Highlights
-
•
Isolated cerebral ventriculomegaly (IVM) is the most common fetal brain anomaly.
-
•
Knowledge of fetal brain development in IVM is limited.
-
•
Quantitative fetal brain MRI analysis revealed increased brain volumes in IVM.
-
•
Novel sulcal developmental analysis discovered altered sulcal position in IVM.
-
•
Volumetric and sulcal pattern measurements may be used as fetal MRI biomarkers.
Abbreviations: IVM, Isolated ventriculomegaly; MRI, Magnetic resonance imaging; SI, Similarity index
Keywords: Fetus, Brain development, Fetal MRI, Volumetrics, Sulcal development
Abstract
Isolated cerebral ventriculomegaly (IVM) is the most common prenatally diagnosed brain anomaly occurs in 0.2–1 % of pregnancies. However, knowledge of fetal brain development in IVM is limited. There is no prenatal predictor for IVM to estimate individual risk of neurodevelopmental disability occurs in 10 % of children. To characterize brain development in fetuses with IVM and delineate their individual neuroanatomical variances, we performed comprehensive post-acquisition quantitative analysis of fetal magnetic resonance imaging (MRI). In volumetric analysis, brain MRI of fetuses with IVM (n = 20, 27.0 ± 4.6 weeks of gestation, mean ± SD) had revealed significantly increased volume in the whole brain, cortical plate, subcortical parenchyma, and cerebrum compared to the typically developing fetuses (controls, n = 28, 26.3 ± 5.0). In the cerebral sulcal developmental pattern analysis, fetuses with IVM had altered sulcal positional (both hemispheres) development and combined features of sulcal positional, depth, basin area, in both hemispheres compared to the controls. When comparing distribution of similarity index of individual fetuses, IVM group had shifted toward to lower values compared to the control. About 30 % of fetuses with IVM had no overlap with the distribution of control fetuses. This proof-of-concept study shows that quantitative analysis of fetal MRI can detect emerging subtle neuroanatomical abnormalities in fetuses with IVM and their individual variations.
1. Introduction
Fetal cerebral ventriculomegaly is the most common prenatally diagnosed fetal brain anomaly occurs in 0.2–1 % of pregnancies (Sethna et al., 2011, Hannon et al., 2012). Ventriculomegaly is defined as enlarged ventricles in the fetal brain with an atrial level of at least 10 mm measured by fetal sonogram or magnetic resonance imaging (MRI) (Sethna et al., 2011). Fetuses with ventriculomegaly have heterogeneous underlying etiologies such as hydrocephalus, intrauterine infections (TORCH infection, etc.), parenchymal volume loss (injury), cerebral malformations (disorders of cortical migration, etc.), or chromosomal anomalies (trisomies 21, 13, etc.) (Sethna et al., 2011, Mehlhorn et al., 2017). Reflecting heterogeneous etiologies, fetuses with ventriculomegaly have a broad spectrum of neurodevelopmental outcomes after birth ranging from normal to severe cognitive, motor, and behavioral disabilities that makes prediction of neurodevelopmental prognosis significantly challenging (Sethna et al., 2011, Hannon et al., 2012, Gaglioti et al., 2005, Beeghly et al., 2010, Tatli et al., 2012, Tonni et al., 2016, Scelsa et al., 2018).
In current clinical practice, postnatal neurodevelopmental prognosis of fetal cerebral ventriculomegaly is predicted based on imaging (obstetric ultrasound and fetal MRI), laboratory (congenital infection), and genetic data (targeted sequencing, chromosomal microarray and non-invasive prenatal testing) (Scelsa et al., 2018). Fetal MRI plays critical role to explore brain anatomy and add clinical information to ultrasound diagnosis as much as in a half of the cases, which may potentially change management (Kandula et al., 2015, van Doorn et al., 2016, Griffiths et al., 2017). The presence of associated anatomical, infections or genetic anomalies is usually associated with poorer neurodevelopmental outcomes (Sethna et al., 2011, Hannon et al., 2012, Beeghly et al., 2010, Tatli et al., 2012, Tonni et al., 2016, Scelsa et al., 2018). In case of isolated ventriculomegaly (IVM), with no other associated anatomical or genetic abnormalities are found, 6 ∼ 10 % of fetuses with IVM can still develop significant neurodevelopmental impairment (Tonni et al., 2016, Scelsa et al., 2018, Scala et al., 2017). There are no fetal markers that can associate with postnatal individual neurodevelopmental function. Such gap in clinical knowledge has resulted in ambiguous prenatal counseling, creates an immense stress on expecting parents. Without reliable neurodevelopmental prognosis predictors, clinicians may not be prepared to rare perinatal complications such as seizures or feeding problems. Clinicians are also deprived the opportunity to provide personalized early intervention. Thus, in the field of fetal neurology, there is a great need for novel approaches to detect prognostic information for fetuses with IVM. More accurate tools to quantify fetal brain development and delineate individual variances may serve as neurodevelopmental predictors for individual fetuses with precision. As the first step, in this study, we applied a new approach to precisely measure regional structural volumetric growth and cerebral sulcal developmental patterns (Im et al., 2017, Tarui et al., 2018, Tarui et al., 2019) in fetuses with IVM.
Knowledge of fetal brain development in IVM is limited and controversial. In the past decade, volumetric analysis of fetal MRI has been developed and evolved to increase their precision to measure fetal brain regional structural volume in three-dimensionally (3D) reconstructed MRI images. However, the results of previous volumetric studies in fetuses with IVM have not been consistent. In recent studies, one study reported increased cortical volume (Kyriakopoulou et al., 2014), while other reported unchanged volume (Scott et al., 2013). This is because in part, brain volumes of fetuses with IVM were measured in different inclusion criteria, 3D reconstruction, and motion correction methods (Kyriakopoulou et al., 2014, Scott et al., 2013, Grossman et al., 2006, Gholipour et al., 2012). We first aimed therefore to determine regional and whole brain volume in fetuses with IVM in our cohort to provide more reference knowledge.
