Highlights
-
•
The Ethos TPS produced comparable automated IMRT plans to manual plans.
-
•
Editing the automated contours was the most time consuming aspect of the oART workflow.
-
•
Significant increase in PTV D98% and decrease to OAR dose were seen with oART.
Keywords: Artificial intelligence, Automated treatment planning, Cervical cancer, Cone-beam computed tomography (CBCT), External beam radiotherapy, Image-guided radiotherapy (IGRT), Online adaptive radiotherapy
Abstract
Background and purpose
Adaptive radiotherapy (ART) in locally advanced cervical cancer (LACC) has shown promising outcomes. This study investigated the feasibility of cone-beam computed tomography (CBCT)-guided online ART (oART) for the treatment of LACC.
Material and methods
The quality of the automated radiotherapy treatment plans and artificial intelligence (AI)-driven contour delineation for LACC on a novel CBCT-guided oART system were assessed. Dosimetric analysis of 200 simulated oART sessions were compared with standard treatment. Feasibility of oART was assessed from the delivery of 132 oART fractions for the first five clinical LACC patients. The simulated and live oART sessions compared a fixed planning target volume (PTV) margin of 1.5 cm around the uterus-cervix clinical target volume (CTV) with an internal target volume-based approach. Workflow timing measurements were recorded.
Results
The automatically-generated 12-field intensity-modulated radiotherapy plans were comparable to manually generated plans. The AI-driven organ-at-risk (OAR) contouring was acceptable requiring, on average, 12.3 min to edit, with the bowel performing least well and rated as unacceptable in 16 % of cases. The treated patients demonstrated a mean PTV D98% (+/-SD) of 96.7 (+/- 0.2)% for the adapted plans and 94.9 (+/- 3.7)% for the non-adapted scheduled plans (p<10−5). The D2cc (+/-SD) for the bowel, bladder and rectum were reduced by 0.07 (+/- 0.03)Gy, 0.04 (+/-0.05)Gy and 0.04 (+/-0.03)Gy per fraction respectively with the adapted plan (p <10−5). In the live.setting, the mean oART session (+/-SD) from CBCT acquisition to beam-on was 29 +/- 5 (range 21–44) minutes.
Conclusion
CBCT-guided oART was shown to be feasible with dosimetric benefits for patients with LACC. Further work to analyse potential reductions in PTV margins is ongoing.
Introduction
Radiotherapy delivery for locally advanced cervical cancer (LACC) has transformed over the last two decades, both with the use of image-guided adaptive brachytherapy [1] and advancements in external beam radiotherapy (EBRT). EBRT has progressed from three-dimensional conformal radiotherapy (3D-CRT) to intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), with associated conformity in treatment volumes and reduction in doses to organs at risk (OAR) [2]. More accurate RT delivery necessitated the use of image-guided radiotherapy (IGRT) moving towards cone-beam computed tomography (CBCT) soft tissue matching [3]. IMRT has shown decreased toxicity compared to 3D-CRT [4], [5], [6], however, with improving survival, long term toxicity, especially chronic gastrointestinal (GI) morbidity, remains a major issue [7].
The requirement for large margins to account for inter- and intra-fractional movement of the utero-cervix complex make adaptive radiotherapy (ART) appealing [8], [9]. ART encompasses a variety of offline and online strategies [10], including scheduled offline replanning [11] and plan of the day (PotD) approach [12], [13]. Technological advancements have seen the realisation of online ART (oART) with daily replanning, using magnetic resonance imaging (MRI) [14], [15], and more recently CBCT guidance [16], using the Ethos™ system (Varian Medical Systems (VMS), Palo Alto, CA) [17]. In non-gynaecologic pelvic cancers, the introduction of CBCT-guided oART has shown improvement in dosimetric outcomes [16], [18], [19], both for tumour coverage and OAR; however, data regarding the implementation, dosimetric and toxicity outcomes for the treatment of LACC with oART is lacking.
A recent survey of 177 centres from 40 countries demonstrated that 32 % of centres utilised some form of offline or online ART for cervical cancer treatment [20]. Two thirds of respondents expressed a desire to implement ART for new treatment sites highlighting the demand for advanced radiotherapy techniques. However technical limitations and human and material resources were the main barriers to implementation [20], [21].
This project explored the implementation of oART on the Ethos system for the treatment of LACC. This covers assessment of automated radiotherapy planning, automated contour quality, and simulated dosimetric analysis of oART. Alongside this, an evaluation of the first five LACC patients treated with this system at our institution. This included timing data, a dosimetric evaluation between our current standard of care using an ITV compared to daily oART without an ITV, and toxicity analysis.
