was extracted with water in a 2L stirred tank, under the following conditions 60 ± 2 °C at 1200 rpm for 4 h. At the end of the extraction, the liquid fraction was separated by filtration. Finally, in step III, the liquid fraction obtained was subjected to precipitation with ethanol at a ratio of 1:4 (v/v) to obtain pectin. The material obtained was centrifuged at 11,000 rpm for 15 min and dried at 70 °C for approximately 24 h (Habibi et al. 2005). The pectin yield obtained in ethanol precipitation was calculated using Eq. 1 proposed by Yuliarti et al. (2015).
| 1 |
Physical and chemical characterization of pectin obtained from Crataeva tapia leaves
Determination of pectin moisture and ash content
The moisture and total ash contents of pectin were determined according to the methodology proposed by Wathoni et al. (2019) and Hossein et al. (2020) with modifications. Initially, the dry matter (moisture) content of pectin was determined by drying the samples in an oven (Tecnal, TE-393/1) at 105 ± 2 ºC for 8 h. The total ash content of pectin was determined by measuring the residue obtained after incineration in a muffle (Thermo Scientific) at 650 ± 5 ºC for 4 h. The analyses were performed in triplicate and the results were obtained using Eqs. 2 and 3, respectively.
| 2 |
| 3 |
Elementary analysis
The carbon, hydrogen and oxygen contents were determined according to the methodology proposed by Waymack et al. (2004) and Coimbra et al. (2011) with few modifications. These were determined on the samples by microanalysis using the Perkin Elmer 2400 Series II CHNS/O Analyzer. The experiments were obtained in triplicate.
Determination of the composition of monosaccharides present in the pectin structure
The pectin was subjected to acid hydrolysis according to the conditions obtained by Lefsih et al. (2017) with modifications. Hydrolysis was performed at 100 ± 5 °C in a thermostatic bath (Nova Instruments-NI 1246) with 2.5 mL of trifluoroacetic acid (4 M) to 25 mg of pectin for 8 h. After removal, the samples were neutralized with 2.5 mL of ammonium hydroxide (NH4OH—2 M), made up to 10 mL with distilled water and filtered through a microporous membrane (0.22 µm).
The identification of monosaccharides (glucose, arabinose, galacturonic acid, xylose and rhamnose) released during hydrolysis was performed using 5 mM H2SO4 as mobile phase, flow 0.6 mL/min, injection volume 5.0 µL, column Aminex HPX87H (Bio-Rad), temperature of 60 °C for 1 h. Monosaccharides were identified and quantified on HPLC (Agilent, series 1100) using a refractive index (IR) detector. The concentration of degradation products, furfural (for C5 monosaccharides) and 5-hydroxymethylfurfural (HMF) (for C6 monosaccharides) was determined using a reversed phase (C-18) column (Agilent Technologies) with a composite mobile compound phase by an 8% acetonitrile–water solution containing 1% acetic acid, flow 0.6 mL/min, injection volume 5.0 µL using a UV/Vis detector (274 nm) at 25 °C on HPLC (Agilent, series 1100). The experiments were obtained in triplicate. From the results of the composition of the monosaccharides, it was possible to determine the percentage of two different polysaccharides present in the structure, these being homogalacturonan (HG) and rhamnogalacturonan-I (RG-I). The percentage of these polysaccharides was determined by Eqs. 4 and 5 proposed by Alba et al. (2015).
| 4 |
| 5 |
Determination of viscosimetric molecular weight
The molecular mass of pectin was determined by viscosity using an Ostwald viscometer. According to the methodology proposed by Santos et al. (2021). The experimental determination was made by flow time. Pectin was solubilized at different concentrations (1.0–9.0 g/L) in water heated to 60 °C. The experiments were obtained in triplicate. The average viscosimetric molecular mass (Mv) of pectin was calculated from the intrinsic viscosity value using the Empirical Mark–Houwink–Sakurada equation (Eq. 6).
| 6 |
where [ƞ] is the intrinsic viscosity K and a are constants corresponding to 1.4 × 10–6 and 1.34, respectively, for pectin (Cheremisinoff 1989; Arslan 1995).
Attenuated total reflectance/Fourier-transform infrared spectroscopy (ATR/FTIR)
ATR–FTIR analysis was used to identify the main functional groups, in addition to helping to determine the degree of esterification of pectin. The experimental procedure was carried out according to Santos et al. (2021) and Melo et al. (2022) with few modifications. For this, a Bruker Tensor 27 accessory spectrometer with attenuated total reflectance (Platinum ATR) was used. The spectra were recorded in the spectral range from 4000 to 500 cm−1, with a resolution of 2 cm−1 and 20 scans. The experiments were obtained in triplicate.
The degree of esterification (DE) was determined according to Guillotin et al. (2007) and Santos et al. (2021) through the integration of the bands of the esterified carboxylic and non-esterified carboxylic groups. The values of the areas under the curves were determined using the Origin 8.0® software and calculated using Eq. 7.
| 7 |
where Aest is the area of the bands of the esterified carboxylic groups and Anest is the area of the bands of non-esterified carboxylic groups.
Nuclear magnetic resonance spectroscopy
The 1H and 13C NMR analysis was used to elucidate the presence of the main functional groups and chemical bonds present in the pectin structure. For this purpose, pectin (20 mg) was dissolved in D2O heated to 60 °C and analyzed in a Bruker Avance 300 spectrometer (Bruker AXS, Inc., Madison, WI, USA) operating at 400 MHz with a 5 mm probe. The experiments were obtained in triplicate.
Thermogravimetric analysis and differential scanning calorimetry
Thermogravimetric analysis (TGA) and differential thermal analysis (DTG) of pectin were performed in a thermogravimetric analyzer TGA-50, Shimadzu, Kyoto, Japan operated with a flow of 20 mL/min nitrogen and heating from 40 to 400 °C, at a rate of 10 °C/min. Measurements were performed in triplicate, using 20 mg of sample. Differential scanning calorimetry (DSC) analysis of pectin was performed using a Perkin Elmer, STA 6000 thermogravimetric analyzer operating with a flow of 20 mL/min nitrogen and heating from 40 to 400 °C at a rate of 10 °C/min. Measurements were performed in triplicate, using 20 mg of sample. The experiments were obtained in triplicate.
X-ray diffraction analysis (DRX)
The DRX test was carried out according to the methodology proposed by Santos et al., (2021) with few modifications, an automatic diffractometer (XRD-6000/Shimadzu) (operating at 40 kV and 40 mA) was used for this over the angular range of 5–70° 2θ (step size = 0.04 and time per step = 353 s) at room temperature. The experiments were obtained in triplicate.
Determination of total phenols
The content of total phenols was determined by the method described by Hosseini et al. (2019) with some modifications. A total of 2.0 mL of Folin and Ciocalteu solution (1:10 v/v) was added to 4.0 mL of pectin dissolved in distilled water (400 μg/mL). After incubation, the assays were protected from light for 3 min and 1.6 mL of 7.5% Na2CO3 was added. Then, the reaction systems were incubated again and protected from light at 25 ºC for 120 min. After the incubation time, they were analyzed in a spectrophotometer (Hewlett-Packard, model 8453) at 765 nm. Distilled water was used as a blank equipment solution. An analytical absorbance curve was constructed as a function of the concentration of gallic acid at concentrations ranging from 0.5 to 1000 μg/mL and presented the following Equation y = 0.0048x + 0.0016, R2 = 0.9999. Phenols were indicated in gallic acid equivalent (mg GAE/g pectin). The experiments were obtained in triplicate.
In vitro antioxidant activity
Phosphomolybdenum complex reduction assay (PCRA)
This method is based on the reduction of molybdenum (VI) to molybdenum (V), this reduction occurs in the presence of certain substances with antioxidant capacity, resulting in the formation of a green complex between phosphate/molybdenum (V), at acidic pH, which is determined by spectrophotometer at 695 nm (Alam et al. 2013). The tests were carried out according to the methodology proposed by Cruz Filho et al. (2019) with modifications. The phosphomolybdenum complex is formed by the reaction of a solution of sodium phosphate (0.1 mol/L) with a solution of ammonium molybdate (0.03 mol/L) and a solution of sulfuric acid (3 mol/L), in an aqueous medium. In this methodology, the phosphomolybdenum complex (3 mL) and distilled water (6.9 mL) were added to aliquots of pectins diluted in water (0.1 mL), in concentrations ranging from 3.12 400 μg/mL. The spectrophotometer blank was obtained using 0.1 mL of distilled water, 3 mL of the reagent and 6.9 mL of distilled water. All tubes were hermetically closed at 95 °C for 90 min, after cooling, absorbance at 695 nm was determined in a Hewlett-Packard model 8453 spectrophotometer. All analyzes were performed in triplicate. A standard curve was drawn from the absorbance readings of ascorbic acid and butylated hydroxytoluene (BHT) at concentrations of 3.12–400 μg/mL, used as a reference to analyze the antioxidant activity. The results of the evaluation of antioxidant activity were expressed as relative antioxidant activity and calculated as obtained by Eq. 8.
| 8 |
The determination of the IC50 (50% Inhibitory Concentration) was obtained by linear regression of the points plotted graphically and through the equation of the straight line obtained the IC50 values.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical capture assay
The scavenging capacity of the stable DPPH radical was determined by the methodology described by Hosseini et al. (2019) and Santos et al. (2021) with modifications. For this, a volume of 0.32 mL of a pectin solution at different concentrations (3.12–400 μg/mL) was added to 2.0 mL of a 1 mM DPPH methanol solution. The system was incubated in the absence of light for a period of 25 min at room temperature. Absorbances were determined at 517 nm in a spectrophotometer (Hewlett-Packard, model 8453). The DPPH solution in methanol was used as a control, the blank was methanol. The experimental pattern used was ascorbic acid and butylated hydroxytoluene (BHT). The tests were carried out in quintuplicate. The scavenging of DPPH radicals was calculated by Eq. 9.
| 9 |
To calculate the IC50, the equation of the straight line was used, replacing the value of y by 50 to obtain the concentration of the sample capable of reducing 50% of the DPPH.
Scavenging activity of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cations
The ABTS radical cation scavenging activity was performed using the method reported by Cruz Filho et al. (2019) and Santos et al. (2021) with slight modifications. Briefly, a stock solution of ABTS (7 mM) in ethanol and a solution of potassium persulfate (140 mM) were prepared. The ABTS radical was prepared from the reaction of 5 mL of ABTS stock solution with 88 μL of potassium persulfate solution. The mixture was kept in the dark at room temperature for 16 h. Then, 1 mL of this mixture was diluted in ethyl alcohol to an Absorbance of 0.70 nm ± 0.05 nm at 734 nm in a spectrophotometer (Hewlett-Packard, model 8453). Using 30 μL of different pectin concentrations ranging from 3.12 and 400 μg/mL and 3.0 mL of ABTS solution, the system was incubated in the absence of light at a temperature of 35 ºC for 5 min. The ABTS solution was used as a control. The blank used was ethanol. The experimental pattern used was ascorbic acid and butylated hydroxytoluene (BHT). Percent inhibition was calculated according to Eq. 10.
| 10 |
To calculate the IC50, the equation of the straight line was used, replacing the value of y by 50 to obtain the concentration of the sample capable of reducing 50% of the ABTS. The experiments were obtained in triplicate.
