Abstract
The current pandemic coronavirus disease-19 (COVID-19) is still a global medical and economic emergency with over 244 million confirmed infections and over 4.95 million deaths by October 2021, in less than 2 years. Severe acute respiratory syndrome (SARS), the Middle East respiratory syndrome coronavirus (MERS), and COVID-19 are three recent coronavirus pandemics with major medical and economic implications. Currently, there is no effective treatment for these infections. One major pathological hallmark of these infections is the so-called ‘cytokine storm,’ which depicts an unregulated production of inflammatory cytokines inducing detrimental inflammation leading to organ injury and multiple organ failure including severe pulmonary, cardiovascular, and kidney failure in COVID-19. Several studies have suggested the potential of curcumin to inhibit the replication of some viruses similar to coronaviruses. Multiple experimental and clinical studies also reported the anti-inflammatory potential of curcumin in multiple infectious and inflammatory disorders. Thus, we hypothesized that curcumin may provide antiviral and anti-inflammatory effects for treating COVID-19. Although these studies suggest that curcumin could serve as an adjuvant treatment for COVID-19, its molecular mechanisms are still debated, especially its potential to modulate the toll-like receptors/TIR-domain-containing adapter-inducing interferon-β/nuclear factor kappa-light-chain-enhancer of activated B cells (TLR/TRIF/NF-κB) pathway. The preliminary results showed that curcumin modulates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, a common pathway controlling cytokine production in multiple infectious and inflammatory disorders. Here, we hypothesize and discuss whether curcumin treatment may provide antiviral and anti-inflammatory clinical advantages for treating COVID-19 by modulating the TLR/TRIF/NF-κB pathway. We also review the current data on curcumin and discuss potential experimental and clinical studies that require defining its potential clinical implications in COVID-19.
Keywords: Coronaviruses, curcumin, cytokine storm, TLRs/TRIF/NF-κB pathway
Introduction
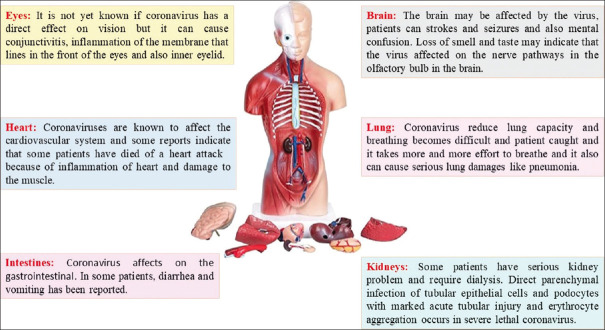
COVID-19 became a global public health challenge in late 2019, and til October 2021, has produced over 219 million infections worldwide and killed 4.55 million patients during the first 16 months.[1,2] Coronaviruses have an average diameter of 100 nm, and they are spherical or oval viruses that stain as negative particles with large spikes of glycoproteins on the surface inducing a typical crown-like shape when observed by electron microscopy.[3,4] Coronavirus is a positive-sense single-stranded RNA virus that harbors the largest genome among currently known RNA viruses with a genome length of about 26–32 kb.[5,6] According to current clinical records, coronaviruses cause mild respiratory tract disorder and also lead to cardiovascular and renal dysfunction[7,8,9,10,11] [Figure 1]. Death in coronavirus infections is normally due to cardiovascular and respiratory failure.[2,12,13,14] Along with the effects of these viruses on various body systems, recent studies reveal that the main cause of death from coronavirus infections is the so-called inflammatory cytokine storm, a chaotic production of inflammatory cytokines causing detrimental inflammation, cytotoxicity, and coagulopathies leading to acute lung, cardiovascular, and kidney failure. This inflammatory storm is a fatal consequence of coronavirus infections and a major clinical target for treating these infectious disorders.[15,16]
Figure 1.
