Abstract
OBJECTIVE
To assess the prevalence and correlates of prescription of sodium–glucose cotransporter 2 inhibitors (SGLT2i) and/or glucagon-like peptide 1 receptor agonists (GLP1-RA) in individuals with type 2 diabetes mellitus (T2DM) with and without chronic kidney disease (CKD).
RESEARCH DESIGN AND METHODS
This was a cross-sectional analyses of SGLT2i and GLP1-RA prescriptions from 1 January 2019 to 31 December 2020 in the Veterans Health Administration System. The likelihood of prescriptions was examined by the presence or absence of CKD and by predicted risks of atherosclerotic cardiovascular disease (ASCVD) and end-stage kidney disease (ESKD).
RESULTS
Of 1,197,880 adults with T2DM, SGLT2i and GLP1-RA were prescribed to 11% and 8% of patients overall, and to 12% and 10% of those with concomitant CKD, respectively. In adjusted models, patients with severe albuminuria were less likely to be prescribed SGLT2i or GLP1-RA versus nonalbuminuric patients with CKD, with odds ratios (ORs) of 0.91 (95% CI 0.89, 0.93) and 0.97 (0.94, 1.00), respectively. Patients with a 10-year ASCVD risk >20% (vs. <5%), had lower odds of SGLT2i use (OR 0.66 [0.61, 0.71]) and GLP1-RA prescription (OR 0.55 [0.52, 0.59]). A 5-year ESKD risk >5%, compared with <1%, was associated with lower likelihood of SGLT2i prescription (OR 0.63 [0.59, 0.67]) but higher likelihood of GLP1-RA prescription (OR 1.53 [1.46, 1.61]).
CONCLUSIONS
Among a large cohort of patients with T2DM, prescription of SGLT2i and GLP1-RA was low in those with CKD. We observed a “risk-treatment paradox,” whereby patients with higher risk of adverse outcomes were less likely to receive these therapies.
Introduction
The sodium–glucose cotransporter 2 inhibitors (SGLT2i) and the glucagon-like peptide 1 receptor agonists (GLP1-RA) afford substantial cardiovascular and kidney-protective effects (1–3). This has triggered a paradigm shift from a glucose-centric approach to type 2 diabetes mellitus (T2DM) management toward a recommended strategy that incorporates therapies based on their ability to lower risk for cardiovascular disease (CVD) and chronic kidney disease (CKD) progression. However, the adoption of this new paradigm may be slow given the traditional practice of using medications for diabetes based on glycemic targets.
As a class, the SGLT2i substantially reduce the risk of major adverse CVD events encompassing atherosclerotic CVD (ASCVD), incident and progressive heart failure, and CVD mortality (1,4). In addition, clinical trials of SGLT2i conducted exclusively in patients with CKD have demonstrated marked reductions in the risk of progression to end-stage kidney disease (ESKD) (5,6). Similarly, the GLP1-RA, including liraglutide, semaglutide, albiglutide, and dulaglutide, have demonstrated marked cardiovascular-protective effects in individuals with T2DM (2). Although dedicated trials of GLP1-RA evaluating primary kidney outcomes have not been conducted, these agents significantly reduce the risk of worsening albuminuria and kidney function decline, both powerful indicators of subsequent adverse kidney and cardiovascular events (3,7).
The protective cardiovascular and kidney effects derived from SGLT2i and GLP1-RA prompted major scientific guidelines, including the American Diabetes Association (ADA) and the Kidney Disease Improving Global Outcomes (KDIGO) in 2020, to recommend their prescription in individuals with T2DM regardless of glycemic control if they have established CVD, are at high risk of ASCVD, or have CKD (8,9). For patients with T2DM and CKD, an SGLT2i is recommended (8,9). For those with an estimated glomerular filtration rate (eGFR) less than adequate for SGLT2i prescription, or in those with high ASCVD risk or established ASCVD, a GLP1-RA may be preferred (9). While the use of these medications in this subset of patients is expected to increase with time, studying baseline prescription patterns is necessary to guide subsequent implementation efforts.
Recent studies have shown that use of SGLT2i and GLP1-RA is low among patients with T2DM and established CVD (10). Yet, less is known about contemporary prescription of these medications among patients with T2DM and concomitant CKD—an exceptionally high-risk population in whom rapid adoption of these therapies could lead to substantial reductions in the burden of CVD and CKD. Accordingly, in this study, we investigated current prescription patterns of SGLT2i and GLP1-RA in >1 million patients with T2DM who received primary care in the Veterans Health Administration (VHA) System during 2019 and 2020. The main objectives were to 1) characterize prescription according to diabetes management and control, including hemoglobin A1c concentrations, and use of other medications for diabetes; 2) evaluate prescription prevalence and correlates among patients with CKD; and 3) assess prescription use according to predicted risks for ASCVD and ESKD.
