Abstract
Inflammatory bowel disease (IBD) is becoming increasingly prevalent with the improvement of people's living standards in recent years, especially in urban areas. The emerging environmental contaminant is a newly-proposed concept in the progress of industrialization and modernization, referring to synthetic chemicals that were not noticed or researched before, which may lead to many chronic diseases, including IBD. The emerging contaminants mainly include microplastics, endocrine-disrupting chemicals, chemical herbicides, heavy metals, and persisting organic pollutants. In this review, we summarize the adverse health effect of these emerging contaminants on humans and their relationships with IBD. Therefore, we can better understand the impact of these new emerging contaminants on IBD, minimize their exposures, and lower the future incidence of IBD.
Keywords: inflammatory bowel disease, emerging contaminant (EC), exposome, adverse health effects (AHEs), gut dysbiosis
Introduction
Inflammatory bowel disease (IBD) refers to chronic, relapsing inflammatory disorders of the gastrointestinal tract, and its pathogenesis includes heredity and environmental factors (1). The two major types of IBD are Crohn's disease (CD) and ulcerative colitis (UC) (2). The pathology of IBD involves impairment of the intestinal mucosal barrier, dysbiosis of the gut microenvironment, and the alteration of the gut immune response (3). Chronic abdominal pain and diarrhea are typical symptoms of IBD. Presently no effective treatments can fully cure IBD, and nearly 30% of IBD patients will require surgery within 10 years after their initial diagnosis (4, 5). The global spread of IBD appears to be associated with industrialization and changes in people's diets and environments, and environmental exposures are closely associated with the increased risk of IBD (6).
The new emerging environmental contaminant is a recently coined term that describes exposome to the environment. The emerging environmental contaminants including but are not limited to microplastics (MPs), endocrine-disrupting chemicals (EDCs), chemical herbicides, heavy metals, and persisting organic pollutants (POPs) (7, 8). The variable composition of these exposomes across regions, and the interaction among these exposures may contribute to the heterogeneous nature of the association between emerging environmental contaminants and IBD (9). These exposomes are not commonly monitored in nature, but have the potential to enter the environment and human body, and cause short-term and long-term adverse health effects. In the immediate dietary intake, the contaminants may cause acute abdominal pain or diarrhea, activating immediate intestinal inflammation (Figure 1). As in long-term exposure, these contaminants will cause chronic diseases like IBD and chronic renal failure, activate a series of chronic inflammation.
Figure 1.
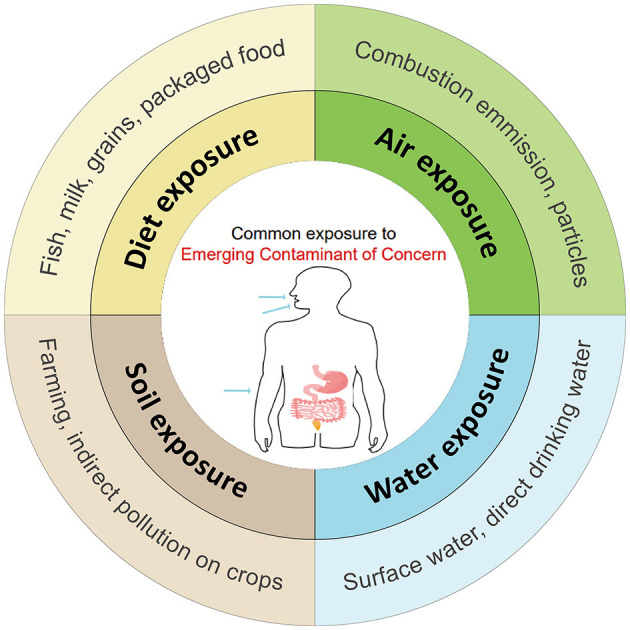
The common way and pollutants that general population in exposure of Emerging Contaminants (ECs) through digestive tract, respiratory tract and skin contact.
In this review, we summarize the current epidemiologic evidence and biological mechanism between new emerging contaminants exposure and the development of IBD. Also, we summarize the common exposure pathways of new emerging contaminants to public generations based on accumulated studies.
Microplastics
Microplastics (MPs) are tiny plastic particles under 5 millimeters in size (10). The primary sources of MPs in human life are plastic bottles, abrasives, and opacifiers (11), and they may be degraded into MPs by various factors like ultraviolet over time (12). The main types of MPs include polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), Polyurethane (PU), etc. (10, 13) (Table 1).
Table 1.
Adverse health effects of microplastics in inflammatory bowel disease.
| Contaminant | References | Experiment model/human group | Exposure time/dose | Route of exposure | Source of dietary intake | Impact on the gut | Other health risks |
|---|---|---|---|---|---|---|---|
| Polystyrene (PS) | Schwarzfischer et al. (14) | Wild type female C57BL/6 mice | 0.2 mg/day for 84 days | Dietary exposure | Drinking water | ° Penetrate the intestinal barrier and accumulate in small intestine, lymphoid organs and liver ° Do not affect intestinal health, nor aggravate acute/chronic DSS colitis | / |
| Luo et al. (15) | 8-week-old male C57BL/6 J mice | 0.5/5 μg for 14 days | Oral gavage | Drinking water | °↓Colonic length °↑Histopathological damage °↓Mucus secretion °↑Colon permeability °↑Colonic inflammatory response | °↑Secondary liver injury associated with inflammatory cell infiltration | |
| Zheng et al. (16) | male C57 mice | 500 μg/L for 28 days | Dietary exposure | Drinking water | °↑Acute colitis and lipid disorders induced by sodium dextran sulfate (DSS) °↑intestinal permeability | ° Intensify liver damage in mice with acute colitis ° Affect lipid metabolism | |
| Lu et al. (17) | Five-week-old mice | 0.5 and 50 μm polystyrene MP, 100 and 1,000 μg/L, 5 weeks | Dietary exposure | Drinking water | °↓Decrease the secretion of mucin in gut ° Induce gut microbiota dysbiosis | ° Induce hepatic lipid metabolism disorder in mice | |
| Polyethylene (PE) | Li et al. (18) | 5-week-old SPF grade male C57BL/6 mice | Respective 6, 60, or 600 μg daily for 5 weeks | Dietary exposure | Special feeds | ° Induce intestinal dysbacteriosis °↑Gut microbial species, bacterial abundance and flora diversity ° Induce inflammation in intestine °↑the secretion of serum pro-inflammatory cytokine IL-1α; °↓the percentage of Th17 and Treg cells among CD4+ cells | / |
| Most Polypropylene (PP) | Schwabl et al. (19) | 8 healthy volunteers aged 33 to 65 from Tokyo | 7 days | Dietary exposure | Seafood; Food is usually wrapped, packaged or stored in plastic; Chewing gum | ° Metastasize to gastrointestinal tissues or other organs and cause harmful effects ° Patients with increased intestinal permeability (e.g., IBD patients) may be more susceptible to microparticle absorption and potential damage. | / |
| Zhang et al. (20) | 26 healthy male students aged 18–25 from Beijing | 8 days | Dietary intake | Packaged water, beverages, milk, dairy products, beer | / | ° Moderate correlation exists between the intake of packaged water and the abundance of microplastics in feces. |
“↑” means increased level or concentration; “↓” means decreased level or concentration.
Human exposures to MPs occur through ingestion, inhalation, and dermal contact (21). MPs exist and exposit in all environments, especially underground and surface water, then the human food chain, and ultimately enter the body through ingestion, which is the major exposure way (11, 13, 22, 23). A recent review has summarized multiple types of food containing MPs, including fruit, vegetables, milk, meat, aquatic food, etc. (12, 24). Besides, the fast spread of takeaway foods accelerates the number of MPs ingested by humans globally, since they are usually packed with plastic products (12). The suggested weekly ingestion range of MPs is within 0.10–5 g/week (5, 7, 8). In a study of a small set of donors, which first measured the mass concentration of the polymetric component of plastic in human blood, the mean of the sum quantifiable concentration of plastic particles in blood was 1.6 μg/ml (25). In human feces, MPs were also detected in the order of 2 MP particles/g (5).
MPs could be detected in the human bloodstream probably due to the absorption of them into the blood by mucosal contact (either ingestion or inhalation) in a size-dependent manner (25). MPs may be transported to organs via the bloodstream, causing intestinal toxicity, metabolic disruption, reproductive toxicity, neurotoxicity, immunotoxicity through oxidative stress, apoptosis, and specific pathways, etc. (26). After dietary intake, MPs are usually absorbed in the digestive tract, liver, kidneys, and spleen (27–31). Once deposited, MPs will induce morphological changes, activate inflammatory responses, inhibit cell differentiation, and affect gene expression (27, 32–34). Several clinical trials found that exposure to MPs impairs the gut epithelial barrier, induces intestinal flora alteration, disturbs lipid metabolism, causes oxidative stress and the release of inflammatory factors (15, 35–37). Apart from passive intake, the intestinal tract absorbs MPs through multiple ways, including endocytosis of enterocytes and specialized M cells, paracellular uptake, and active absorption by the intestinal villus (38).
The increased exposure to MPs will also accelerate the pathogenesis of IBD. A recent study revealed that exposure to MPs may impair the antimicrobial capacity of blood clam by reducing plasma inhibition of bacterial growth, humoral immune effector levels, and chemotactic activity of hemocytes, which showed that MPs imposed significant oxidative stress on hemocytes, causing great immunotoxicity (39).
Researchers found that the average concentrations of MPs in the feces of IBD patients (41.8 items/g dm) were markedly higher than that of healthy people (28.0 items/g dm) (40). In IBD patients, the gut experiences repeating endothelial lesions, which increases the permeability of the intestinal epithelial barrier (41). This means in IBD patients, MPs are more likely to enter the injured intestinal epithelial cells, attach to them, and enhance their translocation to different systems (14, 42). MPs exposure also triggers the over-proliferation of intestinal stem cells, causing the imbalance of colonic epithelial homeostasis, which in turn elevates the occurrence and severity of DSS-induced colitis (43).
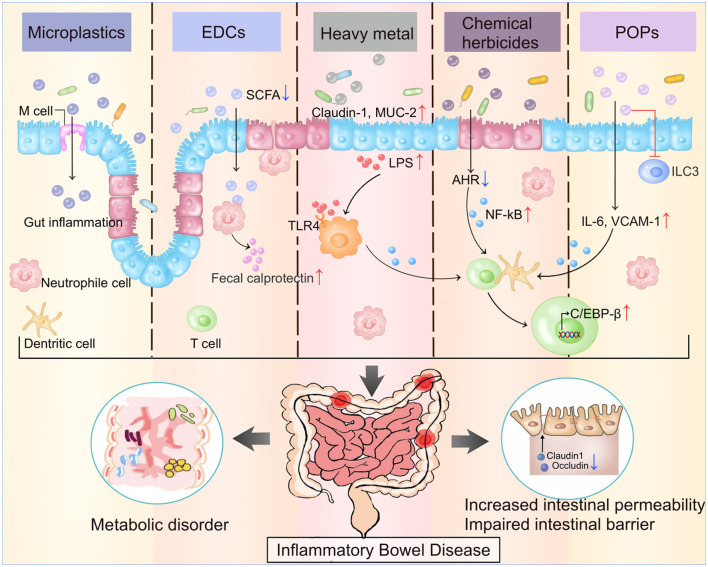
MPs will also alter the composition and diversity of intestinal microflora in animal models, which will trigger multiple follow-up effects, such as changing the ability in the differentiation of Treg cells, and activating the signal transduction pathways associated with intestinal mucosal immune function (17, 18, 44–46). In the DSS mouse model, additional exposure to polystyrene microplastics (PS-MPs) can aggravate the severity of colitis (14, 15) by reducing tight junction proteins such as Claudin1 and Occludin, and increasing intestinal permeability (47–49). Correspondingly, TNF-α, IL-1β, and IL-6 increase significantly in the colitis mice exposed to additional PS-MPs, demonstrating their proinflammatory properties (15, 16). After the exposure, the macrophage infiltration will increase in the liver, which further triggers immune cells to release proinflammatory and immune factors like IL-17α and IL-22 (16).
