Abstract
Background:
Timely incision and drainage (I&D) is first line management for anorectal abscesses. We aimed to define current practices in anorectal abscess management and identify factors associated with abscess recurrence and fistula formation.
Methods:
Index episodes of anorectal abscesses treated with I&D in 2014–2018 at a multi-hospital healthcare system were included. Association with one-year abscess recurrence or fistula formation was evaluated using Cox proportional hazard regression. Fistulae were captured only among patients without fistulae at the index operation.
Results:
A total of 458 patients met study criteria. One-year rate of abscess recurrence or fistula formation was 20.3%. When compared to bedside procedures, drainage in the operating room was associated with a reduced risk of either recurrence or fistula formation (aHR 0.20 [95%CI 0.114–0.367]).
Conclusions:
Improved exposure and patient comfort in the operating room may allow more complete drainage contributing to decreased rates of abscess recurrence or fistula formation.
1. Introduction
Anorectal abscesses are estimated to affect 68,000–96,000 people per year in the United States.1 The majority of these abscesses develop due to the obstruction of glands lining the anal crypts. A smaller percentage of abscesses have etiologies such as inflammatory bowel disease, trauma, or malignancy. Following abscess drainage, recurrent abscesses and fistulae occur in 20–70% of patients; these sequelae cause physical and emotional distress to patients, while incurring significant cost to the healthcare system.1–8
Although there is general consensus that timely incision and drainage (I&D) is first line management for anorectal abscesses, there is no uniform strategy for drain placement or postoperative wound care.9 Previous studies have reported that there were no differences in clinical outcomes when comparing drain placement to packing or no packing.3,10 Indeed, packing the wound was associated with increased pain without any beneficial effect on abscess recurrence and fistula formation.11 There are also conflicting results in the literature regarding postoperative antibiotic use. A prospective, randomized study demonstrated that postoperative antibiotics do not improve abscess recurrence or fistula formation.12 Current guidelines from the American Society of Colon and Rectal Surgeons (ASCRS) provide evidence-based consensus on these the discrepancies and recommend against the routine use of antibiotics in adequately drained abscesses.4 Whether this strategy is feasible in all patients, particularly diabetic or immunocompromised patients, remains unknown as these patients have been excluded from previous studies on the issue.13,14
Despite standardized ASCRS guidelines for timely drainage of anorectal abscesses with or without drain placement and limited antibiotic use, we encountered a significant amount of heterogeneity in treatment within our healthcare system.4 We therefore sought to review index cases of anorectal abscesses that underwent I&D at our institution in order to describe practice patterns and identify factors that contribute to adverse outcomes such as abscess recurrence and fistula formation.
2. Methods
2.1. Patient characteristics
This retrospective study included adult patients ≥18 years of age with an index episode of anorectal abscess treated with I&D between 2014 and 2018 at a multi-hospital healthcare system. Patients presented to one of 14 hospitals in our healthcare system in western Pennsylvania, including tertiary referral centers, community hospitals, and affiliated outpatient clinics. Abscesses were drained by attending surgeons or surgical trainees and rarely emergency medicine providers. Candidate patients were identified using appropriate International Classification of Disease (ICD) version 10 codes K61.0-K61.5 and Current Procedure Terminology (CPT) codes 45990, which represented anorectal exam, surgical, requiring anesthesia and 46040, 46045, and 46050, which represented incision and drainage of ischiorectal, perianal, intramural, intramuscular or submucosal abscesses. Patients with a history of inflammatory bowel disease, hidradenitis, perineal trauma or anorectal surgery, anal or rectal malignancy, pelvic radiation, active sacral ulcer, necrotizing fasciitis, or pregnancy were excluded. Patients with supralevator abscesses were excluded given that these are typically drained intraluminally. Lastly, patients with persistent abscesses that did not have an interval of documented recovery, i.e., intermittent drainage or continued pain at the site of I&D prior to abscess recurrence were also excluded. All demographics, medical comorbidities, preoperative and intraoperative variables were manually abstracted from the electronic health record, including emergency department, inpatient, and outpatient visits for both the index episode and follow-up appointments, as well as imaging, intraoperative, and pathology reports. Medical records included information from all UPMC network hospitals and regional electronic health records from non-governmental facilities available via Epic’s Care Everywhere, an electronic health record exchange with an interoperability platform to view other healthcare facilities’ records. The study was reviewed and determined to be exempt from informed consent by the University of Pittsburgh’s Human Research Protection Office (STUDY20040108).
2.2. Study endpoints
The aim of the study was to delineate practice patterns in the management of anorectal abscesses in a large healthcare system in the United States. The secondary aim was to identify risk factors for abscess recurrence and fistula formation up to one year after index I&D of anorectal abscesses. Adverse outcomes were defined as abscess recurrence or fistula formation. Recurrence was defined as the presence of another anorectal abscess after a period of interval resolution. Post-operative fistula formation was defined as the development of fistula only among those patients who did not have a fistula at the index operation. The primary adverse outcome was a composite of both abscess recurrence or fistula formation. Secondary adverse outcomes included each event in isolation.