Materials and methods
Our centre commissioned an Ethos system and started treating patients with IGRT in August 2020. In January 2021, our first adaptive patient was treated for muscle invasive bladder cancer, and 6 months later our first LACC was treated with CBCT-guided oART. A software emulator of the oART module was utilised to gain additional knowledge of the oART system in a simulated environment before treating patients in the live setting. Prior to implementing oART, the standard procedure for patients undergoing EBRT for LACC at our institution included the creation of an internal target volume (ITV). A bladder drinking protocol was followed: patients voided their bladder followed by drinking 600mls of water, with full and empty bladder CT scans acquired 30 min later. Using the full bladder planning CT scan, three clinical target volumes (CTVs) were contoured, based on the INTERTECC-2 study: CTV1 included the GTV, entire cervix and uterus, CTV2 included the parametrium and vagina, CTV3 included any involved pelvic lymph nodes and elective nodal regions [22]. On the empty bladder planning CT scan, CTV1_empty was contoured. An ITV was created by combining the positions of CTV1 between the full and empty bladder planning scans. Planning target volumes (PTVs) were generated by an isotropic expansion of CTV1 by 1.5 cm, CTV2 by 1 cm, CTV3 by 0.7 cm and ITV by 0.7 cm to create PTV1, PTV2, PTV3 and PTV4 respectively [22]. The 4 PTVs were combined to create a PTV_final which was prescribed 45 Gy in 25 fractions over five weeks for node negative cases or 50.4 Gy in 28 fractions over five and a half weeks for node positive cases.
Online ART workflow
For the oART workflow, the same process as above was undertaken to create a reference plan using standard IGRT contours and margins. A second plan was generated using the full bladder planning scan and utilising the same contours and PTV margins, except there was no ITV or corresponding PTV4, since motion of the uterus would be accounted for via online adaptation. PTV_adapt is the combination of PTV1, PTV2 and PTV3.
The workflow for daily oART using this system has been described in detail elsewhere [17], [18], [23], [24], [25]. Following acquisition of a CBCT, ‘influencer’ structures are automatically generated; these are structures which affect the position of the propagated target volumes, which for cervical cancer comprise bladder, rectum and uterus. Alongside this, bowel loops are generated. Once these contours have been reviewed and edited, the targets are propagated using a structure-guided deformation algorithm from the planning CT. These are reviewed and manually edited before two radiotherapy plans are generated. The scheduled plan is the original reference plan, inclusive of an ITV calculated on the new anatomy. The adapted plan is re-optimised using the new contours. Prior to treatment delivery, plan specific quality assurance (QA) is performed using independent dose calculation software (Mobius3D, VMS), using a global gamma passing criterion, of 95 % within 3 %/3mm. After plan selection, a verification CBCT is acquired to assess intrafractional movement, soft tissue matching is applied if necessary to ensure CTV coverage.
Assessment of automated plan quality
Using retrospective anonymised data for the most recent six consecutive LACC cases at our institution, an initial radiotherapy planning template was iteratively optimised using the Ethos treatment planning system (TPS). A full bladder planning CT scan was used to create an IMRT plan to a median PTV dose of 50.4 Gy in 28 fractions. The template included all institutional standard planning objectives plus additional goals to aid the optimiser.
Automated plan quality was assessed using a further ten retrospective cases. The optimised template was used to generate an automated plan with no additional user input and compared with a newly generated manual plan created in Eclipse (v15.6) by experienced radiotherapy planners. Both automated and manual plans used 12-field IMRT. Dose was calculated using the AcurosXB algorithm (v15.6) reporting dose to medium [26]. The automated plans were exported from the Ethos TPS to Eclipse and assessed using local clinical dose volume histogram (DVH) constraints. A radiation oncologist (RO) performed a blinded evaluation of the plans and rated these as pass/ minor deviation/ fail. Timings for plan setup and optimisation for both the manual and automated plans were recorded.
Evaluation of CBCT-based auto-segmentation
Five consecutive historic anonymised patients were selected from a previous departmental study all received 50.4 Gy in 28 fractions with daily soft tissue matching on a Truebeam (VMS). Each CBCT had OAR (bladder, rectum, bowel, uterus) contoured on Eclipse by a RO, in accordance with Radiation Therapy Oncology Group guidelines [27]. Using the full bladder planning CT scan, a new IMRT plan was created using the Ethos template, and five CBCTs for each patient (one from each week of treatment) were uploaded onto the software emulator. OART was simulated twice: once with the AI-generated contours edited (AIedited) by a single RO and once with the contours left unedited (AIunedited). The time taken to edit the contours in the emulator was recorded. The AIunedited contours were rated qualitatively by a RO as excellent, good, acceptable and unacceptable depending on the extent of modifications required [28],
Simulation of online ART
Ten historic anonymised patients treated with 50.4 Gy in 28 fractions for LACC were selected (five from the auto-segmentation study and a further five consecutive patients treated for LACC using IGRT on the Ethos system). Two Ethos IMRT plans were created using a template as described above, one using the ITV, and one without. For each patient, two courses of ten treatment sessions were simulated on the emulator, using ten CBCTs evenly selected from the initial radiotherapy course. For each fraction, the system contoured influencer structures and propagated target structures onto the CBCT; these were edited by a RO or a trained treatment radiographer (RTT). For the first simulated course of treatment, the re-optimised adapted plan was selected each time to represent oART and for the second course, the re-calculated scheduled plan was chosen each time to represent current practice. Dose accumulation within the Ethos TPS was utilised to compare the dose delivered using the adapted and scheduled plans.