Effects of coronaviruses on multiple body organ systems
Cytokine Storm and Its Role in the Pathophysiology of COVID-19 Disease
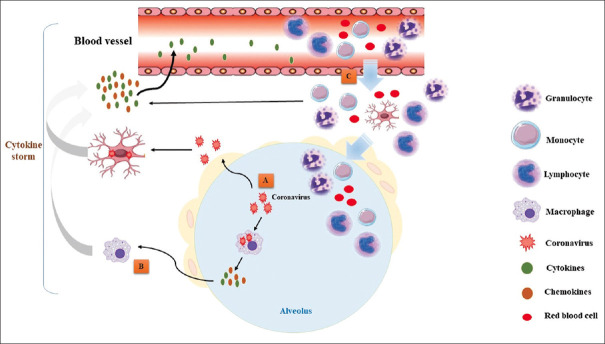
The cytokine storm syndrome (CSS) is a form of systemic inflammatory response syndrome (SIRS) that can be triggered by a variety of clinical conditions including viral infections.[17,18,19] The cytokine storm syndrome occurs when large numbers of immune cells are activated by an infection and release inflammatory cytokines, which in turn, induces the recruitment and activation of additional immune cells and triggers an inflammatory waterfall.[19,20,21] Activated immune cells including monocytes, macrophages, B, T, natural killer, and dendritic cells release inflammatory cytokines, which other immune cells in a positive feedback loop of pathogenic inflammation.[19,22,23] The immune cells are activated by pathogens, stressed, or infected cells through receptor-ligand interactions.[19] Toll-like receptors play a critical role in recognizing pathogens and activating the immune cells to fight the infection. Immune cytokines recruit more effector immune cells such as T-cells and inflammatory monocytes (which differentiate into macrophages) into the site of infection such as the lung tissue.[19,24,25] This process is the critical immune response to fight infections, but when unregulated, overzealous production of inflammatory cytokines becomes life-threatening which can be more dangerous than the original infection and can cause a chaotic inflammatory storm inducing cardiovascular shock, and multiple organ failure.[26,27] During this cytokine storm, in coronavirus infections, large amounts of inflammatory cytokines such as interferons (IFN-α, IFN-γ), interleukins (IL-1β, IL-6, IL-12, IL-18, IL-33), tumor necrosis factor-alpha (TNF-α), etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) attack the lung and produce respiratory failure.[16,28,29] The cytokine storm depicts a chaotic detrimental immune response that causes tissue damage leading to lethal multiple organ failure such as severe pulmonary, cardiovascular, and kidney failure in SARS-CoV-2 infection[30,31] [Figure 2].
Figure 2.
Coronavirus infections can trigger a pulmonary inflammatory ‘cytokine storm’. (A) Coronaviruses infect lung epithelial cells and activate alveolar macrophages to release immune cytokines/chemokines (including interferons). (B) These factors can activate other immune cells triggering positive feedback in a pathogenic inflammatory loop causing a more extensive immune response and the inflammatory cytokine storm. (C) These factors attract more inflammatory cells and cause migration of leukocytes from blood vessels into the site of inflammation (lung), and these cells release additional factors contributing to the cytokine storm leading to lung inflammation and fibrosis
Toll-like Receptors (TLR) Signaling Pathway
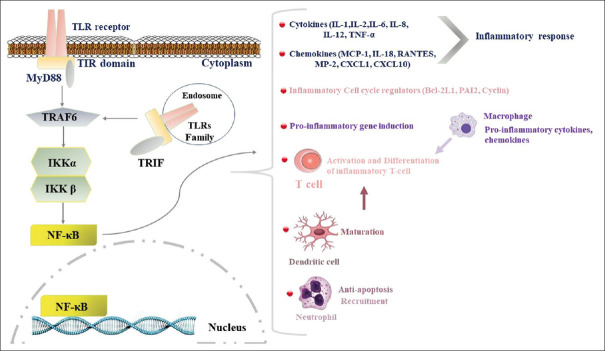
At the molecular level, the TLR/TRIF/NF-κB pathway is a common factor in activating cytokine production in multiple infectious and inflammatory disorders.[32,33] Thus, this pathway can also be a potential therapeutic target to blunt detrimental inflammation in COVID-19. However, the effects of coronaviruses on this pathway are not clarified exactly. However, in recent years, some limited studies evaluated the role of toll-like receptors (TLR) in the pathogenesis of COVID-19 which is discussed below.[34,35] Toll-like receptors (TLR) are a transmembrane protein that belongs to the pattern recognition receptor (PRR) family and it is critical in bacterial infections by recognizing bacterial endotoxin. As part of the innate immune system, TLR4 triggers intracellular signaling activating NF-κB, and thereby, inflammatory cytokine production. NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls DNA transcription for inflammatory cytokines during bacterial or viral infections.[33,36] In between, TIR-domain-containing adapter-inducing interferon-β (TRIF) is a mediator adapter for TLR intracellular signals. It mediates the rather delayed cascade of two TLR-associated signaling cascades such as NF-κB[37,38] [Figure 3].
Figure 3.