2.3. Statistical analyses
Categorical variables are compared using Chi-squared testing and continuous variables are compared with a student’s t-test or Kruskal-Wallis, as appropriate. The risk of composite abscess recurrence or fistula formation among those treated in the operating room or at the bedside was depicted with Kaplan-Meier curves and compared with log-rank testing. The associations between patient, abscess, and treatment factors were evaluated with multivariable Cox proportional hazard regression adjusting for age, body mass index, comorbid conditions, abscess characteristics as well as treatment, and clustered on the year of intervention to generate adjusted hazard ratios (aHR). Proportional hazards assumptions were based on Schoenfeld residuals.15 Survival analysis was censored at the last date of contact among patients in the healthcare system or date of death as determined by the social security death index in combination with hospital system death records.16 All analyses were completed using Stata 17.0 (StataCorp, LLC) with two-sided testing with a p < 0.05 delineating significance.
3. Results
A total of 990 patients with anorectal abscesses treated with I&D were included. Of these, 458 patients met criteria for inclusion in the study (Fig. 1). The majority of patients were white (n = 374, 81.7%) and male (n = 302, 65.9%) with a mean age of 47.5 ± 15.6 (Table 1). Abscesses were most frequently found in the perianal space (n = 304, 66.4%) followed by the ischiorectal fossa (n = 98, 21.4%). A fistula was identified at the index operation in 105 (22.9%) of patients. Most abscesses were drained in the operating room (n = 427, 93.2%) under general anesthesia (n = 360, 78.6%). Postoperative wound care consisted of packing (n = 266, 58.1%), drain placement (n = 112, 24.4%), or neither (n = 80, 17.5%). A total of 310 (67.7%) patients received postoperative antibiotics.
Fig. 1.
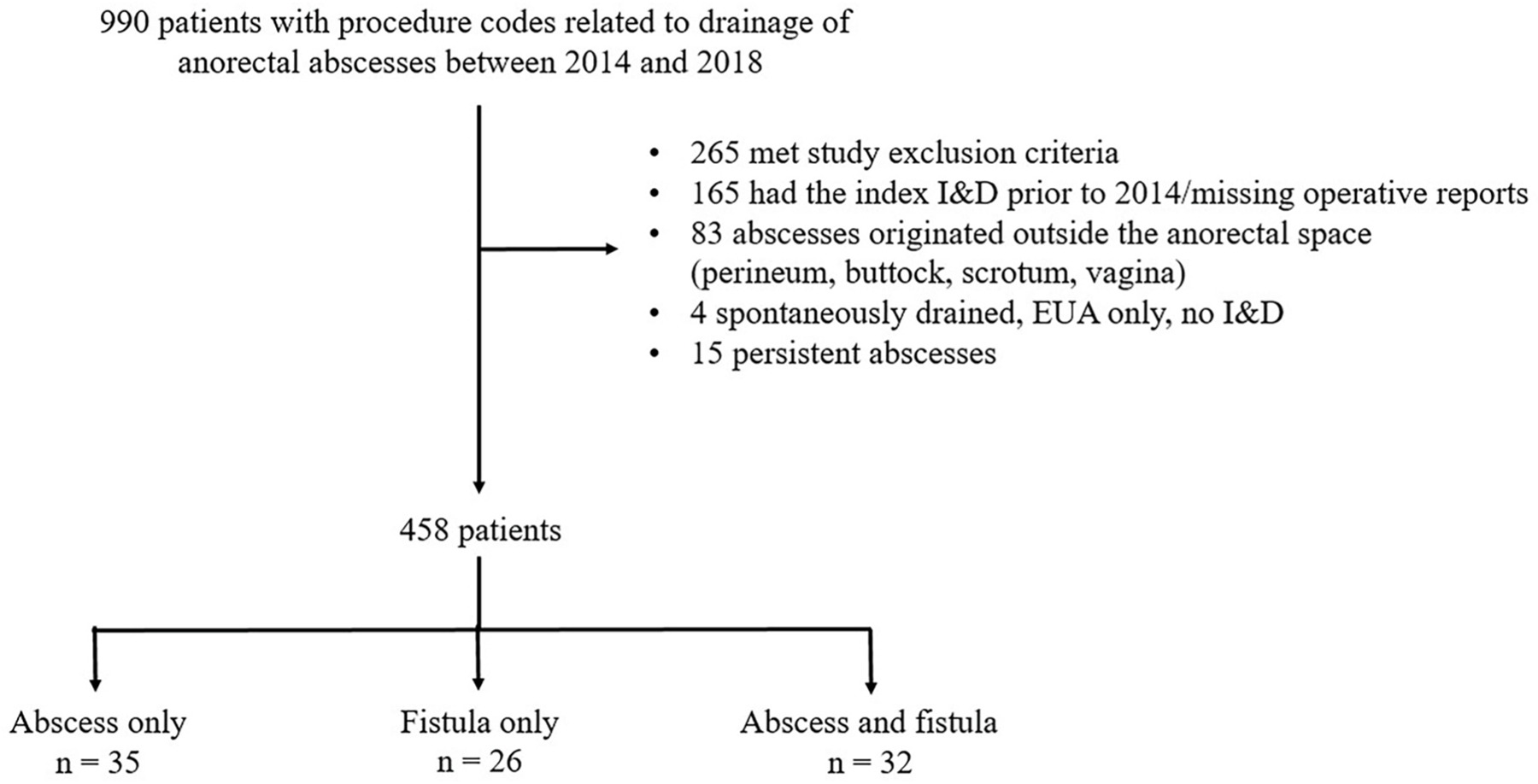
Generation of study cohort and distribution of post-drainage adverse outcomes of abscess recurrence and/or fistula formation.