Nitric oxide (NO) radical scavenging activity
The scavenging test of NO radicals was carried out by the method of Cruz Filho et al. (2019). Pectin was diluted in distilled water at concentrations ranging from 3.12 to 400 μg/mL and submitted to nitrite analysis by the Griess colorimetric method. The NO concentration was estimated using a standard curve (3.12–400.0 µM) reading at 595 nm in a microplate spectrophotometer (Thermo Scientific Multiskan GoW/Curvette, Waltham). The equation obtained was y = 0.0065x + 0.046 R2 = 0.9946. The blank used was distilled water. The control was the Griess reagent with nitrate solution. Percent inhibition was calculated according to Eq. 11. The experiments were obtained in triplicate.
| 11 |
In vitro cytotoxic and immunological assays
Hemolytic and hemagglutinating activity
Human erythrocytes were collected as the experimental protocols were approved by the Ethics Committee Protocol number 1.870,360 of the Federal University of Pernambuco, Recife—PE, Brazil. Blood samples were only collected after all health volunteers signed the “Free and Informed Consent” Term.
The test was carried out in accordance with Amirian et al. (2021) with few modifications. Pectin was evaluated for hemolytic activity by assay performed in 96-well microplates. Each well received 100 µL of a 0.85% NaCl solution containing 10 mM CaCl2. Samples (100 µL) of pectin were added to the first well of the respective treatment, from which 100 µL were transferred to the second to perform a serial dilution (100–1.56 µg/mL). Then, 100 µL of a 2% (v/v) suspension of hamster erythrocytes in saline containing 10 mM CaCl2 was added. The control used was 100 µL of saline solution plus 100 µL of erythrocyte suspension. After centrifugation for 1 h, followed by incubation for 1 h at 27 °C, the supernatant was discarded and released hemoglobin was determined by absorbance at 540 nm. The results of hemolytic activity were expressed by the following Eq. 12. The experiments were obtained in triplicate.
| 12 |
where ABS sample = Sample absorbance; ABS blank = negative control absorbance; ABS saponin = positive control absorbance.
The hemagglutinating activity was performed according to Torralbo et al. (2012) with few modifications. In microtiter plates (96 wells), 50 µL of 0.15 M NaCl were added to each well of the plate and then 50 µL of pectin was added to the second well. After serial dilution (100–1.56 µg/mL), 50 µL of the erythrocyte suspension (2.5% v/v) was added to all wells. The hemagglutinating activity corresponded to the inverse of the highest dilution (title−1) in which total agglutination of erythrocytes was still observed. The experiments were obtained in triplicate.
Preparation, culture and in vitro stimulation of PBMCs
The isolation of lymphocytes and monocytes from peripheral blood mononuclear cells (PBMC) was performed according to Melo et al. (2011) and Santos et al. (2021). The experimental protocols were approved by the Ethics Committee Protocol number 1.870.360 of the Federal University of Pernambuco, Recife—PE, Brazil. Blood samples were only collected after all health volunteers signed the Informed Consent Term. After three centrifugations, cells were counted in a Neubauer chamber, using the trypan blue solution. Cells were used only when viability was > 95%. PBMCs were cultured in RPMI 1640 medium supplemented with 10% (w/v) fetal bovine serum in 24-well plates at a density of 106 cells/well. For viability analysis, cells were treated with pectin at different concentrations and the stimulus was maintained for 24 h.
Cytotoxicity analysis in PBMC
PBMCs (106 cells/well) were treated with pectin (2.5–80 µg/mL). Cell viability assays were performed in 24-well plates where cells were incubated for a period of 24 h. The control used was culture medium and cells. After the incubation time, the cells were centrifuged at 2000 rpm at 25 °C for 10 min. After discarding the supernatant, 1 mL of 1X PBS was added to the precipitate and centrifuged under the same experimental conditions mentioned above. At the end of centrifugation, the supernatant was discarded and the pellet was resuspended in a buffer solution containing Propidium iodide. Assays were analyzed in a FACS Calibur flow cytometer (Becton Dickinson Biosciences). The experiments were carried out in sextuplicate. The results of the analysis were analyzed by dotplots (Dotplot) using Flowing Software 2.5.1®. Cell viability was determined in percentage using Eq. 13.
| 13 |
where VC is the number of viable cells at different concentrations of lignin, TC is the concentration of viable cells in the control (only cells and culture medium) that represents 100% viability.
Measurement of cytokines and nitrite production
Cytokines and NO were quantified in PBMC culture supernatants (106 cells/well) treated with pectin (10 µg/mL) for 24 h of incubation. Cytokine evaluation was performed with the Cytometric Bead Array (CBA) kit Human Th1/Th2 Cytokine (BD Biosciences-USA®) for detection of interleukins IL-2 (y = 0.0057x + 6.2369; R2 = 0.996), IL-4 (y = 0.0033x + 6.165; R2 = 0.9805), IL-6 (y = 0.0043x + 4.4473/R2 = 0.9914), IL-10 (y = 0.0055x + 3.3736/R2 = 0.9966), IL-17 (y = 0.0043x + 4.4473/R2 = 0.9914),TNF-α (y = 0.0036x + 4.0523/R2 = 0.9807) and IFN-γ (y = 0.0072x + 5.1593/R2 = 0.9921) as described by the manufacturer and by Santos et al. (2020). Data were acquired on the FACS Calibur platform (BD Biosciences-USA®) and the results analyzed in Flowing 2.5.1® software, through their respective detection curves. The experiments were carried out in sextuplicate. In addition to the determination of cytokines in the PBMC culture supernatant, the levels of NO produced were determined using the Griess method (Ding et al. 1988). A standard curve for nitrite (y = 0.007x + 0.0256/R2 = 0.9978 /3.12–400 µmol) was performed for the assay. The reading was performed in a microplate spectrophotometer (Thermo Scientific Multiskan FC®, Waltham-USA) at 595 nm. The experiments were carried out in sextuplicate.
Immunophenotyping assay
Lymphocyte immunophenotyping assays were performed according to Santos et al. (2020) and Santos et al. (2021). After 24 h of pectin treatment (10 µg/mL), cells were removed from the plates using ice-cold PBS and transferred to polypropylene tubes (BD Biosciences) with 6 mL PBS for centrifugation (400 × g, 10 min). After discarding the supernatant, cells were washed with 2 mL PBS and centrifuged (400 × g, 10 min). The supernatant was discarded and the surface monoclonal antibodies were added to the tubes and incubated for 30 min. Then, two washing steps were performed with 1 mL of PBS followed by centrifugation (400 × g, 5 min). Supernatants were discarded and cells were fixed for 15 min with 150 mL Cytofix solution (BD Biosciences) and then washed with 2 mL PBS followed by centrifugation (400 × g, 5 min). After discarding the supernatant, 300 μL of PBS was added to each tube and 10.000 events were acquired on the FACS Calibur platform and the results were analyzed using FlowingSoftware version 2.5.1. As experimental control, control cells treated with 1% DMSO were used. The monoclonal antibodies used were anti-CD4-PerCP-Cy5.5 and anti-CD8-FITC (BD®). The experiments were carried out in sextuplicate.
Emulsifying properties
The emulsions were made according to the method used by Schmidt et al. (2017), Yang et al. (2018) and Deng et al. (2020) with some modifications. Pectin was dissolved in distilled water at different concentrations (2.5–80 µg/mL) in order to evaluate the effect of the concentration during the emulsions. In addition to evaluating the effect of pectin concentration, the influence of pH variation (2, 4, 6 and 8) (correction with sodium hydroxide and 3 M phosphoric acid) and of calcium ion concentration, using calcium chloride in different concentrations (0, 20, 40, 60, 80 and 100 mg/g of pectin). The tests were carried out at a ratio of 1:1 (v/v), that is, test solution and soybean oil. As a control, distilled water was used. After that, the samples were homogenized (20,000 rpm for 90 s) and centrifuged at 1300G for 5 min. After centrifugation, the emulsifying activity was determined according to Eq. 14. The stability of the emulsion (Eq. 15) was determined under the same experimental conditions as the emulsifying activity, and for this assay the emulsions were preheated to 80 °C for 3 min.
| 14 |
| 15 |
In the experimental condition that showed the highest emulsification activity (pectin concentration, pH and calcium concentration), the LT-87 molecule (Jacob et al. 2021) was added to the assay at a concentration of 18.7 µM, a concentration capable of eliminating tumor cells by 50%. This molecule was synthesized and patented under the number BR-1020190145030 by our Research Group on Therapeutic Innovation—GPIT/LQIT/UFPE where the anti-inflammatory, COX2 inhibitors, analgesic and anti-tumor activities were described. For the assays, the molecule was dissolved in 1% DMSO. Finally, 1% DMSO solution and distilled water were used as controls. The experiments were carried out in triplicate.
Statistical analysis
The GraphPad Prim 7® software was used for statistical analysis. The Shapiro–Wilk test was applied to test the normality of the hypothesis and the repetition means were analyzed by non-parametric tests. Statistical differences between groups were analyzed by one-way analysis of variance (ANOVA). Differences were considered significant when p < 0.05.
Results and discussion
Physical and chemical characterization of pectin obtained from Crataeva tapia leaves
Physicochemical composition analysis
Table 1 presents the results of the physicochemical composition of pectin obtained from Crataeva tapia leaves, these being the ash contents, protein moisture, phenolics, molecular weight, pectin obtainment yield, elemental analysis, percentage of monosaccharides present in the structure, degradation products obtained during the acid hydrolysis of pectin, in addition to the levels of HG and RGI.
Table 1.