TLR/TRIF/NF-κB pathway. TLRs family recognizes pathogens on the cell surface. Then, these receptors recruit adaptor (TRIF and TRAF6) proteins and activate downstream signaling cascades to activate nuclear factor kappa-B (NF-κB). NF-κB is an inducible transcription factor that activates the transcription of inflammatory factors. NF-κB targets inflammation not only directly by inducing inflammatory cytokines, chemokines, and adhesion molecules, but also by regulating the cell proliferation, apoptosis, morphogenesis, and differentiation
TLRS in the Pathogenesis of SARS-COV-2
Based on the previous studies, TLRs could play a crucial role in the COVID-19 disease. It was suggested that activation of the TLRs in COVID-19 infection could lead to the production of pro-inflammatory cytokines, such as IL-1β.[34,35] Moreover, immunological and pathological concerns of and one of the main causes of death in COVID-19 patients are due to the interaction of TLRs with virus particles.[34] Studies on COVID-19 have indicated that the pathologic role of TLR4 in excessive inflammation in COVID-19 patients as it leads to activation of death signals in infected patients.[39,40] Also, other studies indicated that TLR3/TLR4 adaptor TRIF plays a critical role in the pathogenesis of COVID-19.[34] Also, it was indicated and suggested that TLR3 and TLR-7 may contribute to some inflammation and production of NF-κB in viral diseases such as influenza and COVID-19.[34,41] While about the role of TLR-4 this effect is not direct and after involving patients with secondary bacterial infections in viral diseases the TLR-4 can be a trigger.[40] It was indicated that stimulation of TLR2 leads to activation of the innate immune response during viral diseases such as COVID-19 and similar diseases,[34,42] According to the mentioned concept about the role of multiple types of TLR in the pathogenesis of COVID-19 and based on mentioned limit studies it could conceivably be hypothesized that TLRs have both harmful and possibly beneficial effects in COVID-19 infection. Thus using of Both antagonists and agonists of TLRs, based on the type of the TLR, should be examined to determine the therapeutic and harmful effects in COVID-19 infection.[34]
Suggesting of Therapeutic Approaches Based on TLRs in the Pathogenesis of SARS-COV-2
Recent studies have shown the therapeutic potential of some synthetic and naturally occurring compounds, which are often used in inflammatory pulmonary disorders similar to those produced by coronaviruses.[43,44] Other than flavonoids, phenols such as curcumin (diferuloylmethane) have significant anti-inflammatory and antioxidant properties[45,46,47] [Figure 4]. Curcumin can attenuate inflammatory responses during influenza infections by inhibiting the NF-κB pathway.[48,49,50,51] Curcumin can inhibit virus infection by either inhibiting viral replication or suppressing cellular signaling pathways such as NF-κB.[46,52,53] Despite the antiviral and anti-inflammatory potential of curcumin in some viral infections, it is unknown whether curcumin can affect coronavirus infections or its molecular mechanism of action. However, in recent year efforts for evaluation of the role of the herbal compound such as curcumin for COVID-19 treatment were increased and these studies have been reinforced that curcumin could serve as an adjuvant drug. Despite the absence of specific studies addressing the mechanism of action of curcumin in the treatment of COVID-19, and also because there is no specific study that can accurately identify the exact mechanisms of this compound against COVID-19, especially clarification TLR/TRIF/NF-κB pathway, thus we hypothesize that curcumin may control inflammation by inhibiting the NF-κB pathway. If so, curcumin may provide clinical advantages or critical insights to treat coronavirus infections including the current COVID-19 pandemic.
Figure 4.
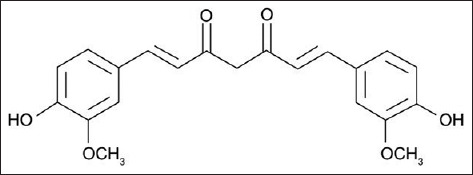
Chemical structures of curcumin
Hypothesis
Given the current COVID-19 pandemic and the lack of effective treatment to either inhibit viral replication or detrimental inflammation, we evaluated potential therapeutic strategies for treating COVID-19 patients and their pulmonary, cardiovascular, and renal dysfunction. Based on the previous studies reporting the potential of curcumin in several viral infections, we hypothesized that curcumin may modulate NF-κB over-activation during COVID-19, and thereby blunt detrimental inflammation and even viral replication.
Evaluation of the Hypothesis
Data bank search such as Scopus, PubMed, Web of Science, Google Scholar, Elsevier, Science Direct, Core Collection, and Cochrane using keywords curcumin and coronavirus such as SARS, MERS, or COVID-19 were performed to evaluate published studies on the antiviral and immunomodulatory role of curcumin in coronavirus infected subjects. We also searched for curcumin plus coronavirus and the TLR/TRIF/NF-κB. Although in recent year efforts for evaluation of the role of herbal compounds such as curcumin for COVID-19 treatment were increased and these studies have been suggested that curcumin could serve as an adjuvant treatment of COVID-19,[54,55,56,57] but there is no specific study that can accurately identify the exact mechanisms of this compound against COVID-19, especially clarification TLR/TRIF/NF-κB pathway, thus we did not find any specific paper or article about the role of curcumin in the management of TLR/TRIF/NF-κB pathway in COVID-19 pathogenesis.
