Abstract
SARS-CoV-2 infection and its clinical manifestations (COVID-19) quickly evolved to a pandemic and a global public health emergency. The limited effectivity of available treatments aimed at reducing virus replication and the lessons learned from other coronavirus infections (SARS-CoV-1 or NL63) that share the internalization process of SARS-CoV-2, led us to revisit the COVID-19 pathogenesis and potential treatments.
Virus protein S binds to the angiotensin-converting enzyme 2 (ACE2) initiating the internalization process. Endosome formation removes ACE2 from the cellular membrane preventing its counter-regulative effect mediated by the metabolism of angiotensin II to angiotensin (1–7). Internalized virus-ACE2 complexes have been identified for these coronaviruses. SARS-CoV-2 presents the highest affinity for ACE2 and produces the most severe symptoms. Assuming ACE2 internalization is the trigger for COVID-19 pathogenesis, accumulation of angiotensin II can be viewed as the potential cause of symptoms. Angiotensin II is a strong vasoconstrictor, but has also important roles in hypertrophy, inflammation, remodeling, and apoptosis. Higher levels of ACE2 in the lungs explain the acute respiratory distress syndrome as primary symptoms. Most of the described findings and clinical manifestations of COVID-19, including increased interleukin levels, endothelial inflammation, hypercoagulability, myocarditis, dysgeusia, inflammatory neuropathies, epileptic seizures and memory disorders can be explained by excessive angiotensin II levels.
Several meta-analyses have demonstrated that previous use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were associated with better prognosis for COVID-19. Therefore, pragmatic trials to assess the potential therapeutic benefits of renin-angiotensin-aldosterone system inhibitors should be urgently promoted by health authorities to widen the therapeutic options for COVID-19.
Keywords: ACE2, SARS-CoV-2, COVID-19, Angiotensin II, AT1 antagonists, Telmisartan
Resumo
A infeção por SARS-CoV-2 e as suas manifestações clínicas (COVID-19) evoluíram rapidamente para uma pandemia e um problema de saúde pública global. A efetividade limitada dos tratamentos existentes orientados apenas para a redução da replicação do vírus e as lições aprendidas com outros coronavírus que utilizam o mesmo recetor de internalização levaram-nos a revisitar a patogénese da Covid-19 e potenciais tratamentos.
A proteína S do vírus liga-se à enzima conversora de angiotensina de tipo 2 (ECA2) iniciando o processo de internalização. A afinidade do SARS-CoV-2 para a ECA2 é superior à dos outros coronavirus, o que se correlaciona com a gravidade dos sintomas. A ECA2 acompanha o vírus no endossoma, tendo sido identificados complexos vírus-ECA2 internalizados dos três coronavírus referidos. A ausência da ECA2 na membrana celular compromete o metabolismo da angiotensina II em angiotensina (1-7), pelo que a acumulação de angiotensina II pode ser vista como a causa potencial dos sintomas. A angiotensina II é um potente vasoconstritor mas também desempenha papéis importantes em processos fisiopatológicos como a inflamação, fibrose, hipertrofia e apoptose. Níveis mais elevados de angiotensina II nos pulmões explicariam a síndrome de insuficiência respiratória aguda como síndrome primária. A maioria das alterações de parâmetros e outras manifestações clínicas, tais como o aumento dos níveis de interleucinas, a inflamação endotelial, a hipercoagulabilidade, a miocardite, a disgeusia, as neuropatias inflamatórias, as convulsões epiléticas e os distúrbios da memória, pode ser explicada por níveis excessivos de angiotensina II.
Várias meta-análises demonstraram que o uso prévio de inibidores da enzima conversora da angiotensina (IECAs) ou antagonistas dos recetores da angiotensina (ARAs) está associado a um melhor prognóstico para a Covid-19. Deste modo, ensaios pragmáticos para avaliar os potenciais benefícios terapêuticos dos inibidores do sistema renina-angiotensina-aldosterona (SRAA) deveriam ser promovidos urgentemente pelas autoridades de saúde para se diversificar as opções terapêuticas para a Covid-19.
Palavras-chave: ECA2, SARS-CoV-2, Covid-19, Angiotensina II, Antagonistas AT1, Telmisartan
Introduction
The potential relationship between the renin-angiotensin-aldosterone system (RAAS), SARS-CoV-2 infection and its clinical manifestation (COVID-19) have been the subject of controversy since the inception of the pandemic. A letter published in the Lancet Respiratory Medicine in March 2020 suggested that patients under treatment with angiotensin II AT1 receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs) would present an increased risk of severe or fatal COVID-19.1 The alarm raised by this commentary among the population using the two most prevalent therapeutic classes for the treatment of hypertension, provoked an extraordinary effort from researchers, health authorities and pharmaceutical industry to restore confidence in these therapeutic classes by gathering clinical evidence on their safety. To date, more than 60 meta-analyses assessing the association of RAAS inhibitors, and several COVID-19 outcomes have been published. These meta-analyses reported no association with increased risk of any of the evaluated outcomes, including risk of infection, progression to severe prognostic, hospitalization, intensive care unit admission, and mortality.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 Four of the 23 meta-analyses presenting estimates of association with progression to severe status of COVID-19 reported a preventive effect of prior exposition to ACEIs/ARBs (Table 1 ). And, more importantly, 16 of the 41 meta-analyses reporting the effect of prior ACEIs/ARBs use on mortality demonstrated a positive effect of these RAAS inhibitors.33 The evidence appears to support the urgent position statement released by the European Society of Cardiology (ESC) as a response to the commentary of Fang et al., indicating that there was no scientific evidence supporting the discontinuation of antihypertensive medication with ARBs or ACEIs in patients with COVID-19.45 Moreover, a recent cohort study further showed that withdrawal of RAAS inhibitors at hospital admission increased acute respiratory distress syndrome (ARDS)/acute lung injury (adjusted odds ratio (OR) 4.54, 95% confidence interval (CI) 1.53–13.44) in patients with COVID-19.46 The initial unfounded alarm may have prevented researchers and healthcare administrations from further exploring the challenge outlined in the ESC position statement about the potential benefits of these drugs on patients with COVID-19.
Table 1.