MPs can alter the structure of intestinal microbiota in mice, which may exacerbate intestinal inflammation. Previous studies showed the overgrowth of Staphylococci is related to IBD. Correspondingly, mice treated with MPs exhibited a marked increase in Staphylococcus genus abundance and a decrease in Parabacteroides genus abundance (P < 0.05) (18). Li et al. observed increased numbers of gut microbial species, bacterial abundance, and flora diversity in mice treated with a high concentration of MPs (50). An increased abundance of Staphylococcus and Bacteroidetes alongside a decreased abundance of Parabacteroides and Firmicutes are also documented in this research (18). Likewise, Lu et al. found that MPs exposure decreased the abundances of Firmicutes and α-Proteobacteria in the feces of mice, and altered the variety and diversity of gut microbes (17).
To sum up, exposure to MPs, either in the blood or digestive tract, may accelerate the occurrence and development of IBD, and cause serious harm to the human body. Therefore, it is necessary to reduce the amount of dietary intake of MPs, such as reducing the use of bottled water and takeaway food (12, 23). The government also needs to pay attention to more efficient ways to degrade MPs. The potential benefits of reducing the pollution of MPs at sources still deserve further exploration.
Endocrine disruptors
Endocrine disruptors (EDCs) are chemicals that interfere with the hormones in the human body through the endocrine system (51). EDCs can affect the way the body reacts to hormones, and change the gut microenvironment, which may cause immune vulnerability, decreased tolerance to food antigens (52), change in gut microbiota, and the occurrence of IBD (53).
EDCs, including phthalates, flame retardants, pharmaceutical agents, and phenols like bisphenol-A (BPA), ethyl-paraben, and methylparaben (54), are massively produced and used for food containers, personal care products, and other plastic objects (51). EDCs enter the human body mainly through dietary ingestion, inhalation, and dermal uptake, and are mostly bioaccumulated in the adipose tissue (55, 56). Most EDCs are lipophilic; therefore, they can induce microbial dysbiosis, and activate xenobiotic pathways and associated genes, enzymes, and metabolites (Table 2).
Table 2.
Adverse health effect of EDCs in inflammatory bowel disease.
| Contaminant | References | Experiment model/Human group | Exposure time/dose | Route of exposure | Source of dietary intake | Impact on the gut | Other health risks |
|---|---|---|---|---|---|---|---|
| PCBs | Min et al. (57) | Female C57BL/6 mice | 5 mg/kg for 6 weeks | Oral gavage | PCB153 dissolved in corn oil | ° Deteriorate the health of gut microbiota. | ° Induce obesity, lipid metabolism disorder, and dyslipidemia. |
| BPA | Javurek et al. (58) | California mice (Peromyscus californium) | BPA (50 mg/kg) or EE (0.1 ppb) | Diet from periconception through weaning | Artificial feeding | ° Increase gut microbiota proportions °↑Bacteroides, Prevotellaceae, Sutterella and etc. | ° Disrupt normal gut flora; ° Induce IBD and colorectal cancer. |
| Lai et al. (59) | Sixteen 3-week-old male CD-1 mice | Bisphenol A (>98% purity) solution (120 mg/mL) | BPA-water feed | Drinking water | ° Reduce gut microbiota diversity ° Induction of Helicobacteraceae ° Reduction of Firmicutes and Clostridia. | ° Alter the gut microbiota; ° Induce IBD. | |
| Yin et al. (60) | Seven-week-old DSS-induced colitis mouse: ICR mice | BPA and its substitute BHPF, 2 weeks | Dietary exposure | Not mentioned | ° Induce inflammatory responses ° Alter gut metabolites ° Deregulate sugar and fatty acid metabolisms. | ° Change the metabolic way of gut microbiota in IBD. | |
| Diamante et al. (61) | Eight-week-old female and male C57BL/6J mice, eight breeding pairs | 0.005 μg/μL BPA and 0.0015% ethanol; | Exposed during gestation, terminated after 19–21 days | Artificial feeding | ° Affect fatty acid metabolism, and the gut microbial composition. | ° Affect the gut microbial composition in an age- and sex-dependent manner. | |
| Linares et al. (62) | 200 CD patients (140 in remission; 60 in active disease) | / | / | Food in EDCs containers; packaged food, seafood. | ° BPA: higher in colonic vs. ileal forms ° Butyrate and tryptophan: lower in exposed patients. | ° Increased in serum of patients with active disease vs. patients in IBD remission period. |
“↑” means increased level or concentration; “↓” means decreased level or concentration.
BPA is the most common and well-known EDC. It is linked with the active type of IBD. The direct intake of BPA-packaged food rarely causes an immediate intestinal response, as it mainly affects humans through chronic exposure and accumulation. BPA level is significantly increased in the serum of patients with the active period of IBD, compared to patients in the remission period, and regarding the disease phenotype, serum BPA levels were higher in colonic vs. ileal forms (62). It can change the level of microbiota and gut metabolites, increase the incidence of IBD, and accelerate IBD development (57, 59, 62).
BPA may change the metabolic way of gut microbiota and increase the risk of IBD. Many bacteria, such as Bacteroides, Mollicutes, Prevotellaceae, Erysipelotrichaceae, Akkermansia, Methanobrevibacter, and Sutterella, whose proportions increase with exposure to BPA, are associated with different diseases, such as IBD and colorectal cancer (58). Another American research shows BPA can affect the gut microbial composition in an age- and sex-dependent manner (61). In the epidemiological studies, dietary exposure to BPA reduces gut microbial diversity, and in the gut microbiota of CD patients, the population of Proteobacteria increased while Clostridium decreased (59). In the plasma of UC patients, the amount of BPA exposure is observed to have correlation with the altered level of plasma proteins involved in lipid-related metabolic processes and cytokine response, indicating BPA may serve as a biomarker in severe UC (63). BPA exposure may also change the level of gut metabolites, reducing butyric acid and tryptophan, and increasing fecal calprotectin, which indicates a correlation between its exposure and the severity of IBD (57, 62).
Aside from BPA and its analogs, many other EDCs also relate to the development of IBD, such as phthalates, triclosan, and perfluoroalkyl substances (64–66). For example, exposure to the BPA substitute, fluorene-9-bisphenol, can alter gut metabolites in mice and deregulates the sugar and fatty acid metabolisms in the gut (60). Several epidemiological studies have demonstrated the correlation between other EDCs and IBD (62, 66, 67). In a study estimating paraben and phenol exposure, approximately one quarter (25.5%) of all participants in this sub-set reported symptoms of chronic diarrhea, which is a typical complication of IBD (67). Notably, in IBD-specific cases, higher mean concentrations of urinary 4-tert-octylphenol were associated with the increased prevalence of IBD (67).
To reduce the adverse health effects of BPA, in 2015, the European Food Safety Authority (EFSA) reduced the tolerable daily intake (TDI) of BPA from 50 μg/kg body weight (bw)/d to 4 μg/kg bw/d (68). The estimated recommended daily intake of BPA ranges from < 1 to 5 μg/kg bw/d (69). To reduce EDCs exposure, it is essential to choose EDCs-free products and not heat food or store hot food in BPA containers marked with the recycling code 3 or 7 (70). From the present situation, it is important to strengthen the food safety policy, and use suitable materials in direct contact with food (56, 71). In the meantime, the necessary action is to reduce waste and use EDCs-free packaging, which may contribute to health improvement in food and reduce the risk of IBD.
Chemical herbicides
Chemical herbicides are herbicides that inhibit the growth of unwanted plants like residential weeds and invasive species (72). Commercially used chemical herbicides can cause substantial mortality of non-target plants and insects. They contaminate soil and reside in water, and may accumulate in the environment over time (Table 3). Glyphosate is the most popular herbicide in America. Its concentration continued to soar in the world, with the level rising from 2 to 430 μg/L in natural water (79). The rising level in water causes severe pollution and threatens food safety.
Table 3.
Adverse health effect of chemical herbicides in inflammatory bowel disease.
| Contaminant | References | Experiment model/human group | Exposure time/dose | Route of exposure | Source of dietary intake | Impact on the gut | Other health risks |
|---|---|---|---|---|---|---|---|
| Glyphosate | Tang et al. (73) | Eight-week-old male Sprague rats, weighing 180–220 g | 0, 5, 50, and 500 mg/kg of weight glyphosate, for 35 days | Oral gavage | Artificial feeding | °↓The relative of Lactobacillus in small intestine | ° Induce inflammatory responses ° Alter gut microbial composition |
| Suppa et al. (74) | Model species Daphnia | 1 mg/L glyphosate, corresponding to the MCL of drinking water | Water surroundings | Not mentioned | ° Induce DNA damage ° Alter the gut microbiota ° Interfere carbon and fat metabolism | ° Dysbiosis of gut and its chronic inflammation | |
| Ding et al. (75) | Six-month-old healthy adult male zebrafish (Danio rerio, AB-wild type) | 3.5 mg/L GLY concentration | Water surroundings | Not mentioned | ° Alter the gut microbiota °↑Fusobacteria ↓Proteobacteria °↓Claudin-5, ZO-1, occludin | ° Gut dysbiosis ° Destroy the intestinal mucosal barrier ° Enhanced intestinal permeability. | |
| 2,4-D | Tu et al. (76) | Specific-pathogen-free (SPF) 8-week-old C57BL/6 male mice | Low-dose 2,4-D exposure: 1 ppm 2,4-D water solution, 13 weeks | Feeding water solution daily | Artificial feeding | ° Influence the homeostasis of gut microbiome °↓ Plasma acylcarnitine. | ° Alter microbiome-related pathways ° Disturb gut-host homeostasis ° Increase risk of IBD |
| Combination of 2,4-D, dicamba and glyphosate | Mesnage et al. (77) | Wild-type mES cells (strain B4418) | The mixture of glyphosate, Dicamba and 2,4-D in water. | Water surroundings | / | ° DNA damage ° Oxidative stress ° Unfolded protein response | ° Carcinogenic effects |
| Propyzamide | Sanmarcro et al. (78) | Zebrafish (7 d.p.f.) | Immersed in TNBS-containing E3 ° Medium for 72 h. | Liquid surroundings | / | ° Upregulate NF-κB-driven C/EBPβ proinflammatory gene expression ° Inhibit AHR signaling ° Boost intestinal inflammation | ° Increase the risk of IBD and other gut diseases |
“↑” means increased level or concentration; “↓” means decreased level or concentration.
Chemical herbicides affect humans mainly through intestinal absorption, skin exposure, and inhalation (69). They may enter the body through contaminated food, like crops, fruits, and vegetables (80). In a German survey, the urinary glyphosate concentrations of 3 to 17-year-old children were above the limit of quantification of 0.1 μg/L, and the overall exposure of the young population may relate to their vegetarian diet or consumption of cereals, pulses, or vegetables (81). Much research hasproven the adverse effects of chemical herbicides on non-human beings, such as mice, zebrafish, and livestock.
Herbicides, including glyphosate, 2,4-Dicholorophenoxy acetic acid (2,4-D), and dicamba, may damage the immune system and cause symptoms of IBD, like diarrhea, bowel inflammation, and maldigestion (77). Especially, people with weakened immune systems are more susceptible to chemical herbicide-related intestinal inflammation (82). Herbicides may disrupt the normal gut flora, and irritate the lining of the digestive tract, which can lead to gut inflammation and gastrointestinal diseases like IBD (83).
Glyphosate is an active ingredient in Roundup, the most widely-used herbicide (84, 85). Glyphosate exposure may be a critical environmental trigger in the etiology of diseases associated with gut microbiota dysbiosis, including IBD (73, 86, 87). Glyphosate can alter the structure of microbiota, interfere with the shikimate pathway in microbiomes, and hinder the production of aromatic amino acids (88, 89). And the dietary intake of aromatic amino acids may alleviate the antimicrobial effect of glyphosate (90). Glyphosate exposure induces inflammatory responses in the small intestine, and alters the gut microbial composition in rats, with the Lactobacillus significantly decreasing (73). It also destroys the intestinal mucosal barrier function, leading to dysbiosis and chronic inflammation.
Other essential elements in chemical herbicides include dicamba, 2,4-D, 2,4-Dinitrofluorobenzene, and 2,4,5-Trichlorophenoxyacetic acid. The sub-chronic low-dose 2,4-D exposure may influence gut microbiome homeostasis, significantly lower the acylcarnitine level, and decrease levels of plasma acylcarnitine (76). An IBD multi-omics research showed that the rising level of acylcarnitine is closely related to the development of IBD (91). But another explanation is that decreasing acylcarnitine levels can also produce toxicity, suggesting the perturbations in the fatty acid beta-oxidation pathway (92). Dicamba, 2,4-D, and glyphosate alone or in combination, account for genotoxicity in patients with gastrointestinal disorders, including DNA damage, oxidative stress, and unfolded protein response, which contributes to the development of IBD (77). Another herbicide, Propyzamide, can boost gut inflammation by upregulating NF-κB-driven C/EBPβ pro-inflammatory gene and inhibiting AHR signaling pathways, and further, inducing the development of IBD (78). Furthermore, an organic diet can reduce the human body's herbicide level, which is a possible solution to reduce herbicide residue and lower the risk of IBD.
