Key Points
Questions
What is the recommended phase 2 dose of regorafenib, ipilimumab, and nivolumab (RIN), and what is its effectiveness against refractory microsatellite stable metastatic colorectal cancer?
Findings
In this nonrandomized clinical trial of 39 patients with metastatic colorectal cancer, the recommended phase 2 dose of RIN had a manageable safety profile and an overall response rate of 27.6%, a median progression-free survival of 4 months, and a median overall survival of 20 months. Responses were limited to patients without liver metastases (overall response rate, 36.4%; median overall survival not reached).
Meaning
Findings suggest that RIN was associated with substantial clinical activity in patients with microsatellite stable metastatic colorectal cancer without liver metastases.
Abstract
Importance
Immunotherapy combinations with activity in patients with microsatellite stable (MSS) metastatic colorectal cancer need to be identified.
Objective
To determine the recommended phase 2 dose (RP2D) of regorafenib, ipilimumab, and nivolumab (RIN) and evaluate its activity in an expansion cohort of patients with MSS metastatic colorectal cancer.
Design, Setting, and Participants
This nonrandomized clinical trial was a single-center 3 + 3 dose de-escalation study with an effectiveness expansion cohort at the RP2D. After the identification of the RP2D, a study amendment was executed to explore a regorafenib dose optimization strategy to mitigate skin-related toxic effects. Study enrollment occurred between May 12, 2020, and January 21, 2022. The trial was conducted at a single academic center. A total of 39 patients with MSS metastatic colorectal cancer whose disease progressed after standard chemotherapy and who had not received prior regorafenib or anti–programmed cell death protein 1 therapy were included.
Interventions
Patients received regorafenib daily for 21 days every 4 weeks; fixed-dose ipilimumab, 1 mg/kg, intravenously every 6 weeks; and fixed-dose nivolumab, 240 mg intravenously every 2 weeks. Patients were treated until progression, unacceptable toxic effects, or completion of 2 years of therapy.
Main Outcomes and Measures
The primary end point was RP2D selection. Secondary end points were safety and overall response rate (ORR) according to the Response Evaluation Criteria in Solid Tumours at the RP2D level.
Results
A total of 39 patients were enrolled, 23 (59.0%) were female, median age was 54 years (range, 25-75 years), 3 were Black (7.7%), and 26 were White (66.7%). No dose-limiting toxic effects were noted in the first 9 patients at the starting dose of RIN, with regorafenib dosed at 80 mg daily. No dose de-escalation was needed. This dose was declared the RP2D. Twenty more patients were enrolled at this level. The ORR, median progression-free survival (PFS), and overall survival (OS) in the RP2D cohort were 27.6%, 4 months (IQR, 2-9 months), and 20 months (IQR, 7 months to not estimable), respectively. For the 22 patients without liver metastases, the ORR, PFS, and OS were 36.4%, 5 months (IQR, 2-11), and greater than 22 months, respectively. A dose optimization cohort with regorafenib at 40 mg/d on cycle 1 and 80 mg/d on cycle 2 and beyond was associated with lower skin and immune toxic effects but had limited activity with stable disease for 5 of 10 patients as the best response.
Conclusions and Relevance
Results of this nonrandomized clinical trial suggest that RIN at the RP2D demonstrated interesting clinical activity in patients with advanced MSS colorectal cancer without liver metastases. These findings should be confirmed in randomized clinical trials.
Trial Registration
ClinicalTrials.gov Identifier: NCT04362839
This nonrandomized clinical trial assesses the recommended phase 2 dose of regorafenib, ipilimumab, and nivolumab immunotherapy for patients with refractory microsatellite stable metastatic colorectal cancer.
Introduction
The anti–programmed cell death protein 1 (PD-1) antibodies nivolumab and pembrolizumab as monotherapy have been ineffective in patients with microsatellite stable (MSS) colorectal cancer, including those with programmed cell death protein ligand 1 (PD-L1)–positive tumors.1,2,3 Studies have shown that the combination of the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab and PD-1 antibodies led to enhanced effector T-cell activity that was greater than that of either agent alone and resulted in enhanced antitumor activity in a variety of tumor types, including melanoma, non–small cell lung cancer, mesothelioma, and microsatellite instable colorectal cancer.4,5,6,7 Regorafenib, a multikinase inhibitor approved for the treatment of refractory metastatic colorectal cancer, modulates the tumor immune microenvironment and synergizes with anti–PD-1 agents in preclinical models.8,9 Despite early promising activity with the combination of regorafenib and nivolumab (REGONIVO) in a phase 1 clinical trial,10 a recent phase 2 study reported limited effectiveness with this combination for patients with MSS metastatic colorectal cancer, with an overall response rate (ORR) of 7.1%. No responses were observed among patients with liver metastases, whereas those without them had an ORR of 21.7%.11
We hypothesized that the addition of the CTLA-4 inhibitor ipilimumab to REGONIVO would be associated with acceptable toxic effects and improved effectiveness against MSS metastatic colorectal cancer. Therefore, we conducted this phase 1 clinical trial to establish the recommended phase 2 dose (RP2D) level and determine the clinical activity of regorafenib, ipilimumab, and nivolumab (RIN) in patients with MSS metastatic colorectal cancer.
