Abstract
Background
Myopia is one of the major eye disorders and the global burden is increasing rapidly. Our purpose is to systematically summarize potential metabolic biomarkers and pathways in myopia to facilitate the understanding of disease mechanisms as well as the discovery of novel therapeutic measures.
Methods
Myopia-related metabolomics studies were searched in electronic databases of PubMed and Web of Science until June 2021. Information regarding clinical and demographic characteristics of included studies and metabolomics findings were extracted. Myopia-related metabolic pathways were analysed for differential metabolic profiles, and the quality of included studies was assessed based on the QUADOMICS tool. Pathway analyses of differential metabolites were performed using bioinformatics tools and online software such as the Metaboanalyst 5.0.
Results
The myopia-related metabolomics studies included in this study consisted of seven human and two animal studies. The results of the study quality assessment showed that studies were all phase I studies and all met the evaluation criteria of 70% or more. The myopia-control serum study identified 23 differential metabolites with the Sphingolipid metabolism pathway beings enriched. The high myopia-cataract aqueous humour study identified 40 differential metabolites with the Arginine biosynthesis pathway being enriched. The high myopia-control serum study identified 43 differential metabolites and 4 pathways were significantly associated with metabolites including Citrate cycle; Alanine, aspartate and glutamate metabolism; Glyoxylate and dicarboxylate metabolism; Biosynthesis of unsaturated fatty acids (all P value < 0.05).
Conclusions
This study summarizes potential metabolic biomarkers and pathways in myopia, providing new clues to elucidate disease mechanisms.
Subject terms: Analytical biochemistry, Biological techniques
Introduction
Myopia is the most common eye abnormality and the global burden of myopia is increasing rapidly in past decades [1]. Holden et al. predicts that the number of people with myopia will reach 4.76 billion worldwide in the year 2050, and individuals affected by high myopia (HM) will increase to nearly 1 billion [2]. As myopia progresses, the anterior and posterior diameters of the eye increase and the wall of the eye thins, resulting in a series of macular changes, including tessellated fundus and further progression of pathological myopia (diffuse chorioretinal atrophy, patchy chorioretinal atrophy, and even macular atrophy) [3]. In addition, individuals with myopia, especially HM, are particularly vulnerable to vision-threatening ocular complications such as open-angle glaucoma and age-related cataract [4]. Thus, clarifying the mechanism of myopia development is highly important for formulating effective myopia prevention strategies. Although the aetiology of myopia has been investigated for more than hundreds of years, the exact mechanisms remain unclear. Currently, it is generally accepted that the development of myopia is influenced by both genetic and environmental factors and joint effect of genetic and environmental exposures also plays a role. Therefore, myopia research needs to find new tools that can elucidate the combined genetic and environmental effects.
Metabolomics, as the end of the “omics” technologies, transmits the final response signals from the interaction of “genetics and environment” and provides direct “functional readouts” of the physiological state of organs, contributing to the understanding of disease complex phenotypes [5, 6]. In ophthalmology, metabolomics has successfully been used for the description of disease progression and mechanism exploration, including age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy (DR), contributing to the understanding of ocular disease mechanisms [7–9]. In this study, we aimed to summarize myopia-related metabolite changes and explore the metabolic pathways involved in the pathogenesis and progression of myopia according to the existing metabolomics studies on myopia. These findings may contribute to the understanding of the mechanisms of myopia and provide new ideas for delaying or treating myopia and its associated complications.
Methods
Search strategy
Studies using metabolomics techniques to analyse metabolic changes associated with myopia were investigated. We searched the electronic database of PubMed and Web of Science for relevant metabolomics studies on myopia published up to June 2021, with the following search terms: (“metabolomics” or “metabonomics” or “metabolome” or “metabolic profiling”) AND (“myopia” or “refractive error”). All the relevant studies, regardless of the use of study species and biological samples, were included in this systematic review. Additional sources were obtained through searching the reference lists of the included studies that met the inclusion criteria. The overall goal was to guarantee the reliability of the search while including a wide range of original studies related to the topic of interest. Additionally, results were limited to papers written in English. Drugs evaluating reports, review articles and abstracts without full texts were excluded. All articles were screened via abstract and full text to determine their suitability for inclusion in the review. Two authors (XWH and YW) assessed the studies and provided independent reports based on their quality and applicability for inclusion. Any differences that emerged were addressed through the discussions with a senior author (CWP).
Quality assessment
Because the quality of the included studies affects the results and conclusions of the review, assessing the quality of the included studies before investigating the relationship between metabolites and myopia using the selected studies is critical to the success of this review. QUADOMICS is an improved quality assessment tool based on QUADAS tool (a quality assessment tool used to systematically evaluate diagnostic accuracy studies), specifically designed for ‘omics’-based diagnostic assessment studies to address the specific challenges in systematic reviews. QUADOMICS divides the study into four clinical validation phases according to the study population and contains four study items, covering sample characteristics, pre-analytical differential conditions, clinical and physiological characteristics, and overfitting during the study and analysis. Two authors (XWH and YW) assessed the quality of included studies using QUADOMICS and addressed discrepancies through discussion.