Clinical online ART for cervical cancer
Five consecutive patients with LACC were treated with daily oART, to a dose of either 45 Gy in 25 fractions or 50.4 Gy in 28 fractions with weekly concomitant Cisplatin, 40 mg/m2, patient characteristics in Table 1. Adjustments to the planning template were made depending on the specific anatomy of the patient. The final template and priority of planning objectives was utilised for the creation of the daily adapted plan. Single isocentre plans were created. Patients with involved pelvic lymph nodes received a non-adaptive pelvic nodal boost of 5.4 Gy in 3 fractions. Timing data for each of the individual phases of the oART process were collected. For each adaptive session there were two trained RTTs, a physicist and a RO present for the entire treatment workflow. Physician reported Common Terminology Criteria for Adverse Events (CTCAE) v5.0 relating to GI and genitourinary (GU) toxicity[29], EORTC patient reported outcome measures (PROMs) cervix module (CX24)[30] and quality of life (QLQ-C30) v3.0[31] were collected in weeks one, three and five during radiotherapy and in week 12 following completion of treatment. Tip of uterus motion was measured between the full and empty bladder planning scan.. Following completion of EBRT, all patients went on to receive MRI-guided high dose rate intracavitary with interstitial needle brachytherapy, up to 28 Gy in 4 fractions, to achieve an equivalent dose in 2 Gy (EQD2) to the high risk CTV D90 of at least 90 Gy. All patients provided informed written consent, and adherence to institutional ‘first use’ policy was maintained.
Table 1.
Patient characteristics of the first 5 clinical patients treated. Adeno; Adenocarcinoma, SCC; Squamous cell carcinoma, oART; online adaptive radiotherapy, fr; fraction, PTV; planning target volume.
| Age | FIGO 2018 staging [32] | Histopathology | Pelvic nodal boost | No. oART fr | Tip of Uterus motion > 2 cm | PTV_adapt volume (cc) | Comment |
|---|---|---|---|---|---|---|---|
| 43 | IIB | Adeno | N | 23 | Y | 1413 | |
| 38 | IIICIr | SCC | Y | 28 | N | 1945 | Ureteric stent, mesorectum treated |
| 48 | IIB | Adeno | N | 25 | Y | 1925 | Replan |
| 38 | IIB | Adeno | N | 28 | Y | 1285 | |
| 66 | IIICIr | SCC | Y | 28 | N | 1326 | Mesorectum treated |
Statistical analysis
For the contouring analysis, both AIedited and AIunedited contours were exported from the Ethos TPS to Eclipse, where they were compared to the original contours quantitatively via dice similarity coefficient (DSC) and 95-percentile Hausdorff distance (HD95%). Adapted and scheduled plans produced in the emulator were exported and transferred to the Eclipse TPS. Dose parameters including PTV D2%(%), PTV D98%(%), PTV V95%(%), PTV D95%(Gy), CTV Dmin(%), CTV Dmax(%) and OAR D2cc(Gy) were recorded and evaluated using custom Python (v3.9) scripts. Boxplots showing the median value and interquartile range (IQR) were created with outliers being values outside of 1.5 × IQR. Wilcoxon signed-rank tests were used to test non-normally distributed data at a statistical significance level of 5 %.
Results
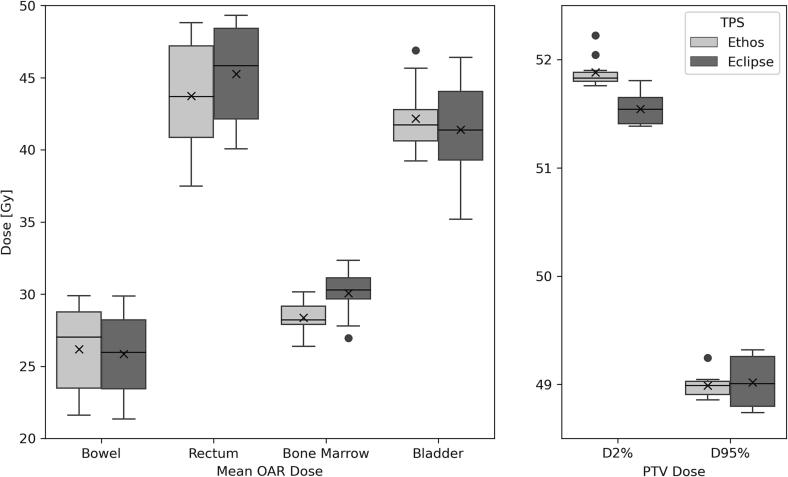
Plan set-up for the manual plans took a mean of 15 (range 9–20) minutes, the number of optimisations ranged from 3 to 15 with a mean total planning time of 133 (range 38–360) minutes. Less than 15 min of user interaction was required to generate the automated plan. The mean PTV D95% was not significantly different between the manual and automated plan. The PTV D2% had a mean increase of 0.3 Gy (p = 0.002) for the automated plan, as shown in Fig. 1, alongside the mean doses for OAR. The mean maximum bladder dose reduced by 0.7 Gy with the automated plan (p = 0.002). All manual plans were clinically acceptable (rated either pass or minor deviation), however 3 out of 10 of the automated plans were deemed unacceptable (2 due to doses of 107 % outside of the PTV and 1 due to poor PTV coverage).