Research Design and Methods
Study Setting
The Kidney Health Research Collaborative (KHRC) data registry is a unified data repository to enhance research initiatives aimed at improving the care of patients with CKD. It has curated data from the VHA Corporate Data Warehouse, which is a comprehensive national repository of data from the VHA electronic health record (EHR). The Corporate Data Warehouse contains individual patient sociodemographic characteristics, outpatient and inpatient clinical encounters, medication prescriptions and fills, medical conditions, procedures, and laboratory results. Data are sourced from >130 hospitals and 1,000 outpatient and skilled nursing facilities, and VA external fee-for-service claims.
Study Population
All VHA patients with T2DM and at least two primary care encounters between 1 January 2019 and 31 December 2020 were included (Supplementary Fig. 1). Ascertainment of T2DM combined ICD-10 codes, hemoglobin A1c values, and prescription of antidiabetes medications following the validated electronic Medical Records and Genomics (e-MERGE) algorithm for ascertainment of T2DM in the EHR (Supplementary Fig. 2 and Supplementary Table 1) (11).
Patients were excluded if they were receiving hospice care, had an invalid date of birth, or did not have a sex classification (n = 20,865). Because SGLT2i and GLP1-RA are not indicated in patients with ESKD and have not been studied in patients who have received a kidney transplant, we excluded patients with an eGFR <15 mL/min/1.73 m2 (stage 5 CKD), those undergoing chronic dialysis treatment, and kidney transplant recipients (n = 32,243). After these exclusions, the final study sample comprised 1,197,880 Veterans Affairs Health Care System patients with T2DM.
Key Covariates
Ascertainment of CKD used the validated e-Phenotype algorithm for the EHR that combines eGFR and urinary albumin-to-creatinine ratio (ACR) values obtained during outpatient clinic visits (12). CKD was defined as at least two measures of eGFR <60 mL/min/1.73 m2 and/or an ACR >30 mg/g obtained >90 days apart. The prevalence of prescription by each CKD stage was assessed using the KDIGO staging system—eGFR stages G1 to G5 and ACR stages A1 to A3 (13). Because the urinary protein-to-creatinine ratio (PCR) is often ordered by providers in lieu of the ACR, we used the recommended KDIGO equivalent values to assign measured PCR values to A1 to A3 categories (Supplementary Table 2). Among patients with CKD, the Kidney Failure Risk Equation was calculated to estimate the 5-year risk of ESKD (14). The Kidney Failure Risk Equation is based on four variables to predict ESKD risk: age, sex, ACR, and eGFR.
We defined atherosclerotic CVD based on the ICD-10 codes for ischemic heart disease or ischemic stroke being present on at least two inpatient and/or outpatient encounters following published methods (Supplementary Table 1) (15). For individuals without prevalent ASCVD, the 10-year ASCVD risk was calculated using the American Heart Association/American College of Cardiology pooled cohort equation excluding patients ≥80 years (16).
SGLT2i and GLP1-RA Prescription
Prevalent SGLT2i or GLP1-RA prescription was defined as any active prescription from 1 January 2019 through 31 December 2020. The SGLT2i empagliflozin and the GLP1-RA semaglutide are included in the VHA national formulary. In addition to these medications, we assessed for the prescription of the SGLT2i ertugliflozin, canagliflozin, and dapagliflozin and for the GLP1-RA liraglutide, albiglutide, and dulaglutide as nonformulary prescriptions. We included only GLP1-RA for which cardiovascular-protective effects have been demonstrated. For each medication class, prevalent medication prescription estimates were calculated overall and stratified by characteristics of diabetes control and management: the most recent hemoglobin A1c value, the prescription of other medications for diabetes, and specialty treatment by endocrinology. In addition, prevalent prescription estimates were stratified by presence or absence of established CKD, by ASCVD and ESKD risk thresholds, and by clinic visits to endocrinology, nephrology, and cardiology.