Data extraction and analyses
For each study, information regarding the title, author, publication date, detection and analysis platforms, sample size, methods of statistical analysis, and repeated reports of biomarkers were extracted and summarized after reading the full articles and supplementary materials. Biochemical interpretation of all altered metabolites was performed using MetaboAnalyst 5.0 (http://www.metaboanalyst.ca/), a software that allows to analyse the impact of particular compounds on biochemical pathways. Pathway analysis was derived from integrating differential metabolites based on Kyoto Encyclopedia of Genes and Genomes and Human Metabolome Database (HMDB). P-values were calculated on the basis of path enrichment analysis, and pathway impact value was derived on the basis of pathway topology analysis.
Results
Study characteristics
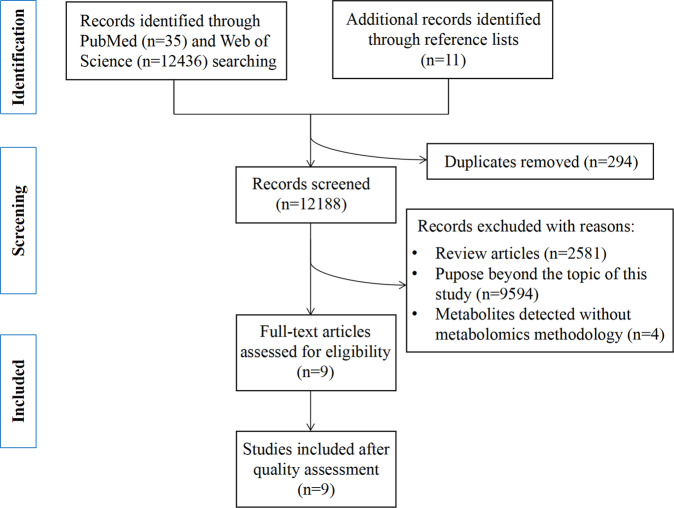
A total of 12482 records were identified by searching electronic databases and references included in the selected studies. After removing duplicate articles (n = 294), the abstracts of the remaining 12188 records were reviewed by two independent authors, excluding 12179 articles that were not relevant to the topic of this study or did not meet the inclusion criteria (review articles, studies that did not utilize metabolomics techniques to detect metabolites). The remaining twelve studies were subjected to full-text review. Finally, nine articles were included in the review. A simplified flow chart of study selection was shown in Fig. 1.
Fig. 1.
Flow diagram of literature search and study selection.
All studies described myopia-related metabolic profiles. Seven studies focused on humans [10–16] and two were animal studies (chicks and guinea pigs) [17, 18]. All studies were performed on appropriately sized groups and the sample size ranged from 38 to 211 subjects. The study with the largest sample size was a school-based case-control study of myopia and non-myopia in Chinese children and adolescents [13]. With regard to seven human studies, four analysed metabolic alterations in HM [10, 11, 14, 15], two focused on metabolic differences in any myopia [12, 13] and the other one investigated myopia-related retinal changes [16]. The most common definition of HM is spherical equivalent −6.0 dioptre (D) or worse, or axial length longer than 26 millimetres. Table 1 shows the basic information of all included studies in this review. A total of four types of biological tissues were involved. Of them, serum was analysed in five studies [12–16], aqueous humour (AH) samples were analysed in two studies [10, 11], and retinas were analysed in two studies (some studies analysed two types of bio-specimens, vitreous and retinas) [17, 18]. With regard to the analytical platforms, mass-spectrometry based metabolomics studies were reported in all studies, including liquid chromatogram-mass spectrometry (LC-MS)(n = 5) [10, 12, 13, 15, 18], gas chromatography - mass spectrometry (GC-MS)(n = 4) [11, 14, 16, 17], whereas one study employed the capillary electrophoresis (CE) & LC-MS platform.
Table 1.
Clinical and demographic characteristics of myopia metabolomics studies.