Heavy metals
Heavy metals are naturally occurring elements with high atomic weight and density (93). They are also called trace elements, usually detected in trace concentrations (ppb range to < 10 ppm) (94). The heavy metals we discuss are those accumulated in the food chain and are highly toxic to living organisms. Most of them come from natural resources and industry (95–98), including lead (Pb), manganese (Mn), arsenic (As), cadmium (Cd), mercury (Hg), and others. They are commonly used in people's daily life with widespread pollution (99, 100).
Human activities may increase the number of heavy metals residing in the environment, including metal processing, and the production of medical waste, plastic products, and electric wastes (101–104). Dietary is the main source of human exposure, with its detrimental effects including cardiovascular, neurological, reproductive, and intestinal disorders (105–108). Here we focus on five heavy metals: Pb, Mn, As, Cd, and Hg. They are not only ubiquitous in the environment, but also associated with gut microbiota dysbiosis and the severity of IBD (Table 4).
Table 4.
Adverse health effect of heavy metals in inflammatory bowel disease.
| Contaminant | References | Experiment model/human group | Exposure time/dose | Route of exposure | Source of dietary intake | Impact on the gut | Other health risks |
|---|---|---|---|---|---|---|---|
| Lead | Yu et al. (11) | C57BL/6 mouse models | 0.1, 0.5, and 1.0 g/L for 8 weeks | Dietary exposure | Drinking water | °↓ Expression of tight junction proteins °↑ Abundance of Marvinbryantia and Ruminococcus °↓Abundance of Lactobacillus and Roseburia ° Induce gut dysbiosis | ° Influence the metabolism of macronutrients, trace elements ° Neurodegenerative injury ° Inhibit CAT activity in kidney and GSH level in liver |
| Xia et al. (109) | Male adult wide type AB strain zebrafish (Danio rerio) | Respective 10 and 30 μg/L for 7 days | / | / | °↑ Gut mucus volume °↓The abundance of α-Proteobacteria °↑ The abundance of Firmicutes | ° Induce hepatic metabolic disorder | |
| Manganese | Choi et al. (110) | Wild type C57BL/6 mice aged 3–4 weeks | Mn: 0–0.5 ppm (deficient), 35–35.5 ppm (adequate), and 300-301 ppm (supplemented). | Dietary exposure | Diets | ° Maintain the intestinal barrier °↑ Morbidity, weight loss, and colon damage °↑ Levels of inflammatory cytokines | / |
| Mitchell et al. (111) | Male C57BL/6 mice | MnCl2 (66 mg/kg) | i.p. injection | / | ° Reduce chronic colitis | / | |
| Arsenic | Zhong et al. (112) | 1-day-old ducks | control group; low ATO group 4 mg/kg; high ATO group 8 mg/kg. | Oral administration and intubation | Drinking water | ° Intestinal injury °↓α diversity of intestinal flora ° Change bacterial composition °↓ Expression of intestinal barrier related proteins | ° Liver inflammatory cell infiltration ° Vesicle steatosis °↑ Pro-inflammatory CKs (IFN-γ TNF-α IL-18 and IL-1β) in the liver |
| Cadmium | Breton et al. (113) | 12-week-old female BALB/c mice | CdCl2 (2.5 and 12.5 mg/kg) for 1, 4, or 6 weeks | Dietary exposure | Drinking water | °↓ Epithelial permeability °↑ Oxidative defense mechanism °↓ Nf-κB and pro-inflammatory cytokine pathways ° Stimulate anti-oxidant pathways | / |
| Mercury | Zhao et al. (114) | Female Kunming mice | 80 mg/L HgCl2 for 90 days | Dietary exposure | Drinking water | ↑ Faecalis, Helicobacter °↓ Halococcus and Bacillus ° intestinal injury | ↑ Pro-apoptotic gene expression |
| Zhao et al. (115) | Eight-week-old female mice | HgCl2 (160 mg/L) for 3 days | Dietary intake | Drinking water | ↓ Growth performance ° Induce oxidative stress °↑ Clostridium, Lactobacillus | / | |
| Seki et al. (116) | Female C57BL/6 mice | MeHg (5 mg/kg) for 14 days | Oral intubation | / | ° Inhibit the growth of lactobacillus | °↓ Gut bacteria after exposure to methylmercury ° Accelerated accumulation in the cerebellum, liver, and lungs |
“↑” means increased level or concentration; “↓” means decreased level or concentration.
Lead
Most Pb emissions in life come from gasoline and enter the environment through burning exhausts. Pb enters the body mainly through dietary exposure, including food (65%) and water (20%) (117). For the Pb in the food, adults can absorb 10–15% of the ingested Pb, while children can absorb up to 50% through the gastrointestinal tract, which indicates children are more susceptible to Pb exposure.
Most Pb ingested accumulates in the kidneys, followed by the liver and other soft organs, like the heart and brain (94). When its dose is over 70 μg/dL, severe consequence happens (118). Pb can cause various disorders by inducing oxidative stress and breaking membrane integrity (119, 120). It also impairs gastrointestinal function and contributes to IBD pathogenies.
Epidemiological findings show that the level of Pb in IBD patients rises significantly (121). In CD patients, Pb in the scalp hair were significantly lower than that in healthy individuals, and its concentration in the serum is lower, which is consistent with the rising level in the tissue (122). In animals exposed to Pb, the amount of intestinal mucus increases, and the diversity and abundance of gut microbiota also change significantly (123, 124). Many metabolites related to glucolipid metabolism, amino acid metabolism, and nucleotide metabolism have changed after Pb exposure (109). Besides, Pb is highly toxic to Escherichia coli and Lactic acid bacteria, and long-term Pb exposure will induce chronic toxicity in a dose-dependent manner (11).
Developed countries have higher centration of Pb emission (125), which corresponds to the fact that IBD in developed countries shows a higher prevalence. Therefore, it is necessary for people in developed countries to take more precautions.
Manganese
Manganese (Mn) is the 12th most abundant element on the Earth. It exists mainly in the chemical oxidation state (126), and it is necessary for normal body functioning (94). It can activate various enzymes in the body and is indispensable for the development of intestinal immune functions (126).
The content of Mn in vegetables is higher than that of animal food (127). Seafood, chocolate, nuts, fruits, rice, and spices are also essential sources of Mn (127). The average concentration of Mn in human tissue is 1 mg/kg (126). Excessive dietary intake may lead to impaired intestinal immune function and over-activate oxidative stress, which is closely associated with the inflammation process in IBD patients.
The serum Mn concentration is different between healthy individuals and IBD patients, with the extent much greater in IBD patients (121, 128, 129). The concentration of Mn in blood is markedly higher in CD patients (121). But animal experiments showed some contradictions. In Mn-deficient mice treated with DSS, the incidence of IBD increases, along with higher inflammatory cytokine levels, oxidative damage, and DNA damage, which indicates the level of Mn may be inversely correlated to the incidence of IBD.
Another two studies also indicate Mn's protective role in gut homeostasis. One shows that a decrease in absorption and accumulation of Mn will trigger the release of proinflammatory factors and exacerbate the severity of inflammation (111). Another study implies Mn can boost the immune system by enhancing the function of intestinal CD8+ T cells (130). Furthermore, some studies have indicated the protective role of Mn for IBD. A study on manganese metal-organic framework (Mn-MOF), is a practical application in the treatment of spontaneous IBD by scavenging ROS to relieve oxidative stress, and protect the intestinal barrier (131). The study on hollow MnO2 (hMnO2) carried out to achieve synergistic IBD therapy, is based on MnO2, which has highlighted SOD-like and CAT-like activities (132, 133). According to the aforementioned viewpoints, the role of Mn for IBD is contradictory. For IBD patients, higher serum Mn concentration may be a protective reaction to avoid producing rapid and excessive ROS. The assumption can be consistent with the fact that IBD is a chronic disease. Experimenting to test the changes in serum Mn concentration with time is valuable. These exposure may increase the risk of IBD through oxidative stress and other mechanism in the gut. It is necessary to come up with the hypotheses to sort out the contradiction.
Arsenic
Arsenic (As) is an essential and poisonous substance commonly found in contaminated soils and water (134). It is also rich in fish and marine mollusks (135). The roots of crops and vegetables also contain high-concentration As (118).
According to World Health Organization, the permissible limit for As in drinking water is 10 μg/L (136). And if the exposure dose is over 50 μg/L, it can lead to gastrointestinal tract dysfunction and multiple organ disorder (136–139). Long-term exposure or high ingestion doses may increase the accumulated As in the gut (140).
Mounting evidence has revealed some intrinsic connections between As exposure and IBD. It enters the body through dietary intake, and metabolites to arsenic trioxide (ATO) in the gut, which is toxic to the gut microenvironment (112). It not only induces intestinal damage and liver inflammatory cell infiltration, but also reduces gut microbiota diversity (112, 141). In ATO exposure, the expression of intestinal barrier-related proteins, such as Claudin-1, MUC2, ZO-1, and occludin, significantly decreases, resulting in increased intestinal permeability (142, 143). However, with exposure to inorganic As, the expression of Claudin-1 reduces, resulting in increased permeability and intestinal barrier disruption. ATO can also activate inflammasome NLRP3, and induce a cascade effect of the LPS/TLR4/NF-κB signaling pathway, which exacerbates the inflammatory severity (112). However, ATO can inhibit NF-κB expression, increase procaspase-3, and induce caspase-3 activation leading to apoptosis to eliminate inflamed cells, which indicates the anti-inflammatory effect of ATO. Another study shows that ATO exposure can alleviate the inflammatory extent in DSS-induced IBD mice by increasing catalase and GSH levels to enhance antioxidants (144). In the epidemiological study, the level of serum As concentration is higher in CD patients compared to healthy adults (121). The causes for the distinct results may include the various metabolism of As in different species, exposure to the diverse form of As (inorganic As or ATO), the various regulation approaches, and the dose of As.
Cadmium
Cadmium (Cd) is mainly used as an anticorrosion agent, and it naturally occurs in ores (93). Cd can enter the human body through contaminated food and water via the gastrointestinal tract, inhalation, and dermal tissues (145). Food is the most important source of Cd exposure in the general non-smoking population, which indicates the risk of dietary exposure. Recent studies have found that Cd is highly enriched in some aquatic animals like zebrafish and crabs. Ingestion of Cd is highly related to gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain. Meanwhile, its chronic exposure can increase risks concerning multiple organ dysfunction, bone deformation, and contribute to cancer cell progression (146).
Cd exposure can also significantly affect the gut microenvironment. It can perturb the diversity and abundance of gut microflora, especially decreasing the number of Firmicutes and γ-proteobacteria (145). In addition, Cd exposure also elevates the level of TNF-α, IFN-γ, and IL-17 in the colon (147). Additionally, Cd exposure can increase intestinal permeability through decreasing mRNA expressions of ZO-1, ZO-2, occludin, and claudin-1 in the jejunum and colon, accompanied by intestinal histological changes (148).
However, research also indicates the potential protective role of Cd. Cd may interfere with LPS signaling, particularly disrupting macrophage inflammation by inhibiting the NF-κB pathway in the gut, inhibiting the pro-inflammatory effect of M1 macrophage (149). Short-term exposure to Cd exacerbates the symptoms of acute DSS- and TNBS-induced colitis, while sub-chronic exposure to Cd significantly alleviates some symptoms in DSS-induced colitis and reduces the severity of colitis in a dose-dependent manner. Its potential mechanisms include reversible reduction in epithelial permeability, stimulation of anti-oxidant pathways, upregulation of oxidative defense mechanism, and downregulation of Nf-κB and pro-inflammatory cytokine pathways (113). Moreover, the study also implies that the outcomes of Cd exposure may vary as a function of dose and exposure time. Along with other common heavy metals like Mn, As, and Pb, the Cd concentration is markedly higher in CD patients (121). Thus, further studies concerning Cd exposure relationship with IBD are needed to clear out the Cd dose, exposure time, and the synthetic effect of Cd regulating in different pathways.
