Abstract
Purpose
To evaluate efficacy, durability, and safety of faricimab in Japanese patients with diabetic macular edema (DME).
Study design
Subgroup analysis of 2 global, multicenter, randomized, double-masked, active-comparator–controlled, phase 3 trials (YOSEMITE, NCT03622580; RHINE, NCT03622593).
Methods
Patients with DME were randomized 1:1:1 to intravitreal faricimab 6.0 mg every 8 weeks (Q8W), faricimab 6.0 mg per personalized treatment interval (PTI), or aflibercept 2.0 mg Q8W through week 100. Primary endpoint was best-corrected visual acuity (BCVA) change from baseline at 1 year, averaged over weeks 48, 52, and 56. This is the first time 1-year outcomes between Japanese patients (only enrolled into YOSEMITE) and the pooled YOSEMITE/RHINE cohort (N = 1891) have been compared.
Results
The YOSEMITE Japan subgroup included 60 patients randomized to faricimab Q8W (n = 21), faricimab PTI (n = 19), or aflibercept Q8W (n = 20). Consistent with global results, the adjusted mean (95.04% confidence interval) BCVA change at 1 year in the Japan subgroup was comparable with faricimab Q8W (+11.1 [7.6–14.6] letters), faricimab PTI (+8.1 [4.4–11.7] letters), and aflibercept Q8W (+6.9 [3.3–10.5] letters). At week 52, 13 (72%) patients in the faricimab PTI arm achieved ≥ Q12W dosing, including 7 (39%) patients receiving Q16W dosing. Anatomic improvements with faricimab were generally consistent between the Japan subgroup and pooled YOSEMITE/RHINE cohort. Faricimab was well tolerated; no new or unexpected safety signals were identified.
Conclusion
Consistent with global results, faricimab up to Q16W offered durable vision gains and improved anatomic and disease-specific outcomes among Japanese patients with DME.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10384-023-00979-8.
Keywords: Angiopoietin-2, Anti-VEGF therapy, Diabetic macular edema, Faricimab, Vascular stability
Introduction
Diabetic retinopathy, including diabetic macular edema (DME), is a vision-threatening complication of diabetes and a leading cause of visual impairment among working-aged people in Japan [1, 2]. Intravitreal anti–vascular endothelial growth factor (VEGF) therapy is the standard first-line treatment for Japanese patients with center-involving DME [1, 3]; however, DME is a multifactorial disease with mechanisms beyond VEGF upregulation that contribute to fluid accumulation in the macula [4]. Of note, the angiopoietin (Ang)-1/Tie2 signaling pathway is a key regulator of vascular stability under physiological conditions which, in DME and other retinal vascular diseases, is disrupted by Ang-2 upregulation [5–7]. Based on the hypothesis that multitargeted treatment strategies for DME may promote vascular stability and durable efficacy beyond VEGF inhibition alone, faricimab was designed as the first bispecific antibody for intraocular use that independently binds and inhibits Ang-2 and VEGF-A [8].
Data from phase 2 and 3 clinical trials of intravitreal faricimab support the notion that dual Ang-2/VEGF-A pathway inhibition may extend treatment durability for patients with DME while maintaining optimal efficacy [9, 10]. In particular, 1-year primary results from the pivotal YOSEMITE and RHINE trials show that faricimab dosed every 8 weeks (Q8W) or according to a treat-and-extend–based personalized treatment interval (PTI) regimen, offered noninferior vision gains and anatomic improvements compared with aflibercept Q8W. The PTI treatment arms also demonstrate the strong durability of faricimab, with > 50% of patients receiving every-16-week (Q16W) dosing at week 52 and > 70% of patients receiving every-12-week (Q12W) dosing or longer. Faricimab was also found to be well tolerated, with an acceptable safety profile comparable with aflibercept [10]. Based on positive 1-year results from YOSEMITE and RHINE, faricimab was first approved for the treatment of patients with DME in the United States in January 2022 [11, 12] and in Japan in March 2022 [13].
Together, YOSEMITE and RHINE are the largest registrational trial program conducted to date on DME, with 1891 patients enrolled across 353 study sites in 31 participating countries [10]. Asia was represented by patients and study sites from Japan in the YOSEMITE trial, whereas patients from China, Hong Kong, Singapore, South Korea, Taiwan, and Thailand participated in RHINE. Although the primary analysis evaluated YOSEMITE and RHINE separately and showed that results were reproducible between trials [10], 1-year outcomes for the pooled YOSEMITE/RHINE cohort and Japanese patient subgroup have not previously been reported or compared. This is particularly important in light of recent reports of intraocular inflammation events among Japanese patients receiving anti-VEGF therapy for neovascular age-related macular degeneration [14, 15]. With this in mind, this subgroup analysis sought to examine whether the efficacy, durability, and safety of faricimab among Japanese patients with DME in YOSEMITE were consistent with the pooled YOSEMITE/RHINE trial population.
Subjects and methods
YOSEMITE and RHINE
The study design, rationale, and primary 1-year results of the YOSEMITE and RHINE trials have been previously described [10, 16]. Briefly, YOSEMITE (ClinicalTrials.gov identifier NCT03622580) and RHINE (NCT03622593) were identical, global, multicenter, randomized, double-masked, active-comparator–controlled, phase 3 trials of faricimab in patients with DME. YOSEMITE and RHINE were conducted in accordance with the International Council for Harmonisation E6 Guideline for Good Clinical Practice; tenets of the Declaration of Helsinki; applicable US Food and Drug Administration regulations; the European Union Clinical Trials Directive (2001/20/EC); and relevant local, state, and federal laws. Study protocols were approved by institutional review boards and ethics committees as applicable, and all patients provided written informed consent.
Key inclusion criteria were patients aged ≥ 18 years with center-involving DME (central subfield thickness [CST] ≥ 325 µm on Spectralis spectral-domain optical coherence tomography [SD-OCT] or CST ≥ 315 μm on Cirrus or Topcon SD-OCT, measured from the internal limiting membrane to Bruch's membrane) and best-corrected visual acuity (BCVA) of 25–73 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (approximate Snellen equivalent, 20/320–20/40). One eye per patient was included in the trial; if both eyes were eligible, the eye with the worse BCVA at screening was designated the study eye. Eyes that had received intravitreal anti-VEGF therapy, panretinal photocoagulation, or macular laser (focal, grid, or micropulse) < 3 months before day 1 were excluded. Anti-VEGF treatment-naïve and previously treated eyes (last treated ≥ 3 months before day 1) were eligible for inclusion; however, previously treated eyes were limited to 25% of the total enrollment.
Enrolled patients were randomized 1:1:1 to intravitreal faricimab 6.0 mg Q8W after 6 initial every-4-week (Q4W) doses, intravitreal faricimab 6.0 mg per PTI after a minimum of 4 initial Q4W doses, or intravitreal aflibercept Q8W after 5 initial Q4W doses. Randomization was performed through an interactive voice- or web-based response system and was stratified by baseline BCVA (< 64 versus ≥ 64 ETDRS letters), previous intravitreal anti-VEGF therapy (yes versus no), and geographic region (US and Canada, Asia, and the rest of the world).
Patients randomized to the faricimab PTI arms followed a treat-and-extend–based regimen that allowed adjustable dosing up to Q16W [10, 16]. Patients initially received faricimab Q4W until they achieved CST < 325 µm (or CST < 315 µm, depending on the SD-OCT device used) at or after week 12. Once achieved, faricimab dosing intervals could be extended by 4 weeks (up to Q16W), maintained, or reduced by 4 or 8 weeks (as low as Q4W) based on CST and BCVA change at active dosing visits.
To maintain masking, all patients were monitored Q4W and received sham injections at nonactive dosing visits. Patients received their assigned treatment (or sham) up to week 96 and attended a final study visit at week 100. Key ocular assessments throughout the trials included BCVA measured by standard ETDRS protocols and ocular images (including SD-OCT and color fundus photography) assessed by masked evaluators at a central reading center.
Subgroup analyses
The present study sought to evaluate 1-year outcomes among Japanese patients enrolled from Japanese study sites in YOSEMITE (no Japanese patients or study sites were included in RHINE), as compared with the pooled YOSEMITE/RHINE trial population. Efficacy and safety endpoints assessed for the YOSEMITE Japan subgroup and pooled YOSEMITE/RHINE cohort were consistent with prespecified endpoints in the primary analysis [10] and included changes in BCVA from baseline at 1 year (averaged over weeks 48, 52, and 56) and over time; the proportion of patients in the faricimab PTI arm on Q4W, Q8W, Q12W, or Q16W dosing at week 52 and over time; change in CST from baseline at 1 year and over time; the proportions of patients with absence of protocol-defined DME (CST < 325 μm or CST < 315 µm, depending on the SD-OCT device used), absence of intraretinal fluid (IRF), and absence of subretinal fluid (SRF) over time; the proportion of patients with ≥ 2-step improvement on the ETDRS Diabetic Retinopathy Severity Scale (DRSS) from baseline at week 52; and the incidence and severity of adverse events (AEs) through week 56.
Statistical analyses
Efficacy and safety analyses for the Japan subgroup were performed as prespecified in the YOSEMITE trial protocol and were consistent with the primary analysis [10]. Continuous efficacy outcomes were assessed using a mixed model for repeated measures, adjusted for treatment group, visit, visit-by-treatment group interaction, baseline BCVA or CST (continuous) as applicable, and randomization factors of baseline BCVA and previous intravitreal anti-VEGF therapy. For binary endpoints, weighted proportions were estimated using the Cochran-Mantel-Haenszel (CMH) method, stratified by baseline BCVA and previous intravitreal anti-VEGF therapy. Efficacy outcomes in the YOSEMITE Japan subgroup are reported with 95.04% confidence intervals (CIs) to adjust for interim safety assessments conducted through to the completion of the primary analysis [10]. In the pooled YOSEMITE/RHINE cohort, efficacy analyses were additionally adjusted or stratified by geographic region and study (YOSEMITE versus RHINE), and 95% CIs are reported. No formal statistical comparisons were made between the YOSEMITE Japan and pooled YOSEMITE/RHINE treatment arms, and results should be interpreted as exploratory and descriptive.
For all efficacy analyses, intercurrent events due to the COVID-19 pandemic (ie, study treatment discontinuation; use of prohibited systemic treatment or prohibited therapy in the study eye; missed doses with potential impact on efficacy [i.e., weeks 44, 48, or 52]; or death due to COVID-19) were handled using a hypothetical strategy where all values were censored after the intercurrent event, and intercurrent events not due to COVID-19 (i.e., study treatment discontinuation due to adverse events or lack of efficacy; or use of prohibited systemic treatment or prohibited therapy in the study eye not due to COVID-19) were handled using a treatment policy strategy where all observed values were used regardless of the intercurrent event. The robustness of these assumptions and the primary results were demonstrated using sensitivity and supplemental analyses as previously described [10]. Safety and tolerability were assessed through descriptive summaries of ocular and systemic AEs (coded using Medical Dictionary for Regulatory Activities thesaurus terms), deaths, and ocular assessments through week 56.
Results
Patient disposition
As previously described, 1891 patients with DME were enrolled in YOSEMITE (N = 940) and RHINE (N = 951) between September 5, 2018 and September 20, 2019 across 353 study sites worldwide [10]. In the pooled YOSEMITE/RHINE cohort, 632 patients were randomized to faricimab Q8W, 632 patients were randomized to faricimab PTI up to Q16W, and 627 patients were randomized to aflibercept Q8W. The Japan subgroup of YOSEMITE comprised 60 patients enrolled from 27 study sites in Japan. Of these, 21 patients were randomized to faricimab Q8W, 19 to faricimab PTI up to Q16W, and 20 to aflibercept Q8W.
Baseline patient characteristics in the YOSEMITE Japan subgroup were generally balanced across treatment arms and consistent with the pooled YOSEMITE/RHINE trial population (Table 1). In the Japan subgroup, mean age at baseline ranged from 63.0–65.8 years across treatment arms (versus 62.1–62.3 years in the pooled YOSEMITE/RHINE cohort), 43–47% of participants were women (versus 37–42%), and mean BCVA was 59.3–60.1 ETDRS letters (versus 61.9–62.2 ETDRS letters). Baseline CST in the Japan subgroup was numerically greater than in the pooled cohort for the faricimab Q8W (mean, 508 µm versus 479 µm) and aflibercept Q8W (497 µm vversus 481 µm) treatment arms but was similar for the faricimab PTI arms (478 µm versus 479 µm). Compared with the pooled cohort, mean time since DME diagnosis was longer in the Japan subgroup (26–28 versus 16–19 months across treatment arms), the proportion of previously anti-VEGF–treated patients was higher (25–33% versus 21–22%), and mean time since last anti-VEGF treatment in these patients was longer (24–41 versus 17–21 months).
