Abstract
Background
Standard stemmed humeral implants have traditionally been utilized for total shoulder arthroplasty (TSA) with a recent trend to implant smaller stems including short and stemless humeral designs. However, the rate of stress shielding after stemless TSA has not been primarily studied. Therefore, the objective of this study is to report the short-term survivorship and radiographic analysis of a stemless humeral implant.
Methods
A retrospective cohort review of a prospectively collected, multicenter database for patients undergoing total shoulder arthroplasty with a stemless humeral design (Equinoxe Stemless; Exactech, Inc., Gainesville, FL, USA) with a minimum of 2 years clinical and radiographic follow-up was performed. The primary outcomes were to report the location and rate of stress shielding from a radiographic analysis of the humeral stem. Additionally, the revision rate of the humeral stem is reported. The secondary outcomes included ASES scores, visual analog scale (VAS) pain scores, and range of motion (ROM). Radiographs (anterior-posterior/Grashey and axillary) were reviewed blindly by two fellowship trained shoulder surgeons. Radiographic analysis included stress shielding (partial or complete cortical resorption) and subsidence or shift in component position.
Results
Fifty four patients were included in this study with an average follow-up of 27 months (range 24-32 months). The average age of this cohort was 65 years (range 57-73 years) with 23 patients (43%) being female. Stress shielding was observed in 4 patients (7%) with the medial calcar being the most common location of stress shielding. Three of the 4 patients (75%) had evidence of partial resorption while 1 patient (25%) had evidence of complete resorption. No humeral component shift or subsidence was observed. There were no revisions due to humeral component complications. There was 1 revision surgery for aseptic glenoid loosening. A significant improvement for all clinical outcome measures was seen including with respect to VAS pain, which improved from 6.2 to 1.8 (P < .05), ASES, which improved from 38.2 to 81.8 (P < .05), and ROM which forward flexion improved from 120 degrees to 153 degrees (P < .05) and external rotation improved from 29 degrees to 49 degrees (P < .05).
Discussion
This ongoing study demonstrates a low rate of stress shielding for a stemless design humeral implant at short-term follow-up without any revision surgery due to humeral component complications. Longer term radiographic and clinical analysis with this cohort will be needed to confirm these findings and theoretical benefits for future revision surgeries.
Keywords: Stemless design, Total shoulder arthroplasty, Radiographic analysis, Stress shielding, Osteolysis, Humeral lucency, Cortical resorption
Total shoulder arthroplasty surgical volume is currently increasing. Standard stemmed (100-150 mm) humeral implants have traditionally been utilized, however there is a trend to implant smaller stems including short (50-100 mm) and stemless (less than 50 mm) humeral designs.8 Stemless humeral designs have many theoretical benefits compared to traditional and short stems. Benefits of stemless humeral designs include less blood loss, ease of removal in revision situations, preservation of humeral bone, decreased operative time, ability to restore glenohumeral joint center of rotation without violating the humeral canal and the ability to perform a reconstruction for proximal humeral deformity cases.1,5,7,8,11,13,15,17,19
Stress shielding is the adaption of cortical bone to changes of load and is regulated by Wolff’s law.2,5,8 Traditional cemented humeral stems create a homogenous stress distribution with the proximal humerus limiting the stress shielding observed.8 However, as surgeons transitioned to press fit traditional humeral stems, greater cortical stress and load bypassing the proximal humerus to the diaphysis via the stem lead to increased stress shielding and cortical thinning of the proximal humerus.8 Furthermore, biomechanical studies and finite element analysis has shown more normal physiologic proximal humerus stress and therefore, predicted lower stress shielding with shorter and stemless humeral implants.8,18,20
Stemless humeral designs have been reported to have decreased stress shielding radiographically, which can be advantageous for future revision surgeries.5, 6, 7, 8,20, 21 However, reports documenting incidence of stress shielding after stemless TSA have had highly variable results.3, 4,9 Stress shielding for stemless humeral designs have been reported to be 0%-42%, which may be due to multiple variables including implant design and implantation technique.3, 4,9,14 Therefore, the primary purpose of this project is to report the short-term survivorship and radiographic outcomes of a stemless humeral press fit design implant for anatomic total shoulder arthroplasty (aTSA) of a particular shoulder system. The secondary outcomes included a clinical evaluation with ASES scores, visual analog scale (VAS) pain scores, and range of motion (ROM).
