Abstract
ZUMA-3 is a phase 1/2 study evaluating KTE-X19, an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). We report the phase 1 results. After fludarabine-cyclophosphamide lymphodepletion, patients received a single infusion of KTE-X19 at 2 × 106, 1 × 106, or 0.5 × 106 cells per kg. The rate of dose-limiting toxicities (DLTs) within 28 days after KTE-X19 infusion was the primary end point. KTE-X19 was manufactured for 54 enrolled patients and administered to 45 (median age, 46 years; range, 18-77 years). No DLTs occurred in the DLT-evaluable cohort. Grade ≥3 cytokine release syndrome (CRS) and neurologic events (NEs) occurred in 31% and 38% of patients, respectively. To optimize the risk-benefit ratio, revised adverse event (AE) management for CRS and NEs (earlier steroid use for NEs and tocilizumab only for CRS) was evaluated at 1 × 106 cells per kg KTE-X19. In the 9 patients treated under revised AE management, 33% had grade 3 CRS and 11% had grade 3 NEs, with no grade 4 or 5 NEs. The overall complete remission rate correlated with CAR T-cell expansion and was 83% in patients treated with 1 × 106 cells per kg and 69% in all patients. Minimal residual disease was undetectable in all responding patients. At a median follow-up of 22.1 months (range, 7.1-36.1 months), the median duration of remission was 17.6 months (95% confidence interval [CI], 5.8-17.6 months) in patients treated with 1 × 106 cells per kg and 14.5 months (95% CI, 5.8-18.1 months) in all patients. KTE-X19 treatment provided a high response rate and tolerable safety in adults with R/R B-ALL. Phase 2 is ongoing at 1 × 106 cells per kg with revised AE management. This trial is registered at www.clinicaltrials.gov as #NCT02614066.
Key Points
-
•
KTE-X19 at 1 × 106 CAR T cells per kg had high manufacturing success and manageable safety that further improved with revised AE management.
-
•
KTE-X19 resulted in high complete remission rates and undetectable residual disease; median response duration (1 × 106 dose) was 18 months.
Introduction
Most adults with B-cell acute lymphoblastic leukemia (B-ALL) respond to initial therapy, but 40% to 50% eventually relapse, after which the 5-year overall survival (OS) rate is 10%.1, 2 Novel immunotherapeutic agents have improved outcomes relative to conventional chemotherapy, yet the median OS in adult relapsed/refractory (R/R) B-ALL is only 6.1 to 7.7 months with blinatumomab3, 4 and 7.7 months with inotuzumab ozogamicin,5 highlighting the need for more effective treatments.
B-ALL cells typically express CD19, and chimeric antigen receptor (CAR) T-cell therapies targeting CD19 are a promising treatment approach in R/R B-ALL.6 An anti-CD19 CAR T-cell therapy containing a CD3ζ and CD28 costimulatory domain developed at the National Cancer Institute7, 8 demonstrated an overall remission rate (ORR) of 70% after a median 10-month follow-up in a phase 1 trial in children and adults age 30 years or younger with R/R B-ALL.9 A similar CAR construct evaluated in a phase 1 trial in adults with R/R B-ALL provided an 83% complete remission (CR) rate and a median 12.9-month OS at a median 29-month follow-up.10 These studies highlight the therapeutic activity of anti-CD19 CAR T-cell therapies with a CD3ζ and CD28 costimulatory domain in adult R/R B-ALL.
The presence of leukemic blasts in peripheral blood may limit the number of T cells available for manufacturing CAR T-cell products, potentially leading to manufacturing failure.11 KTE-X19 is a CAR T-cell product that includes a CD3ζ and CD28 costimulatory domain and a manufacturing process that removes malignant cells.11, 12 This removal reduces the likelihood of activation and exhaustion of anti-CD19 CAR T cells during ex vivo manufacturing.12 KTE-X19 was manufactured at a centralized facility, with coordinated leukapheresis and shipping from multiple centers across the United States and a fast turnaround time, which are critical for patients with rapidly proliferative disease and high tumor burden.13, 14 Here, we report phase 1 results of the phase 1/2, multicenter, single-arm, open-label ZUMA-3 study evaluating the safety and efficacy of KTE-X19 in adults with R/R B-ALL.
Methods
Patients
Eligible patients were age ≥18 with R/R B-ALL, defined as refractory to first-line therapy (ie, primary refractory), relapse ≤12 months after first remission, R/R after ≥2 previous lines of systemic therapy, or relapsed after allogeneic stem cell transplantation (SCT). Patients were required to have ≥5% bone marrow (BM) blasts, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate renal, hepatic, and cardiac function (supplemental Methods, available on the Blood Web site). The first 6 patients enrolled were required to have ≥25% blasts in their BM. For patients who received previous treatment with blinatumomab, leukemic blasts with CD19 expression ≥90% were required. Patients with Philadelphia chromosome–positive (Ph+) disease, concomitant extramedullary disease, central nervous system 2 (CNS-2) disease without neurologic changes, or Down syndrome were eligible.
Study design and treatment
Phase 1 of ZUMA-3 was conducted at 19 sites in the United States (supplemental Methods). The Institutional Review Board at each study site approved the protocol. All patients provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
The phase 1 objective was to evaluate the safety of KTE-X19 and determine the optimal phase 2 dose based on the incidence of dose-limiting toxicities (DLTs) and overall safety profile. DLTs were defined as KTE-X19–related adverse events (AEs) occurring within the first 28 days after KTE-X19 infusion, including grade 3 nonhematologic AEs lasting >7 days, grade 4 nonhematologic AEs regardless of duration except for prespecified expected events (eg, tumor lysis syndrome), and grade 4 hematologic AEs lasting >30 days, except lymphopenia (supplemental Table 1). DLTs were evaluated in the first 3 patients dosed at the starting dose of 2 × 106 CAR T cells per kg (supplemental Figure 1). A safety review team (SRT) reviewed safety data after these patients were observed for 28 days after infusion. On the basis of the incidence of DLTs, additional patients were enrolled at the same dose to assess overall safety, but were not evaluable for DLTs (supplemental Methods). On the basis of SRT review of overall safety data, subsequent patients received 1 × 106 or 0.5 × 106 CAR T cells per kg. At 0.5 × 106 CAR T cells per kg, 2 formulations were explored (68 or 40 mL). To mitigate risk of cytokine release syndrome (CRS) and neurologic events (NEs), AE management guidelines were revised to limit tocilizumab to the treatment of CRS (and not isolated neurotoxicity) and to initiate corticosteroid treatment at grade 2 rather than grade 3 NEs (supplemental Table 2). Revised AE management guidelines were implemented in additional patients treated with 1 × 106 CAR T cells per kg. The SRT reviewed safety and efficacy data on an ongoing basis and made recommendations regarding study conduct and the recommended phase 2 dose according to the protocol and SRT charter.