Table 1.
Baseline patient characteristics in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup
| YOSEMITE/RHINE pooled (N = 1891) | YOSEMITE Japan subgroup (N = 60) | |||||
|---|---|---|---|---|---|---|
| Faricimab Q8W (n = 632) | Faricimab PTI (n = 632) | Aflibercept Q8W (n = 627) | Faricimab Q8W (n = 21) | Faricimab PTI (n = 19) | Aflibercept Q8W (n = 20) | |
| Patient demographics | ||||||
| Age (years), mean (SD)a | 62.1 (9.8) | 62.2 (10.1) | 62.3 (9.8) | 63.9 (10.3) | 63.0 (10.7) | 65.8 (9.4) |
| Female sex, n (%) | 251 (39.7%) | 236 (37.3%) | 263 (41.9%) | 9 (42.9%) | 9 (47.4%) | 9 (45.0%) |
| Geographic region, n (%) | ||||||
| US and Canada | 277 (43.8%) | 279 (44.1%) | 277 (44.2%) | 0 | 0 | 0 |
| Asiab | 50 (7.9%) | 48 (7.6%) | 46 (7.3%) | 21 (100%) | 19 (100%) | 20 (100%) |
| Rest of the worldc | 305 (48.3%) | 305 (48.3%) | 304 (48.5%) | 0 | 0 | 0 |
| Race, n (%)d | ||||||
| White | 491 (77.7%) | 489 (77.4%) | 506 (80.7%) | 0 | 0 | 0 |
| Asian | 65 (10.3%) | 62 (9.8%) | 59 (9.4%) | 21 (100%) | 19 (100%) | 20 (100%) |
| Black or African American | 40 (6.3%) | 48 (7.6%) | 36 (5.7%) | 0 | 0 | 0 |
| American Indian or Alaska Native | 6 (0.9%) | 5 (0.8%) | 8 (1.3%) | 0 | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 4 (0.6%) | 0 | 3 (0.5%) | 0 | 0 | 0 |
| Hispanic or Latinx, n (%) | 93 (14.7%) | 118 (18.7%) | 104 (16.6%) | 0 | 0 | 0 |
| Nonocular characteristics | ||||||
| BMI (kg/m2), mean (SD) | 30.7 (6.5) | 30.4 (6.3) | 30.6 (6.4) | 24.5 (3.6) | 26.2 (5.0) | 25.2 (3.5) |
| HbA1c (%), mean (SD) | 7.6 (1.1) | 7.7 (1.2) | 7.6 (1.2) | 7.2 (0.6) | 7.4 (0.8) | 7.4 (0.9) |
| Type 2 diabetes, n (%) | 588 (93.0%) | 599 (94.8%) | 597 (95.2%) | 21 (100%) | 19 (100%) | 20 (100%) |
| Systolic blood pressure (mmHg), mean (SD) | 137.0 (15.7) | 137.9 (15.8) | 136.9 (16.1) | 135.8 (14.1) | 137.6 (16.6) | 133.7 (14.0) |
| Ocular characteristics | ||||||
| BCVA (ETDRS letters), mean (SD) | 61.9 (10.0) | 62.2 (9.8) | 62.1 (9.5) | 59.3 (10.9) | 60.1 (8.2) | 59.6 (8.7) |
| CST (μm), mean (SD) | 479.2 (128.4) | 478.5 (129.0) | 480.9 (130.2) | 507.6 (130.0) | 478.1 (123.9) | 496.9 (115.9) |
| Macular ischemic nonperfusion, n (%) | 253 (40.0%) | 255 (40.3%) | 254 (40.5%) | 6 (28.6%) | 4 (21.1%) | 8 (40.0%) |
| Macular leakage, n (%) | 605 (95.7%) | 610 (96.5%) | 592 (94.4%) | 21 (100%) | 19 (100%) | 19 (95.0%) |
| Time since DME diagnosis (months), mean (SD) | 16.3 (27.4) | 19.1 (34.7) | 18.9 (32.5) | 27.9 (33.7) | 25.9 (43.4) | 28.2 (37.5) |
| Anti-VEGF treatment naïve, n (%) | 492 (77.8%) | 500 (79.1%) | 490 (78.1%) | 14 (66.7%) | 14 (73.7%) | 15 (75.0%) |
| Previously anti-VEGF treated, n (%) | 140 (22.2%) | 132 (20.9%) | 137 (21.9%) | 7 (33.3%) | 5 (26.3%) | 5 (25.0%) |
| Time since last anti-VEGF treatment (months), mean (SD) | 20.6 (20.5) | 16.6 (18.3) | 18.3 (15.3) | 41.4 (41.7) | 23.7 (19.1) | 24.6 (17.5) |
| Phakic, n (%) | 476 (75.3%) | 474 (75.0%) | 468 (74.6%) | 12 (57.1%) | 14 (73.7%) | 14 (70.0%) |
| ETDRS-DRSS status, n (%) | ||||||
| DR absent/questionable; mild to moderate NPDR (ETDRS-DRSS level 10/12, 14/20, 35, 43) | 357 (56.5%) | 365 (57.8%) | 362 (57.7%) | 12 (57.1%) | 11 (57.9%) | 13 (65.0%) |
| Moderately severe to severe NPDR (ETDRS-DRSS level 47, 53) | 222 (35.1%) | 198 (31.3%) | 208 (33.2%) | 9 (42.9%) | 6 (31.6%) | 5 (25.0%) |
| PDR (ETDRS-DRSS level 61, 65, 71/75) | 42 (6.6%) | 58 (9.2%) | 38 (6.1%) | 0 | 2 (10.5%) | 1 (5.0%) |
| Cannot grade (ETDRS-DRSS level 90) | 6 (0.9%) | 10 (1.6%) | 12 (1.9%) | 0 | 0 | 1 (5.0%) |
| Missing | 5 (0.8%) | 1 (0.2%) | 7 (1.1%) | 0 | 0 | 0 |
BCVA best-corrected visual acuity, BMI body mass index, CST central subfield thickness, DME diabetic macular edema, DR diabetic retinopathy, DRSS Diabetic Retinopathy Severity Scale, ETDRS Early Treatment Diabetic Retinopathy Study, HbA1c glycated hemoglobin, NPDR nonproliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, PTI personalized treatment interval, Q8W every 8 weeks, SD standard deviation, VEGF vascular endothelial growth factor
aAge at randomization
bAsia includes China, Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand
cRest of the world includes Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, Mexico, the Netherlands, New Zealand, Peru, Poland, Portugal, Russia, Slovakia, South Africa, Spain, Switzerland, Turkey, Ukraine, and the United Kingdom
dNot all race categories are listed; therefore, the sum of proportions shown may not equal 100%
Vision outcomes
One-year vision outcomes in the YOSEMITE Japan subgroup were generally consistent with global results (Fig. 1). In the pooled YOSEMITE/RHINE cohort, adjusted mean BCVA change from baseline at 1 year was +11.2 ETDRS letters (95% CI, +10.5 to +12.0) in the faricimab Q8W arm and +11.2 ETDRS letters (+10.4 to +11.9) in the faricimab PTI arm, which were comparable to +10.5 ETDRS letters (+9.8 to +11.3) in the aflibercept Q8W arm (mean difference versus aflibercept Q8W, +0.7 ETDRS letters [−0.4 to +1.7] and +0.6 ETDRS letters [−0.4 to +1.7], respectively). Despite smaller sample sizes and wider CIs in the YOSEMITE Japan subgroup, adjusted mean 1-year vision gains with faricimab Q8W (+11.1 ETDRS letters [95.04% CI, +7.6 to +14.6]) and faricimab PTI (+8.1 ETDRS letters [+4.4 to +11.7]) remained comparable to aflibercept Q8W (+6.9 ETDRS letters [+3.3 to +10.5]; mean difference, +4.2 ETDRS letters [−0.8 to +9.2] and +1.2 ETDRS letters [−3.9 to +6.3], respectively).
Fig. 1.
Adjusted mean change in best-corrected visual acuity (BCVA) a from baseline through week 56 and b at 1 year in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup. Results are based on a mixed model for repeated measures analysis; treatment policy strategy and hypothetical strategy were applied to non–COVID-19–related and COVID-19–related intercurrent events, respectively. Error bars represent 95% confidence intervals (CIs) for the pooled YOSEMITE/RHINE cohort and 95.04% CIs for the YOSEMITE Japan subgroup. aAdjusted mean BCVA change from baseline at 1 year, averaged over weeks 48, 52, and 56. ETDRS Early Treatment Diabetic Retinopathy Study, PTI personalized treatment interval, Q8W every 8 weeks
Durability outcomes
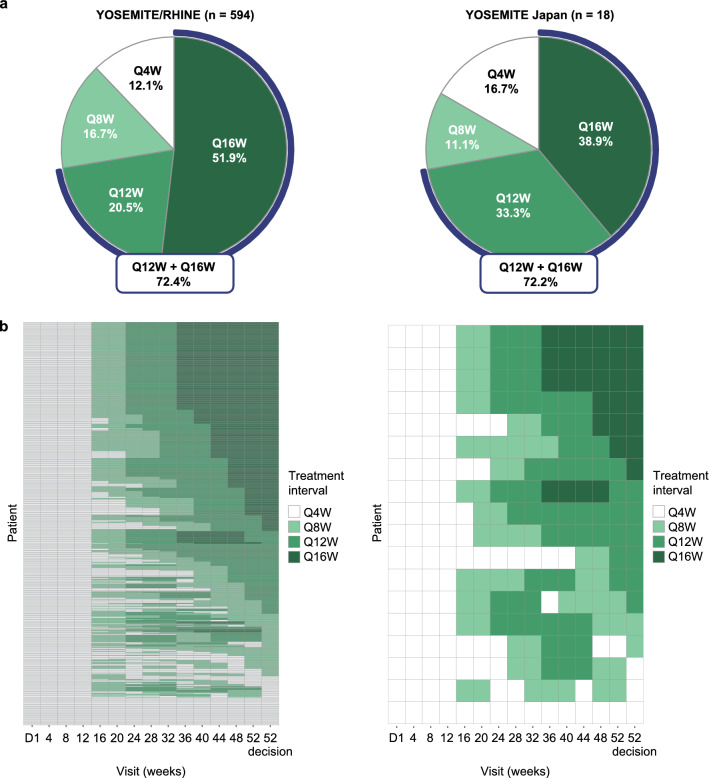
In the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup, durable vision gains with faricimab were achieved with treat-and-extend–based PTI dosing up to Q16W. At week 52, 39% (7/18) of faricimab PTI–treated patients in the Japan subgroup achieved Q16W dosing and 72% (13/18) achieved Q12W dosing or longer compared with 52% and 72% of patients in the pooled YOSEMITE/RHINE cohort, respectively (Fig. 2a). Moreover, 61% (11/18) of Japanese patients achieved Q12W or Q16W dosing without an injection interval decrease below Q12W through week 52 (versus 66% in the pooled cohort).
Fig. 2.
a Proportion of patients in the faricimab personalized treatment interval (PTI) arm who achieved every-4-week (Q4W), every-8-week (Q8W), every-12-week (Q12W), or every-16-week (Q16W) dosing at week 52 and b dosing intervals in the faricimab PTI arms over 1 year in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup. Analyses included patients in the faricimab PTI arms who had not discontinued the study at the week 52 visit. In panel a, treatment interval at week 52 was defined as the treatment interval decision made at that visit. In panel b, treatment interval at a given visit is shown as the interval at the start of the visit. The week 52 decision (calculated/recorded at week 56) is shown in the last column. D day
PTI dosing patterns over time were similar between the YOSEMITE Japan subgroup and the pooled YOSEMITE/RHINE cohort (Fig. 2b). In the Japan subgroup, 43% (3/7) of patients who achieved Q16W dosing at week 52 had already completed a full Q16W dosing cycle compared with 46% in the pooled cohort. Approximately 21% (4/19) of Japanese patients rapidly achieved Q16W dosing at week 32 (versus 25% in the pooled cohort); of those, 75% (3/4) completed a full Q16W cycle and maintained Q16W dosing at week 52 (versus 81%). Among those on Q4W dosing at week 52 (17% [3/18] in the Japan subgroup and 12% in the pooled cohort), 33% (1/3) and 57% of patients, respectively, required Q4W dosing from baseline through week 52.