Table 1.
Demographics and clinical characteristics of patients with and without adverse outcomes of abscess recurrence or fistula formation within one year of I&D of anorectal abscess.
| Variable | Whole cohort (n = 458) | No adverse outcome (n = 365) | Adverse outcome (n = 93) | p value |
|---|---|---|---|---|
| Mean age±SD | 47.5 ± 15.6 | 47.8 ± 16.2 | 46.7 ± 14.6 | 0.56 |
| Male sex, n (%) Race, n (%) | 302 (65.9) | 241 (66.0) | 61 (65.6) | 0.94 |
| Race, n (%) | 0.25 | |||
| White | 374 (81.7) | 293 (80.3) | 81 (87.1) | |
| Black | 62 (13.5) | 55 (15.1) | 7 (7.5) | |
| Other/not specified | 22 (4.8) | 17 (4.7) | 5 (5.4) | |
| BMI ±SD | 31.8 ± 7.7 | 31.8 ± 8.0 | 31.5 ± 6.8 | 0.76 |
| Smoker, n (%) | 0.75 | |||
| Current | 143 (31.2) | 117 (32.1) | 26 (28.0) | |
| Former | 117 (25.5) | 92 (25.2) | 25 (26.9) | |
| Never | 198 (43.2) | 156 (42.7) | 42 (45.2) | |
| Diabetic, n (%) | 94 (20.5) | 76 (20.8) | 18 (19.4) | 0.75 |
| Immunosuppressed, n (%) | 22 (4.8) | 19 (5.2) | 3 (3.2) | 0.43 |
| Median CCI (IQR) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.29 |
| Diagnostic method, n (%) | ||||
| Physical exam | 193 (42.1) | 155 (42.5) | 38 (40.9) | |
| Abscess location, n (%) | ||||
| Perianal | 304 (66.4) | 246 (67.4) | 58 (62.4) | |
| Ischiorectal | 98 (21.4) | 71 (19.5) | 27 (29.0) | |
| Intersphincteric | 31 (6.8) | 26 (7.1) | 5 (5.4) | |
| Horseshoe | 25 (5.5) | 22 (6.0) | 3 (3.2) | |
| Fistula at index operation, n (%) | 105 (22.9) | 96 (26.3) | 9 (9.7) | <0.001 |
| Drainage location, n (%) | <0.001 | |||
| Operating room | 427 (93.2) | 353 (96.7) | 74 (79.6) | |
| Bedside | 31 (6.8) | 12 (3.3) | 19 (20.4) | |
| Anesthesia, n (%) | <0.001 | |||
| General | 360 (78.6) | 304 (83.3) | 56 (60.2) | |
| Spinal | 11 (2.4) | 7 (1.9) | 4 (4.3) | |
| MAC | 55 (12.0) | 42 (11.5) | 13 (14.0) | |
| Local | 32 (7.0) | 12 (3.3) | 20 (21.5) | |
| Wound care, n (%) | ||||
| Packing | 266 (58.1) | 207 (56.7) | 59 (63.4) | 0.097 |
| Drain | 112 (24.4) | 89 (24.4) | 23 (24.7) | |
| No packing/drain | 80 (17.5) | 69 (18.9) | 11 (11.8) | |
| Postoperative antibiotics, n (%) | 310 (67.7) | 243 (66.6) | 67 (72.0) | 0.31 |
SD: standard deviation; BMI: body mass index; CCI: Charlson comorbidity index; IQR: interquartile range; MAC: monitored anesthesia care.