Patients underwent leukapheresis at enrollment, and predefined bridging chemotherapy (supplemental Table 3) was recommended after leukapheresis, particularly for patients with high disease burden at baseline (>25% leukemic blasts in BM or ≥1000 blasts/mm3 in peripheral circulation by local review), after which a BM aspirate was required by day −4. After ≥7 days or 5 half-lives (if shorter) washout from bridging chemotherapy, patients received a lymphodepleting regimen of intravenous fludarabine 25 mg/m2 per day on days −4, −3, and −2, and intravenous cyclophosphamide 900 mg/m2 per day on day −2. On day 0, a single KTE-X19 infusion was administered.
Outcomes and assessments
The primary phase 1 end point was the incidence of DLTs in DLT-evaluable patients. Secondary end points included safety, investigator-assessed ORR (CR + CR with incomplete hematologic recovery [CRi]; supplemental Table 4), duration of remission (DOR), relapse-free survival, OS, and rate of undetectable minimal residual disease (MRD) in BM. Levels of CAR T cells and cytokines in blood were exploratory end points. AEs, including symptoms of CRS and NEs, were graded per the Common Terminology Criteria for Adverse Events (version 4.03). CRS was graded according to the criteria of Lee et al.15 For patients with extramedullary disease, response was assessed according to the response criteria for extramedullary and CNS disease in the revised International Working Group Criteria for malignant lymphoma (supplemental Table 5).16 Undetectable MRD, defined as <1 leukemia cell per 10 000 viable cells, was centrally assessed using flow cytometry (NeoGenomics, Fort Myers, FL).17, 18, 19 Biomarker analyses were performed on blood samples to evaluate predictive pharmacokinetics and pharmacodynamic markers for KTE-X19 as described previously (supplemental Methods).20
Hospitalization after infusion was required for ≥7 days. Patients were evaluated at days 14 and 28 and months 2 and 3 by physical examinations, vital signs measurements, and neurologic and laboratory assessments. BM evaluations and response assessments were conducted at days 7 to 14 (optional) and 28 and months 2 and 3. For patients who underwent SCT after KTE-X19 infusion, BM evaluation was not required during the first 100 days after SCT. Collection and analysis of cerebrospinal fluid was required to confirm CR for patients with baseline CNS-2 disease. Patients completing assessments at month 3 after treatment were observed for survival and disease status every 3 months through month 18, every 6 months during months 24 to 60, and annually for up to 15 years. Patients achieving CR could receive a second KTE-X19 infusion if they progressed >3 months after treatment, provided CD19 expression was retained, and neutralizing antibodies against the CAR were not suspected.
Statistical analysis
The DLT-evaluable cohort included the first 3 patients treated at the 2 × 106 CAR T cells per kg dose level. Safety and efficacy analyses included all patients treated with any dose of KTE-X19. Kaplan-Meier estimates and 2-sided 95% confidence intervals (CIs) were generated for time-to-event end points. DOR was defined as time from CR to relapse or death without documented relapse. The DOR for patients who underwent allogeneic SCT while in remission was censored at the date of transplantation. OS was defined as time from KTE-X19 infusion to date of death as a result of any cause. Data are presented as of 1 April 2019. All statistical analyses were done in SAS (version 9.4).
Results
Patients
Between 9 March 2016 and 12 July 2018, 54 patients were enrolled and underwent leukapheresis in phase 1 (supplemental Figure 2). KTE-X19 was successfully manufactured for all 54 patients; 1 patient required 2 leukapheresis procedures, and 1 patient required 3 procedures for product manufacturing. The median time from leukapheresis to delivery of KTE-X19 to the study site was 15 days (interquartile range [IQR], 14-16 days) and from leukapheresis to KTE-X19 infusion was 27 days (IQR, 22-35 days). Five patients discontinued the trial before lymphodepletion because of AEs (n = 3; supplemental Figure 2), withdrawal of consent (n = 1), or ineligibility after leukapheresis (n = 1). Four additional patients discontinued after lymphodepletion; 3 received no KTE-X19 because of grade 4 sepsis (n = 1), initiation of new therapy (n = 1), or death as a result of grade 5 sepsis (n = 1); and one discontinued before infusion because of deep vein thrombosis (an exclusion criterion) but received KTE-X19 under compassionate use. Forty-five (83%) of 54 patients received KTE-X19 at 2 × 106 (n = 6), 1 × 106 (n = 23), or 0.5 × 106 CAR T cells per kg (n = 16). Nine of 23 patients in the 1 × 106 CAR T cells per kg cohort were treated under revised AE management guidelines which required earlier use of steroids for NEs and reserving tocilizumab only for treating CRS. Forty-four patients received their target dose of KTE-X19; 1 patient enrolled to receive 1 × 106 CAR T cells per kg with revised AE management and was treated with 0.5 × 106 cells per kg but was included in the analysis at the 1 × 106 dose level (supplemental Methods).
The median age of all treated patients was 46 years (range, 18-77 years), and 67% received ≥3 previous lines of therapy (Table 1). Before enrollment, 16 patients (36%) were primary refractory, 13 (29%) relapsed after SCT, and 21 (47%) received previous blinatumomab. Blinatumomab was the last therapy used before study entry in 8 patients (18%), only 1 of whom achieved a response (CR) to blinatumomab. Forty-three treated patients (96%) received bridging therapy per protocol. High disease burden after bridging therapy was confirmed across patients enrolled at different dose levels and different AE management guidelines. Although median levels of BM blasts at preconditioning after bridging therapy seemed higher in patients enrolled at 1 × 106 cells per kg under original vs revised AE management, the difference was not significant (supplemental Table 6).
Table 1.
Patient baseline characteristics
| Characteristic | Value |
|---|---|
| Total no. of patients | 45 |
| Median age (range), y | 46 (18–77) |
| Male sex | 22 (49) |
| ECOG performance status | |
| 0 | 15 (33) |
| 1 | 29 (64) |
| Missing | 1 (2) |
| Philadelphia chromosome–positive | 8 (18) |
| Extramedullary disease | 4 (9) |
| CNS disease at screening | |
| CNS-1 | 42 (93) |
| CNS-2 | 3 (7) |
| Previous regimens | |
| 1 | 6 (13) |
| 2 | 9 (20) |
| ≥3 | 30 (67) |
| Prior blinatumomab | 21 (47) |
| Prior inotuzumab ozogamicin | 6 (13) |
| Refractory | |
| Primary refractory | 16 (36) |
| First relapse with remission ≤12 months | 2 (4) |
| R/R after allogeneic SCT | 13 (29) |
| Median percentage of BM blasts (range) | |
| At screening | 61 (5-100) |
| At preconditioning after bridging | 70 (0-97) |
Data are presented as n (%) unless otherwise specified.
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
Safety
No DLTs were observed among the DLT-evaluable set (n = 3). Ninety-eight percent of patients experienced grade ≥3 AEs (Table 2). The most common AEs of any grade were pyrexia (89%), hypotension (69%), diarrhea (42%), and chills (42%). Common grade ≥3 AEs (≥20% of patients) were pyrexia (42%), hypotension (40%), decreased platelet count (33%), anemia (31%), hypophosphatemia (31%), hypoxia (24%), encephalopathy (22%), febrile neutropenia (22%), and decreased neutrophil count (22%). Serious AEs of any grade occurred in 84% of patients.