Association of ACEI/ARB use and the severity or mortality of COVID-19 as reported in meta-analysis published until 31-jan-2021.
| Author | Online publication | Search update | Severe disease | Mortality |
|---|---|---|---|---|
| Zhang X2 | 15-05-20 | 09-05-20 | OR=0.98 [95% CI 0.87: 1.09] | OR=0.73 [95% CI 0.5: 1.07] |
| Guo X3 | 27-05-20 | 13-05-20 | OR=0.71 [95% CI 0.46: 1.08] | OR=0.57 [95% CI 0.38: 0.84] |
| Usman MS4 | 02-06-20 | 01-05-20 | OR=0.74 [95% CI 0.34: 1.58] | |
| Gao C5 | 04-06-20 | 25-04-20 | RR=0.65 [95% CI 0.45: 0.94] | |
| Grover A6 | 15-06-20 | 01-05-20 | OR=0.81 [95% CI: 0.41–1.58] | |
| Pranata R7 | 27-06-20 | 14-06-20 | OR=0.73 [95% CI 0.38: 1.40] | |
| Flacco ME8 | 01-07-20 | 11-05-20 | OR=0.88 [95% CI 0.68: 1.14] | |
| Greco A9 | 16-07-20 | 07-06-20 | OR=0.88 [95% CI 0.60: 1.31] | OR=0.95 [95% CI 0.57: 1.58] |
| Liu X10 | 05-08-20 | 01-05-20 | OR=0.75 [95% CI: 0.59–0.96] | OR=0.52 [95% CI: 0.35–0.79] |
| Megaly M11 | 17-08-20 | 01-05-20 | OR=0.75 [95% CI 0.36: 1.57] | |
| Baral R12 | 24-08-20 | 17-05-20 | OR=0.664 [95% CI 0.458: 0.964] | |
| Caldeira D13 | 27-08-20 | 08-06-20 | OR=0.90 [95% CI 0.74: 1.11] | OR=0.91 [95% CI 0.74: 1.11] |
| de Almeida-Pititto B14 | 31-08-20 | 06-05-20 | OR=0.76 [95% CI 0.39: 1.49] | |
| Patoulias D15 | 10-09-20 | 19-05-20 | OR=0.86 [95% CI 0.64: 1.16] | OR=1.06 [95% CI 0.63: 1.43] |
| Hasan SS16 | 12-09-20 | 31-08-20 | OR=0.91 [95% CI 0.75: 1.10] | OR=0.73 [95% CI 0.56–0.95] |
| Lo KB17 | 05-10-20 | 30-06-20 | OR=0.94 [95% CI 0.59: 1.50] | OR=1.29 [95% CI 0.89: 1.87] |
| Kurdi A18 | 20-10-20 | 22-05-20 | OR=0.78 [95% CI 0.53: 1.15] | OR=0.97 [95% CI 0.75: 1.27] |
| Xu J19 | 20-10-20 | 18-07-20 | OR=0.95 [95% CI 0.73: 1.24] | OR=0.87 [95% CI 0.66: 1.04] |
| Kerneis M20 | 22-10-20 | 01-07-20 | OR=1.00 [95% CI 0.69: 1.45] | |
| Wang Y21 | 23-10-20 | 12-10-20 | OR=0.624 [95% CI 0.457: 0.852] | |
| Ren L22 | 02-11-20 | 13-07-20 | OR=0.81 [95% CI 0.66: 0.99] | OR=0.77 [95% CI 0.66: 0.91] |
| Ssentongo AE23 | 05-11-20 | 01-09-20 | RR=0.65 [95% CI 0.45: 0.94] | |
| Kashour T24 | 10-11-20 | 18-06-20 | RR=0.63 [95% CI 0.42: 0.94] | |
| Chu C25 | 20-11-20 | 07-09-20 | OR=0.76 [95% CI 0.59: 0.99] | |
| Zhang G26 | 24-11-20 | 30-10-20 | OR=0.65 [95% CI 0.46: 0.85] | |
| Cai XJ27 | 30-11-20 | 30-05-20 | OR=1.06 [95% CI 0.75: 1.50] | |
| Lee MMY28 | 18-12-20 | 12-08-20 | OR=0.75 [95% CI 0.61: 0.92] | |
| Lee HW29 | 27-01-21 | 04-05-20 | OR=0.68 [95% CI 0.44: 1.07] | OR=0.52 [95% CI 0.37: 0.72] |
| Alamer AA30 | 28-Jan-21 | 4-Jul-20 | OR=0.86 [95% CI 0.68: 1.08] | |
| Laurentius A31 | 5-Feb-21 | May-20 | HR=0.55 [95% CI 0.34: 0.90] | |
| Hassib M32 | 4-Feb-21 | 09-Jun-20 | OR=1.30 [95% CI 0.87: 1.94] | OR=0.99 [95% CI 0.48: 2.04] |
| Baral R33 | 1-Mar-21 | 1-Sep-20 | OR=0.68 [95% CI 0.53: 0.88] | OR=0.57 [95% CI 0.43: 0.76] |
| Biswas M34 | 3-Mar-21 | 31-May-20 | RR=1.29 [95% CI 0.81: 2.04] | RR=0.89 [95% CI 0.64: 1.23] |
| Lee T35 | 6-Apr-21 | 19-Aug-20 | OR=0.87 [95% CI 0.71: 1.08] | |
| Ma Z36 | 22-Apr-21 | 15-Aug-20 | OR=0.90 [95% CI 0.55: 1.47] | OR=1.43 [95% CI 0.97: 2.10] |
| Dai XC37 | 26-Apr-21 | 20-Jun-20 | OR=0.95 [95% CI 0.88: 1.02] | OR=0.87 [95% CI 0.75: 1.00] |
| Aparisi A38 | 6-May-21 | Nov-20 | OR=0.60 [95% CI 0.42: 0.84] | |
| Kaur U39 | 14-May-21 | 9-Jul-20 | OR=1.08 [95% CI 0.79: 1.46] | OR=0.91 [95% CI 0.65: 1.26] |
| Kow CS40 | 20-May-21 | 14-Mar-21 | OR=0.84 [95% CI 0.47: 1.50] | |
| Sattar Y41 | 28-Jun-21 | 14-Nov-20 | OR=1.04 [95% CI 0.97: 1.11] | OR=1.07 [95% CI 0.99: 1.15] |
| Asiimwe IG42 | 7-Jul-21 | 1-Nov-20 | OR=1.05 [95% CI 0.81: 1.38] | OR=0.93 [95% CI 0.70: 1.24] |
| Jia N43 | 28-Jul-21 | 21-Jun-20 | OR=1.07 [95% CI 0.76: 1.50] | |
| Fernando ME44 | 7-Sep-21 | 19-Jun-20 | RR=1.23 [95% CI 1.06: 1.42] | RR=1.18 [95% CI 0.92: 1.50] |
Bold represents protective effect of ACEI/ARB use. Italics represent harmful effect of ACEI/ARB use. CI: confidence interval; HR: hazard ratio; OR: odds ratio; RR: relative risk.
The aim of this review was to integrate knowledge gathered on the clinical manifestations of COVID-19 with previous knowledge of the pathophysiology of the coronavirus infection process. In addition, to consider the lessons learned from the failure of theoretically promising treatments such as hydroxychloroquine or remdesivir, and the substantial scientific evidence that is emerging in the literature. This knowledge integration has to be done by starting with a more detailed insight into the infection mechanism at a cellular level, which should serve as foundations for the search for more efficacious pharmacological treatments for COVID-19 or other diseases produced by viruses with a similar internalization system.
