Abstract
In accordance with Article 43 of Regulation (EC) No 396/2005, the Department of Agriculture, Food and the Marine (DAFM) of Ireland requested EFSA to re‐evaluate the acute (short‐term) risk for European consumers related to nicotine exposure via residues in rose hips (code 0154050), teas (Camellia sinensis, 0610000) and capers (0850020) at the level equal to the temporary maximum residue level (tMRL) established in Reg. (EU) 2022/1290 and the tMRL recently voted in the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee). The requestor asked EFSA to perform the calculation with a revised version of EFSA PRIMo rev. 3.1, in which the Irish children consumption data for tea should be replaced with consumption data for tea from another EU Member State, since the Irish consumption data implemented in EFSA PRIMo rev. 3.1 were not confirmed by the Food Safety Authority of Ireland. In the present assessment, EFSA confirmed the previously identified potential acute exposure risks for the intake of nicotine through rose hips jam containing residues at the current EU MRL of 0.3 mg/kg. The new tMRL for rose hips of 0.2 mg/kg is unlikely to pose a risk to consumers. For the consumption of tea and capers containing residues at the levels of the current and the recently voted tMRLs, a consumer health risk is unlikely.
Keywords: nicotine, pesticides, MRL, acute dietary risk assessment, tea, rosehips, capers
Background (as provided by the requestor)
Nicotine is an alkaloid which exhibits insecticidal activity. The compound was previously used as an active substance (a.s.) in plant protection products (PPP). PPPs containing nicotine were withdrawn from the market in June 2009 under Directive 91/414/EEC 1 and the default maximum residue level (MRL) (0.01 mg/kg) was applied to all products. An EFSA opinion (EFSA, 2011) led to specific temporary MRLs (higher than the default MRL) being set in Annex IIIA of Regulation (EC) No 396/2005 2 for nicotine in rose hips, herbs and edible flowers, teas, herbal infusions and spices (Commission Regulation (EU) No 812/2011 3 ). To‐date, the source of nicotine residues in the commodities concerned has not been satisfactorily determined.
Recently, the European Commission requested the European Food Safety Authority (EFSA) to provide a targeted risk assessment for certain maximum residue levels (MRLs) for nicotine.
The risk assessment for nicotine was carried out by EFSA (EFSA, 2022); the conclusions of this assessment were used as the basis for a proposed reduction of the nicotine MRL in tea from 0.6 to 0.5 mg/kg (Item B.09 – Draft Commission Regulation (EU) amending Annexes II, III, IV and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benzalkonium chloride (BAC), chlorpropham, didecyldimethylammonium chloride (DDAC), flutriafol, metazachlor, nicotine, profenofos, quizalofop‐P, sodium aluminium silicate, thiabendazole and triadimenol in or on certain products (SANTE/10090/2022)). This draft Regulation was voted during the Standing Committee (Phytopharmaceuticals – Pesticide Residues) meeting held on 26–27th September 2022. It was voted unanimously in favour by all Member States.
Section 3.2.1 in the 2022 EFSA statement on nicotine cites an exceedance of the Acute Reference Dose (ARfD) for Irish children (115% of the ARfD) assuming consumption of a large portion of tea leaves (Camellia sinensis) containing residues equal to the legal limit (0.6 mg/kg) (EFSA, 2022) The EFSA statement also outlines that the exposure calculations give an indication that the existing temporary MRL for tea is not sufficiently protective for children.
The PRIMo (v3.1) calculator identified an acute exposure risk for Irish children based on a large portion (P97.5) for dried tea leaves (Camellia sinensis) of 30.6 g/person, equivalent to 1.53 g/kg body weight. Footnote 8 in the EFSA statement on nicotine (EFSA, 2022) considers “2.5 g of tea leaves to produce 250 g of tea infusion”. Therefore, using this default dilution factor, the following fluid intake was calculated based on the current large portion in PRIMo (v3.1) for dried tea leaves (Camellia sinesis) for Irish children: 30.6 g/person 2.5 g = 12.24 and 12.24 × 250 = 3,060 mL or 3.06 L. This is an unrealistic fluid consumption rate for a child.
