Abstract
Background
Pain is a common feature of hemophilia, but prevalence of depression and anxiety is less studied. Registry data on prescription drugs can provide an objective measure of the magnitude of these complications.
Objectives
To identify treatment patterns of prescribed pain, antidepressant, and antianxiety medications compared with those of matched controls in 4 Nordic countries.
Methods
The MIND study (NCT03276130) analyzed longitudinal individual-level national data during 2007-2017. People with hemophilia (PwH) were identified from National Health Data Registers by diagnosis or factor replacement treatment and compared with population controls. Three subgroups were defined by the use of factor concentrates and sex (moderate-to-high factor consumption (factor VIII [FVIII] use of ≥40 IU/kg/week or FIX use of ≥10 IU/kg/week), low factor consumption, and women including carriers).
Results
Data of 3246 PwH, representing 30,184 person-years, were analyzed. PwH (including children and adults) used more pain, depression, and anxiety medications compared with controls. This was most accentuated in the moderate-to-high factor consumption group and notably also observed in men with low factor consumption and women including carriers, usually representing a milder phenotype. A higher opioid use was observed across all age groups: 4- to 6-fold higher in the moderate-to-high factor consumption group and 2- to 4-fold higher in the low factor consumption group.
Conclusion
The consistent higher use of pain, depression, and anxiety medications among PwH compared with population controls, regardless of age, sex, or factor consumption, in broad national data suggests a need for improved bleed protection and hemophilia care for all severities including mild hemophilia.
Keywords: anxiety; case-control studies; depression; drug utilization; female; hemophilia A; hemophilia B; analgesics, opioid; pain; prescription drugs
Essentials
-
•
Pain is prevalent in people with hemophilia, but depression and anxiety are less studied.
-
•
National prescription drug data in >3000 Nordic people with hemophilia were compared to population controls.
-
•
PwH had persistently higher use of pain, depression, and anxiety drugs regardless of age or sex.
-
•
Results indicate the need for better bleed protection and care irrespective of severity, age, or sex.
1. Introduction
Pain is a well-known complication of hemophilia [[1], [2], [3], [4], [5], [6]] most often caused by acute bleeds or hemophilic arthropathy, but to date, only few studies have covered aspects of depression and anxiety. [7,8] Previous publications based on surveys may be hampered by bias; for example, recruitment of individuals seeking care alone, nonresponses, recall bias, subjective perceptions, and limited time perspectives. [9,10] Analysis of National Prescription Registry data can give an objective and representative measure of the magnitude of prevalence of pain, depression, and anxiety in hemophilia and its current medical management, which complements earlier survey-based literature. However, there is a lack of large studies with national coverage analyzing broad hemophilia populations with a specific focus on strategies for management of pain, depression, and anxiety.
The long history of nationwide, mandatory health data registries with population coverage in Nordic countries provides unique opportunities to identify patient populations, including those with less frequent contacts with health care, and to analyze the outcomes related to utilization of health care resources with long-term perspectives. Examples of previous research include studies on people with diabetes, [11] cancer, [12] and also bleeding disorders such as von Willebrand disease (VWD). [13] Findings from registry data can reduce knowledge gaps on frequency and volume of use of prescription drugs for pain, depression, and anxiety for people with hemophilia (PwH), and put these outcomes into perspective through comparison with matched controls from the general population.
The MIND study was designed with the overall aim to identify the patterns of prescription of medication for, and perspectives of the overall management of, pain, depression, and anxiety for PwH in a two-part observational study conducted in 4 Nordic countries. Part A aimed to describe patterns of prescription drug utilization among PwH by measuring observed use (yes/no), volume of use, and duration of use based on expected doses during 2007-2017 and to make comparisons with those of matched controls. Part B analyzed the cross-sectional survey data on the perspective of PwH, caregivers, and hemophilia treaters, and was recently published. [14] Here, we present results from part A of the MIND study.
2. Methods
2.1. Study design
The MIND (NCT03276130) study is a descriptive, longitudinal, and retrospective analysis based on individual-level data on filled prescriptions for pain, antidepressant, and antianxiety medication and a set of predefined comorbidity diagnoses. It is a case-control study comparing outcomes for PwH and matched controls from the general population. Information from multiple administrative health data registries on PwH was linked at the individual level using the personal identification numbers in Denmark, Finland, Norway, and Sweden. Study variables were retrieved for years 2007-2017. Using data from National Health Data Registers, we aimed to achieve a high coverage of PwH including subgroups with less frequent health care visits for their hemophilia.
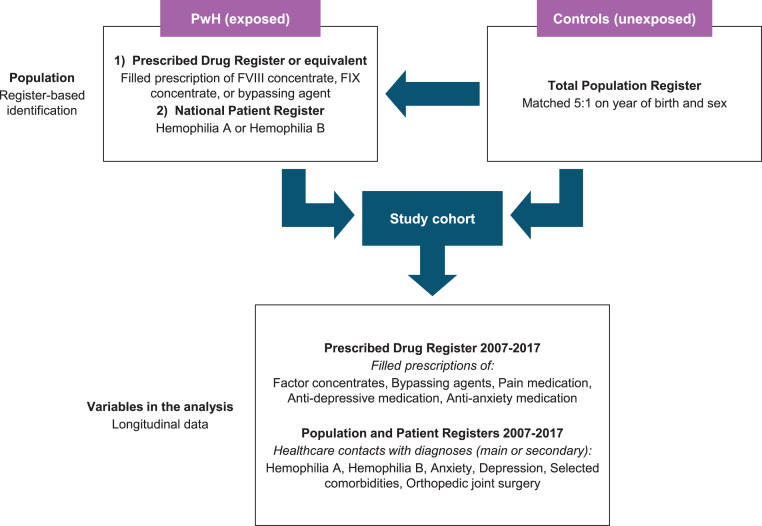
Figure 1 presents an overview of the study design and data collection. PwH were identified as individuals who had (1) ≥1 health care contact with main diagnosis hemophilia A (International Classification of Diseases version 10 [ICD-10] D66.9) or hemophilia B (ICD-10 D67.9) and/or had (2) ≥1 prescription of factor VIII (FVIII) or FIX concentrates (Anatomical Therapeutic Chemical [ATC] codes B02BD02, B02BD04, or B02BD09) or bypassing agents (activated eptacog alfa [ATC code B02BD08], activated prothrombin complex concentrate [ATC code B02BD03]) used in the treatment of PwH during the inclusion period. Nonfactor products, for example, emicizumab, were not available on the market at the time of the study. In Finland, PwH were identified as individuals eligible for the use of factor concentrates or bypassing agents at Kela (also known as the Social Insurance Institution). All individuals were identified in administrative health data registries with national coverage or responsibility.
Figure 1.
Outline of study design by description of the data collection process, including identification of study population and key variables in the analysis. FIX, factor IX; FVIII, factor VIII; PwH, people with hemophilia.
The study design did not prevent inclusion of individuals who, in addition to meeting inclusion criteria, also had registrations of acquired hemophilia or VWD at some point during the study period of 2007-2017. Post-hoc sensitivity analyses excluding patients with ≥1 registration of acquired hemophilia or VWD were performed.
