Abstract
Background.
The phenomenon of alcohol analgesia and tolerance can facilitate misuse and lead to the development of alcohol use disorder (AUD). Numerous alcohol-induced behaviors are genetically influenced; however, it is unknown if alcohol analgesia has a genetic contribution. Rodent studies have shown that alcohol responses differ vastly between two widely studied inbred strains of mice, C57BL/6J (B6) and DBA/2J (D2). Here, we used B6 and D2 mice as an initial behavioral genetic analysis of acute alcohol-induced antinociception.
Methods.
The antinociceptive effect of orally-administered alcohol was characterized using the hot plate test in B6 and D2 mice of both sexes. Using the opioid receptor antagonist naloxone, the involvement of the opioid system was assessed. Locomotor activity and blood alcohol concentrations were also measured. Ovariectomized mice were used to evaluate the influence of ovarian sex hormones on alcohol-induced antinociception.
Results.
Alcohol induced an antinociceptive effect in B6 and D2 male mice in a time- and dose-dependent manner. In addition, D2 male mice were more sensitive to the antinociceptive effect of alcohol than B6 male mice. However, locomotion is not impeded by the tested doses of alcohol in B6 mice. Female D2 and B6 mice failed to show significant antinociceptive effects in alcohol dose-response studies. In addition, alcohol-induced antinociception was still not evident in ovariectomized female mice. Male mice of both strains developed tolerance to this effect after repeated administration of alcohol. Strain differences were found in blood alcohol concentration. Finally, no difference was found in the blockade of alcohol antinociception by 2 mg/kg naloxone.
Conclusion.
Our results indicate that the antinociceptive effects of alcohol in the hot plate test are influenced by strain and sex. These findings support further genetic analysis of alcohol-induced antinociception to identify operative mechanisms and better assess the contribution of this phenotype to AUD.
Keywords: Antinociception, mice, Tolerance, Hot Plate, Sex, Genotype
INTRODUCTION
Alcohol use disorder (AUD) is a debilitating condition that affects 15 million Americans (Tice, 2016). It is marked by chronic overconsumption of alcohol products and can severely disrupt occupational and social function. Long-term overuse of alcohol can contribute to serious medical complications, such as cardiovascular diseases, liver damage, stroke, peripheral neuropathies, cancer and diabetes (Roerecke and Rehm, 2014). Risk factors for AUD include environment, early life experiences, family history and sex. There is a 3 to 4-fold increase the risk of alcohol overconsumption among people with an alcoholic relative (Schuckit, 2014). Human studies reveal a genetic component to AUD, with a heritability of approximately 40% (Wedemeyer et al., 2020). Additionally, rates of AUD are higher in males, with 8.4% of adult men and 4.2% adult females affected in the United States (Tice, 2016). There is also increasing evidence to suggest pain as a risk factor for AUD. Alcohol has been long known to have analgesic properties in humans (James et al., 1978; Woodrow and Eltherington, 1988). The low cost and accessibility of alcohol is a motivating factor for self-medication, resulting in its use by 25% of pain patients (Riley and King, 2009). A higher prevalence of alcohol overconsumption in people suffering from chronic pain has been reported, possibly due to the pain-relieving properties of alcohol. The development of tolerance to alcohol analgesic effects may put patients at an increased risk of alcohol dependence (Egil et al., 2012) and prolonged use of alcohol can also induce hyperalgesia and exacerbate pain (Gatch, 2009; Jochum et al., 2010), perpetuating the cycle of pain and dependence.
Ethanol is a small, water-soluble molecule that is mainly absorbed in the small intestine (Jung and Namkoong, 2014). It is mainly metabolized in the liver by alcohol dehydrogenase in a concentration-dependent manner (Le Daré, Lagente and Gicquel, 2019). Alcohol’s intoxicating effects are seen when it accumulation in the blood outpaces its metabolism, leading to effects in various organ systems, notably the nervous system (Jung and Namkoong, 2014). Alcohol interacts with the opioid system by increasing the release of β-endorphin, thus activating mu opioid receptors in the mesocorticolimbic circuit while simultaneously depressing GABAergic transmission, ultimately stimulating dopamine release (Xiao et al., 2007).
The analgesic-like properties of alcohol have been studied in male rodents using acute thermal tests. Alcohol was reported to induce antinociception in male mice and rats in the tail-flick and hot plate tests (Boada et al., 1981; Pohorecky and Shah, 1987; Yirmiya and Taylor, 1989; Gatch and Lal, 1999a). Alcohol elicits an antinociceptive effect in mouse models of chronic pain as well (Neddenriep et al., 2019). After repeated exposure to alcohol, tolerance to its antinociceptive effects develops in mice (Boada et al., 1981; Malec, Kotlińska and Langwiński, 1987) and in rats (Bell, Olson and Vaccarino, 1998; Sudakov et al., 2016) in the hot plate and tail flick tests.
Clearly, there are a multitude of animal studies that have examined the role of genetics (e.g., inbred strains; selected lines or transgenic) in various alcohol-related traits such as acute effects, tolerance, withdrawal, and intake. However, human and animal studies examining the role of genetic factors in alcohol’s analgesic effects are lacking.
It is well established that behavioral responses to alcohol differ between B6 and D2 strains of mice. For instance, B6 mice have increased voluntary alcohol consumption compared to D2 mice (Belknap et al., 1993), although, D2 mice show greater acute locomotor activation with lower doses of alcohol than B6 strain (Melón and Boehm II, 2011) as well as greater sensitivity to ethanol in a conditioned place preference paradigm (Cunningham, Gremel and Groblewski, 2006). D2 mice show higher sensitivity to alcohol withdrawal compared to B6 mice (Fehr et al.,2003). Clearly, these two strains diverge vastly in these measures of alcohol-induced behaviors, but it is still unknown if they differ in their sensitivity to alcohol antinociception. Families of recombinant inbred mouse lines are routinely used to study the genetics of particular phenotypes of interest such alcohol-related traits. Of particular interest to the alcohol studies is the BXD family of recombinant inbred strains, which was derived by crossing C57BL/6J (B6) and DBA/2J (D2) and inbreeding progeny for 20 or more generations (Belknap et al., 1995).
Therefore, we aimed to compare alcohol-induced antinociception using the hot plate test in these two strains. If a strain difference to alcohol-induced antinociception is observed, this may suggest genetically influenced variations in this particular phenotype. Further studies in the BXD lines would be justified, thus allowing the investigation of genetic contributions to this trait.
This study compared alcohols thermal antinociceptive effect after oral administration in D2 and B6 mouse strains in both male and female animals. Due to differences in voluntary alcohol consumption reported between the two strains, oral gavage was used as the route of administration to standardize the intake. Additionally, the i.p. route of administration fails to account for first-pass metabolism of alcohol. We hypothesized that these two strains will differ in their sensitivity to alcohol’s antinociceptive effects. For this, we used the hot plate test to evoke an acutely noxious thermal stimulus. We generated a time course and dose response curve of alcohol in the hot plate test and assessed the development of tolerance to alcohol-induced antinociception after repeated administration. We also determined the impact of alcohol administration on the locomotor activity of animals. In conjunction with the behavioral measures, we determined the time course of the blood alcohol concentration in the two strains. Finally, we assessed the involvement of opioid receptors in alcohol antinociceptive effects using the opioid antagonist naloxone.
MATERIAL AND METHODS
Animals
Adult male and female C57BL/6J (B6) and DBA/2J (D2) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) at 8–10 weeks of age. Mice were kept in corncob bedding-filled cages of four, separated by strain and sex and were placed on a 12-hour light/dark cycle. Food (#7012, Envigo Teklad, Madison, WI, United States) and water were accessible ad libitum. Experimentation began at 10–12 weeks of age for all subjects. Mice within each cage were randomly assigned to treatment and control groups. For each experiment, all mice were tested in a single session. All testing were done during the light cycle. The same experimenter observed each study and was blinded throughout. All studies were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Ovariectomized adult female D2 mice at 8 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME). Sample size for these studies were determined by a standard power calculation. For the “sham ovariectomy” group of mice, adult female D2 mice obtained from The Jackson Laboratory were used for the “sham ovariectomy” surgery. Mice were anesthetized with an induction dose of 5% isoflurane and maintained with 3% isoflurane. A dorsal midline cutaneous incision was made bilaterally. The skin was then sutured, and antibiotic ointment applied to the surface. The mice were allowed 7 days to heal from the procedure before testing began.