| References | Comparison | Diagnostic criteria | SE | Age | Male% | Source |
|---|---|---|---|---|---|---|
| Liu(2020) [16] | 57 PM | 55.32 ± 14.49 | 28.08 | China | ||
| 81 cataract patients | 65.83 ± 11.94 | 35.80 | ||||
| Ke(2020) [14] | 40 HM | HM(SE < − 0.6D), mild myopia(0 < SE < − 3.0D) | −7.7D | ≥ 60 | 22.00 | Weitang, China |
| 40 mild myopes | −1.7D | match | 22.00 | |||
| Du(2020) [13] | 108 myopes | myopic(SE < − 0.50D) | −2.20 ± 1.59(L), − 2.51 ± 1.28(R) | 10.15 ± 0.99 | 50.93 | 7 schools in a district, China |
| 103control(without myopia) | 3.36 ± 1.15(L), 0.40 ± 0.75(R) | 10.09 ± 1.01 | 60.19 | |||
| Dai(2019) [15] | 30 HM(Discoery) | myopia(SE ≤ − 6.00 D) | −7.8 ± 1.5(L), − 7.9 ± 1.2(R) | 21.0 ± 5.2 | 60.00 | China |
| 30 controls (without myopia)(Discoery) | −0.5~0.5D(L, R) | 23.2 ± 6.9 | 45.00 | |||
| 20HM(Test) | −7.2 ± 1.2(L), − 7.3 ± 1.1(R) | 20.8 ± 3.3 | 36.80 | |||
| 19 controls (without myopia)(Test) | −0.5~0.5D(L,R) | 22.6 ± 5.5 | 45.00 | |||
| Ji(2017) [11] | 20 HM | HM(SE > −6.00D or AL ≥ 26 mm) | 60.35 ± 8.92 | 35.00 | China | |
| 20 cataract patients | 62.75 ± 9.20 | 30.00 | ||||
| Barbas-Bernardos(2016) [10] | 12 HM | HM(AL > 26 mm), low myopia(AL ≤ 26 mm) | Valencia(Spain) | |||
| 26 with low myopia | ||||||
| Kearney(2017) [12] | 25 myopes(Phase 1) | myopia(SE ≤ −0.5D) | −2.37 ± 1.27D | 19.1 ± 0.81 | England | |
| 29 controls(Phase 1) | +0.62 ± 0.89D | |||||
| 22 myopes(Phase 2) | −2.34 ± 1.12D | |||||
| 23 controls(Phase 2) | +0.78 ± 1.16D | |||||
| Yang(2017) [17] | 24 FD guinea pigs(3 d) and 10 controls | FD(3d)−2.73 ± 2.35D | Wenzhou, China | |||
| 24 FD guinea pigs(2 w) and 12 controls | FD(2 weeks)−5.01 ± 2.53D | |||||
| Najjar(2021) [18] | 18 chicks with monocular FD(3900 K SW LED light) | Singapore | ||||
| 18 chicks with monocular FD(BEW light) |
PM Pathological myopia, HM High myopia, FD Form deprivation,
SE Spherical equivalent, D Diopter, AL Axis length,
L Left, R Right.
Quality assessment
The quality of the studies was assessed with the tool described by Lumbreras et al. according to the QUADOMICS [19] and detailed information is presented in Supplementary Table 1. The second and fourteenth items of the QUADOMICS were not applicable in practice to the included studies and, therefore, were not presented. All of the studies included in the review were phase I and all met the evaluation criteria of 70% or more, with one study meeting the criteria of 13/14 (92.86%) [17]. All studies described the sample type (item 3) and selection criteria (item 1), but none met item 12 in the tool, indicating that they interpreted the index test results with knowledge of the reference standard. With regard to item 16, three studies lacked measures to avoid overfitting [12, 13, 15] and one study was not relevant to the item [10].
Metabolic markers and disease prediction
We identified seven studies that compared the metabolic profiles of myopia and controls, most of which used a combination of univariate analysis and multivariate statistical methods as evaluation criteria to identify differential metabolic characteristics (Table 2). Notably, Du et al. identified 256 differential features in metabolomics and lipidomics based on the U test only, and further qualifying criteria for differential features were added in this review, including quantitative identification of endogenous metabolites and fold change over 1.5 or less than 0.5, resulting in 21 metabolic features included in this review for further study [13]. Liu et al. conducted a study related to macular neovascularization in which 14 differential metabolic features were identified between pathological myopia and cataract controls without fundus lesions, and with the help of the backward stepwise-regression selection procedure, the best combination of biomarkers for diagnosis between groups was selected with a model area under receiver operating characteristic value of 0.906 [16]. A summary of studies assessing the ability of differential metabolic characteristics to distinguish myopia-related disorders is shown in Table 3. In the two studies analysing AH, 29 and 21 differential features without duplication between HM and cataract control groups, respectively, were identified, probably due to the inconsistencies in other characteristics of the study demographics between the two groups [10, 11]. Two other studies on myopia and controls were done with targeted and untargeted serum metabolomics, respectively [12, 13]. After standardization of names and assignment of HMDB identity documents, the full study identified 87 compounds with differential variations.