Fig. 1.
Boxplots of the mean doses for key organs at risk (OAR) and planning target volume (PTV) D2% and D95%. The box represents the interquartile range (IQR), the line the median, the whiskers are 1.5 × the IQR, the ‘x’ within the box represents the mean value and circles represent the outliers. The ‘x’ within the boxplot represents the mean value and circles represent the outliers.
A further optimisation removing a rectal constraint was introduced to reduce hotspots outside the PTV for the remainder of the projects involving the automated planning template (the final template Table A1, Supplementary material). The test plans were re-run with the modified template and all automated plans were clinically acceptable. 12-field IMRT plans were used throughout in view of the faster online optimisation time when compared to VMAT plans.
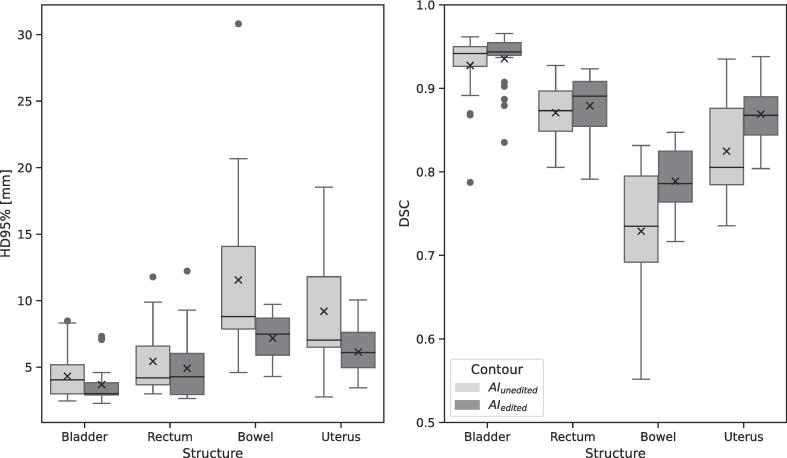
For the auto-generated contour assessment, the AI contours were either left unedited (AIunedited) or edited manually (AIedited) within the Ethos system; these contours were compared with the original manual contours in Eclipse. The DSC and HD95% had statistically significant differences between the AIedited and AIunedited contours for all apart from the rectum structure. Boxplots of the results of each structure are given in Fig. 2. The bladder had the highest DSC for the AIunedited and AIedited contours when compared with the original manual contours, with a median value of 0.94. The bowel was the least accurate AI-generated structure using the DSC and HD95% metrics. Qualitative assessment of the AI generated contours showed that the bladder and rectum required minimal editing, with 42 % rated as excellent and 54 % as good; none were rated unacceptable. In contrast, the bowel contouring was rated as only acceptable in 64 % of cases, with 16 % deemed unacceptable. The mean time taken to edit all four of the AI-generated contours was 12.3 (range 6.3–17.6) minutes, this included the bowel, which took on average 7.5 (range 3.5–12.3) minutes.
Fig. 2.
Boxplots of Hausdorff 95th percentile (HD95%) and dice similarity coefficient (DSC) of the AIedited and AIunedited contours for each structure compared with the original manual contours. The box represents the interquartile range (IQR), the line the median, the whiskers are 1.5 × the IQR, the ‘x’ within the box represents the mean value and circles represent the outliers.
In the simulated environment, using the adapted plan increased the mean CTV Dmin by 6 % compared to current practice (range −0.8 to 24.6) (p = 0.004). The mean CTV Dmax was reduced by 0.7 % (p = 0.04). All OAR differences which were statistically significant were in favour of oART, see Table 2. The average time (+/- SD) taken from loading the CBCT to plan acceptance was 21 +/- 4 (range 12–34) minutes.
Table 2.
Difference in dose between adapted plan and current practice represented by the scheduled plan, (scaled to 50.4 Gy/28 fractions). * highlights significant p-values. SD; standard deviation, CTV; clinical target volume, PTV; planning target volume, LFH; left femoral head, RFH; right femoral head.