Table 2.
AEs
| AE | Dose level | All patients (N = 45) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 × 106 (n = 6) | 1 × 106 (n = 23) | 0.5 × 106 (n = 16) | ||||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any AE | 6 (100) | 6 (100) | 23 (100) | 23 (100) | 16 (100) | 15 (94) | 45 (100) | 44 (98) |
| Pyrexia | 6 (100) | 3 (50) | 22 (96) | 11 (48) | 12 (75) | 5 (31) | 40 (89) | 19 (42) |
| Hypotension | 5 (83) | 3 (50) | 17 (74) | 11 (48) | 9 (56) | 4 (25) | 31 (69) | 18 (40) |
| Chills | 3 (50) | 0 | 13 (57) | 0 | 3 (19) | 0 | 19 (42) | 0 |
| Diarrhea | 3 (50) | 0 | 10 (43) | 1 (4) | 6 (38) | 0 | 19 (42) | 1 (2) |
| Headache | 1 (17) | 0 | 10 (43) | 1 (4) | 7 (44) | 1 (6) | 18 (40) | 2 (4) |
| Anemia | 4 (67) | 4 (67) | 10 (43) | 8 (35) | 3 (19) | 2 (13) | 17 (38) | 14 (31) |
| Encephalopathy | 4 (67) | 2 (33) | 11 (48) | 6 (26) | 2 (13) | 2 (13) | 17 (38) | 10 (22) |
| Hypophosphatemia | 2 (33) | 1 (17) | 12 (52) | 10 (43) | 3 (19) | 3 (19) | 17 (38) | 14 (31) |
| Nausea | 1 (17) | 0 | 13 (57) | 1 (4) | 3 (19) | 0 | 17 (38) | 1 (2) |
| Confusional state | 2 (33) | 1 (17) | 9 (39) | 1 (4) | 5 (31) | 2 (13) | 16 (36) | 4 (9) |
| Hypoxia | 2 (33) | 1 (17) | 8 (35) | 6 (26) | 6 (38) | 4 (25) | 16 (36) | 11 (24) |
| Decreased platelet count | 3 (50) | 3 (50) | 8 (35) | 8 (35) | 5 (31) | 4 (25) | 16 (36) | 15 (33) |
| Constipation | 2 (33) | 0 | 10 (43) | 0 | 2 (13) | 0 | 14 (31) | 0 |
| Fatigue | 1 (17) | 0 | 7 (30) | 1 (4) | 6 (38) | 0 | 14 (31) | 1 (2) |
| Sinus tachycardia | 2 (33) | 0 | 10 (43) | 1 (4) | 2 (13) | 0 | 14 (31) | 1 (2) |
| Hypokalemia | 1 (17) | 0 | 11 (48) | 0 | 1 (6) | 0 | 13 (29) | 0 |
| Tachycardia | 1 (17) | 1 (17) | 6 (26) | 1 (4) | 6 (38) | 0 | 13 (29) | 2 (4) |
| Tremor | 1 (17) | 0 | 8 (35) | 0 | 4 (25) | 0 | 13 (29) | 0 |
| Decreased appetite | 0 | 0 | 9 (39) | 2 (9) | 3 (19) | 0 | 12 (27) | 2 (4) |
| Hyperglycemia | 1 (17) | 0 | 6 (26) | 0 | 5 (31) | 1 (6) | 12 (27) | 1 (2) |
| Hypomagnesemia | 2 (33) | 0 | 8 (35) | 0 | 2 (13) | 0 | 12 (27) | 0 |
| Hyponatremia | 3 (50) | 2 (33) | 7 (30) | 3 (13) | 2 (13) | 0 | 12 (27) | 5 (11) |
| Peripheral edema | 1 (17) | 0 | 7 (30) | 1 (4) | 4 (25) | 0 | 12 (27) | 1 (2) |
Data are presented as n (%). Table includes AEs of any grade occurring in ≥25% of all patients.
CRS was reported in 42 patients (93%); 14 (31%) experienced grade ≥3 CRS (Table 3). Common grade ≥3 symptoms of CRS were pyrexia (40%), hypotension (27%), and hypoxia (16%). Vasopressors were used for the treatment of CRS in 12 patients (27%). The median time to CRS onset after infusion was 2 days (IQR, 1-5 days); the median duration of any grade CRS was 9 days (IQR, 7-14 days), and median duration of grade ≥3 CRS was 4.5 days (IQR, 3.5-9 days). CRS-associated events resolved in all but the 2 patients who experienced grade 5 KTE-X19–related AEs. One patient treated with 2 × 106 CAR T cells per kg had multiorgan failure secondary to CRS (day 6). One patient treated with 0.5 × 106 cells per kg developed cerebrovascular accident (stroke) in the context of grade 2 CRS and grade 4 NEs (day 7). These observations prompted the study of lower doses and the revision of AE management.
Table 3.
CRS and NEs with revised AE management guidelines
| AE management and dose level | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2 × 106 (n = 6) | 1 × 106 original (n = 14) | 1 × 106 revised (n = 9) | 0.5 × 106 (n = 16) | |||||
| Steroids | ||||||||
| For treatment of CRS | 1 (17) | 5 (36) | 5 (56) | 5 (31) | ||||
| For treatment of NEs | 3 (50) | 7 (50) | 5 (56) | 5 (31) | ||||
| Tocilizumab | ||||||||
| For treatment of CRS | 1 (17) | 9 (64) | 9 (100) | 5 (31) | ||||
| For treatment of NEs | 4 (67) | 5 (36) | 4 (44) | 1 (6) | ||||
| Vasopressors for treatment of CRS | 3 (50) | 4 (29) | 2 (22) | 3 (19) | ||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| AE | ||||||||
| CRS | 6 (100) | 3 (50) | 14 (100) | 4 (29) | 9 (100) | 3 (33) | 13 (81) | 4 (25) |
| Pyrexia | 6 (100) | 3 (50) | 12 (86) | 5 (36) | 9 (100) | 6 (67) | 10 (77) | 5 (31) |
| Hypotension | 4 (67) | 3 (50) | 11 (79) | 6 (43) | 6 (67) | 3 (33) | 8 (62) | 3 (19) |
| Sinus tachycardia | 2 (33) | 0 | 6 (43) | 0 | 4 (44) | 1 (11) | 2 (15) | 0 |
| Chills | 1 (17) | 0 | 5 (36) | 0 | 4 (44) | 0 | 2 (15) | 0 |
| Tachycardia | 1 (17) | 1 (17) | 4 (29) | 1 (7) | 2 (22) | 0 | 4 (31) | 0 |
| Tachypnea | 0 | 0 | 4 (29) | 1 (7) | 0 | 0 | 0 | 0 |
| Hypoxia | 2 (33) | 1 (17) | 2 (14) | 2 (14) | 3 (33) | 2 (22) | 3 (23) | 2 (15) |
| Nausea | 0 | 0 | 2 (14) | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 1 (7) | 0 | 3 (33) | 0 | 1 (8) | 0 |
| Headache | 0 | 0 | 1 (7) | 0 | 2 (22) | 0 | 3 (23) | 0 |
| Hyponatremia | 0 | 0 | 1 (7) | 0 | 1 (11) | 0 | 1 (8) | 0 |
| Any NE | 5 (83) | 3 (50) | 13 (93) | 9 (64) | 7 (78) | 1 (11) | 10 (63) | 4 (25) |
| Confusional state | 2 (33) | 1 (17) | 3 (21) | 0 | 6 (67) | 1 (11) | 5 (31) | 2 (13) |
| Tremor | 1 (17) | 0 | 4 (29) | 0 | 4 (44) | 0 | 4 (25) | 0 |
| Aphasia | 0 | 0 | 6 (43) | 4 (29) | 2 (22) | 1 (11) | 2 (13) | 2 (13) |
| Encephalopathy | 4 (67) | 2 (33) | 9 (64) | 6 (43) | 2 (22) | 0 | 2 (13) | 2 (13) |
| Lethargy | 0 | 0 | 1 (7) | 0 | 2 (22) | 0 | 2 (13) | 0 |
| Mental status changes | 0 | 0 | 0 | 0 | 2 (22) | 0 | 0 | 0 |
| Agitation | 0 | 0 | 4 (29) | 1 (7) | 1 (11) | 1 (11) | 2 (13) | 0 |
| Dysarthria | 0 | 0 | 1 (7) | 1 (7) | 1 (11) | 0 | 0 | 0 |
| Restlessness | 0 | 0 | 1 (7) | 1 (7) | 1 (11) | 1 (11) | 0 | 0 |
| Seizure | 1 (17) | 0 | 2 (14) | 2 (14) | 1 (11) | 0 | 1 (6) | 0 |
| Ataxia | 0 | 0 | 1 (7) | 0 | 1 (11) | 0 | 0 | 0 |
Data are presented as n (%).