Chemicals
100% Ethanol obtained from Sigma-Aldrich (St. Louis, MO) was diluted in its vehicle, water, and prepared in a 20:80 ethanol to water volume solution (20%). Administration of alcohol in all tests was done via oral gavage. Alcohol doses with this solution concentration were calculated based on body weight and ranged from 0.5–2 g/kg. Naloxone HCl, purchased from Sigma-Aldrich (St. Louis, MO) was dissolved in 0.9% sodium chloride saline solution. The drug was administered via the subcutaneous route, at a 1 ml/100 g volume-to-body weight ratio. The preparation and route of administration were selected as reported in previous studies (Campbell, Taylor and Tizabi, 2006; Neddenriep et al., 2019) in both sexes of these two strains of mice.
HPLC chemicals: HPLC grade methanol and de-ionized (DI) water, optima grade acetone, ethanol, methanol, n-propanol and isopropanol were purchased from Fisher Scientific (Hanover Park, Illinois). The ethanol 80 and Volatile Controls were purchased from UTACK® Laboratories, Inc. (Valencia, CA). Nitrogen and helium gases were acquired from Praxair and Airgas (Richmond, VA).
Hot Plate Test
The hot plate was used to assess the antinociceptive effects of alcohol as described in our previous study (Neddenriep et al., 2019). The 10-cm wide glass cylinder hot plate (Thermojust Apparatus, Columbus, OH) was set to 55°C. Mice were placed on the plate and timed until signs of nociception were displayed, such as licking and shaking of the paw. Mice were removed immediately after and the cut off time for the test was 20 sec. The percentage of maximum possible effect was calculated based on the response time in order to quantify the antinociception, where %MPE = [(test-control)/(20-control)] × 100. The control response (2–4 s) was determined for each mouse before treatment.
-Time Course Studies
With a separate cohort of naïve male and female mice, this experiment was carried out with an oral gavage of the vehicle (water) or 2 g/kg of 20% alcohol. At different time points (15, 30, 60 and 90 min), the hot plate test was performed in the same mice with 8 mice/group. Latencies (sec) were used to calculate % MPE and a time course of the antinociception was produced.
-Dose Response after Acute Alcohol Exposure
Naïve male and female mice were separated into groups and given vehicle, 1, 1.5 or 2 g/kg of 20% alcohol via oral gavage with 8 mice/group. After 30 min, the hot plate test was performed. Latencies (sec) were used to calculate the % MPE and dose response curves were generated.
-Naloxone Pre-Treatment Studies
Naïve B6 and D2 male mice were separated into four groups, naloxone/alcohol, naloxone/vehicle, vehicle/naloxone and vehicle/vehicle. Each group contained 8 mice. The baseline hot plate latency was recorded prior to experimentation. A pre-treatment of either 2 mg/kg naloxone or vehicle was administered subcutaneously. 30 min after pre-treatment, either 2 g/kg of alcohol or vehicle was administered orally. Following an additional 30 min, the hot plate latency was measured.
-Tolerance studies
In order to induce tolerance to alcohols antinociceptive effects, naïve male mice were initially given the vehicle or a dose of 2 g/kg alcohol orally. This dosing was followed by the hot plate test to assess basal responses to alcohol. An additional group which received only the vehicle, referred to as the control, was added to the study. These naïve mice were purchased within the same time frame and were also 8 weeks of age. The initial study was followed by daily doses of either the vehicle, 0.5, 1 or 2 g/kg of alcohol for 3 consecutive days. Following this repeated exposure, mice were then challenged on the fifth day with 2 g/kg alcohol and assessed again, using the hot plate test 30 min post-gavage (Fig. 3A). %MPE values were determined. The percent tolerance was graphed and is a between-group measure and was calculated as follows:
Figure 3. The Development of Tolerance to Alcohol’s Antinociceptive Effects After Repeated Exposure.
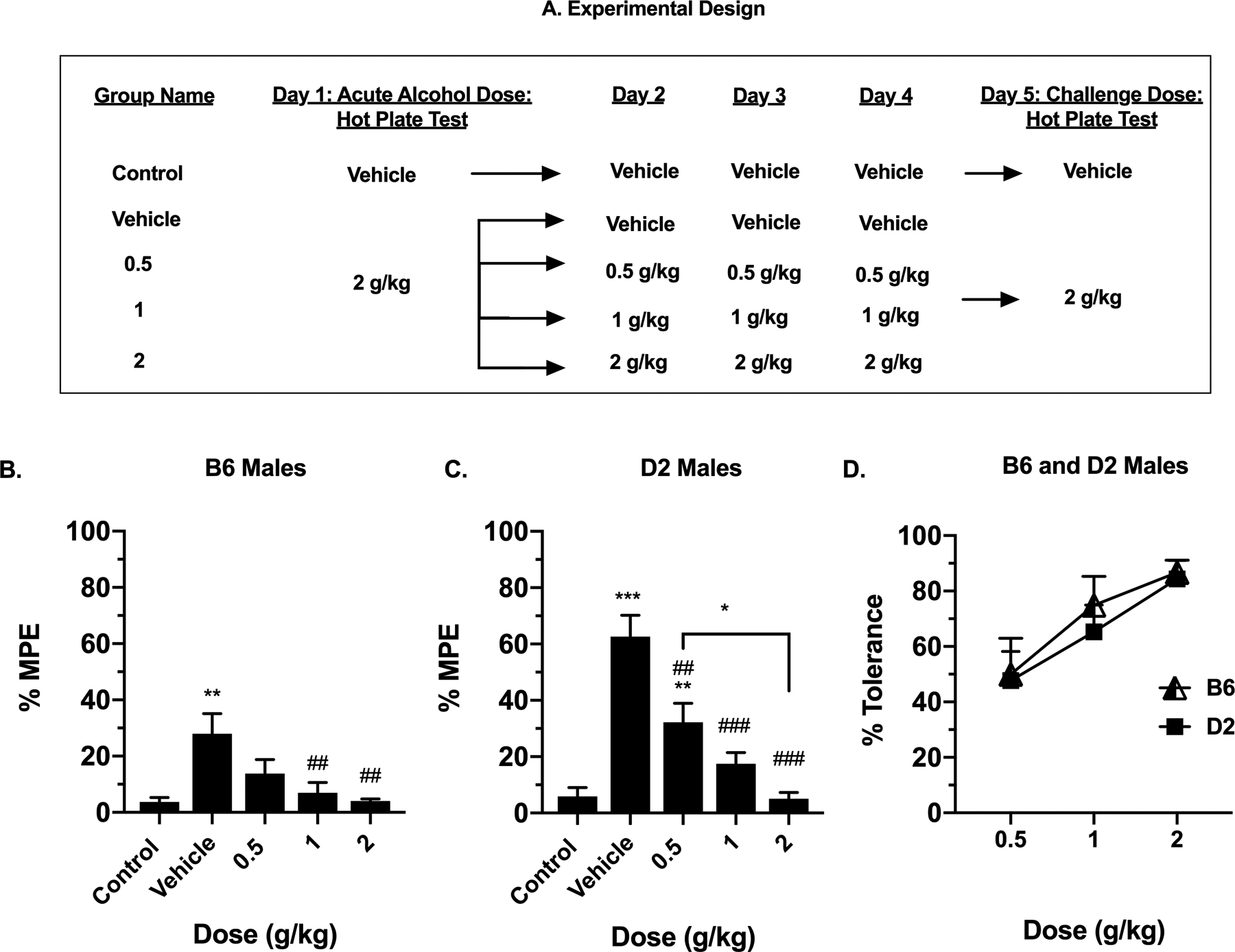
(A) Visual depiction of the experimental design for repeated alcohol exposure. Antinociception effect of alcohol after repeated oral administration, measured by the hot plate test as expressed by %MPE (Maximum possible effect), at different maintenance doses, 0.5, 1 and 2 g/kg, 30 min after alcohol gavage in (B) B6 male mice and (C) D2 male mice. Values are expressed as mean ± SEM. n= 8/group. Results were compared using a one-way ANOVA, followed by a post-hoc Dunnett’s test (*: p<0.05, **: p<0.01 and ***: p<0.001 vs. control) (#: p<0.05, ##: p<0.01 and ###: p<0.001 vs. vehicle). (D) The percent of tolerance measured by dose in B6 and D2 male mice.
-Acute Alcohol Response in Ovariectomized Mice
Naïve ovariectomized and sham mice were given an oral dose of 2 g/kg alcohol. After 30 min, the hot plate test was performed on the mice. Results were converted into %MPE.