| Structure | Dosimetric Parameter [units] | p-value | Difference (Adapted – Scheduled plan) | ||
|---|---|---|---|---|---|
| Mean | Range | SD | |||
| CTV | Dmax [%] | 0.04* | −0.7 | −2.5 to 0.4 | 0.9 |
| Dmin [%] | 0.004* | 6 | −0.8 to 24.6 | 7.1 | |
| Dmean [%] | 0.17 | 0.4 | −1.5 to 1.6 | 0.9 | |
| Bladder | V100% [cc] | 0.18 | −4.8 | −30.4 to 0.7 | 9.6 |
| Dmean [Gy] | 0.01* | −1.3 | −2.5 to 0.4 | 0.4 | |
| Bowel | V100% [cc] | 0.7 | −7 | −65.6 to 2.0 | 20.7 |
| Dmean [Gy] | 0.58 | 0.2 | −2.0 to 2.4 | 0.4 | |
| Rectum | V100% [cc] | 0.26 | −0.5 | −3.7 to 2.5 | 1.6 |
| D50% [Gy] | 0.14 | 1.4 | −0.6 to 6.1 | 0.8 | |
| LFH | D15% [Gy] | 0.02* | −1.5 | −3.1 to 1.7 | 0.5 |
| RFH | D15% [Gy] | 0.02* | −1.6 | −4.2 to 1.5 | 0.5 |
| Bone Marrow | Dmean [Gy] | 0.049* | −0.4 | −0.9 to 0.5 | 0.2 |
| Body-PTV | V50% [cc] | 0.11 | −194.6 | −1066.7 to 198.6 | 359.7 |
| Bowel Bag | V100% [cc] | 0.7 | −7.2 | 146.2 to 42.6 | 52.1 |
| Dmean [Gy] | 0.63 | 0.2 | −1.9 to 2.5 | 0.4 | |
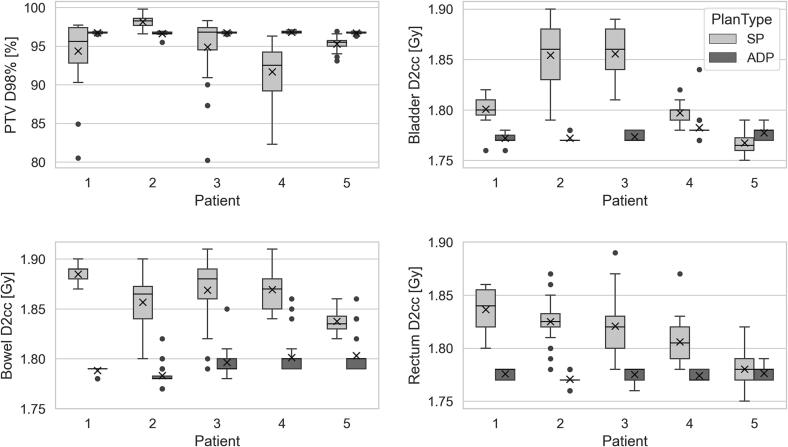
For the treated LACC patients, the mean (+/-SD) oART session from CBCT acquisition to beam-on was 29 +/- 5 (range 21–44) minutes. On average the workflow was divided as follows: influencer review and editing (44 %), target review and editing (15 %) and plan review and online QA (27 %). The remainder of the time was spent on acquisition and review of the initial and verification CBCT. The ureteric stent for patient 2 did not cause difficulty in contouring. One patient was replanned offline after their first oART treatment, as their bladder volume was greater on treatment than on the original full bladder planning CT scan, which impacted the generation of the target contours. As a result of bank holidays and service days, of the planned 137 fractions, 5 treatments were delivered using a standard of care back-up plan on a Truebeam. For the remaining 132 treatments, the adapted plan was chosen in 125/132 (95 %) of treatments, primarily due to better target coverage and improved OAR doses. Of the seven occasions when the scheduled plan was chosen: five were caused by technical difficulties with the online QA system; one was due to improved bowel doses; one was due to there being no physician available to contour. The mean PTV D98% (+/-SD) for the PTV_adapt was 96.7 +/- 0.2 % when treating with the adapted plan and 94.9 +/- 3.7 % if selecting the scheduled plans (p 〈10−5). The D2cc (+/-SD) for the bowel, bladder and rectum were reduced by 0.07 (+/- 0.03)Gy, 0.04 (+/-0.05)Gy and 0.04 (+/-0.03)Gy per fraction respectively with the adapted plan (p 〈10−5), as shown in Fig. 3.
Fig. 3.
Boxplots present PTV D98% (top left) bladder D2cc (top right), bowel D2cc (bottom left) and rectum D2cc (bottom right) showing the difference between the adapted plan (ADP) in dark grey and the scheduled plan (SP) in light grey. The y-axis for the D2cc graphs represents the dose in Gray/fraction. The box represents the interquartile range (IQR), the line the median, the whiskers are 1.5 × the IQR, the ‘x’ within the boxplot represents the mean value and circles represent the outliers.
Incidence of CTCAE grade 2 diarrhoea was 2/5 patients in week 3 and 5, which resolved to grade 0 or 1 in 5/5 patients at 12 weeks following treatment completion. One patient had grade 2 urinary frequency and urgency at 12 weeks. The EORTC PROMs QLQ-C30 module question 17 regarding frequency of diarrhoea showed ‘very much’ diarrhoea in 1/5 patients in week 3 and week 5. By week 12, 1 out of 5 patients reported ‘a little bit’ of diarrhoea and 4 out of 5 patients reported ‘no’ diarrhoea.