The analyses compared drug utilization patterns for pain, depression, and anxiety medications in PwH and control participants from the general population matched 1:5 based on year of birth and sex in Denmark, Finland, and Sweden (Figure 1). All analyses were conducted by country and presented separately to allow for potential differences between countries. Controls were drawn from population registries without reversal by statistical authorities in each country. Control participants were not available from Norway due to legal restrictions for matching controls from other national registries. Supplementary Figures S1–S4 present detailed flowcharts on the data selection process in each country. Permissions, including ethical approvals, were sought in accordance with country-specific regulations (Supplementary Methods). Seven drug types were included in the analysis: opioids, nonopioids, nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, neuroleptics, antidepressants, and antiepileptics.
2.2. Variables and data sources
The following variables from national data sources were included:
-
1.
National prescription drug registries: information on hemophilia treatment (FVIII concentrates, FIX concentrates, and bypassing agents) and that on primary endpoints measuring occurrence, volume, and duration of treatment with (1) pain medications (opioids, nonopioids, NSAIDs, and steroids), (2) depression/anxiety drugs (neuroleptics, antidepressants, and antiepileptics), and (3) indicators of inhibitors (previous or current use of bypassing agents, or FVIII or FIX concentrates at volumes of ≥350 IU/kg/week). Supplementary Table S1 specifies the types of evaluated drugs by their ATC codes.
-
2.
Population and patient registries: year of birth, year of death, sex, and information related to joint complications (current or previous diagnosis of hemophilia arthrosis or joint surgery).
2.3. Subgroup definition
The broad inclusion criteria captured all levels of hemophilia severity and did not exclude women. Factor replacement use and sex were used to characterize the expected heterogeneity among PwH, as the ICD-code system does not identify hemophilia severity (ie, mild, moderate, and severe). Provision of hemophilia care is covered by the health care system in the Nordic countries. Therefore, factor consumption data from filled prescriptions indicating low factor consumption was used as a marker of the disease in PwH with a milder phenotype. The following criteria were used to create subgroups:
-
1.
Moderate-high factor consumption: men or women with filled prescriptions corresponding to FVIII use of ≥40 IU/kg/week or FIX use of ≥10 IU/kg/week.
-
2.
Low factor consumption: men with no filled prescription of factor concentrate or filled prescriptions corresponding to FVIII use of <40 IU/kg/week or FIX <10 IU/kg/week.
-
3.
Women including carriers: with factor deficiency and no or low consumption of factor concentrates.
The low cut-off in factor consumption between “moderate-to-high” and “low” was chosen to ensure that PwH on prophylaxis or on frequent on-demand treatment were not grouped together with PwH with a mild phenotype who do not require prophylactic treatment or frequent on-demand treatment and therefore only have no or a low factor consumption. The lower cut-off for FIX was based on experience from the Nordic hemophilia treaters that some patients with hemophilia B requiring prophylactic treatment may suffice with a relatively low weekly FIX consumption. Thus, the cut-off was well below the lowest recommended prophylactic dose according to the Nordic Guidelines and the subgroup “low factor consumption” aimed to represent a mild phenotype without including people with a frequent need of factor concentrates. [15]
Each individual was classified into one of the subgroups each calendar year. The design allowed switches between subgroups over time and reflected factor concentrate use each year. Control participants followed the PwH at subgroup switch.
2.4. Main analysis endpoints and post-hoc analyses
The study analyzed 3 endpoints for the 7 drug types for pain, depression, and anxiety with comparison to population controls: (1) likelihood of use (yes/no), (2) the volume of use measured by defined daily doses (DDDs) for individuals with ≥1 filled prescription, and (3) duration of use categorized as <2 months, 2-6 months, and >6 months in the observation year.
The main analyses explored the age-adjusted likelihood of use and the volume of use among users for each drug type (endpoints 1 and 2). The duration of use (endpoint 3) is reported for opioids in the main text and for the other drug types in the Supplementary Material. The analyses were carried out in the 3 defined subgroups. The main and post-hoc analyses used the same empirical strategy with regression methods and all 11 years of panel data.
A first set of post-hoc analyses explored the pooled outcome “any prescription drugs for pain” (any opioids, nonopioids, NSAIDs, or steroids) and its association with basic covariates (identified as PwH, age group, use of antidepressant/antianxiety drugs including any neuroleptics or antiepileptics) or the potential impact of an extended set of covariates (identified as current or previous inhibitors and joint complications). Conversely, the impact of use of prescription drugs for pain on the use of antidepressant/antianxiety prescription drugs was explored controlling for the otherwise same covariates (identified as PwH, age group, current or previous inhibitors, and joint complications).
The second set of post-hoc analyses explored factors associated with opioid use among PwH and controls in the 3 subgroups. These regression analyses examined the interaction of all covariates with the PwH covariate. Results are presented as comparisons between PwH and controls for each of the covariates (age group, current or previous inhibitor, joint complications, antidepressant use, neuroleptic use, or antiepileptic use).
A third set of post-hoc analyses described opioid use across age groups as proportions with any use during 2007-2017 by subgroups presented for Sweden in the main text (largest population) and for Finland and Norway in Supplementary Figures S6–S8.
2.5. Statistical analyses
Background characteristics are described by counts and proportions, mean and SD, median and 25th and 75th percentiles, and minimum and maximum values. Population-averaged regression models with logistic or linear specifications adjusting for age (<30, 30-44, 45-59, ≥60 years) were used in longitudinal data on use of the 7 prescription drug types. These regression analyses compared PwH and control participants and aimed to assess the total difference between study groups accounting for expected change in drug use related to age. In cases where the PwH died, controls were censored. Results are presented as odds ratios (ORs) and percentage differences in volume as measured by total number of DDDs during each calendar year among those with ≥1 filled prescription of the medication type. Additional covariates were used in post-hoc analyses as described above.
3. Results
Registry data for 3246 PwH from Denmark (n = 886), Finland (n = 288), Norway (n = 522), and Sweden (n = 1550) over an 11-year study period were included in the analyses. All individuals had ≥1 observation year in the study period 2007-2017 and the panel was dynamic, including births and deaths as observed. There were, in total, 30,184 person-years for PwH. Control participants (n = 14,098) were retrieved in Denmark, Finland, and Sweden, with 135,773 person-years in total. Table 1 summarizes key demographic (PwH and controls) and clinical characteristics (PwH) of study participants.
Table 1.