NEs were reported in 35 patients (78%), and grade ≥3 events occurred in 17 (38%; Table 3). Grade ≥3 NEs that occurred in ≥5% of patients were encephalopathy (22%), aphasia (16%), and confusional state (9%). There were no cases of cerebral edema and no grade 5 NEs. The median time to onset of NEs was 6 days (IQR, 3-8 days) after infusion; the median duration of any grade NE was 12 days (IQR, 4-26 days), and the median duration of grade ≥3 NEs was 9 days (IQR, 4-16 days). NEs resolved in 31 (89%) of 35 patients; 1 patient died as a result of progressive disease and 3 patients died as a result of AEs considered unrelated to KTE-X19 (sepsis, cerebrovascular accident, herpes simplex viremia [n = 1 each]) before NE resolution.
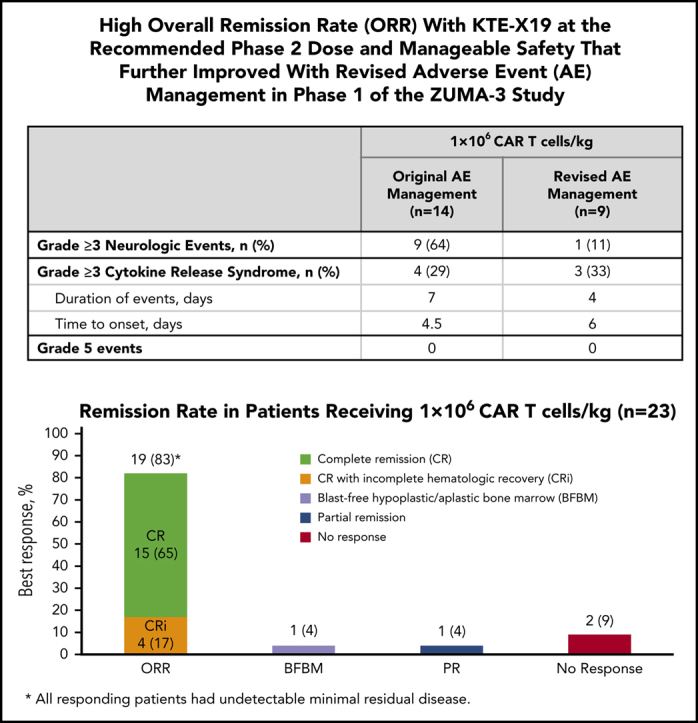
Fifty-three percent of all patients received tocilizumab, and 36% also received steroids for management of CRS; 31% received tocilizumab, and 44% received steroids for NEs. Improved overall safety was observed for the 9 patients treated under revised AE management guidelines relative to the 14 patients treated at the same dose (1 × 106 cells per kg) under the original guidelines (Table 3). Four (29%) of 14 patients treated at 1 × 106 CAR T cells per kg under the original guidelines had grade 3 or 4 CRS. With revised AE management, 3 (33%) of 9 patients treated at 1 × 106 CAR T cells per kg had grade 3 CRS, with no grade 4 CRS reported. These patients also had a median duration of grade ≥3 CRS of 4 days (IQR, 2-8 days) vs 7 days (IQR, 3-13 days) in patients receiving 1 × 106 CAR T cells per kg under original AE guidelines, and onset of grade ≥3 symptoms was 6 days (IQR, 1-10 days) vs 4.5 days (IQR, 2-11 days), respectively. Notably, 9 (64%) of 14 patients in the 1 × 106 CAR T cells per kg dose cohort managed with the original guidelines experienced grade 3 to 4 NEs compared with 1 grade 3 and no grade 4 events in 9 patients receiving the same dose under revised management guidelines (Table 3). On the basis of the review of all available safety and efficacy data, the benefit-risk ratio was considered most favorable at the dose of 1 × 106 CAR T cells per kg, resulting in this dose being the recommended phase 2 dose. All phase 2 patients are being treated under revised AE management guidelines.
Twenty-six treated patients (58%) died as a result of causes that included disease progression in 19 (42%) and AEs in 7 patients (16%), including the 2 above-mentioned treatment-related deaths. The remaining 5 AE-related deaths occurred at a median of 63 days after infusion and were considered unrelated to KTE-X19. They included sepsis (day 50; day 579), cerebrovascular accident (day 48), herpes simplex viremia (day 309), and bacteremia (day 63).
Efficacy
All 45 treated patients were eligible for efficacy analysis. At a median follow-up of 22.1 months (range, 7.1-36.1 months), the ORR was 69%, with 53% of patients achieving CR and 16% achieving CRi (Table 4). In all enrolled patients (intent-to-treat, N = 54), the ORR was 57% (supplemental Table 7). Among the 23 patients treated with 1 × 106 CAR T cells per kg, the ORR was 83%, with 15 (65%) achieving CR and 4 (17%) achieving CRi. Six of 9 patients who received revised AE management achieved CR/CRi (4 CR, 2 CRi). The median time to CR/CRi across dose levels was 30 days (range, 26-192 days), which included 1 patient with blast-free hypoplastic or aplastic BM (BFBM) at day 28 who did not meet CR criteria until month 6. ORR was generally consistent across key covariates, including refractory patients (56%), previous transplantation (77%), previous treatment with blinatumomab (57%) or inotuzumab ozogamicin (50%), and Ph+ disease patients (100%) (supplemental Figure 3). Undetectable BM MRD was achieved at day 28 in 100% of responders, including the 31 patients with CR/CRi, 1 patient with partial response, and 1 with BFBM. Residual disease assessment was unavailable in 1 patient with BFBM. Two of 6 patients who underwent the optional BM assessment at day 7 to 14 had undetectable MRD; the 5 patients with data available at day 30 had undetectable MRD.