The Irish University Nutrition Alliance (IUNA), 4 with responsibility for the development of national databases of dietary intake in Ireland, has confirmed that a discrepancy in the data exists. Analysis of acute consumption of black tea infusion, per person per day, for consuming days only (n = 594) was carried out on the data from the Irish National Children's Food Survey (2003–2004), which underpins the Irish data for children in PRIMo. This assessment showed the 97.5th percentile (P97.5) for black tea infusion to be 511.4 g per person per day. Assuming 200 g infusion = 2 g tea leaves, this is equivalent to 5.1 g of tea leaves per person, much lower than the estimate in the EFSA statement (30.6 g per person). This discrepancy has been acknowledged by the Food Safety Authority of Ireland (FSAI) and will be updated as part of the next PRIMo revision.
The difference in the large portion of dried tea leaves (Camellia sinensis) for children, (5.1 g per person versus EFSA estimate 30.6 g per person) resulted in a significant over‐estimation of the acute risk to Irish children. It is anticipated that an updated risk assessment will decrease the estimated dietary exposure compared to the original risk assessment. Moreover, as the consumption amount of tea leaves (Camellia sinensis) for Irish children is 6‐fold lower than the value considered in the EFSA statement, there appears to be no issue of public health concern for the nicotine intake for Irish children. As previous risk assessments have overestimated the risks, public safety has not been compromised.
Terms of reference (as provided by the requestor)
EFSA is requested, according to Article 43 of Regulation (EC) No 396/2005, to re‐evaluate the acute (short‐term) risk for European consumers related to nicotine exposure via residues in rose hips (code 0154050), teas (Camellia sinensis, 0610000) and capers (0850020) at the level equal to the MRL established in Reg. (EU) 2022/1290 and the MRL recently voted in the Standing Committee on Plants, Animals, Food and Feed (PAFF Committee) (i.e. 0.6 and 0.5 mg/kg for teas).
The risk assessment shall be performed with the newest version of the PRIMo model, based on the available residue definitions, using the acute reference dose derived by EFSA in 2009 (EFSA, 2009).
With due consideration to the reasons outlined above, the large portion of dried tea leaves (Camellia sinensis) for Irish children in PRIMo should be excluded and instead the second highest large portion for this commodity considered from another Member State as appropriate.
Assessment
As requested, EFSA performed the acute (short‐term) dietary risk assessment for the three food commodities, i.e. rose hips, tea and capers. The exposure calculations for rose hips and capers were performed with the standard setting of EFSA PRIMo rev. 3.1; for the exposure calculation for tea, the EFSA PRIMo rev. 3.1 was modified, replacing the consumption data for children as outlined in Section 3.
In addition, EFSA performed a calculation, reporting the amount of tea containing residues at the level of the current and the new tMRL that could be consumed leading to an exposure equal to the ARfD for nicotine.
EFSA did not re‐assess the following elements:
Toxicological reference values for nicotine;
The existing residue definitions for enforcement and risk assessment;
The origin of nicotine residues in the three food commodities under assessment;
The origin of the existing and the recently voted MRLs for nicotine in the commodities under assessment (rose hips, tea and capers);
The acute and chronic consumer risk related to the MRLs on nicotine established for other commodities.
1. Toxicological reference values
In 2009, EFSA derived the following toxicological reference values for nicotine (EFSA, 2009):
Acceptable daily intake (ADI): 0.0008 mg/kg body weight per day.
ARfD: 0.0008 mg/kg body weight.
More details on the toxicological assessment can be retrieved in the previous EFSA assessments (EFSA, 2009, 2022).
2. Residue levels and residue definition for enforcement
The enforcement residue definition established in Regulation (EC) No 396/2005 comprises the parent compound nicotine only.
The current temporary MRLs established by Regulation (EU) 2022/1290 5 for rose hips, teas and capers and the relevant descriptions of the part of the crop to which the MRL refers to are reported in Table 1.
Table 1.