During the first two years of COVID-19, many pharmacologic strategies were focused on the disease as a direct consequence of the virus replication and paid less attention to the consequences of angiotensin-converting enzyme 2 (ACE2) internalization, the SARS-CoV-2 receptor. The loss of ACE2 function at the membrane may not only be the origin of many of COVID-19 symptoms, but may also reduce the capacity of COVID-19 vaccines to provide long-term protection.
Angiotensin-converting enzyme: not just a receptor
Angiotensin-converting enzyme has been identified as the receptor for SARS-CoV-2 internalization.47, 48 This role of ACE2 is not exclusive to the SARS-CoV-2 virus, as it also functions as a receptor for other coronaviruses such as SARS-CoV-1 or NL63.49, 50 The affinity of coronaviruses for ACE2, which reflects the amount of virus needed to mobilize the same amount of ACE2, is different among these three viruses. SARS-CoV-1 has higher affinity than NL63 but lower than SARS-CoV-2.50, 51 This affinity ranking for ACE2 correlates with the severity of the clinical manifestations of the viruses and their transmission capacity. An infection with NL63 only produces severe manifestations in immunocompromised patients. However, infection by SARS-CoV-1 caused an epidemic responsible for about 800 deaths, whereas SARS-CoV-2 is responsible for a pandemic with more than five million deaths to date.52
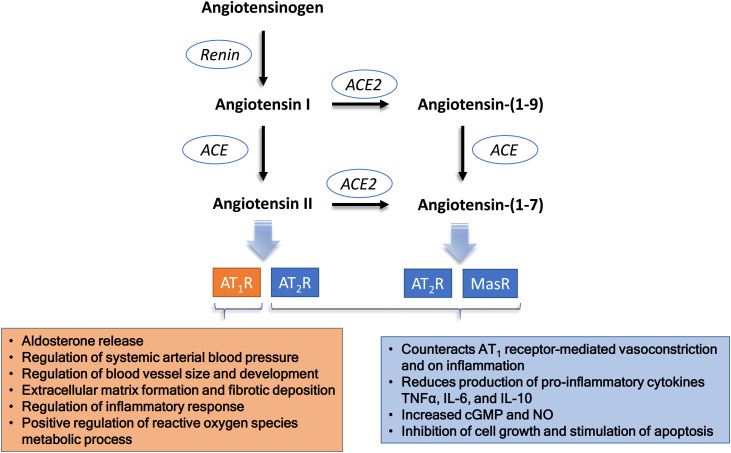
The role of ACE2 as a receptor for virus internalization is accessory to its physiological functions. The main physiological role of ACE2 is related to the regulation of the RAAS, preventing the excess of angiotensin II.53 ACE2 exerts this counter-regulative effect in two different ways: (i) through the metabolization of angiotensin I into angiotensin (1–9), which removes substrate from the ACE1 pathway, with a consequent reduction of angiotensin II production; and (ii) through the metabolization of angiotensin II into angiotensin (1–7) (Figure 1 ). Consequently, ACE2 presents two simultaneous and synergic effects: reduced angiotensin II and increased angiotensin (1–7), which presents opposite effects to those mediated by angiotensin II.54 The activity of ACE2 to metabolize angiotensin II is about 400 times higher than its activity to metabolize angiotensin I.55 This means that the pathway that converts angiotensin II into angiotensin (1–7) is more relevant as a counter-regulative mechanism than the metabolism of angiotensin I into angiotensin (1–9). Regardless of the relative importance of the two metabolic pathways, the loss of function of ACE2 results in increased levels of angiotensin II as a consequence of the inhibition of both pathways (Figure 2 ).
Figure 1.
The main players of the renin-angiotensin system that may be disturbed in COVID-19. Schematic representation of the steps that may be relevant in the lungs, the major organ for the conversion of plasma angiotensin I into angiotensin II. Although ACE2 may also participate in the metabolism of des-(Arg)-bradykinin, its lower concentration in the lungs and the lower affinity to ACE2 makes its putative contribution less relevant.
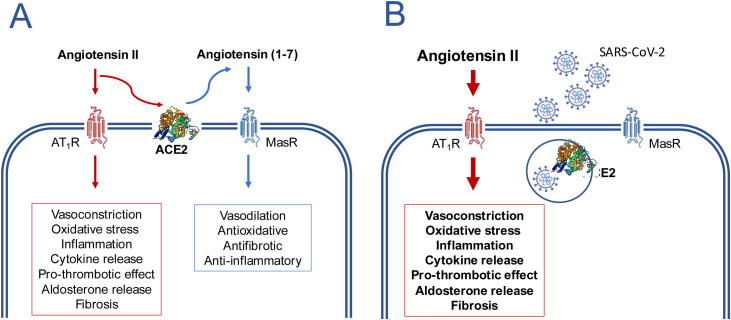
Figure 2.
Disturbance of the balance angiotensin II/angiotensin (1–7) caused by the SARS-CoV-2 virus as a potential mechanism for inducing the clinical manifestations of COVID-19.
In the absence of the virus (A) the AT1-receptor (AT1R) mediated effects of angiotensin II are counter balanced by the effects of angiotensin (1–7) formed by the action of ACE2. In the presence of SARS-CoV-2 (B), ACE2 is internalized along with virus and its absence from the cytoplasmic membrane causes a loss of its metabolic function, the consequent accumulation of angiotensin II and an exacerbation of the AT1R mediated effects. This imbalance occurs more markedly in the lungs, the major organ where plasma angiotensin II is formed. The formed angiotensin II will be released into the circulation and may cause a systemic inflammatory response even if there has been no systemic exposure to the virus.
The mechanisms of interaction between SARS-CoV-2 and ACE2 are known. It depends on the binding of the protein S of the viral capsule and ACE2. The affinity of protein S for ACE2 is increased by the action of the serine protease TMPRSS2, which cleaves protein S and exposes a residue with greater affinity for ACE2.56 This mechanism is similar to that previously described for SARS-CoV-1 and seems to be crucial for the viral tropism and pathogenesis.57 The similarities in the interaction and internalization processes between SARS-CoV-2 and SARS-CoV-1 or NL63 enable us to use studies conducted over many years with these two coronaviruses to understand better the sequence of events after the virus binds to ACE2.