Anatomic outcomes
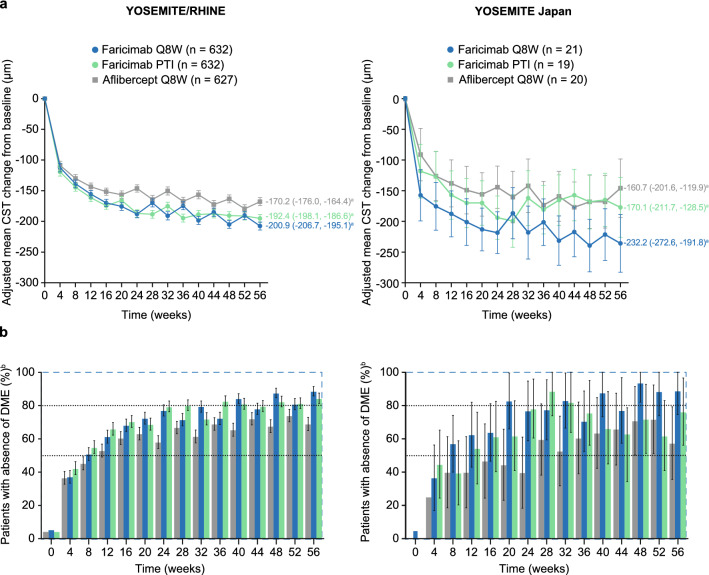
In the pooled YOSEMITE/RHINE cohort, mean reductions in CST and patients with absence of protocol-defined DME through week 56 were consistently greater with faricimab Q8W and PTI versus aflibercept Q8W. In the YOSEMITE Japan subgroup, change in CST and absence of DME tended to favor faricimab Q8W over aflibercept Q8W through week 56, whereas results with faricimab PTI were comparable to aflibercept Q8W but achieved with up to Q16W dosing. Adjusted mean CST change from baseline at 1 year was −232.2 μm among Japanese patients in the faricimab Q8W arm (versus −200.9 μm in the pooled YOSEMITE/RHINE cohort), −170.1 μm in the faricimab PTI arm (versus −192.4 μm), and −160.7 μm in the aflibercept Q8W arm (versus −170.2 μm) (Fig. 3a). In CMH-weighted estimates, 89–94% of faricimab Q8W–treated patients in the YOSEMITE Japan subgroup and 62–76% of those in the faricimab PTI arm achieved absence of DME at weeks 48–56 compared with 57–72% in the aflibercept Q8W arm. Corresponding estimates in the pooled YOSEMITE/RHINE cohort were 81–89%, 82–85%, and 68–74%, respectively (Fig. 3b).
Fig. 3.
a Adjusted mean change in central subfield thickness (CST) from baseline and b proportion of patients with absence of diabetic macular edema (DME) through week 56 in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup. In panel a, results are based on a mixed model for repeated measures analysis. In panel b, weighted proportions were estimated using the Cochran-Mantel-Haenszel method; weighted proportions for the aflibercept every 8 weeks (Q8W) arms are presented for the faricimab Q8W versus aflibercept Q8W comparison. Baseline values (defined as the last available measurement obtained on or before randomization) are based on observed data. Treatment policy strategy and hypothetical strategy were applied to non–COVID-19–related and COVID-19–related intercurrent events, respectively. Error bars represent 95% confidence intervals (CIs) for the pooled YOSEMITE/RHINE cohort and 95.04% CIs for the YOSEMITE Japan subgroup; estimates < 0% or > 100% were imputed as 0% or 100%, respectively. aAdjusted mean CST change from baseline at 1 year, averaged over weeks 48, 52, and 56. bAbsence of DME was defined as CST < 325 μm on Spectralis spectral-domain optical coherence tomography (SD-OCT) or CST < 315 μm on Cirrus or Topcon SD-OCT, and measured as the average thickness between the internal limiting membrane and Bruch’s membrane in the central 1-mm diameter of the Early Treatment Diabetic Retinopathy Study grid. PTI personalized treatment interval
Consistent with the pooled YOSEMITE/RHINE cohort, we observed a trend for more patients with absence of IRF among the faricimab versus aflibercept arms of the YOSEMITE Japan subgroup (Fig. 4a). At weeks 48–56, absence of IRF was achieved by 31–40% and 24–36% of Japanese patients in the faricimab Q8W and PTI arms, respectively, compared with 17–28% of those in the aflibercept Q8W arm (pooled YOSEMITE/RHINE cohort, 41–46% and 33–42% versus 22–27%, respectively). Absence of SRF through week 56 was high and comparable across treatment arms and trial populations; 58–76% of patients in the Japan subgroup and 62–66% of those in the pooled cohort had absence of SRF at baseline, which increased to near 100% for all groups through week 56 (Fig. 4b).
Fig. 4.
Proportion of patients with a absence of intraretinal fluid (IRF) and b absence of subretinal fluid (SRF) through week 56 in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup. IRF and SRF were measured in the central 1-mm diameter of the Early Treatment Diabetic Retinopathy Study grid. Weighted proportions were estimated using the Cochran-Mantel-Haenszel method; weighted proportions for the aflibercept every 8 weeks (Q8W) arms are presented for the faricimab Q8W versus aflibercept Q8W comparison. Baseline values (defined as the last available measurement obtained on or before randomization) are based on observed data. Treatment policy strategy and hypothetical strategy were applied to non–COVID-19–related and COVID-19–related intercurrent events, respectively. Error bars represent 95% confidence intervals (CIs) for the pooled YOSEMITE/RHINE cohort and 95.04% CIs for the YOSEMITE Japan subgroup; estimates < 0% or > 100% were imputed as 0% or 100%, respectively. PTI personalized treatment interval
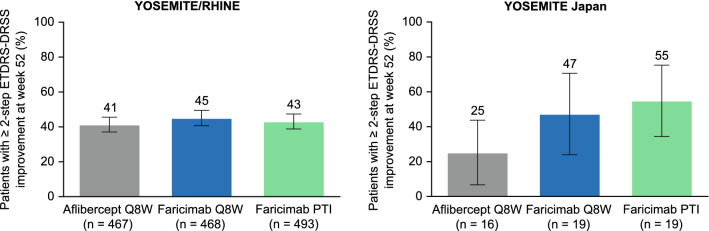
The weighted proportion of patients in the pooled YOSEMITE/RHINE cohort who achieved ≥ 2-step ETDRS-DRSS improvement at week 52 was comparable between the faricimab Q8W (45.1% [95% CI, 40.7–49.5%]), faricimab PTI (43.1% [38.8–47.4%]), and aflibercept Q8W (41.3% [37.1–45.6%]) treatment arms (Fig. 5). In the YOSEMITE Japan subgroup, patients with ≥ 2-step ETDRS-DRSS improvement at week 52 were consistent between faricimab treatment arms and numerically greater than the aflibercept Q8W arm. The weighted proportion of Japanese patients who achieved ≥ 2-step ETDRS-DRSS improvement at week 52 was 47.3% (95.04% CI, 23.9–70.6%) in the faricimab Q8W arm and 54.9% (34.4–75.3%) in the faricimab PTI arm, compared with 25.2% (6.7–43.7%) in the aflibercept Q8W arm.
Fig. 5.
Proportion of patients with at ≥ 2-step Early Treatment Diabetic Retinopathy Study (ETDRS)–Diabetic Retinopathy Severity Scale (DRSS) improvement from baseline at week 52 in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup. Analyses included patients with evaluable color fundus photographs at baseline and week 52. Weighted proportions were estimated using the Cochran-Mantel-Haenszel method; weighted proportions for the aflibercept every 8 weeks (Q8W) arms are presented for the faricimab Q8W versus aflibercept Q8W comparison. Treatment policy strategy and hypothetical strategy were applied to non–COVID-19–related and COVID-19–related intercurrent events, respectively. Error bars represent 95% confidence intervals (CIs) for the pooled YOSEMITE/RHINE cohort and 95.04% CIs for the YOSEMITE Japan subgroup. PTI personalized treatment interval
Safety outcomes
Consistent with the pooled YOSEMITE/RHINE cohort, faricimab was well tolerated in the YOSEMITE Japan subgroup and displayed an acceptable safety profile comparable with aflibercept (Table 2). In the Japan subgroup and the pooled cohort, the incidence of ocular AEs through week 56 was comparable between patients receiving faricimab Q8W (24% and 37%, respectively), faricimab PTI (32% and 36%), and aflibercept Q8W (40% and 34%), with most events being mild or moderate in severity. Serious ocular AEs in the Japan subgroup and the pooled cohort were low and comparable between the faricimab Q8W (0% and 2%, respectively), faricimab PTI (5% and 3%), and aflibercept Q8W (5% and 1%) treatment arms. Nonocular AEs were also comparable across treatment arms and similar between the Japan subgroup and the pooled cohort, and no Antiplatelet Trialists’ Collaboration events were reported in the Japan subgroup through week 56.
Table 2.
Summary of key AEs through week 56 in the pooled YOSEMITE/RHINE cohort and the YOSEMITE Japan subgroup
| YOSEMITE/RHINE pooled (N = 1887) | YOSEMITE Japan subgroup (N = 60) | |||||
|---|---|---|---|---|---|---|
| Faricimab Q8W (n = 630) | Faricimab PTI (n = 632) | Aflibercept Q8W (n = 625) | Faricimab Q8W (n = 21) | Faricimab PTI (n = 19) | Aflibercept Q8W (n = 20) | |
| Summary of AEs, n (%) | ||||||
| Total number of AEsa | 2169 | 1891 | 1852 | 55 | 47 | 47 |
| Total number of SAEsa | 272 | 193 | 191 | 3 | 5 | 4 |
| Patients with ≥ 1 ocular AEb | 235 (37.3%) | 225 (35.6%) | 215 (34.4%) | 5 (23.8%) | 6 (31.6%) | 8 (40.0%) |
| Patients with ≥ 1 ocular SAEb | 15 (2.4%) | 19 (3.0%) | 8 (1.3%) | 0 | 1 (5.3%) | 1 (5.0%) |
| Patients with ≥ 1 nonocular AE | 393 (62.4%) | 385 (60.9%) | 390 (62.4%) | 13 (61.9%) | 11 (57.9%) | 14 (70.0%) |
| Patients with ≥ 1 nonocular SAE | 127 (20.2%) | 103 (16.3%) | 102 (16.3%) | 2 (9.5%) | 3 (15.8%) | 1 (5.0%) |
| Patients with ≥ 1 treatment-related ocular AEb | 19 (3.0%) | 16 (2.5%) | 19 (3.0%) | 3 (14.3%) | 1 (5.3%) | 0 |
| Patients with ≥ 1 treatment-related ocular SAEb | 0 | 5 (0.8%) | 0 | 0 | 1 (5.3%) | 0 |
| Patients with ≥ 1 ocular AE of special interestb,c | 15 (2.4%) | 17 (2.7%) | 6 (1.0%) | 0 | 1 (5.3%) | 0 |
| IOI events, n (%)b,d | ||||||
| Patients with ≥ 1 IOI event | 8 (1.3%) | 9 (1.4%) | 4 (0.6%) | 1 (4.8%) | 1 (5.3%) | 0 |
| Anterior chamber inflammation | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| Chorioretinitis | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| Iridocyclitis | 2 (0.3%) | 2 (0.3%) | 0 | 0 | 0 | 0 |
| Iritis | 2 (0.3%) | 3 (0.5%) | 2 (0.3%) | 0 | 0 | 0 |
| Keratic precipitates | 0 | 1 (0.2%) | 0 | 0 | 1 (5.3%) | 0 |
| Keratouveitis | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| Uveitis | 2 (0.3%) | 4 (0.6%) | 0 | 1 (4.8%) | 1 (5.3%) | 0 |
| Vitritis | 3 (0.5%) | 1 (0.2%) | 2 (0.3%) | 0 | 0 | 0 |
| Ocular SAEs associated with intravitreal anti-VEGF therapy, n (%)b | ||||||
| Endophthalmitis | 2 (0.3%) | 2 (0.3%) | 1 (0.2%) | 0 | 0 | 0 |
| Intraocular pressure increased | 0 | 1 (0.2%) | 0 | 0 | 0 | 0 |
| Retinal tear | 0 | 2 (0.3%) | 0 | 0 | 0 | 0 |
| Rhegmatogenous retinal detachment | 1 (0.2%) | 0 | 0 | 0 | 0 | 0 |
| Traumatic cataract | 0 | 0 | 0 | 0 | 0 | 0 |
| Retinal vasculitis or occlusive events, n (%)b | ||||||
| Retinal vasculitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Retinal vein occlusion | 1 (0.2%) | 2 (0.3%) | 0 | 0 | 1 (5.3%) | 0 |
| Retinal artery occlusion | 0 | 0 | 1 (0.2%) | 0 | 0 | 0 |
| Retinal artery embolism | 0 | 0 | 1 (0.2%) | 0 | 0 | 0 |
| APTC events, n (%)e | ||||||
| Patients with ≥ 1 APTC event | 13 (2.1%) | 12 (1.9%) | 14 (2.2%) | 0 | 0 | 0 |
| Nonfatal myocardial infarction | 4 (0.6%) | 2 (0.3%) | 6 (1.0%) | 0 | 0 | 0 |
| Nonfatal stroke | 4 (0.6%) | 4 (0.6%) | 4 (0.6%) | 0 | 0 | 0 |
| Death | 5 (0.8%) | 6 (0.9%) | 4 (0.6%) | 0 | 0 | 0 |
AE adverse event, APTC Antiplatelet Trialists’ Collaboration, BCVA best-corrected visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, IOI intraocular inflammation, PTI personalized treatment interval, Q4W every 4 weeks, Q8W every 8 weeks, SAE serious adverse event, VEGF vascular endothelial growth factor
Safety analyses included all patients who received ≥ 1 dose of active study treatment (faricimab or aflibercept), grouped by the actual treatment received. Includes AEs with onset up to day 405 (last day of week 56 analysis visit window); percentages are based on n values in the column headings. Multiple occurrences of the same AE in 1 individual are counted only once, except for the “Total number of events” rows, in which multiple occurrences of the same AE are counted separately.