Discussion
The current COVID-19 outbreak is an international health emergency affecting millions of people worldwide. Coronaviruses are a group of viruses that cause respiratory diseases in mammals and birds.[58,59] In humans, coronaviruses cause respiratory tract infections that are typically mild, such as the common cold, though rarer forms such as SARS (Severe Acute Respiratory Syndrome), Middle East respiratory syndrome coronavirus (MERS), and COVID-19 (coronavirus disease-19) can be lethal.[58,59] Symptoms vary in humans, but according to current data, they cause upper respiratory tract diseases, and around 3-5% of infected patients can die especially elderly or patients with comorbidities. Also, there is significant information about the long-term respiratory sequels of the infection, but its pathologic mechanism remains unknown. Recent studies show that the pathogenesis of coronaviruses is mediated by an inflammatory cytokine storm.[15,60] Coronavirus infections induce an overwhelming inflammatory response in some patients, activating the production of inflammatory cytokines and progressively recruiting new hordes of immune cells, which further exacerbate the inflammatory response.[61,62,63] The mechanisms inducing this “cytokine storm” and their contribution to fatal COVID-19 are still under investigation.[63,64] In many infected patients, the “cytokine storm” caused severe acute respiratory distress syndrome, when the lungs are unable to provide enough oxygenation.[65,66] The cytokine storm is associated with a dramatic increase of inflammatory cytokines, particularly those that control migration and activation of macrophages.[65,67,68] Large amounts of inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) are produced in the lung,[65,68,69] and they induce detrimental inflammation that can cause organ dysfunction and lethal multiple organ failure[70] [Figure 2]. Also, these inflammatory cytokines bind their cognate receptors on immune cells to further induce cytotoxic effects.[70,71] The molecular mechanism leading to this inflammatory storm during coronavirus infections is unknown,[71] but it is expected that NF-κB, a key intracellular pathway controlling cytokine production, can play a critical role in COVID-19.[72] Recent studies have reported that the TLR/TRIF/NF-κB pathway plays a critical role in inducing a cytokine storm in other viral infections such as influenza,[73,74] suggesting that this pathway can be a pivotal target to control this detrimental inflammatory process in diverse conditions.[27,75,76] However, the clinical implication of the NF-κB pathway in coronavirus infections is unknown. Previous studies reported that SARS-CoV infections induce IL-6 and TNF-α production via NF-κB, which is activated through I-κBα degradation. These results suggest that the SARS-CoV spike protein may play an important role in the pathogenesis to induce cytokine production.[77,78,79,80] These studies also provided comprehensive insights that host cell transcriptome changes after coronavirus infection are induced by NF-κB,[77,78,79,80] and also the critical role of TLRs contributing to highly pathogenic coronavirus infections,[81,82,83] but their clinical role is still unknown. TLRs, modulate multiple inflammatory pathways and cytokines during coronavirus and other similar viral infections.[84] TLRs family is a transmembrane protein that belongs to the pattern recognition receptor (PRR) family. TLRs trigger intracellular signaling activating NF-κB and inducing inflammatory cytokine production to initiate the innate immune response to pathogens. NF-κB is a protein complex that controls DNA transcription for inflammatory cytokines to trigger a defensive response to bacterial or viral infections.[84,85] TRIF is an adapter contributing to the intracellular signaling of TLRs. TRIF mediates TLR-associated signaling cascades such as NF-κB.[86,87] These studies reveals that the TLR/TRIF/NF-κB pathway plays a critical role inducing the innate inflammatory response to fight infections, but at the same time over-activation of this pathway can cause an overwhelming production of multiple inflammatory factors leading to organ failure. The specific inhibition of all these factors represents a major clinical challenge, but NF-κB may represent a specific target to control the complex inflammatory response of diverse factors. Thus, it is important to study NF-κB in coronavirus infections to determine the pathological factors inducing this inflammatory storm but also to gain insights for new treatments against infectious disorders such as COVID-19.
Of course, it is important to mention this point according to current knowledge about the role of multiple types of TLR in the pathogenesis of COVID-19 studies hypothesized that TLRs have both harmful and possibly beneficial effects in COVID-19 infection. It suggested that TLR3 and TLR-7 may contribute to some inflammation and production of NF-κB in viral diseases such as influenza and COVID-19, but this was not approved yet.[72,88,89] While about the role of TLR-4 this effect is not direct and after involving patients with secondary bacterial infections in viral diseases the TLR-4 can be a trigger.[40] Also, it was suggested that TLR2 leads to activation of the innate immune response during viral diseases such as COVID-19 and similar diseases.[34,42]
Because there is no specific study that clarified and accurately identifies the exact role of the TLR/TRIF/NF-κB signaling pathway on the pathogenesis of COVID-19, thus it seems that doing a study with this goal of evaluation of TLR/TRIF/NF-κB signaling pathway on the pathogenesis of COVID-19 is necessitates.