Other Covariates
VHA does not systematically collect individual-level information on socioeconomic status in structured data fields. Therefore, we used the median per capita income of residential ZIP Code and the ZIP Code-level social deprivation index as proxies for socioeconomic status using data derived from the American Community Survey (17,18). Patients with >50% health coverage for service-connected conditions and those in whom diabetes is a service-connected condition do not have copayments for their medications for diabetes. Thus, we were able to assess prescriptions in the subset of patients who did not have any cost-sharing for these medications. Rurality was assessed using Rural-Urban Commuting Area codes, which consider population density as well as how closely a community is linked socioeconomically to large urban centers (19). Disparities in diabetes care have been described for patients with substance use disorder, including unhealthy alcohol use, and for patients with underlying mental illness (20,21). Therefore, we assessed alcohol with the Alcohol Use Disorder Identification Test Consumption (AUDIT-C), with unhealthy alcohol use defined as an AUDIT-C score ≥3 for women and ≥4 for men (22). Smoking status in the VA Health Care System is recorded as part of routine care under “health factors” (23). A mental health diagnosis comprised the presence of an ICD-10 code encompassing posttraumatic health disorder or other severe mental illness following published methods (24). Frailty was assessed with the validated VA frailty index, which encompasses variables related to mobility, functional status, cognition and mood, sensory impairment (e.g., hearing, or visual impairment), and other geriatric syndromes (e.g., incontinence) (25).
Statistical Analysis
Frequency and percentage are reported for categorical variables, and mean and SD are reported for continuous variables. We constructed crude and multivariable logistic regression models to investigate the associations of the following variables with prescription of SGLT2i and GLP1-RA: diabetes control and management (hemoglobin A1c concentration and concomitant prescription of other antidiabetes medications), presence and stage of CKD, and predicted ASCVD and ESKD risk. Multivariable models adjusted for age, sex, race/ethnicity, ZIP Code median income, ZIP Code social deprivation index, service-connected disability and diabetes, rurality, smoking status, unhealthy alcohol use, hypertension, BMI, mental health diagnosis, frailty, and coronavirus disease 2019 (COVID-19) diagnosis. Because of overlap, separate models were constructed to evaluate the association of a visit to an endocrinology provider (yes/no) and the number of endocrinology visits with prescription for SGLT2i and GLP1-RA. Similarly, separate models were constructed to assess the association of CKD and KDIGO CKD stages with each prescription. Multivariable models simultaneously assessed the associations between eGFR and ACR category with each medication class. To account for the correlation among individuals within VA facilities, robust SEs were estimated with the empirical (“sandwich”) estimator.
All statistical analyses were conducted using SAS version 8.1 software (SAS Institute, Cary, NC) for Unix.
Results
Between 1 January 2019 and 31 December 2020, we identified 1,197,880 patients with T2DM who met the inclusion and exclusion criteria. Of these, 11% and 8% were prescribed an SGLT2i or a GLP1-RA, respectively (Supplementary Fig. 1). Nearly all of these prescriptions (>99%) were filled. Close to 99% of SGLT2i prescriptions were for empagliflozin specifically. For GLP1-RA, 41% of prescriptions were for liraglutide, 28% for dulaglutide, 26% for semaglutide, and 5% for albiglutide. Most patients (57%) without a prescription for an SGLT2i or a GLP1-RA had a hemoglobin A1c <7% compared with <30% who were prescribed an SGLT2i, a GLP1-RA, or both medications. Compared with patients without a prescription, patients with prescriptions for either or both medications were more likely to have hypertension, obesity (BMI ≥30 kg/m2), ASCVD, heart failure, and CKD (Table 1).
Table 1.