Discussion
The 12-field IMRT automated plan generation produced clinically acceptable plans for the majority of cases. The PTV coverage, bowel and bladder doses were comparable to the manual plan. In the two cases with hotspots outside the PTV, removal of a rectal constraint ensured the plans were clinically acceptable. One automated plan had poor PTV coverage due to a large volume of bowel within the PTV; this patient had also been a challenge for the manual plan creation requiring significant compromise to the mandatory bowel constraints.
Our initial experience has shown that the auto-generated contours for the female pelvis influencer structures are satisfactory, and the time required for manual editing is acceptable. The bowel was the least acceptable structure, requiring considerable adjustment. The edited AI bowel contours were considered acceptable; however, the mean DSC was 0.79 compared to > 0.85 for all other structures. It is noted that some of the differences seen between contours are due to the inherent variability in manual contour generation; similar DSC values have been shown when comparing bowel loop contouring between clinicians on different imaging modalities [33]. Additionally some centres using oART for the pelvis, have implemented a bowel bag structure, such that editing bowel as an influencer is not performed [16]. Moreover, the CBCTs used for this part of the study were acquired from a Truebeam and may differ in quality than those available on the Ethos system.
The dosimetric outcomes for simulated oART treatments showed small benefits for oART, with increased CTV coverage and reduced dose to OAR. However, the dosimetric changes showed large inter-patient variation (see Table 2), highlighting that patient factors will be important for appropriate oART case selection. Similar results have been described by Yock et al. who simulated 149 adaptive radiotherapy fractions for cervical cancer on an emulator. They discovered improved target coverage (CTV and PTV V95%) and decreased maximum OAR dose with the adapted plan. Their average duration of workflow (influencer generation through to adapted plan approval) was 24.4 min [23], which is similar to that achieved both in our simulated and live environments, 21 and 29 min respectively. In our live environment, the workflow included the time required for acquisition and review of the initial and verification CBCT as well as the online QA. These phases are not possible within the simulated environment and may account for some of the differences in time seen between these studies.
Similarly, Sibolt et al. demonstrated promising dosimetric outcomes for 20 CBCT-guided oART treatments for pelvic cancers, with a procedure duration (acceptance of CBCT to beam on) of 17.6 min [16], although their study did not include any LACC patients. Limited data are available regarding treatment with daily oART in LACC. Early abstract publications from Magnetic Resonance Linacs (MRL) have reported treatment times of 32 min in post-operative cervical cases [14] to 60 min (from patient entering the treatment room to beam off) for a patient with LACC [15]. In post operative cervical cancer oART, the contouring requirements would be different to those patients having radical chemoradiation to an intact uterus.
During the oART treatments, a multi-disciplinary team (MDT) was available with the RTTs contouring under direct supervision of a RO. The departmental aim is to create a predominantly RTT-led oART service; consequently, there is a likelihood that with increased experience the timings for the workflow will reduce further. As highlighted by other centres using this system for other pelvic sites, the majority of the workflow is spent reviewing and editing the influencers [25].
It is noticeable that our live patient dosimetric results show a greater benefit for oART for both target coverage and OAR sparing than was seen in our simulated treatments. This could be partly due to patient-related differences between the two cohorts. On analysis of the ten simulated patients, only two had any significant movement of the cervix-uterus complex with bladder filling. However, three of the five clinical patients had a tip of uterus motion>2 cm. Removal of the ITV was therefore able to deliver dosimetric gains, even without the reduction of margins. Further studies to establish safe margin reduction are ongoing. Furthermore, 1 patient had a substantial reduction (43 %) in her PTV1 volume from 905 cc to 518 cc, (Fig. A2, Supplementary material), during the course of EBRT, which could be corrected for using oART.
Our study was not designed to establish treatment-related toxicity; however, data from these five patients have raised no concerns and similar toxicity has been reported in other studies [6]. Georg et al. showed that, when comparing three EBRT techniques, dose-volume effects for diarrhoea were found, specifically highlighting the significance of bowel V40Gy less than 250 cc [34]. One anticipates that utilising oART will further reduce the dose to surrounding OAR, and clinical trials are required to establish this.
Limitations for our study include that for our simulated dosimetric analysis, we assessed 10 fractions of radiotherapy rather than the full course of 28 treatment that the patients had received, although the 10 CBCTs for each patient were taken from time points evenly spread throughout their radiotherapy course. Similarly, with the simulated patients, contours had to be drawn twice, to treat with both the adapted and scheduled plans, although the same team of RO and RTTs performed the contouring. Alongside this, assumptions have been made when applying the dose accumulation function for our simulated study [35]. Lastly, the dosimetric analysis for our live LACC patients compared the doses for the adapted and scheduled plans at the plan selection phase of the oART workflow and has not taken into consideration the change in anatomy seen on the verification CBCT and any additional shifts that might have been necessary. However, despite these considerations, the overall trends observed are expected to be valid and have demonstrated clear dosimetric benefits in some cases.