As mentioned above, COVID-19 is a global medical challenge without effective treatment, potential therapeutic approaches and protective agents with antiviral and/or anti-inflammatory potential are critical and urgent needs. Curcumin is a suitable candidate that may provide both antiviral and anti-inflammatory advantages for COVID-19.[46,48,49,51,53] Curcumin (diferuloylmethane) the most abundant component of turmeric is extracted from rhizomes of the plant Curcuma longa.[90,91,92] This non-nutritive yellow pigment is an established nutraceutical dietary phenol and has significant medicinal and pharmacological values.[93,94,95] Curcumin exerts biological effects through its antioxidant, anti-apoptotic, and anti-inflammatory activity.[96] Recent studies performed in vertebrate and invertebrate experimental models revealed that curcumin can provide therapeutic advantages in multiple metabolic and infectious diseases.[93,97,98,99] Curcumin treatment can blunt inflammation by reducing inflammatory biomarkers and improving the symptoms of patients with inflammatory and autoimmune diseases.[100,101] Furthermore, chronic curcumin administration reduces TNF-α, IL-1β, and Tumor Growth Factor-Beta1 (TGF-β1) levels in different experimental models of inflammatory disorders such as sepsis-induced acute lung injury and.[101,102] The potential of curcumin to inhibit different viral infections can provide critical information about the common mechanism regulating these infections and the potential to design curcumin derivatives that can increase its effectiveness.[45,47] Some of the most significant results about curcumin in viral infections show its potential to control both viral replication and inflammation in influenza infections.[47,103] These studies strongly suggest the potential of curcumin for treating other viral infections affecting the respiratory tract.
Recent studies also reported the potential of curcumin and artemisinin combination therapy as an anti-microbial strategy in malaria, it is thus tempting to propose similar curcumin and artemisinin-based oral spray for COVID-19.[104,105] Multiple studies have shown the anti-inflammatory potential of curcumin.[106,107] Curcumin is a highly pleiotropic molecule capable of interacting with numerous targets involved in inflammation. Multiple studies including cell culture, animal research, and clinical trials concur in showing that curcumin has beneficial effects in multiple disorders such as inflammatory bowel diseases, pancreatitis, arthritis, chronic anterior uveitis, and certain types of cancer.[100,107] Consistent with its anti-inflammatory potential, curcumin blocks cytokine production including critical inflammatory factors such as IL-1, IL-6, and TNFα in different experimental models. These effects correlate with the potential of curcumin to blunt the inflammatory cytokine storm and thereby the clinical symptoms of patients with viral infections such as influenza or Ebola.[46,108] Although curcumin has been investigated in some viral infections, its effects on coronavirus infectious are unknown.[46,108] The potential of curcumin to modulate the TLR/TRIF/NF-κB pathway was clarified in some studies about autoimmune human and experimental models.[109,110] However, most of these studies focused on the potential of curcumin to blunt inflammation,[110,111] but its potential role on COVID-19 infection and the possible role of this herbal compound on the management of TLR/TRIF/NF-κB signaling pathway during the pathogenesis of COVID-19 is still not well characterized and need for assessment.
Conclusions
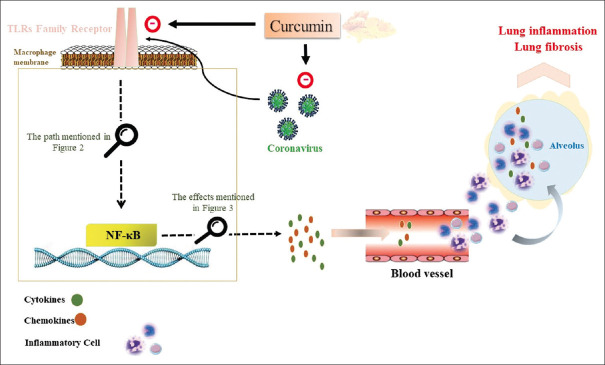
In summary, scientific literature shows the antiviral and anti-inflammatory potential of curcumin in multiple experimental and clinical studies. Although some initial results suggest that curcumin can control influenza virus replication, it is uncertain the molecular mechanism of action and whether it applies to other viruses. The stronger results suggest that curcumin can provide clinical advantages to control inflammation in multiple experimental and clinical studies. These studies suggest that curcumin may regulate a common pathway to all these disorders, and preliminary results strongly suggest the potential of curcumin to regulate the TLR/TRIF/NF-κB pathway [Figure 5]. According to these studies, curcumin may provide clinical advantages for its antiviral and anti-inflammatory potential to control detrimental inflammation in COVID-19 patients. However, the specific target(s) of curcumin and its molecular mechanism of action and specificity are still unknown, and especially its role on TLR/TRIF/NF-κB signaling pathway during the pathogenesis of COVID-19 is still not well defined and thus its clinical implications for treating COVID-19 infectious and its inflammatory consequences need for evaluation in an experimental and or clinical study.