aTotal number of AEs and SAEs includes nonocular events and ocular events in the study or fellow eye
bOcular AEs in the study eye only are presented
cOcular AEs of special interest were defined as events associated with severe IOI, events requiring surgical or medical intervention to prevent permanent loss of sight, or events associated with BCVA loss of ≥ 30 ETDRS letters for > 1 hour
dIncludes serious and nonserious IOI events; excludes endophthalmitis events. Most IOI events occurred after the initial Q4W dosing phase for each treatment arm, and approximately 4–6 weeks after the most recent dose of faricimab or aflibercept
eAPTC events were externally adjudicated; all other events were investigator reported
In the YOSEMITE Japan subgroup and the pooled YOSEMITE/RHINE cohort, intraocular inflammation AEs through week 56 were low across the faricimab Q8W (5% [1/21] and 1% [8/630], respectively), faricimab PTI (5% [1/19] and 1% [9/632]), and aflibercept Q8W (0% and 1% [4/625]) treatment arms. In this subgroup, 1 Japanese patient in the faricimab Q8W arm had moderate uveitis that led to study drug interruption and 1 patient in the faricimab PTI arm had mild keratic precipitates and severe uveitis that led to treatment withdrawal; both cases of uveitis were resolving at week 56. Noninflammatory retinal occlusive events through week 56 were low and comparable across treatment arms and trial populations, and no cases of retinal vasculitis or occlusive retinal vasculitis were reported.
Discussion
This subgroup analysis of YOSEMITE and RHINE was the first to evaluate the efficacy, durability, and safety of faricimab in the pooled trial population specifically among Japanese patients with DME. Consistent with the primary analysis in which YOSEMITE and RHINE were evaluated separately [10], 1-year results from the YOSEMITE Japan subgroup and the pooled YOSEMITE/RHINE cohort suggest that faricimab Q8W or PTI may offer comparable vision gains to aflibercept Q8W, improved anatomic outcomes, and extended dosing up to Q16W. Together, these data support the hypothesis that dual Ang-2/VEGF-A pathway inhibition with faricimab may promote vascular stability and durable efficacy beyond current intravitreal anti-VEGF therapies for patients with DME.
Despite the small sample size of the YOSEMITE Japan subgroup, we observed similar 1-year vision gains and trends for improved anatomic outcomes (particularly change in CST, absence of DME, absence of IRF, and ≥ 2-step ETDRS-DRSS improvement) among Japanese patients receiving faricimab Q8W compared with aflibercept Q8W. In the faricimab PTI arm, trends for improved anatomic outcomes versus aflibercept Q8W were less pronounced for some endpoints (eg, change in CST and absence of DME) but were achieved by almost 40% of Japanese patients on Q16W dosing at week 52 and > 70% on Q12W dosing or longer. This level of treatment durability is beyond that reported in the phase 3b RETAIN study of treat-and-extend versus as-needed ranibizumab 0.5 mg for DME, which found that the median injection interval after reaching BCVA stability was 2.1–2.5 months across treat-and-extend and as-needed treatment arms [17]. The binding affinity of faricimab to VEGF-A is comparable to ranibizumab [8], and although the injected molar dose of faricimab 6.0 mg is approximately 4 times greater than ranibizumab 0.5 mg [9], previous DME studies have shown no significant durability advantages with higher doses of anti-VEGF [18, 19]. Therefore, extended dosing up to Q16W in the faricimab PTI arm of YOSEMITE/RHINE suggests that additional Ang-2 pathway inhibition with faricimab may confer durability benefits over VEGF inhibition alone.
Although 1-year outcomes were generally comparable between the YOSEMITE Japan subgroup and the global trial cohort, we observed numerical differences in some endpoints, including BCVA and CST changes over time and the proportion of patients with ≥ 2-step ETDRS-DRSS improvement at week 52. Variation between groups may be due to differences in baseline patient characteristics (eg, time since DME diagnosis and proportion of previously anti-VEGF–treated patients); however, it is more likely that observed differences are due to the small sample size of the YOSEMITE Japan subgroup. In addition to year 2 YOSEMITE/RHINE data, the RHONE-X open-label long-term extension study (NCT04432831) and future real-world studies will continue to assess whether the efficacy, durability, and safety of faricimab in Japanese patients remain consistent with the wider DME population.
Previous studies in neovascular age-related macular degeneration report that Japanese patients are at increased risk of intraocular inflammation AEs following anti-VEGF therapy [14, 15]. In our subgroup analysis, faricimab was well tolerated among Japanese patients with DME, and its acceptable safety profile was consistent with global results. The incidence of intraocular inflammation events in the Japan subgroup was low and comparable across treatment arms, and no cases of retinal vasculitis or occlusive retinal vasculitis were reported.
As stated previously, 1 limitation of our study is the small sample size of the YOSEMITE Japan subgroup, and data from this group should be interpreted with appropriate caution. The PTI dosing regimen in YOSEMITE/RHINE was designed to evaluate the durability of faricimab using treat-and-extend–based methods common in clinical practice [16]; however, real-world studies are needed to explore whether faricimab may reduce treatment burdens and optimize outcomes in Japan, where more flexible dosing regimens are tolerated [1, 20, 21]. As previously described [10], YOSEMITE and RHINE were not designed to compare the durability of faricimab with aflibercept, which was administered according to a fixed Q8W regimen consistent with its globally aligned label [22]. The present study describes relatively short-term results from a small subgroup of Japanese patients with DME; therefore, longer-term data from YOSEMITE/RHINE, RHONE-X, and future real-world studies will be important to monitor the ongoing efficacy, durability, and safety of faricimab in this population.
In conclusion, our subgroup analysis of the phase 3 YOSEMITE/RHINE trials showed that faricimab offered durable vision gains and improved anatomic and disease-specific outcomes among Japanese patients with DME, all of which were achievable with adjustable dosing up to Q16W. These results were consistent with findings from the global trial cohort and demonstrate the potential for faricimab, via its novel mechanism of dual Ang-2/VEGF-A inhibition, to address a significant unmet need for durable therapies that improve real-world outcomes for patients with DME.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
F. Hoffmann-La Roche Ltd. (Basel, Switzerland); Genentech, Inc. (South San Francisco, California, USA); and Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan) provided financial support for the study. Funding was provided by Chugai Pharmaceutical Co., Ltd. for third-party writing assistance, which was provided by Karina D. Hamilton-Peel, PhD, CMPP, of Envision Pharma Group.