Existing/new temporary MRLs for nicotine
| Code (a) | Commodity | Existing tMRL (b) | new tMRL (c) | Part of the product to which MRLs apply |
|---|---|---|---|---|
| Residue definition for enforcement: Nicotine | ||||
| 0154050 | Rose hips | 0.3 (f) | 0.2 (d) | Whole product after removal of caps, crown and stems (description for the group of berries and small fruits) |
| 0610000 | Teas | 0.6 (f) | 0.5 (e) | Dried leaves, stalk and flowers, whether fermented or otherwise treated |
| 0850020 | Capers | 4 (f) | 0.07 (d) | Dried product whole, crushed or ground (description for spices) |
MRL: maximum residue level.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
tMRL: temporary MRL established by Regulation (EU) 2022/1290.
New tMRLs voted in Standing Committee on Plants, Animals, Food and Feed (PAFF), Section Phytopharmaceuticals – Pesticide Residues (document SANTE/10090/2022), hold on 26–27 September 2022; new MRLs not yet applicable.
Footnote to the new tMRL established in the Regulation voted in September 2022: Scientific evidence is not conclusive to demonstrate that nicotine occurs naturally in the concerned crop and to elucidate its mechanism of formation. When re‐viewing the MRL, the Commission will take into account the information, if it is submitted by 27/9/2029, or, if that information is not submitted by that date, the lack of it.
Footnote to the new tMRL established in the Regulation voted in September 2022: Scientific evidence is not conclusive to demonstrate that nicotine occurs naturally in the concerned crop and to elucidate its mechanism of formation. Temporary MRL valid until 27/09/2025. After this date, the MRL will be 0,4 mg/kg unless further modified by a Regulation in light of new information provided by 30 June 2025 at the latest.
Footnote to the existing tMRL established in Regulation (EU) 2022/1290: Scientific evidence is not conclusive to demonstrate that nicotine occurs naturally in the concerned crop and to elucidate its mechanism of formation. When re‐viewing the MRL, the Commission will take into account the information, if it is submitted by 19 October 2021, or, if that information is not submitted by that date, the lack of it.
In September 2022, the Standing Committee on Plants, Animals, Food and Feed voted on a Regulation to amend the temporary MRLs for nicotine, to take into account new information and the assessment performed by EFSA (2022). As EFSA identified a risk for the existing temporary MRLs for rose hips and tea (0.3 and 0.6 mg/kg, respectively), the MRLs were lowered to 0.2 and 0.5 mg/kg, respectively, to protect consumers. For tea, in addition to the lowering from 0.6 to 0.5 mg/kg, a specific provision foresees an automatic decline from 0.5 to 0.4 mg/kg in 2025, unless data supporting the need to maintain the higher level of 0.5 would be submitted by 30 June 2025.
3. Acute (short‐term) exposure assessment
The consumer exposure assessment was performed with a modified version of PRIMo rev. 3.1. for nicotine. As requested in the Terms of Reference, the consumption data of tea (unprocessed dry leaves) for Irish children used in the acute exposure assessment (1.53 g/kg body weight), was replaced.
To identify the alternative consumption data, the information notified to EFSA in the framework of the development of PRIMo 3 and PRIMo 3.1. was reviewed. For tea the following large portion consumption data for EU children (reported as dry tea leaves) were provided to EFSA (listed in descending order):
IE children: 1.53 g/kg bw.
FR children (11–14 years): 1.1 g/kg bw.
DE children: 0.23 g/kg bw.
IT children: 0.11 g/kg bw.
Hence, the second highest consumption data for acute exposure assessment of children is the large portion (LP) for French children. It is noted that the French consumption data are also likely to overestimate the actual consumption. Considering the mean body weight of 46.3 kg notified together with the consumption data for the French children of 11–14 years, and a default dilution factor of 100 for tea infusion, the tea consumption data would result in a consumption of 5 L of tea infusion per day. EFSA recommends that in the context of the development of a new version of the EFSA PRIMo tool the available information in the comprehensive food consumption database should be carefully analysed to identify realistic, robust information for the exposure assessment for tea. However, for the purpose of the current request, the French LP was used, noting that the results are likely to lead to an overestimation of the exposure.
For the current assessment, the following changes were introduced in PRIMo rev. 3.1, compared to the version of PRIMo that is currently made available on the EFSA website: 6
The LP for the acute exposure assessment for children (1.53 g/kg bw) was replaced with consumption data from France (i.e. 1.1 g tea/kg body weight for 11–14 years old French children);
The body weight of Irish children (20 kg) was replaced by the body weight of French children (11–14 years), i.e. 46.3 kg.