Materials and methods
Study design and humeral implant
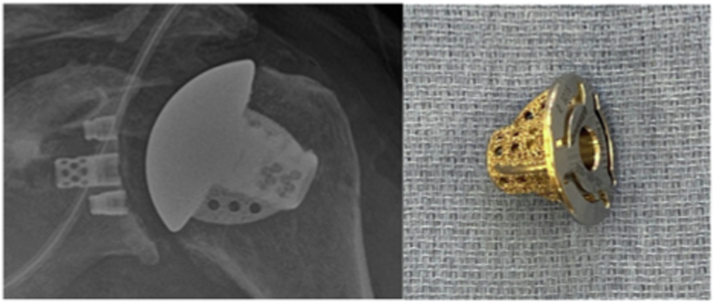
We performed a retrospective cohort review of a prospectively collected, multicenter database for patients undergoing total shoulder arthroplasty with a stemless humeral design (Equinoxe Stemles; Exactech Inc., Gainesville, FL, USA) with a minimum of 2 years clinical and radiographic follow-up. Indications for the procedure included osteoarthritis, rheumatoid arthritis, avascular necrosis and proximal humerus malunion with arthritis. The humeral implant utilized with these patients is a tapered, three-fined caged implant. The humeral implant is a press-fit design into the metaphyseal bone via the three highly porous fins and a core fenestrated nucleus. (Fig. 1).
Figure 1.
Equinoxe stemless humeral design. (Exactech, Inc., Gainesville, Florida).
Surgical technique
All surgical procedures were performed by shoulder reconstruction specialist through a standard deltopectoral approach. A lesser tuberosity osteotomy, subscapularis peel, subscapularis tenotomy or subscapularis sparring approach were performed based on surgeon’s preference. An anatomical neck osteotomy was performed with respect to the humerus native retroversion. Postoperatively, patients were placed in a sling and passive motion was initiated according to surgeon’s preference. Formal physical therapy for active and passive motion was initiated within six weeks postoperatively.
Radiographic evaluation
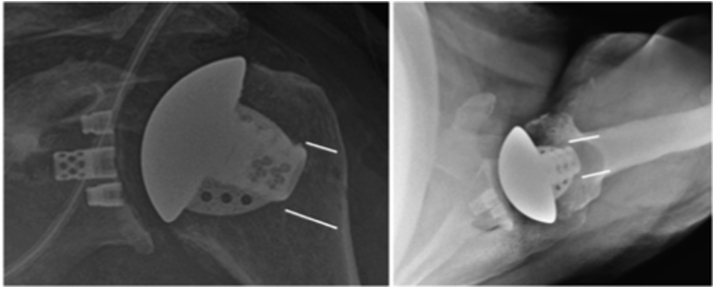
Anteroposterior (Grashey) and axillary radiographs were routinely taken during the initial postoperative period and during various intervals. All radiographs were reviewed independently by two fellowship trained shoulder surgeons. Latest radiographs (minimum 2 years postoperative) were compared to initial postoperative radiographs for decreased bone mineral density (cancellous changes), partial or complete stress shielding (cortical changes), and implant position change (subsidence or shift). Previously described anatomic locations for grading of the bony changes were utilized for this study.8,15 (Fig. 2) Additionally, a previously described classification by Denard et al was utilized for the grading of bony changes. Grade 1 is considered decreased bone mineral density without cortical resorption (cancellous changes), grade 2 is considered humeral lucency with partial cortical resorption, and grade 3 is considered humeral lucency with complete cortical resorption.8 Stress shielding was considered grade 2 or grade 3 humeral lucencies.8 All discrepancies were evaluated and discussed to arrive at a consensus.
Figure 2.
Previously described anatomic locations for stress shielding with a stemless humeral designed implant. On the AP view, there are three locations including greater tuberosity, tip and medical calcar. On the axillary view, there are three additional locations including anterior, tip and posterior; AP, anteroposterior.
Patient demographics and clinical evaluation
Clinical outcomes including baseline demographics (age, gender, body mass index) and patient reported outcomes were collected. Patient reported outcomes included pain (visual analog score/VAS), range of motion, specifically forward flexion (FF) and external rotation (ER), and ASES functional scores. Revision surgeries and complications were also collected.
Statistical analysis
Standard descriptive summaries (means, standard deviations, and percentages) were utilized for baseline demographics and radiographic characteristics. For clinical preoperative to postoperative comparisons, chi-squared test and Student’s t-test were utilized for categorical and numerical data. A P-value of < .05 was considered statistically significant. All statistical analyses were completed using Excel software (Microsoft Corporation, Redmond, WA, USA).