Summary presentation of demographic and clinical characteristics of the study population by country. PwH with eligible data for ≥1 year in the study period of 2007-2017 and controls as applicable.
| Variable | Denmarka | Finlandb | Norwayc | Swedend |
|---|---|---|---|---|
| PwH, n (person-years) | 886 (6204) | 288 (2911) | 522 (5263) | 1550 (15,806) |
| Control, n (person-years) | 4529 (37,107) | 1411 (14,365) | NA | 8158 (84,310) |
| Demographic information of PwH | ||||
| Men, women, n | 591, 295 | 280, 8 | 446, 76 | 1158, 392 |
| Year of birth | ||||
| Mean (min, max) | 1973 (1911, 2016)e | 1982 (1931, 2015) | 1978 (1919, 2017) | 1975 (1913, 2016) |
| Median (25th, 75th percentile) | 1975 (1952, 1993)e | 1987.5 (1966, 2000) | 1981 (1959, 1998) | 1978 (1956, 1995) |
| Age, mean (SD) in 2012 (mid-year of study period)f | NA | 30.1 (20.3) | 34.7 (21.8) | 36.5 (23.4) |
| Number of years with observations, mean (SD, min, max) | NA | 10.1 (2.1, 2, 11) | 10.1 (2.2, 1, 11) | 10.2 (2.1, 1, 11) |
| Mortality data—PwH vs. controls | ||||
| Deaths during 2007-2017, n (%) | ||||
| PwH | 127 (14.3) | 17 (5.9) | 47 (9.0) | 157 (10.1) |
| Control | 452 (10.0) | 55 (3.9) | NA | 634 (7.8) |
| Age at death, years, mean (min, max) | ||||
| PwH | 76 (11, 101)e | 66 (44, 80) | 64 (3, 97) | 75 (29, 96) |
| Control | 74 (1, 102)e | 58 (1, 85) | NA | 78 (1, 102) |
| Age at death, years, median (25th, 75th percentile) | ||||
| PwH | 77 (68, 86)e | 69 (59, 73) | 67 (55, 78) | 76 (67, 84) |
| Control | 78 (65, 87)e | 65 (49, 72) | NA | 82 (72, 89) |
| Clinical characteristics of PwH | Denmark | Finland | Norway | Sweden |
| Type of bleeding disorder, n (%) | ||||
| Hemophilia A | 776e (79.3) | 226 (78.5) | 421 (80.7) | 1108 (71.5) |
| Hemophilia B | 203e (20.7) | 50 (17.4) | 101 (19.3) | 242 (15.6) |
| No information during the study period | 12g (4.2) | 200h (12.9) | ||
| PwH with ≥1 filled prescription of FVIII or FIX concentrates or bypassing agents during 2007-2017, n | NA | 276 | 318 | 757 |
| Inhibitor, PwH with ≥1 filled prescription of bypassing agents or FVIII or FIX concentrates at volumes of ≥350 IU/kg/week during 2007-2017, n (%) | 51 (5.8) | 23 (8.0) | 23 (4.4) | 90 (5.8) |
FIX, factor IX; FVIII, factor VIII; Max, maximum; Min, minimum; NA, not available/applicable; PwH, people with hemophilia.
Inclusion from national patient registry (NPR; 1995-2017) and hospital pharmacy registry (2007-2017).
Inclusion if entitlement for factor concentrates (2000-2017) or filled prescription of factor concentrates (1995-2017).
Inclusion from Norwegian Prescription Database (NorPD; 2004-2017).
Inclusion from NPR (1987-2017) and prescription registry (2005-2017).
Statistics Denmark reported year of birth, age at death, and type of hemophilia for all PwH selected during 1995-2017 (n = 979, of which 88 were deceased before 2007 and 5 did not have any observation in Danish population registries during 2007-2017; see also Supplementary Figure S1).
Post-hoc analysis. Data not available for Denmark. Number of individuals with data in year 2012. Finland: n = 265; Norway: n = 483; and Sweden: n = 1459.
Finland: n = 12 insufficient information for determining the type of hemophilia in the data during 2007-2017. Eleven of them had an index year before 2007 and had filled prescriptions of desmopressin or tranexamic acid.
Sweden: n = 200 had insufficient information for determining type of hemophilia from the data during 2007-2017 (107 men, 93 women). 179/200 (89.5%) had an index year before 2007. 10 individuals of the 200 had ≥1 filled prescription of desmopressin during 2007-2017.
The age-adjusted ORs for most types of pain medications and several prescription drugs for depression/anxiety showed higher likelihood of use in PwH compared to matched controls. The likelihood of opioid use was 4- to 6-fold higher in the moderate-to-high factor consumption group, 2- to 4-fold higher in the low factor consumption groups, and 2- to 8-fold higher in women including carriers compared to controls in Denmark, Finland, and Sweden (Table 2). In addition, a higher volume of opioid use among users was observed to range from +26% to +127% in all but 2 subgroups.
Table 2.
Age-adjusted comparison between PwH and controls for annual use of drug types for treatment of pain, depression, and anxiety. Results presented by subgroups. Longitudinal regression analysis of annual data on the use of drug type (OR [95% CI]) and volume of drug types (% difference [P value] if use > 0) among people with ≥1 filled prescription from Denmark (2007-2016) and Finland and Sweden (2007-2017).
| Drug type | Denmark |
|||||
|---|---|---|---|---|---|---|
| Moderate-high factor consumption PwH: n = 221; person-years = 1293 Control: n = 1049; person-years = 6085 |
Low factor consumption PwH: n = 554; person-years = 3826 Control: n = 2677; person-years = 17,902 |
Women including carriers PwH: n = 295; person-years = 1765 Control: n = 1448; person-years = 8384 |
||||
| Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | |
| Opioids | 6.57 (4.69-9.19) | 51.2 (.008) | 2.33 (1.89-2.88) | 21.8 (.19) | 2.58 (1.97-3.39) | 57.8 (.003) |
| Nonopioids | 3.43 (2.45-4.79) | 22.1 (.13) | 1.64 (1.34-2.02) | 7.8 (.31) | 1.40 (1.10-1.80) | 17.9 (.07) |
| NSAIDs | 1.14 (0.81-1.60) | 61.2 (<.001) | 0.71 (0.59-0.87) | 24.4 (.03) | 0.77 (0.60-0.98) | −3.4 (.71) |
| Steroids | 1.26 (0.68-2.33) | −10.4 (.79) | 1.77 (1.30-2.42) | 57.8 (.001) | 2.26 (1.56-3.27) | 79.1 (<.001) |
| Neuroleptics | 1.36 (0.83-2.22) | 14.5 (.65) | 1.65 (1.29-2.12) | 2.4 (.90) | 1.61 (1.17-2.21) | 18.2 (.28) |
| Antidepressants | 2.26 (1.34-3.81) | 39.4 (.07) | 1.48 (1.09-2.01) | 19.0 (.16) | 1.29 (0.92-1.81) | −9.6 (.49) |
| Antiepileptics | 2.01 (0.99-4.08) | −21.6 (.60) | 0.80 (0.48-1.34) | 13.1 (.67) | 1.24 (0.70-2.19) | −61.3 (.03) |
| Drug type | Finland |
|||||
|---|---|---|---|---|---|---|