To utilize quantitative measurements of fetal MRI as neurodevelopmental predictors, we need to extract measures of individual variances in fetal brain development that can be relevant to future neurodevelopmental function. Our group has developed a similarity index-based sulcal developmental pattern analysis that has a significantly high sensitivity in detecting developmental alterations in sulcal position and size (basin area and depth) in fetuses with isolated agenesis of corpus callosum (Tarui et al., 2018); polymicrogyria (Im et al., 2017), and congenital heart diseases (Ortinau et al., 2018). While other surface analytic methods such as curvature-based surface analysis or gyrification index-based analysis use a single feature, sulcal developmental pattern analysis can comprehensively evaluate complex geometric and topological patterns using multiple features such as sulcal position, depth, sulcal basin area, and intersulcal relationships of those features. Importantly, this method revealed individual fetal variations in sulcal developmental aberration with the normalized index which ranges from 0 to 1 (Tarui et al., 2018). We applied this novel analytic method to delineate individual variances in sulcal developmental patterns. To our knowledge (as of September 2022), no study has measured individual variances in fetal brain development in IVM.
2. Material and methods
2.1. Subjects
This study was conducted at three institutions Tufts Medical Center (TMC), Boston Children’s Hospital (BCH), Maine Medical Center (MMC), and Jikei University School of Medicine (JU) to increase study recruitment. The study was approved by the Institutional Review Boards of all participating institutions. MR images from fetuses with isolated ventriculomegaly (IVM) were prospectively identified and recruited with informed consent at three institutions. Postnatal MRIs obtained within two months of life confirmed the diagnosis of IVM. The Inclusion criteria for both case (IVM) and typical developing (control) fetuses were maternal age 18–45 years; singleton pregnancy; gestational age 18–32 gestational weeks (GW); and both sexes. The gestational age of each subject was determined by utilizing sonographic biometric measurements of the fetus in the first or second trimesters (crown–rump length, biparietal diameter, etc.) per standard care at obstetric clinics. The inclusion criteria for the case fetuses (IVM) was sonographic and/or MRI diagnosis of ventriculomegaly with atrial diameter of 10 mm ∼ 15 mm. Exclusion criteria were multiple pregnancies, fetuses with other brain and/or other organ anomalies, known chromosomal abnormalities, known congenital infections, or progressive ventriculomegaly (hydrocephalus or progressive parenchymal injury). We excluded fetuses with ventriculomegaly evolved to hydrocephalus.
For volumetric analysis, we included every fetus included to the study (18–32 GW). For sulcal developmental pattern analysis, we excluded fetuses younger than 21 GW because there were too little sulci to analyze (21–32 GW).
Healthy control subjects were identified and recruited at TMC by approaching healthy pregnant women with uncomplicated singleton pregnancies from the Obstetric clinic or women with fetuses suspected for brain anomalies in ultrasound but confirmed to be normal in fetal MRI. The clinical imaging diagnoses were confirmed by a pediatric neurologist (T.T.) and two neuroradiologist (N.M.). Images were excluded if significant motion or other artifacts that degraded image quality were present. Healthy controls were excluded if the fetal MRI identified any abnormality.
2.2. MRI acquisition and post-acquisition processing
Using MRI scanners at each institution, fetal brains were scanned using a single shot fast spin echo T2 technique (single shot turbo spin echo on Philips). The following MRI sequences were used in a Phillips 1.5 T scanner. Time repetition = 12.5 s, time echo = 180 ms, field of view = 256 mm, in-plane resolution = 1 mm, slice thickness = 2–3 mm. The HASTE acquisition was acquired at least three times in different orthogonal orientations. In addition to these, 3–12 HASTE scans (depending on the severity of the motion artifacts), orientated at ∼30°–45° relative to the original three orthogonal scans, were acquired for reliable image post-processing. At initial review, MRI studies without severe motion or other artifacts in the brain region were included in the study.
Raw MR images had motion artifacts and were not aligned reflecting fetal head positions at the time of scans. Fetal head motion correction and isotropic high-resolution volume reconstruction (voxel size: 0.75 × 0.75 × 0.75 [mm]) were performed from the multiple planes repeatedly acquired from the same fetus (Tarui et al., 2018, Tarui et al., 2019, Kuklisova-Murgasova et al., 2012). Subsequently, fetal heads were aligned to same direction using anterior and posterior commissures (AC-PC) as landmarks using AFNI (afni.nimh.nih.gov/afni) (Fig. 1a–c) (Tarui et al., 2018, Tarui et al., 2019, Cox, 2012).
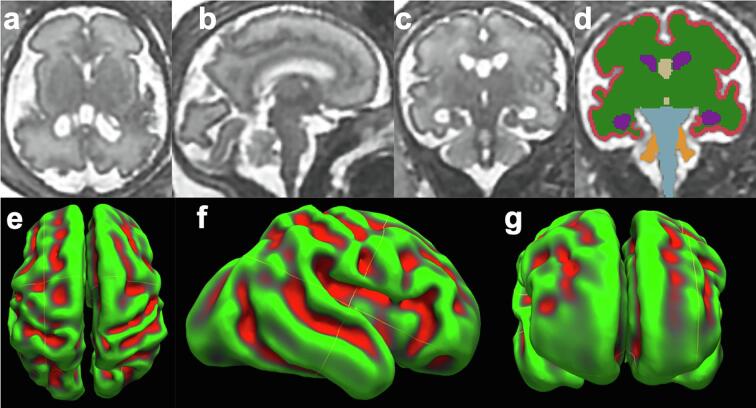
Fig. 1.