Figure 5.
Curcumin may inhibit coronaviruses replication, and inhibit the TLRs/TRIF/NF-κB pathway to blunt cytokine production and deleterious inflammation in the infected patients
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Infection Prevention and Control Guidance for Long-term Care Facilities in the Context of COVID-19: Interim Guidance, 21 March 2020. World Health Organization. 2020 [Google Scholar]
- 2.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak? J Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. doi: 10.1016/j.jaut. 2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satija N, Lal SK. The molecular biology of SARS coronavirus. Ann N Y Acad Sci. 2007;1102:26–38. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer K-A, Dee M, Britton P, Hiscox JA. Role of phosphorylation clusters in the biology of the coronavirus infectious bronchitis virus nucleocapsid protein. Virology. 2008;370:373–81. doi: 10.1016/j.virol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai MM. Coronavirus: Organization, replication and expression of genome. Ann Rev Microbiol. 1990;44:303. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- 6.Prentice E, Denison MR. The Nidoviruses. Springer Berlin/Heidelberg; 2001. The Cell Biology of Coronavirus Infection; pp. 609–14. [DOI] [PubMed] [Google Scholar]
- 7.de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, Feldmann F, Brining DL, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci. 2013;110:16598–603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–4. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis? Int J Infect Dis. 2020;10 doi: 10.1016/j.ijid.2020.03.017. doi: 10.1016/j.ijid. 2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferonγ-related cytokine storm in SARS patients. J Med Virol. 2005;75:185–94. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netea MG, van der Meer JW, van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: Not enough, or too much of a good thing? Trends Immunol. 2003;24:254–8. doi: 10.1016/s1471-4906(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome? J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–31. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel? J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [Google Scholar]
- 22.Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–40. doi: 10.1093/cid/ciy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50–2. doi: 10.1016/j.retram.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 25.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Us D. [Cytokine storm in avian influenza] Mikrobiyol Bul. 2008;42:365–80. [PubMed] [Google Scholar]
- 29.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci. 2014;111:3799–804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–5. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation? Signal Transduct Target Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dash P, Thomas PG. II. Springer; 2014. Host detection and the stealthy phenotype in influenza virus infection. Influenza Pathogenesis and Control; pp. 121–47. [DOI] [PubMed] [Google Scholar]
- 34.Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93:2735–9. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onofrio L, Caraglia M, Facchini G, Margherita V, Placido SD, Buonerba C. Toll-like receptors and COVID-19: A two-faced story with an exciting ending. Future Sci. 2020;6:FSO605. doi: 10.2144/fsoa-2020-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen J, et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity. 2014;40:501–14. doi: 10.1016/j.immuni.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Park S-J, Youn H-S. Isoliquiritigenin suppresses the toll − interleukin-1 receptor domain-containing adapter inducing interferon-β (TRIF)-dependent signaling pathway of toll-like receptors by targeting TBK1. J Agric Food Chem. 2010;58:4701–5. doi: 10.1021/jf100484r. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 39.Aboudounya MM, Heads RJ. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation? Mediators Inflamm 2021. 2021:8874339. doi: 10.1155/2021/8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandão SC, Ramos JdOX, Dompieri LT, Godoi ET, Figueiredo JL, Sarinho ESC, et al. Is toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021;58:102–10. doi: 10.1016/j.cytogfr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bortolotti D, Gentili V, Rizzo S, Schiuma G, Beltrami S, Strazzabosco G, et al. TLR3 and TLR7 RNA sensor activation during SARS-COV-2 infection? Microorganisms. 2021;9:1820. doi: 10.3390/microorganisms9091820. doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. NatImmunol. 2021;22:1–10. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) Statpearls: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 44.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 45.Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin? BioMed Res Int 2014. 2014 doi: 10.1155/2014/186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D-Y, Shien J-H, Tiley L, Chiou S-S, Wang S-Y, Chang T-J, et al. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119:1346–51. [Google Scholar]
- 47.Mathew D, Hsu W-L. Antiviral potential of curcumin. J Funct Foods. 2018;40:692–9. [Google Scholar]