Locomotor Activity
To assess the effect of various doses of alcohol on locomotion, separate groups of naïve male mice with 10 mice per group were gavaged with the vehicle, 0.5, 1 or 2 g/kg of alcohol. Similarly, a separate group of naïve female mice with 10 mice per group were gavaged with the vehicle or 1.5 and 2 g/kg of alcohol. Mice were placed in the experimental room to acclimate for 30 min before testing. The test was performed 30 min after gavage of the vehicle or alcohol. This time was selected based on previous work indicating that blood alcohol concentrations peak after the first 30 min of oral administration (Livy, Parnell and West, 2003). Mice were placed individually into photocell activity cages (Omnitech Electronics, Columbus, OH, USA) which measured 28 × 16.5 cm. Locomotor activity score was defined by the total number of photocell beam interruptions in 30 min. Data were expressed as mean of the number of photocell interruptions.
Blood Alcohol Concentration (BAC) Analysis
Naïve male and female mice were gavaged with 2 g/kg. At 15, 30, 60, and 90 min, blood was repeatedly taken via retro-orbital vein bleeds and stored in heparinized tubes at −80 °C. The alcohol content was of the whole blood samples was determined using a modified method routinely employed for the analysis of clinical, forensic and types other samples for ethanol, acetone, isopropanol and methanol (Poklis et al., 2017). In brief, one hundred microliter aliquots of the samples, calibrator, and ethanol free control consisting of DI water and the ethanol 80 and Volatile controls, were added to 900 μL of 234mg/L n-propanol working internal standard. Analysis was performed using a Tekmar HT3 Headspace sampler attached to a Shimadzu 2014 Gas Chromatograph (GC) with a flame ionization detector (FID). The chromatographic separation was performed on an RTX-BAC1, 30 m × 0.32 mm id × 1.80 μM column (Restek Corp, Bellefonte, PA). The headspace oven and transfer line temperatures were set to 160 °C with a standby flow rate of 200 mL/min. The platen sample temperature was 80 °C with the mixer on. The sample equilibrium time was 3.5 min with a sample injection time of 0.5 min. The GC had an oven temperature of 50 °C (isothermal) and an injection temperature of 200 °C run in split mode with a 1:20 ratio. The column flow rate was 6.85mL/min with purge flow of 0.5 mL/min. The detector temperature was 225 °C with both hydrogen and air set at 50 KPa. The LOD for all volatiles was 50 mg/L with a determined linear range of 50 to 8000 mg/L for the assay.
Statistical Analysis
All data are expressed as mean ± SEM (Standard Error of the Mean). Normality and equal variance have been verified respectively by Shapiro-Wilk and Brown-Forsythe tests. Data were then compared using either one-way or two-way repeated analysis of variance (ANOVAs), followed by post-hoc Tukey (Three-way ANOVA), Sidak (Two-way ANOVA), Dunnett’s (One-way ANOVA) comparison tests, where appropriate. All statistical analyses were performed with GraphPad statistical software (GraphPad Software, Inc., La Jolla, CA, USA). Differences were considered significant when p was <0.05*, <0.01** or <0.001***. ED50 (effective dose 50%) values with 95% confidence limits (CL) were calculated by unweighted least-squares linear regression as described by Tallarida and Murray (1987).
RESULTS
Alcohol Time Course in The Hot Plate Test
To determine the time-course of alcohol antinociceptive responses in B6 and D2 mice, an acute oral dose of alcohol (2 g/kg) was administered, and the hot plate test was performed at multiple time points (Fig. 1). The average basal latency for B6 male and female mice was 9.7 ± 0.40 sec and 9.3 ± 0.42 sec, respectively, and was not significantly different (p=0.45). For D2 male and female mice, the basal latencies were 6.2 ± 0.78 and 10.1 ± 0.47, respectively, and differed significantly (p<0.0001). In a two-way ANOVA analysis, there was an interaction between time and treatment after alcohol administration in male B6 mice (Fig. 1A; F(3, 42) = 2.77, p=0.05) as well as significant time F(2, 42) = 2.90, p=0.05) and treatment F(1, 14) = 41.13, p<0.0001) factors. A post-hoc analysis resulted in a significant difference compared to the control at 15 min (p=0.0278), 30 min (p=0.0327), 60 min (p0.0159) and 90 min (p=0.0056). In addition, our results showed a clear interaction between time and treatment in male D2 mice (Fig. 1B; F(3, 42) = 5.31, p=0.0034) as well as significant time F(2, 42) = 4.67, p=0.015) and treatment F(1, 14) = 126.1, p<0.0001) factors, with post-hoc analysis indicating a significant difference compared to the control at 15 min (p=0.0103), 30 min (p=0.0001), 60 min (p=0.0067) and 90 min (0.0263) reflecting a thermal antinociceptive effect of alcohol in male mice.
Figure 1. Time-Course of Alcohol-Induced Antinociception in The Hot Plate Test.
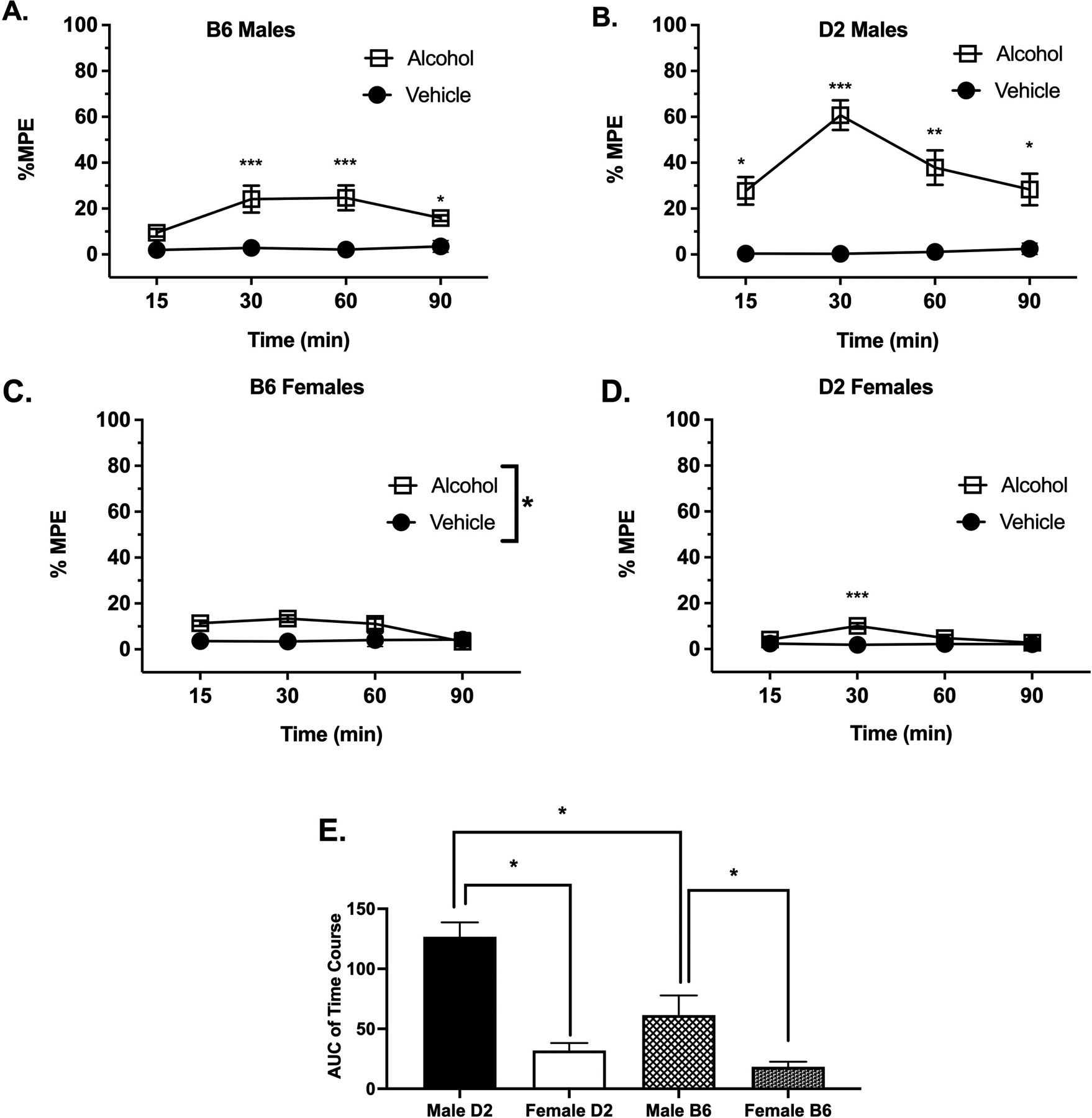
Antinociception effect of alcohol was measured by the hot plate test expressed as %MPE (Maximum possible effect), at different time points 15, 30, 60, 90 and 120 min after alcohol gavage was measured in (A) B6 male mice, (B) D2 male mice, (C) B6 female mice and (D) D2 female mice. (E) Comparison of the AUC of time-course of antinociceptive effect of alcohol measured by hot plate in all mice (Results were compared using two-way ANOVA with sex and strain as a factor and post-hoc Sidak’s test). Values are expressed as mean ± SEM. n= 8/group- (*p<0.05; **p<0.01 and ***p<0.001 vs. vehicle).