Imaging processing, volumetric and cerebral surface reconstruction Axial (a), sagittal (b) and coronal (c) view of motion corrected and three dimensionally reconstructed brain of 29 weeks gestational age fetus with ventriculomegaly. Series of coronal section was manually segmented to regional structures (d). Axial (e), sagittal (f) and coronal (g) views of three-dimensional inner cortical plate surface reconstructed based on the same segmented images. Green and red color reflects positive and negative curvature values on the surface (green = gyri, red = sulci). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Segmentation and volumetric analysis
Motion-corrected and AC-PC alignment processes provide seamless, reconstructed 3D volumes of fetal MR images that we used for further quantitative analyses. First, using Freeview (surfer.nmr.mgh.harvard.edu), regional structures such as the cortical plate, subcortical parenchyma, lateral ventricles, third ventricles, brainstem, cerebellar hemispheres, vermis, and fourth ventricle were semi-automatically segmented on coronal planes by three raters (R.K., S.A., E.T.) who completed the training with at least three sample brains. All segmentations were finally curated by pediatric neurologist (T.T.) (Fig. 1c, d). Subcortical parenchyma represents the sum of all substructures in cerebrum except for the cortical plate and ventricles. In the second and third trimesters, the region should contain grey matter structures (basal ganglia, various nuclei) and developing white matter. As resolution of fetal MR images only allowed consistent segmentation as a sum, but not for each individual structure, we collectively segmented and measured the volume of this region. Axial and sagittal planes were also used to confirm borders of these structures. Once segmentation was completed, regional structures were three dimensionally reconstructed as a whole structure. Using Slicer (slicer.org), the volume of each structure was reconstructed and measured. Our volumetric analysis is described in more detail in Tarui et al. (2019) and Akiyama et al. (2022). The previous study also showed high inter-rater reliability (0.94 ∼ 0.99) of volumetric analysis tested by Dice’s coefficients of two investigators’ regional volume segmentations (Tarui et al., 2019).
2.4. Cerebral surface sulcal pattern analysis
Using the same segmented images, we reconstructed the 3D inner cortical plate surface using the isosurface function from the Matlab software (Im et al., 2017, Tarui et al., 2018, Tarui et al., 2019) for further sulcal matching and pattern analysis (Fig. 1e–g). First, we generated mean curvature and depth maps on a cortical surface using the FreeSurfer software (surfer.nmr.mgh.harvard.edu). Sulcal basins were automatically identified using a watershed algorithm based on the smoothed curvature map (Im et al., 2010, Im et al., 2011, Im et al., 2013, Im et al., 2016, Tarui et al., 2018). To enable quantitative analysis of sulcal position patterns, a minimum bounding box for a given surface model was created to determine an average 3D relative position (x: left – right [0 – 1], y: posterior – anterior [0 – 1], z: inferior – superior [0 – 1]) for each sulcal basin. We also computed the normalized surface area (s) and mean sulcal depth (d) of sulcal basins to capture their geometry (Im et al., 2017, Tarui et al., 2018). To assess how similar to normal an individual’s sulcal patterns are, we used 9 fetal brain templates from 23 to 31 GW as a reference, which was previously published (brain-development.org/brain-atlases) (Serag et al., 2012) and used in our former studies (Im et al., 2017, Tarui et al., 2018).
We assessed the pattern similarities of the sulcal position, basin area, depth, or the combination of all three features between the case and template brains. For cases, controls, and template brains, the whole sulcal pattern set including the local sulcal features (feature vector F(i) = (xi, yi, zi, si, di): 3D position, area, and depth of sulcal basin i) and the inter-sulcal geometric relationships, F(i) – F(u) = (xi – xu, yi – yu, zi – zu, si – su, di – du), were determined in the left and right whole hemispheres (Im et al., 2017, Tarui et al., 2018). The sulcal pattern set of individual fetuses was matched and compared with the set of template brains and their similarity index was computed using a spectral matching technique (Im et al., 2011). In addition, the similarities of the matched corresponding sulcal basins and the inter-sulcal relationships were separately measured (Tarui et al., 2018). Our sulcal pattern analysis is described in more detail in Tarui et al. (2018).
2.5. Statistical analysis
First, to compare subject demographics between IVM and control groups, an independent sample t-test was performed to test if gestational age distribution was statistically matched between two groups. Group differences in sex ratio were assessed using a Fisher’s exact test.
To compare regional volume growth patterns between fetuses with IVM and controls over fetal developmental period, we plotted measured regional volumes in function of gestational age. We used non-linear regression model to represent growth curves of regional structures (Fig. 2). We compared growth curves of the whole brain; cortical plate; subcortical parenchyma; lateral, third, and fourth ventricles; cerebellar hemispheres; and vermis between groups. We tested if one curve fits to datasets from both IVM and control groups (null hypothesis) by extra sum-of-squares F test with significance of p value <0.05.
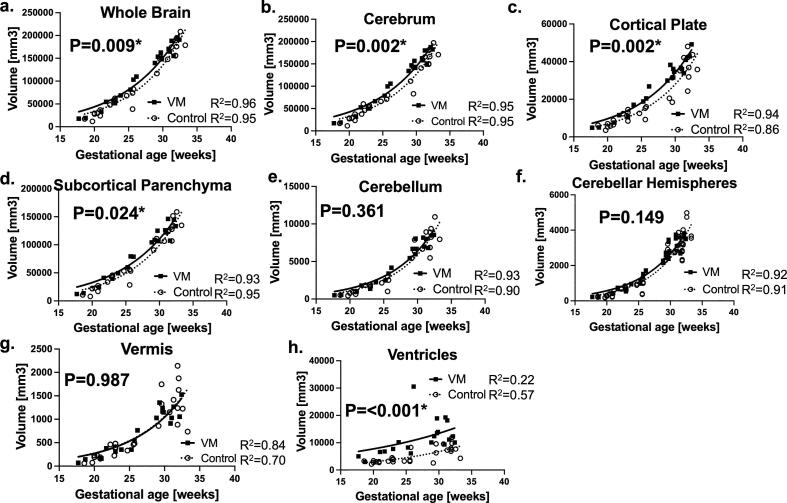
Fig. 2.