For the remaining commodities – rose hips and capers – the acute exposure calculation was updated by performing an exposure calculation with new temporary MRLs.
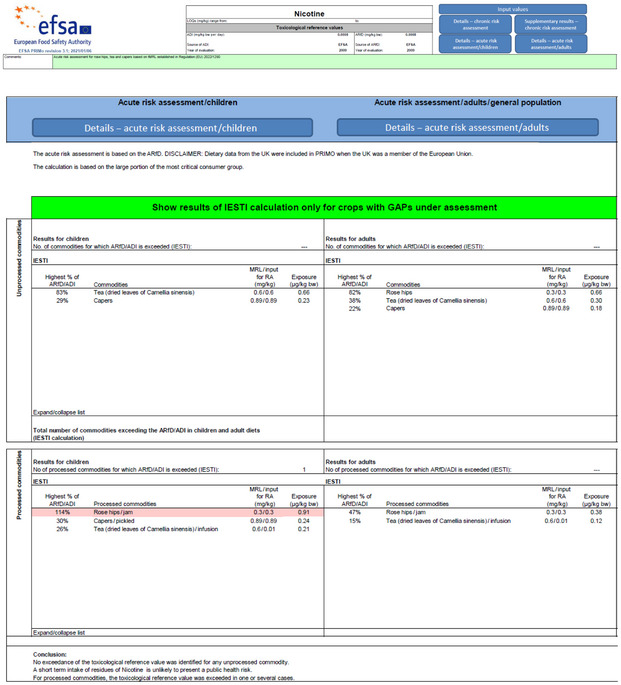
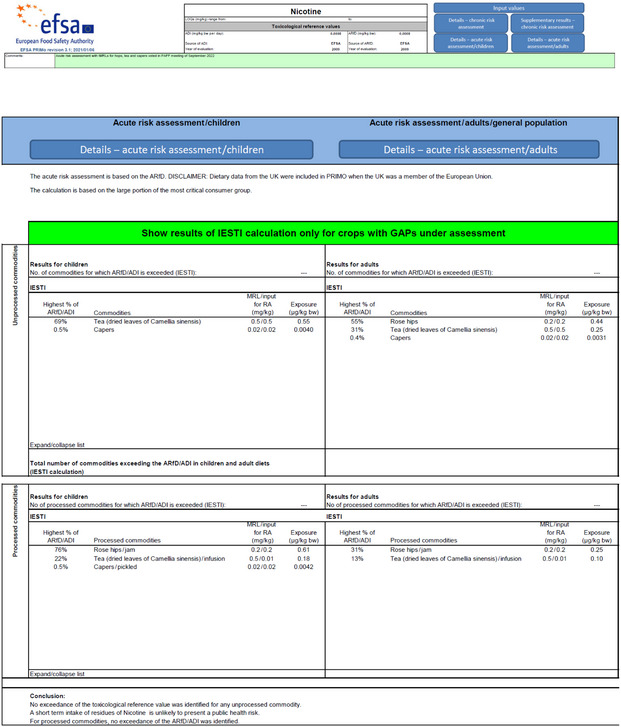
The screenshots of the acute risk assessments for the existing and the new tMRLs performed with the modified PRIMo version are presented in Appendix A.
3.1. Rose hips
The previously performed exposure/risk assessment for Dutch children, Dutch and Finnish adults with regard to rose hips jam containing residues at the current tMRL of 0.3 mg/kg (EFSA, 2022) is still valid.
An additional exposure/risk assessment was performed for the new tMRL of 0.2 mg/kg.
Table 2 provides a summary of the results for the exposure/risk assessment for the current tMRL and the new tMRL.
Table 2.