Of the 458 patients, 93 (20.3%) developed adverse outcomes of either abscess recurrence or fistula formation within one year of I&D. The median time to either outcome was 92 days (IQR 24–221); the median time to abscess recurrence was 113 days (IQR 43–225) (Fig. 2A) while the median time to fistula formation was 90 days (IQR 52–168) (Fig. 2B). There were no major differences in the demographics or preoperative clinical characteristics of patients with and without adverse outcomes (Table 1). However, patients who did not develop adverse outcomes were drained in the operating room more frequently than patients who developed recurrent abscesses or fistulae (n = 353, 96.7% vs. n = 74, 79.6%, p < 0.001).
Fig. 2. Frequency of abscess recurrence or fistula formation within one year of I&D of anorectal abscess.
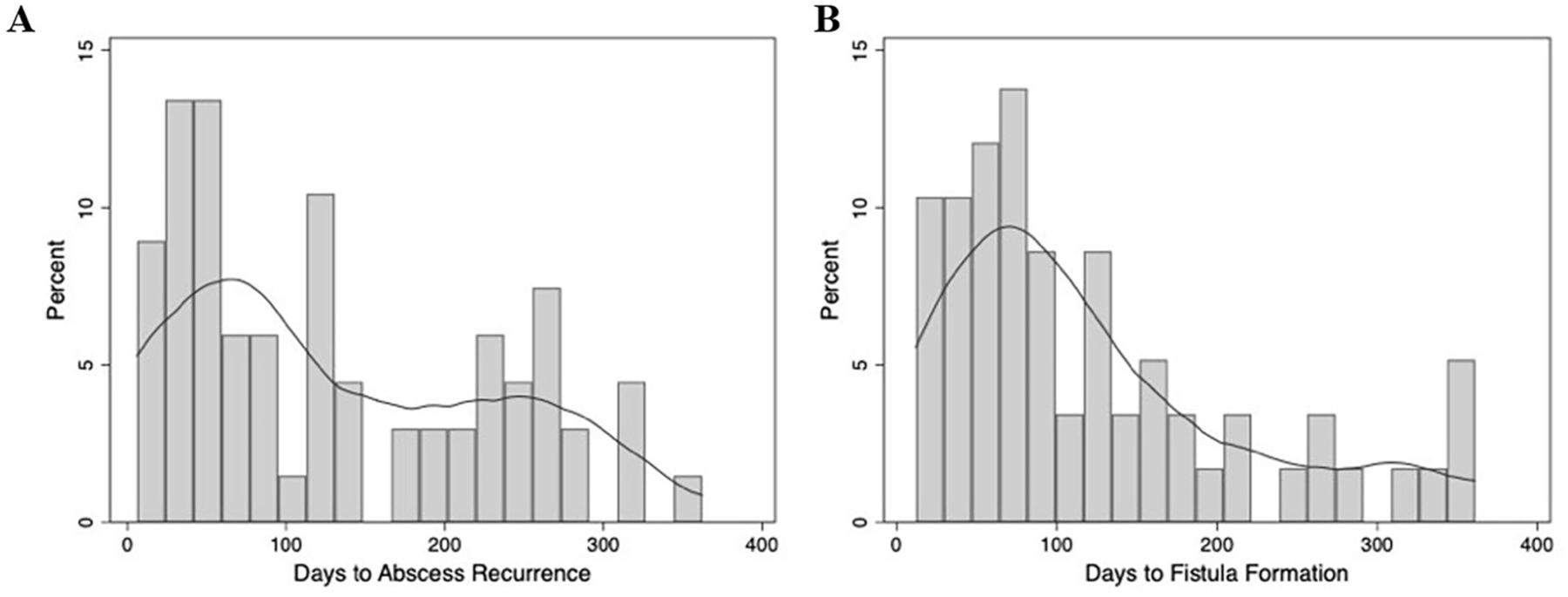
Histogram demonstrating days to development of (A) abscess recurrence (whole cohort) or (B) fistula among patients who did not have a fistula at the index operation. The overlying curves represent univariate kernel density estimation.
Given that the procedure location (bedside or operating room) of abscess drainage is a modifiable factor, we sought to further understand how it impacts abscess recurrence and fistula formation. When stratified by procedure location, the median time to recurrence or fistula formation was 92 days (IQR 44–189) in patients drained in the operating room and 117 days (IQR 27–161) in patients drained at the bedside. However, the cumulative risk of either adverse outcome after I&D was significantly higher in patients drained at the bedside compared to those drained in the operating room (Fig. 3; p < 0.001). Use of local anesthesia was also more frequent in the adverse outcome group (n = 20, 21.5% vs. n = 12, 3.3%). On multivariable analysis, operating room drainage was associated with a decreased risk of abscess recurrence or fistula formation (aHR = 0.20, 0.114–0.367 CI, p < 0.0001) (Table 2). Although age, abscess location within the ischiorectal space, and placing neither packing nor drain reached significance in the composite model, only operating room drainage remained significant when the risk for either adverse outcome was analyzed independently (Table 3). Charlson comorbidity index (CCI) was associated with a slightly reduced composite risk of abscess recurrence and fistula formation; this decrease in risk persisted when each outcome was assessed independently but did not reach statistical significance.