Our results with B6 female mice (Fig. 1C) treated with alcohol also show an interaction between time and treatment (F(3, 42) = 3.480; p=0.0024) as well as significant time F(2, 42) = 2.085, p=0.05) and treatment F(1, 14) = 27.42, p<0.0001) factors, but post-hoc analysis only showed a significant difference from the mean at 30 min (p=0.0004) in comparison to the control group. Finally, the data observed in D2 female mice showed no interaction between time and treatment (Fig. 1D, F (3, 42) = 2.64, p=0.0617), no significant time factor F (3, 42) = 1.970, p=0.1332) but significant treatment F(1, 14) = 23.06, p=0.0003) factor.
In addition, a two-way ANOVA of AUC analysis of alcohol treatment (Fig. 1E) comparing sex and strain showed significant sex (F(1, 14) = 109.9 p<0.0001) and strain (F(1, 14) = 18.30 p=0.0008) and interaction between strain × treatment (F(1, 14) = 14.68; p=0.0018). Taken together, these results show that the time-course of alcohol’s antinociceptive effects after the oral dose of 2 g/kg differed between sexes and strains.
Alcohol Dose Response in the Hot Plate Test
To probe the impact of dose on the thermal antinociceptive effects of alcohol, alcohol was administered orally in B6 and D2 mouse strains at different doses (1, 1.5, and 2 g/kg) (Fig. 2). Our previous results above indicated that performing the hot plate test 30 min post-gavage produced a peak antinociceptive effect in both strains. This time point (30 min post-gavage) was therefore chosen for the dose-response study.
Figure 2. Acute Alcohol Dose-Response Curve in The Hot Plate Test.
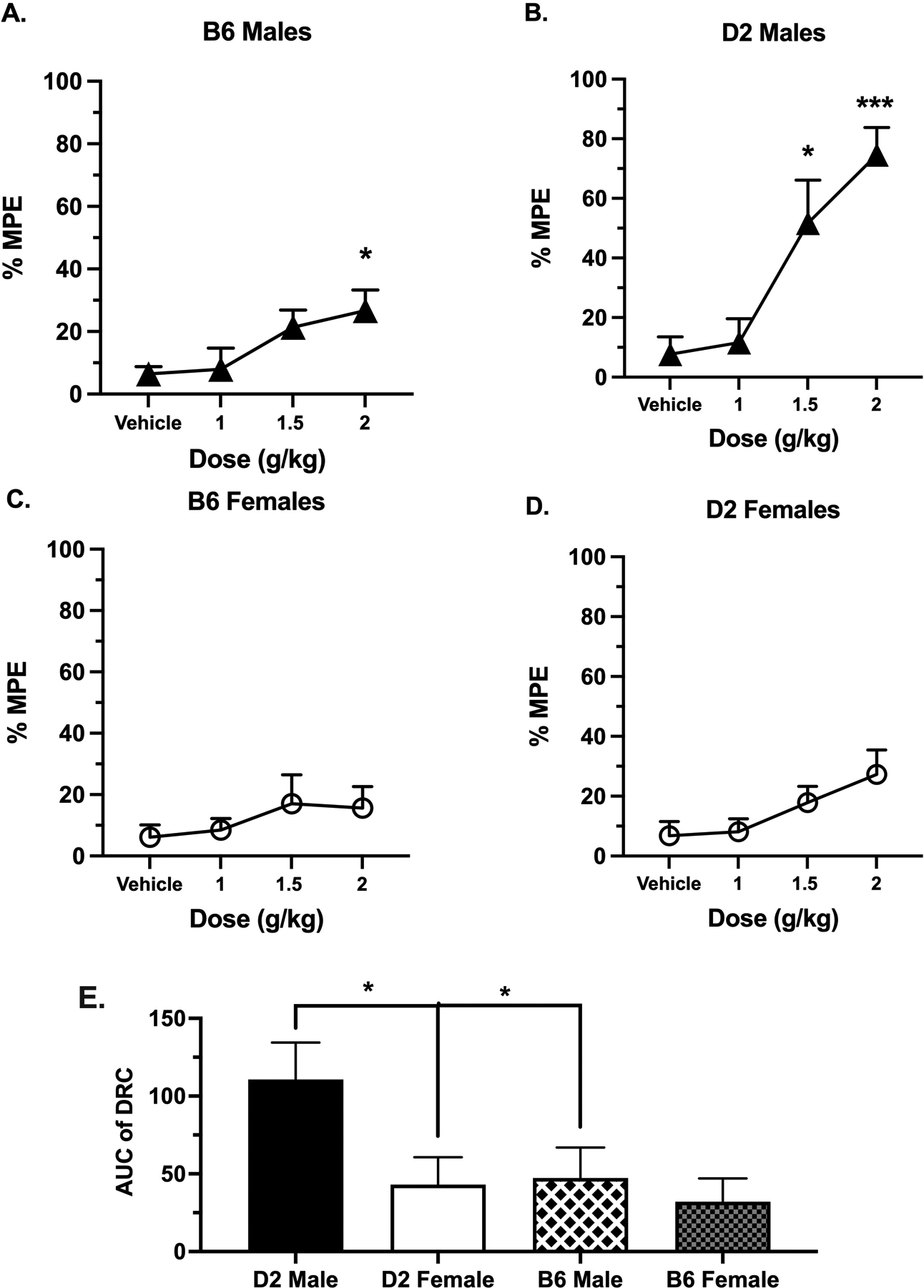
Antinociception effect of alcohol measured by the hot plate test expressed as %MPE (Maximum possible effect), at different doses, 1, 1.5 and 2 g/kg, 30 min after alcohol gavage in (A) B6 male mice, (B) D2 male mice, (C) B6 female mice and (D) D2 female mice. Values are expressed as mean ± SEM. n= 8/group. Results were compared using one-way ANOVA with treatment as the factor and post-hoc Dunnett’s test (*: p<0.05, ** and p<0.01 vs. vehicle). E) Comparison of the AUC of time-course of antinociceptive effect of alcohol measured by hot plate in all mice (Results were compared using two-way ANOVA with sex and strain as a factor and post-hoc Sidak’s test).
In a one-way ANOVA, alcohol induced a significant effect on %MPE in B6 male mice (Fig. 2A; F (3, 28) = 3.196, p=0.0386). A post-hoc analysis indicated that only the dose of 2 g/kg was significantly different as compared to vehicle-treated mice (p=0.0418). Similar to B6 males, alcohol produced a significant change in %MPE in D2 mice (Fig. 2B; F= 10.72 p<0.0001). However, post-hoc analysis showed a significant difference from the control at two doses, 1.5 g/kg (p=0.0105) and 2 g/kg (p<0001). In contrast, alcohol induced no effect on the %MPE at all aforementioned doses in female B6 mice (Fig. 2C; F (3, 28) = 0.6825, p=0.5702) or female D2 mice (Fig. 2B; F (3, 28) = 2.003, p=0.1364).
In addition, the determination of ED50 value in male D2 showed a potency of 1.5 (1.31–1.73) g/kg. Since none of the other DRCs of alcohol reached > 50% MPE, we were not able to determine their ED50 values accurately. However, alcohol response in male D2 mice was more efficacious than male B6 (74.6% vs 30% MPE), female D2 (74.6% vs 27% MPE) and female B6 (74.6% vs 15% MPE) mice at the highest dose of 2g/kg of alcohol. Finally, a two-way ANOVA of AUC analysis of alcohol doses (Fig. 2E) comparing sex and strain showed significant sex (F(1, 14) = 31.99; p<0.0001) and strain (F(1, 14) = 35.02; p<0.0001) and interaction between strain × treatment (F(1, 14) = 12.78; p=0.003). Taken together, these results show that alcohol’s antinociceptive dose-response effects differed between sexes and strains.