Conclusion
This study is the first to describe the successful implementation of a novel CBCT-based oART system for the treatment of LACC. Further work is required to identify appropriate patient groups, the possibility of PTV margin reduction, clinical outcome data and how the workforce can be adapted to incorporate this technique more widely.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The Royal Surrey Hospital has a professional services agreement with Varian Medical Systems (VMS). VMS had no role in study design, data collection and analysis, and decisions on preparation of the manuscript. None of the authors have any affiliation with VMS. There are no other conflicts of interests to declare from all authors.
Acknowledgements
The authors wish to thank all the radiotherapy planners and treatment radiographers who have been involved in this project. We would also like to thank Kyah Howard for her support with data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100596.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Potter R., Georg P., Dimopoulos J.C., Grimm M., Berger D., Nesvacil N., et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forrest J., Presutti J., Davidson M., Hamilton P., Kiss A., Thomas G. A dosimetric planning study comparing intensity-modulated radiotherapy with four-field conformal pelvic radiotherapy for the definitive treatment of cervical carcinoma. Clin Oncol (R Coll Radiol) 2012;24:e63–e70. doi: 10.1016/j.clon.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Webster A., Appelt A.L., Eminowicz G. Image-Guided Radiotherapy for Pelvic Cancers: A Review of Current Evidence and Clinical Utilisation. Clin Oncol (R Coll Radiol) 2020;32:805–816. doi: 10.1016/j.clon.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi A.K., Sharma D.N., Rath G.K., Julka P.K., Subramani V., Sharma S., et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiat Oncol Biol Phys. 2013;87:542–548. doi: 10.1016/j.ijrobp.2013.06.2059. [DOI] [PubMed] [Google Scholar]
- 5.Naik A., Gurjar O.P., Gupta K.L., Singh K., Nag P., Bhandari V. Comparison of dosimetric parameters and acute toxicity of intensity-modulated and three-dimensional radiotherapy in patients with cervix carcinoma: A randomized prospective study. CancerRadiother. 2016;20:370–376. doi: 10.1016/j.canrad.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Yeung A.R., Pugh S.L., Klopp A.H., Gil K.M., Wenzel L., Westin S.N., et al. Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study. J Clin Oncol. 2020;38:1685–1692. doi: 10.1200/JCO.19.02381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kj N.B., Potter R., Spampinato S., Fokdal L.U., Chargari C., Lindegaard J.C., et al. Dose-Volume Effects and Risk Factors for Late Diarrhea in Cervix Cancer Patients After Radiochemotherapy With Image Guided Adaptive Brachytherapy in the EMBRACE I Study. Int J Radiat Oncol Biol Phys. 2021;109:688–700. doi: 10.1016/j.ijrobp.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Chan P., Dinniwell R., Haider M.A., Cho Y.B., Jaffray D., Lockwood G., et al. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys. 2008;70:1507–1515. doi: 10.1016/j.ijrobp.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 9.Heijkoop S.T., Langerak T.R., Quint S., Mens J.W., Zolnay A.G., Heijmen B.J., et al. Quantification of intra-fraction changes during radiotherapy of cervical cancer assessed with pre- and post-fraction Cone Beam CT scans. Radiother Oncol. 2015;117:536–541. doi: 10.1016/j.radonc.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Shelley C.E., Barraclough L.H., Nelder C.L., Otter S.J., Stewart A.J. Adaptive Radiotherapy in the Management of Cervical Cancer: Review of Strategies and Clinical Implementation. Clin Oncol (R Coll Radiol) 2021;33:579–590. doi: 10.1016/j.clon.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Lim K., Stewart J., Kelly V., Xie J., Brock K.K., Moseley J., et al. Dosimetrically triggered adaptive intensity modulated radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2014;90:147–154. doi: 10.1016/j.ijrobp.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad R., Bondar L., Voet P., Mens J.W., Quint S., Dhawtal G., et al. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: a dosimetric evaluation. Acta Oncol. 2013;52:1430–1436. doi: 10.3109/0284186X.2013.813640. [DOI] [PubMed] [Google Scholar]
- 13.Novakova E., Heijkoop S.T., Quint S., Zolnay A.G., Mens J.W.M., Godart J., et al. What is the optimal number of library plans in ART for locally advanced cervical cancer? Radiother Oncol. 2017;125:470–477. doi: 10.1016/j.radonc.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 14.J. Li Y. Ouyang X. Cao First Postoperative Cervical Cancer Patients Treated with a 1.5 Unity MR-Linac and Analysis of Treatment Safety and Acute Toxicity (Conference Abstract) Int J of Radiat Oncol Biol Phys 2020;108:e485. 10.1016/j.ijrobp.2020.07.1542 .