Results
Cohort demographics
There were 54 patients included in this retrospective study with a minimum of 2 years clinical and radiographic follow-up. The average follow-up was 27 months (range 24-32 months). The average age was 65 years (range 57-73 years) with 23 patients (43%) being female and 31 patients (57%) being male. The average BMI for this cohort was 31 (range 24-38) (Table I).
Table I.
Cohort analysis of 54 patients included in this retrospective review.
| N | 54 patients |
| Average follow up | 27 mo (24-32 mo) |
| Average age | 65 y (57-73 y) |
| Gender | 23 (43%) Female: 31 (57%) Male |
| Average body mass index | 31 (24-38) |
Clinical evaluation
At the final postoperative evaluation, on average all clinical measures improved significantly. Specifically, pain (visual analog scale) scores improved from 6.2 to 1.8 (−4.4, P < .05). Functional scores (ASES) improved preoperatively from 38.2 to 81.8 (+43.5) post operatively (P < .05). Range of motion, specifically forward flexion and external rotation also significantly improved. Forward flexion improved from 120 degrees to 153 degrees (+34 degrees, P < .05) and external rotation increased from 29 degrees to 49 degrees (+20 degrees, P < .05) (Table II).
Table II.
Preoperative and postoperative clinical evaluations including Pain or Visual Analog Score, ASES function score, forward flexion and external rotation measurements.
| Clinical assessment | Preoperative | Postoperative | Change | P value |
|---|---|---|---|---|
| VAS | 6.2 | 1.8 | −4.4 | <.05 |
| ASES | 38.2 | 81.8 | +43.5 | <.05 |
| Forward flexion (degrees) | 120 | 153 | +34 | <.05 |
| External rotation (degrees) | 29 | 49 | +20 | <.05 |
VAS, visual analog scale; ASES, American Shoulder and Elbow Surgeons.
Revisions and complications
Two of the 54 patients (3.7%) had a surgical complication during this study period. One patient (2%) sustained a fall and now has limited active motion despite no fracture. Currently, no surgical intervention for this patient has been performed. The second complication with this cohort includes 1 patient (2%) who underwent a revision surgery for aseptic glenoid loosening. There were no revision surgeries or complications related to the humeral implant (Table III).
Table III.
Revision and complications during the short term follow-up with this study. No revision surgery noted due to humeral component complications within the short term follow-up.
| Complications | 2 (3.7%) | Fall, aseptic glenoid loosening |
| Revisions | 1 (2%) | Aseptic Glenoid Loosening |
| Revisions due to humeral component | 0 (0%) |
Radiographic analysis
Four of 54 patients (7%) within this cohort exhibited radiographic evidence of proximal humeral stress shielding (partial or complete cortical bone changes). Of these 4 patients, all exhibited radiographic stress shielding in the medical calcar region (100%). Two of these patients (50%) also exhibited radiograph stress shielding in the anterior location on the axillary view. No other locations of stress shielding were observed. For the medical calcar location, 3 patients (75%) had partial (grade 2) cortical resorption while 1 patient (25%) had complete (grade 3) cortical resorption. The 2 patients with stress shielding anteriorly, they also displayed partial or grade 2 stress shielding in the anterior location. No humeral implant (0%) demonstrated a change in position (shift or subsidence) radiographically (Table IV).
Table IV.
Short term radiographic analysis revealing the medial calcar and partial resorption (grade 2 stress shielding) is the most common location and severity of stress shielding with this cohort.
| Stress shielding | 4 (7%) | |
| Location | Medial Calcar (4) | 3 (75%) Grade 2 (partial) 1 (25%) Grade 3 (complete) |
| Anterior (2) | 2 (100%) Grade 2 (partial) | |
| Humeral Component Change in Position (Shift or Subsidence) | 0 (0%) |
Discussion
The primary objective for this retrospective cohort study was to report the survivorship and radiographic analysis for a particular stemless humeral design utilized in aTSA, which has not been reported prior in literature. This cohort did not have any revision surgeries due to humeral component complications. Additionally, no change in humeral component position (shift or subsidence) was observed. However, stress shielding was observed in 7% of the patients with this particular implant. Stress shielding was primarily observed in the medial calcar location and was more likely to be associated with partial (75%) rather than complete (25%) cortical resorption. These findings appear to be similar to previously published articles regarding stemless humeral implants. Stress shielding with other stemless humeral designs range from 0% to 42%.3, 4,9 However, the majority of radiographic studies reveal a relatively low rate of stress shielding with stemless humeral press fit implants.15 Only one study reported a revision surgery with short term follow-up due to a humeral component complication with a stemless humeral design, which was noted to be aseptic loosening.3 This may indicate that while humeral loosening following stemless TSA is rare, vigilance is still needed when determining if the bone quality is adequate for this type of implant.