- 48.Fan Z, Yao J, Li Y, Hu X, Shao H, Tian X. Anti-inflammatory and antioxidant effects of curcumin on acute lung injury in a rodent model of intestinal ischemia reperfusion by inhibiting the pathway of NF-Kb. Int J Clin Exp Pathol. 2015;8:3451–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Lubbad A, Oriowo M, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–35. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 50.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern Med Rev. 2009;14:141–53. [PubMed] [Google Scholar]
- 51.Menon VP, Sudheer AR. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; 2007. Antioxidant and anti-inflammatory properties of curcumin; pp. 105–25. [DOI] [PubMed] [Google Scholar]
- 52.Ou JL, Mizushina Y, Wang SY, Chuang DY, Nadar M, Hsu WL. Structure–activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013;280:5829–40. doi: 10.1111/febs.12503. [DOI] [PubMed] [Google Scholar]
- 53.Yadav V, Mishra K, Singh D, Mehrotra S, Singh V. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–97. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 54.Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–20. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soni VK, Mehta A, Ratre YK, Tiwari AK, Amit A, Singh RP, et al. Curcumin, a traditional spice component, can hold the promise against COVID-19?? Eur J Pharmacol. 2020;886:173551. doi: 10.1016/j.ejphar.2020.173551. doi: 10.1016/j.ejphar.2020.173551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Sci Nutr. 2020;8:5215–27. doi: 10.1002/fsn3.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocha FA, de Assis MR. Curcumin as a potential treatment for COVID-19. Phytother Res. 2020;34:2085–7. doi: 10.1002/ptr.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience. 2020;42:1387–410. doi: 10.1007/s11357-020-00233-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velavan TP, Meyer CG. The Covid-19 epidemic. Trop Med Int Health. 2020;25:278–80. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okabayashi T, Kariwa H, Yokota Si, Iki S, Indoh T, Yokosawa N, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–24. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi Y, Lagniton PN, Ye S, Li E, Xu R-H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–66. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiel V, Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine Growth Factor Rev. 2008;19:121–32. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–32. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S, Zhang Y, Lui S, Peng H, Mackey V. Coronaviruses and the associated potential therapeutics for the viral infections. J Infect Dis Ther. 2020. [Last accessed on 2022 Mar 30]. p. 8. Available from: https://www.researchgate.net/profile/Lichun-Sun/publication/339796674_Coronaviruses_and_the_Associated_Potential_Therapeutics_for_the_Viral_Infections/links/5e6662d6a6fdcc37dd126b52/Coronaviruses-and-the-Associated-Potential-Therapeutics-for-the-Viral-Infections.pdf.
- 65.Ye Q, Wang B, Mao J. Cytokine storm in COVID-19 and treatment. J Infect. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L, Song K, Huang Y. Coronavirus disease 2019 (COVID-19) and cardiovascular complications. J Cardiothorac Vasc Anesth. 2020;35:1860–5. doi: 10.1053/j.jvca.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minoia F, Davì S, Alongi A, Ravelli A. Criteria for Cytokine Storm Syndromes.Cytokine Storm Syndrome. Berlin/Heidelberg: Springer; 2019. pp. 61–79. [Google Scholar]
- 68.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons? Cytokine Growth Factor Rev. 2020;85:104502. doi: 10.1016/j.cytogfr.2020.05.002. 10.1016/j.meegid.2020.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulert GS, Zhang K. Cytokine Storm Syndrome. Berlin/Heidelberg: Springer; 2019. Genetics of Acquired Cytokine Storm Syndromes; pp. 113–29. [DOI] [PubMed] [Google Scholar]
- 70.Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J Infect. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta KK, Khan MA, Singh SK. Constitutive inflammatory cytokine storm: A major threat to human health. J Interferon Cytokine Res. 2020;40:19–23. doi: 10.1089/jir.2019.0085. [DOI] [PubMed] [Google Scholar]
- 72.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007;13:460–9. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q-W, Su Y, Sheng J-T, Gu L-M, Zhao Y, Chen X-X, et al. Anti-influenza a virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways? PloS One. 2018;13:e0191793. doi: 10.1371/journal.pone.0191793. doi: 10.1371/journal.pone.0191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Planz O. Influenza viruses and intracellular signalling pathways. Berl Munch Tierarztl Wochenschrift. 2006;119:101–11. [PubMed] [Google Scholar]
- 75.Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci. 2011;108:12018–23. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xi-zhi JG, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol Berlin/Heidelberg. 2017;39:541–50. doi: 10.1007/s00281-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-κB. Virology. 2007;365:324–35. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, Regla-Nava JA, Castaño-Rodriguez C, Fernandez-Delgado R, et al. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–37. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro . Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeDiego ML, Nieto-Torres JL, Regla-Nava JA, Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88:913–24. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuk J-M, Jo E-K. Toll-like receptors and innate immunity. J Bacteriol Virol. 2011;41:225–35. [Google Scholar]