Results of the analysis of the physicochemical composition of pectin obtained from Crataeva tapia leaves
| Physical and chemical composition | |
|---|---|
| Ashes (%) | 1.50 ± 0.0 |
| Moisture (%) | 6.75 ± 0.1 |
| Yield of obtaining pectin (%) | 2.3 ± 0.01 |
| Molecular mass (Mw) (kDa) | 37.2 ± 0.3 |
| Total phenolics (mg GAE/g pectin) | 1.87 ± 0.3 |
| Elementary analysis | % |
| Carbon | 41.0 ± 0.1 |
| Hydrogen | 6.0 ± 0.4 |
| Oxygen | 53.0 ± 0.2 |
| Monosaccharides and degradation products | % |
| Rhamnose | 2.85 ± 0.3 |
| Arabinose | 2.50 ± 0.4 |
| Xylose | 6.60 ± 0.1 |
| Glucose | 26.5 ± 0.0 |
| Galacturonic acid | 47.7 ± 0.3 |
| 5-Hydroxymethylfurfural | 7.50 ± 0.3 |
| Furfural | 5.50 ± 0.0 |
| Total | 99.15 ± 0.3 |
| Polymers present in pectin | % |
| Homogalacturonan (HG) | 44.8 ± 0.1 |
| Rhamnogalacturonan I (RGI) | 55.9 ± 0.1 |
*Mean ± standard deviation
The literature presents different values for the analysis of the physical and chemical composition of pectins. Yields, ash and moisture contents ranged from 1.2 to 32%, 1.0 to 3.9% and 6.0 to 9.8%, respectively, for pectins from soybean hulls (Kalapathy and Proctor 2001), mulberry branch bark (Liu et al. 2010), golden kiwi pomace (Yuliarti et al. 2015), Ubá mango husk (Oliveira et al. 2018), commercial (Oliveira et al. 2018), cocoa pod husks (Priyangini et al. 2018) of Indonesian mangosteen (Wathoni et al. 2019) and cherry pomace (Hosseini et al. 2020).
The molecular weight of pectins and different polysaccharides is a highly variable parameter. Pectins have different molecular weight values and these can vary between 20 and 360 kDa (Gonzalez and Rosso 2011). Therefore, the pectin in this study is considered to have a low molecular weight. During the obtaining of pectin, different phenolic compounds are also extracted (since many of these are soluble in water or ethanol), these can be found adhered to the structure of pectin. The levels of phenolics present vary according to the method of extraction and obtaining the polysaccharide. The literature presents different values of total phenols for different pectins: Fractions of Parkia speciosa 74.5–382.8 mg of GAE/g. Gan et al. (2010), eggplant husks and stems 161 and 16 mg of GAE/g Kazemi et al. (2019b), aerial parts of Equisetum arvense L 0.23 mg of GAE/g, aerial parts of Equisetum sylvaticum 0.11 mg of GAE/g (Patova et al. 2019) and sour orange peel 39.9 mg GAE/g (Kazemi et al. 2019a). The content of total phenols present in pectin was 1.87 ± 0.3 mg of GAE/g. The low content of phenolics and ash are related to the purity of pectins. That is, the lower they indicate the greater purity of the polysaccharide.
The results of the elemental analysis obtained are close to those found for other pectins reported by several authors. Waymack et al. (2004) obtained contents of carbon (38.4%), hydrogen (5.80%) and oxygen (50.7%) (commercial citrus pectin). Bae et al. (2009) obtained carbon (39.59%), hydrogen (6.05%) and oxygen (54.36%) and Coimbra et al. (2011) obtained carbon (40.8%), hydrogen (6.01%), oxygen (52.49%) and nitrogen (0.7%) contents for citrus pectin.
In addition to the carbon, hydrogen and oxygen contents, it was also possible to determine the presence of different monosaccharides present in the chemical structure. Other pectins also had these same monosaccharides identified in our study (rhamnose, arabinose, xylose, glucose and galacturonic acid). Among these we can mention pectins obtained from different pectic fractions of Mesembryanthenum crystallinum fruits (M’Sakni et al. 2006), from the early harvested fruits of the golden kiwi and the main fruit harvested of the golden kiwi (Yuliarti et al. 2015), from okra pods obtained at different pHs (Alba et al. 2015), potato pulp (Yang et al. 2019) and gabiroba fruit pulp (Barbieri et al. 2019).
Through this compositional result, it was possible to determine the percentage of different fragments of the polysaccharides HG (44.8%) and RGI (55.9%) present in the pectin structure.
The differences found for the physical and chemical composition of pectins are directly related to the different extraction methods, maturation stage and extraction source (Kalapathy and Proctor 2001; Liu et al. 2010; Yuliarti et al. 2015; Priyangini et al 2018; Oliveira et al. 2018; Wathoni et al. 2019; Hosseini et al. 2020).
Attenuated total reflectance/Fourier-transform infrared spectroscopy (ATR/FTIR)
The functional groups found by the spectroscopic analysis of ATR/FTIR for pectin obtained from Crataeva tapia leaves are also reported by different pectins which we can cite those extracted from orange, passion fruit mesocarp, broccoli, carrot, sugar beet, tomato, mango, apple and pectin-like polysaccharide obtained from the leaves of Conocarpus erectus (Lima et al. 2010; Kyomugasho et al. 2015; Santos et al. 2021). The chemical behavior of pectins depends on the number of ionic groups attached and their distribution along the main chain (Santos et al. 2021). Figure 1 shows the ATR/FTIR spectrum for pectin from Crataeva tapia leaves in the regions 4000–500 cm−1.
Fig. 1.
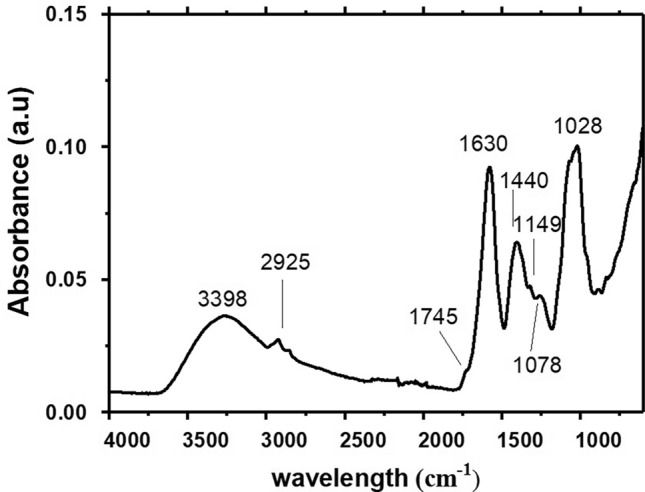
FTIR spectrum by pectin from Crataeva tapia leaves
Through the spectrum, it was possible to observe a broad and strong intensity band at 3398 cm−1 referring to the elongation of the hydroxyl groups (OH), associated with the presence of water, as well as the inter and intramolecular hydrogen interactions. The bands at 2925 cm−1 corresponding to the C–H stretch of the CH2 groups. The vibrational bands at 1745 and 1637 cm−1 are attributed to stretches of esterified carboxylic methyl groups (C=O) and carboxylate groups (COO–), respectively. The bands at 1630 cm−1 were accompanied by the signals at 1440 cm−1 corresponding to the asymmetric and symmetrical stretches of the carboxyl group. Vibrational bands, attributed to the pyranoside ring, were found in the regions of 1149, 1078 and 1028 cm−1, and refer to the region called “fingerprint” of the polysaccharide (below 2000 cm−1).
Pectins are subdivided according to the degree of esterification into two groups. Those with high methoxylation content present (DE) greater than 50% and are characterized by the ease of gel formation in the presence of sugar and low pH (2.5–3.8). Pectins with (DE) values below 50% are classified as low in methoxylation and gel-forming capacity in the presence of divalent cations, such as calcium (Ca2+), over a wider pH range (from 2.5 to 7) without the need for large amounts of sugars (Guillotin et al. 2007). The degree of pectin esterification in this study was 25.7 ± 0.2%. Here, pectin is classified as low-esterification pectin. The literature presents different values for the degree of pectin esterification and these differences are related to the form of extraction, characterization and source of obtainment (Lima et al. 2010; Kyomugasho et al. 2015; Kazemi et al. 2019a, b).
Commercial pectin showed a degree of esterification ranging from 63 to 92% (Guillotin et al. 2007), that of passion fruit mesocarps from 20 to 69% (Lima et al. 2010; Kyomugasho et al. 2015), that of Opuntia ficus-indica 41.4% (Bayar et al. 2017), those obtained from eggplant stem and skin showed a degree of esterification of 60.74 and 68.18% respectively (Kazemi et al. 2019a, b) and pectin polysaccharide similar to Conocarpus leaves erectus presented 37.5 ± 0.3% (Santos et al. 2021).
Nuclear magnetic resonance spectroscopy (1H and 13C NMR)
The 1H and 13C NMR spectrum for pectin from C. tapia showed characteristic signals present in different pectins, these being apple pomace pectins (Marcon et al. 2005), Comarum palustre L. pectin (Ovodova et al. 2005), isolated from Bergenia crassifolia leaves (Golovchenko et al. 2007), pectin extracted from the pulp of Tamarindus indica L. (Sharma et al. 2013), from cherry pomace (Hosseini et al. 2019), eggplant peel and stem pectin (Kazemi et al. 2019a, b). Figure 2 shows the proton (1H) NMR spectra for C. tapia pectin.
Fig. 2.
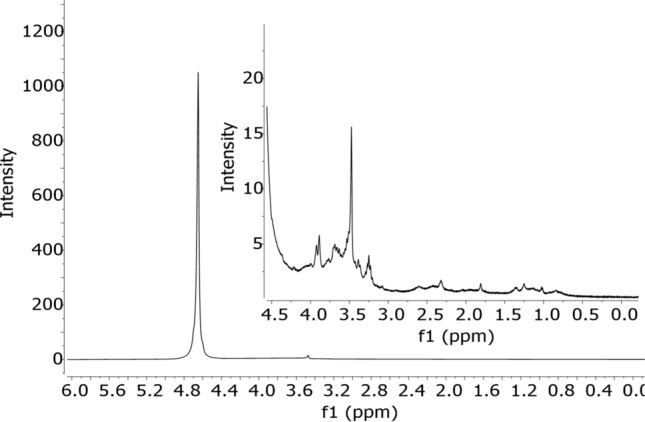
Spectrum of 1H NMR by pectin extracted from Crataeva tapia leaves
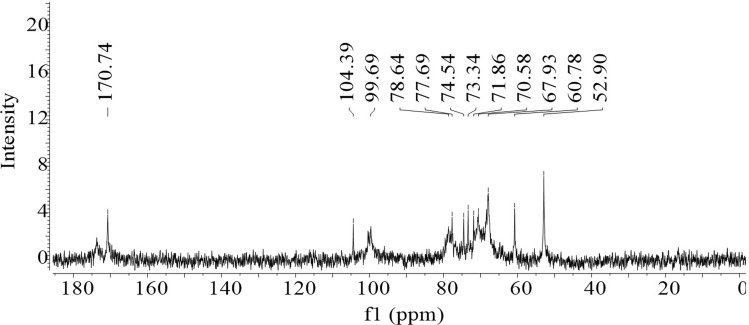
The 1H NMR spectrum (Fig. 2) showed a signal relative to the solvent (D2O) in the region between 4.68 and 5.23 ppm. The H-3 hydrogen specific signal for arabinose residues was 4.02 ppm. Signals at 3.67 ppm and 3.87 ppm correspond to protons H-2 and H-3 of methyl groups of esterified galacturonic acids, respectively. It is also observed in Fig. 2 at 4.40 ppm a signal referring to the H-4 proton. The signal at 1.14 ppm refers to methylated L-Ramnose groups. Around 2.1 ppm, both signs refer to galacturonic acid units that can be acetylated to O-2 or O-3 or esterified with methyl at the carboxyl groups. The intense signal at 3.70 ppm relative to methyl groups attached to carboxylic groups of galacturonic acid. Figure 3 shows the 13C NMR spectrum of the pectin under study.