Fig. 3. One-year composite risk of abscess recurrence or fistula formation in patients drained in the operating room versus at the bedside.
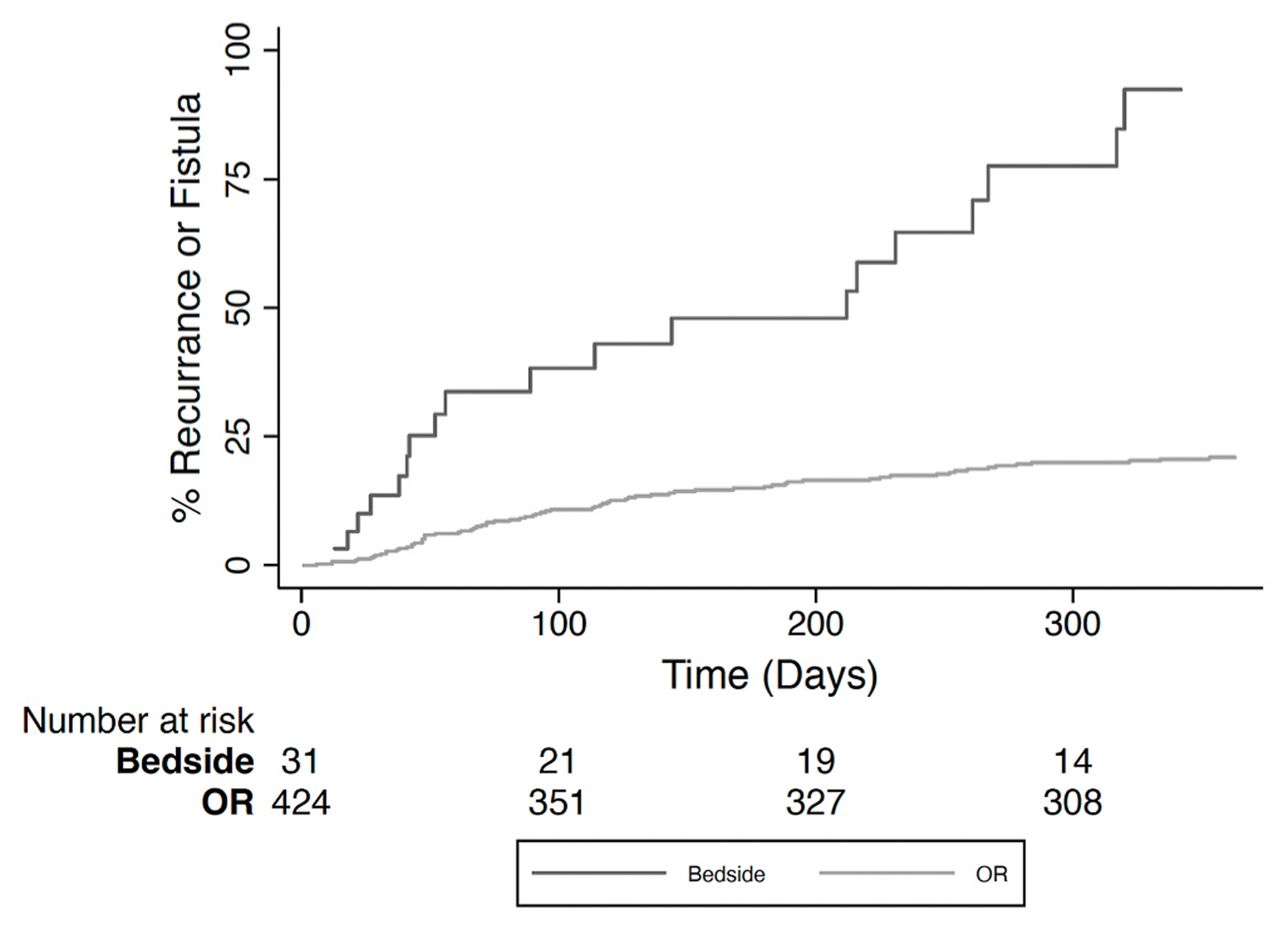
OR, operating room.
Table 2.
Associations between abscess recurrence or fistula formation within one year of I&D of anorectal abscess.