Alcohol’s Influence on Locomotor Activity
To investigate if locomotor impairment due to alcohol influenced its antinociceptive effects at the doses tested, B6 and D2 mice underwent locomotor activity measurement after drug administration (Fig. 1Suppl.). Subjects were given the vehicle or alcohol at various doses (0.5, 1.5 and 2 g/kg for males; 1.5 and 2 g/kg for females) and assessed 30 min post-gavage for their levels of spontaneous activity over a period of 30 min. In a one-way ANOVA test, alcohol did not modify the locomotion in B6 male mice (Fig. 1S-A) (F (3, 24) = 2.172, p=0.1176). However, the locomotor activity of D2 male mice (Fig. 1S-B) was increased by alcohol (F (3, 28) = 4.917, p=0.0072). A post-hoc test showed that there was a significant difference in activity, as compared to the vehicle, at the dose of 2 g/kg (p=0.0101). In female B6 mice, alcohol did not significantly modify the locomotion of animals (Fig. 1S-C) (F (2, 21) = 2.83, p=0.0811). Finally, the locomotor activity of D2 female mice (Fig. 1S-D) was increased by alcohol (F (2, 21) = 3.614, p=0.0448). A post-hoc test showed that there was a significant difference in activity, as compared to the vehicle, at the dose of 2 g/kg (p=0.025).
Tolerance Studies After Repeated Alcohol Exposure
As a continuation of our initial study of the response to acute alcohol in the hot plate test, we examined how repeated alcohol exposure subsequently influenced alcohol-induced antinociception in the same mice (Fig. 3). Females were not included in this study due to a lack of significant antinociceptive effect of alcohol. Male B6 and D2 mice initially received either the vehicle or 2 g/kg of alcohol. For 3 days, these mice were repeatedly administered the vehicle or alcohol at various doses (0.5, 1 and 2 g/kg), as well as an additional group, which received the vehicle throughout the study, as shown in the experimental design (Fig. 3A). On the fifth day, the mice were challenged with 2 g/kg of alcohol. One control group was administered the vehicle throughout the entire experiment. As shown in Figures 3B and 3C tolerance to the antinociceptive effects of alcohol developed after repeated administration in both strains. A one-way ANOVA analysis in B6 male mice detected a significant effect, with treatment as the factor [F(4, 69) = 7.36; p=0.0027]. In the post-hoc test, B6 mice were significant different from the vehicle at 1 g/kg (p= 0.0062) and 2 g/kg (p= 0.0012). Similarly, a significant effect was observed in D2 male mice [F(4, 69) = 16.39; p<0.0001], with the post-hoc test signifying a difference from the vehicle at 0.5 g/kg and 2 g/kg (p=0.0384). The calculated percent of tolerance to alcohol after repeated administration of the drug increased with increasing dose of alcohol (Fig. 3D). At a dose of 0.5 g/kg, the percent tolerance was 49.3% in B6 and 47.7% for D2 male mice. At 1 g/kg, the percent tolerance increased to 74.8% in B6 and 65.3% in D2 male mice. At 2 g/kg, the percent tolerance was 86.7% in B6 and 84.3% in D2 mice. Overall, the percent tolerance was not different between the two strains (F(1, 27) = 0.05302, p=0.8196).
Naloxone Blockade of Alcohol-Induced Antinociception
To assess the role of opioid receptors in the alcohol antinociception, a pre-treatment of 2 mg/kg of naloxone was administered to B6 and D2 mice 30 min prior to an oral dose of alcohol at 2 g/kg (Fig. 4). Male mice were again selected in this study due to the presence of alcohol-induce antinociception. Using the hot plate test and two-way ANOVA analysis, it was observed that the dose of 2 mg/kg of naloxone caused a sharp decline in %MPE induced by alcohol in both B6 (Fig. 4A; F(3, 28) = 20.93, p<0.0001) and D2 male mice (Fig. 4B; F(3, 28) = 98.02, p<0.0001).
Figure 4. The Effect of Naloxone Pre-Treatment on Alcohol-Induced Antinociception.
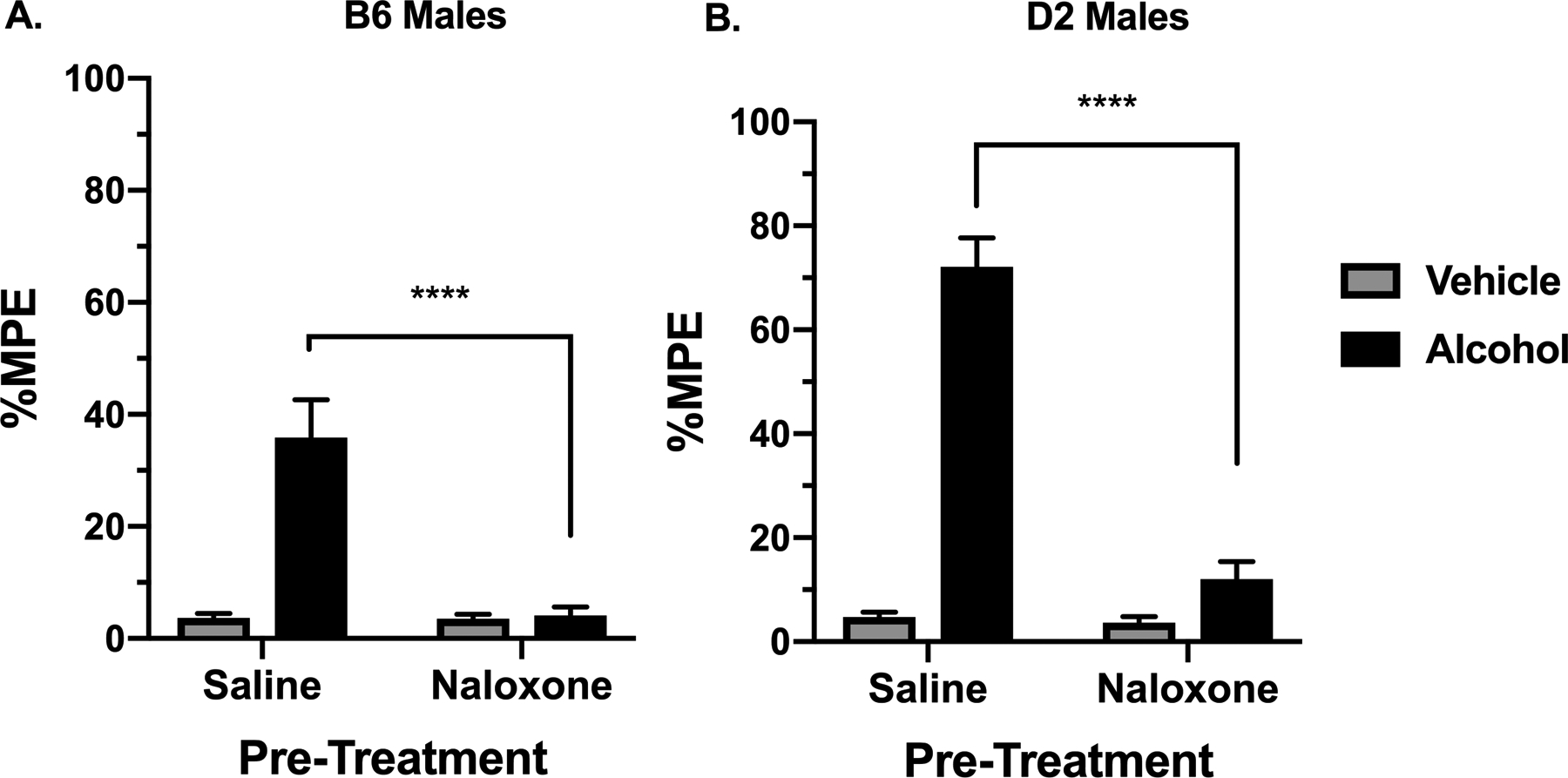
Antinociception effect of 2 g/kg alcohol after pre-treatment with 2 mg/kg naloxone, measured in the hot plate test as expressed by %MPE (Maximum possible effect) 30 min after alcohol gavage in (A) in B6 male mice and (B) D2 male mice. Values are expressed as mean ± SEM. n= 8/group. Results were compared using one-way ANOVA with treatment as the factor and post-hoc Tukey’s test (****p<0.0001 vs. vehicle).