YOSEMITE and RHINE Investigators: YOSEMITE (NCT03622580): Bolz, Matthias: Kepler Universitätskliniken GmbH, Abteilung für Augenheilkunde, Linz, AUSTRIA. Findl, Oliver: Hanusch Krankenhaus, Abteilung für Augenkrankheiten mit Augen-Tagesklinik, Wien, AUSTRIA. Pollreisz, Andreas: Medizinische Universität Wien, Universitätsklinik für Augenheilkunde und Optometrie, Wien, AUSTRIA. Weger, Martin: LKH-Universitätsklinikum Graz, Universitäts-Augenklinik, Graz, AUSTRIA. Daskalov, Vesselin: Pentagram Eye Hospital (Medical Center Pentagram), Sofia, BULGARIA. Misheva, Aneta: Medical Center for Eye Health (Focus Ltd), Sofia, BULGARIA. Petkova, Iva: Specialized Hospital for Active Treatment of Eye Diseases (Zora), Sofia, BULGARIA. Tosheva Guneva, Daniela: Medical Center for Specialized Medical Assistance (Eye Clinic Sveta Petka Ltd), Varna, BULGARIA. Vassileva, Petja: Specialized Eye Hospital for Active Treatment (Academic Pashev), Sofia, BULGARIA. Cornut, Pierre Loic: Pole Vision Val d'Ouest, Ophtalmologie, Ecully, FRANCE. Korobelnik, Jean Francois: Hopital Pellegrin, Ophtalmologie, Bordeaux, FRANCE. Lebreton, Olivier: CHU de Nantes, Hôtel Dieu, Ophthalmology, Nantes, FRANCE. Tadayoni, Ramin: Hopital Lariboisiere, Ophtalmologie, Paris, FRANCE. Eter, Nicole: Universitätsklinikum Münster, Augenheilkunde, Münster, GERMANY. Feltgen, Nicolas: Universitätsmedizin Göttingen Georg-August-Universität, Klinik für Augenheilkunde, Göttingen, GERMANY. Framme, Carsten: Medizinische Hochschule Hannover, Klinik für Augenheilkunde, Hannover, GERMANY. Lorenz, Katrin: Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Augenklinik und Poliklinik, Mainz, GERMANY. Spital, Georg: Augenabteilung am St Franziskus-Hospital, Münster, GERMANY. Bator, Gyorgy: Markusovszky Egyetemi Oktatokorhaz, Szemészet, Szombathely, HUNGARY. Seres, András: Budapest Retina Associates Kft, Budapest, HUNGARY. Szalczer, Lajos: Zala Megyei Kórház, Szemészet, Zalaegerszeg, HUNGARY. Toth-Molnar, Edit: Szegedi Tudományegyetem ÁOK, Department of Ophtalmology, Szeged, HUNGARY. Vajas, Attila: Debreceni Egyetem Klinikai Kozpont, Szemeszeti Klinika, Debrecem, HUNGARY. Varsanyi, Balazs: Ganglion Medial Center, Pécs, HUNGARY. Goldstein, Michaella: Tel Aviv Sourasky Medical Center, Ophthalmology, Tel Aviv, ISRAEL. Levy, Jaime: Hadassah Medical Center, Ophthalmology, Jerusalem, ISRAEL. Morori-Katz, Haia: Kaplan Medical Center, Rehovot, ISRAEL. Rosenblatt, Irit: Rabin Medical Center, Ophthalmology, Petach Tikva, ISRAEL. Yoreh, Barak: Rambam Medical Center, Ophthalmology, Haifa, ISRAEL. Bandello, Francesco: IRCCS Ospedale San Raffaele, UO Oculistica, Milano, ITALY. Cagini, Carlo: Azienda Ospedaliera di Perugia Ospedale S Maria Della Misericordia, Clinica Oculistica, Perugia, ITALY. Mastropasqua, Leonardo: Ospedale Clinicizzato SS Annunziata, Clinica Oftalmologica, Chieti, ITALY. Nicolo, Massimo: Università degli Studi di Genova, Clinica Oculistica, Genova, ITALY. Parravano, Maria Cristina: Fondazione IRCCS GB Bietti per lo Studio e la Ricerca in Oftalmologia, Presidio Ospedaliero Britannico, Roma, ITALY. Viola, Francesco: Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Clinica Regina Elena, UOC Oculistica, Milano, ITALY. Fukutomi, Akira: Aichi Medical University Hospital, Aichi, JAPAN. Hayashi, Ken: Hayashi Eye Hospital, Fukuoka, JAPAN. Hirakata, Akito: Kyorin University Hospital, Tokyo, JAPAN. Honda, Shigeru: Osaka City University Hospital, Osaka, JAPAN. Ikeda, Yasuhiro: University of Miyazaki Hospital, Miyazaki, JAPAN. Ito, Yasuki: Nagoya University Hospital, Aichi, JAPAN. Kawasaki, Tsutomu: Ideta Eye Hospital, Kumamoto, JAPAN. Kimura, Kazuhiro: Yamaguchi University Hospital, Yamaguchi, JAPAN. Kishino, Genichiro: Kozawa Eye Hospital and Diabetes Center, Ibaraki, JAPAN. Kitano, Shigehiko: Tokyo Women's Medical University Hospital, Tokyo, JAPAN. Maeno, Takatoshi: Toho University Sakura Medical Center, Chiba, JAPAN. Mitamura, Yoshinori: Tokushima University Hospital, Tokushima, JAPAN. Murakami, Tomoaki: Kyoto University Hospital, Kyoto, JAPAN. Noda, Kousuke: Hokkaido University Hospital, Hokkaido, JAPAN. Obana, Akira: Seirei Hamamatsu General Hospital, Shizuoka, JAPAN. Ogata, Nahoko: Nara Medical University Hospital, Nara, JAPAN. Oh, Hideyasu: Hyogo Prefectural Amagasaki General Medical Center, Hyogo, JAPAN. Sawada, Osamu: Shiga University of Medical Science Hospital, Shiga, JAPAN. Shimouchi, Akito: Asahikawa Medical University Hospital, Hokkaido, JAPAN. Shimura, Masahiko: Tokyo Medical University Hachioji Medical Center, Tokyo, JAPAN. Sugimoto, Masahiko: Mie University Hospital, Mie, JAPAN. Sugita, Iichiro: Sugita Eye Hospital, Aichi, JAPAN. Takagi, Hitoshi: St Marianna University School of Medicine Hospital, Kanagawa, JAPAN. Takayama, Kei: National Defense Medical College Hospital, Saitama, JAPAN. Tanabe, Teruyo: Kitano Hospital, Osaka, JAPAN. Yasukawa, Tsutomu: Nagoya City University Hospital, Aichi, JAPAN. Yoshida, Shigeo: Kurume University Hospital, Fukuoka, JAPAN. Garcia, Renata: Instituto Mexicano de Oftalmología IAP, Querétaro, MEXICO. Lozano Rechy, David: Macula Retina Consultores, Mexico City, MEXICO. Morales Canton, Virgilio: Hospital de la Ceguera APEC, Mexico City, MEXICO. Ramirez Estudillo, Juan: Centro Oftalmológico Mira SC, Mexico City, MEXICO. Barraza, Karen: Oftalmosalud SRL, Lima, PERU. Fernandez, Carlos: Oftalmolaser, Lima, PERU. Guzman, Miguel: TG Laser Oftalmica, Lima, PERU. Lujan, Silvio: Mácula D&T, Lima, PERU. Gawecki, Maciej: Dobry Wzrok, Gdańsk, POLAND. Herba, Ewa: Szpital Specjalistyczny Nr 1, Oddzial Okulistykiul, Bytom, POLAND. Michalska-Malecka, Katarzyna: Optomed, Rybnik, POLAND. Muzyka-Wozniak, Maria: SPEKTRUM Osrodek Okulistyki Klinicznej, Wroclaw, POLAND. Nester-Ostrowska, Kamila: Kliniczny Szpital Wojewódzki Nr 1 im F Chopina, Klinika Okulistyki, Rzeszów, POLAND. Oleksy, Piotr: Centrum Medyczne UNO-MED, Krakow, POLAND. Wowra, Bogumil: Szpital św Łukasza, Bielsko-Biała, POLAND. Wylęgała, Edward: Gabinet Okulistyczny Prof Edward Wylęgała, Katowice, POLAND. Budzinskaya, Maria: FSBI Scientific Research Institute of Eye Diseases, Moscow, RUSSIAN FEDERATION. Kulikov, Alexey: SM Kirov Medical Military Academy, St Petersburg, RUSSIAN FEDERATION. Morugova, Tatiana: Clinic Optimed, Ufa, RUSSIAN FEDERATION. Hurcikova, Maria: Nemocnica s poliklinikou Trebišov, Trebišov, SLOVAKIA. Kacerík, Marek: Fakultná nemocnica Trenčín, Očná klinika, Trenčín, SLOVAKIA. Lipkova, Blandina: Fakultná nemocnica s poliklinikou Žilina, Očné oddelenie, Žilina, SLOVAKIA. Abengoechea, Santiago: Centro de Oftalmologia Barraquer, Servicio Oftalmologia, Barcelona, SPAIN. Adan Civera, Alfredo: Hospital Clinic Barcelona, Servicio Oftalmologia, Barcelona, SPAIN. Amat, Pedro: VISSUM Instituto Oftalmológico de Alicante, Alicante, SPAIN. Cabrera, Francisco: Hospital Universitario de Gran Canaria, Servicio de Oftalmologia, Las Palmas de Gran Canaria, SPAIN. Cava, Carlos: Complejo Hospitalario Universitario Albacete, Servicio de Oftalmologia, Albacete, SPAIN. Garcia-Layana, Alfredo: Clinica Universitaria de Navarra, Servicio de Oftalmologia, Pamplona, SPAIN. Gomez Ulla, Francisco: Instituto Oftalmologico Gomez Ulla, Servicio de Oftalmologia, Santiago de Compostela, SPAIN. Ruiz Moreno, Jose Maria: Hospital Universitario Puerta de Hierro, Servicio de Oftalmologia, Majadahonda, SPAIN. Vela, Jose Ignacio: Hospital de Santa Creu I Sant Pau, Servicio de Oftalmologia, Barcelona, SPAIN. Eldem, Bora: Hacettepe University Medical Faculty, Department of Ophthalmology, Ankara, TURKEY. Mentes, Jale: Ege University Medical Faculty, Department of Ophthalmology, Izmir, TURKEY. Ozturk, Banu: Selcuk University Faculty of Medicine, Department of Ophthalmology, Konya, TURKEY. Aaberg Jr, Thomas: Foundation for Vision Research, Grand Rapids, MI, UNITED STATES. Abbey, Ashkan (Csaky, Karl): Texas Retina Associates, Dallas, TX, UNITED STATES. Abraham, Prema: Black Hills Eye Institute, Rapid City, SD, UNITED STATES. Alam, Suhail: Barnet Dulaney Perkins Eye Center, Sun City, AZ, UNITED STATES. Almony, Arghavan: Carolina Eye Associates, Southern Pines, NC, UNITED STATES. Amini, Payam: California Eye Specialists, Pasadena, CA, UNITED STATES. Antoszyk, Andrew: Char Eye Ear & Throat Association, Charlotte, NC, UNITED STATES. Baker, Carl: Paducah Retinal Center, Paducah, KY, UNITED STATES. Bertolucci, George: Eye Medical Center, Fresno, CA, UNITED STATES. Bochow, Thomas: Eye Care Associates of East Texas, Tyler, TX, UNITED STATES. Brown, Jamin: Retina-Vitreous Surgeons of Central New York, Liverpool, NY, UNITED STATES. Busquets, Miguel (Isernhagen, Ricky): Retina Associates of Kentucky, Lexington, KY, UNITED STATES. Campochiaro, Peter: Johns Hopkins Medicine, Wilmer Eye Institute, Baltimore, MD, UNITED STATES. Carlson, John: Retina Consultants of Southern California, Redlands, CA, UNITED STATES. Chan, Clement: Southern California Desert Retina Consultants, Palm Desert, CA, UNITED STATES. Chang, Emmanuel: Retina & Vitreous of Texas, Houston, TX, UNITED STATES. Chang, Jonathan: University of Wisconsin, Madison, WI, UNITED STATES. Charles, Steve (Calzada, Jorge): Charles Retina Institute, Germantown, TN, UNITED STATES. Chen, Judy (Fu, Arthur): West Coast Retina Medical Group Inc, San Francisco, CA, UNITED STATES. Danzig, Carl: Rand Eye, Deerfield Beach, FL, UNITED STATES. Das, Arup: University of New Mexico, Albuquerque, NM, UNITED STATES. Dessouki, Amr: Retinal Diagnostic Center, Campbell, CA, UNITED STATES. Do, Brian (Johnson, Mark): Retina Group of Washington, Chevy Chase, MD, UNITED STATES. Feiner, Leonard: Retina Associates of NJ, Teaneck, NJ, UNITED STATES. Ferrone, Philip: Long Island Vitreoretinal Consultants, Great Neck, NY, UNITED STATES. Fine, Howard: New Jersey Retina Research