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Age | 1.01 | 1.010–1.015 | <0.0001 |
| BMI | 1.00 | 0.967–1.044 | 0.80 |
| CCI | 0.85 | 0.687–1.056 | 0.14 |
| Abscess location; ref, perianal | |||
| Ischiorectal | 1.61 | 1.029–2.522 | 0.04 |
| Intersphincteric | 1.34 | 0.913–1.979 | 0.13 |
| Horseshoe | 0.80 | 0.159–4.009 | 0.79 |
| Drainage location; ref, bedside | 0.20 | 0.114–0.367 | <0.0001 |
| Wound care; ref, packing | |||
| Drain | 1.09 | 0.821–1.444 | 0.55 |
| Nothing | 0.63 | 0.406–0.978 | 0.04 |
CI: confidence interval; BMI: body mass index; CCI: Charlson comorbidity index; ref: value of reference.
Table 3.
Distinct associations between abscess recurrence or fistula formation within one year of I&D of anorectal abscess.
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Abscess recurrence | |||
| Age | 1.00 | 0.992–1.012 | 0.69 |
| BMI | 1.01 | 0.976–1.042 | 0.60 |
| CCI | 0.93 | 0.758–1.145 | 0.49 |
| Abscess location; ref, perianal | |||
| Ischiorectal | 1.56 | 0.995–2.456 | 0.05 |
| Intersphincteric | 1.52 | 0.657–3.493 | 0.33 |
| Horseshoe | 0.73 | 0.133–4.017 | 0.72 |
| Drainage location; ref, bedside | 0.23 | 0.108–0.472 | <0.0001 |
| Wound care; ref, packing | |||
| Drain | 1.30 | 0.910–1.850 | 0.15 |
| Nothing | 0.53 | 0.233–1.195 | 0.13 |
| Fistula formation | |||
| Age | 1.02 | 1.012–1.038 | <0.0001 |
| BMI | 0.99 | 0.946–1.044 | 0.80 |
| CCI | 0.78 | 0.613–1.000 | 0.05 |
| Abscess location; ref, perianal | |||
| Ischiorectal | 1.43 | 0.857–2.394 | 0.17 |
| Intersphincteric | 1.11 | 0.379–3.224 | 0.85 |
| Horseshoe | 2.41 | 0.859–6.782 | 0.10 |
| Drainage location; ref, bedside | 0.22 | 0.108–0.449 | <0.0001 |
| Wound care; ref, packing | |||
| Drain | 0.92 | 0.440–1.936 | 0.83 |
| Nothing | 1.08 | 0.344–3.384 | 0.90 |
CI: confidence interval; BMI: body mass index; CCI: Charlson comorbidity index; ref: value of reference.
4. Discussion
We evaluated patterns in current management of anorectal abscesses at a large healthcare system. The majority of patients had abscesses in the perianal space, were treated with drain placement or packing, and received postoperative antibiotics. In congruence with prior studies, BMI, anatomic location of abscess, postoperative wound care, and antibiotic use did not correlate or associate with the risk of abscess recurrence or fistula formation within one year of I&D.3,4,6,11–13,17–22 In contrast to the above studies, we evaluated the impact of procedure location and discovered that drainage in the operating room was associated with a reduction in abscess recurrence or fistula formation at one year when compared to I&D at the bedside.
Abscess recurrence and fistula formation rates vary widely between 20 and 70%.1–8 The wide range in the reported rates of these adverse outcomes is complicated by the ability to detect their presence, especially that of anorectal fistulae. In our study, 105 (22.9%) patients were noted to have a fistula at the index operation. Only one of these patients was drained at the bedside, which underscores the importance of adequate exposure in the operating room and thorough examination of the anal canal in detecting fistulae. It is possible that more than one of the 31 patients who were drained at the bedside had a fistula that was not identified at the time of initial I&D, which could explain their higher rates of adverse postoperative outcomes. Interestingly, comorbidities such as diabetes have been associated with a similar or decreased risk of abscess recurrence and fistula formation.3,23 We did not find any differences in the frequency of diabetics or CCI when comparing patients who had no adverse outcomes with those that developed abscess recurrence or fistulae. CCI was associated with a lower hazard ratio in the composite model and both independent models of recurrence and fistula formation. CCI did not reach statistical significance, but perhaps this trend seen in both our cohort and in previous studies indicates that the biology of anorectal abscesses in medically complex patients is different from that of the otherwise healthy patient. It is important to note, however, that our study excluded patients with necrotizing soft tissue infections (NSTIs) as it was difficult to identify the origin of these infections due to their rapid spread by the time of presentation. As diabetics are at an increased risk of NSTIs, it is possible that we did not capture the full breadth of adverse outcomes in diabetics with anorectal abscesses.