Demographic and clinical characteristics of patients with type 2 diabetes mellitus in the VHA health care system from 2019 to 2020 by prescription of SGLT2i and GLP1-RA agents
| Characteristics | None n = 1,011,176 | SGLT2i n = 128,523 | GLP1-RA n = 92,496 | GLP1-RA and SGLT2i n = 34,315 |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age, mean (SD), years | 68 (11) | 65 (10) | 68 (11) | 64 (9) |
| Female sex | 40,037 (4) | 4,750 (4) | 5,312 (6) | 1,685 (5) |
| Race | ||||
| White | 710,980 (70) | 95,959 (75) | 69,733 (75) | 26,050 (76) |
| Black | 204,954 (20) | 20,618 (16) | 14,371 (16) | 5,017 (15) |
| Asian or Asian Pacific Islander | 20,615 (2) | 2,921 (2) | 1,975 (2) | 849 (2) |
| American Indian or Alaska Native | 8,462 (1) | 1,117 (1) | 852 (1) | 304 (1) |
| Ethnicity | ||||
| Hispanic/Latino | 71,956 (7) | 9,317 (7) | 6,043 (7) | 2,287 (7) |
| Service-connected disability >50% | 422,886 (42) | 61,186 (48) | 45,876 (50) | 17,405 (51) |
| Diabetes service connection | 241,043 (24) | 38,252 (30) | 28,775 (31) | 10,769 (31) |
| Lowest ZIP Code median income quartile (<$44,818) | 255,797 (25) | 28,098 (22) | 19,595 (21) | 7,033 (20) |
| Highest social deprivation index quartile (score >73) | 24,6047 (24) | 28,459 (22) | 20,539 (22) | 7,304 (21) |
| Rural or highly rural ZIP Code | 377,858 (37) | 47,649 (37) | 34,746 (38) | 12,724 (37) |
| Lifestyle | ||||
| Unhealthy alcohol use | 79,504 (8) | 7,648 (6) | 4,207 (5) | 1,618 (5) |
| Current smoking | 155,768 (15) | 17,635 (14) | 11,080 (12) | 3,936 (11) |
| Diabetes management and control | ||||
| Hemoglobin A1c ≤7% (53 mmol/mol) | 572,691 (57) | 32,602 (25) | 25,900 (28) | 7,842 (23) |
| Diabetes medications other than SGLT2i or GLP1-RA | ||||
| None | 235,455 (23) | 3,602 (3) | 2,154 (2) | 424 (1) |
| One | 409,144 (40) | 23,582 (18) | 18,454 (20) | 5,106 (15) |
| Two | 248,427 (25) | 55,501 (43) | 41,486 (45) | 15,671 (46) |
| Three or more | 118,150 (12) | 45,838 (36) | 30,402 (33) | 13,114 (38) |
| Endocrinology visit | 105,801 (10) | 34,961 (27) | 32,544 (35) | 13,409 (39) |
| Clinical characteristics | ||||
| Hypertension | 985,389 (97) | 126,414 (98) | 91,256 (99) | 33,881 (99) |
| BMI ≥30 kg/m2 | 549,224 (54) | 82,354 (64) | 66,313 (72) | 24,531 (71) |
| ASCVD | 317,094 (31) | 55,128 (43) | 36,581 (40) | 14,761 (43) |
| Heart failure | 97,496 (10) | 18,514 (14) | 14,191 (15) | 5,264 (15) |
| CKD | 345,322 (34) | 50,337 (39) | 43,648 (47) | 14,627 (43) |
| Mental health diagnosis | 218,381 (22) | 29,984 (23) | 23,413 (25) | 8,707 (25) |
| COVID-19 diagnosis | 26,629 (3) | 4,636 (4) | 3,506 (4) | 1,374 (4) |
Data are presented as n (%) unless otherwise specified.
Prescription of SGLT2i and GLP1-RA According to Diabetes Management and Control
All variables related to diabetes management and control were strongly associated with SGLT2i or GLP1-RA prescription. Compared with patients who were not prescribed additional medications for diabetes, those with three or more prescriptions (other than prescriptions for SGLT2i or GLP1-RA) were more than four times as likely to be prescribed an SGLT2i or a GLP1-RA in multivariable analyses (Supplementary Table 3). Patients with higher hemoglobin A1c were more likely to be prescribed both medication classes compared with patients with hemoglobin A1c <7%. Patients who were seen by an endocrinologist (13%) and those with a greater number of visits were substantially more likely to be prescribed an SGLT2i or a GLP1-RA compared with patients without endocrinology visits during the study period. Of specific medications, prescription of insulin was associated with nearly two- and threefold higher odds of being prescribed an SGLT2i or a GLP1-RA, respectively (Supplementary Table 4).
Prescription of SGLT2i and GLP1-RA Among Patients With CKD
Overall, 35% of patients had CKD, the great majority of whom were not prescribed an SGLT2i or a GLP1-RA (Table 2). Of all patients with T2DM, 658,632 (55%) had ACR or PCR ordered during the study period. The strongest correlates of albuminuria testing were BMI, hypertension diagnosis, and a nephrology visit (Supplementary Table 5).
Table 2.