Fig. 3.
Spectrum of 13C NMR by pectin extracted from Crataeva tapia leaves
Through the 13C NMR spectrum of Fig. 3, it was possible to observe the chemical shifts corresponding to the anomeric region of d-α-galacturonic acid, evidenced by the signs at δ 99.69 ppm and δ 104.39 ppm equivalent to C-1 carbon of esterified and non-esterified units, and a very low field signal at δ 170.74 ppm corresponding to the C-6 of the methylesterified and free carboxylic groups, respectively. At δ 52.9 ppm it is assigned to the C-6 O-methyl ester (OMe) group of the pyranoside ring. The signals at δ 60.78, 67.93, 70. 58, δ 71.86, 74.54, 73.34, 77.69 and 78.64 ppm are relative to carbons C-2, C-3, C-5 and C-4 of galacturonic acid, respectively.
Thermogravimetric analysis and differential scanning calorimetry
Figure 4 shows the results of thermal analysis for pectin extracted from Crataeva tapia leaves. Figure 4A and B show the results of thermogravimetry (TGA), derived thermogravimetry (DTG) and Fig. 4C shows the differential scanning calorimetry curve.
Fig. 4.
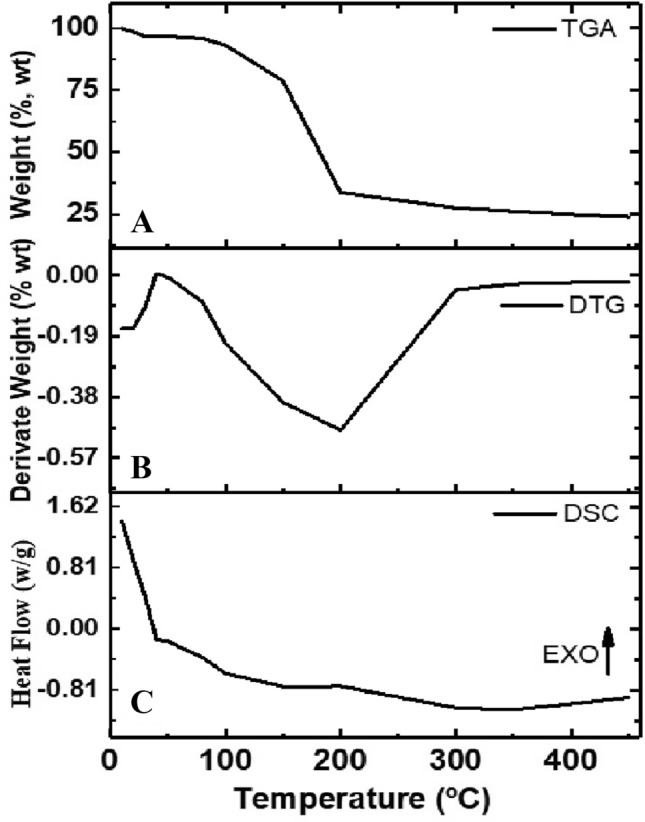
Thermogravimetric analysis (TGA) (A), derived thermogravimetric curves (DTG) (B) and differential scanning calorimetry (DSC) thermogram (C) for pectin extracted from Crataeva tapia leaves
The TGA/DTG pectin curve (Fig. 4A and B) showed three stages of mass loss. The first step, in the temperature range of 50–100 °C (mass loss of 6.0%); the second, in the temperature range of 100–210 °C (mass loss around 65%) and the third in the range from 210 to 400 °C, with mass loss around 5%, resulting in an ash content of 23%. The sample mass loss in nitrogen atmosphere was studied to investigate the main stages of thermal degradation of pectin (Tan et al. 2020). The pectin obtained from C. tapia leaves showed thermal degradation in three stages, which was also reported by other authors, Einhorn-Stoll et al. (2007), Wang et al. (2016) and Tan et al. (2020). The first stage of degradation is related to water loss. The second referent of pectin which is due to primary and secondary decarboxylation involving the acid side group and a ring carbon. This step is confirmed by the DTG curve that shows a more intense peak indicating a higher rate of pectin decomposition. Finally, the third step involves degrading the entire exposure to ash.
Figure 4C shows the results of the DSC analysis between 40 and 400 °C. The results of the DSC curves show an endothermic peak (around 50 °C) with an enthalpy of + 12.4 ± 0.1 J/g characteristic of water scavenging and an exothermic peak that suggests pectin degradation (around 200 °C) with an enthalpy of − 301 ± 0.5 J/g. The results of the DSC curves showed two peaks, the first endothermic, corresponding to the dehydration process and the second, exothermic, corresponding to the decomposition process. The two events are consistent with the events observed in the TGA curve and agree with what was observed by Iijima et al. (2000), Einhorn-Stoll et al. (2007) and Nisar et al. (2018) who described two thermal events for pectin samples under N2 in DSC curves.
X-Ray diffraction analysis (DRX)
DRX analysis was used to provide more information about the structure of pectin (amorphous and crystalline) (Kazemi et al. 2019a, b; Misra and Yadav 2020). Pectins have peaks at different angles. Nisar et al. (2018) obtained peaks for citrus pectin in the range 12.72º–40.14º. Kazemi et al. (2019a, b) characterizing eggplant food processing residues as a source of pectin obtained peaks in a range from 13.4 º to 44.8º. Misra and Yadav (2020) evaluating carrot pectins they obtained peaks in the range from 14.3 º to 51.6º.
Figure 5 shows the DRX spectrum for pectin extracted from Crataeva tapia leaves. The pectin X-ray diffractogram shows the 2θ peaks equal to 16.2 and 23, 25, 30 and 40. The pectin under study showed an amorphous structure. This characteristic was observed in other pectins, among which we can mention the pectins extracted from Akebia trifoliata var. australis bark (Jiang et al. 2012), Indonesian mangosteen pectins and commercial pectin (Wathoni et al. 2019), pectin-like polysaccharide from leaves of Conocarpus erectus (Santos et al. 2021). The amorphous or crystalline behavior of different pectins is related to the origin and extraction methods (Wathoni et al. 2019; Kazemi et al. 2019a, b; Misra and Yadav 2020; Santos et al. 2021).
Fig. 5.
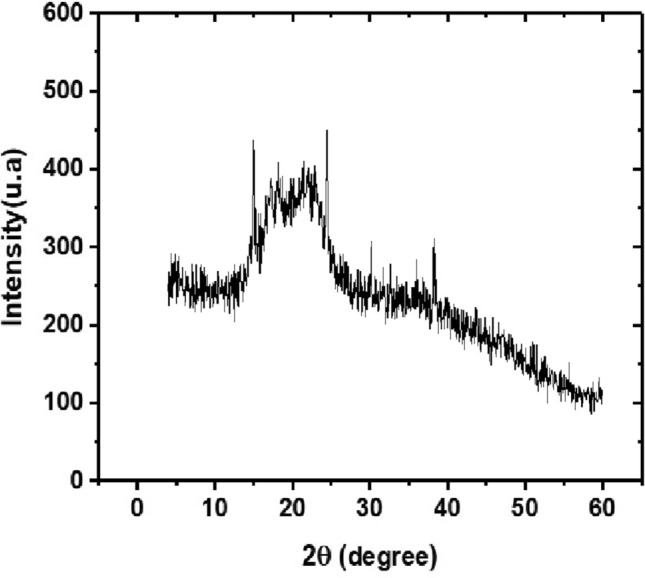
XRD spectra for extracted from Crataeva tapia leaves
In vitro antioxidant activity
The antioxidant activity promoted by different pectins is directly related to their chemical structure, that is, monosaccharide composition, galacturonic acid content, molecular weight, type of glycosidic link in the main chain, degree of esterification and flexibility of the configurations of the chains associated with the presence of phenolic compounds due to the extraction process (Chen et al. 2019; Wathoni et al. 2019; Kazemi et al. 2019a, b; Hosseini et al. 2019; Santos et al. 2021). The results of antioxidant activity are shown in Fig. 6.
Fig. 6.
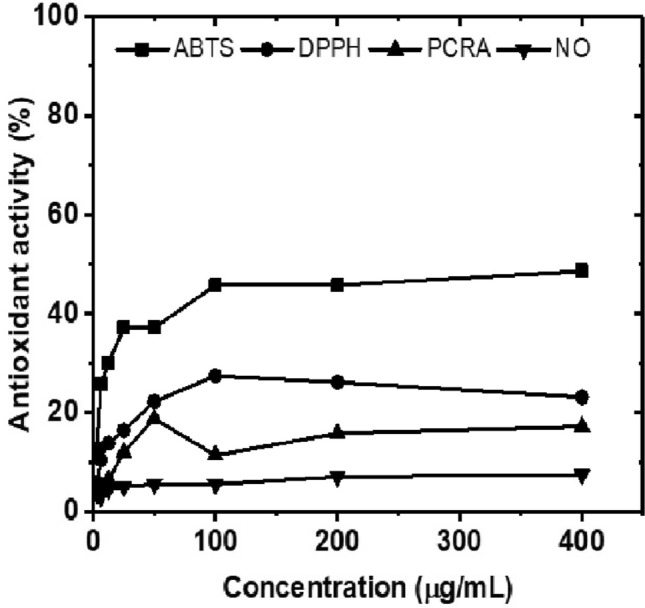
Results of antioxidant activity in percentage promoted by pectin obtained from Crataeva tapia leaves in different assays of ABTS, DPPH, PCRA and NO
The curves obtained for each assay showed an increase in antioxidant activity with increasing concentration for all assays. A similar profile was obtained by Wathoni et al. (2019) evaluating Indonesian mangosteen pectin for the DPPH assay. Chen et al. (2019) evaluating hawthorn pectin obtained an increase in antioxidant activity with increasing concentration for DPPH, OH and reducing power elimination assays. Santos et al. (2021) evaluating the antioxidant activity of a pectin-like polysaccharide obtained from leaves of Conocarpus erectus, the authors performed the ABTS and DPPH assays.
Table 2 presents the results in percentage of antioxidant activity for pectin in this study compared to ascorbic acid and BHT standards. In none of the test’s pectin showed IC50 (50% capture/reduction concentration).
Table 2.