Foundation, Edison, NJ, UNITED STATES. Fox, Gregory M: Retina Associates, Shawnee Mission, KS, UNITED STATES. Foxman, Scott: Retinal & Ophthalmic Consultants PC, Northfield, NJ, UNITED STATES. Ghorayeb, Ghassan: West Virginia University Eye Institute, Morgantown, WV, UNITED STATES. Gonzalez, Victor: Valley Retina Institute PA, McAllen, TX, UNITED STATES. Greven, Craig: Wake Forest Baptist Medical Center, Winston Salem, NC, UNITED STATES. Gupta, Sunil: Retina Specialty Institute, Pensacola, FL, UNITED STATES. Hau, Vivienne (Hau, Vincent): Kaiser Permanente Riverside Medical Center, Riverside, CA, UNITED STATES. Heier, Jeffrey: Ophthalmic Consultants of Boston, Boston, MA, UNITED STATES. Holekamp, Nancy: Midwest Vision Research Foundation, Chesterfield, MO, UNITED STATES. Hsu, Jason (Regillo, Carl): Mid Atlantic Retina, Wills Eye Hospital, Cherry Hill, NJ, UNITED STATES. Hu, Allen: Cumberland Valley Retina PC, Hagerstown, MD, UNITED STATES. Jacoby, Rachael: University of Utah, Division of Gastroenterology/Hepatology, Salt Lake City, UT, UNITED STATES. Javey, Golnaz: Piedmont Eye Center, Lynchburg, VA, UNITED STATES. Javid, Cameron: Retina Associates Southwest PC, Tucson, AZ, UNITED STATES. Kapoor, Kapil: Wagner Macula & Retina Center, Virginia Beach, VA, UNITED STATES. Khanani, Arshad: Sierra Eye Associates, Reno, NV, UNITED STATES. Kim, Brian: University of Vermont Medical Center, Burlington, VT, UNITED STATES. Kwun, Robert: Retina Associates of Utah, Salt Lake City, UT, UNITED STATES. Laird, Philip: Retina Care Specialists, Palm Beach Gardens, FL, UNITED STATES. Lee, Seong: Strategic Clinical Research Group LLC, Willow Park, TX, UNITED STATES. Liu, Mimi: Colorado Retina Associates PC, Golden, CO, UNITED STATES. London, Nikolas: Retina Consultants San Diego, Poway, CA, UNITED STATES. Makkouk, Fuad (Jhaveri, Chirag): Retina Research Center, Austin, TX, UNITED STATES. Malik, Khurram: Emerson Clinical Research Institute, Falls Church, VA, UNITED STATES. Maturi, Raj: Midwest Eye Institute, Indianapolis, IN, UNITED STATES. McCabe, Frank: Vitreo-Retinal Associates PC, Worcester, MA, UNITED STATES. Moore, Jeffrey: Maine Eye Center, Portland, ME, UNITED STATES. Newell, Charles: Southern Vitreoretinal Association, Tallahassee, FL, UNITED STATES. Nielsen, Jared: Wolfe Eye Clinic, West Des Moines, IA, UNITED STATES. Oh, Kean: Associated Retinal Consultants PC, Traverse City, MI, UNITED STATES. Ohr, Matthew: OSU Eye Physicians & Surgeons, Columbus, OH, UNITED STATES. Osher, James: Cincinnati Eye Institute, Cincinnati, OH, UNITED STATES. Parke, D Wilkin: VitreoRetinal Surgery, Minneapolis, MN, UNITED STATES. Patel, Sugat: Midwest Retina, Dublin, OH, UNITED STATES. Patel, Sunil: Retina Research Institute of Texas, Abilene, TX, UNITED STATES. Rathod, Rajiv: Orange County Retina Medical Group, Santa Ana, CA, UNITED STATES. Rofagha, Soraya: East Bay Retina Consultants, Oakland, CA, UNITED STATES. Rosberger, Daniel: MaculaCare PLLC, New York, NY, UNITED STATES. Schadlu, Ramin: Arizona Retina and Vitreous Consultants, Phoenix, AZ, UNITED STATES. Shah, Sandeep: Retina Vitreous Center, Edmond, OK, UNITED STATES. Singer, Michael: Medical Center Ophthalmology Associates, San Antonio, TX, UNITED STATES. Singerman, Lawrence: Retina Associates of Cleveland Inc, Cleveland, OH, UNITED STATES. Stern, Jeffrey: Capital Region Retina, Albany, NY, UNITED STATES. Stoltz, Robert: Georgia Retina PC, Marietta, GA, UNITED STATES. Stone, Cameron: Western Carolina Retinal Associates PA, Asheville, NC, UNITED STATES. Suan, Eric: The Retina Care Center, Baltimore, MD, UNITED STATES. Sun, Jennifer: Beetham Eye Institute, Joslin Diabetes Center, Boston, MA, UNITED STATES. Suner, Ivan: Retina Associates of Florida LLC, Tampa, FL, UNITED STATES. Tlucek, Paul: Retina Northwest, Portland, OR, UNITED STATES. Torti, Robert: Retina Specialists, Plano, TX, UNITED STATES. Weber, Pamela: Island Retina, Shirley, NY, UNITED STATES. Wee, Raymond (Kokame, Gregg): Retina Consultants of Hawaii, Aiea, HI, UNITED STATES. Weishaar, Paul: Vitreo-Retinal Consultants, Wichita, KS, UNITED STATES. Williams, Thomas Reginald (Williams, Thomas): Graystone Eye, Hickory, NC, UNITED STATES. Wolfe, Jeremy: Associated Retinal Consultants PC, Royal Oak, MI, UNITED STATES. Wykoff, Charles C: Retina Consultants of Houston, The Woodlands, TX, UNITED STATES. Yiu, Glenn: UC Davis Eye Center, Sacramento, CA, UNITED STATES. RHINE (NCT03622593): Alezzandrini, Arturo: Oftalmos, Buenos Aires, ARGENTINA. Bafalluy, Joaquin: Centro Oftalmólogos Especialistas, Rosario, ARGENTINA. Furno Sola, Federico: Grupo Laser Vision, Rosario, ARGENTINA. Schlottmann, Patricio: Organización Médica de Investigación, Buenos Aires, ARGENTINA. Zambrano, Alberto: Fundación Zambrano, Buenos Aires, ARGENTINA. Zeolite, Carlos: Oftar, Mendoza, ARGENTINA. Chang, Andrew: Sydney Retina Clinic and Day Surgery, Sydney, AUSTRALIA. Chen, Fred: The Lions Eye Institute, Nedlands, AUSTRALIA. Fraser-Bell, Samantha: Sydney Eye Hospital, Sydney, AUSTRALIA. Mitchell, Paul: Sydney West Retina, Westmead, AUSTRALIA. Sandhu, Sukhpal: Retina Specialists Victoria, Rowville, AUSTRALIA. Wickremasinghe, Sanjeewa: Centre For Eye Research Australia, East Melbourne, AUSTRALIA. Wong, James: Strathfield Retina Clinic, Strathfield, AUSTRALIA. Avila, Marcos: Centro Brasileiro de Cirurgia de Olhos, Goiânia, BRAZIL. Belfort Jr, Rubens: Universidade Federal de São Paulo, Oftalmologia, São Paulo, BRAZIL. Bordon, Arnaldo: Hospital de Olhos de Sorocaba, Sorocaba, BRAZIL. Lavinsky, Daniel: Hospital de Clínicas de Porto Alegre, UFRGS, Porto Alegre, BRAZIL. Neto, Julio: Faculdade de Medicina do ABC, Santo André, BRAZIL. Penha, Fernando: Botelho Hospital da Visão, Blumenau, BRAZIL. Salomão, Gustavo: Cemape Centro Médico, São Paulo, BRAZIL. Taleb, Alexandre: Hospital de Olhos de Aparecida, Aparecida de Goiânia, BRAZIL. Zacharias, Leandro: Hospital das Clínicas da FMUSP, São Paulo, BRAZIL. Brent, Michael: University Health Network Toronto Western Hospital, Toronto, CANADA. Chow, David: Toronto Retina Institute, Toronto, CANADA. Dickinson, John: Queen Elizabeth II Health Sciences Centre, Department of Ophthalmology, Halifax, CANADA. Dollin, Michael: University of Ottawa Eye Institute, Ottawa, CANADA. Lalonde, Laurent: Institut De L'Oeil Des Laurentides, Boisbriand, CANADA. Ma, Patrick: University of British Columbia, Vancouver Coastal Health Authority, Vancouver, CANADA. Olivier, Sebastien: Hôpital Maisonneuve-Rosemont, Montreal, CANADA. Sheidow, Thomas: Ivey Eye Institute, London, CANADA. Williams, Geoff: Calgary Retina Consultants, Calgary, CANADA. Wong, David: Unity Health Toronto, Toronto,CANADA. Sun, Xiaodong: Shanghai First People's Hospital, Shanghai City, CHINA. Dusova, Jaroslava: Fakultní nemocnice Hradec Králové, Oční klinika, Hradec Králové, CZECH REPUBLIC. Ernest, Jan: Axon Clinical, Prague, CZECH REPUBLIC. Farkas, Andrej: Nemocnice Sokolov, Sokolov, CZECH REPUBLIC. Nemcansky, Jan: Fakultní nemocnice Ostrava, Ophthalmology clinic, Ostrava, CZECH REPUBLIC. Veith, Miroslav: Fakultní nemocnice Královské Vinohrady, Ophthalmology clinic, Prague, CZECH REPUBLIC. Larsen, Michael: Rigshospitalet Glostrup, Øjenafdelingen, Glostrup, DENMARK. Laugesen, Caroline: Sjællands Universitetshospital Roskilde, Øjenafdelingen, Roskilde, DENMARK. Vorum, Henrik: Aalborg Universitetshospital, Øjenafdelingen, Aalborg, DENMARK. Buffet, Sylvia: Centre Ophtalmologique d' Imagerie et de Laser, Paris, FRANCE. Razavi, Hessam: Centre Ophtalmologique St Exupéry, Ophtalmologie, Tours, FRANCE. Souied, Eric: CHI de Créteil, Ophtalmologie, Créteil, FRANCE. Agostini, Hansjurgen: Universitätsklinikum Freiburg, Klinik für Augenheilkunde, Freiburg, GERMANY. Kampik, Daniel (Ach, Thomas): Universitätsklinikum Würzburg, Augenklinik und Poliklinik, Würzburg, GERMANY. Lohmann, Chris P: Klinikum rechts der Isar der Technischen Universität München, Augenklinik, München, GERMANY. Priglinger, Siegfried (Wolf, Armin): LMU Klinikum der Universität, Augenklinik, München, GERMANY. Sandner, Dirk: Universitätsklinikum Carl Gustav Carus, Klinik und Poliklinik für Augenheilkunde, Dresden, GERMANY. Schuart, Claudia (Wecke, Thoalf): Universitätsklinikum Magdeburg AöR, Universitätsaugenklinik, Magdeburg, GERMANY. Seitz, Berthold: Universitätsklinikum des Saarlandes, Klinik für Augenheilkunde, Homburg, GERMANY. Fung, Nicholas (Wong, Ian): Queen Mary Hospital, Department of Ophthalmology, Aberdeen, HONG KONG. Lai, Timothy: Hong Kong Eye Hospital, CUHK Eye Centre, Mong Kok, HONG KONG. Kerenyi, Agnes: Bajcsy-Zsilinszky Hospital, Budapest, HUNGARY. Papp, Andras: Semmelweis Egyetem Szemészeti Vizsgálóhely, Budapest, HUNGARY. Szecsko, Timea: Péterfy Sándor Utcai Kórház-Rendelőintézet és Baleseti Központ, Szemészet KR, Budapest, HUNGARY. Vogt, Gábor: Magyar Honvédség Egészségügyi Központ, Szemészeti Osztály, Budapest, HUNGARY. Lanzetta, Paolo: Azienda Ospedaliero Universitaria S Maria della Misericordia di Udine, Clinica Oculistica, Udine, ITALY. Nardi, Marco: Nuovo Ospedale Santa Chiara, AOUP Presidio Ospedaliero di Cisanello, UO Oculistica Universitaria, Pisa, ITALY. Pertile, Grazia: Ospedale Classificato Equiparato Sacro Cuore Don Calabria, Dipartimento Oculistica, Negrar, ITALY. Ricci, Federico: Fondazione Policlinico Tor Vergata di Roma, UOSD Patologie Renitiche, Roma, ITALY. Virgili, Gianni: Azienda Ospedaliero Universitaria Careggi, SOD Oculistica, Firenze, ITALY. Kang, Se Woong: Samsung Medical Center, Seoul, REPUBLIC OF KOREA. Park, Kyu Hyung: Seoul National University Bundang Hospital, Gyeonggi-do, REPUBLIC OF KOREA. Yoon, Young Hee: Asan Medical Center, Seoul, REPUBLIC OF KOREA. Yu, HyeongGon: Seoul National University Hospital, Seoul, REPUBLIC OF KOREA. Yu, Seung Young: Kyung Hee