Table 2.
Summary of myopia metabolomics study results.
| References | Biofluid/Technique/Standard | Altered metabolites ↑ | ↓ |
|---|---|---|---|
| Liu(2020) [16] | Serum | (8)Oleic acid, Pyruvic acid, Pipecolic acid, Petroselinic acid, Linoleic acid, Glycerol, Palmitic acid, Stearic acid | (6)Maleic acid, Beta-Alanine, Ribonolactone, D-Maltose, Hypoxanthine, L-2-Amino-3-(1-pyrazolyl)propanoic acid |
| GC-TOF-MS | |||
| OPLS-DA(VIP > 1.0), t-test(P < 0.05), and FC > 1.2 or <0.8 | |||
| Ke(2020) [14] | Serum | (12)Citric acid, Aminomalonic acid, Palmitoleic acid, Conduritol b epoxide, Shikimic acid, 4-Hydroxyphenylacetic acid, Hesperitin, Anandamide, Oxalacetic acid, Pimelic acid, 2-Ketoadipate, N-Ethylmaleamic acid | (8)Alanine, Mannose, Itaconic acid, Aconitic acid, O-Acetylserine, Phthalic acid, Abietic acid, Salicin |
| GC-TOF-MS | |||
| PLS-DA(VIP > 1.0) and t-test(P < 0.05) | |||
| Du(2020) [13] | Serum | (12)DG(16:1(9Z)/16:1(9Z)/0:0), Sphingosine 1-phosphate, PC(24:0/P-18:1(11Z)), Allopregnanolone, DG(18:1(9Z)/18:2(9Z,12Z)/0:0), Leukotriene D4, Ceramide (d18:1/18:0), Thiamine monophosphate, TG(16:0/16:0/18:0), DG(16:0/20:4(5Z,8Z,11Z,14Z)/0:0), Deoxyuridine, Hydrocinnamic acid | (9)Uridine diphosphate-N-acetylglucosamine, PC(18:2(9Z,12Z)/P-18:1(11Z)), Taurocyamine, Phosphoserine, DG(18:2(9Z,12Z)/20:4(5Z,8Z,11Z,14Z)/0:0), SM(d18:0/22:0), Tyramine, all-trans-Retinoic acid, Docosapentaenoic acid (22n-3) |
| UHPLC-MS | |||
| U test(P < 0.001),FC > 1.5 or <0.5,quantified, Endogenous | |||
| Dai(2019) [15] | Serum | (7)seryltryptophan, DG (8:0/17:0/0:0), phosphatidylethanolamine (PE) (20:3/22:6), lysophosphatidylethanolamine (LysoPE) (22:4/0:0), 25-hydroxyvitamin D2-25-glucuronide, γ-glutamyltyrosine, and 5-hydroxytryptamine | (2)5-Methyltetrahydrofolic acid, 12-Oxo-20-trihydroxy-leukotriene B4 |
| LC-QTOF/MS | |||
| PLS-DA(VIP > 1.5) and t-test(P < 0.05) | |||
| Ji(2017) [11] | AH | (27)glutamine 1, N-alpha-Acetyl-L-ornithine 3, Nicotinoylglycine 2, o-Hydroxyhippuric acid 2, oxalacetic acid, oxalic acid, ribose, cis-gondoic acid, Linoleic acid methyl ester, thymidine 3, phosphate, indole-3-acetamide 4, 2-aminophenol 2, 2-ketoadipate 2, 3-Phenyllactic acid, cis-Phytol, conduritol b epoxide 2, salicin, 3 analytes and 6 unknowns | (2)Analyte 420, Analyte 76 |
| GC/ TOF MS | |||
| PLS-DA(VIP ≥ 1) and t-test(p ≤ 0.05) | |||
| Barbas-Bernardos(2016) [10] | AH | (9)HM vs LM: L-Arginine, Citrulline, Butyryl-L-carnitine, Pantothenic Acid (VITAMIN B5), Sphinganine, Histidinyl-Phenylalanine, PC(O-32:2)//PC(P-32:1), C24 Sulfatide, LacCer(d40:0) | (13)Aminocyclohexanecarboxylic acid, Aminooctanoic acid, Aminoundecanoic acid, Dodecanedioic acid, Trihydroxyphenyl-gamma-valerolactone, Didehydro-Retinoic acid, L-Cysteinylglycine disulfide, Dihydropteroic acid, Dimethylnonanoyl carnitine, PC (42:6), PC(P-42:2)//PC(O-42:3), Trihexosylceramide (d36:2), NeuAcaGalCer(d42:2) |
| CE-MS & LC-MS | |||
| OPLS-DA(VIP > 1) & RSD | |||
| Kearney(2017) [12] | Serum | (1)Melatonin(Phase 1&2) | (1)Dopamine(Phase 2) |
| LC-SPE-MS/MS | |||
| Logistic regression model | |||
| Yang(2017) [17] | Retina | 2-Ketoglutaric acid, glucose, mannose | urea, arabinose, tyrosine, glutamic acid, Treonine, valine, isoleucine, alanine, malic acid, Arachidic acid (20:0), Octadecenoic acid (18:1), Octadecanoic acid (18:0), Arachidonic acid (20:4), Hexadecanoic acid (16:0), Tetradecanoic acid (14:0), Octadecadienoic acid (18:2), Cholesterol, Ethanolamine, GABA |
| GC-TOF/MS | |||
| PLS-DA、OPLS(VIP > 1.0) | |||
| Najjar(2021) [18] | Vitreous & Retinas | dihydroxyphenylalanine, ornithine, methionine sulfoxide, total dimethylarginine, arginine, lysine,tryptophan,sphingolipids, glycerophospholipids, symmetric dimethylarginine, glutamate, serotonin, taurine, trans-4-Hydroxyproline, tyrosine, proline, histidine, phenylalanine, alanine, threonine, valine and isoleucine, monosaccharides, glycerophospholipids, sphingolipids | asparagine, glycerophospholipids, carnitine, Spermine, acylcarnitines |
| LC-MS | |||
| PLS-DA、OPLS-DA |
GC Gas chromatography, TOF Time of flight, UHPLC Ultra Performance Liquid Chromatography, CE Capillary electrophoresis, SPE Solid phase extraction, MS Mass spectrometry;
OPLS-DA Orthogonal partial least squares discriminant analysis, PLS-DA Partial least squares discriminant analysis, FC Fold change, VIP Variable importance projection, RSD Relative standard deviation,
AH Aqueous humor.
Table 3.