Mercury
Mercury (Hg) is a well-known component in medical apparatus like thermometers and other medical instruments (93). The absorptivity of Hg is 8–15% in the gastrointestinal tract (104). Human's primary exposure to Hg is dietary intake (125, 150–152).
Hg can accumulate in untreated wastewater from factory and agricultural runoff, which directly contaminates the crops and fish. Correspondingly, methyl mercury, a chemical substance converted from Hg, is discovered to be highly enriched in vegetables and fish, which implies the ability of Hg to accumulate in the food chain.
The toxicity of Hg can induce multiple organ failures when the dose of Hg exceeds 10 μg/L in blood or 20 μg/L in urine, such as lung injury, intestinal damage, proteinuria, allergies, and chronic poisoning.
Exposure to Hg is closely associated with IBD. Methyl mercury can accumulate in organs, and change the composition of gut microbes (116). Moreover, dietary exposure to Hg affects the growth of mice, partly due to changed gut microbiota (114). Mice exposed to Hg have a decreased abundance of Bacteroidetes and Proteobacteria, and an increased abundance of Clostridium, Lactobacillus, Treponema, and Helicobacter in the gut (115). The toxicity of Hg may also contribute to the development of IBD (115). It can directly break the calcium homeostasis and activate multiple enzymes by affecting the electron transport chains in mitochondria, producing superfluous reactive oxygen species (ROS) (115, 153, 154). Besides, ROS promotes mitosis, polyploid aberration, and susceptibility to DNA damage in the gut (155–157). Epidemiological studies have also shown altered enzyme activity in people exposed to Hg (29).
In conclusion, diet exposure is the common exposure route for heavy metals. Along with the food chain, heavy metals enter the human body, generating adverse health effects by various mechanisms. Gut injury caused by heavy metals is highly associated with the occurrence and development of IBD. Heavy metals can alter the gut microbiota by increasing some flora and decreasing other flora, then causing gut dysbiosis. In addition, some uncommon impacts include damaged intestinal barrier function, increased levels of inflammation cytokines, oxidative stress, etc. Meanwhile, other organ dysfunction can occur due to heavy metals exposure and accumulation. As heavy metals tend to accumulate in fish (107), people with a disease or hypo-immunity should reduce their eating frequency. And IBD patients should avoid dietary exposure to heavy metals. For relevant authorities, they should supervise factories' proper treatment of sewage and sludge to reduce heavy metals accumulation in crops (158–160). Bioremediation can also work by changing pollutants into food and energy (161, 162). Some novel ways also focus on dealing with heavy metal contamination, such as Particle Capture Systems, soil displacement/isolation, and Soil-flow-electrode capacitive deionization.
Persistent organic pollutants
Persistent organic pollutants (POPs) are chemicals of global concern with the potential to persist in the environment. They can bio-accumulate and bio-magnify in ecosystems and threaten human health (71). POPs mainly include new pesticides, chemicals, and by-products of industrial production, which may lead to multiple effects on immune response and alter gut function (Table 5).
Table 5.
Adverse health effects of POPs in inflammatory bowel disease.
| Study | References | Experiment model/human group | Exposure time/dose | Route of exposure | Source of dietary intake | Impact on the gut | Other health risks |
|---|---|---|---|---|---|---|---|
| New pesticide | |||||||
| Chlorpyrifos (CPF) | Huang et al. (163) | Eight-week-old DSS-induced male C57BL/6 mice | AIN-93 diet at doses of 1, 2.5, or 5 mg/kg/day CPF,6 days | Diet exposure | Artificial feeding | ° Affect immune-cell populations °↑Inflammatory responses | ° Lead to severe tissue injury ° Exert adverse effect on the gut microenvironment |
| Imidacloprid (IMI) | Luo et al. (164) | Adult male zebrafish | IMI (100 and 1,000 μg/L) in water solution, 3 weeks | Water surroundings | Not mentioned | °↑Superoxide dismutase and catalase (CAT) levels °↑LPS levels and inflammatory factors | ° Cause intestinal barrier injury, oxidative stress, inflammatory response and gut microbiota dysbiosis |
| Fu et al. (165) | The Pacific white shrimp L. vannamei | IMI (50, 100, 200, 400, and 800 μg/L) in water solution, 28 days | Water surroundings | Not mentioned | ° Reshape the structure and interaction of gut microbiota °↑Gut pathogenic microbiota abundance ° Function disorders | °↓Growth performance ° Gause tissue damage in shrimp ° Cause disorder of differential gene expression | |
| Chemicals and by-products of industrial production | |||||||
| TCDD, TCDF, and PCBs | Tian et al. (166) | Cecal contents isolated from 7-week-old C57BL/6 J male wild type mice | TCDD (0.6, 0.06 μM), TCDF and PCBs (6, 0.6 μM), 37°C for 4 h | Direct contact: incubation | / | °↓Metabolic activity (dose-dependent) °↑Low nucleic acid (LNA) bacteria °↓High nucleic acid (HNA) bacteria | ° Alter transcriptional and metabolic pathways in cecal bacterial mixtures ° Affect the physiological metabolism of individual bacteria |
| PBDD/F | Fernandes and Falandysz (167) | / | Some groups, particularly young children, may exceed the tolerable limit (2 pg TEQ/kg bw/week) | Present not only in soil, but also in plant foliage; Accumulate in the food chain | Mainly from dietary intakes: plant-based foods contain more PBDD/Fs | / | ° Bind to the AhR, having carcinogenesis, immunotoxicity, enzyme induction and reproductive effects |
| TCDD | Li et al. (168) | C57BL/6 mice, 8–10 weeks old | 0.1 and 10 μg/kg bw TCDD on embryonic day 0.5, ED 12.5, and post-natal 7 days | Oral gavage | Dissolved in dimethyl sulfoxide (DMSO) and diluted in olive oil | ° Affect the structure and composition of the colonic microbiota ° Do not change the community diversity and richness ° Change the functional pathways of the colonic microbiota | / |
“↑” means increased level or concentration; “↓” means decreased level or concentration.
New pesticide
New pesticide is widely used to wipe out indoor and outdoor pests, such as imidacloprid, pyrethroids, and β-ketonitrile derivatives (169). It harms humans by taking the contaminated food and water (170), and causing intoxication through its accumulation in the food chain (171). Its exposure mainly includes the intake of vegetables, fruits, and grains. Among these, pesticide can residue more easily in grains. A food survey of Swedish adolescents showed that secondary school students who consumed grains had a higher exposure to pesticides than those who consumed vegetables and fruits, lending support to the findings (172).
Emerging links between pesticide residue and changes in the gut have emerged in recent years (173). Exposure to low-dose pesticide in diet seldom causes immediate health effect. Long-term exposure to chemicals in the pesticide can induce gut microbiota dysbiosis, alter the immune response in the gut, and contribute to the development of IBD (174, 175). Research on dietary exposure to chlorpyrifos, a widely used pesticide, suggests that dietary exposure can affect the population of immune-cell, induce inflammatory responses, and lead to severe tissue injury in DSS-induced colitis mice.
Another popular pesticide, imidacloprid (IMI), also adversely affects the gut microbiota (164, 165). IMI exposure can induce intestinal injury and oxidative stress in the gut of zebrafish (164). Additionally, it also results in a higher intestinal LPS level and the overexpression of inflammatory factors in the gut, as well as a rising level of the biochemical responses, transcriptome, and gut microbiota in the Pacific white shrimp. Human exposure to IMI is also observed in recent years. Currently, the maximum estimated daily intake of IMI [34.8 μg/kg bw/d] was lower than the chronic reference dose of IMI (57 μg/kg-bw/d) recommended by the United States Environmental Protection Agency (176). But IMI is already reported to have adverse effects on human semen quality parameters and the activation of macrophages in the body, which may increase the permeability of the intestine and impair the immune system (177, 178).
Chemicals and by-products of industrial production
Another type of POPs is chemicals and by-products derivate from industrial production (179). These pollutants include Polychlorinated biphenyls (PCBs), Polybrominated dibenzo-p-dioxins and furans (PBDD/Fs), 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), etc. (167). They can accumulate in the environment and exert a long-term adverse effect on human health (180). The food chain and the food web are the primary pathways of human exposure (180). Human exposure to them in diets mainly includes ingesting contaminated food, like fruits, vegetables, and grains, and eating polluted meat, milk, eggs, and fish, which is closely associated with IBD.
Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are synthetic organochlorine chemicals, which are mixtures of 209 different components (181). And PCBs are among the 12 initial POPs listed under the Stockholm Convention.
PCBs are mainly formed as by-products in manufacturing industries (182). They can reserve in soil and transfer to water surroundings, increasing the risk of human exposure via food chains (183, 184). Since PCBs are lipid-soluble, people who frequently eat animal fats can easily access PCBs (185). Contaminated meat and milk also show high concentrations of PCBs (186), and aquatic product consumption also increases the risk of PCBs exposure (181).
PCBs play a pro-inflammatory role in various diseases, which adversely affect IBD. They can induce oxidative stress by uncoupling CYP1A1 dose-dependently, and disrupt the normal endothelial barrier function (110, 187). Meanwhile, PCBs induce proinflammatory factors like IL-6 and vascular adhesion molecules such as VCAM-1, and then activate the NF-κB pathway (187–189). The expression of these molecules facilitates the recognition and migration of leukocytes, which are critical events of inflammatory responses (187). When exposed to PCBs, hosts can show disorders of gut microbiota, with reduced gut microbial diversity and variety (190, 191). In mice exposed to PCBs, the amount of Bacteroidales, Erysipelotrichales, Lactobacillales, Bifidobacteriales, Phyla Proteobacteria, Actinobacteria, Saccharibacteria, Deferribacteres, Firmicutes, and Verrucomicrobia increases significantly, while the level of Bacteroidetes decreases. Research in humans also shows that exposure to PCBs may interfere with the DNA hypomethylation of peripheral blood monocytes, inducing chronic inflammation (192).
PBDD/Fs and TCDD
Apart from PCBs, other POPs such as PBDD/Fs and TCDD are also related to IBD (193). These organics are by-products of industry, which are classified into unintentional POPs (194). Their primary exposure pathway is dietary intake.
Plant-based food is reported to show higher PBDD/F, and the overly dietary intakes of PBDD/F suggest some population groups, particularly young children, will exceed the tolerable weekly intake (2 pg TEQ/kg bw/week) (167). The metabolic mechanism of PBDD includes causing oxidative stress, apoptosis, and cell damage. It can induce gut inflammation and dysbiosis of the gut microenvironment, leading to IBD development.
TCDD is the most potent chemical carcinogen evaluated by the US Environmental Protection Agency (195). It has a long half-life of 5–10 years in humans, due to its high lipophilicity and low metabolism (195). Most TCDD released into the atmosphere eventually settles onto the plant, soil, and water surfaces. After being taken, it accumulates in blood serum and adipose tissue, which leads to further damage in the body (195). An animal experiment showed that maternal exposure to TCDD suppresses the differentiation of Type 3 innate lymphoid cells (ILC3s) in the offspring, and distinctly affects colonic ILC3 function (196). Since ILC3s play a significant role in the mucosal immune response in the pathogenesis of IBD, there is a close connection between TCDD exposure and the occurrence and development of IBD (197). Moreover, TCDD can impact the gut microbiota and metabolic pathways, such as upregulating harmful bacteria and downregulating beneficial bacteria (168).
In sum, people are exposed to POPs primarily through dietary intake. POPs can alter transcriptional and metabolic pathways in cecal bacterial mixtures, modify gut microbiota-host homeostasis, and affect the metabolism of individual bacteria in vitro (166). POPs can also alter the microbial community structure and metabolic activities, leading to host disorders (198). Some POPs can increase the amount of Proteus and proportion of Firmicutes/Bacteroidetes, and increase the synthesis of short-chain fatty acid, which is related to the inflammatory changes of IBD (191). Epidemiological studies also present the adverse health effects of POPs. The interplays between POPs and gut microbiota lead to intestinal inflammatory changes and resultant toxicity (198). That is to say, the variations in the microbial communities partially indicate the body's exposure to these pollutants. Therefore, it is essential to formulate some daily dietary interventions such as prebiotics, probiotics, or symbiotics, which could impede or alleviate detrimental impacts induced by exposure to POPs.