The formation of the complex virus-ACE2 triggers an internalization process.58 This process does not only facilitate the diffusion of the virus across the cellular membrane but also encompasses a more complex five-step mechanism: (i) the development of an endocytic vesicle, (ii) followed by the creation of an endosome, (iii) its transport by interacting with the microtubule network, (iv) the placement of the endosome in the cytoplasmic compartment, and (v) the release of the endosomal content. During this process the endosome content is exposed to lysosomal enzymes (creating endolysosome), which metabolize the viral proteins in a process that requires endosome acidification. The fate of ACE2 is unknown. It can be spared from degradation and return to the membrane to resume its physiological functions or it can be degraded and the physiological functions can only be restored by de novo synthesized proteins. The internalization of ACE2/virus complexes has been demonstrated for NL63, SARS-CoV-1 and more recently for SARS-CoV-2.58, 59, 60, 61
Temporary ACE2 loss from the cellular membrane caused by the coronavirus internalization process will disrupt angiotensin II metabolism. In SARS-CoV-1, ACE2 “absence” from the membrane can occur for several hours.58 It is thought that the time ACE2 stays inside the cell is associated with the affinity of different coronaviruses for ACE2. This suggests that the ACE2/SARS-CoV-2 complex can remain inside the cell longer than the ACE2/SARS-CoV-1 complex and, therefore, produce more severe clinical manifestations with similar viral loads. Clinical manifestations of SARS-CoV-2 infection vary depending on the viral load affecting the different organs.
Angiotensin II can be produced almost ubiquitously, but lungs are the main organ where the conversion of angiotensin I into angiotensin II occurs.62 Therefore, lungs are the organ that suffers more drastic consequences of the removal of ACE2 from the cellular membrane. Symptom variability in infected individuals (which varies from the absence of symptoms to acute respiratory failure) could be associated with the viral load that reaches the lungs and hampers their ability to manage an excess of angiotensin II. Therefore, an alternative pathogenic model for COVID-19 is proposed in which the severity of clinical manifestations may be more dependent on the accumulation of angiotensin II than on overall viral load.
Unsuccessful therapeutic attempts
Since the emergence of COVID-19, several drugs have been proposed to treat the condition and reduce the replication of the SARS-CoV-2 in infected patients. Hydroxychloroquine was one of these drugs, supported by the rationale that the clinical manifestations of COVID-19 were mainly dependent on the viral load and, therefore, the inhibition of virus replication would reduce these clinical manifestations. Similar to chloroquine, hydroxychloroquine is part of the lysosomotropic group of drugs.63 These drugs present high affinity for the acidified endolysosome, inhibiting the action of viral protein metabolizing enzymes increasing the viral retention within the endolysosome. The expected outcome of hydroxychloroquine was to block the access of the virus to the cell replication machinery. This hypothesis was originally confirmed with hydroxychloroquine inhibiting SARS-CoV-2 replication in vitro.64 Later clinical trials showed that hydroxychloroquine was not effective at improving clinical manifestations of COVID-19 in outpatient patients or after hospital admission.65, 66
Remdesivir is an inhibitor of RNA-dependent RNA polymerase (RdRp), one of the proteins of the SARS-CoV-2 viral replication complex.67 The antiviral activity of remdesivir was explored in recent viral outbreaks (Ebola, MERS-CoV and SARS-CoV-2).68, 69, 70 The efficacy of remdesivir in inhibiting SARS-CoV-2 replication was demonstrated in cell culture in vitro assays, showing that remdesivir was as potent as lopinavir in inhibiting SARS-CoV-2 replication, but more potent than flavipiravir, galidesivir, ritonavir, oseltamivir, and baloxivir.71 Lopinavir failed to demonstrate clinical benefits in COVID-19.72 Despite the promising in vitro results, remdesivir showed only marginal clinical benefits in patients with COVID-19.73, 74, 75, 76 A living systematic review first performed in August 2020 by the American College of Physicians reported significant improvements in recovery rate and mortality, but not in hospital length of stay. While the review update performed in December 2020 reported no reduction in mortality but a small reduction in the need for mechanical ventilation.77, 78 Poor correlation between in vitro antiviral activity against SARS-CoV-2 and clinical efficacy in COVID-19 patients was also observed for other antiviral drugs.79
The dissociation between antiviral effects on SARS-CoV-2 and clinical effects on COVID-19 was also confirmed by the studies aiming to repurpose other drugs as COVID-19 therapeutic options, such as in the case of ivermectin. Ivermectin inhibited the replication of SARS-CoV-2 with concentrations needed to inhibit replication (IC50) in the micromolar range (between 2.2 and 2.8 μM).80 These concentrations are much higher than the peak plasmatic levels that can be achieved with the doses used in the clinical study that reported clinical effect of ivermectin in hospitalized patients with COVID-19.81
More recently, the UK and U.S. drug regulatory agencies issued emergency use authorizations for molnupiravir and nirmatrelvir/ritonavir, two antiviral drugs with the advantage of having oral bioavailability. However, the real therapeutic potential of these drugs is far from being defined. Molnupiravir is an RdRp inhibitor, developed as anti-influenza drug,82 shown to markedly reduce replication of SARS-CoV-2 variants in different experimental models.83, 84, 85, 86 Still, available evidence of molnupiravir's clinical benefits is limited. In the clinical trial available to date (a phase 3 clinical study sponsored by the proprietary company), molnupiravir reduced the need of hospitalization and deaths to 6.8% compared with 9.7% in the placebo group. This produced a treatment difference CI very close to the null effect (no differences).87 Additionally, molnupiravir showed no difference from placebo at WHO Clinical Progression Scale at day 29 (OR=1.04; 95% CI 0.84, 1.29), no difference in incidence of hospitalization or death at day 29 for the majority of the subgroups, no difference in time to sustained improvement or resolution of self-reported COVID-19 for the majority of the signs/symptoms, and also no difference in the time to progression of any self-reported COVID-19 sign/symptom through day 29.87 A co-packaged association of nirmatrelvir/ritonavir (Paxlovid™) is the newest antiviral candidate against SARS-CoV-2. Nirmatrelvir is a 3C-like protease (3CLpro) inhibitor that undergoes a marked first-pass metabolism by CYP450, the reason why the company decided to associate ritonavir.88 The company is claiming in a press release that this association is 89% effective at reducing the need of hospitalization and number of deaths89 but this information was not independently validated nor has it been reproduced. However, some doubts remain about the mechanisms of action involved. Nirmatrelvir was shown to inhibit 3CLpro in concentrations much lower than those reached during its clinical use, raising the possibility that nirmatrelvir may also be acting in host proteases that participate in viral infection, similar to other virus protease inhibitors.90, 91 Additionally, ritonavir may also contribute to the therapeutic effect not only by inhibiting CYP450 enzymes but also by inhibiting TMPRSS2,92 a host protease crucial for SARS-CoV-2 infection.56 Whatever the clinical benefits future studies may confirm for these drugs in COVID-19, the results obtained so far indicate that any potential therapeutic benefit in reducing clinical manifestations of COVID-19 requires their administration in a narrow time frame, at the very beginning of the infection course.