Stress shielding when using a standard length (100-150 mm) humeral press fit stem has been reported to range from 6% to 43%.6,10,16 Short (50-100 mm) humeral press fit stem stress shielding rates have been reported to range from 20% to 25% (M. Virk, unpublished data, 2022).8,12 Therefore, the results of this study combined with other previous studies with similar short term follow-up indicate that the stemless design is associated with lower rates of stress shielding in comparison to stemmed implants. However, the clinical ramifications are yet to be determined and longer-term radiographic studies are needed.
Our study is not without limitations. First, it is a retrospective study which presents inherent weaknesses, such as selection bias and particular patients that were lost to follow-up. Second, inaccuracies in radiographic findings are possible. The multicenter nature of this study may have introduced variations in radiographic views and techniques that could affect the analysis of x-rays and hence our results. Finally, our study could benefit from a larger sample size; however, with the recent trend to implant more stemless design components, this ongoing analysis will be able to include larger cohort numbers with future studies.
Conclusion
This ongoing radiographic analysis demonstrates a low rate of stress shielding for a stemless humeral design at short-term follow-up without any revision surgery due to humeral component complications. Additionally, compared to stress shielding rates for short and standard humeral stems, a stemless design appears to have a lower rate of stress shielding compared to uncemented stemmed implants as historically reported. Larger and longer term radiographic and clinical studies are needed to confirm our findings and elucidate the clinical implications.
Disclaimers
Funding: No outside funding or grant was received for the completion of this work.
Conflicts of interest: Felix Savoie - Dr. Savoie is a paid consultant for Exactech, which is related to the subject of this work. Curtis Noel - Dr. Noel is a paid consultant for Exactech, which is related to the subject of this work. Ryan Simovitch - Dr. Simovitch is a paid consultant for Exactech, which is related to the subject of this work. Alexander Greene - is a former employee of Exactech which is related to the subject of this work. Oke Anakwenze - Dr. Anakwenze is a paid consultant for Exactech, which is related to the subject of this work. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
This study was approved by WCG/WIRB. Study number: 1133601; IRB tracking number: 20091791.
References
- 1.Aibinder W.R., Bartels D.W., Sperling J.W., Sanchez-Sotelo J. Mid-term radiological results of a cementless short humeral component in anatomical and reverse shoulder arthroplasty. Bone Joint J. 2019;101-B:610–614. doi: 10.1302/0301-620X.101B5.BJJ-2018-1374.R1. [DOI] [PubMed] [Google Scholar]
- 2.Beck S., Patsalis T., Busch A., Dittrich F., Wegner A., Landgraeber S., et al. Long-term radiographic changes in stemless press-fit total shoulder arthroplasty. Z Orthop Unfall. 2021;159:274–280. doi: 10.1055/a-1079-6549. [DOI] [PubMed] [Google Scholar]
- 3.Brunner U.H., Fruth M., Rückl K., Magosch P., Tauber M., Resch H., et al. The stemless eclipse prosthesis – indications and mid-term results: a prospective multicenter study. Obere Extremitat. 2012;7:22–28. doi: 10.1007/s11678-011-0152-y. [DOI] [Google Scholar]
- 4.Churchill R.S., Chuinard C., Wiater J.M., Friedman R., Freehill M., Jacobson S., et al. Clinical and radiographic outcomes of the simpliciti canal-sparing shoulder arthroplasty system: a prospective two-year multicenter study. J Bone Joint Surg Am. 2016;98:552–560. doi: 10.2106/JBJS.15.00181. [DOI] [PubMed] [Google Scholar]