- 15.Freear L.B.J., Chuter R., Budgell G., Whitehurst P. Lessons learnt from the the first radical cervix treatment on the MR-Linac (Conference Abstract) Radiother Oncol. 2021;161:S1297–S1298. doi: 10.1016/S0167-8140(21)08023-3. [DOI] [Google Scholar]
- 16.Sibolt P., Andersson L.M., Calmels L., Sjöström D., Bjelkengren U., Geertsen P., et al. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region. Physics and Imaging in Radiation Oncology. 2021;17:1–7. doi: 10.1016/j.phro.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archambault Y., Boylan C., Bullock D., Morgas T., Peltola J., Ruokokoski E., et al. Making on-Line Adaptive Radiotherapy Possible Using Artificial Intelligence and Machine Learning for Efficient Daily Re-Planning. Med Phys. 2020;8:77–86. [Google Scholar]
- 18.de Jong R., Visser J., van Wieringen N., Wiersma J., Geijsen D., Bel A. Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer. Radiat Oncol. 2021;16:136. doi: 10.1186/s13014-021-01866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwart L.G.M., Ong F., Ten Asbroek L.A., van Dieren E.B., Koch S.A., Bhawanie A., et al. Cone-beam computed tomography-guided online adaptive radiotherapy is feasible for prostate cancer patients. Phys Imaging Radiat Oncol. 2022;22:98–103. doi: 10.1016/j.phro.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertholet J., Anastasi G., Noble D., Bel A., van Leeuwen R., Roggen T., et al. Patterns of practice for adaptive and real-time radiation therapy (POP-ART RT) part II: Offline and online plan adaption for interfractional changes. Radiother Oncol. 2020;153:88–96. doi: 10.1016/j.radonc.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lievens Y., Borras J.M., Grau C., Aggarwal A. Value-based radiotherapy: A new chapter of the ESTRO-HERO project. Radiother Oncol. 2021;160:236–239. doi: 10.1016/j.radonc.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Mell L.K., Sirak I., Wei L., Tarnawski R., Mahantshetty U., Yashar C.M., et al. Bone Marrow-sparing Intensity Modulated Radiation Therapy With Concurrent Cisplatin For Stage IB-IVA Cervical Cancer: An International Multicenter Phase II Clinical Trial (INTERTECC-2) Int J Radiat Oncol Biol Phys. 2017;97:536–545. doi: 10.1016/j.ijrobp.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Yock A.D., Ahmed M., Ayala-Peacock D., Chakravarthy A.B., Price M. Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum. J Appl Clin Med Phys. 2021;22:210–221. doi: 10.1002/acm2.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon S.W., Lin H., Alonso-Basanta M., Anderson N., Apinorasethkul O., Cooper K., et al. Initial Evaluation of a Novel Cone-Beam CT-Based Semi-Automated Online Adaptive Radiotherapy System for Head and Neck Cancer Treatment - A Timing and Automation Quality Study. Cureus. 2020;12 doi: 10.7759/cureus.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astrom L.M., Behrens C.P., Calmels L., Sjostrom D., Geertsen P., Mouritsen L.S., et al. Online adaptive radiotherapy of urinary bladder cancer with full re-optimization to the anatomy of the day: Initial experience and dosimetric benefits. Radiother Oncol. 2022;171:37–42. doi: 10.1016/j.radonc.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Vassiliev O.N., Wareing T.A., McGhee J., Failla G., Salehpour M.R., Mourtada F. Validation of a new grid-based Boltzmann equation solver for dose calculation in radiotherapy with photon beams. Phys Med Biol. 2010;55:581–598. doi: 10.1088/0031-9155/55/3/002. [DOI] [PubMed] [Google Scholar]
- 27.Gay H.A., Barthold H.J., O'Meara E., Bosch W.R., El Naqa I., Al-Lozi R., et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huyskens D.P., Maingon P., Vanuytsel L., Remouchamps V., Roques T., Dubray B., et al. A qualitative and a quantitative analysis of an auto-segmentation module for prostate cancer. Radiother Oncol. 2009;90:337–345. doi: 10.1016/j.radonc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 29.[29] NIH National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf [accessed May 2022].
- 30.Greimel E.R., Kuljanic Vlasic K., Waldenstrom A.C., Duric V.M., Jensen P.T., Singer S., et al. The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer. 2006;107:1812–1822. doi: 10.1002/cncr.22217. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.I., Atri M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology. 2019;292:15–24. doi: 10.1148/radiol.2019190088. [DOI] [PubMed] [Google Scholar]
- 33.Perna L., Sini C., Cozzarini C., Agnello G., Cattaneo G.M., Hysing L.B., et al. Deformable registration-based segmentation of the bowel on Megavoltage CT during pelvic radiotherapy. Phys Med. 2016;32:898–904. doi: 10.1016/j.ejmp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Seppenwoolde Y., Majercakova K., Buschmann M., Dorr E., Sturdza A.E., Schmid M.P., et al. Early morbidity and dose-volume effects in definitive radiochemotherapy for locally advanced cervical cancer: a prospective cohort study covering modern treatment techniques. Strahlenther Onkol. 2021;197:505–519. doi: 10.1007/s00066-021-01781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chetty I.J., Rosu-Bubulac M. Deformable Registration for Dose Accumulation. Semin Radiat Oncol. 2019;29:198–208. doi: 10.1016/j.semradonc.2019.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.