Table 4.
Response to KTE-X19
| Response category | Dose level | Total (N = 45) | ||
|---|---|---|---|---|
| 2 × 106 (n = 6) | 1 × 106 (n = 23) | 0.5 × 106 (n = 16) | ||
| CR | 4 (67) | 19 (83) | 8 (50) | 31 (69) |
| CR | 3 (50) | 15 (65) | 6 (38) | 24 (53) |
| CRi | 1 (17) | 4 (17) | 2 (13) | 7 (16) |
| BFBM | 0 | 1 (4) | 1 (6) | 2 (4) |
| Partial remission | 0 | 1 (4)* | 0 | 1 (2) |
| No response | 1 (17) | 2 (9) | 6 (38) | 9 (20) |
| Unknown or not evaluable | 1 (17)† | 0 | 1 (6)‡ | 2 (4) |
Data are presented as n (%).
Patient had extramedullary disease at response assessment. Of the 3 other patients with extramedullary disease, 2 achieved a CR, and 1 had stable disease (no response); all had extramedullary disease present at response assessment.
Patient died on day 6 as a result of multiorgan failure secondary to CRS.
Patient died on day 7 as a result of cerebrovascular accident (stroke) in the context of CRS and neurologic events.
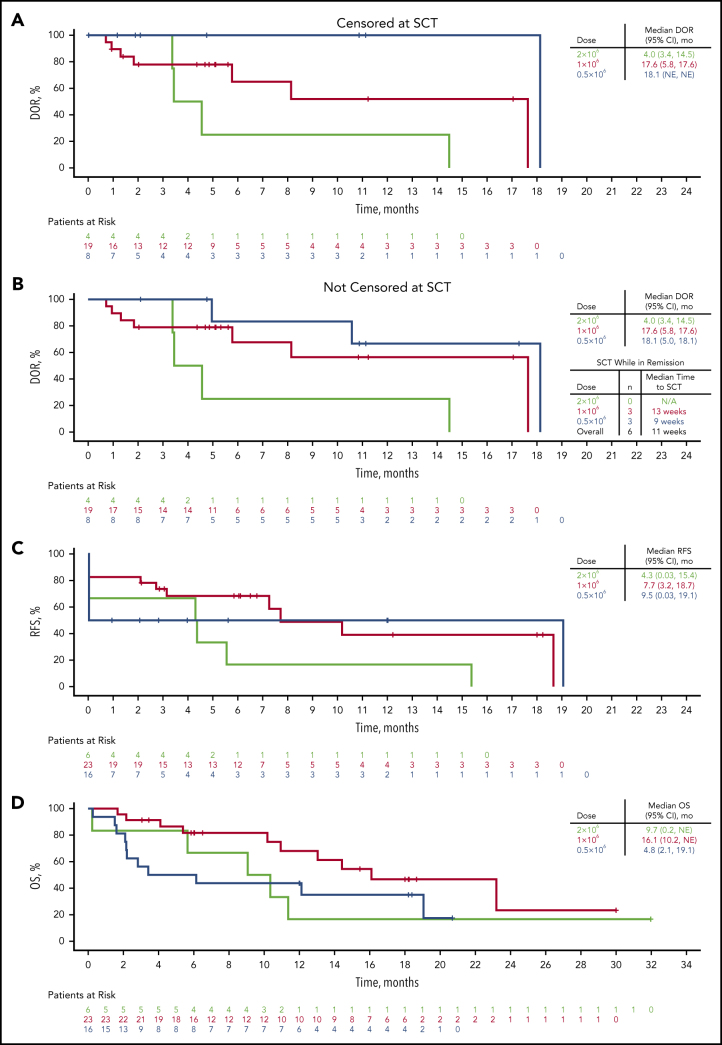
The median DOR for the 31 patients achieving CR/CRi was 14.5 months (95% CI, 5.8-18.1 months; Figure 1A), and 17.6 months (95% CI, 5.8-17.6 months) in patients treated with 1 × 106 CAR T cells per kg. Median DOR was similar regardless of censoring for SCT after treatment with KTE-X19 (Figure 1B). As of the data cutoff, 8 patients (26%) had ongoing CRs, including 2 who received 0.5 × 106 and 6 who received 1 × 106 CAR T cells per kg, with a median follow-up of 6.3 months (range, 5.9-18.2 months); 5 of these patients did not receive SCT after treatment with KTE-X19. A total of 6 patients (2 with CR and 1 with a partial response treated with 1 × 106 CAR T cells per kg; 3 with CR treated with 0.5 × 106 cells per kg) underwent SCT at a median of 2.7 months (range, 1.7-4.3 months) after infusion. As of this analysis, 3 of these remained in CR (2 treated with 1 × 106 and 1 treated with 0.5 × 106 cells per kg). Across all dose levels, the median duration of relapse-free survival was 7.3 months (95% CI, 2.7-18.7 months) vs 7.7 months (95% CI, 3.2-18.7 months) in patients receiving 1 × 106 CAR T cells per kg (Figure 1C). The median OS was 12.1 months (95% CI, 6.1-19.1 months) across all dose levels and 16.1 months (95% CI, 10.2 to not estimable) with 1 × 106 CAR T cells per kg (Figure 1D).
Figure 1.
DOR, relapse-free survival (RFS), and OS by dose level. (A-B) Kaplan-Meier curves of DOR in patients achieving CR/CRi censored at SCT (A) or not censored at SCT (B). (C-D) Kaplan-Meier curves of RFS (C) and OS (D). Patients who were alive at the time of data cutoff were censored at their last date of contact. N/A, not applicable; NE, not estimable.
As of the data cutoff, 1 patient (2%) withdrew consent, 1 (2%) was lost to follow-up, and 17 (38%) were alive, including 11 (50%) of 23 patients treated with 1 × 106 cells per kg. Four patients received a second infusion of KTE-X19; 1 was in CR at 15 months after redosing, 2 had relapsed by the month-3 assessment, and 1 withdrew consent before the first response assessment.