Blood Alcohol Concentration Time Course
With the strain and sex differences observed after acute alcohol administration in the hot plate test, it was of interest to investigate if the blood alcohol concentration (BAC) time-course in both sexes of B6 and D2 mouse strains differ. An oral dose of alcohol (2 g/kg) was administered, and the blood alcohol concentrations were measured at several time points (Fig. 5). We performed a two-way ANOVA of AUC analysis of BAC doses (Fig. 5E) comparing sex and strain that showed significant sex (F(1, 10) = 5.035; p=0.0487) and strain (F(1, 10) = 31.63; p<0.0002) and interaction between strain × treatment (F(1, 10) = 11.00; p=0.0078). In general, B6 mice showed significantly higher AUC than D2 mice. In addition, only D2 female mice showed significantly higher AUC than D2 male mice. Taken together, these results show that alcohol’s BAC differed between sexes and strains.
Figure 5. Blood Alcohol Concentration (BAC) Time-Course After Alcohol Administration.
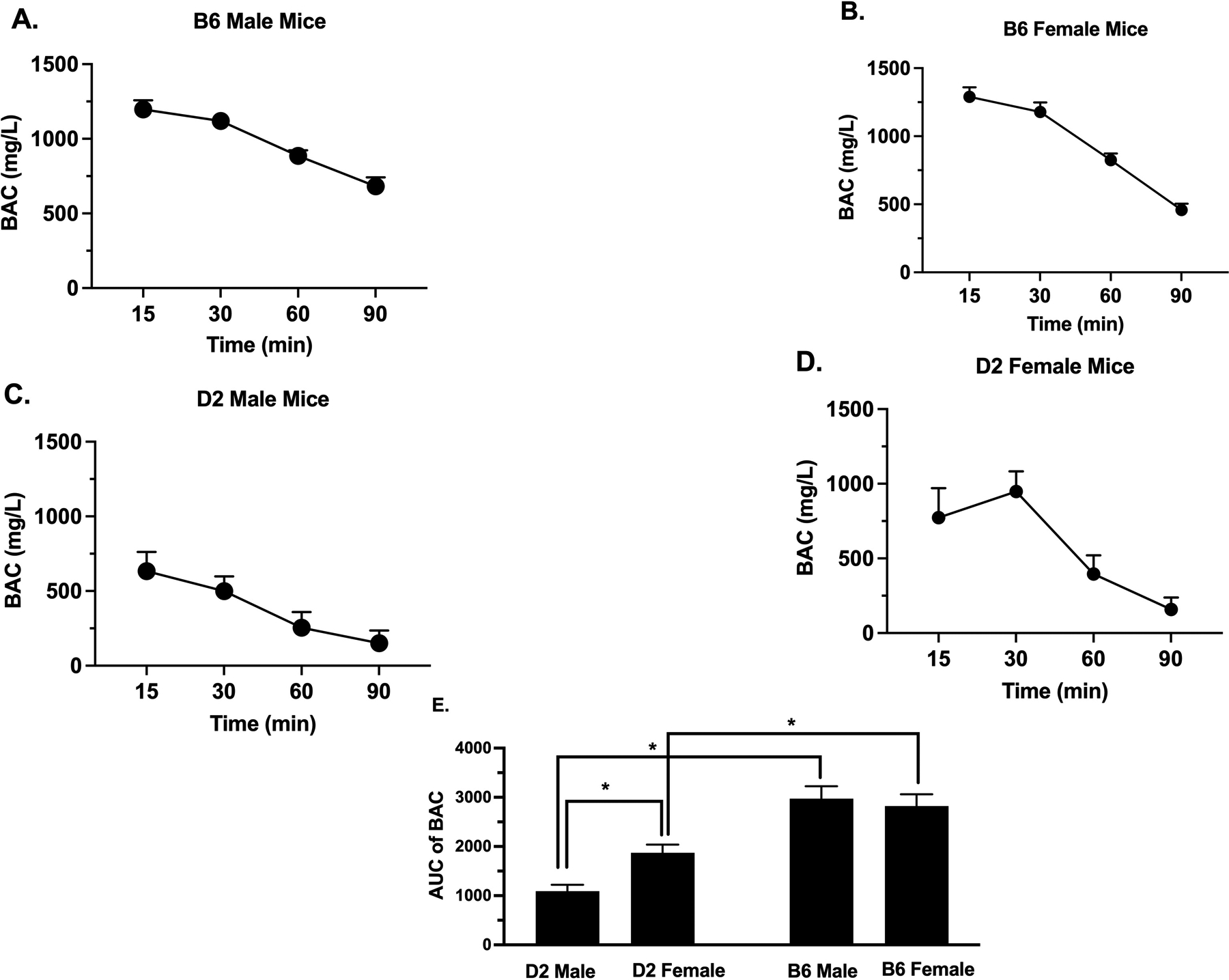
BAC (mg/L) in (A) B6 male, (B) B6 female, (C) D2 male and (D) D2 female mice at various time points, 15, 30, 60 and 90 min after oral gavage of 2 g/kg alcohol. Values are expressed as mean ± SEM. n= 10/group. E) Comparison of the AUC of time-course of BAC in all mice (Results were compared using two-way ANOVA with sex and strain as a factor and post-hoc Sidak’s test) (*p<0.05).
Alcohol Effects in the Hot Plate Test in Ovariectomized Female Mice
Our results above show that the sex difference in alcohol antinociception most likely could not be attributed to differences in alcohol pharmacokinetics. We therefore examined the role of gonadal female sex hormones in the lack of alcohol-induced antinociceptive responses in females. For this, ovariectomized female D2 mice were tested on the hot plate test after oral administration of 2 g/kg of alcohol or vehicle. Hot plate basal latencies were 7.74 ±1.15 for the ovariectomized mice and 7.09 ±0.68 for the sham mice. The basal latency did not differ between the two groups (F(8,7)= 3.16 p=0.1471). In a two-way ANOVA analysis comparing treatment and surgery, no effect of surgery was seen [F (1, 33) = 1.578; P=0.2179] and no interaction (surgery × treatment) was found in mice [F (1, 33) = 0.3468; P=0.5600] (Fig. 6).
Figure 6. Assessment of Alcohol’s Antinociceptive Effects in Female Mice with the Absence of Ovarian Sex Hormones.