Fetuses with VM had distinct regional growth trajectories compared to control. Growth trajectories of regional volume were modeled according to non-linear regression models and compared between fetuses with VM and typically developing fetuses (control). Growth trajectory of (a) whole brain, (b) cerebrum, (c) cortical plate, (d) subcortical parenchyma was significantly larger in fetuses with VM compared to the controls. No Measures from left and right cortical plate, subcortical parenchyma, and cerebellar hemispheres were separately plotted. Growth trajectory of (e) cerebellum, (f) cerebellar hemispheres, (g) vermis is in different between VM and CT. (h) As expected, fetuses with VM had larger ventricular volume compared to the controls. Volumes of cortical plate, subcortical parenchyma, and cerebellar hemispheres from each hemisphere were plotted separately.
The sulcal pattern similarities to the templates were compared between control fetuses and fetuses with IVM using t-test analysis and linear regression model. In linear regression model, the similarity index was a dependent variable, and the group and gestational age were independent variables. The group effects were statistically examined by comparing the slopes and intercepts between linear regression models of VM and controls. Significant difference in this analysis would reflect that intergroup difference in sulcal feature was apparent across the whole age range.
3. Results
3.1. Subjects
Subjects demographics of this study is summarized in Table 1. Twenty fetuses with IVM and 25 typically developing control fetuses were recruited. Maternal age (27.2 ± 6.4, 30.7 ± 4.5, P = 0.06, Mann-Whitney test) were not significantly different between two groups (Table 1). Ethnicity of the IVM and control groups were Asian 5, 4, Black 1, 2, Hispanic 2, 2, and White 12 and 17, respectively (p = 0.86. Chi-square test) (Table 1).
Table 1.
Subjects demographics are indifferent between VM and controls.
| #of subjects (MRI data) | Gestational age [weeks] average ± SD (min – max) | Fetal sex F: M | Maternal age [years] average ± SD (min – max) | |
|---|---|---|---|---|
| Volumetric study | ||||
| Isolated ventriculomegaly | 20 (20) | 27.0 ± 4.6 (17.7–32.4) | 7: 12* | 27.2 ± 6.4 (15–38) |
| Typically developing control | 25 (28) | 26.3 ± 5.0 (18.6–33.3) | 11: 14 | 30.7 ± 4.5 (22–37) |
| Cerebral surface study | ||||
| Isolated ventriculomegaly | 16 (16) | 29.5 ± 4.2 (21.7–37.7) | 4: 11* | 28.8 ± 6.0 (15–36) |
| Typically developing control | 17 (20) | 28.0 ± 4.3 (21.9–35.6) | 7: 10 | 31.2 ± 4.2 (22–37) |
*One fetal sex unknown.
All subjects had one fetal MRI study except three controls volunteered to have two MRI studies, that made 28 control MRIs. The gestational ages of the MRI studies (IVM, control; mean ± SD (range)) were, respectively, 27.0 ± 4.6 (17.7–32.4) and 26.3 ± 5.0 (18.6–33.3) GW (P = 0.62, Mann-Whitney test). Fetal sex (female IVM 36.8 %, control 44.0 %, P = 0.76, Fisher’s exact test) was not significantly different between two groups (Table 1).
Since further cerebral sulcal pattern analysis requires accurate reconstruction of cortical plate surface, we visually inspected the quality of reconstructed volume and surface in focus of continuity and accuracy of cortical plate boundary. From above fetal brain images, two IVM and two control surfaces were excluded due to poor reconstruction. We excluded 2 IVM and 6 control surfaces since their gestational age was younger than 21 weeks, because there were too little sulci to analyze. Therefore, included 16 of 20 surfaces in IVM group and all 20 of 28 surfaces in control group for the cerebral surface analysis that showed high quality at cortical surface reconstruction. The gestational ages of the surface MRI studies (IVM, control; mean ± SD (range)) were, respectively, 29.5 ± 4.2 (21.7–37.7) and 28.0 ± 4.3 (21.9–35.6) GW (P = 0.31, Mann-Whitney test). Fetal sex (female IVM 26.70 %, control 41.2 %, P = 0.47, Fisher’s exact test) and maternal age (28.8 ± 6.0, 31.2 ± 4.2, P = 0.19, t-test) were not significantly different between fetuses with IVM and control. Ethnicity of the IVM and control groups were Asian 4, 3, Black 1, 0, Hispanic 1, 1, and White 10 and 12, respectively (p = 0.72. Chi-square test).
Following clinical MRI review, IVM cases were diagnosed as “isolated” VM without other central nervous system malformations.
3.2. Volumetric analysis
We found an exponential curve model was the best fit model for most of the data (R2 = 0.90 ∼ 0.95) except vermis (R2 = 0.84, 0.70, IVM and controls respectively) and ventricles (R2 = 0.22, 0.57) (Fig. 2). Fetuses with IVM had increased volume in the whole brain (Fig. 2a, p = 0.009), cerebrum (2b, p = 0.002) including cortical plate (2c, p = 0.002), and subcortical parenchyma (2d, p = 0.024). Cerebellar hemispheres appear to have increased volume though not significant (2f, p = 0.149). As expected, ventricular size was also larger in IVM (2h, p < 0.001) compared to control. Volume growth curves of cortical plate in IVM has already appeared to take distinct growth trajectory to control fetuses as early as 20 GW. Growth curves of ventricles appeared to be different as early as 18 GW. We did not see any difference in growth curves of cerebellum (2e, p = 0.361) or vermis (2g, p = 0.987). (Fig. 2) In this study, the four raters’ segmentation results had been consistent with Dice coefficient of 0.926 ± 0.081 and 0.922 ± 0.092 for the left and right cortical plate, 0.973 ± 0.027 and 0.984 ± 0.017 for the left and right subcortical parenchyma, and 0.925 ± 0.046 0.914 ± 0.089 for left and right cerebellar hemispheres, respectively.