Association between the presence of CKD and prescription of SGLT2i and GLP1-RA among VA patients with T2DM during the years 2019 and 2020
| SGLT2i prescription | GLP1-RA prescription | ||||
|---|---|---|---|---|---|
| n | % Prescribed | Multivariable model* | % Prescribed | Multivariable model* | |
| OR (95% CI) | OR (95% CI) | ||||
| CKD† | |||||
| Absent | 610,528 | 11 | Reference | 7 | Reference |
| Present | 424,680 | 12 | 0.98 (0.97, 1.00) | 10 | 1.13 (1.12, 1.15) |
| Unknown | 162,672 | 8 | 0.85 (0.81, 0.90) | 5 | 0.81 (0.75, 0.86) |
| KDIGO CKD stage‡ | |||||
| eGFR, mL/min/1.73 m2 | |||||
| G1: ≥90 | 88,959 | 16 | Reference | 11 | Reference |
| G2: 60–89 | 192,723 | 15 | 1.02 (0.99, 1.05) | 11 | 0.99 (0.94, 1.04) |
| G3a: 45–59 | 94,564 | 12 | 0.93 (0.89, 0.96) | 9 | 1.02 (0.98, 1.07) |
| G3b: 30–44 | 24,056 | 9 | 0.72 (0.69, 0.76) | 12 | 1.17 (1.11, 1.22) |
| G4:15–29 | 23,588 | 4 | 0.42 (0.39, 0.45) | 12 | 1.09 (1.03, 1.15) |
| ACR, mg/g | |||||
| A1: <30 | 125,732 | 11 | Reference | 9 | Reference |
| A2: 30–300 | 185,413 | 13 | 0.96 (0.95, 0.98) | 11 | 1.01 (0.98, 1.03) |
| A3: >300 | 71,935 | 12 | 0.91 (0.89, 0.93) | 13 | 0.97 (0.94, 1.00) |
| Unknown ACR/PCR | 41,600 | 7 | 0.76 (0.72, 0.79) | 7 | 0.80 (0.73, 0.87) |
| Nephrology visit | |||||
| No | 1,133,361 | 11 | Reference | 7 | Reference |
| Yes | 64,519 | 13 | 1.05 (1.01, 1.09) | 15 | 1.18 (1.14, 1.23) |
Multivariable model adjusted for age, sex, self-identified race/ethnicity, ZIP Code median income, ZIP Code area social deprivation index, VA diabetes and service connection, rurality, smoking status, unhealthy alcohol use, hypertension, BMI, mental health diagnosis, hemoglobin A1c, antidiabetes medications, endocrinology visit, cardiology visit, nephrology visit, frailty, and COVID-19 diagnosis.
Separate models were fitted for CKD and CKD stage.
For eGFR category, models simultaneously adjust for ACR. For ACR category, models simultaneously adjust for eGFR.
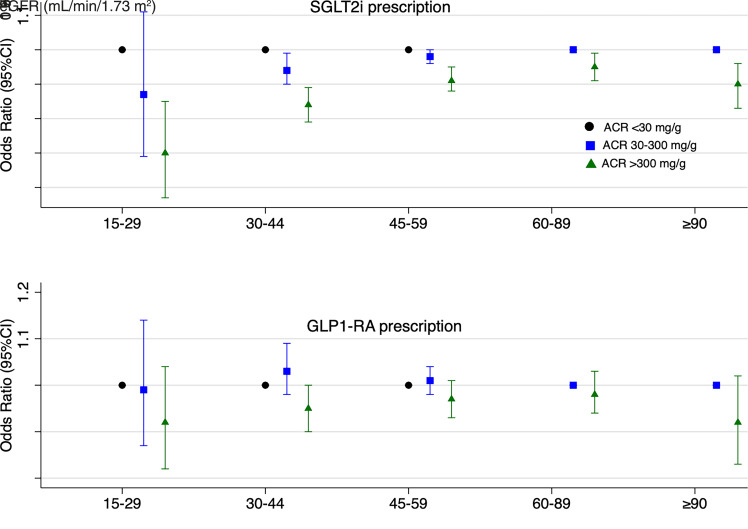
Among patients with CKD, lower eGFR (for SGLT2i) and higher ACR (for both SGLT2i and GLP1-RA) were associated with lower prescription of these medications (Table 2). The inverse associations between ACR and prescription of SGLT2i and GLP1-RA were observed across all eGFR categories for SGLT2i but not for GLP1-RA (Fig. 1).
Figure 1.
Association of albuminuria with SGLT2i and GLP1-RA prescription across eGFR categories among patients with CKD. Multivariable model adjusted for age, sex, self-identified race/ethnicity, ZIP Code median income, ZIP Code area social deprivation index, VA diabetes and service connection, rurality, smoking status, unhealthy alcohol use, hypertension, BMI, mental health diagnosis, hemoglobin A1c, antidiabetes medications, endocrinology visit, cardiology visit, nephrology visit, frailty, and COVID-19 diagnosis.