The fact that these drugs that have proved to inhibit SARS-CoV-2 in vitro but were not able to prevent all clinical manifestations of COVID-19 suggests that pathogenesis of the disease may not have been properly established.
Angiotensin II as a suspect
The hypothesis of angiotensin II being a determinant for the clinical manifestations of SARS-CoV-2 infection is supported by three lines of evidence: (i) the limited efficacy of available viral replication inhibitors; (ii) the experience gathered with severe acute respiratory syndrome (SARS) and (iii) the similarities between the clinical manifestations of COVID-19 and those expected from the exposure to increased levels of angiotensin II.
SARS was the clinical manifestation of an epidemic caused by the SARS-CoV-1 that mainly affected China, Singapore and Canada during 2002 and 2003.93 Several studies were conducted during the SARS emergency to clarify its pathogenesis, and the role of ACE2 in the internalization of SARS-CoV-1 and its subsequent involvement in the origin of the acute respiratory insufficiency. At that time, the potential role of RAAS inhibition was already being proposed as a possibility for treating/preventing SARS.94 The end of the SARS epidemic precluded further studies to confirm this hypothesis.
COVID-19 is a complex syndrome with multiple clinical manifestations coincident with that expected in response to exposure to angiotensin II excess.
Acute respiratory distress syndrome is probably the most severe of the COVID-19 clinical manifestations. Patients with ARDS suffer from hypoxia caused by alterations in pulmonary perfusion with hypoxia associated with pulmonary vasoconstriction and endothelial inflammation, both compatible with RAAS deregulation.95, 96, 97 These effects are in line with the well-known vasoconstrictive effect of angiotensin II on pulmonary circulation but also with the recognized pulmonary pro-inflammatory effects resulting from a dysregulation of the balance between angiotensin II and ACE2 function.98, 99, 100
Increased angiotensin II production in the lungs can affect organs situated downstream in the circulatory system, in particular the heart. The inflammatory effects of angiotensin II, and not the viral load, could be the origin of the myocarditis described in COVID-19 patients.101, 102, 103 This hypothesis is reinforced by the fact that in fatal cardiac COVID-19 cases, no evidence was found of the presence of viruses in the heart.104
Coagulation changes are also among the main causes of COVID-19-associated morbidity and mortality.105 Hypercoagulation is also compatible with RAAS over-activation as angiotensin II can stimulate coagulation either by increasing the expression of coagulation factors or by stimulating platelet aggregation.106, 107, 108
The most severe clinical manifestations of COVID-19 are accompanied by changes in cytokine levels, particularly IL-6, IL-8 and TNF-alpha, which were compared to cytokine storm.109 These interleukins are considered inducers of the inflammatory response observed in COVID-19. IL-6 has been given special emphasis not only for its potential role in inducing inflammation, but also for its ability to cause a lymphopenia that precludes an effective immunologic response to the virus.110, 111 Increased levels of interleukins have steered the use of anti-cytokine treatments, mainly IL-6 antagonists, to reduce the effects of the cytokine storm associated to severe COVID-19.112 However, it might be important to bear in mind that angiotensin II can induce the release of any of these interleukins and that the clinical use of angiotensin receptor blockers (ARBs) reduces plasma levels of IL-6.10, 113
The coincidence between the clinical manifestations of COVID-19 and the expected effects of angiotensin II – aldosterone over-activation can also be found in other manifestations like dysgeusia, the risk of inflammatory neuropathies, epileptic seizures and memory disorders.114, 115, 116, 117, 118, 119, 120, 121 Even less frequent COVID-19 manifestations such as Kawasaki-like syndrome may also be related to angiotensin II; one of the genes known to be altered in patients with Kawasaki syndrome is that of ACE.122 Exposure to SARS-CoV-2 in individuals who have this ACE polymorphism can create a combination of effects that increase angiotensin II levels, a higher conversion rate of angiotensin I into angiotensin II, and a lower metabolism of angiotensin II.
The cumulative knowledge gathered from these mechanistic bases for all the clinical manifestations of COVID-19 points to a more than plausible influence of RAAS dysregulation, with an excessive activation of AT1 receptors, as the main pathogenic mechanism of this syndrome. Additionally, the observation that COVID-19 patients present higher levels of plasma angiotensin II confirmed that the physiological mechanisms to control angiotensin II levels are impaired during the SARS-CoV-2 infection.123
The challenge of clinical translation
The initial unfounded alert about the risk of using RAAS inhibitors was more than sufficiently rejected by the evidence produced in the meta-analyses shown in Table 1. Primary studies included in these meta-analyses evaluated the use of ACEI/ARBs prior to the SARS-CoV-2 infection, and the meta-analyses aimed to assess the potentially increased risks of their use in COVID-19 patients, and not their use as therapeutic agents in this condition. However, the results of several of these meta-analyses demonstrated a protective effect of the previous use of RAAS inhibitors, even used at antihypertensive doses, in COVID-19 clinical manifestations.
Several clinical studies are now registered in ClinicalTrials.org aiming to evaluate the hypothesis that RAAS inhibition can be efficacious in reducing inflammatory markers and clinical manifestations of COVID-19. Few of these trials [e.g., NCT04606563; NCT04355936; NCT04335786] aim to evaluate the effect of higher doses than those used as antihypertensives and include only patients after hospital admission. NCT04355936 included 158 patients (78 in the telmisartan group and 80 in the control group), admitted into hospital in less than five days after the onset of symptoms and with no need for intensive care admission, before randomization. The results showed that the administration of telmisartan 160 mg/day achieved a significant reduction in C-reactive protein both at day five and day eight. More importantly, it caused a significant reduction in hospital length of stay (from 15 days in control group to nine days in the telmisartan group) and reduced mortality at day 30 (4.3% in telmisartan group and 22.5% in control group).124
Although these preliminary results help to confirm the hypothesis that therapeutic RAAS inhibition may produce significant benefits in COVID-19 patients, additional efforts need to be made. An early initiation of ARB treatment, soon after confirmation of infection and even in the absence of symptoms, could reduce the consequences of excessive angiotensin II exposure and avoid not only hospitalizations, but also mild symptoms when future the consequences are not yet foreseen.
There is no doubt that randomized controlled trials and, ultimately, systematic reviews are the milestones that should guide evidence-based medical practice, including the use of RAAS inhibitors in COVID-19. However, the global emergency resulting from the successive waves of SARS-CoV-2 infections, together with the emergence of virus variants with uncertain effects on vaccine effectiveness, might remind regulatory bodies to consider more pragmatic study designs to evaluate pharmacological alternatives, such as RAAS inhibitors. These drugs, now at low cost after the patent expired, present vast clinical experience that confirms their safety profile. However, the patent expired status reduces pharmaceutical industry's interest in funding complex and expensive studies commonly used to evaluate new indications for old drugs.