3.3. Sulcal developmental pattern analysis
We analyzed cerebral surfaces from 16 IVM and 20 control fetal brain surfaces (Table 2). We compared the sulcal pattern similarities between controls and IVM fetuses for each cerebral hemisphere.
Table 2.
Comparison of linear regression models of VM and controls for slopes and intercepts.
| LEFT |
RIGHT |
||
|---|---|---|---|
| P-value | P-value | ||
| Comparison of whole pattern | |||
| Combined feature | 0.001* | Combined feature | 0.012* |
| 3D position | 0.001* | 3D position | 0.041* |
| Sulcal basin area | 0.241 | Sulcal basin area | 0.544 |
| Sulcal depth | 0.060 | Sulcal depth | 0.008* |
| Comparison of corresponding sulcal regions | |||
| Combined feature | 0.222 | Combined feature | 0.237 |
| 3D position | <0.001* | 3D position | 0.011* |
| Sulcal basin area | 0.943 | Sulcal basin area | 0.830 |
| Sulcal depth | 0.060 | Sulcal depth | 0.025* |
| Comparison of inter-sulcal relationship | |||
| Combined feature | 0.016* | Combined feature | 0.032* |
| 3D position | 0.009* | 3D position | 0.088 |
| Sulcal basin area | 0.751 | Sulcal basin area | 0.945 |
| Sulcal depth | 0.500 | Sulcal depth | 0.042* |
* P-value < 0.05 significance.
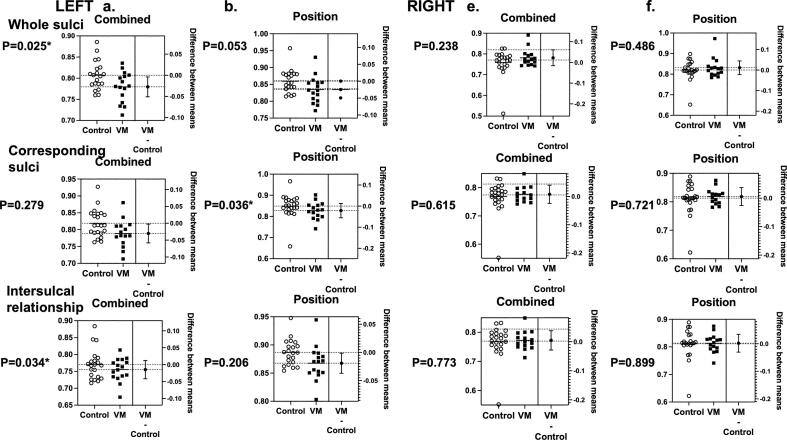
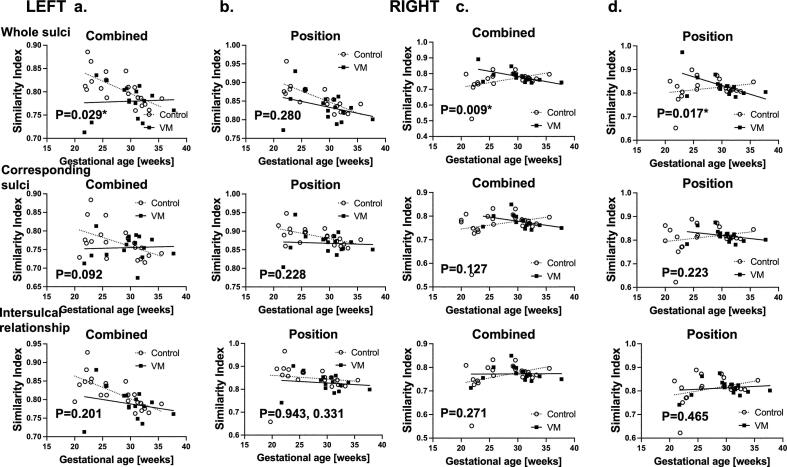
Left hemisphere: In t-test analysis of combined feature of 3D position, sulcal basin area and depth, the similarity-based sulcal developmental pattern analyses identified that fetuses with IVM had lower similarity indices compared to the controls in whole pattern (p-value = 0.025, t-test) and inter-sulcal relationship (p = 0.034), but not in corresponding sulcal region analyses (p = 0.279) (Fig. 3a). Linear regression model analysis also found significant difference in slope, suggesting that fetuses with IVM had lower similarity indices throughout the pregnancy (Fig. 4a).
Fig. 3.
Fetuses with VM had distinct sulcal positional developmental patterns compared to control. Similarity analyses of left hemisphere (a, b) and right hemisphere (c, d) were presented. Similarity indices of combined feature (a, c) and sulcal position (b, d) were compared between fetuses with VM and controls using t-test. Similarity indices were compared as all sulci in a hemisphere as a whole, matched corresponding sulci, and the inter-sulcal relationships. In the left cerebral hemispheres, fetuses with VM had lower similarity indices in sulcal position and combined feature. In the right cerebral hemispheres, no differences were seen in sulcal developmental patterns. * Significance p-value <0.05.
Fig. 4.
Fetuses with VM had distinct sulcal positional developmental trajectories throughout the gestation compared to control. We also compared linear regression models of fetuses with VM and controls in combined feature (a, c) and sulcal position (b, d) in the left (a, b) and right hemispheres (c, d), respectively. In both cerebral hemispheres, combined sulcal features were different between fetuses with VM and controls. * Significance p-value <0.05.