Antioxidant activities of Crataeva tapia pectin compared to ascorbic acid and BHT standards at a concentration of 400 µg/mL
| Antioxidant assays | C. tapia pectin | Ascorbic acid | BHT |
|---|---|---|---|
| ABTS | 41.16 ± 0.30 | 90.47 ± 0.34 | 95.01 ± 0.1 |
| DPPH | 25.9 ± 0.01 | 91.05 ± 0.17 | 92.41 ± 0.4 |
| PCRA | 20.4 ± 0.20 | 100 ± 0.00 | 99 ± 0.2 |
| NO | 7.6 ± 1.95 | 95.42 ± 0.80 | 73.1 ± 0.5 |
Mean ± Standard deviation
DPPH 2,2-diphenyl-1-picrylhydrazyl radical, ABTS 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid radical, PCRA Total antioxidant activity, NO nitric oxide
The results show that the antioxidant activity promoted by pectin was higher for the ABTS assays, followed by DPPH, PCRA and NO and that this polysaccharide presents moderate to low antioxidant activity when compared to the standards used (ascorbic acid and BHT). Similar to our results, pectin isolated from eggplant and sour orange peel showed antioxidant potential at concentrations above 400 µg/mL (Kazemi et al. 2019a, b; Hosseini et al. 2019). The studies by Patova et al. (2019) which showed that pectins isolated from the leaves of Equisetum arvense L. and Equisetum sylvaticum L. were efficient in scavenging the hydroxide peroxide radical and inhibiting the hydroxyl radical in concentrations above 400 µg/mL. Thus, the antioxidant activity is directly associated with the chemical structure of pectin, concentration and phenolic content associated with the structure (Kazemi et al. 2019a, b; Hosseini et al. 2019; Patova et al. 2019; Misra and Yadav 2020; Santos et al. 2021).
Cytotoxicity assessment, cytokine production and immunophenotyping investigation
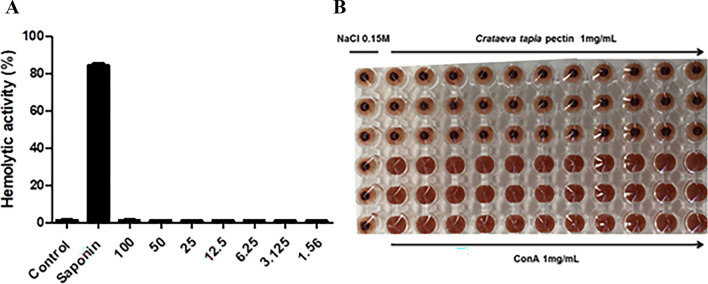
Cytotoxicity assays on polysaccharides is an important step for their biomedical and pharmaceutical application (Chen et al. 2016a, b; Ktari et al. 2017; Slima et al. 2019). In vitro hemolysis assays have been routinely used in toxicity studies of different polysaccharides (Ktari et al. 2017; Slima et al. 2019). Therefore, this study aimed to evaluate the cytotoxic capacity of C. tapia pectin against erythrocyte cells. Hemolysis is characterized by rupture of the erythrocyte with the release of hemoglobin, as erythrocytes are simple cells, their interaction with different bioactive molecules is carried out by the plasma membrane (Kumar et al. 2011; Jeswani et al. 2015). Figure 7 shows the results of hemolytic activity in percentage (A) and hemagglutination (B) promoted by the pectin under study.
Fig. 7.
Hemolytic and hemagglutination activity promoted by pectin from Crataeva tapia at different concentrations
The results showed that pectin was not able to promote hemolytic activity at high concentrations. As well as the pectins from Cola cordifolia obtained by Austarheim et al. (2014) were also not toxic in erythrocytes. Kothandaraman et al. (2017), evaluating this activity for citrus pectin conjugated to different drugs, at concentrations ranging from 50 to 500 µg/mL, they obtained percentage of hemolysis lower than 20%. Kodoth et al. (2019) evaluating the cytotoxic effect of pectin-based silver nanocomposite film also verified that the material associated with pectin also did not promote cytotoxic effect against erythrocytes. Regarding hemagglutinating activity, the pectin in our study was not able to promote hematoagglutination when compared to the control, that is, the ability to unite erythrocytes in the form of bridges, promoting loss of cell function, the hemagglutinate activity was recently observed by the PgTeL protein (Silva et al. 2021).
In parallel, assays with erythrocytes were performed with assays with peripheral blood mononuclear cells (PBMC). The viability of pectin-treated PBMC was investigated by flow cytometry, using as propidium iodide a fluorescent agent used to show cell damage (Melo et al. 2011; Santos et al. 2021). Figure 8 shows the results in percentage for the viability of PBMC, after incubation with different concentrations of pectin (2.5–80 µg/mL) for a period of 24 h.
Fig. 8.
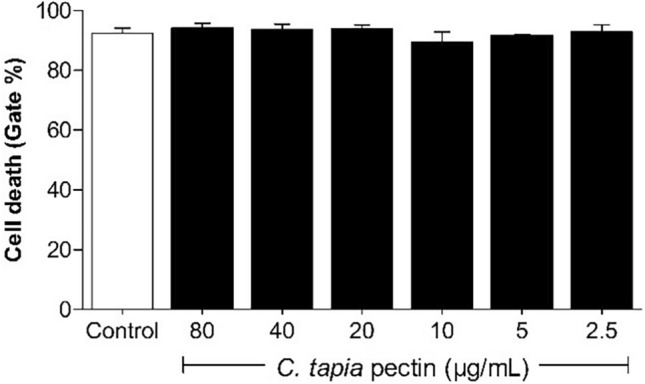
Viability of human PBMCs treated with C. tapia pectin at different concentrations. Pectin did not promote significant cell death. Vertical bars represent the mean of two independent experiments performed in triplicate
Pectin from Crataeva tapia leaves was not cytotoxic (viability > 95%) at the concentrations studied. Behavior similar to that observed by Salman et al. (2008) and Merheb et al. (2019) investigating citrus pectin cytotoxicity in PBMC and mouse splenocytes, respectively. Wang et al. (2005) investigating Centella asiatica pectin, Amorim et al. (2016), studying the effects of three pectin fractions (OP, MOP and PG) extracted from Theobroma cacao at different concentrations (25, 50, 100, 200 and 400 μg/mL) against macrophage cells. Lefsih et al. (2017), with water-soluble pectin from Opuntia ficus-indica in NIH-3T3 cells, and Santos et al. (2021), evaluating the cytotoxic effect of a pectin-like polysaccharide obtained from Conocarpus erectus leaves against PBMC cells, found that the evaluated pectins did not present cytotoxic effects against the evaluated cells.
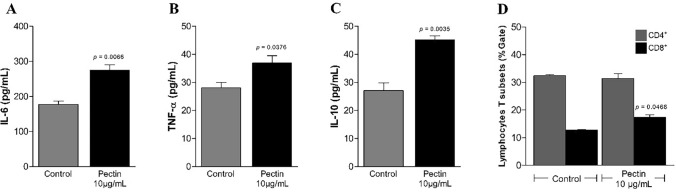
Immunomodulation assays were performed with pectin at a low, non-toxic concentration of 10 μg/mL. A study has shown that this concentration is capable of immunostimulating different cells (Nergard et al. 2005; Merheb et al. 2019; Santos et al. 2021). Figure 9 shows the profile of cytokines produced and lymphocyte activation. Pectin was able to induce a high production of IL-6 (275 ± 0.1 pg/mL) (Fig. 9A), IL-10 (38.7 ± 0.3 pg/mL) (Fig. 9B) and TNF-α (44.7 ± 1.0 pg/mL) (Fig. 9C). The other immunological mediators, such as NO levels, IFN production, IL-2 IL-4 and IL-17 cytokines, did not show significant results. Furthermore, elevated levels of CD8 + T lymphocytes were found in pectin-stimulated cells (Fig. 9D).
Fig. 9.
Production of cytokines IL-6 (A), TNF-α (B), IL-10 (C) and activation of CD8 + T lymphocytes (D) in cultures of human PBMCs stimulated for 24 h with C. tapia pectin. Vertical bars represent the mean of two independent experiments performed in triplicate
The immunomodulatory potential of pectins is also related to the presence of methyl or acetyl groups and the content of galacturonic acid (< 75%). Thus, pectins obtained by different methods and sources promote different stimuli in cells (Popov and Ovodov 2013; Amorim et al. 2016; Merheb et al. 2019).
This fact was also observed by Santos et al. (2021) evaluating a pectin-like polysaccharide from leaves of Conocarpus erectus. Similar to our results, pectins from Lycium ruthenicum (Peng et al. 2014), Sambuci fructus L (Ho et al. 2015), Hovenia dulcis (Wang et al. 2017) and Punica granatum L. (Gavlighi et al. 2018) also were able to promote immunostimulation in animal cells (Seyfried et al. 2016). Furthermore, results from C. tapia pectin suggest its use as a pro-inflammatory agent (Khan et al. 2017).
The pectin from our study proliferation and activation of TCD8 + lymphocytes reduced activation of TCD4 + lymphocytes. Santos et al. (2021) obtained results contrary to our proliferation and activation of TCD4 + lymphocytes, reduction of activation of TCD8 + lymphocytes for the pectin-like polysaccharide of Conocarpus erectus leaves. According to Lim et al. (1997, 2003), apple pectin increased the proportion of CD4 + and CD8 + T lymphocytes in the mesenteric lymph node. Yaneva et al. (2002) evaluated the immunomodulatory activity of Aronia in combination with apple pectin in breast cancer patients undergoing postoperative radiotherapy and showed that CD4 + and CD8 + T cell counts increased significantly. These results show that the chemical structure directly interferes with the immunomodulatory activity (Popov and Ovodov 2013; Amorim et al. 2016; Merheb et al. 2019).
Emulsifying activity
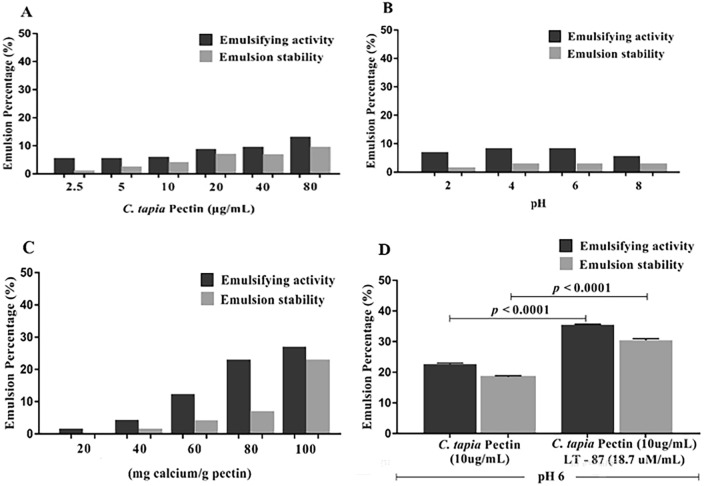
The emulsifying properties of pectins are related to the methyl ester (hydrophobic) groups present in the chains of this polysaccharide. Generally, the greater the number of hydrophobic groups, the greater the results of emulsification (Chen et al. 2016a, b). The pectin obtained in this work was subjected to different emulsion tests that evaluated the influence of variation in pectin concentration, pH and calcium ion concentration. The results of this study are shown in Fig. 10.