The variation in fistula identification when comparing bedside and operative drainage at the time of index drainage begs the question: does identification and treatment of fistula at that time impact outcomes? A 2010 Cochrane systematic review of randomized control trials found that identification and management of fistula-in-ano at the time of abscess incision and drainage resulted in a significant reduction in abscess recurrence or persistence when compared to abscess drainage alone.24 The impact on clinically significant incontinence from this review is less clear, but is more prevalent in the management of high trans-sphincteric fistulae. The ASCRS Clinical Practice Guidelines acknowledges that this is a controversial topic, but ultimately recommends proceeding with primary fistulotomy in patients with simple fistulas if the perceived risk of incontinence is low.4 Note is made of the risk for creation of a false passage in the setting of active inflammation, and an alternative management option presented in the guidelines is staged fistulotomy with initial seton placement. In our study, the method of fistula identification was not uniform as operative descriptions of using a fistula probe or instilling hydrogen peroxide varied. Our colorectal division’s practice is to first interrogate the abscess cavity gently with a fistula probe. If a potential tract is suspected and not easily identified, we confirm an internal os using hydrogen peroxide. Setons are placed only if the internal os is widely patent.
Lastly, the importance of adequate anesthesia during drainage of any type of abscess is self-evident. Previous studies have differing suggestions on the type of anesthesia to be used during I&D, although this has not been formally investigated.6,17 The majority of patients in our cohort were drained under general or regional anesthesia or conscious sedation. We attempted to include type of anesthesia in our composite risk model, but any iteration in which both location of drainage and anesthesia were jointly included resulted in failure of the model. We suspect that because all bedside drainage procedures were performed under local anesthesia, for the purposes of computation, they served as the same variable. Our data would therefore argue that more than local anesthesia is necessary to decrease the risk of abscess recurrence and fistula formation.
Our study has several limitations. Although it spanned multiple hospitals, the study cohort was generated from a single healthcare system that serves patients with similar demographics in western Pennsylvania. As a result, our findings may not be generalizable to a more diverse population. We were also restricted to the information that was available in the electronic medical record due to the retrospective study design. Abscess size was therefore excluded from the analysis because a large proportion of patients did not have preoperative imaging and the abscess cavity’s size was not consistently recorded in operative reports. It is also possible that some of the study patients developed a recurrent abscess or fistula and presented to another institution for evaluation. We abstracted all data available through the institutional electronic medical record, which includes the Care Everywhere network with access to records outside of our institution through an interoperability platform, and censored patients at the date of last known contact in our analysis; nonetheless, our rates of one-year abscess recurrence of fistula formation could still be higher than 20.3%. Similarly, the definition of persistent abscess excluded patients from the study; however as most of these patients follow up due to discomfort from symptoms, most should be correctly categorized as persistent rather than recurrent abscesses. The large catchment area of our health system and the ability to view electronic medical records, including many outside of our health system, likely minimized the risk of patients being evaluated without our knowledge and misclassified as recurrent rather than persistent abscess. Our findings were further limited by coding as most patients with both an ICD code for anorectal abscess and a CPT code for I&D were inpatients. This strategy was necessary given the retrospective nature of our study. However, it is likely that we missed several bedside I&Ds, especially those performed by trainees or overnight. It remains unclear whether the bias towards operating room drainage was reflective of practice patterns in our healthcare system or due to inadequate capture of bedside and outpatient I&Ds.
In conclusion, anorectal abscesses are best treated with prompt and adequate drainage. We found that despite consensus guidelines on optimal management of anorectal abscesses, there was significant heterogeneity in the management of these abscesses at our institution. We also demonstrate that abscesses drained in the operating room, as opposed to the bedside, are associated with a decreased risk of recurrence and fistula formation. Improved exposure and patient comfort in the operating room likely allow more complete drainage contributing to this favorable association. Further studies are needed to evaluate whether this association is secondary to better fistula identification at the index operation and if operative drainage should in fact be the standard in anorectal abscess management.
Funding
This work was supported by the National Institutes of Health grants 5T32CA113263 (S.N.), 5T32HL098036 (L.H.A.), 5T32HL0098036 (K.M.R.), and L30AG064730 (K.M.R.).
Footnotes
Declaration of competing interest
The authors do not have any conflicts of interest related to this publication.