| Moderate-high factor consumption PwH: n = 200; person-years = 1167 Control: n = 972; person-years = 5661 |
Low factor consumption PwH: n = 239; person-years = 1678 Control: n = 1170; person-years = 8079 |
Women including carriers (unadjusted) PwH: n = 7; person-years = 66 Control: n = 35; person-years = 323 |
||||
| Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | |
| Opioids | 5.64 (3.75-8.50) | 127 (<.001) | 4.33 (3.17-5.91) | 89.9 (<.001) | 8.13 (2.05-32.22) | 85.6 (.34) |
| Nonopioids | 3.35 (2.35-4.79) | 23.7 (.006) | 2.84 (2.11-3.82) | −2.1 (.79) | 4.42 (1.75-11.12) | 42.4 (.27) |
| NSAIDs | 1.48 (1.10-2.00) | 65.6 (<.001) | 1.06 (0.83-1.36) | 58.3 (<.001) | 1.82 (0.73-4.53) | 69.1 (.001) |
| Steroids | 1.01 (0.52-1.97) | −14.1 (.51) | 1.02 (0.56-1.88) | 27.3 (.14) | 0.48 (0.12-1.91) | −57.2 (.31) |
| Neuroleptics | 1.97 (1.11-3.50) | 40.2 (.260) | 1.27 (0.79-2.04) | 2.2 (.95) | 1.81 (0.39-8.34) | 29.2 (.71) |
| Antidepressants | 0.78 (0.39-1.56) | −31.5 (.185) | 0.95 (0.55-1.63) | 23.6 (.26) | 0.60 (0.20-1.79) | −157 (.007) |
| Antiepileptics | 3.08 (1.27-7.47) | 19.0 (.676) | 1.28 (0.58-2.83) | −49.8 (.21) | 0.66 (0.11-3.90) | −218 (.03) |
| Drug type | Sweden |
|||||
|---|---|---|---|---|---|---|
| Moderate-high factor consumption PwH: n = 419; person-years = 2960 Control: n = 2072; person-years = 14,565 |
Low factor consumption PwH: n = 1016; person-years = 8869 Control: n = 4968; person-years = 42,649 |
Women including carriers PwH: n = 389; person-years = 3977 Control: n = 1920; person-years = 19,438 |
||||
| Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | Use; OR (95% CI) | % Difference in volume (P value) if use > 0 | |
| Opioids | 4.70 (3.69-6.00) | 105.8 (<.001) | 2.51 (2.17-2.90) | 25.6 (.02) | 2.40 (1.93-2.99) | 33.9 (.04) |
| Nonopioids | 5.64 (4.44-7.17) | 19.2 (.09) | 2.19 (1.89-2.54) | 10.9 (.02) | 2.13 (1.74-2.60) | 18.1 (.01) |
| NSAIDs | 3.20 (2.59-3.96) | 68.2 (<.001) | 1.26 (1.09-1.45) | 38.7 (<.001) | 1.17 (0.96-1.41) | 21.3 (.05) |
| Steroids | 3.00 (2.28-3.96) | 2.3 (.90) | 1.62 (1.32-1.97) | 19.2 (.05) | 2.42 (1.86-3.16) | 35.6 (.002) |
| Neuroleptics | 2.08 (1.55-2.80) | −6.0 (.75) | 1.61 (1.36-1.92) | 19.8 (.04) | 1.56 (1.24-1.95) | 11.3 (.37) |
| Antidepressants | 1.51 (1.00-2.30) | −4.2 (.81) | 1.30 (1.03-1.62) | −17.6 (.06) | 1.41 (1.09-1.82) | −0.8 (.94) |
| Antiepileptics | 2.87 (1.65-5.00) | 45.4 (.13) | 1.84 (1.31-2.58) | 1.2 (.95) | 1.59 (0.96-2.63) | 17.2 (.60) |
NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio; PwH, people with hemophilia.
The use of nonopioids was consistently higher in all hemophilia subgroups in the 3 countries with OR ranging from 1.40 to 5.64 and data indicated a higher volume of use in 3 out of 9 subgroups. A more mixed picture was observed for the other prescription drugs for pain. The likelihood of steroid use did not differ between PwH and controls in Finland but was higher in Sweden (all subgroups) and Denmark (low factor consumption and women including carriers).
Table 2 also shows a higher likelihood of use of neuroleptics, antidepressants, and antiepileptics in all but one subgroup in Sweden (OR, 1.30 to 2.87) and in all but 2 subgroups in Denmark (OR, 1.48-2.26). In Finland, only the moderate-to-high factor consumption subgroup had a higher likelihood of using neuroleptics (OR, 1.97), and there was no difference for antidepressants. The moderate-to-high factor consumption had a higher likelihood of use of antiepileptics in Sweden and Finland, with Danish results indicating a similar tendency. However, the data do not indicate that PwH with at least some use of neuroleptics and antidepressants differed from controls in annual volume of use. Supplementary Figure S5 supplements results in Table 2 by showing the proportion of PwH by country (including results from Norway) with ≥1 filled prescription per drug type in each subgroup during the study period. The highest proportion of opioid users was found in women and carriers (46%-72%) in Denmark, Norway, and Sweden. This subgroup also had a high proportion using nonopioid analgetic drugs (49%-54%), NSAID (47%-70%), steroids (32%-39%), neuroleptics (34%-58%), and antidepressants (27%-37%) and a relatively high proportion using antiepileptics (9%-22%).
Table 3 shows post-hoc analyses for Sweden evaluating the impact of additional covariates on the use of any pain medication. Use of any prescription drug for pain increases with age and was higher for the PwH group and for those with concomitant medication for depression or anxiety. Adding covariates for inhibitors and joint health (extended set of covariates) has an impact on estimates of OR and percentage difference in volumes for PwH. For example, PwH with low factor consumption had an OR of 1.89 with the basic set of covariates and an OR of 1.76 for the basic and extended sets of covariates compared with controls. Both inhibitors and joint complications (extended set of covariates) were associated with a higher likelihood of use, an OR of 2.84, and an OR of 2.39. Part of the higher OR for PwH in the estimation with the basic set of covariates may thus be attributed to presence of joint or inhibitor complications.
Table 3.
Basic set of covariates (age groups, antidepressant/antianxiety prescription drugs) and extended set of covariates (basic + inhibitors, joint complications) associated with annual use (yes/no; OR [95% CI]) and annual volume (% difference [P value] if use > 0) of any pain medication (opioids, nonopioids, NSAIDs, and/or steroids) in years with ≥1 filled prescription for PwH compared with that for controls. Longitudinal regression analysis of annual data on the use of 7 prescription drug types and volume of 7 prescription drug types for years 2007-2017 in Sweden by subgroups such as moderate-to-high factor consumption, low factor consumption, and women including carriers.