Fig. 10.
Emulsion tests varying the concentration (A), pH (B), calcium concentration (C) and evaluation of the emulsion properties with the insertion of the drug LT-87 (D). Vertical bars represent the average of two independent experiments performed in triplicate
Figure 10A shows that both the activity and stability of the emulsion did not differ significantly, but increasing the concentration leads to better results. Pectin showed low emulsification activity at a concentration of 80 µg/mL (12.9 ± 0.06%) and stability of (9.33 ± 0.06%). This low activity and stability result may be associated with a low degree of esterification, since the methyl ester groups are responsible for these properties (Chen et al. 2016a, b; Schmidt et al. 2017; Yang et al. 2018).
A limiting factor in choosing the best concentration for emulsion testing is viscosity (Chen et al. 2016a, b). At higher concentrations, there is a higher viscosity resulting in less sample homogenization (Yang et al. 2018). Therefore, for this study, the concentration of 10 µg/mL equivalent to 1% was chosen. This concentration has already been used by other authors studying different pectins (Schmidt et al. 2017; Yang et al. 2018; Deng et al. 2020). In addition to being a non-cytotoxic concentration, it was able to stimulate immune system cells.
Figure 10B shows the results of emulsifying the pectin solution (1%) at different pHs. The activity and stability of the emulsion did not show significant differences; however, it increased with increasing pH (2 to 6) having greater activity (8.0 ± 0.01%) and emulsion stability (2.89 ± 0.03%) at pH 6.0. Values of pH above this value decrease the activity and stability of the emulsion. Similar results were found by Yang et al. (2018) found that stability and emulsifying activity decrease at pH above 8. Guzey et al. (2004) observed loss of stability at basic pHs.
Figure 10C shows the effect of calcium chloride concentration with a pectin solution (1%) and pH 6.0. The values show that with the increase in the concentration of calcium ions there was an increase in the activity and stability of the emulsion. These properties did not show significant differences. Low esterification pectins form gels more easily in the presence of calcium ions by Guillotin et al. (2007). Similar behavior was observed by Yang et al. (2018), Zhao et al. (2018) and Ren et al. (2020) where they found that the emulsifying capacity was relatively stable, with an increase in the concentration of calcium chloride.
The condition used for the activity and stability of the emulsion was: pectin solution (1%), pH 6.0 and calcium ion concentration (100 mg/g) and the results were shown in Fig. 10D. The results showed that the insertion of the LT-87 molecule in the emulsion test promoted greater activity and stability (35.5 ± 0.0%; 30.4 ± 0.06%) when compared to the test that presented only pectin (32 ± 0.1%); 21.33 ± 0.01%). The experiments showed a significant difference of p < 0.0001.
Emulsions are procedures used in the pharmaceutical industry to facilitate the transport of drugs to increase the oral bioavailability of poorly water-soluble drugs (Burapapadh et al. 2010; Sriamornsak 2011). The literature reports different studies using pectin-based emulsions as carriers of different drugs, including Itraconazole (Burapapadh et al. 2010), Metformin hydrochloride (Banerjee et al. 2012), Metronidazole (Vaidya et al. 2015). Thus, emulsions with greater activity and stability promote better solubilization of the molecule to be transported, in addition to increasing the shelf life of the product (Xiang et al. 2015).
Conclusion
The pectin extracted from Crataeva tapia leaves did not show in vitro toxicity in blood cells and promoted a pro-inflammatory and antioxidant immune response. Its physicochemical properties allow its application as an emulsifying agent, that is, acting as supporting vehicles in pharmaceutical manipulations.
Acknowledgements
We would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel) and the Pernambuco State Research Support Foundation—FACEPE (Process APQ-0498-4.03/19), researcher fixation grant—FACEPE (Process BFP-0038-04.03/21) and grant from the National Council for Science and Technology Development—CNPq (Process 306865/2020-3) for granting financial assistance during the research period. The authors also thank the Center for Technological Platforms at the Aggeu Magalhães Research Center for using the flow cytometer (FIOCRUZ Pernambuco).
Data availability
The authors make available the data.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Simone da Paz Leôncio Alves, Email: simone.leoncio@hotmail.com.
Iris Trindade Tenório Jacob, Email: iristrindadetj@hotmail.com.
Marcela Daniela Muniz Arruda, Email: marceladanielamuniz@outlook.com.
Abdênego Rodrigues da Silva, Email: rodriguesabdenego@gmail.com.
Georon Ferreira de Sousa, Email: Georon.sousa@gmail.com.
Guilherme Antônio de Souza, Email: guilherme.assufpe@gmail.com.
Maria do Carmo Alves de Lima, Email: maria.calima@ufpe.br.
Ivone Antônia de Souza, Email: ivone.souza@ufpe.br.
Cristiane Moutinho Lagos de Melo, Email: cristianemout@gmail.com.
Iranildo José da Cruz Filho, Email: iranildoj@gmail.com.
Dayane Kelly Dias do Nascimento Santos, Email: Nascimento.d.k.d@gmail.com.
References
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. SPJ. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba K, Laws AP, Kontogiorgos V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015;43:726–735. [Google Scholar]
- Alves EU, Santos-Moura SDS, Moura MFD, Silva RDS, Galindo EA. Drying on the germination and vigor of Crataeva tapia L. seeds. Ciência Rural. 2017 doi: 10.1590/0103-8478cr20150338. [DOI] [Google Scholar]
- Amirian J, Zeng Y, Shekh MI, Sharma G, Stadler FJ, Song J, Zhu Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr Polym. 2021;251:117005. doi: 10.1016/j.carbpol.2020.117005. [DOI] [PubMed] [Google Scholar]
- Amorim JC, Vriesmann LC, Petkowicz CL, Martinez GR, Noleto GR. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int J Biol Macromol. 2016;92:1040–1048. doi: 10.1016/j.ijbiomac.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Araújo RMS, Silva Ferreira R, Napoleão TH, Graças CCM, Coelho LCBB, Santos CMT, Paiva PMG. Crataeva tapia bark lectin is an affinity adsorbent and insecticidal agent. Plant Sci. 2012;183:20–26. doi: 10.1016/j.plantsci.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Arruda MDM, Alves SDPL, da Cruz Filho IJ, de Sousa GF, de Souza Silva GA, do Nascimento Santos DKD, et al. Characterization of a lignin from Crataeva tapia leaves and potential applications in medicinal and cosmetic formulations. Int J Biol Macromol. 2021;180:286–298. doi: 10.1016/j.ijbiomac.2021.03.077. [DOI] [PubMed] [Google Scholar]
- Arslan N. Extraction of pectin from sugar-beet pulp and intrinsic viscosity molecular weight relationship of pectin solutions. J Food Sci Technol. 1995;32(5):381–385. [Google Scholar]
- Austarheim I, Christensen BE, Aas HTN, Thöle C, Diallo D, Paulsen BS. Chemical characterization and complement fixation of pectins from Cola cordifolia leaves. Carbohydr Polym. 2014;102:472–480. doi: 10.1016/j.carbpol.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Bae IY, Joe YN, Rha HJ, Lee S, Yoo SH, Lee HG. Effect of sulfation on the physicochemical and biological properties of citrus pectins. Food Hydrocoll. 2009;23(7):1980–1983. [Google Scholar]
- Banerjee P, Deb J, Roy A, Ghosh A, Chakraborty P. Fabrication and development of pectin microsphere of metformin hydrochloride. Int Sch Res Notices. 2012 doi: 10.5402/2012/230621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri SF, Amaral SC, Ruthes AC, Oliveira PCL, Kerkhoven NC, Silva ERA, et al. Pectins from the pulp of gabiroba (Campomanesia xanthocarpa Berg): structural characterization and rheological behavior. Carbohydr Polym. 2019;214:250–258. doi: 10.1016/j.carbpol.2019.03.045. [DOI] [PubMed] [Google Scholar]
- Bayar N, Bouallegue T, Achour M, Kriaa M, Bougatef A, Kammoun R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: optimization of experimental conditions and evaluation of chemical and functional properties. Food Hydrocoll. 2017;235:275–282. doi: 10.1016/j.foodchem.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Burapapadh K, Kumpugdee-Vollrath M, Chantasart D, Sriamornsak P. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application. Carbohydr Polym. 2010;82(2):384–393. [Google Scholar]
- Cabral DLDV, Castro VT, Coutinho HDM, Tintino SR, Menezes CDA, Menezes IR, Amorim ELC. Modulatory activity and chemical profile of a hydroalcoholic extract of Crateva tapia L. Afr J Microbiol Res. 2015;9(5):326–331. [Google Scholar]
- Campos JLA, Albuquerque UP. Indicators of conservation priorities for medicinal plants from seasonal dry forests of northeastern Brazil. Ecol Indic. 2021;121:106993. doi: 10.1016/j.ecolind.2020.106993. [DOI] [Google Scholar]
- Chen HM, Fu X, Luo ZG. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016;54:99–106. [Google Scholar]
- Chen T, Wang Y, Li J, Su N, Surhio MM, Yang L, Ye M. Phthaloyl modification of a polysaccharide from Lachnum YM262 and immunomodulatory activity. Process Biochem. 2016;51(10):1599–1609. [Google Scholar]
- Chen X, Qi Y, Zhu C, Wang Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int J Biol Macromol. 2019;131:273–281. doi: 10.1016/j.ijbiomac.2019.03.077. [DOI] [PubMed] [Google Scholar]
- Cheremisinoff NP. Designing EPDM products for extrusion applications. J Macromol Sci Pure Appl Chem. 1989;26(8):1231–1259. [Google Scholar]
- Coimbra P, Ferreira P, Sousa HC, Batista P, Rodrigues MA, Correia IJ, Gil MH. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol. 2011;48(1):112–118. doi: 10.1016/j.ijbiomac.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Cornejo X, Iltis HH. A revision of the American species of the genus Crateva (Capparaceae) Harv Pap Bot. 2008;13(1):121–135. [Google Scholar]