University Hospital, Seoul, REPUBLIC OF KOREA. Borcz, Emilia: Ośrodek Chirurgii Oka Prof Zagórskiego, Rzeszów, POLAND. Kaluzny, Jakub: Oftalmika, Bydgoszcz, POLAND. Raczynska, Dorota: OPTIMUM Profesorskie Centrum Okulistyki, Gdańsk, POLAND. Romanczak, Dominika: Centrum Zdrowia MDM, Warszawa, POLAND. Romanowska-Dixon, Bożena: Szpital Uniwersytecki w Krakowie, Oddział Kliniczny Okulistyki i Onkologii Okulistycznej, Kraków, POLAND. Sikorski, Bartosz: Specjalistyczny Ośrodek Okulistyczny Oculomedica, Bydgoszcz, POLAND. Zaczek Zakrzewska, Karolina: PRYZMAT Poradnia Okulistyczna i Salon Optyczny w Gliwicach, Gliwice, POLAND. Zatorska, Barbara: CaminoMed, Tarnowskie Góry, POLAND. Figueira, Joao: Association for Innovation and Biomedical Research on Light, Coimbra, PORTUGAL. Gomes, Nuno: Hospital de Braga, Servico de Oftalmologia, Braga, PORTUGAL. Silva, Rufino: Espaço Médico de Coimbra, Coimbra, PORTUGAL. Vaz-Pereira, Sara: Hospital de Santa Maria, Servico de Oftalmologia, Lisboa, PORTUGAL. Abdulaeva, Elmira: Kuzlyar Ltd, Kazan, RUSSIAN FEDERATION. Bratko, Galina: Intersec Research and Technology Complex, Eye Microsurgery n a SN Fyodorov, Novosibirsk, RUSSIAN FEDERATION. Pozdeyeva, Nadezhda: Intersec Research and Technology Complex, Eye Microsurgery n a SN Fyodorov, Cheboksary, RUSSIAN FEDERATION. Yurieva, Tatiana: Intersec Research and Technology Complex, Eye Microsurgery n a SN Fyodorov, Irkutsk, RUSSIAN FEDERATION. Chee, Caroline: National University Hospital, Ophthalmology Department, Singapore, SINGAPORE. Rajagopalan, Rajesh: Tan Tock Seng Hospital, Ophthalmology Department, Singapore, SINGAPORE. Tan, Gavin: Singapore Eye Research Institute, Singapore, SINGAPORE. Aliseda, Daniel: Complejo Hospitalario de Navarra, Servicio de Oftalmología, Pamplona, SPAIN. Arias, Luis: Hospital Universitari de Bellvitge, Servicio de Oftalmología, L'Hospitalet de Llobregat, SPAIN. Desco, Carmen: FISABIO-Oftalmología Médica, Servicio de Oftalmología, Valencia, SPAIN. Escobar, Joan Josep: Hospital Dos de Maig, Pharmacy Service, Barcelona, SPAIN. Fernandez Vega, Alvaro: Instituto Oftalmológico Fernández-Vega, Servicio de Oftalmología, Oviedo, SPAIN. Figueroa, Marta: VISSUM Madrid, Servicio de Oftalmología, Madrid, SPAIN. Gallego-Pinazo, Roberto: Oftalvist Valencia, Valencia, SPAIN. Montero, Javier: Hospital Universitario Rio Hortega, Servicio de Oftalmología, Valladolid, SPAIN. Sararols, Laura: Hospital General de Catalunya, Servicio de Oftalmología, Sant Cugat del Vallès, SPAIN. Hatz, Katja: Vista Klinik Binningen, Binningen, SWITZERLAND. Chen, Shih-Jen: Taipei Veterans General Hospital, Ophthalmology, Taipei City, TAIWAN. Lai, Chi-Chun: Chang Gung Medical Foundation, Ophthalmology, Taoyuan, TAIWAN. Yang, Chang-Hao: National Taiwan University Hospital, Ophthalmology, Taipei City, TAIWAN. Chaikitmongkol, Voraporn: Maharaj Nakorn Chiang Mai Hospital, Ophthalmology Department, Chiang Mai, THAILAND. Pongsachareonnont, Pear: King Chulalongkorn Memorial Hospital, Ophthalmology Department, Bangkok, THAILAND. Ruamviboonsuk, Paisan: Rajavithi Hospital, Ophthalmology Department, Bangkok, THAILAND. Karabas, Levent: Kocaeli Üniversitesi Tıp Fakültesi, Department of Ophthalmology, Kocaeli, TURKEY. Ozcalışkan, Sehnaz (Perente, Irfan): Beyoğlu Göz Training and Research Hospital; Department of Ophthalmology, Istanbul, TURKEY. Sermet, Figen: Ankara Üniversitesi Tıp Fakültesi, Department of Ophthalmology, Ankara, TURKEY. Yilmaz, Gursel: Başkent Üniversitesi Tıp Fakültesi, Department of Ophthalmology, Ankara, TURKEY. Asaria, Riaz: Royal Free Hospital, London, UNITED KINGDOM. Burton, Ben: James Paget University Hospitals NHS Foundation Trust, Norfolk, UNITED KINGDOM. Cheong-Leen, Richard (George, Sheen): Hillingdon Hospital, Uxbridge, UNITED KINGDOM. Esposti, Simona: Moorfields Eye Hospital NHS Foundation Trust, London, UNITED KINGDOM. Ghanchi, Faruque: Bradford Royal Infirmary, Bradford, UNITED KINGDOM. Harris, Martin (Mehta, Hemal): Barnet Hospital, Royal Free London NHS Foundation Trust, Barnet, UNITED KINGDOM. Jackson, Tim: Kings College Hospital, London, UNITED KINGDOM. Jafree, Afsar: East Kent Hospitals University NHS Foundation Trust, Canterbury, UNITED KINGDOM. Lotery, Andrew: University Hospital Southampton NHS Foundation Trust, Southampton Eye Unit, Southampton, UNITED KINGDOM. McKibbin, Martin: St James University Hospital, Leeds, UNITED KINGDOM. Menon, Geeta: Frimley Park Hospital, Frimley, UNITED KINGDOM. Mohamed, Quresh: Gloucestershire Hospitals NHS Foundation Trust, Gloucester, UNITED KINGDOM. Pearce, Ian: Royal Liverpool University Hospital, St Paul's Clinical Eye Research Centre, Liverpool, UNITED KINGDOM. Peto, Tunde: Belfast Health and Social Care Trust, Royal Victoria Hospital, Belfast, UNITED KINGDOM. Ross, Adam: University Hospitals Bristol NHS Foundation Trust, Bristol Eye Hospital, Bristol, UNITED KINGDOM. Stone, Amy (Mahmood, Sajjad): Manchester Royal Eye Hospital, Manchester, UNITED KINGDOM. Talks, James: Royal Victoria Infirmary, Newcastle upon Tyne, UNITED KINGDOM. Varma, Deepali: Sunderland Eye Infirmary, Sunderland, UNITED KINGDOM. Adams, Serrhel: Northwest Arkansas Retina Associates, Springdale, AR, UNITED STATES. Adrean, Sean: Retina Consultants of Orange County, Fullerton, CA, UNITED STATES. Alfaro, Virgil: Charleston Neuroscience Institute, Ladson, SC, UNITED STATES. Awh, Carl C: Tennessee Retina PC, Nashville, TN, UNITED STATES. Barakat, Mark (Dugel, Pravin): Retinal Research Institute LLC, Phoenix, AZ, UNITED STATES. Baumal, Caroline: Tufts Medical Center Ophthalmology, Boston, MA, UNITED STATES. Bergstrom, Chris: Retina Consultants Of Carolina, Greenville, SC, UNITED STATES. Boyer, David: Retina-Vitreous Associates Medical Group, Beverly Hills, CA, UNITED STATES. Brown, David M: Retina Consultants of Houston, Houston, TX, UNITED STATES. Burgess, Stuart: Fort Lauderdale Eye Institute, Plantation, FL, UNITED STATES. Castellarin, Alessandro: California Retina Consultants, Santa Barbara, CA, UNITED STATES. Chaudhry, Nauman: Retina Group of New England, Waterford, CT, UNITED STATES. Chiang, Allen (Ho, Allen): Mid Atlantic Retina, Wills Eye Hospital, Philadelphia, PA, UNITED STATES. Connolly, Brian: Retina Associates of Western New York, Rochester, NY, UNITED STATES. Eichenbaum, David: Retina Vitreous Associates of Florida, Saint Petersburg, FL, UNITED STATES. Engstrom, Robert: The Retina Partners, Encino, CA, UNITED STATES. Falk, Naomi: The Retina Consultants, Slingerlands, NY, UNITED STATES. Fortun, Jorge: Bascom Palmer Eye Institute, Palm Beach Gardens, FL, UNITED STATES. Goldberg, Roger: Bay Area Retina Associates, Walnut Creek, CA, UNITED STATES. Hershberger, Vrinda: Florida Eye Associates, Melbourne, FL, UNITED STATES. Higgins, Patrick: Retina Center of New Jersey, Bloomfield, NJ, UNITED STATES. Khurana, Rahul: Northern California Retina Vitreous Associates, Mountain View, CA, UNITED STATES. Kuriyan, Ajay: University of Rochester Flaum Eye Institute, Rochester, NY, UNITED STATES. Kwong, Henry (Klein-Mascia, Kendra): Associated Retina Consultants, Phoenix, AZ, UNITED STATES. Liu, Judy (Thach, Allen): Retina Consultants of Nevada, Henderson, NV, UNITED STATES. Marcus, Dennis: Southeast Retina Center, Augusta, GA, UNITED STATES. Margherio, Alan: Associated Retinal Consultants, Grand Rapids, MI, UNITED STATES. Modi, Yasha: New York University, New York, NY, UNITED STATES. Oliver, Scott: University of Colorado, Department of Ophthalmology, Aurora, CO, UNITED STATES. Pearlman, Joel: Retinal Consultants Medical Group, Sacramento, CA, UNITED STATES. Perkins, Stephen: Southeastern Retina Associates, Knoxville, TN, UNITED STATES. Pieramici, Dante: California Retina Consultants, Santa Maria, CA UNITED STATES. Qureshi, Jawad: Retina Center of Texas, Grapevine, TX, UNITED STATES. Raskauskas, Paul: National Ophthalmic Research Institute, Fort Myers, FL, UNITED STATES. Rosenblatt, Brett: Long Island Vitreoretinal Consultants, Hauppauge, NY, UNITED STATES. Shah, Ankur: Prairie Retina Center, Springfield, IL, UNITED STATES. Shah, Rohan: Southeastern Retina Associates, Chattanooga, TN, UNITED STATES. Sharma, Sumit (Singh, Rishi): Cleveland Clinic Foundation, Cole Eye Institute, Cleveland, OH, UNITED STATES. Sheth, Veeral: University Retina and Macula Associates PC, Oak Forest, IL, UNITED STATES. Spinak, David J: Retina Center Northwest, Silverdale, WA, UNITED STATES. Steinle, Nathan: California Retina Consultants, Bakersfield, CA, UNITED STATES. Stoller, Glenn: Ophthalmic Consultants of Long Island, Oceanside, NY, UNITED STATES. Tabassian, Ali: Retina Institute of Virginia, Richmond, VA, UNITED STATES. Taylor, Stanford (Chittum, Mark): Retina Consultants of Southern Colorado, Colorado Springs, CO, UNITED STATES. Thompson, John: Retina Specialists, Towson, MD UNITED STATES. Uchiyama, Eduardo: Retina Group of Florida, Fort Lauderdale, FL, UNITED STATES. Wells, John A: Palmetto Retina Center, West Columbia, SC, UNITED STATES. Wong, Robert: Austin Retina Associates, Austin, TX, UNITED STATES. Yates, Paul: University of Virginia Ophthalmology, Charlottesville, VA, UNITED STATES. Zheutlin, Jeffrey: Vitreo-Retinal Associates, Grand Rapids, MI, UNITED STATES.
Data sharing statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://go.roche.com/data_sharing). Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Conflicts of interest
M. Shimura, Consulting fees (Chugai, Roche), Lecture fees (Chugai); S. Kitano, Consulting fees (Chugai), Speaker fees (Bayer, Novartis); N. Ogata, None; Y. Mitamura, Grants (Santen), Speaker fee (Bayer, Chugai, Novartis, Santen); H. Oh, Grants (Chugai), Lecture fees (Alcon, Bayer, Kowa, Novartis, Santen); H. Ochi, Employee (Chugai); S. Ohsawa, Employee (Chugai); A. Hirakata, None.