Summary of exposure/risk assessment for rose hips
| Population, LP (a) | Residue concentration used in exposure calculation | Exposure (in μg/kg bw) | Exposure (in % of the ARfD for nicotine) | Comments |
|---|---|---|---|---|
|
Dutch children (18.4 kg bw): LP of rose hip jam of 3.03 g/kg bw No processing factor available. |
existing tMRL: 0.3 mg/kg | 0.91 | 114% |
Consumption of a large portion of processed rose hips in a form of rose hips jam containing residues at the level of 0.3 mg/kg leads to an exposure exceeding the ARfD. Threshold residue concentration (expressed as mg/kg rose hips) leading to an exposure equivalent to the ARfD: 0.26 mg/kg. More details in EFSA (2022). |
| new tMRL: 0.2 mg/kg | 0.61 | 76% | The new tMRL is unlikely to pose a consumer health concern for children. | |
|
Finnish women: LP (unprocessed rosehips) 2.2 g/kg bw |
existing tMRL: 0.3 mg/kg | 0.66 | 82% | The existing tMRL is unlikely to pose a consumer health concern for adults (EFSA, 2022. |
| new tMRL: 0.2 mg/kg | 0.44 | 55% | The new tMRL is unlikely to pose a consumer health concern for adults. | |
|
Dutch adults: LP of rose hip jam of 1.25 g/kg bw No processing factor available. |
existing tMRL: 0.3 mg/kg | 0.38 | 47% | The existing tMRL is unlikely to pose a consumer health concern for adults (EFSA, 2022. |
| new tMRL: 0.2 mg/kg | 0.25 | 31% | The new tMRL is unlikely to pose a consumer health concern for adults. |
3.2. Tea (Camellia sinensis)
The previously performed exposure/risk assessment for tea was updated for children, considering alternative consumption data for children.
The calculations were performed for the existing and the new tMRL for tea.
Table 3 provides a summary of the results for the exposure/risk assessment for the current tMRL and the new tMRL.
Table 3.
Summary of exposure/risk assessment for tea
| Population, LP (a) | Residue concentration used in exposure calculation | Exposure (in μg/kg bw) | Exposure (in % of the ARfD for nicotine) | Comments |
|---|---|---|---|---|
|
French children (11–14 years, 46.3 kg bw): LP of 1.1 g/kg bw (reported as unprocessed dry leaves) |
existing tMRL: 0.6 mg/kg (dry leaves) | 0.66 | 83% | The existing tMRL is unlikely to pose a consumer health concern for children. |
| new tMRL: 0.5 mg/kg (dry leaves) | 0.55 | 69% | The new tMRL is unlikely to pose a consumer health concern for children. | |
|
Dutch children (18.4 kg bw): LP 35.09 g/kg bw; (reported as tea infusion) Default processing/dilution factor: 0.01 (b) |
existing tMRL: 0.6 mg/kg (dry leaves) | 0.21 | 26% |
The existing tMRL is unlikely to pose a consumer health concern for children. More details in EFSA (2022). |
| new tMRL: 0.5 mg/kg (dry leaves) | 0.18 | 22% | The new tMRL is unlikely to pose a consumer health concern for children. | |
|
German women: LP: 0.5 g/kg bw (reported as unprocessed dry leaves) |
existing tMRL: 0.6 mg/kg (dry leaves) | 0.3 | 38% |
The existing tMRL is unlikely to pose a consumer health concern for children. More details in EFSA (2022). |
| new tMRL: 0.5 mg/kg (dry leaves), | 0.25 | 31% | The new tMRL is unlikely to pose a consumer health concern for adults. | |
|
Dutch general population: LP: 20.29 g/kg bw Default processing/dilution factor: 0.01 (b) |
existing tMRL: 0.6 mg/kg (dry leaves) | 0.12 | 15% |
The existing tMRL is unlikely to pose a consumer health concern for adults. More details in EFSA (2022). |
| new tMRL: 0.5 mg/kg (dry leaves) | 0.1 | 13% | The new tMRL is unlikely to pose a consumer health concern for adults. |
bw: body weight; ARfD: acute reference dose; tMRL; temporary maximum residue level.
LP: large portion, used in the exposure calculation in EFSA PRIMo rev. 3.1 (EFSA, 2018, 2019), except the LP for French children (see Section 3).
The default processing/dilution factor is based on the assumption that 1 g of dry tea leaves is used to produce 100 ml (g) of tea infusion.