- Cortes UAB, Gutiérrez MC, Mendoza DG, Salitre LG, Vargas AS, Catzim CEA, Valenzuela BEL. Microencapsulation and antimicrobial activity of extract acetone-methanol of Hibiscus sabdariffa L. using a blend modified starch and pectin as a wall material. Ind Crops Prod. 2021;170:113725. [Google Scholar]
- Cruz Filho IJ, Silva BBR, Souza ALM, Navarro CDC, Ruas JS, Lorena VMB, Maior AMS. Lignins isolated from Prickly pear cladodes of the species Opuntia fícus-indica (Linnaeus) Miller and Opuntia cochenillifera (Linnaeus) Miller induces mice splenocytes activation, proliferation and cytokines production. Int J Biol Macromol. 2019;123:1331–1339. doi: 10.1016/j.ijbiomac.2018.09.120. [DOI] [PubMed] [Google Scholar]
- Deng Z, Pan Y, Chen W, Chen W, Yun Y, Zhong Q, Chen H. Effects of cultivar and growth region on the structural, emulsifying and rheological characteristic of mango peel pectin. Food Hydrocoll. 2020;103:105707. [Google Scholar]
- Deshmukh R. Bridging the gap of drug delivery in colon cancer: the role of chitosan and pectin based nanocarriers system. Curr Drug Deliv. 2020;17(10):911–924. doi: 10.2174/1567201817666200717090623. [DOI] [PubMed] [Google Scholar]
- Devasvaran K, Lim V. Green synthesis of metallic nanoparticles using pectin as a reducing agent: a systematic review of the biological activities. Pharm Biol. 2021;59(1):494–503. doi: 10.1080/13880209.2021.1910716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141(7):2407–2412. [PubMed] [Google Scholar]
- Einhorn-Stoll U, Kunzek H, Dongowski G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007;21(7):1101–1112. [Google Scholar]
- Gan CY, Manaf NHA, Latiff AA. Physico-chemical properties of alcohol precipitate pectin-like polysaccharides from Parkia speciosa pod. Food Hydrocoll. 2010;24(5):471–478. [Google Scholar]
- Gavlighi HA, Tabarsa M, You S, Surayot U, Ghaderi-Ghahfarokhi M. Extraction, characterization and immunomodulatory property of pectic polysaccharide from pomegranate peels: enzymatic vs conventional approach. Int J Biol Macromol. 2018;116:698–706. doi: 10.1016/j.ijbiomac.2018.05.083. [DOI] [PubMed] [Google Scholar]
- Golovchenko VV, Bushneva OA, Ovodova RG, Shashkov AS, Chizhov AO, Ovodov YS. Structural study of bergenan, a pectin from Bergenia crassifolia. Russ J Bioorganic Chem. 2007;33:47–56. doi: 10.1134/s1068162007010050. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Rosso ND. Determination of pectin methylesterase activity in commercial pectinases and study of the inactivation kinetics through two potentiometric procedures. Food Sci Technol. 2011;31:412–417. [Google Scholar]
- Govindaraj D, Rajan M, Hatamleh AA, Munusamy MA. From waste to high-value product: jackfruit peel derived pectin/apatite bionanocomposites for bone healing applications. Int J Biol Macromol. 2018;106:293–301. doi: 10.1016/j.ijbiomac.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Guedes RS, Alves EU, Gonçalves EP, Colares PNQ, Medeiros MSD, Viana JS. Germination and vigor of Myracrodruon urundeuva Allemão seeds in different substrates and temperatures. Revista Árvore. 2011;35:975–982. [Google Scholar]
- Guillotin SE, Bakx EJ, Boulenguer P, Schols HA, Voragen AGJ. Determination of the degree of substitution, degree of amidation and degree of blockiness of commercial pectins by using capillary electrophoresis. Food Hydrocoll. 2007;21:444–451. [Google Scholar]
- Guzey D, Kim HJ, McClements DJ. Factors influencing the production of o/w emulsions stabilized by β-lactoglobulin–pectin membranes. Food Hydrocoll. 2004;18:967–975. [Google Scholar]
- Habibi Y, Mahrouz M, Vignon MR. Isolation and structural characterization of protopectin from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr Polym. 2005;60:205–213. [Google Scholar]
- Ho GTT, Ahmed A, Zou YF, Aslaksen T, Wangensteen H, Barsett H. Structure–activity relationship of immunomodulating pectins from elderberries. Carbohydr Polym. 2015;125:314–322. doi: 10.1016/j.carbpol.2015.02.057. [DOI] [PubMed] [Google Scholar]
- Hosseini SS, Khodaiyan F, Kazemi M, Najari Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int J Biol Macromol. 2019;125:621–629. doi: 10.1016/j.ijbiomac.2018.12.096. [DOI] [PubMed] [Google Scholar]
- Hosseini S, Parastouei K, Khodaiyan F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int J Biol Macromol. 2020;158:911–921. doi: 10.1016/j.ijbiomac.2020.04.241. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nakamura K, Hatakeyama T, Hatakeyama H. Phase transition of pectin with sorbed water. Carbohydr Polym. 2000;41:101–106. [Google Scholar]
- Jacob ÍT, Gomes FO, Miranda MD, Almeida SM, Cruz-Filho IJ, Peixoto CA, Lima MC. Anti-inflammatory activity of novel thiosemicarbazone compounds indole-based as COX inhibitors. Pharmacol Rep. 2021 doi: 10.1007/s43440-021-00221-7. [DOI] [PubMed] [Google Scholar]
- Jeswani G, Alexander A, Saraf S, Saraf S, Qureshi A. Recent approaches for reducing hemolytic activity of chemotherapeutic agents. J Control Release. 2015;21:10–21. doi: 10.1016/j.jconrel.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Du Y, Zhu X, Xiong H, Woo MW, Hu J. Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohydr Polym. 2012;87(2):1663–1669. [Google Scholar]
- Kalapathy U, Proctor A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001;73:393–396. [Google Scholar]
- Kazemi M, Khodaiyan F, Hosseini SS. Eggplant peel as a high potential source of high methylated pectin: ultrasonic extraction optimization and characterization. LWT. 2019;105:182–189. [Google Scholar]
- Kazemi M, Khodaiyan F, Hosseini SS. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019;294:339–346. doi: 10.1016/j.foodchem.2019.05.063. [DOI] [PubMed] [Google Scholar]
- Khan J, Noboru N, Young A, Thomas D. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology. 2017;24:155–159. doi: 10.1016/j.pathophys.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Kodoth AK, Ghate VM, Lewis SA, Prakash B, Badalamoole V. Pectin-based silver nanocomposite film for transdermal delivery of Donepezil. Int J Biol Macromol. 2019;134:269–279. doi: 10.1016/j.ijbiomac.2019.04.191. [DOI] [PubMed] [Google Scholar]
- Kothandaraman GP, Ravichandran V, Bories C, Loiseau PM, Jayakrishnan A. Anti-fungal and anti-leishmanial activities of pectin-amphotericin B conjugates. J Drug Deliv Sci Technol. 2017;39:1–7. [Google Scholar]
- Ktari N, Trabelsi I, Bardaa S, Triki M, Bkhairia I, Salem RBSB, Salah RB. Antioxidant and hemolytic activities, and effects in rat cutaneous wound healing of a novel polysaccharide from fenugreek (Trigonella foenum-graecum) seeds. Int J Biol Macromol. 2017;95:625–634. doi: 10.1016/j.ijbiomac.2016.11.091. [DOI] [PubMed] [Google Scholar]
- Kumar G, Karthik L, Rao KVB. Hemolytic activity of Indian medicinal plants towards human erythrocytes: an in vitro study. Elixir Appl Botany. 2011;40(5534):e5537. [Google Scholar]
- Kyomugasho C, Christiaens S, Shpigelman A, Van Loey AM, Hendrickx M. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chem. 2015;176:82–90. doi: 10.1016/j.foodchem.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Langhout DJ, Schutte JB, Van Leeuwen P, Wiebenga J, Tamminga S. Effect of dietary high-and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br Poult Sci. 1999;40:340–347. doi: 10.1080/00071669987421. [DOI] [PubMed] [Google Scholar]
- Lefsih K, Giacomazza D, Dahmoune F, Mangione MR, Bulone D, San Biagio PL, Madani K. Pectin from Opuntia ficus indica: optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017;221:91–99. doi: 10.1016/j.foodchem.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Lim BO, Yamada K, Nonaka M, Kuramoto Y, Hung P, Sugano M. Dietary fibers modulate indices of intestinal immune function in rats. J Nutr. 1997;127:663–667. doi: 10.1093/jn/127.5.663. [DOI] [PubMed] [Google Scholar]
- Lim BO, Lee SH, Park DK, Choue RW. Effect of dietary pectin on the production of immunoglobulins and cytokines by mesenteric lymph node lymphocytes in mouse colitis induced with dextran sulfate sodium. Biosci Biotechnol Biochem. 2003;67:1706–1712. doi: 10.1271/bbb.67.1706. [DOI] [PubMed] [Google Scholar]
- Lima MS, Paiva EP, Andrade SAC, Paixão JA. Fruit pectins–A suitable tool for screening gelling properties using infrared spectroscopy. Food Hydrocoll. 2010;24:1–7. [Google Scholar]
- Liu L, Cao J, Huang J, Cai Y, Yao J. Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresour Technol. 2010;101:3268–3273. doi: 10.1016/j.biortech.2009.12.062. [DOI] [PubMed] [Google Scholar]
- Lupi FR, Gabriele D, Seta L, Baldino N, de Cindio B, Marino R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol Acta. 2015;54:41–52. [Google Scholar]
- M’sakni NH, Majdoub H, Roudesli S, Picton L, Le Cerf D, Rihouey C, Morvan C. Composition, structure and solution properties of polysaccharides extracted from leaves of Mesembryanthenum crystallinum. Eur Polym J. 2006;42:786–795. [Google Scholar]
- Marcon MV, Carneiro PIB, Wosiacki G, Beleski-Carneiro E, Petkowicz CLO. Pectins from apple pomace–characterization by 13C and 1H NMR spectroscopy. Ann Magn Reson. 2005;4:56–63. [Google Scholar]
- Melo CML, Melo H, Correia MT, Coelho LCBB, Da Silva MB, Pereira VRA. Mitogenic response and cytokine production induced by cramoll 1, 4 lectins in splenocytes of inoculated mice. Scand J Immunol. 2011;73:112–121. doi: 10.1111/j.1365-3083.2010.02490.x. [DOI] [PubMed] [Google Scholar]
- Melo CMLD, Sousa GFD, Silva GADS, Silva RSD, Bezerra Júnior NDS, Santos DKDDN, et al. Pectin-like polysaccharide extracted from the leaves Caesalpinia pulcherrima is a promising antioxidant and immunomodulator agent. Braz Arch Biol Technol. 2022 doi: 10.1590/1678-4324-2022200718. [DOI] [Google Scholar]