In analysis of sulcal position, we found that fetuses with IVM had lower similarity indices compared to the controls in corresponding sulcal region analysis (p = 0.036, t-test). It seemed to be true for whole pattern and intersulcal relationship analyses, but not statistically significant (p = 0.053 and 0.206, respectively) (Fig. 3b). Linear regression model analysis appeared to have difference in slope, though statistically not significant (Fig. 4b).
In analysis of sulcal depth and area, we did not find any differences between fetuses with IVM and controls, regardless of analytic methods.
Right hemisphere: In t-test analysis of combined feature, position, sulcal basin area and depth, the similarity-based sulcal developmental pattern analyses did not find any differences between fetuses with IVM and controls (Fig. 3c, d).
In linear regression model analysis, both combined feature and position analyses found significant difference in slope (p = 0.009 and 0.017, respectively) (Fig. 4c, d). There were no differences in sulcal depth or area.
3.4. Individual variations of sulcal developmental pattern
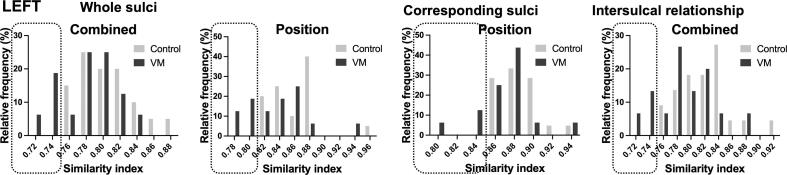
In review of sulcal developmental patterns in each individual fetus, both in IVM and control groups, a frequency histogram was formulated with similarity index (SI) on the x-axis and percentage of cases within the group which showed corresponding SI on the y-axis. In Fig. 5, we presented the results of regional analysis of combined sulcal feature and sulcal position as examples of individually variant features of sulcal developmental patterns. As shown in the Fig. 5, there is a variation how similar the combined sulcal feature or sulcal positional patterns are in each fetus with IVM or controls. In the analysis of both sulcal position and depth, overall distribution of SI was shifted toward the lower values for fetuses with IVM when compared to that of controls. In the left hemisphere, while 0 % of control fetuses had SI lower than 0.82, 31 % of fetuses with IVM distributed in such low SI of whole positional pattern. Therefore, ∼30 % of fetuses with IVM had no overlap with control fetuses. Similarly, when compared distribution of combined sulcal feature similarity indices, while 0 % of control fetuses had SI lower than 0.76, 25 % of fetuses with IVM distributed in such low SI of whole positional pattern (Fig. 5).
Fig. 5.
Fetuses with VM had distinct distribution of Individual variances in sulcal developmental patterns compared to the control. A frequency histogram of sulcal positional and depth developmental similarity indices reflects percentage of cases within the group that had specific similarity indices. In fetuses with VM, distribution of similarity indices was shifted toward to the lower values compared to controls, suggestive for overall poorer sulcal positional development and more fetuses having lower SIs.
In the corresponding sulcal positional pattern analysis, while 0 % of control fetuses had SI lower than 0.84, 19 % of fetuses with IVM distributed in such low SI. In the intersulcal relationship analysis of combined feature, while 0 % of control fetuses had SI lower than 0.76, 20 % of fetuses with IVM distributed in such low SI (Fig. 5).
4. Discussion
In this prospective fetal cohort study, we performed volumetric and cerebral sulcal pattern analysis on fetuses with IVM. We found increased regional growth in the whole brain, cortical plate, and subcortical parenchyma, and cerebrum, which becomes apparent between 20 and 25 weeks of gestation. As the novel finding, we found that fetuses with IVM have distinct sulcal developmental patterns in sulcal position and depth compared to controls in the second and third trimesters.
4.1. Accelerated brain growth in isolated ventriculomegaly
In this study, regional volumetric analyses have shown accelerated brain growth in fetuses with IVM. Previous studies reported controversial results on volumetric analysis of the fetal brain with IVM (Kyriakopoulou et al., 2014, Scott et al., 2013). Our study found increased volume of cortical plate as well as subcortical parenchymal and cerebellar hemisphere volumes consistent with Kyriakopoulou et al. (2014) which support reproducible findings of cortical overgrowth in fetuses with IVM. It is noticed that our growth models fit best to non-linear (exponential) growth models rather than linear models. This is the same for another fetal neurological condition (fetuses with Down syndrome (Tarui et al., 2019)). Underlying pathology or disease mechanism of IVM is unknown. Possible explanation may be accelerated neurogenesis and neuronal production that may result into increased volumes of cortical plate and basal ganglia or increased axonal production in developing white matter. We are not able to determine if increased subcortical parenchymal volume is caused by increased volumes in developing white matter or basal ganglia or both. Because we were not able to segment basal ganglia from subcortical parenchyma due to insufficient tissue resolution to differentiate those structures. Higher resolution of fetal MRI study or fetal histopathological study may be needed to make conclusion.
4.2. Unique sulcal developmental patterns in isolated ventriculomegaly
Little was known about sulcal development in fetuses with IVM. Benkarim et al. (2018) has found local (insula, posterior part of the temporal lobe and occipital lobe) and global decreased cortical folding by curvature-based measures (Benkarim et al., 2018). In our study, we observed geometric and topological patterning of early sulcal folds including 3D positions, sulcal basin surface area and depth. We found significant changes in sulcal positional and depth patterns. Our findings are novel as prior studies assessed overall regional curvature values on the cortical surface and did not assess the geometric and topological patterns of sulcal position, depth, and basin area specifically that we measured. This suggests that anatomical changes in IVM are not only limited to ventricular dilatation, but also extend to cerebral parenchymal anatomy both in size and shape. We consider that in our sulcal developmental pattern analysis, the sulcal positional alteration is not affected by volumetric alteration. When compared the sulcal developmental features, we measured a 3D relative position from a bounding box for a spatially aligned cortical surface model. In addition, we conducted a regression analysis to test the correlations between the sulcal pattern indices and ventricular size, which were not significant. Therefore, significant sulcal positional changes may be independent of the volumetric alterations and be unique developmental alterations rather than artifacts. When analyzed individual variances in sulcal positional and depth developmental patterns, about 10 % of fetuses with IVM had no overlap with control fetuses. This may suggest that some fetuses with IVM may have significantly altered sulcal development while others fall into the range of “normal variation”. It is necessary to examine underlying pathology or disease mechanism to explain altered sulcal developmental patterns in future studies.