As the consumption data for children were affected by uncertainties, EFSA performed an additional assessment, where EFSA calculated the amount of tea containing residues at the level of the current and the new tMRL that could be consumed without exceeding the ARfD for nicotine. According to this inverse exposure calculation, the consumption of 1.33 g tea (dry leaves) per kg body weight containing residues at the existing level of the MRL (0.6 mg/kg) results in an exposure equal to the ARfD (100% of the ARfD). The calculation is performed under the assumption that a complete transfer of the nicotine residues to the tea infusion occurs. For the new temporary MRL of 0.5 mg/kg, this ‘threshold consumption’ (i.e. the consumption that leads to an exposure equal to the ARfD is 1.6 g tea (dry tea leaves) per kg body weight).
These results would mean that, e.g. a child of 10 kg bw consuming 1.33 L of tea infusion with residues at the current level of 0.6 mg/kg would be exposed to 0.0008 mg/kg (equivalent to the ARfD) of nicotine. For the new tMRL the consumption is 1.6 L of tea infusion.
3.3. Capers
The previously performed exposure/risk assessments for capers (unprocessed and processed capers) containing residues at the current tMRL of 4 mg/kg (EFSA, 2022) is still valid.
An additional exposure/risk assessment was requested for the new tMRL of 0.07 mg/kg.
Table 4 provides a summary of the results for the exposure/risk assessment for the current tMRL and the new tMRL.
Table 4.
Summary of exposure/risk assessment for capers
| Population, LP (a) | Residue concentration used in exposure calculation | Exposure (in μg/kg bw) | Exposure (in % of the ARfD for nicotine) | Comments |
|---|---|---|---|---|
| German children: LP of 0.26 g/kg bw (expressed for fresh capers) | existing tMRL: 4 mg/kg (dry capers), corresponding 0.89 mg/kg for fresh capers (b) | 0.23 | 29% |
The existing tMRL is unlikely to pose a consumer health concern for children. More details in EFSA (2022). |
| new tMRL: 0.07 mg/kg (dry capers), corresponding 0.016 mg/kg for fresh capers (b) | 0.004 | 0.5% | The new tMRL is unlikely to pose a consumer health concern for children. | |
|
Dutch children: LP (pickled capers) 0.27 g/kg bw |
existing tMRL: 4 mg/kg (dry capers), corresponding 0.89 mg/kg for pickled capers (c) | 0.24 | 30% |
The existing tMRL is unlikely to pose a consumer health concern for children. More details in EFSA (2022). |
| new tMRL: 0.07 mg/kg corresponding 0.016 mg/kg for pickled capers (c) | 0.0042 | 0.5% | The new tMRL is unlikely to pose a consumer health concern for children. | |
|
German adults: LP of 0.2 g/kg bw (expressed for fresh capers) |
existing tMRL: 4 mg/kg (dry capers), corresponding 0.89 mg/kg for fresh capers (b) | 0.18 | 22% |
The existing tMRL is unlikely to pose a consumer health concern for adults. More details in EFSA (2022). |
| new tMRL: 0.07 mg/kg corresponding 0.016 mg/kg for fresh capers (b) | 0.0031 | 0.4% | The new tMRL is unlikely to pose a consumer health concern for adults. |
bw: body weight; ARfD: acute reference dose; tMRL: temporary maximum residue level.
The MRL established for dry capers had to be recalculated to fresh capers, to match with the German consumption data (see EFSA, 2022).
Default processing factor of 1 was used (PF fresh capers to pickled capers).
4. Conclusions
The acute exposure calculations performed by EFSA give an indication that the existing tMRL for rose hips of 0.3 mg/kg may not be sufficiently protective, if a large portion of rose hips jam is consumed which contains residues at the level of the tMRL set for unprocessed rose hips. The new tMRL voted in the in the Standing Committee on Plants, Animals, Food and Feed in September (0.2 mg/kg) is unlikely to pose an acute consumer health concern.
No acute intake concerns were identified for the existing and the new tMRLs for tea and capers.
The results of the acute exposure assessment and the recommendations are summarised below (Table 5).
Table 5.