- 5.Comenda M., Quental C., Folgado J., Sarmento M., Monteiro J. Bone adaptation impact of stemless shoulder implants: a computational analysis. J Shoulder Elbow Surg. 2019;28:1886–1896. doi: 10.1016/j.jse.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Denard P.J., Haidamous G., Gobezie R., Romeo A.A., Lederman E. Short-term evaluation of humeral stress shielding following reverse shoulder arthroplasty using press-fit fixation compared with cemented fixation. J Shoulder Elbow Surg. 2020;29:906–912. doi: 10.1016/j.jse.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Denard P.J., Noyes M.P., Walker J.B., Shishani Y., Gobezie R., Romeo A.A., et al. Radiographic changes differ between two different short press-fit humeral stem designs in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:217–223. doi: 10.1016/j.jse.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Denard P.J., Raiss P., Gobezie R., Edwards T.B., Lederman E. Stress shielding of the humerus in press-fit anatomic shoulder arthroplasty: review and recommendations for evaluation. J Shoulder Elbow Surg. 2018;27:1139–1147. doi: 10.1016/j.jse.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Engler I.D., Hart P.A., Swanson D.P., Kirsch J.M., Murphy J.P., Wright M.A., et al. High prevalence of early stress shielding in stemless shoulder arthroplasty. Semin Arthroplasty JSES. 2022;32:751–756. doi: 10.1053/j.sart.2022.07.001. [DOI] [Google Scholar]
- 10.Erickson B.J., Denard P.J., Griffin J.W., Gobezie R., Lederman E., Werner B.C. Initial and 1-year radiographic comparison of reverse total shoulder arthroplasty with a short versus standard length stem. J Am Acad Orthop Surg. 2022;30:e968–e978. doi: 10.5435/JAAOS-D-21-01032. [DOI] [PubMed] [Google Scholar]
- 11.Gallacher S., Williams H.L.M., King A., Kitson J., Smith C.D., Thomas W.J. Clinical and radiologic outcomes following total shoulder arthroplasty using Arthrex Eclipse stemless humeral component with minimum 2 years' follow-up. J Shoulder Elbow Surg. 2018;27:2191–2197. doi: 10.1016/j.jse.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Gilot G., Alvarez-Pinzon A.M., Wright T.W., Flurin P.H., Krill M., Routman H.D., et al. The incidence of radiographic aseptic loosening of the humeral component in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1555–1559. doi: 10.1016/j.jse.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Gruson K.I., Lo Y., Stallone S., Qawasmi F., Lee S., Shah P. A comparison of operative time and intraoperative blood volume loss between stemless and short-stem anatomic total shoulder arthroplasty: a single institution's experience. J Am Acad Orthop Surg Glob Res Rev. 2022;6:e22.00141. doi: 10.5435/JAAOSGlobal-D-22-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habermeyer P., Lichtenberg S., Tauber M., Magosch P. Midterm results of stemless shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2015;24:1463–1472. doi: 10.1016/j.jse.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Hawi N., Tauber M., Messina M.J., Habermeyer P., Martetschläger F. Anatomic stemless shoulder arthroplasty and related outcomes: a systematic review. BMC Musculoskelet Disord. 2016;17:376. doi: 10.1186/s12891-016-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King J.J., Farmer K.W., Struk A.M., Wright T.W. Uncemented versus cemented humeral stem fixation in reverse shoulder arthroplasty. Int Orthop. 2015;39:291–298. doi: 10.1007/s00264-014-2593-6. [DOI] [PubMed] [Google Scholar]
- 17.Nixon R.A., Dang K.H., Haberli J.E., O'Donnell E.A. Surgical time and outcomes of stemmed versus stemless total shoulder arthroplasty. J Shoulder Elbow Surg. 2022;31:S83–S89. doi: 10.1016/j.jse.2022.01.129. [DOI] [PubMed] [Google Scholar]
- 18.Reeves J.M., Athwal G.S., Johnson J.A., Langohr G.D.G. The effect of inhomogeneous trabecular stiffness relationship selection on finite element outcomes for shoulder arthroplasty. J Biomech Eng. 2019;141:034501 doi: 10.1115/1.4042172. [DOI] [PubMed] [Google Scholar]
- 19.Smith T., Horstmann H., Karkosch R., Tsamassiotis S., Bowsher N., Ellwein A., et al. Short-term results of a new anatomic stemless shoulder arthroplasty - a prospective multicentre study. Orthop Rev (Pavia) 2022;14:37042. doi: 10.52965/001c.37042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synnott S., Langohr G.D.G., Reeves J.M., Johnson J.A., Athwal G.S. The effect of humeral implant thickness and canal fill on interface contact and bone stresses in the proximal humerus. JSES Int. 2021;5:881–888. doi: 10.1016/j.jseint.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uschok S., Magosch P., Moe M., Lichtenberg S., Habermeyer P. Is the stemless humeral head replacement clinically and radiographically a secure equivalent to standard stem humeral head replacement in the long-term follow-up? A prospective randomized trial. J Shoulder Elbow Surg. 2017;26:225–232. doi: 10.1016/j.jse.2016.09.001. [DOI] [PubMed] [Google Scholar]