Prescription of SGLT2i and GLP1-RA According to ASCVD and ESKD Risk
Among patients without established ASCVD, higher ASCVD risk was associated with lower prescription of SGLT2i and GLP1-RA (Table 3). Indeed, for patients with a 10-year ASCVD risk >20%, the prevalence odds of prescription for an SGLT2i or a GLP1-RA were lower compared with a 10-year ASCVD risk <5%. Among patients with CKD, those with higher ESKD risk were less likely to be prescribed an SGLT2i but more likely to be prescribed a GLP1-RA (Table 3). Sensitivity analyses that assessed these associations across age strata yielded results consistent with the main analyses (Supplementary Table 6A and B).
Table 3.
Association of ASCVD and ESKD risk and prescription of SGLT2i and GLP1-RA among VHA patients with T2DM during the years 2019 and 2020
| SGLT2i prescription | GLP1-RA prescription | ||||
|---|---|---|---|---|---|
| n | % Prescribed | Multivariable model* | % Prescribed | Multivariable model* | |
| OR (95% CI) | OR (95% CI) | ||||
| 10-year ASCVD risk† | |||||
| <5% | 35,500 | 13 | Reference | 11 | Reference |
| 5 to 7.4% | 23,390 | 13 | 1.02 (0.96, 1.07) | 11 | 0.99 (0.93, 1.06) |
| 7.5 to 19.9% | 146,616 | 12 | 0.91 (0.86, 0.96) | 9 | 0.84 (0.79, 0.89) |
| >20% | 538,036 | 8 | 0.66 (0.61, 0.71) | 6 | 0.55 (0.52, 0.59) |
| Unknown | 60,296 | 9 | 0.70 (0.65, 0.75) | 6 | 0.54 (0.5, 0.59) |
| 5-year ESKD risk‡ | |||||
| <1% | 165,955 | 14 | Reference | 10 | Reference |
| 1 to 2.9% | 71,129 | 13 | 0.92 (0.89, 0.96) | 11 | 1.20 (1.16, 1.24) |
| 3 to 4.9% | 23,186 | 12 | 0.86 (0.81, 0.92) | 13 | 1.36 (1.3, 1.42) |
| >5% | 54,719 | 9 | 0.63 (0.59, 0.67) | 14 | 1.53 (1.46, 1.61) |
| Unknown | 62,255 | 8 | 0.51 (0.45, 0.59) | 9 | 0.81 (0.73, 0.89) |
Multivariable model adjusted for age, sex, self-identified race/ethnicity, ZIP Code median income, ZIP Code area social deprivation index, VA diabetes and service connection, rurality, smoking status, unhealthy alcohol use, hypertension, BMI, mental health diagnosis, hemoglobin A1c, antidiabetes medications, endocrinology visit, cardiology visit, nephrology visit, frailty, and COVID-19 diagnosis.
ASCVD risk calculation excluded patients ≥80 years.
ESKD only calculated among patients with CKD and available ACR measurements.
Sensitivity analyses that excluded 299 patients and 945 patients with prescriptions for SGLT2i and GLP1-RA, respectively, prior to 1 January 2019, but no prescriptions during the study period, yielded results consistent with the main findings (Supplementary Table 7).
Conclusions
In this study, we present a detailed analysis of contemporary prescription patterns of SGLT2i and GLP1-RA among a large cohort of patients with T2DM receiving primary care in the VHA, a large integrated health care system in the U.S. For patients with CKD, we found lower prevalent odds of prescription of SGLT2i with lower eGFR, and of SGLT2i and GLP1-RA with worsening albuminuria. Higher risks for ASCVD (for SGLT2i and GLP1-RA) and ESKD (for SGLT2i) were inversely associated with these prescriptions; these results remained robust across age-groups.
The results from this study should be contextualized with respect to clinical trial results demonstrating the cardiorenal-protective effects of these medications, which have informed current guidelines. For SGLT2i, the first trial in patients with T2DM demonstrating the protective cardiorenal effects of SGLT2i was the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG) trial, which was published in 2015 (26). Among patients with albuminuric CKD, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and the Dapagliflozin in Patients with Chronic Kidney Disease (DAPA-CKD) were published in 2019 and 2020, respectively (5,6). Evidence of the kidney-protective effects of GLP1-RA stems from meta-analyses of landmark trials among patients with diabetes, including Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) and Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6), both published in 2016 (27,28). Responsive ADA and KDIGO guidelines recommending prescription of these medications to lower cardiac and kidney risk were only published in 2020. As such, the observed low prescription rates among patients with CKD may be expected. Indeed, recent studies suggest that while low, the prescription of these medications has increased incrementally over the past decade (29). Nonetheless, the impetus for this study was to establish a baseline and to motivate ongoing and future efforts to improve prescription among patients who would derive the largest benefit.