Summary table
| Code (a) | Commodity | Existing tMRL (b) | New tMRL (c) | Threshold residue concentration (d) | Outcome of the review/Comment |
|---|---|---|---|---|---|
| Residue definition for enforcement: Nicotine | |||||
| 0154050 | Rose hips | 0.3 | 0.2 | 0.26 |
The recommendation derived in the previous EFSA statement remains unchanged where EFSA recommended the lowering of the existing tMRL to a level equal or lower than the threshold concentration of 0.26 mg/kg for rose hips jam, since a potential consumer health risk could not be excluded for children consuming rose hips jam containing residues at the level of 0.3 mg/kg (EFSA, 2022). The new tMRL of 0.2 mg/kg is unlikely to pose a consumer health concern neither for children nor for adults. |
| 0610000 | Teas | 0.6 | 0.5 | > 0.6 |
The previously performed exposure calculations for children were updated, replacing the Irish consumption data for children, with the next highest consumption data reported for French children. Based on the calculations performed with the modified version of EFSA PRIMo rev. 3.1, EFSA concludes that the existing and the new tMRLs for tea are unlikely to pose a consumer health concern for children and for adults. |
| 0850020 | Capers | 4 | 0.07 | > 4 | The existing and the new tMRL are unlikely to pose a consumer health concern neither for children nor for adults. |
MRL: maximum residue level; ARfD: acute reference dose.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing temporary MRL (in mg/kg) established by Regulation (EU) 2022/1290.
New temporary MRLs voted in Standing Committee on Plants, Animals, Food and Feed (PAFF), Section. Phytopharmaceuticals – Pesticide Residues (document SANTE/10090/2022), hold on 26–27 September 2022; new MRLs not yet applicable.
Threshold residue concentration (in mg/kg) is the residue level that leads to a short‐term exposure equal to the ARfD.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- FAO
Food and Agriculture Organization of the United Nations
- LOQ
limit of quantification
- LP
large portion
- MRL
maximum residue level
- PAFF
Standing Committee on Plants, Animals, Food and Feed
- PF
processing factor
- PPP
plant protection products
- PRIMo
(EFSA) Pesticide Residues Intake Model
- SCoPAFF
Standing Committee on Plants, Animals, Food and Feed, (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- tMRL
temporary MRL
Appendix A – Pesticide Residue Intake Model (PRIMo)
• Acute risk assessment for existing tMRLs established in Regulation (EU) 2022/1290
• Acute risk assessment for existing tMRLs voted in the Standing Committee for Plants, Animals, Food and Feed (PAFF) in September 2022
Suggested citation: EFSA (European Food Safety Authority) , 2023. Statement on the revised targeted risk assessment for certain maximum residue levels for nicotine. EFSA Journal 2023;21(3):7883, 13 pp. 10.2903/j.efsa.2023.7883
Requestor Department of Agriculture, Food and the Marine (DAFM) of Ireland
Question number EFSA‐Q‐2022‐00870
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 7 February 2023
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) No 812/2011 of 10 August 2011 amending Annex Ill to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for dimethomorph, fluopicolide, mandipropamid, metrafenone, nicotine and spirotetramat in or on certain products. OJ L208, 13.8.2011, p. 1–22.
Commission Regulation (EU) 2022/1290 of 22 July 2022 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for ametoctradin, chlormequat, dodine, nicotine, profenofos and Spodoptera exigua multicapsid nucleopolyhedrovirus (SeMNPV) isolate BV‐0004 in or on certain products. OJ L 196, 25.7.2022, p. 74–114.
References
- EFSA (European Food Safety Authority) , 2009. Statement on the potential risks for public health due to the presence of nicotine in wild mushrooms. EFSA Journal 2009;7(5):RN‐286, 47 pp. 10.2903/j.efsa.2009.286r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Reasoned opinion on the setting of temporary MRLs for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs. EFSA Journal 2011;9(3):2098, 50 pp. 10.2903/j.efsa.2011.2098 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A, Verani A, 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Statement on the short‐term(acute) dietary risk assessment for the temporary maximum residue levels for nicotine in rose hips, teas and capers. EFSA Journal 2022;20(9):7566, 13 pp. 10.2903/j.efsa.2022.7566 [DOI] [PMC free article] [PubMed] [Google Scholar]