Classification potential of the information of metabolomics.
| References | Method | Discriminant models | Discriminant group;Precision |
|---|---|---|---|
| Liu(2020) [16] | Logistic Regression | Demographic characteristics and panel metabolites(hypoxanthine, L-2-amino-3-(1-pyrazolyl)propanoic acid, linoleic acid, maleic acid, ribonolactone) | AUC = 0.906; Sen = 0.877; Spe = 0.684 |
| Ke(2020) [14] | AUC | Altered metabolites | AUC: 0.59~0.71 |
| Dai(2019) [15] | Logistic regression | γ-glutamyltyrosine and 12-oxo-20-trihydroxy-leukotriene B4 | (Discovery)AUC = 0.983; Sen = 97%; Spe = 90%; (Test)AUC = 0.958; Sen = 80%; Spe = 95% |
AUC Area under receiver operating characteristic, Sen Sensitivity, Spe Specificity.
In addition, animal studies are an important part of myopia-related metabolomics research, and animal models make it possible to track changes in retinal metabolic profiles and changes in ocular tissue metabolism associated with environmental alterations. Two animal model studies were included in the review, in which Yang et al. established a retinal metabolism model for form-deprived guinea pigs and found differences in retinal metabolism at different time points [17]; Najjar et al. studied the effects of moderate levels of ambient standard white (Standard white light: 233.1 lux, 3900 K) and blue-enriched white (Blue-enriched white: 223.8 lux, 9700 K) lights on the growth and metabolism of the chicken-model of form-deprivation myopia [18]. This study showed that the metabolic structure of the vitreous and retina in restored form-deprived eyes differed from that of control eyes and was dependent on the spectral content of ambient light, providing new insights into light-dependent regulation in ocular growth and metabolomics.
Pathway analysis
Based on the source of the biological samples and study subjects, we divided the included studies into three parts: including serum metabolomics study of myopia-controls [12, 13], AH metabolomics study of HM-cataract controls [10, 11], and serum metabolomics study of HM-controls [14–16] for separate pathway analysis. Only the sphingolipid metabolic pathway was statistically significantly enriched in the myopia-control serum metabolomics study, whereas only the arginine biosynthetic pathway was statistically significantly enriched in the AH metabolomics study in the HM-cataract control. In a HM-control serum metabolomics study, 39 differential metabolites were imported in MetaboAnalyst 5.0 software for pathway analysis. As is shown in Fig. 2, myopia-altered metabolites belonged to 29 biochemical pathways, four of which had statistically significant effects (Supplementary Fig. 1). In this pathway analysis, the Citrate cycle (TCA cycle) pathway had the largest effect values and was the most statistically significant pathway, which included four altered metabolites (Oxaloacetate; cis-Aconitate; Citrate; Pyruvate). Other pathways were also enriched (P < 0.05), including Alanine, aspartate, and glutamate metabolism; Glyoxylate and dicarboxylate metabolism; Biosynthesis of unsaturated fatty acids. Additional details are available in the Supplementary Tables 2, 3.
Fig. 2. Results of the pathway analysis of high myopia-control serum metabolomics study.
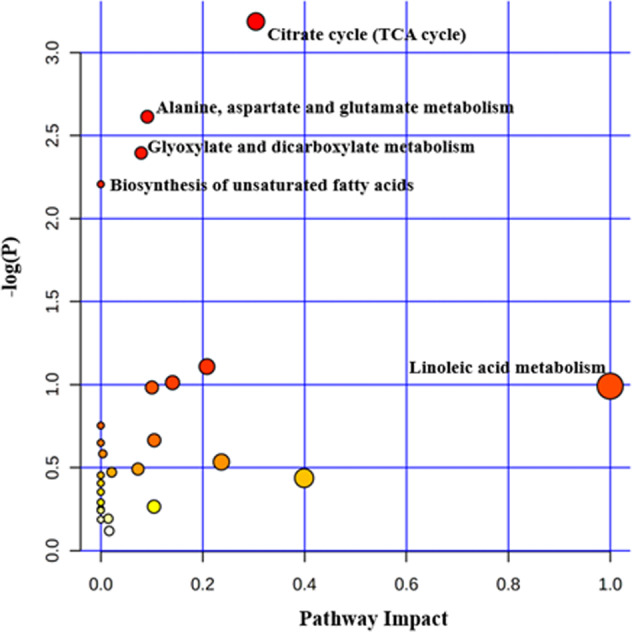
The colour of the circle indicates the significance level in the enrichment analysis, the darker the colour (more red) indicates more significant; the size of the circle reflects the pathway impact value in the topology analysis, the larger the circle, the larger the impact value. The x-axis is the pathway impact value calculated based on topology analysis.
Discussion
Nine myopia metabolomics studies were included in this review, including both human and animal studies. The human studies included three types of study samples: comparison of highly myopic and non-myopic controls, comparison of highly myopic and mildly myopic, and comparison of myopic and non-myopic, covering both serum and AH biological samples. Limitations in the number of included review studies and the use of different methods (GC, LC, etc) and differential metabolite evaluation criteria resulted in duplicate biomarkers not being obtained. However, the inclusion of diversity studies was complementary to each other. Pathway analysis revealed four statistically significant metabolic pathways enriched in the HM serum metabolomics study. Only the sphingolipid metabolic pathway was statistically significantly affected in the myopia-control serum metabolomics study, whereas only the arginine biosynthetic pathway was statistically significantly affected in the AH metabolomics study in the HM-cataract controls.