Commonly used animal models (e.g. mice or rats) are not suitable to study gyral or sulcal development due to the absence of those structures which may be one of the reasons that developmental neurobiological studies using animals with gyrification (e.g. ferret or macaque) or humans are needed. To study living human fetuses, our method may provide sensitive and precise measures for fetal cerebral development in other conditions as well.
4.2.1. Structural and etiological heterogeneity of isolated ventriculomegaly
Prognostic counseling of fetal IVM can be challenging. Without any recognizable comorbidities, absence of additional fetal brain MRI anomalies, negative TORCH and genetic work up, the majority of these cases have relatively favorable neurodevelopmental prognosis (Beeghly et al., 2010, Scala et al., 2017). However, 6 ∼ 10 % of fetuses with IVM still could have postnatal neurodevelopmental impairments such as normal to moderate intellectual and/or motor impairments (Tonni et al., 2016, Scelsa et al., 2018, Scala et al., 2017). Our post acquisition quantitative analysis of fetal MRI has shown aberrant regional cerebral growth and heterogeneous sulcal developmental patterns in fetuses with IVM. We also discovered individual variances in sulcal developmental patterns. While SI of some fetuses overlap with distribution of control subjects, SI of others distribute out of range representing such heterogeneity within the groups. Our study also shows 20 ∼ 30 % of fetuses with IVM had no overlap with the control fetuses (Fig. 5). Our study may provide potential fetal brain MRI biomarkers to predict neurodevelopmental prognosis of fetuses with IVM, which would impact perinatal and postnatal management of the affected children. It is our future task to determine its associations with underlying etiologies and clinical relevancy especially its implication on the postnatal neurodevelopmental function.
Limitations of this study are small sample size and limited MRI tissue resolution to differentiate basal ganglia. Over the year, our fetal MRI acquisition and tissue resolution has improved with introduction of 3T MRI and newly developed coils. We continue to acquire images to further increase study size in higher resolution images. In this study, we have successfully delineated individual variances in sulcal developmental patterns in the second and third trimester fetuses with IVM and typically developing control fetuses. Such measurements can be used as fetal MRI biomarkers to associate individual’s future neurodevelopmental functionality. In future study, we will determine clinical relevancy of such individual variances in sulcal developmental patterns by following up those subjects’ neurodevelopmental outcomes. To determine, whether those individual sulcal developmental patterns correlates with postnatal neurodevelopmental outcomes, postnatal follow up study is ongoing.
Funding
This work was supported by National Institutes of Health K23HD079605 (TT), R01HD100009 (EG), R01EB032708 (EG), R21HD083956 (KI), the Susan Saltonstall Foundation (TT), and Tufts Pilot Fund (TT) and Clinical Translational Science Institution support from National Center for Advancing Translational Sciences through UL1TR002544 (Tufts Medical Center/Tufts University).
5. Notes
The authors thank Michael Stanley, Annie Felhofer, Jiyeon Janice Jang, and Esther Muradov, for their help with the post-acquisition segmentation of fetal brain MRI, Drs Ada Taymoori and Vidya Iyer for helping identification and recruitment of the participants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Tomo Tarui, Email: tomo_tarui@brown.edu.
Kiho Im, Email: Kiho.Im@childrens.harvard.edu.
Data availability
Data will be made available on request.
References
- Akiyama S., Madan N., Graham G., et al. Regional brain development in fetuses with Dandy-Walker malformation: A volumetric fetal brain magnetic resonance imaging study. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263535. PMC8870580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeghly M., Ware J., Soul J., et al. Neurodevelopmental outcome of fetuses referred for ventriculomegaly. Ultrasound Obstet. Gynecol. 2010;35(4):405–416. doi: 10.1002/uog.7554. PMC2892836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkarim O.M., Hahner N., Piella G., et al. Cortical folding alterations in fetuses with isolated non-severe ventriculomegaly. Neuroimage Clin. 2018;18:103–114. doi: 10.1016/j.nicl.2018.01.006. PMC5790022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: what a long strange trip it's been. Neuroimage. 2012;62(2):743–747. doi: 10.1016/j.neuroimage.2011.08.056. 3246532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglioti P., Danelon D., Bontempo S., Mombro M., Cardaropoli S., Todros T. Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol. 2005;25(4):372–377. doi: 10.1002/uog.1857. [DOI] [PubMed] [Google Scholar]
- Gholipour A., Akhondi-Asl A., Estroff J.A., Warfield S.K. Multi-atlas multi-shape segmentation of fetal brain MRI for volumetric and morphometric analysis of ventriculomegaly. Neuroimage. 2012;60(3):1819–1831. doi: 10.1016/j.neuroimage.2012.01.128. PMC3329183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P.D., Bradburn M., Campbell M.J., et al. Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet. 2017;389(10068):538–546. doi: 10.1016/S0140-6736(16)31723-8. [DOI] [PubMed] [Google Scholar]