| Moderate-high factor consumption | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 419; person-years = 2960 Control: n = 2072; person-years = 14,565 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| Basic covariates | Basic + extended set of covariates | Basic covariates | Basic + extended set of covariates | |
| PwH group | 4.55 (3.84-5.39) | 3.99 (3.35-4.75) | 63.4 (<.001) | 50.6 (<.001) |
| Age group (y) | ||||
| 30-44 | 2.04 (1.72-2.43) | 2.06 (1.73-2.45) | 58.8 (<.001) | 58.7 (<.001) |
| 45-59 | 3.88 (3.25-4.63) | 3.85 (3.22-4.60) | 114 (<.001) | 109 (<.001) |
| ≥60 | 5.21 (4.20-6.46) | 5.12 (4.13-6.35) | 114 (<.001) | 112 (<.001) |
| Antidepressant/antianxiety prescription drugsa | 2.16 (1.83-2.56) | 2.18 (1.84-2.58) | 72.4 (<.001) | 72.0 (<.001) |
| Inhibitors, current or previousb | 2.24 (1.39-3.60) | 22.5 (.20) | ||
| Joint complicationc | 2.77 (1.85-4.14) | 67.8 (<.001) | ||
| Low factor consumption | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 1016; person-years = 8869 Control: n = 4968; person-years = 42,649 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| Basic covariates | Basic + extended set of covariates | Basic covariates | Basic + extended set of covariates | |
| PwH group | 1.89 (1.70-2.11) | 1.76 (1.58-1.96) | 16.7 (.006) | 6.66 (.25) |
| Age group (years) | ||||
| 30-44 | 1.85 (1.66-2.06) | 1.86 (1.67-2.07) | 41.3 (<.001) | 44.7 (<.001) |
| 45-59 | 2.88 (2.59-3.20) | 2.87 (2.58-3.20) | 78.9 (<.001) | 81.8 (<.001) |
| ≥60 | 4.90 (4.42-5.43) | 4.85 (4.38-5.38) | 105 (<.001) | 107 (<.001) |
| Antidepressant/antianxiety prescription drugsa | 2.34 (2.14-2.57) | 2.34 (2.14-2.57) | 56.7 (<.001) | 55.3 (<.001) |
| Inhibitors, current or previousb | 2.84 (1.73-4.68) | 106 (<.001) | ||
| Joint complicationc | 2.39 (1.84-3.11) | 52.7 (<.001) | ||
| Women including carriers | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 389; person-years = 3977 Control: n = 1920; person-years = 19,438 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| Basic covariates | Basic + extended set of covariates | Basic covariates | Basic + extended set of covariates | |
| PwH group | 1.89 (1.61-2.21) | 1.86 (1.59-2.18) | 29.1 (.001) | 24.5 (.004) |
| Age group (y) | ||||
| 30-44 | 1.87 (1.63-2.13) | 1.87 (1.64-2.13) | 34.5 (<.001) | 34.6 (<.001) |
| 45-59 | 3.19 (2.74-3.71) | 3.18 (2.73-3.69) | 77.3 (<.001) | 76.7 (<.001) |
| ≥60 | 5.30 (4.52-6.21) | 5.24 (4.46-6.14) | 104 (<.001) | 101 (<.001) |
| Antidepressant/antianxiety prescription drugsa | 2.15 (1.93-2.40) | 2.15 (1.93-2.40) | 71.4 (<.001) | 71.1 (<.001) |
| Inhibitors, current or previousb | 1.16 (0.55-2.43) | 80.6 (.01) | ||
| Joint complicationc | 1.64 (1.03-2.60) | 70.1 (.001) | ||
Note: The reference person for comparison of regression estimates is a control <30 years old not using antidepressant or antianxiety prescription drugs, and without joint complications (and no inhibitors by definition).
NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio; PwH, people with hemophilia.
Filled prescription (≥1) during the calendar year of any antidepressant or antianxiety prescription drugs.
Use of bypassing agent or FVIII or FIX at a dosage of ≥350 IU/kg/week in current or any previous year.
Orthopaedic joint surgery and/or registration of the health care contact with hemarthrosis (M25.0, M36.2). Registration of the surgery indicator in the current or past years.
Corresponding analyses exploring how the basic and extended sets of covariates affect the use of any antidepressant or antianxiety prescription drugs are presented in Supplementary Table S2. These analyses show that concomitant use of pain medication was associated with a higher likelihood of use (OR around 1.6 in all subgroups) of antidepressant or antianxiety prescription drugs. For women including carriers, pain medications also increased the volume of antidepressant and antianxiety drugs (about +26%), a pattern not observed for subgroups of moderate-to-high (mainly men) or low factor consumption (men only).
Figure 2 shows the distribution of duration of opioid use by subgroup in Finland (panel A) and Sweden (panel C) compared with that of controls, and that in Norway (panel B) for PwH only. Data indicated that a higher proportion of PwH with moderate-to-high factor consumption than controls had long periods of opioid use (>6 months) in Finland and Sweden (29% vs. 9% and 27% vs. 9% of person-years) and a corresponding high proportion was found in Norway (35%). A high proportion of long duration (>6 months) was also observed in the low factor consumption group in Finland (25% v 8%), whereas in Sweden, the low factor consumption group and women and carriers did not differ from controls. Data from Denmark had too many censored annual observations for graphical illustration. The corresponding data for the other 6 prescription drug types are shown in Supplementary Figures S6–S11.
Figure 2.
Percentage distribution of the duration of opioids for individuals with ≥1 filled prescription of opioids during 2007-2017 in (A) Finland, (B) Norway, and (C) Sweden. Figure shows PwH vs. control for moderate-to-high factor consumption, low factor consumption, and women including carriers depending on the data availability. Brackets denote the number of person-years. P values indicate the Kruskal-Wallis difference in distribution between PwH and control groups. Percentages may not sum to 100 due to rounding. The number of women in Finland was low and thereby did not permit subgrouping. Matched controls from population registries could not be obtained in Norway due to national regulations. DDD, defined daily dose; PwH, people with hemophilia; PY, person-years.
Results from post-hoc analyses of opioid use based on data from Sweden show a persistent higher likelihood of opioid use in PwH than controls across ages in all subgroups (Figure 3) despite the nonnegligible use among controls. Opioid use increases with age for both PwH and controls and to a higher degree for PwH as shown in the regression analyses of interactions between hemophilia and all other covariates (Table 4), with OR of 5.41-20.0 in the 3 subgroups compared to the reference group aged <30 years. Concomitant use of other prescription drugs, presence of inhibitors, and joint complications are all associated with increased opioid use. The OR remains around 3 for the 3 PwH groups despite adding demographic and clinical characteristics to the regression analysis.
Figure 3.
Proportion of individuals with ≥1 filled prescription of opioids during 2007-2017 by age category for PwH and controls in Sweden: (A) moderate-to-high factor consumption, (B) low factor consumption, and (C) women including carriers. Age category measured as first observed age in years during study period. Five-year age categories are shown unless pooled; categories were pooled for (A) 0-10 years and 70+ years, (B) 85+ years, and (C) 85+ years. P values indicate Pearson’s chi-square difference between PwH and control for each age group. ∗P = .003. No data were available from Denmark for post-hoc analysis across age groups. PwH, people with hemophilia.
Table 4.
Factors associated with annual use (yes/no; OR [95% CI]) of opioids and annual volume (% difference [P value] if use > 0) of opioids in years with ≥1 filled prescription for PwH compared with that for controls in Sweden during 2007-2017. Predictions for PwH and controls, respectively, compared with a reference person as described in the table footnote. Longitudinal regression analyses in the model with interaction terms between the covariate PwH and all other covariates.