The body abnormally alters its original metabolic state, and identifying metabolic differences between disease and healthy states may lead to a better understanding of metabolic changes associated with disease [20]. Pathway analysis is the combination of altered metabolites with biological a priori knowledge to simplify the interpretability of data analysis [21]. The 23 differential metabolites identified by the myopic-control serum metabolomics study were mainly enriched in the Sphingolipid metabolism pathway and contained three altered metabolites (Sphingosine 1-phosphate; Sphingomyelin; N-Acylsphingosine) [12, 13]. Sphingolipids are one of the major components of the phospholipid bilayer of eukaryotic biological cell membranes and act as signalling molecules regulating inflammation, cell migration [22]. Sphingomyelin is a sphingolipid with phosphocholine as a polar headgroup, which can be hydrolyzed to ceramides and phosphocholine [23]. Ceramide regulates various cellular processes including apoptosis and senescence [24]. Ceramide hydrolysis is the only synthetic pathway for sphingosine, and then sphingosine undergoes phosphorylation to produce sphingosine 1-phosphate (S1P) [25]. Blood levels of S1P are relatively higher than intracellular levels, and the retina also produces S1P, which plays an important role in retinal angiogenesis [26]. As myopia progresses, HM is often accompanied by retinopathy. The present study found serum changes in sphingomyelin between myopic-control groups, which may be a pre-warning of retinal changes, and further validations are needed in future studies.
TCA cycle and Glyoxylate and dicarboxylate metabolism pathways were significantly enriched in the serum metabolism of HM, suggesting that glycometabolism disorder may play an important role in the pathogenesis of HM. This observation is supported by both genomics and proteomics. Expression of metabolic genes associated with mitochondrial metabolism pathways have been demonstrated to be correlated with ocular axial length and refraction [27], providing evidence of the abnormal energy metabolism during myopia development. Yu et al. performed quantitative analysis of myopic retinal proteins and found that many glucose-related metabolic enzymes (including pyruvate kinase and serine/threonine phosphatase) were upregulated, which supports the hypothesis of a hypoxic environment in animal models of myopia [28]. Abnormal retinal glucose metabolism was also found in the Yang et al. animal model, with glucose accumulating in the form-deprived retina, indicating a decrease in aerobic glycolysis and an abnormal TCA cycle [17]. Briefly, hypoxia could result in hyperactivation of adenosine signalling [29], one of the key signal pathways related to energy metabolism. Therefore, it could be inferred that the development of myopia was related to energy metabolism disorder and oxidative stress induced by anoxic environment. In addition, in this review, we observed that the metabolic regulatory mechanisms controlling progression of myopia and DR and AMD appear to be related [9, 30], since AMD and DR have the same abnormal energy metabolism feature as HM. Because choroidal neovascularization is a common pathology in many vision-threatening retinal diseases [31, 32], biochemical changes in the choroid might underlie progression of eye diseases. Neovascular tissues are prone to be incompetent and leaky, thus presumably altering the availability of metabolites. One of the pathological mechanisms expected to be involved is the choroid energy metabolism. Emerging evidence has displayed that glucose metabolism could control endothelial cell neovascularization [33]. VEGF has emerged as a key molecular regulator of retinal and choroidal neovascularization. Over the past decade, the advent of injectable antiangiogenic agents into the vitreous cavity of the eye has achieved considerable success [34]. Many therapeutic compounds with parameters and antibodies that bind VEGF have placed emphasis on inhibiting the VEGF signalling pathway. In oxygen-induced retinopathy (OIR) mouse models, oral administration or intraperitoneal injections of arginine-glutamine increased retinal Docose Hexaenoie Acid (DHA) and inhibited retinal neovascularization, which was also associated with decreased retinal VEGF mRNA levels [35]. Vascular dysfunction may also lead to oxidative stress and excessive production of reactive oxygen species (ROS), resulting in oxidative damage [36]. The corresponding hypoxia-inducible factor-1α (HIF-1α) is a specific mediator in adaptation to hypoxic environments and pathological responses [37]. Therefore, the link between the role of disorders of glucose metabolism, and VEGF-related hypoxic metabolism in retinopathy may be a common potential therapeutic target for these vision-threatening diseases and needs to be further investigated.