Conclusion
Mounting evidence of the underlying hazards of emerging contaminant exposures in IBD arouses increasing attention. However, the correlation of timing and frequency of such contaminant exposures to IBD incidence and disease phenotype was not specified. Environmental exposure may contribute to the pathogenesis of IBD through diet intake and the metabolic mechanism in the body. The currently accepted pathogenesis of IBD includes the interplay between genetic susceptibility and environmental factors, as well as the gut microbiome and the immune system.
The further relationship between these emerging contaminants and the risk of IBD deserves everyone's attention. Currently, these contaminants mainly exert long-term adverse effects by accumulating in the body and inducing chronic inflammation. Research in this field should focus more on the direct relationship and mechanism of these contaminants and the health of the human gut. And more epidemiological research about new emerging contaminants and their adverse health effects is needed.
In this review, we have summarized the standard ways of exposure and inclusion of emerging contaminants, and their adverse effects on IBD patients through various underlying mechanisms (Figure 2). Exposure to these pollutants will increase the risk of IBD in healthy individuals. And people with IBD should pay special attention to preventing daily exposure to these contaminants, as they may cause adverse health effects regardless of age and exposure time. Exposure to these new emerging contaminants may increase the risk of IBD and accelerate the process of IBD. Therefore, understanding the role of these contaminants, including how they enter the body, how they induce immune-related reactions, and how they affect certain inflammatory diseases like IBD, will enable the more comprehensive formation of policies concerning the prevention and control, and will reduce medical expenses and burdens on the families and countries.
Figure 2.
The main ECs and their impacts on gut microenvironment. ECs damage the intestine by regulating the intensity of immune response, releasing proinflammatory factors, impairing the intestinal barrier, and increasing the intestinal permeability through various mechanism. The figure illustrates the potential mechanism of five ECs mentioned in the review. ECs, Emerging contaminants; EDCs, Endocrine-disrupting chemicals; POPs, Persistent organic pollutants; SCFA, Short-chain fatty acid; TLR4, Toll-like receptors 4; ILC-3, Innate lymphoid cells; VCAM 1, Vascular cell adhesion molecule 1.
Author contributions
XC: conceptualization, design, draft writing, and writing—review and editing. SW: design, draft writing, review, and writing—review and editing. XM: draft writing, review, and visualization. XX, AZ, YM, XY, and SP: methodology and review. SY: review and visualization. JC: methodology and writing—review and editing. XW: conceptualization, funding acquisition, and writing—review and editing. MD: conceptualization, design, methodology, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81970494) and Key Project of Research and Development Plan of Hunan Province (2019SK2041).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Kaplan GG. The global burden of Ibd: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. (2015) 12:720–7. 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. (2007) 369:1627–40. 10.1016/S0140-6736(07)60750-8 [DOI] [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. (2011) 474:307–17. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn's disease. Gut. (2012) 61:622–9. 10.1136/gutjnl-2011-301397 [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Feuerstein JD, Binion DG, Tremaine WJ. Aga technical review on the management of mild-to-moderate ulcerative colitis. Gastroenterology. (2019) 156:769–808.e29. 10.1053/j.gastro.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. (2019) 157:647–59.e4. 10.1053/j.gastro.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Bilal M, Adeel M, Rasheed T, Zhao Y, Iqbal HMN. Emerging contaminants of high concern and their enzyme-assisted biodegradation - a review. Environ Int. (2019) 124:336–53. 10.1016/j.envint.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 8.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, et al. The lancet commission on pollution and health. Lancet. (2018) 391:462–512. 10.1016/s0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- 9.Ryu H, Li B, De Guise S, McCutcheon J, Lei Y. Recent progress in the detection of emerging contaminants Pfass. J Hazard Mater. (2021) 408:124437. 10.1016/j.jhazmat.2020.124437 [DOI] [PubMed] [Google Scholar]
- 10.Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, et al. Lost at sea: where is all the plastic? Science. (2004) 304:838. 10.1126/science.1094559 [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Duan H, Yu Y, Zhang Q, Zhao J, Zhang H, et al. Dose-dependent effects of chronic lead toxicity in vivo: focusing on trace elements and gut microbiota. Chemosphere. (2022) 301:134670. 10.1016/j.chemosphere.2022.134670 [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Chen Z, Chen Y, Yang F, Yao W, Xie Y. Microplastics and nanoplastics: emerging contaminants in food. J Agric Food Chem. (2021) 69:10450–68. 10.1021/acs.jafc.1c04199 [DOI] [PubMed] [Google Scholar]
- 13.Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. (2021) 12:724989. 10.3389/fendo.2021.724989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzfischer M, Niechcial A, Lee SS, Sinnet B, Wawrzyniak M, Laimbacher A, et al. Ingested nano- and microsized polystyrene particles surpass the intestinal barrier and accumulate in the body. Nanoimpact. (2022) 25:100374. 10.1016/j.impact.2021.100374 [DOI] [PubMed] [Google Scholar]
- 15.Luo T, Wang D, Zhao Y, Li X, Yang G, Jin Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci Total Environ. (2022) 844:156884. 10.1016/j.scitotenv.2022.156884 [DOI] [PubMed] [Google Scholar]
- 16.Zheng H, Wang J, Wei X, Chang L, Liu S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci Total Environ. (2021) 750:143085. 10.1016/j.scitotenv.2020.143085 [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. (2018) 631–2:449–58. 10.1016/j.scitotenv.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 18.Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. (2020) 244:125492. 10.1016/j.chemosphere.2019.125492 [DOI] [PubMed] [Google Scholar]
- 19.Schwabl P, Koppel S, Konigshofer P, Bucsics T, Trauner M, Reiberger T, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. (2019) 171:453–7. 10.7326/M19-0618 [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Li YB, He HR, Zhang JF, Ma GS. You are what you eat: microplastics in the feces of young men living in Beijing. Sci Total Environ. (2021) 767:144345. 10.1016/j.scitotenv.2020.144345 [DOI] [PubMed] [Google Scholar]
- 21.Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. (2020) 17:57. 10.1186/s12989-020-00387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu K, Yang Y, Zuo J, Tian W, Wang Y, Duan X, et al. Emerging microplastics in the environment: properties, distributions, and impacts. Chemosphere. (2022) 297:134118. 10.1016/j.chemosphere.2022.134118 [DOI] [PubMed] [Google Scholar]
- 23.Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. Estimation of the mass of microplastics ingested - a pivotal first step towards human health risk assessment. J Hazard Mater. (2021) 404(Pt B):124004. 10.1016/j.jhazmat.2020.124004 [DOI] [PubMed] [Google Scholar]
- 24.Kwon JH, Kim JW, Pham TD, Tarafdar A, Hong S, Chun SH, et al. Microplastics in food: a review on analytical methods and challenges. Int J Environ Res Public Health. (2020) 17:6710. 10.3390/ijerph17186710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. (2022) 163:107199. 10.1016/j.envint.2022.107199 [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Liu J, Xiong F, Xu K, Pu Y, Huang J, et al. Research advances of microplastics and potential health risks of microplastics on terrestrial higher mammals: a bibliometric analysis and literature review. Environ Geochem Health. (2023):1–36. 10.1007/s10653-022-01458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. (2020) 702:134455. 10.1016/j.scitotenv.2019.134455 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal KC, Vinayak VK, Ganguly NK, Kumar M, Chhuttani PN. Ecological effects of production of biogas from human excreta on the enteric pathogens. Indian J Med Res. (1978) 67:737–43. [PubMed] [Google Scholar]
- 29.Atis S, Tutluoglu B, Levent E, Ozturk C, Tunaci A, Sahin K, et al. The respiratory effects of occupational polypropylene flock exposure. Eur Respir J. (2005) 25:110–7. 10.1183/09031936.04.00138403 [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. (2016) 50:4054–60. 10.1021/acs.est.6b00183 [DOI] [PubMed] [Google Scholar]
- 31.Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. (2013) 10:3886–907. 10.3390/ijerph10093886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, et al. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol. (2018) 195:49–57. 10.1016/j.aquatox.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 33.Canesi L, Ciacci C, Bergami E, Monopoli MP, Dawson KA, Papa S, et al. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve mytilus. Mar Environ Res. (2015) 111:34–40. 10.1016/j.marenvres.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 34.Forte M, Iachetta G, Tussellino M, Carotenuto R, Prisco M, De Falco M, et al. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol In Vitro. (2016) 31:126–36. 10.1016/j.tiv.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 35.Powell JJ, Thoree V, Pele LC. Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br J Nutr. (2007) 98(Suppl. 1):S59–63. 10.1017/S0007114507832922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. (2014) 5:215–9. 10.4161/gmic.27251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu D, Chen Q-L, An X-L, Yang X-R, Christie P, Ke X, et al. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol Biochem. (2018) 116:302–10. 10.1016/j.soilbio.2017.10.027 [DOI] [Google Scholar]
- 38.Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. (2010) 34:J226–33. 10.1016/j.jaut.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 39.Tang Y, Han Y, Zhang W, Yu Y, Huang L, Zhou W, et al. Bisphenol a and microplastics weaken the antimicrobial ability of blood clams by disrupting humoral immune responses and suppressing hemocyte chemotactic activity. Environ Pollut. (2022) 307:119497. 10.1016/j.envpol.2022.119497 [DOI] [PubMed] [Google Scholar]
- 40.Yan Z, Liu Y, Zhang T, Zhang F, Ren H, Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. (2022) 56:414–21. 10.1021/acs.est.1c03924 [DOI] [PubMed] [Google Scholar]
- 41.Feakins R, Torres J, Borralho-Nunes P, Burisch J, Cúrdia Gonçalves T, De Ridder L, et al. Ecco topical review on clinicopathological spectrum and differential diagnosis of inflammatory bowel disease. J Crohns Colitis. (2022) 16:343–68. 10.1093/ecco-jcc/jjab141 [DOI] [PubMed] [Google Scholar]
- 42.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environmental Sci Technol. (2017) 51:6634–47. 10.1021/acs.est.7b00423 [DOI] [PubMed] [Google Scholar]
- 43.Xie S, Zhang R, Li Z, Liu C, Chen Y, Yu Q. Microplastics perturb colonic epithelial homeostasis associated with intestinal overproliferation, exacerbating the severity of colitis. Environ Res. (2023) 217:114861. 10.1016/j.envres.2022.114861 [DOI] [PubMed] [Google Scholar]
- 44.Qiao R, Sheng C, Lu Y, Zhang Y, Ren H, Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci Total Environ. (2019) 662:246–53. 10.1016/j.scitotenv.2019.01.245 [DOI] [PubMed] [Google Scholar]
- 45.Fackelmann G, Sommer S. Microplastics and the gut microbiome: how chronically exposed species may suffer from gut dysbiosis. Mar Pollut Bull. (2019) 143:193–203. 10.1016/j.marpolbul.2019.04.030 [DOI] [PubMed] [Google Scholar]
- 46.Kang HM, Byeon E, Jeong H, Kim MS, Chen Q, Lee JS. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka oryzias melastigma. J Hazard Mater. (2021) 405:124207. 10.1016/j.jhazmat.2020.124207 [DOI] [PubMed] [Google Scholar]