Clinical pharmacology
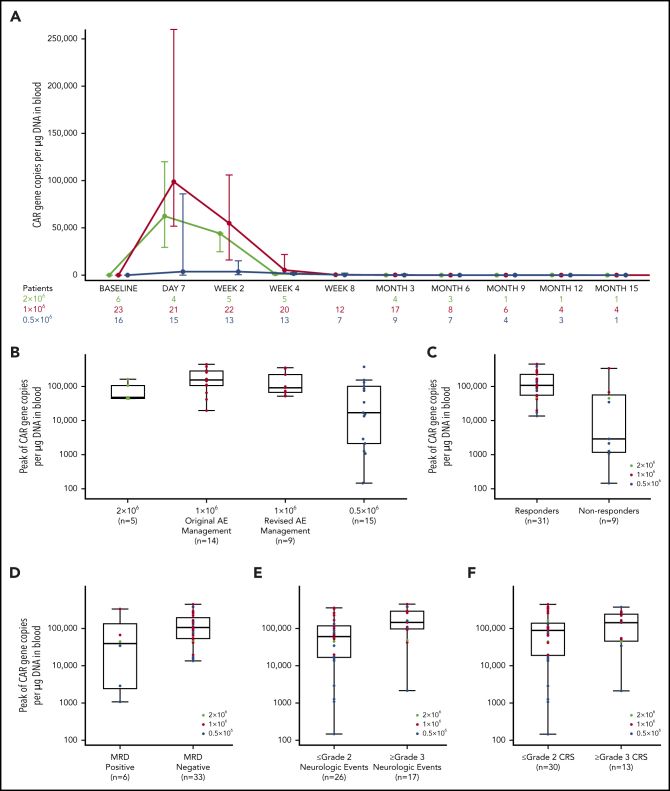
CAR T-cell levels measured by CAR gene copies per µg DNA in blood peaked 7 to 14 days after KTE-X19 infusion for most patients and remained detectable in 2 of 12 evaluable patients at 12 months, both of whom were in CR (Figure 2A; supplemental Table 8). CAR T cells were undetectable in the 5 patients with data available at relapse. CAR T-cell expansion was highest with 1 × 106 CAR T cells per kg and was similar between patients who received original vs revised AE management (Figure 2B; supplemental Figure 4A). Patients achieving CR/CRi had greater median peak expansion than nonresponders, as did patients with undetectable vs detectable MRD (Figure 2C-D; supplemental Figure 4B-C). Higher median peak expansion was also observed in patients with grade ≥3 vs those with grade ≤2 NEs, regardless of dose level, with similar results observed in patients with grade ≥3 vs grade ≤2 CRS (Figure 2E-F; supplemental Table 9; supplemental Figure 4D-E). No relationship between median peak expansion by median BM blast percentage at screening for enrollment or at preconditioning after bridging therapy was observed in any dose group (supplemental Table 10). In an assessment of the patients in the 1 × 106 CAR T cells per kg dose group by BM blast percentage split into quartiles, a potential trend toward increased expansion with higher BM disease burden was observed, but the relationship was not linear (supplemental Table 10; supplemental Figure 5). Of 13 patients who relapsed, 7 had detectable CD19+ cells at relapse (median, 168 days after infusion [range, 82-348 days after infusion]), 3 had no detectable CD19+ cells (median, 178 days after infusion [range, 95-220 days after infusion]), and 3 had no data available.
Figure 2.
Peak CAR T-cell expansion and associations with response, MRD, and toxicity. (A) Expansion and persistence of CAR T cells depicted as medians and IQRs. (B) Peak CAR T-cell levels by dose level, including in original vs revised AE management (1 × 106 cells per kg dose level). (C-F) Association between peak CAR T-cell expansion and ORR (C), MRD (D), grade ≥3 NEs (E), and grade ≥3 CRS (F). The horizontal line within each box represents the median, the lower and upper borders of each box represent the 25th and the 75th percentiles, respectively, and the error bars represent the 95% CI.
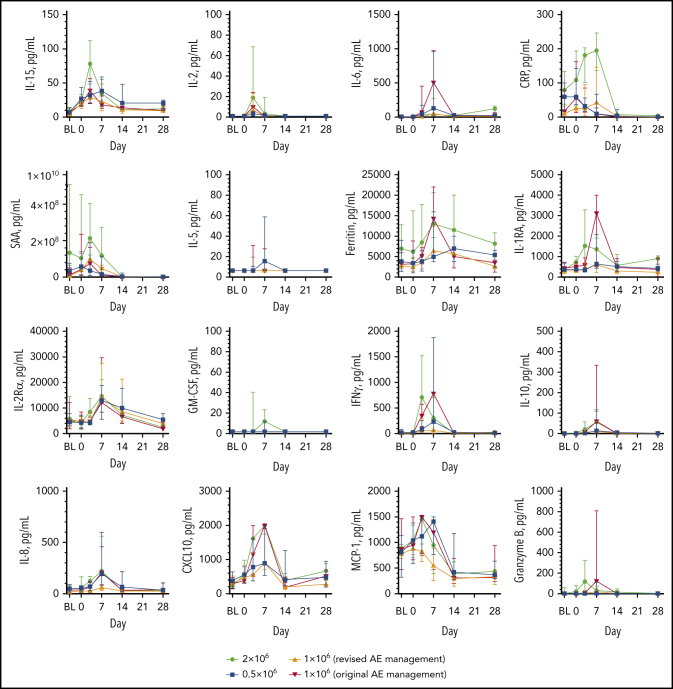
Peak levels of key cytokines, chemokines, and pro-inflammatory markers occurred by day 7, with some trending higher in patients dosed with 2 × 106 compared with 1 × 106 CAR T cells per kg (interleukin-15 [IL-15], C-reactive protein [CRP], serum amyloid A [SAA], C-X-C motif chemokine ligand 10 [CXCL10], interferon-γ [IFN-γ]) or lower in those with revised AE management vs those with original AE management (IL-6, ferritin, IL-1RA, IFN-γ, IL-8, CXCL10, monocyte chemoattractant protein-1 [MCP-1]) (Figure 3; supplemental Table 11). Although peak IL-15 serum levels were surprisingly lower in patients with grade ≥3 CRS, median peak levels of several biomarkers trended higher in patients with grade ≥3 CRS and those with grade ≥3 NEs (IFN-γ, IL-8, granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-1RA, granzyme B; supplemental Table 12).
Figure 3.
Cytokine and inflammatory marker levels over time. Serum biomarker levels by dose over the first 28 days after KTE-X19 infusion. BL, baseline; Rα, receptor α; RA, receptor antagonist.
Four patients tested positive for anti-CAR antibodies during screening assays, but all were negative in confirmatory assays at leukapheresis. Characteristics of manufactured CAR T-cell products were as anticipated and as previously reported (supplemental Table 13).
Discussion
ZUMA-3 is the first multicenter study evaluating CAR T-cell therapy in adult R/R B-ALL to complete phase 1. In phase 1, no protocol-defined DLTs were observed with KTE-X19, and the AEs reported were consistent with those in previous studies of anti-CD19 CAR T-cell therapies.21, 22 The overall safety observations prompted the evaluation of lower doses and implementation of revised AE management guidelines. The 1 × 106 CAR T cells per kg dose coupled with revised AE management guidelines had the most favorable risk-benefit ratio without compromising activity. Although patients had high disease burden and were heavily pretreated, high rates of remission and undetectable BM MRD were achieved, particularly in those treated at the 1 × 106 dose level; the ORR was 83%, including 65% CR and 17% CRi, all of whom had undetectable MRD. Based on these results demonstrating that KTE-X19 is safe and has promising efficacy, the 1 × 106 CAR T cells per kg dose was chosen for further evaluation in phase 2 of ZUMA-3.