Limitations and research directions
Myopia is a risk factor for numerous ocular diseases, especially in patients with HM, and increases the risk of a range of secondary sequelae and irreversible vision loss, including myopic macular degeneration, glaucoma, retinal detachment, and cataract [38]. A summary of current myopia metabolomics studies shows that there are few reproducible validated results, mainly for the following reasons that limit the extrapolation of findings: First, due to the diverse metabolic status of the population [39], with age, gender, race, and lifestyle affecting metabolic levels, most studies do not describe quality control prior to sample collection, which may affect the extent to which differential metabolites are identified. Therefore, in experimental design sessions, study subjects should fast overnight or for at least one or two hours prior to sample collection; a complete study presentation should include a brief description of food intake over the past 12–24 h; [40] and reduce the possibility of differences due to sex, age, race, BMI and lifestyle factors. Most of the studies in this review matched subjects for age and gender, but did not describe in detail the pre-sampling medication and dietary status of the subjects. Myopia is a disease with a complex aetiology, and the study population includes patients with HM, low myopia, and concomitant retinal changes, etc., and because the eye is a relatively independent organ, the difficulty in obtaining ocular tissue, controls and myopes are often derived from non-healthy controls operated on for cataract or other eye diseases, which limits the range of myopia-related biomarkers. Second, the different evaluation criteria for metabolite detection platforms and differential metabolites in the study also contributed to the lack of duplicate identification of metabolites. For example, Liu et al. conducted experiments using a GC-TOF-MS metabolic detection platform, where biological samples need to be derivatized to be volatile for analysis [16], while the study by Barbas-Bernardos et al. adopted a dual platform combination strategy to increase metabolite coverage [10]. The characteristics contained in the preprocessed data are static during data analysis, so it is important to choose a suitable statistical method [41]. The quality of studies evaluated in this review found studies in which only five studies considered avoiding overfitting [11, 14, 16–18], except for the study by Barbas-Bernardos et al. which did not involve overfitting issues [10]. Our inability to access the original assay data for most of the studies also limited the reuse of the study results. Finally, the pathway-based analysis approach combines the results of data analysis with a priori knowledge of biology by combining a series of altered metabolic features revealed by statistical analysis in metabolomics studies [21]. However, due to the limited number of studies, only the Sphingolipid metabolism pathway was enriched in serum metabolomics studies in children and adolescents, and only the Arginine biosynthesis pathway was enriched in AH metabolomics studies in HM. We believe that NMR metabolomics studies on myopia will be a promising tool for analysis. In the future, metabolomics studies of complex diseases need to develop in a comprehensive (multi-platform approach, multi-omics, multi-tissue, and multi-time-point) direction to gradually map the complete myopia mechanism network and provide a reference basis for myopia prevention and control.
In conclusions, myopia has metabolic alterations in serum, AH, and retina, and the predictive value of a metabolic biomarker panel suggests the value of metabolomics in the management of myopia disease. Abnormal carbohydrate metabolism is present in HM, and the same pathways of change exist as in AMD and DR. In the future, standardized myopia metabolomics studies are needed to further unravel the mystery of myopia.
Supplementary information is available at Eye’s website
Summary Table
What was known before
Myopia is the most common eye abnormality and the global burden of myopia is increasing rapidly in past decades. In ophthalmology, metabolomics has successfully been used for the description of disease progression and mechanism exploration, including age-related macular degeneration, glaucoma, and diabetic retinopathy, contributing to the unveiling of ophthalmic diseases.
What this study adds
This study found significant metabolic alterations in serum, aqueous humorous fluid and retina in myopia, and found that the same metabolic changes exist in myopia as in other eye diseases.
Supplementary information
Author contributions
Conception or design of the work: XWH. Data collection: XWH and YW. Data analysis and interpretation: XWH and CK. Article drafting: XWH and YW. Critical revision of the article: CK and CWP. Final approval of the version to be published: XWH, YW, CK, and CWP.
Funding
This study was supported by the National Key R&D Program of China (2021YFC2702100, 2021YFC2702103, and 2021YFC2702104), the National Natural Science Foundation of China (82122059 and 81973061), the Tang Scholar of Soochow University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02019-0.
References
- 1.Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007;114:216–20. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–83.e877. doi: 10.1016/j.ajo.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Pan CW, Cheung CY, Aung T, Cheung CM, Zheng YF, Wu RY, et al. Differential associations of myopia with major age-related eye diseases: The Singapore Indian Eye Study. Ophthalmology. 2013;120:284–91. doi: 10.1016/j.ophtha.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 5.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–7. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 7.Lauwen S, de Jong EK, Lefeber DJ, den Hollander A. Omics Biomarkers in Ophthalmology. Invest Ophthalmol Vis Sci. 2017;58:BIO88–BIO98. doi: 10.1167/iovs.17-21809. [DOI] [PubMed] [Google Scholar]
- 8.Grochowski ET, Pietrowska K, Kowalczyk T, Mariak Z, Kretowski A, Ciborowski M, et al. Omics in Myopia. J Clin Med. 2020;9:3464. doi: 10.3390/jcm9113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou XW, Wang Y, Pan CW. Metabolomics in age-related macular degeneration: A systematic review. Invest Ophthalmol Vis Sci. 2020;61:13. doi: 10.1167/iovs.61.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbas-Bernardos C, Armitage EG, Garcia A, Merida S, Navea A, Bosch-Morell F, et al. Looking into aqueous humor through metabolomics spectacles - exploring its metabolic characteristics in relation to myopia. J Pharm Biomed Anal. 2016;127:18–25. doi: 10.1016/j.jpba.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Rao J, Rong X, Lou S, Zheng Z, Lu Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147–55. doi: 10.1016/j.exer.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kearney S, O’Donoghue L, Pourshahidi LK, Cobice D, Saunders KJ. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt. 2017;37:557–67. doi: 10.1111/opo.12396. [DOI] [PubMed] [Google Scholar]