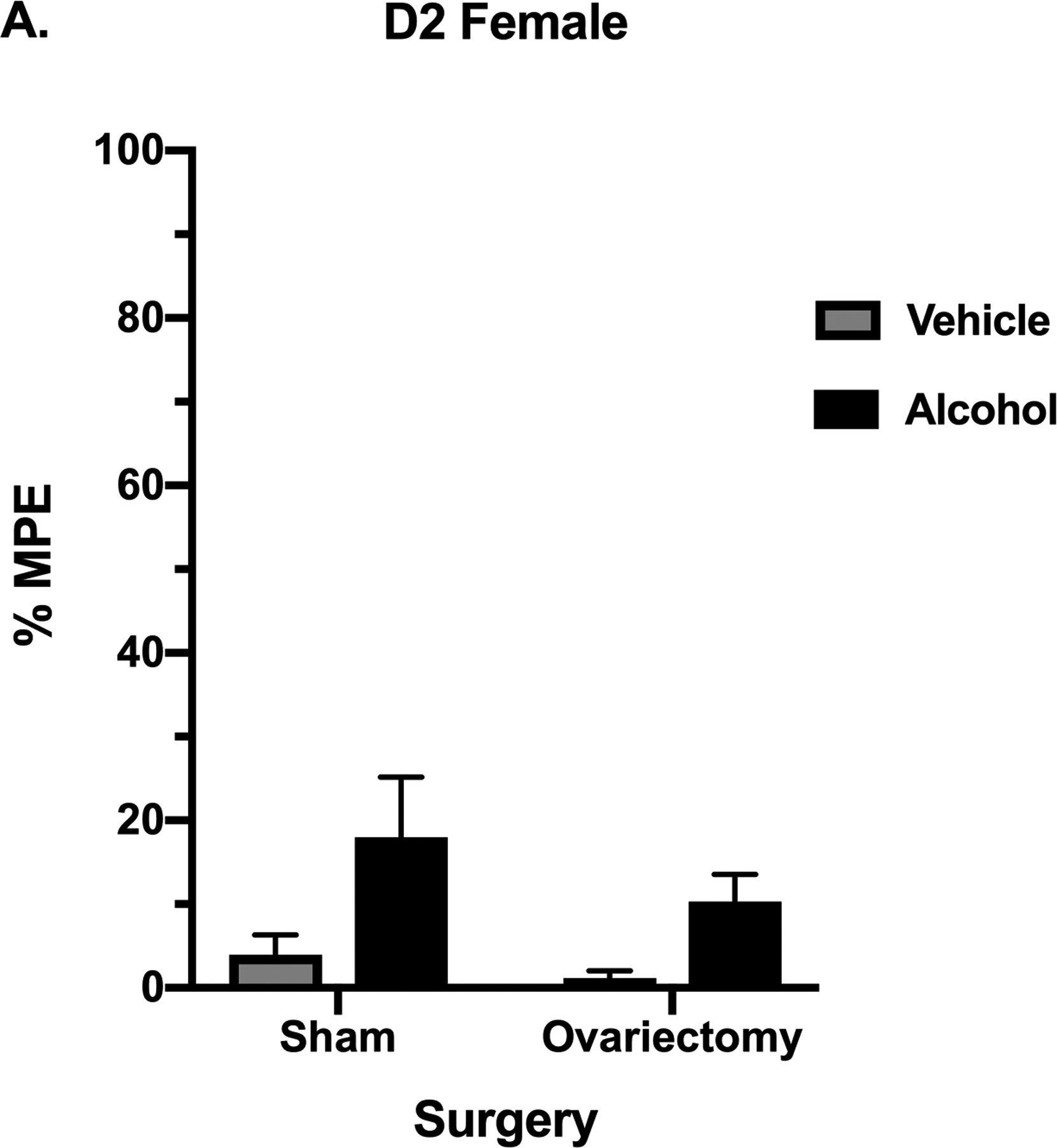
Antinociception effect of 2 g/kg alcohol in ovariectomized and sham mice, measured by the hot plate test in D2 females as expressed by %MPE (Maximum possible effect) 30 min after alcohol gavage. Values are expressed as mean ± SEM. n= 10/group.
DISCUSSION
The focus of our study was to characterize alcohol’s antinociceptive effect in mice. For that, genotype and sex following oral administration of alcohol in the hot plate test were examined. Here, we report that alcohol given by gavage elicits an antinociceptive effect in a time- and dose-dependent manner in both male B6 and D2 mice. The peak response was recorded at 30 min and a dose of 2 g/kg produced the greatest effect. In addition, alcohol-induced antinociception is higher in D2 compared to B6 male mice. Previous work has also shown that alcohol elicits an antinociceptive effect after systemic administration in the hot plate and tail flick tests in male rats, as well as Swiss Albino, B6 and BALB/cJ male mice at the same range of doses used in our study (Boada et al., 1981; Pohorecky and Shah, 1987; Yirmiya and Taylor, 1989; Gatch and Lal, 1999b). However, our studies were conducted only in the hot-plate test and it is possible that a different effect of alcohol can be observed in other acute thermal tests such as the tail-flick assay. The hot-plate test is predominantly supraspinal while the tail-flick test predominantly elicits spinal response (Langerman et al., 1995).
One concern about these results is alcohol’s sedative properties observed at high doses (Stevenson, Besheer and Hodge, 2008). If alcohol elicits a significant locomotor impairment, then the hot-plate response latency may be affected. For that, we assessed the effects of alcohol on the locomotor activity assay of mice. In B6 mice, no significant change in activity was found. A modest, but significant increase in activity was observed at the highest dose of 2 g/kg in D2 male and female mice. This observation is supported by previous studies with D2 mice after i.p. alcohol administration (Kiianmaa and Tabakoff, 1983; Stevenson et al., 2008; Laverne et al., 2011).
The BAC profiles observed in our studies may serve as an explanation for the differences in alcohol-induced antinociception between D2 and B6 mice. B6 mice overall had higher concentrations than D2 mice. It is therefore possible that acute tolerance may have developed at faster rate in male B6 mice, explaining alcohol lower potency in the hot plate test compared to D2 mice. Few reports compared BAC in the two strains after oral gavage. D2 animals displayed significantly lower BAC than B6 after i.p. injection of 4 g/kg of alcohol (Linsenbardt et al., 2009) but no difference after i.p administration of a low dose of 0.5 g/kg alcohol (Rose et al., 2013). In addition, previous work demonstrated differences in BAC after oral and i.p administration of 3.8 g/kg of alcohol in B6 mice (Livy et al., 2003). Furthermore studies showed that there are differences in the acetaldehyde, with higher concentrations in D2 mice, suggesting that these differences in BAC are dependent upon first-pass metabolism (Eriksson et al., 1984).
As with other effects of alcohol (sedative, ataxic, hypothermic and anxiolytic), our results showed that tolerance developed to alcohol antinociceptive effects in mice. However, it developed at similar degree in both strains after repeated administration of alcohol (Fig. 3). We would have predicted that D2 mice, with a higher sensitivity than B6 mice, would show the largest degree of tolerance within a particular dose. While it is not clear why the two strains did not differ in the degree of chronic tolerance to alcohol-induced antinociception, it is possible that pharmacokinetic (rate of ethanol absorption and/or metabolism after repeated gavage) and non- pharmacokinetic factors played an important role. Additional doses of alcohol will be necessary to more adequately investigate possible differences.
It is well established that the opioid system is involved in the activation and reinforcement of alcohol consumption. Alcohol is involved in the release of opioid endogenous ligands and also alters the synthesis and properties of its receptors (Sanchis-Segura et al., 2005). Some of these ligands contribute to pain perception and analgesia (Konig et al., 1996). We considered the possibility of strain differences in the opioid circuit being involved in the alcohol-induced antinociception differences observed between B6 and D2 mice. Alcohol-induced antinociception is reversed upon pre-treatment of the non-selective opioid antagonist naloxone in Swiss mice in the tail-flick test (Boada et al., 1981) and B6 male mice in the hot plate and tail-flick tests (Campbell et al., 2006; Neddenriep et al., 2019). We replicated these studies in both B6 and D2 mice and found similarly that alcohol effects in the hot plate test were blocked by pre-treatment with naloxone in both strains. However, since one dose of naloxone was used in our study, it cannot be known whether there were differences in sensitivity to the effect of naloxone between the strains. A dose response curve of naloxone blockade of alcohol-induced antinociception would need to be generated.
An important finding of our study is the sex differences to alcohol-induced antinociception observed in both B6 and D2 strains. Female mice were found to be much less sensitive to alcohol in the hot plate test. However, in the hot plate time course of alcohol, our results show a modest, yet significant effect of repeated testing on the hot plate, although the mean %MPE are similar to our other findings. Our results complement previous literature on sex differences reported for alcohol consumption, acute withdrawal and effects on locomotion in rodents. For example, reported data shows both the stimulatory and the depressive locomotor effects of alcohol were greater in male than female B6 mice (Laverne et al., 2011). The sex differences observed in our study were unrelated to BAC measured between male and female B6 and D2 mice, suggesting that a difference in alcohol pharmacokinetics is an unlikely explanation of sex differences in alcohol-induced antinociception. In addition, our results with ovariectomy suggest that sex differences in alcohol-induced antinociception are not related to gonadal hormones. Further studies are needed to clarify the mechanisms of sex differences observed in our results.
In conclusion, these results have determined that the antinociceptive effects of alcohol in the hot plate test are influenced by genotype and sex. Our original findings show that B6 and D2 mice differ in their sensitivity to the antinociceptive effects of alcohol. These findings indicate a potential genetic component to alcohol-induced antinociception. These findings support further examination of possible genetic factors involved in alcohol analgesic-like properties, as in recombinant inbred strains of mice such as the BXD lines derived from crosses between B6 and D2 strains.
Supplementary Material
Locomotor activity after acute oral administration of alcohol in B6 and D2 male and female mice. (A) & (B) The locomotor activity of B6 and D2 male mice, measured in number of interruptions per 30 min, following oral administration of 0.5, 1.5 and 2 g/kg. (n= 10/group). (C) & (D) The locomotor activity of B6 and D2 female mice, measured in number of interruptions per 30 min, following oral administration of 1.5 and 2 g/kg. (n= 10/group). Results were compared using one-way ANOVA with treatment as the factor separately for each strain and post-hoc Tukey’s test (*p<0.05 vs. vehicle).