Use of anti-CD19 CAR T cells to treat adult R/R B-ALL has proven difficult owing to the highly proliferative nature of B-ALL and the inability of patients to tolerate treatment-related AEs. A previous CAR T-cell trial in this population was closed early because of fatal NEs, including 5 cases of cerebral edema.23 Under the original AE management guidelines in ZUMA-3, 2 patients died as a result of grade 5 AEs considered related to KTE-X19 either secondary to CRS or in the context of CRS and NEs outside the DLT-assessment time frame. In addition to evaluating multiple doses to identify the dose with the most manageable toxicities, revised AE management guidelines requiring earlier steroid administration for neurotoxicity and the use of tocilizumab only for CRS were implemented in 9 patients enrolled at the 1 × 106 CAR T cells per kg dose level. This resulted in a shorter duration of CRS events and lower incidence, severity, and duration of NEs compared with 14 patients treated at the same dose under the original guidelines.
At a median follow-up of 22.1 months, 26% of patients were in ongoing CR, most of whom received 1 × 106 CAR T cells per kg (32% ongoing CR/CRi). Responses tended to occur early after treatment. Most occurred within the first month, although 1 patient with extramedullary disease achieved CR at month 6. High response rates were observed across all prespecified subgroups, including a 100% CR rate in patients with Ph+ disease. Response (CR/CRi) was associated with higher expansion of CAR T cells measured within 2 weeks after treatment. Similarly, in a single-center, phase 1 study using an anti-CD19 CAR T-cell therapy also containing a CD3ζ and CD28 costimulatory domain,10 the overall CR rate was 83%; however, at the time of infusion, 51% of treated patients had ≥5% blasts in the BM, 28% had MRD, and 11% had undetectable MRD. Nevertheless, those trial results largely paralleled the results of this study, further supporting the potential utility of anti-CD19 CAR T-cell therapies using a CD3ζ and CD28 costimulatory domain in adult R/R B-ALL.
Evaluation of cellular products with CD28 vs 4-1BB costimulatory domains in adult R/R B-ALL is ongoing, although interpretation of findings is challenging because of differences in patient populations across trials and the lack of head-to-head data. Tisagenlecleucel, an anti-CD19 CAR T-cell therapy containing a CD3ζ T-cell activation domain and a 4-1BB costimulatory domain is approved for the treatment of R/R B-ALL in children and young adults (age 25 years or younger).22, 24 However, the dosing regimen for tisagenlecleucel used in younger patients resulted in substantial toxicity and CRS-related deaths in adults with R/R B-ALL.25 In a single-center study in adult R/R B-ALL across 2 clinical trials, administering tisagenlecleucel in dose fractions resulted in manageable CRS and a 90% CR rate.25 Another single-center study evaluating 4-1BB CAR T cells in a series of adults with B-ALL reported high rates of severe toxicities that were mitigated with risk-stratified dosing.26 Similar to observations in the ZUMA-3 trial, optimized dosing and toxicity management strategies may enable patients vulnerable to life-threatening treatment-related toxicities to benefit from CAR T-cell therapy.
Preclinical and clinical studies have suggested longer CAR T-cell persistence with 4-1BB signaling than that with CD28 in B-ALL.25, 27, 28 Although hypothesized to allow for extended antitumor surveillance in the absence of SCT, more than one-third of adult patients with B-ALL who achieved CR with tisagenlecleucel underwent subsequent SCT in the single-center study mentioned previously.25, 28 Although survival was improved with SCT consolidation after treatment with tisagenlecleucel in that study, a similar benefit has not been observed with CD28-based CAR T cells in adult patients.10, 25 Additional studies that directly compare the impact of 4-1BB vs CD28 costimulation for anti-CD19 CAR T-cell therapy are needed to assess the impact on CAR T-cell persistence and long-term outcomes in R/R adult B-ALL.
Despite differences in trial designs, patient populations, and OS methodology, the median OS with 1 × 106 CAR T cells per kg in this study was 16.1 months, whereas the median OS previously reported with blinatumomab, which also targets CD19, was 6.1 to 7.7 months in adult R/R B-ALL.3, 4 Of 10 patients evaluable for CD19 blast expression at relapse, 3 showed lack of CD19 expression, reminiscent of other reports that attributed target loss to selection of exon splice variants and mutations.29 In the ZUMA-3 trial, only 1 (13%) of 8 patients with blinatumomab as last previous therapy responded to blinatumomab. Through ex vivo activation and expansion of large numbers of biologically active T cells redirected to CD19, CAR T-cell therapy may overcome a suboptimal state of the immune system and primary or secondary treatment resistance to bispecific engagers, as supported by this study. Of the 21 patients with previous treatment with blinatumomab in any line, 12 (57%) achieved CR/CRi after therapy with KTE-X19. As previously reported,30 responses to KTE-X19 were similar regardless of previous blinatumomab exposure in patients with continued CD19 positivity. Overall, 6 patients achieving CR underwent SCT and were censored at the time of SCT; 3 remain in remission.
Adults with R/R B-ALL achieved high rates of CR and undetectable BM MRD with a tolerable safety profile after treatment with KTE-X19. The successful manufacture for all enrolled patients and the relatively rapid turnaround time support the feasibility of providing this cellular therapy to patients with rapidly progressing disease who need prompt treatment. By carefully evaluating a range of doses and adopting safety strategies, including use of tocilizumab or steroids and conditions under which they should be administered to manage AEs, we were able to transition the study from phase 1 to an international phase 2 study. Phase 2 of ZUMA-3 is ongoing at the 1 × 106 CAR T cells per kg dose with revised AE management guidelines.
Acknowledgments
The authors thank the patients who participated in the study and their families, friends, and caregivers and the study staff and health care providers at all the study sites. Medical writing support was provided by Stephanie Morgan of Nexus Global Group Science.
This study was supported by Kite, a Gilead Company.
Footnotes
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Shah et al report results of a phase 1 trial of KTE-X19 anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in 45 adults with relapsed/refractory acute lymphoblastic leukemia (ALL). Complete remissions, all of which were minimal residual disease negative, were achieved in 69% of patients, and in 83% of patients receiving the highest dose, which is the dose in the ongoing phase 2 trial.
Authorship
Contribution: B.D.S., W.G.W., J.M.R., A.B., T.S., L.G., R.K.J., and R.V. designed the study; B.D.S., M.R. Bishop, O.O.O., A.C.L., M.R. Baer, W.B.D., K.M.O., H.H., M.L.A., A.G., J.M.P., Y.L., R.D.C., J.E.C., D.J.D., J.H.P., A.K.M., R.M., G.J.S., M.A., and W.G.W. enrolled and treated patients and gathered data; and all authors participated in the analysis and interpretation of the data and manuscript writing, had full access to the data, and approved the final submitted version.