- 13.Du B, Jin N, Zhu X, Lu D, Jin C, Li Z, et al. A prospective study of serum metabolomic and lipidomic changes in myopic children and adolescents. Exp Eye Res. 2020;199:108182. doi: 10.1016/j.exer.2020.108182. [DOI] [PubMed] [Google Scholar]
- 14.Ke C, Xu H, Chen Q, Zhong H, Pan CW. Serum metabolic signatures of high myopia among older Chinese adults. Eye (Lond) 2021;35:817–24. doi: 10.1038/s41433-020-0968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai L, Yang W, Qin X, Li Y, Cao H, Zhou C, et al. Serum metabolomics profiling and potential biomarkers of myopia using LC-QTOF/MS. Exp Eye Res. 2019;186:107737. doi: 10.1016/j.exer.2019.107737. [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Fang J, Jin J, Zhu S, Xu X, Xu Y, et al. Serum metabolomics reveals personalized metabolic patterns for macular neovascular disease patient stratification. J Proteome Res. 2020;19:699–707. doi: 10.1021/acs.jproteome.9b00574. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Reinach PS, Zhang S, Pan M, Sun W, Liu B, et al. Changes in retinal metabolic profiles associated with form deprivation myopia development in guinea pigs. Sci Rep. 2017;7:2777. doi: 10.1038/s41598-017-03075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Najjar RP, Chao De La Barca JM, Barathi VA, Ho CEH, Lock JZ, Muralidharan AR, et al. Ocular growth and metabolomics are dependent upon the spectral content of ambient white light. Sci Rep. 2021;11:7586. doi: 10.1038/s41598-021-87201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumbreras B, Porta M, Marquez S, Pollan M, Parker LA, Hernandez-Aguado I. QUADOMICS: An adaptation of the quality assessment of diagnostic accuracy assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of ‘-omics’-based technologies. Clin Biochem. 2008;41:1316–25. doi: 10.1016/j.clinbiochem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Lindon JC, Holmes E, Nicholson JK. So what’s the deal with metabonomics? Anal Chem. 2003;75:384A–391A. doi: 10.1021/ac031386+. [DOI] [PubMed] [Google Scholar]
- 21.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protocols Bioinform. 2016; 55: 14.10.11-14.10.91. [DOI] [PubMed]
- 22.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol Rev. 2008;60:181–95. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandet CL, Tan-Chen S, Bourron O, Le Stunff H, Hajduch E. Sphingolipid metabolism: New insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci. 2019;20:479. doi: 10.3390/ijms20030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 25.Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul. 2017;63:122–31. doi: 10.1016/j.jbior.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Tan Y, Wang L, Su X, Shi Y. Serum sphingosine-1-phosphate levels and Sphingosine-1-Phosphate gene polymorphisms in acute respiratory distress syndrome: a multicenter prospective study. J Transl Med. 2020;18:156. doi: 10.1186/s12967-020-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Riddell N, Giummarra L, Hall NE, Crewther SG. Bidirectional Expression of Metabolic, Structural, and Immune Pathways in Early Myopia and Hyperopia. Front Neurosci. 2016;10:390. doi: 10.3389/fnins.2016.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu FJ, Lam TC, Sze AY, Li KK, Chun RK, Shan SW, et al. Alteration of retinal metabolism and oxidative stress may implicate myopic eye growth: Evidence from discovery and targeted proteomics in an animal model. J Proteom. 2020;221:103684. doi: 10.1016/j.jprot.2020.103684. [DOI] [PubMed] [Google Scholar]
- 29.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Disco. 2013;12:265–86. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou XW, Wang Y, Pan CW. Metabolomics in diabetic retinopathy: A systematic review. Invest Ophthalmol Vis Sci. 2021;62:4. doi: 10.1167/iovs.62.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullins RF, Grassi MA, Skeie JM. Glycoconjugates of choroidal neovascular membranes in age-related macular degeneration. Mol Vis. 2005;11:509–17. [PubMed] [Google Scholar]
- 32.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22:307–38. doi: 10.1016/S1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 33.Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol. 2015;35:137–45. doi: 10.1161/ATVBAHA.114.304277. [DOI] [PubMed] [Google Scholar]
- 34.Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: Therapeutic opportunities. Microvascular Res. 2007;74:131–44. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Shaw LC, Li Calzi S, Li N, Moldovan L, Sengupta-Caballero N, Quigley JL, et al. Enteral Arg-Gln Dipeptide Administration Increases Retinal Docosahexaenoic Acid and Neuroprotectin D1 in a Murine model of retinopathy of prematurity. Investigative Ophthalmol Vis Sci. 2018;59:858–69. doi: 10.1167/iovs.17-23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth F, Bindewald A, Holz FG. Keypathophysiologic pathways in age-related macular disease. Graefe’s Arch Clin Exp Ophthalmol = Albrecht von Graefes Arch fur klinische und experimentelle Ophthalmologie. 2004;242:710–6. doi: 10.1007/s00417-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima T, Nakajima E, Shearer TR, Azuma M. Concerted inhibition of HIF-1α and -2α expression markedly suppresses angiogenesis in cultured RPE cells. Mol Cell Biochem. 2013;383:113–22. doi: 10.1007/s11010-013-1760-1. [DOI] [PubMed] [Google Scholar]
- 38.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 39.Tan SZ, Begley P, Mullard G, Hollywood KA, Bishop PN. Introduction to metabolomics and its applications in ophthalmology. Eye (Lond) 2016;30:773–83. doi: 10.1038/eye.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, et al. Mass-spectrometry-based metabolomics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5:435–58. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen AH, Gash DM, Avison MJ. Principal component analysis of the dynamic response measured by fMRI: A generalized linear systems framework. Magn Reson Imaging. 1999;17:795–815. doi: 10.1016/S0730-725X(99)00028-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.