- 47.Yin K, Wang D, Zhao H, Wang Y, Zhang Y, Liu Y, et al. Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environ Pollut. (2022) 307:119449. 10.1016/j.envpol.2022.119449 [DOI] [PubMed] [Google Scholar]
- 48.Huang Z, Weng Y, Shen Q, Zhao Y, Jin Y. Microplastic: a potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci Total Environ. (2021) 785:147365. 10.1016/j.scitotenv.2021.147365 [DOI] [PubMed] [Google Scholar]
- 49.Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. (2019) 236:124334. 10.1016/j.chemosphere.2019.07.065 [DOI] [PubMed] [Google Scholar]
- 50.Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. (2013) 69:42–51. 10.1016/j.phrs.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 51.The Lancet Oncology . Endocrine disruptors—the lessons (not) learned. Lancet Oncol. (2021) 22:1483. 10.1016/S1470-2045(21)00597-0 [DOI] [PubMed] [Google Scholar]
- 52.Casas M, Gascon M. Prenatal exposure to endocrine-disrupting chemicals and asthma and allergic diseases. J Investig Allergol Clin Immunol. (2020) 30:215–28. 10.18176/jiaci.0580 [DOI] [PubMed] [Google Scholar]
- 53.Arbuckle TE, Agarwal A, MacPherson SH, Fraser WD, Sathyanarayana S, Ramsay T, et al. Prenatal exposure to phthalates and phenols and infant endocrine-sensitive outcomes: the mirec study. Environ Int. (2018) 120:572–83. 10.1016/j.envint.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 54.Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Prakash A, et al. Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front Public Health. (2020) 8:553850. 10.3389/fpubh.2020.553850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. (2020) 21:127–47. 10.1007/s11154-019-09521-z [DOI] [PubMed] [Google Scholar]
- 56.Ismanto A, Hadibarata T, Kristanti RA, Maslukah L, Safinatunnajah N, Kusumastuti W. Endocrine disrupting chemicals (Edcs) in environmental matrices: occurrence, fate, health impact, physio-chemical and bioremediation technology. Environ Pollut. (2022) 302:119061. 10.1016/j.envpol.2022.119061 [DOI] [PubMed] [Google Scholar]
- 57.Malaise Y, Lencina C, Placide F, Bacquie V, Cartier C, Olier M, et al. Oral exposure to bisphenols induced food intolerance and colitis in vivo by modulating immune response in adult mice. Food Chem Toxicol. (2020) 146:111773. 10.1016/j.fct.2020.111773 [DOI] [PubMed] [Google Scholar]
- 58.Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, et al. Effects of exposure to bisphenol a and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. (2016) 7:471–85. 10.1080/19490976.2016.1234657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai KP, Chung YT, Li R, Wan HT, Wong CK. Bisphenol a alters gut microbiome: comparative metagenomics analysis. Environ Pollut. (2016) 218:923–30. 10.1016/j.envpol.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 60.Yin F, Huang X, Lin X, Chan TF, Lai KP, Li R. Analyzing the synergistic adverse effects of bpa and its substitute, bhpf, on ulcerative colitis through comparative metabolomics. Chemosphere. (2022) 287(Pt 2):132160. 10.1016/j.chemosphere.2021.132160 [DOI] [PubMed] [Google Scholar]
- 61.Diamante G, Cely I, Zamora Z, Ding J, Blencowe M, Lang J, et al. Systems toxicogenomics of prenatal low-dose bpa exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ Int. (2021) 146:106260. 10.1016/j.envint.2020.106260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linares R, Fernandez MF, Gutierrez A, Garcia-Villalba R, Suarez B, Zapater P, et al. Endocrine disruption in crohn's disease: bisphenol a enhances systemic inflammatory response in patients with gut barrier translocation of dysbiotic microbiota products. FASEB J. (2021) 35:e21697. 10.1096/fj.202100481R [DOI] [PubMed] [Google Scholar]
- 63.Huang C, Wang Y, Lin X, Chan TF, Lai KP, Li R. Uncovering the functions of plasma proteins in ulcerative colitis and identifying biomarkers for bpa-induced severe ulcerative colitis: a plasma proteome analysis. Ecotoxicol Environ Saf. (2022) 242:113897. 10.1016/j.ecoenv.2022.113897 [DOI] [PubMed] [Google Scholar]
- 64.Xu Y, Li Y, Scott K, Lindh CH, Jakobsson K, Fletcher T, et al. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ Res. (2020) 181:108923. 10.1016/j.envres.2019.108923 [DOI] [PubMed] [Google Scholar]
- 65.Lochhead P, Khalili H, Ananthakrishnan AN, Burke KE, Richter JM, Sun Q, et al. Plasma concentrations of perfluoroalkyl substances and risk of inflammatory bowel diseases in women: a nested case control analysis in the nurses' health study cohorts. Environ Res. (2022) 207:112222. 10.1016/j.envres.2021.112222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braun JM. Early-life exposure to edcs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. (2017) 13:161–73. 10.1038/nrendo.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Silva PS, Yang X, Korzenik JR, Goldman RH, Arheart KL, Caban-Martinez AJ. Association of urinary phenolic compounds, inflammatory bowel disease and chronic diarrheal symptoms: evidence from the national health and nutrition examination survey. Environ Pollut. (2017) 229:621–6. 10.1016/j.envpol.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 68.Lopardo L, Petrie B, Proctor K, Youdan J, Barden R, Kasprzyk-Hordern B. Estimation of community-wide exposure to bisphenol a via water fingerprinting. Environ Int. (2019) 125:1–8. 10.1016/j.envint.2018.12.048 [DOI] [PubMed] [Google Scholar]
- 69.Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, et al. Bisphenol analogues other than bpa: environmental occurrence, human exposure, and toxicity-a review. Environ Sci Technol. (2016) 50:5438–53. 10.1021/acs.est.5b05387 [DOI] [PubMed] [Google Scholar]
- 70.Sokal A, Jarmakiewicz-Czaja S, Tabarkiewicz J, Filip R. Dietary intake of endocrine disrupting substances presents in environment and their impact on thyroid function. Nutrients. (2021) 13:867. 10.3390/nu13030867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casas G, Martinez-Varela A, Vila-Costa M, Jimenez B, Dachs J. Rain amplification of persistent organic pollutants. Environ Sci Technol. (2021) 55:12961–72. 10.1021/acs.est.1c03295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyohemba RL, Pillay L, Humphries MS. Bioaccumulation of current-use herbicides in fish from a global biodiversity hotspot: Lake St Lucia, South Africa. Chemosphere. (2021) 284:131407. 10.1016/j.chemosphere.2021.131407 [DOI] [PubMed] [Google Scholar]
- 73.Tang Q, Tang J, Ren X, Li C. Glyphosate exposure induces inflammatory responses in the small intestine and alters gut microbial composition in rats. Environ Pollut. (2020) 261:114129. 10.1016/j.envpol.2020.114129 [DOI] [PubMed] [Google Scholar]
- 74.Suppa A, Kvist J, Li X, Dhandapani V, Almulla H, Tian AY, et al. Roundup causes embryonic development failure and alters metabolic pathways and gut microbiota functionality in non-target species. Microbiome. (2020) 8:170. 10.1186/s40168-020-00943-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding W, Shangguan Y, Zhu Y, Sultan Y, Feng Y, Zhang B, et al. Negative impacts of microcystin-Lr and glyphosate on zebrafish intestine: Linked with gut microbiota and micrornas? Environ Pollut. (2021) 286:117685. 10.1016/j.envpol.2021.117685 [DOI] [PubMed] [Google Scholar]
- 76.Tu P, Gao B, Chi L, Lai Y, Bian X, Ru H, et al. Subchronic low-dose 2,4-D exposure changed plasma acylcarnitine levels and induced gut microbiome perturbations in mice. Sci Rep. (2019) 9:4363. 10.1038/s41598-019-40776-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mesnage R, Brandsma I, Moelijker N, Zhang G, Antoniou MN. Genotoxicity Evaluation of 2,4-D, dicamba and glyphosate alone or in combination with cell reporter assays for DNA damage, oxidative stress and unfolded protein response. Food Chem Toxicol. (2021) 157:112601. 10.1016/j.fct.2021.112601 [DOI] [PubMed] [Google Scholar]
- 78.Sanmarco LM, Chao CC, Wang YC, Kenison JE, Li Z, Rone JM, et al. Identification of environmental factors that promote intestinal inflammation. Nature (2022) 611:801–9. 10.1038/s41586-022-05308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Bruggen AHC, He MM, Shin K, Mai V, Jeong KC, Finckh MR, et al. Environmental and health effects of the herbicide glyphosate. Sci Total Environ. (2018) 616–7:255–68. 10.1016/j.scitotenv.2017.10.309 [DOI] [PubMed] [Google Scholar]
- 80.Supe Tulcan RX, Ouyang W, Gu X, Lin C, Tysklind M, Wang B. Typical herbicide residues, trophic transfer, bioconcentration, and health risk of marine organisms. Environ Int. (2021) 152:106500. 10.1016/j.envint.2021.106500 [DOI] [PubMed] [Google Scholar]
- 81.Fagan J, Bohlen L, Patton S, Klein K. Organic diet intervention significantly reduces urinary glyphosate levels in U.S. children and adults. Environ Res. (2020) 189:109898. 10.1016/j.envres.2020.109898 [DOI] [PubMed] [Google Scholar]
- 82.Belsey NA, Cordery SF, Bunge AL, Guy RH. Assessment of dermal exposure to pesticide residues during re-entry. Environ Sci Technol. (2011) 45:4609–15. 10.1021/es200172q [DOI] [PubMed] [Google Scholar]
- 83.Kruger M, Shehata AA, Schrodl W, Rodloff A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on clostridium botulinum. Anaerobe. (2013) 20:74–8. 10.1016/j.anaerobe.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 84.Zhou C, Luo X, Chen N, Zhang L, Gao J. C–P natural products as next-generation herbicides: chemistry and biology of glufosinate. J Agric Food Chem. (2020) 68:3344–53. 10.1021/acs.jafc.0c00052 [DOI] [PubMed] [Google Scholar]
- 85.Sandermann H. Plant biotechnology: ecological case studies on herbicide resistance. Trends Plant Sci. (2006) 11:324–8. 10.1016/j.tplants.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 86.Matich EK, Laryea JA, Seely KA, Stahr S, Su LJ, Hsu PC. Association between pesticide exposure and colorectal cancer risk and incidence: a systematic review. Ecotoxicol Environ Saf. (2021) 219:112327. 10.1016/j.ecoenv.2021.112327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu S, Fu H, Zhou R, Yang Z, Bai G, Shi B. Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotoxicol Environ Saf. (2020) 187:109846. 10.1016/j.ecoenv.2019.109846 [DOI] [PubMed] [Google Scholar]
- 88.Samsel A, Seneff S. Glyphosate, pathways to modern diseases III: manganese, neurological diseases, and associated pathologies. Surg Neurol Int. (2015) 6:45. 10.4103/2152-7806.153876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Del Castilo I, Neumann AS, Lemos FS, De Bastiani MA, Oliveira FL, Zimmer ER, et al. Lifelong exposure to a low-dose of the glyphosate-based herbicide roundup((R)) causes intestinal damage, gut dysbiosis, and behavioral changes in mice. Int J Mol Sci. (2022) 23:5583. 10.3390/ijms23105583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nielsen LN, Roager HM, Casas ME, Frandsen HL, Gosewinkel U, Bester K, et al. Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environ Pollut. (2018) 233:364–76. 10.1016/j.envpol.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 91.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. (2019) 569:655–62. 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCoin CS, Knotts TA, Adams SH. Acylcarnitines–old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol. (2015) 11:617–25. 10.1038/nrendo.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Järup L. Hazards of heavy metal contamination. Br Med Bull. (2003) 68:167–82. 10.1093/bmb/ldg032 [DOI] [PubMed] [Google Scholar]
- 94.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. (2012) 101:133–64. 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dietler D, Babu M, Cissé G, Halage AA, Malambala E, Fuhrimann S. Daily variation of heavy metal contamination and its potential sources along the major urban wastewater channel in Kampala, Uganda. Environ Monit Assess. (2019) 191:52. 10.1007/s10661-018-7175-4 [DOI] [PubMed] [Google Scholar]