We observed that higher rates of prescription occur among patients with higher hemoglobin A1c concentrations and that these medications are mainly prescribed as third- or fourth-line diabetes medications. In addition, we found that while only 13% of patients visited an endocrinologist during the study period, these patients were substantially more likely to be prescribed an SGLT2i or a GLP1-RA. For SGLT2i, these results align with prescription trends in other health care systems that have shown that use rates are low for this medication class and that prescriptions are concentrated among subspecialty providers (30,31). From an equity perspective, the observed reliance on subspecialists to prescribe these medications may adversely disadvantage patients who rely on medical practices that lack readily available access to subspecialty care. To improve equitable medication access and to maximize population-level impacts of CVD and CKD risk reduction, implementation efforts should focus on improving prescriptions from primary care providers who care for the bulk of patients with T2DM.
The observed lower prevalence of SGLT2i prescription among patients with the highest severity of CKD is consistent with recently published studies in non-VHA health systems (32). Although the low prescription rates of SGLT2i are expected among patients with an eGFR <30 mL/min/1.73 m2 in whom prior guidelines advised against their initiation, we found low prescription rates at CKD stages G3a and G3b, wherein prescription of SGLT2i could substantially prevent further CKD progression. Conversely, ACR is a major risk predictor of CVD and CKD progression independent of the GFR and is an essential component of CKD staging (33,34). The main SGLT2i trials among patients with diabetic kidney disease have used albuminuria as the main criterion for inclusion. Yet, although yearly testing for albuminuria is indicated among all patients with T2DM, and testing for albuminuria is integral in guiding SGLT2i or GLP1-RA selection, only 55% of patients had an ACR or PCR test over the 2-year study period. Furthermore, higher ACR was inversely associated with prescription of SGLT2i across all eGFR categories. The low rates of testing for albuminuria are consistent with those in other health systems (35). Because treatment of CKD at early stages is paramount both for the primary prevention of CVD and prevention of CKD progression to ESKD, overcoming the albuminuria detection and treatment gaps among patients with T2DM and CKD may lead to substantial population-level benefits in cardiovascular and kidney health.
Limitations of this study include that the VHA is an integrated health system with uniform pharmacy benefits offering discounted or free medications to its patients. Therefore, our results may not be generalizable to other health care systems where the substantial copayments or costs for these medications may be a major barrier to prescription (36); however, we anticipate that such a barrier may lead to even lower SGLT2i and GLP1-RA prescriptions in other settings. In addition, the observational and cross-sectional nature of this study precludes drawing causal inferences from the observed associations. Furthermore, we did not study prescription of other novel therapies that are now recommended for cardiorenal prevention. The 2022 ADA guidelines recommend that for patients with T2DM and CKD who are at increased risk for CVD events or CKD progression or who are unable to use an SGLT2i, the nonsteroidal mineralocorticoid receptor agonist finerenone should be used to reduce the risk of CKD progression and cardiovascular events (37).
In conclusion, we found low rates of prescription of SGLT2i and GLP1-RA among patients with CKD and in those at high ASCVD and ESKD risk. Considering the overwhelming evidence of cardiovascular and kidney protection, these results call for accelerated efforts to improve delivery of these medications to the highest-risk patients.
Article Information
Funding. J.A.L.-M. is supported by National Heart, Lung and Blood Institute grant 1K99HL157721-01A1. E.M., M.G.S., and M.M.E. are supported by VA Health Services Research and Development grant SDR 20-387. C.A.P. was supported by an American Heart Association Established Investigator Award.