| Moderate-high factor consumption | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 419; person-years = 2960 Control: n = 2072; person-years = 14,565 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| PwH | Control | PwH | Control | |
| PwH group | 3.10 (2.11-4.54) | NA | 50.3 (0.08) | NA |
| Age group (y) | ||||
| 30-44 | 8.09 (5.26-12.5) | 2.26 (1.62-3.16) | 134 (<.001) | 8.9 (.61) |
| 45-59 | 20.0 (13.3-29.9) | 3.74 (2.73-5.13) | 147 (<.001) | 44.9 (.03) |
| ≥60 | 14.8 (8.40-26.2) | 5.58 (4.04-7.72) | 151 (.002) | 33.6 (.14) |
| Inhibitors, current or previousa | 2.77 (1.67-4.58) | NA | −104 (.007) | NA |
| Joint complicationb | 7.93 (4.58-13.7) | 5.89 (1.69-20.6) | 27.6 (.51) | 118 (.43) |
| Antidepressants | 7.01 (3.58-13.7) | 1.08 (0.66-1.77) | 230 (<.001) | 45.4 (.15) |
| Neuroleptics | 7.32 (4.27-12.5) | 2.73 (1.98-3.77) | 83.3 (.05) | 89.1 (<.001) |
| Antiepileptics | 3.77 (1.68-8.48) | 4.25 (2.48-7.27) | 66.5 (.30) | 93.4 (.04) |
| Low factor consumption | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 1016; person-years = 8869 Control: n = 4968; person-years = 42,649 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| PwH | Control | PwH | Control | |
| PwH group | 2.70 (2.08-3.52) | NA | 63.5 (.001) | NA |
| Age group (y) | ||||
| 30-44 | 5.41 (4.15-7.06) | 2.14 (1.76-2.62) | 44.9 (.04) | 20.2 (.15) |
| 45-59 | 7.44 (5.61-9.87) | 3.67 (3.01-4.46) | 107 (<.001) | 81.3 (<.001) |
| ≥60 | 9.24 (7.15-12.0) | 5.23 (4.36-6.27) | 62.4 (.001) | 67.8 (<.001) |
| Inhibitors, current or previousa | 3.37 (2.13-5.33) | NA | 19.0 (.46) | NA |
| Joint complicationb | 6.41 (3.71-11.1) | 5.27 (3.52-7.89) | 84.9 (.02) | −9.5 (.69) |
| Antidepressants | 4.37 (2.84-6.72) | 1.59 (1.30-1.94) | 103 (<.001) | 55.8 (<.001) |
| Neuroleptics | 7.39 (5.28-10.4) | 2.36 (1.97-2.84) | 104 (<.001) | 48.5 (<.001) |
| Antiepileptics | 5.49 (3.15-9.56) | 2.36 (1.78-3.14) | 142 (<.001) | 41.9 (.07) |
| Women including carriers | ||||
|---|---|---|---|---|
| Regression covariates | PwH: n = 389; person-years = 3977 Control: n = 1920; person-years = 19,438 |
|||
| OR (95% CI) |
% Difference in volume (P value) if use > 0 |
|||
| PwH | Control | PwH | Control | |
| PwH group | 2.80 (1.95-4.02) | NA | 61.1 (.013) | NA |
| Age group (y) | ||||
| 30-44 | 5.68 (4.00-8.07) | 2.05 (1.59-2.63) | 111 (<.001) | 51.1 (.02) |
| 45-59 | 9.36 (6.46-13.5) | 3.59 (2.78-4.65) | 124 (<.001) | 78.5 (<.001) |
| ≥60 | 8.73 (5.92-12.9) | 5.15 (3.98-6.67) | 72.9 (.004) | 86.9 (<.001) |
| Inhibitors, current or previousa | 1.22 (0.58-2.56) | NA | 64.7 (.06) | NA |
| Joint complicationb | 4.98 (2.81-8.82) | 3.46 (1.91-6.26) | 93.6 (.02) | 38.5 (.37) |
| Antidepressants | 4.64 (2.91-7.41) | 1.79 (1.46-2.20) | 109 (.001) | 47.4 (.007) |
| Neuroleptics | 5.79 (3.78-8.88) | 2.31 (1.90-2.79) | 111 (<.001) | 76.2 (<.001) |
| Antiepileptics | 9.4 (4.89-18.1) | 2.32 (1.68-3.20) | 175 (<.001) | 88.5 (<.001) |
Note: The reference person for the comparison of regression estimates is a control aged <30 years who does not use antidepressant or antianxiety prescription drugs and has no joint complications (and no inhibitors by definition).
OR, odds ratio; NA, not available/applicable; PwH, people with hemophilia.
Use of bypassing agent or a FVIII or FIX dosage of ≥350 IU/kg/week in the current or any previous year.
Orthopaedic joint surgery and/or registration of the health care contact with hemarthrosis (M25.0, M36.2). Registration of the surgery indicator in current or past years.
Similarly, higher likelihood of opioid use in ages of ≥30 years was found in all 3 PwH groups in Denmark (Supplementary Table S3), as well as in the moderate-to-high and the low factor consumption groups in Finland (Supplementary Table S4, too few women for the analyses in Finland). For Norway, the patterns of opioid use among PwH across ages in the 3 study groups were similar to the observations from Finland and Sweden and in some cases higher (Supplementary Figures S12 and S13). No data were available from Denmark for this post-hoc descriptive statistical analysis across age groups.
Sensitivity analyses in the Swedish data showed that the main outcomes—likelihood of use and volume of use—were robust to excluding the subset of PwH with likely VWD (n = 24; 1.5%) or acquired hemophilia (n = 25, 1,6%; refer to section “Post-hoc analysis of population selection strategy” in the Supplementary Material; results available on request).
4. Discussion
PwH from 4 Nordic countries (n > 3000) had a higher use and longer duration of pain drugs compared with matched population controls. This pattern was most evident for PwH with moderate-to-high factor use, but similar differences were found also for male PwH with low factor use and women including carriers compared to matched population controls. The population-based study design provides an objective way of describing the presence of pain, depression, and anxiety through analyzing registry data on filled prescriptions for 7 types of drugs over a long study period (11 years). The data also showed a higher use of antidepressant, antianxiety, neuroleptics, and antiepileptic drugs in many subgroups of hemophilia with the strongest tendency in Sweden. In addition to their primary indication, these drugs may be used as adjuvants to pain medication. [[16], [17], [18], [19]]
Although opioids are an important tool in the short-term treatment of severe pain, increasing prescriptions of opioids have raised concerns related to risk of dependency among patient groups exposed to pain. [20] PwH is one such group at an increased risk. The higher proportion of opioid use observed across all ages in our data highlights the need for person-centered approaches to pain and hemophilia management (Table 2). Even when adjusting for inhibitors and joint complications, there was a remaining higher likelihood of use and volume of use of pain medications associated with PwH group, as shown in the post-hoc analysis on opioid use based on Swedish data (Table 4). In moderate-to-high factor use, the highest difference in age-related likelihood of opioid use between PwH and controls was observed for middle-aged adults, consistent with an early burden from joints in PwH and an increase in work- or lifestyle-related causes of pain in later life in the general population. In addition, age-related diseases, such as cancer, may lead to increased opioid use in both groups later in life. The results from the registry data analyses of part A of the MIND study adds further evidence to the findings from recently published survey data of part B, which aimed to obtain the perspective of PwH and treaters from Denmark, Finland, and Sweden. The part B study showed patient- and treater-reported issues with pain, depression, and anxiety in PwH with mild, moderate, and severe phenotypes. [14] The pattern of self-reported pain and depression/anxiety in part B of the study MIND is consistent with the observed differences in drug utilization in part A of MIND study shown here despite different subgroup definitions.