- 96.Shifaw E. Review of heavy metals pollution in china in agricultural and urban soils. J Health Pollut. (2018) 8:180607. 10.5696/2156-9614-8.18.180607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar V, Sharma A, Kaur P, Singh Sidhu GP, Bali AS, Bhardwaj R, et al. Pollution assessment of heavy metals in soils of india and ecological risk assessment: a state-of-the-art. Chemosphere. (2019) 216:449–62. 10.1016/j.chemosphere.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 98.Shammi SA, Salam A, Khan MAH. Assessment of heavy metal pollution in the agricultural soils, plants, and in the atmospheric particulate matter of a suburban industrial region in Dhaka, Bangladesh. Environ Monit Assess. (2021) 193:104. 10.1007/s10661-021-08848-y [DOI] [PubMed] [Google Scholar]
- 99.Suvarapu LN, Baek SO. Determination of heavy metals in the ambient atmosphere. Toxicol Ind Health. (2017) 33:79–96. 10.1177/0748233716654827 [DOI] [PubMed] [Google Scholar]
- 100.Sall ML, Diaw AKD, Gningue-Sall D, Efremova Aaron S, Aaron JJ. Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res Int. (2020) 27:29927–42. 10.1007/s11356-020-09354-3 [DOI] [PubMed] [Google Scholar]
- 101.He ZL, Yang XE, Stoffella PJ. Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol. (2005) 19:125–40. 10.1016/j.jtemb.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 102.Shallari S, Schwartz C, Hasko A, Morel JL. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci Total Environ. (1998) 209:133–42. 10.1016/S0048-9697(98)80104-6 [DOI] [PubMed] [Google Scholar]
- 103.Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. (2020) 6:e04691. 10.1016/j.heliyon.2020.e04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witkowska D, Słowik J, Chilicka K. Heavy metals and human health: possible exposure pathways and the competition for protein binding sites. Molecules. (2021) 26:6060. 10.3390/molecules26196060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye F, Li X, Li F, Li J, Chang W, Yuan J, et al. Cyclosporin a protects against lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology. (2016) 57:203–13. 10.1016/j.neuro.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 106.Ladizinski B, Mistry N, Kundu RV. Widespread use of toxic skin lightening compounds: medical and psychosocial aspects. Dermatol Clin. (2011) 29:111–23. 10.1016/j.det.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 107.Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between eralpha and C-Jun. Mol Endocrinol. (2010) 24:981–92. 10.1210/me.2009-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Copan L, Fowles J, Barreau T, McGee N. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int J Environ Res Public Health. (2015) 12:10943–54. 10.3390/ijerph120910943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia J, Lu L, Jin C, Wang S, Zhou J, Ni Y, et al. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. (2018) 209:1–8. 10.1016/j.cbpc.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 110.Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, et al. Polychlorinated biphenyls disrupt intestinal integrity via nadph oxidase-induced alterations of tight junction protein expression. Environ Health Perspect. (2010) 118:976–81. 10.1289/ehp.0901751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitchell J, Kim SJ, Howe C, Lee S, Her JY, Patel M, et al. Chronic intestinal inflammation suppresses brain activity by inducing neuroinflammation in mice. Am J Pathol. (2022) 192:72–86. 10.1016/j.ajpath.2021.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhong G, Wan F, Lan J, Jiang X, Wu S, Pan J, et al. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci Total Environ. (2021) 788:147780. 10.1016/j.scitotenv.2021.147780 [DOI] [PubMed] [Google Scholar]
- 113.Breton J, Daniel C, Vignal C, Body-Malapel M, Garat A, Plé C, et al. Does oral exposure to cadmium and lead mediate susceptibility to colitis? the dark-and-bright sides of heavy metals in gut ecology. Sci Rep. (2016) 6:19200. 10.1038/srep19200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y, Zhou C, Wu C, Guo X, Hu G, Wu Q, et al. Subchronic oral mercury caused intestinal injury and changed gut microbiota in mice. Sci Total Environ. (2020) 721:137639. 10.1016/j.scitotenv.2020.137639 [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y, Zhou C, Guo X, Hu G, Li G, Zhuang Y, et al. Exposed to mercury-induced oxidative stress, changes of intestinal microflora, and association between them in mice. Biol Trace Elem Res. (2021) 199:1900–7. 10.1007/s12011-020-02300-x [DOI] [PubMed] [Google Scholar]
- 116.Seki N, Akiyama M, Yamakawa H, Hase K, Kumagai Y, Kim YG. Adverse effects of methylmercury on gut bacteria and accelerated accumulation of mercury in organs due to disruption of gut microbiota. J Toxicol Sci. (2021) 46:91–7. 10.2131/jts.46.91 [DOI] [PubMed] [Google Scholar]
- 117.Pratush A, Kumar A, Hu Z. Adverse effect of heavy metals (as, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int Microbiol. (2018) 21:97–106. 10.1007/s10123-018-0012-3 [DOI] [PubMed] [Google Scholar]
- 118.Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int. (2019) 125:365–85. 10.1016/j.envint.2019.01.067 [DOI] [PubMed] [Google Scholar]
- 119.Zhou F, Yin G, Gao Y, Ouyang L, Liu S, Jia Q, et al. Insights into cognitive deficits caused by low-dose toxic heavy metal mixtures and their remediation through a postnatal enriched environment in rats. J Hazard Mater. (2020) 388:122081. 10.1016/j.jhazmat.2020.122081 [DOI] [PubMed] [Google Scholar]
- 120.Annabi Berrahal A, Nehdi A, Hajjaji N, Gharbi N, El-Fazaa S. Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol. (2007) 330:581–8. 10.1016/j.crvi.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 121.Stojsavljević A, Sokić-Milutinović A, Rovčanin B, Tončev L, Manojlović D. Profiling of circulatory elements reveals alteration of essential and toxic trace metals in Crohn's disease. Biol Trace Elem Res. (2022) 200:2572–80. 10.1007/s12011-021-02862-4 [DOI] [PubMed] [Google Scholar]
- 122.Ogasawara H, Hayasaka M, Maemoto A, Furukawa S, Ito T, Kimura O, et al. Levels of major and trace metals in the scalp hair of Crohn's disease patients: Correlations among transition metals. Biometals. (2021) 34:197–210. 10.1007/s10534-020-00272-y [DOI] [PubMed] [Google Scholar]
- 123.Breton J, Daniel C, Dewulf J, Pothion S, Froux N, Sauty M, et al. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol Lett. (2013) 222:132–8. 10.1016/j.toxlet.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 124.Chi L, Xue J, Tu P, Lai Y, Ru H, Lu K. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Arch Toxicol. (2019) 93:25–35. 10.1007/s00204-018-2332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cordier S, Grasmick C, Paquier-Passelaigue M, Mandereau L, Weber JP, Jouan M. Mercury exposure in french guiana: levels and determinants. Arch Environ Health. (1998) 53:299–303. 10.1080/00039899809605712 [DOI] [PubMed] [Google Scholar]
- 126.O'Neal SL, Zheng W. Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep. (2015) 2:315–28. 10.1007/s40572-015-0056-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martins AC, Krum BN, Queirós L, Tinkov AA, Skalny AV, Bowman AB, et al. Manganese in the diet: bioaccessibility, adequate intake, and neurotoxicological effects. J Agric Food Chem. (2020) 68:12893–903. 10.1021/acs.jafc.0c00641 [DOI] [PubMed] [Google Scholar]
- 128.Stochel-Gaudyn A, Fyderek K, Kościelniak P. Serum trace elements profile in the pediatric inflammatory bowel disease progress evaluation. J Trace Elem Med Biol. (2019) 55:121–6. 10.1016/j.jtemb.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 129.Cho JM, Yang HR. Hair mineral and trace element contents as reliable markers of nutritional status compared to serum levels of these elements in children newly diagnosed with inflammatory bowel disease. Biol Trace Elem Res. (2018) 185:20–9. 10.1007/s12011-017-1225-6 [DOI] [PubMed] [Google Scholar]
- 130.Song Y, Liu Y, Teo HY, Hanafi ZB, Mei Y, Zhu Y, et al. Manganese enhances the antitumor function of Cd8(+) T cells by inducing type I interferon production. Cell Mol Immunol. (2021) 18:1571–4. 10.1038/s41423-020-00524-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen G, Yu Y, Fu X, Wang G, Wang Z, Wu X, et al. Microfluidic encapsulated manganese organic frameworks as enzyme mimetics for inflammatory bowel disease treatment. J Colloid Interface Sci. (2022) 607(Pt 2):1382–90. 10.1016/j.jcis.2021.09.016 [DOI] [PubMed] [Google Scholar]
- 132.Qiu H, Gong H, Bao Y, Jiang H, Tong W. Reactive oxygen species-scavenging hollow Mno(2) nanozymes as carriers to deliver budesonide for synergistic inflammatory bowel disease Therapy. Biomater Sci. (2022) 10:457–66. 10.1039/D1BM01525G [DOI] [PubMed] [Google Scholar]
- 133.Li W, Liu Z, Liu C, Guan Y, Ren J, Qu X. Manganese dioxide nanozymes as responsive cytoprotective shells for individual living cell encapsulation. Angew Chem Int Ed Engl. (2017) 56:13661–5. 10.1002/anie.201706910 [DOI] [PubMed] [Google Scholar]
- 134.Missimer TM, Teaf CM, Beeson WT, Maliva RG, Woolschlager J, Covert DJ. Natural background and anthropogenic arsenic enrichment in florida soils, surface water, and groundwater: a review with a discussion on public health risk. Int J Environ Res Public Health. (2018) 15:2278. 10.3390/ijerph15102278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. (2011) 123:305–32. 10.1093/toxsci/kfr184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. (2018) 119:157–84. 10.1002/jcb.26234 [DOI] [PubMed] [Google Scholar]
- 137.Wang C-H, Hsiao CK, Chen C-L, Hsu L-I, Chiou H-Y, Chen S-Y, et al. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol. (2007) 222:315–26. 10.1016/j.taap.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 138.Civantos DP, López Rodríguez A, Aguado-Borruey JM, Narvaez JA. Fulminant malignant arrythmia and multiorgan failure in acute arsenic poisoning. Chest. (1995) 108:1774–5. 10.1378/chest.108.6.1774-a [DOI] [PubMed] [Google Scholar]
- 139.Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the health effects of arsenic longitudinal study (heals) in Bangladesh. Toxicol Appl Pharmacol. (2009) 239:184–92. 10.1016/j.taap.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palma-Lara I, Martinez-Castillo M, Quintana-Perez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limon OL, et al. Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol. (2020) 110:104539. 10.1016/j.yrtph.2019.104539 [DOI] [PubMed] [Google Scholar]
- 141.Chiocchetti GM, Domene A, Kühl AA, Zúñiga M, Vélez D, Devesa V, et al. In vivo evaluation of the effect of arsenite on the intestinal epithelium and associated microbiota in mice. Arch Toxicol. (2019) 93:2127–39. 10.1007/s00204-019-02510-w [DOI] [PubMed] [Google Scholar]
- 142.Slifer ZM, Blikslager AT. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int J Mol Sci. (2020) 21:972. 10.3390/ijms21030972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie S-Z, Liu B, Ye H-Y, Li Q-M, Pan L-H, Zha X-Q, et al. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polym. (2019) 206:149–62. 10.1016/j.carbpol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 144.Moulahoum H, Boumaza BMA, Ferrat M, Bounaama A, Djerdjouri B. Arsenic trioxide ameliorates murine colon inflammation through inflammatory cell enzymatic modulation. Naunyn Schmiedebergs Arch Pharmacol. (2019) 392:259–70. 10.1007/s00210-018-1578-1 [DOI] [PubMed] [Google Scholar]
- 145.Zhang S, Jin Y, Zeng Z, Liu Z, Fu Z. Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem Res Toxicol. (2015) 28:2000–9. 10.1021/acs.chemrestox.5b00237 [DOI] [PubMed] [Google Scholar]
- 146.Junejo SH, Baig JA, Kazi TG, Afridi HI. Cadmium and lead hazardous impact assessment of pond fish species. Biol Trace Elem Res. (2019) 191:502–11. 10.1007/s12011-018-1628-z [DOI] [PubMed] [Google Scholar]
- 147.Ninkov M, Popov Aleksandrov A, Demenesku J, Mirkov I, Mileusnic D, Petrovic A, et al. Toxicity of oral cadmium intake: impact on gut immunity. Toxicol Lett. (2015) 237:89–99. 10.1016/j.toxlet.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 148.Zhai Q, Tian F, Zhao J, Zhang H, Narbad A, Chen W. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl Environ Microbiol. (2016) 82:4429–40. 10.1128/AEM.00695-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cox JN, Rahman MA, Bao S, Liu M, Wheeler SE, Knoell DL. Cadmium attenuates the macrophage response to lps through inhibition of the Nf-Kb pathway. Am J Physiol Lung Cell Mol Physiol. (2016) 311:L754–65. 10.1152/ajplung.00022.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. (2006) 36:609–62. 10.1080/10408440600845619 [DOI] [PubMed] [Google Scholar]