A shortsighted vision of the concept of evidence-based medicine during a global emergency like the COVID-19 pandemic may delay the implementation of solutions and cost many lives. Evidence is much more than introducing data into a computer and obtaining pooled effect sizes. In fact, this simplistic concept led to an enormous proportion of useless articles reporting irrelevant and poor meta-analyses.125 The original papers in the genesis of the evidence-based medicine concept recognized that the “clinically relevant research” that supports evidence, often comes from the “basic sciences of medicine”.126 In global emergency situations, it is important to recall the third principle of evidence: “evidence never determines decisions; it is always in the context of values and preferences”.127 This might be the reason why the World Health Organization, when promoting evidence-informed policy-making, affirms that “if no evidence is found, the alternative measures available may require consideration”.128
In summary, the current knowledge on the mechanism of SARS-CoV-2 infection, the marginal benefits of treatments with drugs that inhibit viral replication, the similarities between the clinical manifestations of COVID-19 and the effects of excessive angiotensin II exposure and the clinical evidence that drugs that inhibit RAAS improve the clinical manifestations of COVID-19 are solid scientific arguments that support the hypothesis that RAAS inhibitor drugs can have an effect in preventing clinical manifestations of COVID-19 if administered at early stages of the infection with SARS-CoV-2. The enormous clinical experience obtained after years of using RAAS inhibitors gives regulatory bodies the opportunity to initiate pragmatic trials aiming to test the potential benefits of these drugs in minimizing the clinical manifestations of SAR-CoV-2 infection, supported not only by mechanistic considerations but also by meta-analyses of observational studies.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X., Yu J., Pan L.Y., et al. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo X., Zhu Y., Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020;76:e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572. [DOI] [PubMed] [Google Scholar]
- 4.Usman M.S., Siddiqi T.J., Khan M.S., et al. A meta-analysis of the relationship between renin-angiotensin-aldosterone system inhibitors and COVID-19. Am J Cardiol. 2020;130:159–161. doi: 10.1016/j.amjcard.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao C., Cai Y., Zhang K., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover A., Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2021;7:148–157. doi: 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pranata R., Permana H., Huang I., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flacco M.E., Acuti Martellucci C., Bravi F., et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020;106:1519–1524. doi: 10.1136/heartjnl-2020-317336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco A., Buccheri S., D’Arrigo P., et al. Outcomes of renin-angiotensin-aldosterone system blockers in patients with COVID-19: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020;6:335–337. doi: 10.1093/ehjcvp/pvaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Long C., Xiong Q., et al. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID-19, inflammation level, severity, and death in patients with COVID-19: a rapid systematic review and meta-analysis. Clin Cardiol. 2020 doi: 10.1016/10.1002/clc.23421. [Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megaly M., Glogoza M. Renin-angiotensin system antagonists are associated with lower mortality in hypertensive patients with COVID-19. Scott Med J. 2020;65:123–126. doi: 10.1177/0036933020949219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baral R., White M., Vassiliou V.S. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22:61. doi: 10.1007/s11883-020-00880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldeira D., Alves M., Gouveia E.M.R., et al. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and the risk of COVID-19 infection or severe disease: systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2020;31:100627. doi: 10.1016/j.ijcha.2020.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patoulias D., Katsimardou A., Stavropoulos K., et al. Renin-angiotensin system inhibitors and COVID-19: a systematic review and meta-analysis. Evidence for significant geographical disparities. Curr Hypertens Rep. 2020;22:90. doi: 10.1007/s11906-020-01101-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan S.S., Kow C.S., Hadi M.A., et al. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20:571–590. doi: 10.1007/s40256-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo K.B., Bhargav R., Salacup G., et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID-19: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2020;18:919–930. doi: 10.1080/14779072.2020.1826308. [DOI] [PubMed] [Google Scholar]
- 18.Kurdi A., Abutheraa N., Akil L., et al. A systematic review and meta-analysis of the use of renin-angiotensin system drugs and COVID-19 clinical outcomes: what is the evidence so far? Pharmacol Res Perspect. 2020;8:e00666. doi: 10.1002/prp2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Teng Y., Shang L., et al. The effect of prior ACEI/ARB treatment on COVID-19 susceptibility and outcome: a systematic review and meta-analysis. Clin Infect Dis. 2021;72:e901–e913. doi: 10.1093/cid/ciaa1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerneis M., Ferrante A., Guedeney P., et al. Severe acute respiratory syndrome coronavirus 2 and renin-angiotensin system blockers: a review and pooled analysis. Arch Cardiovasc Dis. 2020;113:797–810. doi: 10.1016/j.acvd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Chen B., Li Y., et al. The use of renin-angiotensin-aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2021;93:1370–1377. doi: 10.1002/jmv.26625. [DOI] [PubMed] [Google Scholar]
- 22.Ren L., Yu S., Xu W., et al. Lack of association of antihypertensive drugs with the risk and severity of COVID-19: a meta-analysis. J Cardiol. 2021;77:482–491. doi: 10.1016/j.jjcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ssentongo A.E., Ssentongo P., Heilbrunn E.S., et al. Renin-angiotensin-aldosterone system inhibitors and the risk of mortality in patients with hypertension hospitalised for COVID-19: systematic review and meta-analysis. Open Heart. 2020;7:e001353. doi: 10.1136/openhrt-2020-001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashour T., Bin Abdulhak A.A., Tlayjeh H., et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and mortality among COVID-19 patients: a systematic review and meta-analysis. Am J Ther. 2020 doi: 10.1097/MJT.0000000000001281. [Ahead of Print] [DOI] [PubMed] [Google Scholar]
- 25.Chu C., Zeng S., Hasan A.A., et al. Comparison of infection risks and clinical outcomes in patients with and without SARS-CoV-2 lung infection under renin-angiotensin-aldosterone system blockade: systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:2475–2492. doi: 10.1111/bcp.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang G., Wu Y., Xu R., et al. Effects of renin-angiotensin-aldosterone system inhibitors on disease severity and mortality in patients with COVID-19: a meta-analysis. J Med Virol. 2021;93:2287–2300. doi: 10.1002/jmv.26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai X.J., Tay J.C.K., Kui S.L., et al. Impact of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on in-hospital mortality in COVID-19 patients: a systematic review and meta-analysis. Singapore Med J. 2021;62:563–567. doi: 10.11622/smedj.2020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M.M.Y., Docherty K.F., Sattar N., et al. Renin-angiotensin system blockers, risk of SARS-CoV-2 infection and outcomes fromCoViD-19: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2022;8:165–178. doi: 10.1093/ehjcvp/pvaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H.W., Yoon C.H., Jang E.J., et al. Renin-angiotensin system blocker and outcomes of COVID-19: a systematic review and meta-analysis. Thorax. 2021;76:479–486. doi: 10.1136/thoraxjnl-2020-215322. [DOI] [PubMed] [Google Scholar]