In line with MIND study data, recent surveys have highlighted links between pain, functional impairment, depression, and anxiety in PwH, [7,21,22] the importance of health-related quality of life for PwH, [23] and their wider impact on caregivers in the family. [22] A systematic review and meta-analysis concluded that PwH are at about 2-fold or higher increased risk of reporting depression, anxiety, or either anxiety or depression compared with the general population. [24] In addition, a study enrolling predominantly men with severe hemophilia suggested that a history of depression may also negatively affect adherence to factor replacement. [25]
Prevention of bleeds, especially in joints, has been a longstanding objective in hemophilia care, [2,26,27] and the favorable outcome of regular prophylactic treatment for severe hemophilia has been established in observational [28,29] and trial data [30] for decades. In addition to continued efforts to reduce the burden of bleeds, our findings call for an increased awareness of pain, depression, and anxiety in all severities of PwH, including women and men with low, no, or irregular factor consumption. A recent publication from the US found that mild and moderate hemophilia among men and women is associated with physical and psychosocial impacts. [31] There is thus growing evidence supporting increased attention to problems of pain, as well as depression and anxiety, and the importance of hemophilia treatment centers reaching out to all severities and both sexes. It is important to not only manage these symptoms but also understand and prevent their root cause.
Several limitations of the MIND study need to be acknowledged. The broad registry-based inclusion criteria risked including individuals with other bleeding disorders due to single registrations of hemophilia diagnosis by mistake. However, sensitivity analyses of the Swedish data set showed that the potential inclusion of individuals with other bleeding disorders was likely small, with negligible impact on the outcomes addressed in the study (see Supplementary Material). Extended half-life factor products were only introduced toward the end of the study period and therefore probably had minimal influence on results of this study.
Although all 4 Nordic countries allow research based on health data registries with complete national coverage and have personal identity numbers, which enable tracking of individuals over time and cross-linking between administrative registries, country-specific regulations and practices required some adaptations of the research plan in each country. For instance, analyses in Denmark were commissioned through Statistics Denmark and fewer post-hoc analyses could therefore be conducted on Danish data. Furthermore, at the time of data collection, matched controls from population registries could not be obtained in Norway due to national regulations, nor was it possible to link individual-level information from the patient registries to Norwegian prescription data in smaller populations such as PwH. The Finnish data set was identified through eligibility of reimbursement of factor concentrates, likely resulting in a less broad coverage of people with mild hemophilia and of women including carriers in Finland than those in the other countries.
Another limitation is that National Health Data Registers do not hold information on laboratory values allowing for the classification of hemophilia severity; the subgroup classification was instead based on observed filled prescriptions of factor concentrates. However, as prophylaxis is available to all PwH who need it through public health insurance in the 4 Nordic countries, factor use may in this case correlate reasonably well with severity. In particular, PwH with mild phenotypes and limited or no need for regular factor concentrate use are expected to have been captured in the low factor consumption group in this study. We have applied a low cut-off threshold for a weekly volume of factor concentrate per kg bodyweight, which is lower than standard recommendations for prophylactic treatment in order to ensure that people with recurrent on-demand treatment or those who are not fully compliant with prophylaxis were included in the moderate-to-high factor consumption group. Although Nordic national registry data do not include information on race and ethnicity, which are variables used as sociocultural indicators in other countries, the public health insurance in the Nordic countries limits the concerns of affordability as a barrier to access of prophylaxis treatment and health care in general.
A strength of the study is that information on all filled prescriptions for 7 drugs used in the management of pain, depression, and anxiety was available during the 11-year study period, providing an extensive insight into treatment patterns in the broad PwH population, with the possibility to compare it to population controls in 3 countries.
In summary, our data show that PwH use more pain, depression, and anxiety drugs compared with population controls. Notably, this was also observed in men with low factor consumption and women with hemophilia, usually representing a milder phenotype. The increased use of these drugs is a sign of possible insufficient treatment and follow-up of hemophilia, irrespective of sex and hemophilia severity, even in those with a milder hemophilia phenotype.
Acknowledgments
This research was conducted by Swedish Institute for Health Economics. Analyses of Danish data were conducted by Louise Rasmussen and Caroline Østerholm Jørgensen at Statistics Denmark. Editorial assistance for this article was provided by Costello Medical, UK.
Funding
This research was funded by Swedish Orphan Biovitrum AB. Editorial assistance for this article was funded by Swedish Orphan Biovitrum AB in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author contributions
K.S.C., B.W., S.B., and S.L. contributed substantially to the study concept and design. K.S.C., B.W., J.A., F.B., E.B., E.F., M.H., K.Ö., S.B., and S.L. made substantial contributions to the analysis and interpretation of data. K.S.C., B.W., J.A., F.B., E.B., E.F., M.H., K.Ö., S.B., and S.L. contributed to critical writing or revising of the intellectual content. All authors have read and approved the final version of the article. K.S.C. had full access to all data from Finland, Norway, and Sweden, whereas Statistics Denmark analyzed individual-level data from Denmark on behalf of the study. All authors had full access to summary data as required from all 4 countries.
Relationship Disclosure
K. Steen Carlsson: Grant/research support from Bayer, Cancerfonden, Indivior, Medtronic, Novo Nordisk, Pfizer, Region Skåne, and the Swedish National Board of Health and Welfare, and is an employee of the Swedish Institute for Health Economics (IHE), a consulting company that has received funding from Sobi for the study. B.W. is an employee of Sobi. J.A. received grant/research support from Bayer, Biogen, CSL Behring, Sobi, and Shire and is a consultant for Bayer, BioMarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Shire, Sobi, Sparks, and UniQure. F.B. received honoraria as a member of the advisory board and/or speaker from Bayer, BioMarin, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, Sobi, and UniQure. E.B. received grant/research support from Sobi. E.F. is a speaker bureau of Roche, Shire/Takeda, and Sobi. M.H. participated in clinical trials from Novo Nordisk, Roche, Sobi, and Takeda. K.O. received grant/research support from Sobi; is a speaker bureau of Bayer, CSL Behring, and Sobi; and received honoraria as a member of the advisory board for Pfizer. S.B. is an employee of Sobi. S.L. is an employee and shareholder of Sobi.
Data availability
Individual-level data hosted by national authorities are available for research after formal evaluation of the research protocol by the ethical review authorities and the respective national authorities providing data. Permission to conduct research is granted on a case-by-case basis to a limited number of named persons. The secondary individual-level data of the MIND study cannot be shared by the authors for this reason. The aggregated summary data in the current study are available from the corresponding author on reasonable request. Informed consent is not required for registry data research in the Nordic countries studied.