- 82.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 83.Turvey SE, Broide DH. Innate immunity. J Allergy Clin Immunol. 2010;125:S24–32. doi: 10.1016/j.jaci.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–20. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 85.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186:1199–208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 87.Takeda K, Akira S. TLR signaling pathways. Semin Immunol Berlin/Heidelberg. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front Immunol. 2020;11:3446. doi: 10.3389/fimmu.2020.598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: Potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:561–7. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng Y-F, Guo L, Xie Y-S, Liu Y-S, Zhang J, Wu Q-W, et al. Curcumin rescues aging-related loss of hippocampal synapse input specificity of long term potentiation in mice. Neurochem Res. 2013;38:98–107. doi: 10.1007/s11064-012-0894-y. [DOI] [PubMed] [Google Scholar]
- 91.Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, et al. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35:374–9. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 92.Panchal HD, Vranizan K, Lee CY, Ho J, Ngai J, Timiras PS. Early anti-oxidative and anti-proliferative curcumin effects on neuroglioma cells suggest therapeutic targets. Neurochem Res. 2008;33:1701–10. doi: 10.1007/s11064-008-9608-x. [DOI] [PubMed] [Google Scholar]
- 93.Cole GM, Teter B, Frautschy SA. Berlin/Heidelberg: Springer; 2007. Neuroprotective effects of curcumin.The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; pp. 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Motaghinejad M, Karimian M, Motaghinejad O, Shabab B, Yazdani I, Fatima S. Protective effects of various dosage of curcumin against morphine induced apoptosis and oxidative stress in rat isolated hippocampus. Pharmacol Rep. 2015;67:230–5. doi: 10.1016/j.pharep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 95.Shojaii A, Motaghinejad M, Norouzi S, Motevalian M. Evaluation of anti-inflammatory and analgesic activity of the extract and fractions of Astragalus hamosus in animal models. Iran J Pharm Res. 2015;14:263–9. [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ. Curcumin and neurodegenerative diseases: A perspective. Expert Opin Invest Drugs. 2012;21:1123–40. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- 98.Motaghinejad M, Motevalian M, Fatima S, Hashemi H, Gholami M. Curcumin confers neuroprotection against alcohol-induced hippocampal neurodegeneration via CREB-BDNF pathway in rats. Biomed Pharmacother. 2017;87:721–40. doi: 10.1016/j.biopha.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Huang H-C, Chang P, Dai X-L, Jiang Z-F. Protective effects of curcumin on amyloid-β-induced neuronal oxidative damage. Neurochem Res. 2012;37:1584–97. doi: 10.1007/s11064-012-0754-9. [DOI] [PubMed] [Google Scholar]
- 100.Akram M, Shahab-Uddin AA, Usmanghani K, Hannan A, Mohiuddin E, Asif M. Curcuma longa and curcumin: A review article. Rom J Biol Plant Biol. 2010;55:65–70. [Google Scholar]
- 101.Xiao X, Yang M, Sun D, Sun S. Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res. 2012;176:e31–9. doi: 10.1016/j.jss.2011.11.1032. [DOI] [PubMed] [Google Scholar]
- 102.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;3:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang M, Lee G, Si J, Lee S-J, You HJ, Ko G. Curcumin shows antiviral properties against norovirus? Molecules. 2016;21:1401. doi: 10.3390/molecules21101401. doi: 10.3390/molecules21101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nandakumar DN, Nagaraj VA, Vathsala PG, Rangarajan P, Padmanaban G. Curcumin-artemisinin combination therapy for malaria. Antimicro Agents Chemother. 2006;50:1859–60. doi: 10.1128/AAC.50.5.1859-1860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Isacchi B, Bergonzi MC, Grazioso M, Righeschi C, Pietretti A, Severini C, et al. Artemisinin and artemisinin plus curcumin liposomal formulations: Enhanced antimalarial efficacy against Plasmodium berghei-infected mice. Eur J Pharm Biopharm. 2012;80:528–34. doi: 10.1016/j.ejpb.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 106.Rao TS, Basu N, Siddiqui H. Anti-inflammatory activity of curcumin analogues. Indian J Med Res. 2013;137:574–8. [PubMed] [Google Scholar]
- 107.Araujo C, Leon L. Biological activities of curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–8. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 108.Sordillo PP, Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo. 2015;29:1–4. [PubMed] [Google Scholar]
- 109.Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang J, et al. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med. 2015;38:199–206. doi: 10.1179/2045772313Y.0000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dai J, Gu L, Su Y, Wang Q, Zhao Y, Chen X, et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol. 2018;54:177–87. doi: 10.1016/j.intimp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 111.Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–46. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]