Funding:
This work was funded by RO1AA027175 (NIH) to MID and MFM and P30DA033934 (NIH) to William Dewey.
References
- Belknap JK et al. (1995) ‘Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/ and DBA/2 strains’, Life Sciences, 57(10), pp. 117–124. doi: 10.1016/0024-3205(95)02040-P. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC and Young ER (1993) ‘Voluntary consumption of ethanol in 15 inbred mouse strains’, Psychopharmacology, 112(4), pp. 503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bell RL, Olson RD and Vaccarino AL (1998) ‘Tolerance to ethanol analgesia is not accompanied by cross-tolerance to morphine analgesia in rats’, Pharmacology Biochemistry and Behavior, 59(1), pp. 123–127. doi: 10.1016/S0091-3057(97)00380-8. [DOI] [PubMed] [Google Scholar]
- Boada J, Feria M and Sanx E (1981) ‘Inhibitory Effect of Naloxone On The Ethanol-Induce Antinociception in Mice’, Pharmacologica/ Research Communications, 13(7). [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE and Tizabi Y (2006) ‘Antinociceptive effects of alcohol and nicotine: Involvement of the opioid system’, Brain Research, 1097(1), pp. 71–77. doi: 10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM and Groblewski PA (2006) ‘Drug-induced conditioned place preference and aversion in mice’, Nature Protocols, 1(4), pp. 1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Egil M, Koob GF and Edwards S (2012) ‘Alcohol dependence as a chronic pain disorder’, Neurosci Biobehav, 36(10), pp. 2179–2192. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson CJP et al. (1984) ‘BLOOD AND LIVER ACETALDEHYDE CONCENTRATIONS DURING ETHANOL OXIDATION IN C57 AND DBA MICE’, 33(14), pp. 2213–2216. [DOI] [PubMed] [Google Scholar]
- Fehr C, Rademacher BLS and Buck KJ (2003) ‘Evaluation of the glutamate decarboxylase genes Gad1 and Gad2 as candidate genes for acute ethanol withdrawal severity in mice’, Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27(3), pp. 467–472.. [DOI] [PubMed] [Google Scholar]
- Gatch MB (2009) ‘Ethanol withdrawal and hyperalgesia’, Current Drug Abuse Reviews, 2(1), pp. 41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- Gatch MB and Lal H (1999a) ‘Effects of ethanol and ethanol withdrawal on nociception in rats’, Alcoholism: Clinical and Experimental Research, 23(2), pp. 328–333. doi: 10.1111/j.1530-0277.1999.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB and Lal H (1999b) ‘Effects of ethanol and ethanol withdrawal on nociception in rats’, Alcoholism: Clinical and Experimental Research, 23(2), pp. 328–333. doi: 10.1111/j.1530-0277.1999.tb04118.x. [DOI] [PubMed] [Google Scholar]
- James MFM et al. (1978) ‘Analgesic effect of ethyl alcohol’, British Journal of Anaesthesia. British Journal of Anaesthesia, 50(2), pp. 139–141. doi: 10.1093/bja/50.2.139. [DOI] [PubMed] [Google Scholar]
- Jochum T et al. (2010) ‘Increased pain sensitivity in alcohol withdrawal syndrome’, European Journal of Pain. European Federation of International Association for the Study of Pain Chapters, 14(7), pp. 713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Jung Y. chul and Namkoong K (2014) ‘Alcohol: Intoxication and poisoning - diagnosis and treatment’, in Handbook of Clinical Neurology. doi: 10.1016/B978-0-444-62619-6.00007-0. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K and Tabakoff B (1983) ‘Neurochemical correlates of tolerance and strain differences in the neurochemical effects of ethanol’, Pharmacology, Biochemistry and Behavior, 18(SUPPL. 1), pp. 383–388. doi: 10.1016/0091-3057(83)90204-6. [DOI] [PubMed] [Google Scholar]
- Konig M et al. (1996) ‘Pain response, anxiety and aggression in mice deficient in pre-proenkephalin’. Nature, 1996 Oct 10;383(6600):535–8. [DOI] [PubMed] [Google Scholar]
- Langerman L, Zakowski MI, Piskoun B, Grant GJ. (1995). Hot plate versus tail flick: evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Methods. Sep;34(1):23–7. [DOI] [PubMed] [Google Scholar]
- Melón Laverne C., Boehm Stephen L. II. (2011) ‘Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/ 2J and C57BL/6J inbred mouse strains’, Alcohol Clin Exp Res, 35(7), pp. 1351–1360. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE and West JR (2003) ‘Blood ethanol concentration profiles: A comparison between rats and mice’, Alcohol, 29(3), pp. 165–171. doi: 10.1016/S0741-8329(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Malec D, Kotlińska J and Langwiński R (1987) ‘Cross-tolerance between morphine and ethanol and their antinociceptive effects.’, Journal of Studies on Alcohol and Drugs, 48(5), pp. 507–510. [DOI] [PubMed] [Google Scholar]
- Neddenriep B et al. (2019) ‘Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain’, Neuropharmacology. Elsevier, 160(July), p. 107793. doi: 10.1016/j.neuropharm.2019.107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA and Shah P (1987) ‘Ethanol-Induced Analgesia’, Life Sciences, 41, pp. 1289–1295. doi: 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- Poklis JL, Wolf CE and Peace MR (2017) ‘Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID)’, Drug Testing and Analysis, 9(10), pp. 1637–1640. doi: 10.1002/dta.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL and King C (2009) ‘Self-Report of Alcohol Use for Pain in a Multi-Ethnic Community Sample’, Journal of Pain. Elsevier Ltd, 10(9), pp. 944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M and Rehm J (2014) ‘Cause-specific mortality risk in alcohol use disorder treatment patients: A systematic review and meta-analysis’, International Journal of Epidemiology, 43(3), pp. 906–919. doi: 10.1093/ije/dyu018. [DOI] [PubMed] [Google Scholar]
- Rose JH et al. (2013) ‘Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics’, PLoS ONE, 8(12), pp. 1–10. doi: 10.1371/journal.pone.0083852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C et al. (2005) ‘Role of the endogenous opioid system on the neuropsychopharmacological effects of ethanol: New insights about an old question’, Alcoholism: Clinical and Experimental Research, 29(8), pp. 1522–1527. doi: 10.1097/01.alc.0000174913.60384.e8. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (2014) ‘A brief history of research on the genetics of alcohol and other drug use disorders.’, Journal of studies on alcohol and drugs. Supplement, 75 Suppl 1, pp. 59–67. doi: 10.15288/jsads.2014.75.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J and Hodge CW (2008) ‘Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice’, Bone, 197(3), pp. 361–370. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakov SK et al. (2016) ‘Anxiolytic, Psychostimulant, and Analgesic Effects of Various Volumes of Ethanol Solution in Different Concentrations, but in the Same Dose’, Bulletin of Experimental Biology and Medicine, 161(1), pp. 1–3. doi: 10.1007/s10517-016-3330-5. [DOI] [PubMed] [Google Scholar]
- Tice P (2016) ‘Results from the 2015 national survey on drug use and health’, Center for Behavioral Health Statistics and Quality, p. 3263. doi: 10.1016/j.jphotobiol.2013.11.022. [DOI] [Google Scholar]
- Woodrow KM and Eltherington LG (1988) ‘Feeling no pain: Alcohol as an analgesic’, Pain, 32(2), pp. 159–163. doi: 10.1016/0304-3959(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Xiao C et al. (2007) ‘Effects of ethanol on midbrain neurons: Role of opioid receptors’, Alcoholism: Clinical and Experimental Research. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R and Taylor AN (1989) ‘Genetic differences in opiate receptor concentration and sensitivity to ethanol’s effects’, Pharmacology, Biochemistry and Behavior, 33(4), pp. 793–796. doi: 10.1016/0091-3057(89)90472-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locomotor activity after acute oral administration of alcohol in B6 and D2 male and female mice. (A) & (B) The locomotor activity of B6 and D2 male mice, measured in number of interruptions per 30 min, following oral administration of 0.5, 1.5 and 2 g/kg. (n= 10/group). (C) & (D) The locomotor activity of B6 and D2 female mice, measured in number of interruptions per 30 min, following oral administration of 1.5 and 2 g/kg. (n= 10/group). Results were compared using one-way ANOVA with treatment as the factor separately for each strain and post-hoc Tukey’s test (*p<0.05 vs. vehicle).