Conflict-of-interest disclosure: B.D.S. reports honoraria from Pharmacyclics, Janssen, Acrotech, Spectrum, BeiGene, and Gilead Sciences; a consultancy or advisory role for Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, and Kite, a Gilead Company; research funding from Incyte, Jazz Pharmaceuticals, Gilead Sciences, and Kite; and travel support from Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, Stemline Therapeutics, and Kite. M.R. Bishop reports honoraria from Kite, Incyte, Celgene, Sanofi, Novartis, Bristol Myers Squibb, and Agios; a consulting or advisory role with Novartis, Kite, CRISPR Therapeutics, Agios, Iovance, Bluebird Bio, WindMil Therapeutics, and Acellx; speakers bureau service with Kite, Agios, Incyte, Sanofi, and Bristol Myers Squibb; research funding from Kite, Novartis, CRISPR Therapeutics, Arcellx, Autolus, Immatics, Triumvera, and Tmunity; and travel support from Kite, Novartis, Bristol Myers Squibb, Agios, and Incyte. O.O.O. reports a consultancy or advisory role for Kite, Pfizer, Spectrum Pharmaceuticals, Legend, and Bayer. A.C.L. reports a consultancy or advisory role for Amgen, Pfizer, Bristol Myers Squibb, Agios, Novartis, and Incyte; and research funding from Astellas, Jazz, Kite, Amphivena, Kadmon, Autolus, and Pharmacyclics. M.R. Baer reports research funding from the University of Maryland Greenebaum Comprehensive Cancer Center. H.H. reports a consultancy or advisory role for Kite, Gilead Sciences, Bayer, Rigel, Janssen, and Juno; speakers' bureau participation for Kite, Seattle Genetics, Rigel, Dova, and Karyo‐pharm, research funding from Kite, Unum, Juno, Novartis, Genentech, ADC Therapeutics, Seattle Genetics, Bluebird, Incyte, and Janssen; and patents, royalties, or other intellectual property from a pending patent. M.L.A. reports a consultancy or advisory role for Gilead Sciences and Kite and research funding from Emory University. A.G. reports a consulting or advisory role, honoraria, and research funding from Kite and a consulting and advisory role with Amgen, Atara, Wugen, and Celgene. J.M.P. reports a consultancy or advisory role for Kite, Pharmacyclics, and Actinium. Y.L. reports a consultancy or advisory role for Kite, Janssen, Novartis, Celgene, Bluebird Bio, Juno, Legend, Sorrento, Gamida Cells, and Vineti and research funding from Kite, Janssen, Celgene, Bluebird Bio, Merck, and Takeda. R.D.C. reports employment and stock or other ownership with Seagen; a consultancy or advisory role for Kite, Pfizer, and Amgen; and research funding from Pfizer, Merck, Amgen, Kite, and Vanda Pharmaceuticals. J.H.P. reports a consultancy or advisory role for Kite, Novartis, Amgen, InCyte, Allogene, Autolus, Artiva, Intellia, Servier, Pfizer, Takeda, and AstraZeneca and research funding from Genentech, Amgen, and Juno Therapeutics. M.A. reports consultancy or advisory role for Celgene, Kite, and Takeda, research funding from Amgen, Celgene, and CIRM, and speakers' bureau participation for Bristol Myers Squibb, AbbVie, Celgene, Gilead, Seattle Genetics, and Takeda. D.J.D. reports honoraria from Autolos, Agios, Blueprint, Forty-Seven, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda; research funding from AbbVie, Glycomimetics, Novartis, and Blueprint Pharmaceuticals; and travel support from Glycomimetics, Pfizer, and Blueprint Pharmaceuticals. G.J.S. reports speakers' bureau participation with Kite. A.B. reports employment with Kite, stock or other ownership in Gilead Sciences, a consultancy or advisory role with Gilead Sciences, and travel support from Gilead Sciences. T.S. reports employment, stock or other ownership, honoraria, and travel support with Kite. J.M.R. reports employment with Kite. L.G. reports employment with Kite, and stock or other ownership in Gilead. R.K.J. reports employment with Kite and Vida Ventures, stock or other ownership in Kite and Amgen, patents, royalties, or other intellectual property from CAR T field, and travel support from Kite. R.V. reports employment with Kite. W.G.W. reports consultancy or advisory role for Sanofi and Genzyme, and research funding from GlaxoSmithKline, Novartis, AbbVie, Genentech, Karyopharm, Pharmacyclics, Acerta, Gilead Sciences, Juno Therapeutics, Sunesis, Miragen, Oncternal Therapeutics, Cyclacel, Loxo Oncology, Janssen, and Xencor. The remaining authors declare no competing financial interests.
Supplementary Material
REFERENCES
- 1.Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–1666. doi: 10.1016/j.mayocp.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Oriol A, Vives S, Hernández-Rivas JM, et al. Programa Español de Tratamiento en Hematologia Group Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: Transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13(5):396–406. doi: 10.1007/s11899-018-0470-x. [DOI] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabatino M, Choi K, Chiruvolu V, Better M. Production of anti-CD19 CAR T cells for ZUMA-3 and -4: Phase 1/2 multicenter studies evaluating KTE-C19 in patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL) [abstract] Blood. 2016;128(22) Abstract 1227. [Google Scholar]
- 12.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah B, Huynh V, Sender LS, et al. High rates of minimal residual disease-negative (MRD−) complete responses (CR) in adult and pediatric and patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treated with KTE-C19 (anti-CD19 chimeric antigen receptor [CAR] T cells): Preliminary results of the ZUMA-3 and ZUMA-4 trials [abstract] Blood. 2016;128(22) Abstract 2803. [Google Scholar]
- 14.Lee DW, Wayne AS, Huynh V, et al. ZUMA-4 preliminary results: phase 1 study of KTE-C19 chimeric antigen receptor T cell therapy in pediatric and adolescent patients (pts) with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) [abstract] Ann Oncol. 2017;28(suppl 5) Abstract 1008PD. [Google Scholar]
- 15.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126(8):964–971. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brüggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1(25):2456–2466. doi: 10.1182/bloodadvances.2017009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Devidas M, Loh ML, et al. Flow-cytometric vs. -morphologic assessment of remission in childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group (COG) Leukemia. 2018;32(6):1370–1379. doi: 10.1038/s41375-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeAngelo DJ, Ghobadi A, Park JH, et al. Clinical outcomes for the phase 2, single-arm, multicenter trial of JCAR015 in adult B-ALL (ROCKET Study) [abstract] J Immunother Cancer. 2017;5(suppl 2) Abstract 217. [Google Scholar]
- 24.US Food and Drug Administration KYMRIAH. (tisagenlecleucel), package insert. 2018. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert-KYMRIAH.pdf
- 25.Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2020;38(5):415–422. doi: 10.1200/JCO.19.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philipson BI, O'Connor RS, May MJ, June CH, Albelda SM, Milone MC. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci Signal. 2020;13(625):eaay8248. doi: 10.1126/scisignal.aay8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Yang J, Zhang X, et al. Efficacy and safety of CD28- or 4-1BB-based CD19 CAR-T cells in B cell acute lymphoblastic leukemia. Mol Ther Oncolytics. 2020;18:272–281. doi: 10.1016/j.omto.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah BD, Oluwole OO, Baer MR, et al. Outcomes of patients (pts) treated with prior blinatumomab (Blin) in ZUMA-3: a study of KTE-C19, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) [abstract] J Clin Oncol. 2018;36(15_suppl) Abstract 7006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.