- Grossman R., Hoffman C., Mardor Y., Biegon A. Quantitative MRI measurements of human fetal brain development in utero. Neuroimage. 2006;33(2):463–470. doi: 10.1016/j.neuroimage.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hannon T., Tennant P.W., Rankin J., Robson S.C. Epidemiology, natural history, progression, and postnatal outcome of severe fetal ventriculomegaly. Obstet Gynecol. 2012;120(6):1345–1353. doi: 10.1097/aog.0b013e3182732b53. [DOI] [PubMed] [Google Scholar]
- Im K., Jo H.J., Mangin J.F., Evans A.C., Kim S.I., Lee J.M. Spatial distribution of deep sulcal landmarks and hemispherical asymmetry on the cortical surface. Cereb. Cortex. 2010;20(3):602–611. doi: 10.1093/cercor/bhp127. [DOI] [PubMed] [Google Scholar]
- Im K., Pienaar R., Lee J.M., et al. Quantitative comparison and analysis of sulcal patterns using sulcal graph matching: a twin study. Neuroimage. 2011;57(3):1077–1086. doi: 10.1016/j.neuroimage.2011.04.062. [DOI] [PubMed] [Google Scholar]
- Im K., Pienaar R., Paldino M.J., Gaab N., Galaburda A.M., Grant P.E. Quantification and discrimination of abnormal sulcal patterns in polymicrogyria. Cereb. Cortex. 2013;23(12):3007–3015. doi: 10.1093/cercor/bhs292. PMC3888213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K., Raschle N.M., Smith S.A., Ellen Grant P., Gaab N. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb. Cortex. 2016;26(3):1138–1148. doi: 10.1093/cercor/bhu305. PMC4757938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K., Guimaraes A., Kim Y., et al. Quantitative folding pattern analysis of early primary sulci in human fetuses with brain abnormalities. AJNR Am. J. Neuroradiol. 2017;38(7):1449–1455. doi: 10.3174/ajnr.A5217. PMC5509490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandula T., Fahey M., Chalmers R., et al. Isolated ventriculomegaly on prenatal ultrasound: what does fetal MRI add? J. Med. Imaging Radiat. Oncol. 2015;59(2):154–162. doi: 10.1111/1754-9485.12287. [DOI] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M., Quaghebeur G., Rutherford M.A., Hajnal J.V., Schnabel J.A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 2012;16(8):1550–1564. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulou V., Vatansever D., Elkommos S., et al. Cortical overgrowth in fetuses with isolated ventriculomegaly. Cereb. Cortex. 2014;24(8):2141–2150. doi: 10.1093/cercor/bht062. [DOI] [PubMed] [Google Scholar]
- Mehlhorn A.J., Morin C.E., Wong-You-Cheong J.J., Contag S.A. Mild fetal cerebral ventriculomegaly: prevalence, characteristics, and utility of ancillary testing in cases presenting to a tertiary referral center. Prenat. Diagn. 2017;37(7):647–657. doi: 10.1002/pd.5057. [DOI] [PubMed] [Google Scholar]
- Ortinau C.M., Rollins C.K., Gholipour A., et al. Early-emerging sulcal patterns are atypical in fetuses with congenital heart disease. Cereb Cortex. 2018 doi: 10.1093/cercor/bhy235. PMC6644862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala C., Familiari A., Pinas A., et al. Perinatal and long-term outcomes in fetuses diagnosed with isolated unilateral ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017;49(4):450–459. doi: 10.1002/uog.15943. [DOI] [PubMed] [Google Scholar]
- Scelsa B., Rustico M., Righini A., et al. Mild ventriculomegaly from fetal consultation to neurodevelopmental assessment: A single center experience and review of the literature. Eur. J. Paediatr. Neurol. 2018;22(6):919–928. doi: 10.1016/j.ejpn.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Habas P.A., Rajagopalan V., et al. Volumetric and surface-based 3D MRI analyses of fetal isolated mild ventriculomegaly: brain morphometry in ventriculomegaly. Brain Struct. Funct. 2013;218(3):645–655. doi: 10.1007/s00429-012-0418-1. [DOI] [PubMed] [Google Scholar]
- Serag A., Aljabar P., Ball G., et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59(3):2255–2265. doi: 10.1016/j.neuroimage.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Sethna F., Tennant P.W., Rankin J., Robson S.C. Prevalence, natural history, and clinical outcome of mild to moderate ventriculomegaly. Obstet. Gynecol. 2011;117(4):867–876. doi: 10.1097/AOG.0b013e3182117471. [DOI] [PubMed] [Google Scholar]
- Tarui T., Madan N., Farhat N., et al. Disorganized patterns of sulcal position in fetal brains with agenesis of corpus callosum. Cereb Cortex. 2018;28(9):3192–3203. doi: 10.1093/cercor/bhx191. PMC6095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T., Im K., Madan N., et al. Quantitative MRI analyses of regional brain growth in living fetuses with Down syndrome. Cereb. Cortex. 2019 doi: 10.1093/cercor/bhz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatli B., Ozer I., Ekici B., et al. Neurodevelopmental outcome of 31 patients with borderline fetal ventriculomegaly. Clin. Neurol. Neurosurg. 2012;114(7):969–971. doi: 10.1016/j.clineuro.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Tonni G., Vito I., Palmisano M., Martins W.P., Araujo J.E. Neurological outcome in fetuses with mild and moderate ventriculomegaly. Rev. Bras. Ginecol. Obstet. 2016;38(9):436–442. doi: 10.1055/s-0036-1592315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn M., Oude Rengerink K., Newsum E.A., Reneman L., Majoie C.B., Pajkrt E. Added value of fetal MRI in fetuses with suspected brain abnormalities on neurosonography: a systematic review and meta-analysis. J Matern. Fetal Neonatal. Med. 2016;29(18):2949–2961. doi: 10.3109/14767058.2015.1109621. [DOI] [PubMed] [Google Scholar]
Further reading
- Yun H.J., Chung A.W., Vasung L., et al. Automatic labeling of cortical sulci for the human fetal brain based on spatio-temporal information of gyrification. Neuroimage. 2019;188:473–482. doi: 10.1016/j.neuroimage.2018.12.023. PMC6452886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.