- 151.Larose C, Canuel R, Lucotte M, Di Giulio RT. Toxicological effects of methylmercury on walleye (Sander vitreus) and perch (Perca flavescens) from lakes of the boreal forest. Comp Biochem Physiol C Toxicol Pharmacol. (2008) 147:139–49. 10.1016/j.cbpc.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 152.Webb J. Use of the ecosystem approach to population health: the case of mercury contamination in aquatic environments and Riparian populations, Andean Amazon, Napo River Valley, Ecuador. Can J Public Health. (2005) 96:44–6. 10.1007/BF03404015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, et al. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of Akt/Nrf2 and Nf-Kb pathways. Food Chem Toxicol. (2018) 113:296–302. 10.1016/j.fct.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 154.Ye F, Li X, Li L, Lyu L, Yuan J, Chen J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in Sh-Sy5y cells. Food Chem Toxicol. (2015) 86:191–201. 10.1016/j.fct.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 155.Vieira HC, Bordalo MD, Rodrigues ACM, Pires SFS, Rocha RJM, Soares A, et al. Water temperature modulates mercury accumulation and oxidative stress status of common goby (Pomatoschistus microps). Environ Res. (2021) 193:110585. 10.1016/j.envres.2020.110585 [DOI] [PubMed] [Google Scholar]
- 156.Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharmacol. (2008) 233:92–9. 10.1016/j.taap.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 157.Soares FA, Farina M, Santos FW, Souza D, Rocha JB, Nogueira CW. Interaction between metals and chelating agents affects glutamate binding on brain synaptic membranes. Neurochem Res. (2003) 28:1859–65. 10.1023/a:1026175825871 [DOI] [PubMed] [Google Scholar]
- 158.Duan C, Fang L, Yang C, Chen W, Cui Y, Li S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol Environ Saf. (2018) 156:106–15. 10.1016/j.ecoenv.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 159.Singh RP, Agrawal M. Potential benefits and risks of land application of sewage sludge. Waste Manag. (2008) 28:347–58. 10.1016/j.wasman.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 160.Pathak A, Dastidar MG, Sreekrishnan TR. Bioleaching of heavy metals from sewage sludge: a review. J Environ Manag. (2009) 90:2343–53. 10.1016/j.jenvman.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 161.Achal V, Pan X, Fu Q, Zhang D. Biomineralization based remediation of as(III) contaminated soil by sporosarcina ginsengisoli. J Hazard Mater. (2012) 201–2:178–84. 10.1016/j.jhazmat.2011.11.067 [DOI] [PubMed] [Google Scholar]
- 162.Li M, Cheng X, Guo H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad. (2013) 76:81–5. 10.1016/j.ibiod.2012.06.01630669299 [DOI] [Google Scholar]
- 163.Huang HM, Pai MH, Liu JJ, Yeh SL, Hou YC. Effects of dietary exposure to chlorpyrifos on immune cell populations and inflammatory responses in mice with dextran sulfate sodium-induced colitis. Food Chem Toxicol. (2019) 131:110596. 10.1016/j.fct.2019.110596 [DOI] [PubMed] [Google Scholar]
- 164.Luo T, Wang X, Jin Y. Low concentrations of imidacloprid exposure induced gut toxicity in adult zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol. (2021) 241:108972. 10.1016/j.cbpc.2020.108972 [DOI] [PubMed] [Google Scholar]
- 165.Fu Z, Han F, Huang K, Zhang J, Qin JG, Chen L, et al. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the pacific white shrimp Litopenaeus vannamei. J Hazard Mater. (2022) 424(Pt B):127513. 10.1016/j.jhazmat.2021.127513 [DOI] [PubMed] [Google Scholar]
- 166.Tian Y, Gui W, Rimal B, Koo I, Smith PB, Nichols RG, et al. Metabolic impact of persistent organic pollutants on gut microbiota. Gut Microbes. (2020) 12:1–16. 10.1080/19490976.2020.1848209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fernandes AR, Falandysz J. Polybrominated dibenzo-P-dioxins and furans (Pbdd/Fs): contamination in food, humans and dietary exposure. Sci Total Environ. (2021) 761:143191. 10.1016/j.scitotenv.2020.143191 [DOI] [PubMed] [Google Scholar]
- 168.Li J, Li Y, Sha R, Zheng L, Xu L, Xie HQ, et al. Effects of perinatal tcdd exposure on colonic microbiota and metabolism in offspring and mother mice. Sci Total Environ. (2022) 832:154762. 10.1016/j.scitotenv.2022.154762 [DOI] [PubMed] [Google Scholar]
- 169.Ge M, Wang X, Yang G, Wang Z, Li Z, Zhang X, et al. Persistent organic pollutants (pops) in deep-sea sediments of the tropical western pacific ocean. Chemosphere. (2021) 277:130267. 10.1016/j.chemosphere.2021.130267 [DOI] [PubMed] [Google Scholar]
- 170.El-Nahhal Y, El-Nahhal I. Cardiotoxicity of some pesticides and their amelioration. Environ Sci Pollut Res Int. (2021) 28:44726–54. 10.1007/s11356-021-14999-9 [DOI] [PubMed] [Google Scholar]
- 171.Knauer K, Homazava N, Junghans M, Werner I. The influence of particles on bioavailability and toxicity of pesticides in surface water. Integr Environ Assess Manag. (2017) 13:585–600. 10.1002/ieam.1867 [DOI] [PubMed] [Google Scholar]
- 172.Noren E, Lindh C, Rylander L, Glynn A, Axelsson J, Littorin M, et al. Concentrations and temporal trends in pesticide biomarkers in urine of swedish adolescents, 2000–2017. J Expo Sci Environ Epidemiol. (2020) 30:756–67. 10.1038/s41370-020-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hurtado-Barroso S, Tresserra-Rimbau A, Vallverdu-Queralt A, Lamuela-Raventos RM. Organic food and the impact on human health. Crit Rev Food Sci Nutr. (2019) 59:704–14. 10.1080/10408398.2017.1394815 [DOI] [PubMed] [Google Scholar]
- 174.Yuan X, Pan Z, Jin C, Ni Y, Fu Z, Jin Y. Gut microbiota: an underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere. (2019) 227:425–34. 10.1016/j.chemosphere.2019.04.088 [DOI] [PubMed] [Google Scholar]
- 175.Liang Y, Zhan J, Liu D, Luo M, Han J, Liu X, et al. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. (2019) 7:19. 10.1186/s40168-019-0635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mahai G, Wan Y, Xia W, Wang A, Qian X, Li Y, et al. Exposure assessment of neonicotinoid insecticides and their metabolites in Chinese women during pregnancy: a longitudinal study. Sci Total Environ. (2022) 818:151806. 10.1016/j.scitotenv.2021.151806 [DOI] [PubMed] [Google Scholar]
- 177.Wang A, Wan Y, Zhou L, Xia W, Guo Y, Mahai G, et al. Neonicotinoid insecticide metabolites in seminal plasma: associations with semen quality. Sci Total Environ. (2022) 811:151407. 10.1016/j.scitotenv.2021.151407 [DOI] [PubMed] [Google Scholar]
- 178.Walderdorff L, Laval-Gilly P, Wechtler L, Bonnefoy A, Falla-Angel J. Phagocytic activity of human macrophages and drosophila hemocytes after exposure to the neonicotinoid imidacloprid. Pestic Biochem Physiol. (2019) 160:95–101. 10.1016/j.pestbp.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 179.Jones KC. Persistent Organic Pollutants (Pops) and Related Chemicals in the Global Environment: Some Personal Reflections. Environ Sci Technol. (2021) 55:9400–12. 10.1021/acs.est.0c08093 [DOI] [PubMed] [Google Scholar]
- 180.Castro-Jimenez J, Banaru D, Chen CT, Jimenez B, Munoz-Arnanz J, Deviller G, et al. Persistent organic pollutants burden, trophic magnification and risk in a pelagic food web from coastal Nw Mediterranean Sea. Environ Sci Technol. (2021) 55:9557–68. 10.1021/acs.est.1c00904 [DOI] [PubMed] [Google Scholar]
- 181.Carpenter DO. Polychlorinated biphenyls (Pcbs): routes of exposure and effects on human health. Rev Environ Health. (2006) 21:1–23. 10.1515/REVEH.2006.21.1.1 [DOI] [PubMed] [Google Scholar]
- 182.Mao S, Liu S, Zhou Y, An Q, Zhou X, Mao Z, et al. The occurrence and sources of polychlorinated biphenyls (Pcbs) in agricultural soils across China with an emphasis on unintentionally produced Pcbs. Environ Pollut. (2021) 271:116171. 10.1016/j.envpol.2020.116171 [DOI] [PubMed] [Google Scholar]
- 183.Ravanipour M, Nabipour I, Yunesian M, Rastkari N, Mahvi AH. Exposure Sources of polychlorinated biphenyls (Pcbs) and health risk assessment: a systematic review in Iran. Environ Sci Pollut Res Int. (2022) 29:55437–56. 10.1007/s11356-022-21274-y [DOI] [PubMed] [Google Scholar]
- 184.Lü H, Cai Q-Y, Jones KC, Zeng Q-Y, Katsoyiannis A. Levels of organic pollutants in vegetables and human exposure through diet: a review. Crit Rev Environ Sci Technol. (2013) 44:1–33. 10.1080/10643389.2012.710428 [DOI] [Google Scholar]
- 185.Wang S-L, Tsai P-C, Yang C-Y, Leon Guo Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the yucheng cohort. Diabetes Care. (2008) 31:1574–9. 10.2337/dc07-2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fernández-González R, Yebra-Pimentel I, Martínez-Carballo E, Simal-Gándara J. A critical review about human exposure to polychlorinated dibenzo-P-dioxins (Pcdds), polychlorinated dibenzofurans (pcdfs) and polychlorinated biphenyls (Pcbs) through foods. Crit Rev Food Sci Nutr. (2015) 55:1590–617. 10.1080/10408398.2012.710279 [DOI] [PubMed] [Google Scholar]
- 187.Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, et al. Proinflammatory properties of coplanar Pcbs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. (2002) 181:174–83. 10.1006/taap.2002.9408 [DOI] [PubMed] [Google Scholar]
- 188.Leijs M, Fietkau K, Merk HF, Schettgen T, Kraus T, Esser A. Upregulation of Ccl7, Ccl20, Cxcl2, Il-1β, Il-6 and Mmp-9 in skin samples of Pcb exposed individuals-a preliminary study. Int J Environ Res Public Health. (2021) 18:9711. 10.3390/ijerph18189711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leijs MM, Esser A, Amann PM, Schettgen T, Heise R, Fietkau K, et al. Expression of Cyp1a1, Cyp1b1 and Il-1β in Pbmcs and skin samples of Pcb exposed individuals. Sci Total Environ. (2018) 642:1429–38. 10.1016/j.scitotenv.2018.06.136 [DOI] [PubMed] [Google Scholar]
- 190.Min L, Chi Y, Dong S. Gut microbiota health closely associates with Pcb153-derived risk of host diseases. Ecotoxicol Environ Saf. (2020) 203:111041. 10.1016/j.ecoenv.2020.111041 [DOI] [PubMed] [Google Scholar]
- 191.Rude KM, Pusceddu MM, Keogh CE, Sladek JA, Rabasa G, Miller EN, et al. Developmental exposure to polychlorinated biphenyls (Pcbs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ Pollut. (2019) 253:708–21. 10.1016/j.envpol.2019.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vidali MS, Dailianis S, Vlastos D, Georgiadis P. Pcb cause global DNA hypomethylation of human peripheral blood monocytes in vitro. Environ Toxicol Pharmacol. (2021) 87:103696. 10.1016/j.etap.2021.103696 [DOI] [PubMed] [Google Scholar]
- 193.Patrizi B, Siciliani de Cumis M. Tcdd toxicity mediated by epigenetic mechanisms. Int J Mol Sci. (2018) 19:4101. 10.3390/ijms19124101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Popli S, Badgujar PC, Agarwal T, Bhushan B, Mishra V. Persistent organic pollutants in foods, their interplay with gut microbiota and resultant toxicity. Sci Total Environ. (2022) 832:155084. 10.1016/j.scitotenv.2022.155084 [DOI] [PubMed] [Google Scholar]
- 195.Sorg O, Zennegg M, Schmid P, Fedosyuk R, Valikhnovskyi R, Gaide O, et al. 2,3,7,8-tetrachlorodibenzo-P-dioxin (Tcdd) poisoning in victor yushchenko: identification and measurement of Tcdd metabolites. Lancet. (2009) 374:1179–85. 10.1016/S0140-6736(09)60912-0 [DOI] [PubMed] [Google Scholar]
- 196.Li Y, Xie HQ, Zhang W, Wei Y, Sha R, Xu L, et al. Type 3 innate lymphoid cells are altered in colons of C57bl/6 mice with dioxin exposure. Sci Total Environ. (2019) 662:639–45. 10.1016/j.scitotenv.2019.01.139 [DOI] [PubMed] [Google Scholar]
- 197.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. (2015) 21:698–708. 10.1038/nm.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jin Y, Wu S, Zeng Z, Fu Z. Effects of environmental pollutants on gut microbiota. Environ Pollut. (2017) 222:1–9. 10.1016/j.envpol.2016.11.045 [DOI] [PubMed] [Google Scholar]