References
- 1.Abcarian H Anorectal infection: abscess-fistula. Clin Colon Rectal Surg 2011. Mar;24 (1):14–21. Epub 2012/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu D, Lu L, Cao B, et al. Relationship between body mass index and recurrence/anal fistula formation following initial operation for anorectal abscess. Med Sci Mon Int Med J Exp Clin Res 2019. Oct 23;25:7942–7950. Epub 2019/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano T, Asano M, Matsuda Y, Kawakami, Nakai, Nonaka. Prognostic factors for recurrence following the initial drainage of an anorectal abscess. Int J Colorectal Dis 2010. Dec;25(12):1495–1498. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 4.Gaertner WB, Burgess PL, Davids JS, et al. The American society of Colon and rectal surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum 2022. Aug 1;65(8): 964–985. Epub 2022/06/23. [DOI] [PubMed] [Google Scholar]
- 5.Onaca N, Hirshberg A, Adar R. Early reoperation for perirectal abscess: a preventable complication. Dis Colon Rectum 2001. Oct;44(10):1469–1473. Epub 2001/10/13. [DOI] [PubMed] [Google Scholar]
- 6.Vasilevsky CA, Gordon PH. The incidence of recurrent abscesses or fistula-in-ano following anorectal suppuration. Dis Colon Rectum 1984. Feb;27(2):126–130. Epub 1984/02/01. [DOI] [PubMed] [Google Scholar]
- 7.Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg 1997. Aug;63(8): 686–689. Epub 1997/08/01. [PubMed] [Google Scholar]
- 8.Hämäläinen KP, Sainio AP.Incidence of fistulas after drainage of acute anorectal abscesses. Dis Colon Rectum 1998. Nov;41(11):1357–1362. ; discussion 61–2. Epub 1998/11/21. [DOI] [PubMed] [Google Scholar]
- 9.Sahnan K, Adegbola SO, Tozer PJ, Watfah Phillips. Perianal abscess. BMJ 2017. Feb 21;356:j475. Epub 2017/02/23. [DOI] [PubMed] [Google Scholar]
- 10.Zhu DA, Houlihan LM, Mohan HM, McCourt Andrews. Packing versus mushroom catheters following incision and drainage in anorectal abscess. Ir J Med Sci 2019. Nov;188(4):1343–1348. Epub 2019/01/25. [DOI] [PubMed] [Google Scholar]
- 11.Perera AP, Howell AM, Sodergren MH, et al. A pilot randomised controlled trial evaluating postoperative packing of the perianal abscess. Langenbeck’s Arch Surg 2015. Feb;400(2):267–271. Epub 2014/07/24. [DOI] [PubMed] [Google Scholar]
- 12.Sözener U, Gedik E, Kessaf Aslar A, et al. Does adjuvant antibiotic treatment after drainage of anorectal abscess prevent development of anal fistulas? A randomized, placebo-controlled, double-blind, multicenter study. Dis Colon Rectum 2011. Aug;54 (8):923–929. Epub 2011/07/07. [DOI] [PubMed] [Google Scholar]
- 13.Seow-En I, Ngu J. Routine operative swab cultures and post-operative antibiotic use for uncomplicated perianal abscesses are unnecessary. ANZ J Surg 2017. May;87(5): 356–359. Epub 2014/11/22. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PS, Moreno C. A multidisciplinary approach to perianal and intra-abdominal infections in the neutropenic cancer patient. Oncology 2015. Aug;29(8): 581–590. Epub 2015/08/19. [PubMed] [Google Scholar]
- 15.Zhang Z, Reinikainen J, Adeleke KA, Pieterse, Groothuis-Oudshoorn. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018. Apr;6(7): 121. Epub 2018/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reitz KM, Marroquin OC, Zenati MS, et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg 2020. Jun 1;155(6), e200416. Epub 2020/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanujam PS, Prasad ML, Abcarian H, Tan AB. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum 1984. Sep;27(9):593–597. Epub 1984/09/01. [DOI] [PubMed] [Google Scholar]
- 18.Beck DE, Fazio VW, Lavery IC, Jagelman Weakley. Catheter drainage of ischiorectal abscesses. South Med J 1988. Apr;81(4):444–446. Epub 1988/04/01. [DOI] [PubMed] [Google Scholar]
- 19.Millan M, García-Granero E, Esclápez P, Flor-Lorente, Espí Lledó. Management of intersphincteric abscesses. Colorectal Dis 2006. Nov;8(9):777–780. Epub 2006/10/13. [DOI] [PubMed] [Google Scholar]
- 20.Albright JB, Pidala MJ, Cali JR, Snyder, Voloyiannis, Bailey. MRSA-related perianal abscesses: an underrecognized disease entity. Dis Colon Rectum 2007. Jul;50(7): 996–1003. Epub 2007/05/26. [DOI] [PubMed] [Google Scholar]
- 21.Tonkin DM, Murphy E, Brooke-Smith M, et al. Perianal abscess: a pilot study comparing packing with nonpacking of the abscess cavity. Dis Colon Rectum 2004. Sep;47(9):1510–1514. Epub 2004/10/16. [DOI] [PubMed] [Google Scholar]
- 22.Isbister WH. A simple method for the management of anorectal abscess. Aust N Z J Surg 1987. Oct;57(10):771–774. Epub 1987/10/01. [DOI] [PubMed] [Google Scholar]
- 23.Hamadani A, Haigh PI, Liu IL, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis Colon Rectum 2009. Feb;52(2):217–221. Epub 2009/03/13. [DOI] [PubMed] [Google Scholar]
- 24.Malik AI, Nelson RL, Tou S. Incision and drainage of perianal abscess with or without treatment of anal fistula. Cochrane Database Syst Rev 2010. Jul 7;(7): CD006827. Epub 2010/07/09. [DOI] [PubMed] [Google Scholar]