Duality of Interest. This work was supported by Bayer, Inc. J.A.L.-M., E.M., C.D.C., M.G.S., and M.M.E. receive research funding from Bayer, Inc. M.G.S. and M.M.E. have received an honorarium from Boehringer-Ingelheim, Inc. S.L.T. received consulting fees from Bayer AG and research funding from Scanwell Health. C.A.P. is Chief Medical Officer at Cricket Health. Y.D., R.S., and S.X.K. are employees of Bayer Healthcare U.S. LLC. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.A.L.-.M., E.M., S.L.T., C.D.C., C.A.P., M.G.S., and M.M.E. contributed to conception, design, and interpretation of data, drafting and revising of the manuscript, and final approval of the manuscript submitted. Y.D., R.S., S.X.K., and D.S.T., contributed to analysis and interpretation of data and final approval of the manuscript submitted. J.A.L.-M. and M.M.E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as an oral presentation at the 82nd Scientific Sessions of the American Diabetes Association, virtual and at New Orleans, LA, 3–7 June 2022.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20816365.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. Johansen ME, Argyropoulos C. The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use sodium glucose cotransporter 2 inhibitors is now. Clin Cardiol 2020;43:1376–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785 [DOI] [PubMed] [Google Scholar]
- 3. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: a pooled analysis of SUSTAIN 6 and LEADER Trials. Circulation 2022;145:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:2097–2099 [DOI] [PubMed] [Google Scholar]
- 6. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 7. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662 [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020;98(Suppl.):S1–S115 [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . 16. Diabetes advocacy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2020;43(Suppl. 1):S203–S204 [DOI] [PubMed] [Google Scholar]
- 10. Mahtta D, Ramsey DJ, Lee MT, et al. Utilization rates of SGLT2 inhibitors and GLP-1 receptor agonists and their facility-level variation among patients with atherosclerotic cardiovascular disease and type 2 diabetes: insights from the Department of Veterans Affairs. Diabetes Care 2022;45:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newton KM, Peissig PL, Kho AN, et al. Validation of electronic medical record-based phenotyping algorithms: results and lessons learned from the eMERGE network. J Am Med Inform Assoc 2013;20:e147–e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norton JM, Ali K, Jurkovitz CT, et al. Development and validation of a pragmatic electronic phenotype for CKD. Clin J Am Soc Nephrol 2019;14:1306–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:5–14 [DOI] [PubMed] [Google Scholar]
- 14. Tangri N, Grams ME, Levey AS, et al.; CKD Prognosis Consortium . Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016;315:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Groeneveld PW, Medvedeva EL, Walker L, Segal AG, Richardson DM, Epstein AJ. Outcomes of care for ischemic heart disease and chronic heart failure in the Veterans Health Administration. JAMA Cardiol 2018;3:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goff DC, Lloyd-Jones DM, Bennett G, Coady S. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guideline [published correction appears in J Am Coll Cardiol. 2014;63:3026]. J Am Coll Cardiol 2014;63:2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Research Council of the National Academies . Using the American Community Survey: Benefits and Challenges. Citro CF, Kalton G, Eds. National Academies Press, 2007 [Google Scholar]
- 18. Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res 2013;48:539–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health 2006;83:162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frayne SM, Halanych JH, Miller DR, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med 2005;165:2631–2638 [DOI] [PubMed] [Google Scholar]
- 21. Hoggatt KJ, Frayne SM, Saechao FS, Yano EM, Washington DL. Substance use disorder-related disparities in patient experiences of primary care. Health Equity 2019;3:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med 2003;163:821–829 [DOI] [PubMed] [Google Scholar]
- 23. Melzer AC, Pinsker EA, Clothier B, et al. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol 2018;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krein SL, Bingham CR, McCarthy JF, Mitchinson A, Payes J, Valenstein M. Diabetes treatment among VA patients with comorbid serious mental illness. Psychiatr Serv 2006;57:1016–1021 [DOI] [PubMed] [Google Scholar]
- 25. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning from ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci 2021;76:1318–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 27. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 29. Funck KL, Knudsen JS, Hansen TK, Thomsen RW, Grove EL. Real-world use of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: a Danish nationwide cohort study, 2012 to 2019. Diabetes Obes Metab 2021;23:520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eberly LA, Yang L, Eneanya ND, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 2021;4:e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–3372 [DOI] [PubMed] [Google Scholar]
- 32. Zhuo M, Li J, Buckley LF, et al. Prescribing patterns of sodium-glucose cotransporter-2 inhibitors in patients with chronic kidney disease. Kidney360 2022;3:455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsushita K, Coresh J, Sang Y, et al.; CKD Prognosis Consortium . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coresh J, Heerspink HJL, Sang Y, et al.; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration . Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 2019;7:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care 2021;44:2000–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tummalapalli SL, Montealegre JL, Warnock N, Green M, Ibrahim SA, Estrella MM. Coverage, formulary restrictions, and affordability of sodium-glucose cotransporter 2 inhibitors by US Insurance plan types. JAMA Health Forum 2021;2:e214205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 11. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S175–S184 [DOI] [PubMed] [Google Scholar]