Footnotes
Funding information Swedish Orphan Biovitrum AB
Handling Editor: Johnny Mahlangu
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100061
Supporting Information
References
- 1.Auerswald G., Dolan G., Duffy A., Hermans C., Jiménez-Yuste V., Ljung R., et al. Pain and pain management in haemophilia. Blood Coagul Fibrinolysis. 2016;27:845–854. doi: 10.1097/MBC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphries T.J., Kessler C.M. Managing chronic pain in adults with haemophilia: current status and call to action. Haemophilia. 2015;21:41–51. doi: 10.1111/hae.12526. [DOI] [PubMed] [Google Scholar]
- 3.Witkop M., Neff A., Buckner T.W., Wang M., Batt K., Kessler C.M., et al. Self-reported prevalence, description and management of pain in adults with haemophilia: methods, demographics and results from the Pain, Functional Impairment, and Quality of Life (P-FiQ) study. Haemophilia. 2017;23:556–565. doi: 10.1111/hae.13214. [DOI] [PubMed] [Google Scholar]
- 4.Witkop M., Santaella M., Nichols C.D., Lambing A.Y., Baumann K., Curtis R.G., et al. Understanding the pain management landscape within the US bleeding disorder community: a multi-center survey. Pain Med. 2022;23:269–279. doi: 10.1093/pm/pnab196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roussel N.A. Gaining insight into the complexity of pain in patients with haemophilia: state-of-the-art review on pain processing. Haemophilia. 2018;24:3–8. doi: 10.1111/hae.13509. [DOI] [PubMed] [Google Scholar]
- 6.Pinto P.R., Paredes A.C., Almeida A. Pain prevalence, characteristics, and impact among people with hemophilia: findings from the first Portuguese survey and implications for pain management. Pain Med. 2020;21:458–471. doi: 10.1093/pm/pny309. [DOI] [PubMed] [Google Scholar]
- 7.Buckner T.W., Batt K., Quon D., Witkop M., Recht M., Kessler C., et al. Assessments of pain, functional impairment, anxiety, and depression in US adults with hemophilia across patient-reported outcome instruments in the Pain, Functional Impairment, and Quality of Life (P-FiQ) study. Eur J Haematol. 2018;100:5–13. doi: 10.1111/ejh.13027. [DOI] [PubMed] [Google Scholar]
- 8.Iannone M., Pennick L., Tom A., Cui H., Gilbert M., Weihs K., et al. Prevalence of depression in adults with haemophilia. Haemophilia. 2012;18:868–874. doi: 10.1111/j.1365-2516.2012.02863.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheung K.L., ten Klooster P.M., Smit C., de Vries H., Pieterse M.E. The impact of non-response bias due to sampling in public health studies: a comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC Public Health. 2017;17:276. doi: 10.1186/s12889-017-4189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjellsson G., Clarke P., Gerdtham U.G. Forgetting to remember or remembering to forget: a study of the recall period length in health care survey questions. J Health Econ. 2014;35:34–46. doi: 10.1016/j.jhealeco.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Norhammar A., Bodegård J., Nyström T., Thuresson M., Nathanson D., Eriksson J.W. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21:1136–1145. doi: 10.1111/dom.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulder F.I., Horváth-Puhó E., van Es N., van Laarhoven H.W.M., Pedersen L., Moik F., et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137:1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 13.Holm E., Osooli M., Steen Carlsson K., Berntorp E. Cardiovascular disease-related hospitalization and mortality among persons with von Willebrand disease: a nationwide register study in Sweden. Haemophilia. 2019;25:109–115. doi: 10.1111/hae.13642. [DOI] [PubMed] [Google Scholar]
- 14.Steen Carlsson K., Winding B., Astermark J., Baghaei F., Brodin E., Funding E., et al. Pain, depression and anxiety in people with haemophilia from three Nordic countries: cross-sectional survey data from the MIND study. Haemophilia. 2022;28:557–567. doi: 10.1111/hae.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordic Hemophilia Council Nordic hemophilia guidelines. https://www.nordhemophilia.org/frontpage/guidelines/ 2020 [accessed June 27, 2022]
- 16.Watson C.P. Antidepressant drugs as adjuvant analgesics. J Pain Symptom Manag. 1994;9:392–405. doi: 10.1016/0885-3924(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 17.Tomić M., Pecikoza U., Micov A., Vučković S., Stepanović-Petrović R. Antiepileptic drugs as analgesics/adjuvants in inflammatory pain: current preclinical evidence. Pharmacol Ther. 2018;192:42–64. doi: 10.1016/j.pharmthera.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Seidel S., Aigner M., Ossege M., Pernicka E., Wildner B., Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2013:CD004844. doi: 10.1002/14651858.CD004844.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright S.L. Limited utility for benzodiazepines in chronic pain management: a narrative review. Adv Ther. 2020;37:2604–2619. doi: 10.1007/s12325-020-01354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller A.E., Clausen T., Sjøgren P., Odsbu I., Skurtveit S. Prescribed opioid analgesic use developments in three Nordic countries, 2006–2017. Scand J Pain. 2019;19:345–353. doi: 10.1515/sjpain-2018-0307. [DOI] [PubMed] [Google Scholar]
- 21.Buckner T.W., Witkop M., Guelcher C., Frey M.J., Hunter S., Peltier S., et al. Management of US men, women, and children with hemophilia and methods and demographics of the Bridging Hemophilia B Experiences, Results and Opportunities into Solutions (B-HERO-S) study. Eur J Haematol. 2017;98:5–17. doi: 10.1111/ejh.12854. [DOI] [PubMed] [Google Scholar]
- 22.Buckner T.W., Witkop M., Guelcher C., Sidonio R., Kessler C.M., Clark D.B., et al. Impact of hemophilia B on quality of life in affected men, women, and caregivers-assessment of patient-reported outcomes in the B-HERO-S study. Eur J Haematol. 2018;100:592–602. doi: 10.1111/ejh.13055. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth A.L., Witkop M., Lambing A., Garrido C., Dunn S., Cooper D.L., et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Preference Adherence. 2015;9:1549–1560. doi: 10.2147/PPA.S87659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Huniti A., Reyes Hernandez M., Ten Eyck P., Staber J.M. Mental health disorders in haemophilia: systematic literature review and meta-analysis. Haemophilia. 2020;26:431–442. doi: 10.1111/hae.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran D.Q., Barry V., Antun A., Ribeiro M., Stein S., Kempton C.L. Physician trust and depression influence adherence to factor replacement: a single-centre cross-sectional study. Haemophilia. 2017;23:98–104. doi: 10.1111/hae.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis R., Baker J., Riske B., Ullman M., Niu X., Norton K., et al. Young adults with hemophilia in the U.S.: demographics, comorbidities, and health status. Am J Hematol. 2015;90:S11–S16. doi: 10.1002/ajh.24218. [DOI] [PubMed] [Google Scholar]
- 27.Rambod M., Forsyth K., Sharif F., Khair K. Assessment and management of pain in children and adolescents with bleeding disorders: a cross-sectional study from three haemophilia centres. Haemophilia. 2016;22:65–71. doi: 10.1111/hae.12765. [DOI] [PubMed] [Google Scholar]
- 28.Fischer K., Steen Carlsson K., Petrini P., Holmström M., Ljung R., van den Berg H.M., et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129–1136. doi: 10.1182/blood-2012-12-470898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson I.M., Berntorp E., Löfqvist T., Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 30.Manco-Johnson M.J., Abshire T.C., Shapiro A.D., Riske B., Hacker M.R., Kilcoyne R., et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 31.Witkop M., Wang M., Hernandez G., Recht M., Baumann K., Cooper D.L. Impact of haemophilia on patients with mild-to-moderate disease: results from the P-FiQ and B-HERO-S studies. Haemophilia. 2021;27:8–16. doi: 10.1111/hae.14251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual-level data hosted by national authorities are available for research after formal evaluation of the research protocol by the ethical review authorities and the respective national authorities providing data. Permission to conduct research is granted on a case-by-case basis to a limited number of named persons. The secondary individual-level data of the MIND study cannot be shared by the authors for this reason. The aggregated summary data in the current study are available from the corresponding author on reasonable request. Informed consent is not required for registry data research in the Nordic countries studied.