Footnotes
Corresponding Author: Masahiko Shimura
Study group investigators: The YOSEMITE and RHINE investigators and study sites are listed in acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masahiko Shimura, Email: masahiko@v101.vaio.ne.jp.
on behalf of the YOSEMITE and RHINE Investigators:
Matthias Bolz, Oliver Findl, Andreas Pollreisz, Martin Weger, Vesselin Daskalov, Aneta Misheva, Iva Petkova, Daniela Tosheva Guneva, Petja Vassileva, Pierre Loic Cornut, Jean Francois Korobelnik, Olivier Lebreton, Ramin Tadayoni, Nicole Eter, Nicolas Feltgen, Carsten Framme, Katrin Lorenz, Georg Spital, Gyorgy Bator, András Seres, Lajos Szalczer, Edit Toth-Molnar, Attila Vajas, Balazs Varsanyi, Michaella Goldstein, Jaime Levy, Haia Morori-Katz, Irit Rosenblatt, Barak Yoreh, Francesco Bandello, Carlo Cagini, Leonardo Mastropasqua, Massimo Nicolo, Maria Cristina Parravano, Francesco Viola, Akira Fukutomi, Ken Hayashi, Akito Hirakata, Shigeru Honda, Yasuhiro Ikeda, Yasuki Ito, Tsutomu Kawasaki, Kazuhiro Kimura, Genichiro Kishino, Shigehiko Kitano, Takatoshi Maeno, Yoshinori Mitamura, Tomoaki Murakami, Kousuke Noda, Akira Obana, Nahoko Ogata, Hideyasu Oh, Osamu Sawada, Akito Shimouchi, Masahiko Shimura, Masahiko Sugimoto, Iichiro Sugita, Hitoshi Takagi, Kei Takayama, Teruyo Tanabe, Tsutomu Yasukawa, Shigeo Yoshida, Renata Garcia, David Lozano Rechy, Virgilio Morales Canton, Juan Ramirez Estudillo, Karen Barraza, Carlos Fernandez, Miguel Guzman, Silvio Lujan, Maciej Gawecki, Ewa Herba, Katarzyna Michalska-Malecka, Maria Muzyka-Wozniak, Kamila Nester-Ostrowska, Piotr Oleksy, Bogumil Wowra, Edward Wylęgała, Maria Budzinskaya, Alexey Kulikov, Tatiana Morugova, Maria Hurcikova, Marek Kacerík, Blandina Lipkova, Santiago Abengoechea, Alfredo Adan Civera, Pedro Amat, Francisco Cabrera, Carlos Cava, Alfredo Garcia-Layana, Francisco Gomez Ulla, Jose Maria Ruiz Moreno, Jose Ignacio Vela, Bora Eldem, Jale Mentes, Banu Ozturk, Thomas Aaberg Jr, Ashkan (Csaky, Karl) Abbey, Prema Abraham, Suhail Alam, Arghavan Almony, Payam Amini, Andrew Antoszyk, Carl Baker, George Bertolucci, Thomas Bochow, Jamin Brown, Miguel (Isernhagen, Ricky) Busquets, Peter Campochiaro, John Carlson, Clement Chan, Emmanuel Chang, Jonathan Chang, Steve (Calzada, Jorge) Charles, Judy (Fu, Arthur) Chen, Carl Danzig, Arup Das, Amr Dessouki, Brian (Johnson, Mark) Do, Leonard Feiner, Philip Ferrone, Howard Fine, Gregory M Fox, Scott Foxman, Ghassan Ghorayeb, Victor Gonzalez, Craig Greven, Sunil Gupta, Vivienne (Hau, Vincent) Hau, Jeffrey Heier, Nancy Holekamp, Jason (Regillo, Carl) Hsu, Allen Hu, Rachael Jacoby, Golnaz Javey, Cameron Javid, Kapil Kapoor, Arshad Khanani, Brian Kim, Robert Kwun, Philip Laird, Seong Lee, Mimi Liu, Nikolas London, Fuad (Jhaveri, Chirag) Makkouk, Khurram Malik, Raj Maturi, Frank McCabe, Jeffrey Moore, Charles Newell, Jared Nielsen, Kean Oh, Matthew Ohr, James Osher, D Wilkin Parke, Sugat Patel, Sunil Patel, Rajiv Rathod, Soraya Rofagha, Daniel Rosberger, Ramin Schadlu, Sandeep Shah, Michael Singer, Lawrence Singerman, Jeffrey Stern, Robert Stoltz, Cameron Stone, Eric Suan, Jennifer Sun, Ivan Suner, Paul Tlucek, Robert Torti, Pamela Weber, Raymond (Kokame, Gregg) Wee, Paul Weishaar, Thomas Reginald (Williams, Thomas) Williams, Jeremy Wolfe, Charles C Wykoff, Glenn Yiu, Arturo Alezzandrini, Joaquin Bafalluy, Federico Furno Sola, Patricio Schlottmann, Alberto Zambrano, Carlos Zeolite, Andrew Chang, Fred Chen, Samantha Fraser-Bell, Paul Mitchell, Sukhpal Sandhu, Sanjeewa Wickremasinghe, James Wong, Marcos Avila, Rubens Belfort Jr, Arnaldo Bordon, Daniel Lavinsky, Julio Neto, Fernando Penha, Gustavo Salomão, Alexandre Taleb, Leandro Zacharias, Michael Brent, David Chow, John Dickinson, Michael Dollin, Laurent Lalonde, Patrick Ma, Sebastien Olivier, Thomas Sheidow, Geoff Williams, David Wong, Xiaodong Sun, Jaroslava Dusova, Jan Ernest, Andrej Farkas, Jan Nemcansky, Miroslav Veith, Michael Larsen, Caroline Laugesen, Henrik Vorum, Sylvia Buffet, Hessam Razavi, Eric Souied, Hansjurgen Agostini, Daniel (Ach, Thomas) Kampik, Chris P Lohmann, Siegfried (Wolf, Armin) Priglinger, Dirk Sandner, Claudia (Wecke, Thoalf) Schuart, Berthold Seitz, Nicholas (Wong, Ian) Fung, Timothy Lai, Agnes Kerenyi, Andras Papp, Timea Szecsko, Gábor Vogt, Paolo Lanzetta, Marco Nardi, Grazia Pertile, Federico Ricci, Gianni Virgili, Se Woong Kang, Kyu Hyung Park, Young Hee Yoon, HyeongGon Yu, Seung Young Yu, Emilia Borcz, Jakub Kaluzny, Dorota Raczynska, Dominika Romanczak, Bożena Romanowska-Dixon, Bartosz Sikorski, Karolina Zaczek Zakrzewska, Barbara Zatorska, Joao Figueira, Nuno Gomes, Rufino Silva, Sara Vaz-Pereira, Elmira Abdulaeva, Galina Bratko, Nadezhda Pozdeyeva, Tatiana Yurieva, Caroline Chee, Rajesh Rajagopalan, Gavin Tan, Daniel Aliseda, Luis Arias, Carmen Desco, Joan Josep Escobar, Alvaro Fernandez Vega, Marta Figueroa, Roberto Gallego-Pinazo, Javier Montero, Laura Sararols, Katja Hatz, Shih-Jen Chen, Chi-Chun Lai, Chang-Hao Yang, Voraporn Chaikitmongkol, Pear Pongsachareonnont, Paisan Ruamviboonsuk, Levent Karabas, Sehnaz (Perente, Irfan) Ozcalışkan, Figen Sermet, Gursel Yilmaz, Riaz Asaria, Ben Burton, Richard (George, Sheen) Cheong-Leen, Simona Esposti, Faruque Ghanchi, Martin (Mehta, Hemal) Harris, Tim Jackson, Afsar Jafree, Andrew Lotery, Martin McKibbin, Geeta Menon, Quresh Mohamed, Ian Pearce, Tunde Peto, Adam Ross, Amy (Mahmood, Sajjad) Stone, James Talks, Deepali Varma, Serrhel Adams, Sean Adrean, Virgil Alfaro, Carl C Awh, Mark (Dugel, Pravin) Barakat, Caroline Baumal, Chris Bergstrom, David Boyer, David M Brown, Stuart Burgess, Alessandro Castellarin, Nauman Chaudhry, Allen (Ho, Allen) Chiang, Brian Connolly, David Eichenbaum, Robert Engstrom, Naomi Falk, Jorge Fortun, Roger Goldberg, Vrinda Hershberger, Patrick Higgins, Rahul Khurana, Ajay Kuriyan, Henry (Klein-Mascia, Kendra) Kwong, Judy (Thach, Allen) Liu, Dennis Marcus, Alan Margherio, Yasha Modi, Scott Oliver, Joel Pearlman, Stephen Perkins, Dante Pieramici, Jawad Qureshi, Paul Raskauskas, Brett Rosenblatt, Ankur Shah, Rohan Shah, Sumit (Singh, Rishi) Sharma, Veeral Sheth, David J Spinak, Nathan Steinle, Glenn Stoller, Ali Tabassian, Stanford (Chittum, Mark) Taylor, John Thompson, Eduardo Uchiyama, John A Wells, Robert Wong, Paul Yates, and Jeffrey Zheutlin
References
- 1.Terasaki H, Ogura Y, Kitano S, Sakamoto T, Murata T, Hirakata A, et al. Management of diabetic macular edema in Japan: a review and expert opinion. Jpn J Ophthalmol. 2018;62:1–23. doi: 10.1007/s10384-017-0537-6. [DOI] [PubMed] [Google Scholar]
- 2.Morizane Y, Morimoto N, Fujiwara A, Kawasaki R, Yamashita H, Ogura Y, et al. Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol. 2019;63:26–33. doi: 10.1007/s10384-018-0623-4. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida S, Murakami T, Nozaki M, Suzuma K, Baba T, Hirano T, et al. Review of clinical studies and recommendation for a therapeutic flow chart for diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2021;259:815–836. doi: 10.1007/s00417-020-04936-w. [DOI] [PubMed] [Google Scholar]
- 4.Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68. doi: 10.1016/j.preteyeres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat Rev Drug Discov. 2017;16:635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 6.Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TY, Loewenstein A, et al. The angiopoietin/Tie pathway in retinal vascular diseases: a review. Retina. 2021;41:1–19. doi: 10.1097/IAE.0000000000003003. [DOI] [PubMed] [Google Scholar]
- 7.Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond) 2021;35:1305–1316. doi: 10.1038/s41433-020-01377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regula JT, Lundh-von-Leithner P, Foxton R, Barathi VA, Cheung CMG, Bo-Tun SB, et al. Targeting key angiogenic pathways with a bispecific Cross MAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8:1265–1288. doi: 10.15252/emmm.201505889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741–755. doi: 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]
- 11.Genentech Inc. FDA approves Genentech’s Vabysmo, the first bispecific antibody for the eye, to treat two leading causes of vision loss. 2022. https://www.gene.com/media/press-releases/14943/2022-01-28/fda-approves-genentechs-vabysmo-the-firs. Accessed 20 Apr 2022.
- 12.Vabysmo. Package insert. Genentech Inc; 2022.
- 13.Chugai Pharmaceutical Co., Ltd. Chugai obtains regulatory approval for Vabysmo, the first bispecific antibody in ophthalmology, for neovascular age-related macular degeneration and diabetic macular edema. 2022. https://www.chugai-pharm.co.jp/english/news/detail/20220328160002_909.html. Accessed 20 Apr 2022.
- 14.Maruko I, Okada AA, Iida T, Hasegawa T, Izumi T, Kawai M, et al. Brolucizumab-related intraocular inflammation in Japanese patients with age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol. 2021;259:2857–2859. doi: 10.1007/s00417-021-05136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka K, Koizumi H, Tamashiro T, Itagaki K, Nakayama M, Maruko I, et al. Short-term results for brolucizumab in treatment-naïve neovascular age-related macular degeneration: a Japanese multicenter study. Jpn J Ophthalmol. 2022;66:379–385. doi: 10.1007/s10384-022-00922-3. [DOI] [PubMed] [Google Scholar]
- 16.Eter N, Singh RP, Abreu F, Asik K, Basu K, Baumal C, et al. YOSEMITE and RHINE: phase 3 randomized clinical trials of faricimab for diabetic macular edema: study design and rationale. Ophthalmol Sci. 2021;2:100111. doi: 10.1016/j.xops.2021.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100:787–795. doi: 10.1136/bjophthalmol-2015-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepah YJ, Sadiq MA, Boyer D, Callanan D, Gallemore R, Bennett M, et al. Twenty-four–month outcomes of the ranibizumab for edema of the macula in diabetes–protocol 3 with high dose (READ-3) study. Ophthalmology. 2016;123:2581–2587. doi: 10.1016/j.ophtha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Brown DM, Emanuelli A, Bandello F, Barranco JJE, Figueira J, Souied E, et al. KESTREL and KITE: 52-week results from two phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol. 2022;238:157–172. doi: 10.1016/j.ajo.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto M, Tsukitome H, Okamoto F, Oshika T, Ueda T, Niki M, et al. Clinical preferences and trends of anti–vascular endothelial growth factor treatments for diabetic macular edema in Japan. J Diabetes Investig. 2019;10:475–483. doi: 10.1111/jdi.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogura Y, Shiraga F, Terasaki H, Ohji M, Ishida S, Sakamoto T, et al. Clinical practice pattern in management of diabetic macular edema in Japan: survey results of Japanese retinal specialists. Jpn J Ophthalmol. 2017;61:43–50. doi: 10.1007/s10384-016-0481-x. [DOI] [PubMed] [Google Scholar]
- 22.Eylea. Package insert. Regeneron Pharmaceuticals, Inc; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://go.roche.com/data_sharing). Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.