- 30.Alamer A.A., Almulhim A.S., Alrashed A.A., et al. Mortality, severity, and hospital admission among COVID-19 patients with ACEI/ARB use: a meta-analysis stratifying countries based on response to the first wave of the pandemic. Healthcare (Basel) 2021;9:127. doi: 10.3390/healthcare9020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurentius A., Mendel B., Prakoso R. Clinical outcome of renin-angiotensin-aldosterone system blockers in treatment of hypertensive patients with COVID-19: a systematic review and meta-analysis. Egypt Heart J. 2021;73:13. doi: 10.1186/s43044-021-00135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassib M., Hamilton S., Elkhouly A., et al. Renin-angiotensin-aldosterone system inhibitors and COVID-19: a meta-analysis and systematic review. Cureus. 2021;13:e13124. doi: 10.7759/cureus.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baral R., Tsampasian V., Debski M., et al. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e213594. doi: 10.1001/jamanetworkopen.2021.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas M., Kali M.S.K. Association of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers with risk of mortality, severity or SARS-CoV-2 test positivity in COVID-19 patients: meta-analysis. Sci Rep. 2021;11:5012. doi: 10.1038/s41598-021-84678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee T., Cau A., Cheng M.P., et al. Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors in COVID-19: meta-analysis/meta-regression adjusted for confounding factors. CJC Open. 2021;3:965–975. doi: 10.1016/j.cjco.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Z., Wang M.P., Liu L., et al. Does taking an angiotensin inhibitor increase the risk for COVID-19? A systematic review and meta-analysis. Aging (Albany NY) 2021;13:10853–10865. doi: 10.18632/aging.202902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai X.C., An Z.Y., Wang Z.Y., et al. Associations between the use of renin-angiotensin system inhibitors and the risks of severe COVID-19 and mortality in COVID-19 patients with hypertension: a meta-analysis of observational studies. Front Cardiovasc Med. 2021;8:609857. doi: 10.3389/fcvm.2021.609857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aparisi A., Catala P., Amat-Santos I.J., et al. Chronic use of renin-angiotensin-aldosterone inhibitors in hypertensive COVID-19 patients: results from a Spanish registry and meta-analysis. Med Clin (Barc) 2022;158:315–323. doi: 10.1016/j.medcli.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur U., Chakrabarti S.S., Patel T.K. Renin-angiotensin-aldosterone system blockers and region-specific variations in COVID-19 outcomes: findings from a systematic review and meta-analysis. Ther Adv Drug Saf. 2021;12 doi: 10.1177/20420986211011345. 20420986211011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kow C.S., Ming L.C., Hasan S.S. Renin-angiotensin system inhibitor use and the risk of mortality in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. Hypertens Res. 2021;44:1042–1045. doi: 10.1038/s41440-021-00670-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattar Y., Mukuntharaj P., Zghouzi M., et al. Safety and efficacy of renin-angiotensin-aldosterone system inhibitors in COVID-19 population. High Blood Press Cardiovasc Prev. 2021;28:405–416. doi: 10.1007/s40292-021-00462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asiimwe I.G., Pushpakom S., Turner R.M., et al. Cardiovascular drugs and COVID-19 clinical outcomes: a living systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:4534–4545. doi: 10.1111/bcp.14927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia N., Zhang G., Sun X., et al. Influence of angiotensin converting enzyme inhibitors/angiotensin receptor blockers on the risk of all-cause mortality and other clinical outcomes in patients with confirmed COVID-19: a systemic review and meta-analysis. J Clin Hypertens (Greenwich) 2021;23:1651–1663. doi: 10.1111/jch.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernando M.E., Drovandi A., Golledge J. Meta-analysis of the association between angiotensin pathway inhibitors and COVID-19 severity and mortality. Syst Rev. 2021;10:243. doi: 10.1186/s13643-021-01802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Society of Cardiology . 2020. Position statement of the ESC council on hypertension on ACE-inhibitors and angiotensin receptor blockers. Available from: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [accessed 14.04.20] [Google Scholar]
- 46.Gaspar P., Parreira I., Antunes Meireles P., et al. The effect of chronic and inhospital exposure to renin-angiotensin system inhibitors on the outcome and inflammatory state of coronavirus disease 2019 adult inpatients. Int J Hypertens. 2021;2021:5517441. doi: 10.1155/2021/5517441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan Y., Shang J., Graham R., et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann H., Pyrc K., van der Hoek L., et al. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin M., Sorour M.K., Kasry A. Comparing the binding interactions in the receptor binding domains of SARS-CoV-2 and SARS-CoV. J Phys Chem Lett. 2020;11:4897–4900. doi: 10.1021/acs.jpclett.0c01064. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization . 2015. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at: http://www.who.int/csr/sars/country/table2004_04_21/en/ [accessed 15.02.21] [Google Scholar]
- 53.Santos R.A., Ferreira A.J., Verano-Braga T., et al. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 54.Paz Ocaranza M., Riquelme J.A., Garcia L., et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vickers C., Hales P., Kaushik V., et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuyama S., Nagata N., Shirato K., et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Yang P., Liu K., et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milewska A., Nowak P., Owczarek K., et al. Entry of human coronavirus NL63 into the cell. J Virol. 2018;92:e01933-17. doi: 10.1128/JVI.01933-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bayati A., Kumar R., Francis V., et al. SARS-CoV-2 infects cells following viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021;296:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gheblawi M., Wang K., Viveiros A., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oparil S., Sanders C.A., Haber E. In-vivo and in-vitro conversion of angiotensin I to angiotensin II in dog blood. Circ Res. 1970;26:591–599. doi: 10.1161/01.res.26.5.591. [DOI] [PubMed] [Google Scholar]
- 63.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J., Cao R., Xu M., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skipper C.P., Pastick K.A., Engen N.W., et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horby P., Mafham M., Linsell L., et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon C.J., Tchesnokov E.P., Woolner E., et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madelain V., Baize S., Jacquot F., et al. Ebola viral dynamics in nonhuman primates provides insights into virus immuno-pathogenesis and antiviral strategies. Nat Commun. 2018;9:4013. doi: 10.1038/s41467-018-06215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Wit E., Feldmann F., Cronin J., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eastman R.T., Roth J.S., Brimacombe K.R., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choy K.T., Wong A.Y., Kaewpreedee P., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang P., Tekwani S., Martin G.S. In COVID-19, adding lopinavir-ritonavir to usual care did not shorten time to clinical improvement. Ann Intern Med. 2020;172:JC63. doi: 10.7326/ACPJ202006160-063. [DOI] [PubMed] [Google Scholar]