- Merheb R, Abdel-Massih RM, Karam MC. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int J Biol Macromol. 2019;121:1–5. doi: 10.1016/j.ijbiomac.2018.09.189. [DOI] [PubMed] [Google Scholar]
- Misra NN, Yadav SK. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: kinetics, characterization and process economics. Food Hydrocoll. 2020;102:105592. [Google Scholar]
- Mzoughi Z, Abdelhamid A, Rihouey C, Le Cerf D, Bouraoui A, Majdoub H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr Polym. 2018;185:127–137. doi: 10.1016/j.carbpol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Nergard CS, Matsumoto T, Inngjerdingen M, Inngjerdingen K, Hokputsa S, Harding SE, Yamada H. Structural and immunological studies of a pectin and a pectic arabinogalactan from Vernonia kotschyana Sch. Bip. ex Walp. (Asteraceae) Carbohydr Res. 2005;340:115–130. doi: 10.1016/j.carres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Nisar T, Wang ZC, Yang X, Tian Y, Iqbal M, Guo Y. Characterization of citrus pectin films integrated with clove bud essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Int J Biol Macromol. 2018;106:670–680. doi: 10.1016/j.ijbiomac.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Oliveira AN, Paula DA, Oliveira EB, Saraiva SH, Stringheta PC, Ramos AM. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int J Biol Macromol. 2018;113:395–402. doi: 10.1016/j.ijbiomac.2018.02.154. [DOI] [PubMed] [Google Scholar]
- Ovodova RG, Bushneva OA, Shashkov AS, Chizhov AO, Ovodov YS. Structural studies on pectin from marsh cinquefoil Comarum palustre L. Biochem Mosc. 2005;70:867–877. doi: 10.1007/s10541-005-0196-y. [DOI] [PubMed] [Google Scholar]
- Patova OA, Smirnov VV, Golovchenko VV, Vityazev FV, Shashkov AS, Popov SV. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr Polym. 2019;209:239–249. doi: 10.1016/j.carbpol.2018.12.098. [DOI] [PubMed] [Google Scholar]
- Peng Q, Xu Q, Yin H, Huang L, Du Y. Characterization of an immunologically active pectin from the fruits of Lycium ruthenicum. Int J Biol Macromol. 2014;64:69–75. doi: 10.1016/j.ijbiomac.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Popov SV, Ovodov YS. Polypotency of the immunomodulatory effect of pectins. Biochemistry-Moscow. 2013;78:823–835. doi: 10.1134/S0006297913070134. [DOI] [PubMed] [Google Scholar]
- Pratissoli D, Polanczyk RA, Dalvi LP, Cocheto JG, Melo DG. Occurrence of Ascia monuste orseis (Lepidoptera: Pieridae) damaging seedlings of Crataeva tapia. Cienc Rural. 2007;37:874–875. [Google Scholar]
- Priyangini F, Walde SG, Chidambaram R. Extraction optimization of pectin from cocoa pod husks (Theobroma cacao L.) with ascorbic acid using response surface methodology. Carbohydr Polym. 2018;202:497–503. doi: 10.1016/j.carbpol.2018.08.103. [DOI] [PubMed] [Google Scholar]
- Ranieri G, Mazzei R, Poerio T, Bazzarelli F, Wu Z, Li K, Giorno L. Biorefinery of olive leaves to produce dry oleuropein aglycone: use of homemade ceramic capillary biocatalytic membranes in a multiphase system. Chem Eng Sci. 2018;185:149–156. [Google Scholar]
- Ren W, Zhao S, Lian Y, Yang Y, Tian G, Zhao C, Zheng J. Effects of hydrosoluble calcium ions and organic acids on citrus oil emulsions stabilized with citrus pectin. Food Hydrocoll. 2020;100:105413. [Google Scholar]
- Salman H, Bergman M, Djaldetti M, Orlin J, Bessler H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed Pharmacother. 2008;62:579–582. doi: 10.1016/j.biopha.2008.07.058. [DOI] [PubMed] [Google Scholar]
- Santos DKDN, Silva Barros BR, Souza Aguiar LM, Cruz Filho IJ, Lorena VMB, Melo CML, Napoleão TH. Immunostimulatory and antioxidant activities of a lignin isolated from Conocarpus erectus leaves. Int J Biol Macromol. 2020;150:169–177. doi: 10.1016/j.ijbiomac.2020.02.052. [DOI] [PubMed] [Google Scholar]
- Santos DKDN, Barros BRS, Filho IJC, Júnior NDSB, Silva PR, Nascimento PHB, Melo CML. Pectin-like polysaccharide extracted from the leaves of Conocarpus erectus Linnaeus promotes antioxidant, immunomodulatory and prebiotic effects. Bioact Carbohydr Diet Fibre. 2021;26:100263. [Google Scholar]
- Santos-Moura SDS, Alves EU, Galindo EA, Moura MFD, Melo PAFRD. Qualidade fisiológica de sementes de Crataeva tapia L. submetidas a diferentes métodos de extração da mucilagem. Rev Bras Frutic. 2014;36:686–692. [Google Scholar]
- Schmidt US, Schütz L, Schuchmann HP. Interfacial and emulsifying properties of citrus pectin: interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017;62:288–298. [Google Scholar]
- Seyfried M, Soldera-Silva A, Bovo F, Stevan-Hancke FR, Maurer JBB, Zawadzki-Baggio SF. Pectinas de plantas medicinais: características estruturais e atividades imunomoduladoras. Rev Brasi De Plant Med. 2016;18:201–214. [Google Scholar]
- Sharma P, Patil D, Patil A. Crataeva tapia Linn—an important medicinal plant: a review of its traditional uses, phytochemistry and pharmacological Properties. Int J Pharm Sci Res. 2013;4:582–589. [Google Scholar]
- Silva AR, Oliveira WF, Silva PM, Patriota LLS, Alves RRV, Oliveira APS, Correia MTS, Paiva PMG, Vainstein MH, Filho PEC, Fontes A, Napoleão TH. Quantum dots conjugated to lectins from Schinus terebinthifolia leaves (SteLL) and Punica granatum sarcotesta (PgTeL) as potential fluorescent nanotools for investigating Cryptococcus neoformans. Int J Biol Macromol. 2021;192:232–240. doi: 10.1016/j.ijbiomac.2021.10.002. [DOI] [PubMed] [Google Scholar]
- Slima SB, Trabelsi I, Ktari N, Bardaa S, Elkaroui K, Bouaziz M, Salah RB. Novel Sorghum bicolor (L.) seed polysaccharide structure, hemolytic and antioxidant activities, and laser burn wound healing effect. Int J Biol Macromol. 2019;132:87–96. doi: 10.1016/j.ijbiomac.2019.03.192. [DOI] [PubMed] [Google Scholar]
- Souza MAD (2014) Elaboração e caracterização de biscoitos obtidos a partir da farinha do fruto do trapiá (Crataeva tapia L.)
- Sriamornsak P. Application of pectin in oral drug delivery Expert Opin. Drug Deliv. 2011;8:1009–1023. doi: 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- Tahmouzi S, Nejat MRS. New infertility therapy effects of polysaccharides from Althaea officinalis leaf with emphasis on characterization, antioxidant and anti-pathogenic activity. Int J Biol Macromol. 2020;145:777–787. doi: 10.1016/j.ijbiomac.2019.12.224. [DOI] [PubMed] [Google Scholar]
- Tan J, Hua X, Liu J, Wang M, Liu Y, Yang R, Cao Y. Extraction of sunflower head pectin with superfine grinding pretreatment. Food Chem. 2020;320:126631. doi: 10.1016/j.foodchem.2020.126631. [DOI] [PubMed] [Google Scholar]
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Torralbo DF, Batista KA, Di-Medeiros MCB, Fernandes KF. Extraction and partial characterization of Solanum lycocarpum pectin. Food Hydrocoll. 2012;27(2):378–383. [Google Scholar]
- Vaidya A, Jain S, Agrawal RK, Jain SK. Pectin–metronidazole prodrug bearing microspheres for colon targeting. J Saudi Chem Soc. 2015;19:257–264. [Google Scholar]
- Wang XS, Liu L, Fang JN. Immunological activities and structure of pectin from Centella asiatica. Carbohydr Polym. 2005;60:95–101. [Google Scholar]
- Wang W, Ma X, Jiang P, Hu L, Zhi Z, Chen J, Ding T, Ye X, Liu D. Characterization of pectin from grapefruit peel: a comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016;61:730–739. [Google Scholar]
- Wang M, Liu Y, Qiang M, Wang J. Structural elucidation of a pectin–type polysaccharide from Hovenia dulcis peduncles and its proliferative activity on RAW264.7 cells. Int J Biol. 2017;104:1246–1253. doi: 10.1016/j.ijbiomac.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Wathoni N, Shan CY, Shan WY, Rostinawati T, Indradi RB, Pratiwi R, Muchtaridi M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon. 2019;5:02299. doi: 10.1016/j.heliyon.2019.e02299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymack BE, Belote JL, Baliga VL, Hajaligol MR. Effects of metal salts on char oxidation in pectins/uronic acids and other acid derivative carbohydrates. Fuel. 2004;83:1505–2151. [Google Scholar]
- Xavier MEV, Silva DCG, Silva Macedo E, Souza MA, Santos AF, Costa JG. Potencial antioxidante e alelopático de Crataeva tapia L. Diversitas Journal. 2019;4(1):306–318. [Google Scholar]
- Xia YG, Huang YX, Liang J, Kuang HX. Comparable studies of two polysaccharides from leaves of Acanthopanax senticosus: structure and antioxidation. Int J Biol Macromol. 2020;147:350–362. doi: 10.1016/j.ijbiomac.2019.12.244. [DOI] [PubMed] [Google Scholar]
- Xiang J, Liu F, Fan R, Gao Y. Physicochemical stability of citral emulsions stabilized by milk proteins (lactoferrin, α-lactalbumin, β-lactoglobulin) and beet pectin. Colloids Surf A Physicochem Eng Asp. 2015;487:104–112. [Google Scholar]
- Yaneva M, Botushanova AD, Grigorov LA, Kokov JL, Todorova EP, Krachanova MG. Evaluation of the immunomodulatory activity of Aronia in combination with apple pectin in patients with breast cancer undergoing postoperative radiation therapy. Folia Med. 2002;44:22–25. [PubMed] [Google Scholar]
- Yang Y, Wang Z, Hu D, Xiao K, Wu JY. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018;79:189–196. [Google Scholar]
- Yang JS, Mu TH, Ma MM. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019;289:351–359. doi: 10.1016/j.foodchem.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Yuliarti O, Matia-Merino L, Goh KK, Mawson J, Williams MA, Brennan C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015;166:479–485. doi: 10.1016/j.foodchem.2014.06.055. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gao W, Tian G, Zhao C, DiMarco-Crook C, Fan B, Zheng J. Citrus oil emulsions stabilized by citrus pectin: the influence mechanism of citrus variety and acid treatment. J Agric Food Chem. 2018;66:12978–12988. doi: 10.1021/acs.jafc.8b04711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors make available the data.