- 73.Paladugu S., Donato A.A. Remdesivir improved time to recovery in adults hospitalized with COVID-19 and lower respiratory tract involvement. Ann Intern Med. 2020;173:JC4. doi: 10.7326/ACPJ202007210-005. [DOI] [PubMed] [Google Scholar]
- 74.Rezagholizadeh A., Khiali S., Sarbakhsh P., et al. Remdesivir for treatment of COVID-19: an updated systematic review and meta-analysis. Eur J Pharmacol. 2021;897:173926. doi: 10.1016/j.ejphar.2021.173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Abdouh A., Bizanti A., Barbarawi M., et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Contemp Clin Trials. 2021;101:106272. doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reddy Vegivinti C.T., Pederson J.M., Saravu K., et al. Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond) 2021;62:43–48. doi: 10.1016/j.amsu.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilt T.J., Kaka A.S., MacDonald R., et al. Remdesivir for adults with COVID-19: a living systematic review for American College of Physicians Practice Points. Ann Intern Med. 2021;174:209–220. doi: 10.7326/M20-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaka A.S., MacDonald R., Greer N., et al. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2021;174:663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lou Y., Liu L., Yao H., et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157:105631. doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caly L., Druce J.D., Catton M.G., et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajter J.C., Sherman M.S., Fatteh N., et al. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021;159:85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toots M., Yoon J.J., Cox R.M., et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11:515. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheahan T.P., Sims A.C., Zhou S., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12:541. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wahl A., Gralinski L.E., Johnson C.E., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdelnabi R., Foo C.S., De Jonghe S., et al. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224:749–753. doi: 10.1093/infdis/jiab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Owen D.R., Allerton C.M.N., Anderson A.S., et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 89.Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 90.Vandyck K., Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol. 2021;49:36–40. doi: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ullrich S., Sasi V.M., Mahawaththa M.C., et al. Challenges of short substrate analogues as SARS-CoV-2 main protease inhibitors. Bioorg Med Chem Lett. 2021;50:128333. doi: 10.1016/j.bmcl.2021.128333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abhinand C.S., Nair A.S., Krishnamurthy A., et al. Potential protease inhibitors and their combinations to block SARS-CoV-2. J Biomol Struct Dyn. 2022;40:903–917. doi: 10.1080/07391102.2020.1819881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8(Suppl. 1) doi: 10.1046/j.1440-1843.2003.00518.x. S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Habashi N.M., Camporota L., Gatto L.A., et al. Functional pathophysiology of SARS-CoV-2 induced acute lung injury and clinical implications. J Appl Physiol (1985) 2021;130:877–891. doi: 10.1152/japplphysiol.00742.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarzani R., Giulietti F., Di Pentima C., et al. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am J Physiol Lung Cell Mol Physiol. 2020;319:L325–L336. doi: 10.1152/ajplung.00189.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Y., Packer C.S., Rhoades R.A. Chronic hypoxia impairs pulmonary venous smooth muscle contraction. Respir Physiol. 1995;100:75–82. doi: 10.1016/0034-5687(94)00117-i. [DOI] [PubMed] [Google Scholar]
- 99.Tan W.S.D., Liao W., Zhou S., et al. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17. doi: 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 101.Balakumar P., Jagadeesh G. A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26:2147–2160. doi: 10.1016/j.cellsig.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 102.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuomo G., Menozzi M., Carli F., et al. Acute myocarditis as the main clinical manifestation of SARS-CoV 2 infection. Infect Dis Rep. 2020;12:8609. doi: 10.4081/idr.2020.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McFadyen J.D., Stevens H., Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620938149. 1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Celi A., Cianchetti S., Dell’Omo G., et al. Angiotensin II tissue factor and the thrombotic paradox of hypertension. Expert Rev Cardiovasc Ther. 2010;8:1723–1729. doi: 10.1586/erc.10.161. [DOI] [PubMed] [Google Scholar]
- 108.Brown N.J., Vaughan D.E. Prothrombotic effects of angiotensin. Adv Intern Med. 2000;45:419–429. [PubMed] [Google Scholar]
- 109.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu B., Li M., Zhou Z., et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGonagle D., Sharif K., O’Regan A., et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klahr S., Morrissey J. Angiotensin II and gene expression in the kidney. Am J Kidney Dis. 1998;31:171–176. doi: 10.1053/ajkd.1998.v31.pm9428470. [DOI] [PubMed] [Google Scholar]
- 114.Sriramula S., Haque M., Majid D.S., et al. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shigemura N., Iwata S., Yasumatsu K., et al. Angiotensin II modulates salty and sweet taste sensitivities. J Neurosci. 2013;33:6267–6277. doi: 10.1523/JNEUROSCI.5599-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mosimann R., Imboden H., Felix D. The neuronal role of angiotensin II in thirst, sodium appetite, cognition and memory. Biol Rev Camb Philos Soc. 1996;71:545–559. doi: 10.1111/j.1469-185x.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 117.Labandeira-Garcia J.L., Rodriguez-Perez A.I., Garrido-Gil P., et al. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129. doi: 10.3389/fnagi.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asadi-Pooya A.A., Simani L., Shahisavandi M., et al. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2021;42:415–431. doi: 10.1007/s10072-020-04932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Bundel D., Smolders I., Vanderheyden P., et al. Ang II and Ang IV: unraveling the mechanism of action on synaptic plasticity, memory, and epilepsy. CNS Neurosci Ther. 2008;14:315–339. doi: 10.1111/j.1755-5949.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Denny J.B., Polan-Curtain J., Wayner M.J., et al. Angiotensin II blocks hippocampal long-term potentiation. Brain Res. 1991;567:321–324. doi: 10.1016/0006-8993(91)90812-a. [DOI] [PubMed] [Google Scholar]
- 122.Xie X., Shi X., Liu M. The roles of genetic factors in kawasaki disease: a systematic review and meta-analysis of genetic association studies. Pediatr Cardiol. 2018;39:207–225. doi: 10.1007/s00246-017-1760-0. [DOI] [PubMed] [Google Scholar]
- 123.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duarte M., Pelorosso F., Nicolosi L.N., et al. Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962. doi: 10.1016/j.eclinm.2021.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ioannidis J.P. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94:485–514. doi: 10.1111/1468-0009.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sackett D.L., Rosenberg W.M., Gray J.A., et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Djulbegovic B., Guyatt G.H. Progress in evidence-based medicine: a quarter century on. Lancet. 2017;390:415–423. doi: 10.1016/S0140-6736(16)31592-6. [DOI] [PubMed] [Google Scholar]
- 128.WHO-Europe. Evidence-informed policy-making. Available from: https://www.euro.who.int/en/data-and-evidence/evidence-informed-policy-making/about-us [accessed 15.02.21].