Methods
Patient Eligibility
Patients who had not received prior regorafenib or anti–PD-1 therapy and who had MSS metastatic colorectal cancer that had progressed on or after treatment with a fluoropyrimidine, oxaliplatin, irinotecan, and anti–epidermal growth factor receptor therapy if the patient has a RAS/BRAF wild type with left-sided colon primary tumor were enrolled in this nonrandomized clinical trial. Full eligibility criteria are detailed in section 4 of the trial protocol in Supplement 1. Race and ethnicity were self-reported and captured in an electronic database at enrollment.
Study Design
This single-center trial followed a 3 + 3 dose de-escalation design, with a treatment expansion cohort (n = 20) at the RP2D. Regorafenib was given at the starting dose level of 80 mg once daily for 21 days every 28-day cycle, along with ipilimumab, 1 mg/kg, intravenously every 6 weeks and nivolumab, 240 mg, intravenously every 2 weeks. If 1 or none of the first 3 patients experienced a dose-limiting toxic effect (DLT), 3 additional patients were to be enrolled in this cohort. Of 6 evaluable patients, if 1 or none had a DLT, this dose would be considered the RP2D. No escalation beyond this starting dose was allowed, given the known RP2D of regorafenib (80 mg) and nivolumab (240 mg).10 The fixed dose of nivolumab and ipilimumab was based on the effectiveness of this combination against microsatellite instable colorectal cancer in CheckMate 142.12 If 2 or more patients of 6 or fewer patients at the first dose level had a DLT, lower doses of regorafenib were to be explored. Treatment beyond progression was allowed if the treating physician suspected clinical benefit based on symptomatic patient improvement or favorable trends in tumor markers. Patients were treated until progression, unacceptable toxic effects, or completion of 2 years of therapy.
The primary end point of this study was to determine the RP2D of RIN. Secondary end points included safety, ORR, duration of response, progression-free survival (PFS), and overall survival (OS) at the RP2D.
After completion of enrollment in the RP2D expansion cohort and given a high rate of transient G3 skin toxic effects on cycle 1, the protocol was amended to investigate the safety of a dose optimization regimen of regorafenib of 40 mg/d on cycle 1 followed by 80 mg/d on cycle 2 and beyond in an additional 10-patient cohort (section 12 of the trial protocol in Supplement 1).
This trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol and amendment were approved by the City of Hope Comprehensive Cancer Center institutional review board. All patients provided written consent before enrollment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Dose-Limiting Toxic Effects
Dose-limiting toxic effects were evaluated during the primary DLT observation period (4 weeks, from the time of first administration of RIN [cycle 1, day 1] until the planned administration of the second cycle of regorafenib [cycle 2, day 1]). Adverse events defining DLTs are detailed in section 11 of the trial protocol in Supplement 1.
Assessment
Tumors were assessed with computed tomography or magnetic resonance imaging within 14 days before the first dose and every 8 weeks until progression or discontinuation. Secondary end points of ORR, PFS, OS, and duration of response were assessed by Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1.13 Patients treated beyond progression were assessed by the modified RECIST guideline for immunotherapy (iRECIST).14 All study-related radiology studies were read and interpreted according to RECIST and iRECIST guidelines by board-certified radiologists at the City of Hope Comprehensive Cancer Center, and results were confirmed by the supervising investigator (M.F.). The overall response rate included both confirmed and unconfirmed responses. Adverse events were evaluated at baseline and throughout the treatment period with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Analysis
In prior studies of regorafenib alone or combinations of PD-L1 and CTLA-4 antibodies, the historic ORR was less than 5%.15,16 We estimated that a minimum of 26 patients needed to be enrolled at the RP2D. This sample size would provide greater than 80% power to rule out an ORR less than 5% with more than 95% confidence if at least 5 of 26 patients responded.
Progression-free survival and OS were estimated with the Kaplan-Meier method, and the corresponding 95% CIs were calculated with the Brookmeyer-Crowley method. Analyses were performed with R, version 4.0.3 (R Foundation for Statistical Computing).
Results
Patient Characteristics and Dose Finding
A total of 39 patients were enrolled between May 12, 2020, and January 21, 2022. The median age was 54 years (range, 25-75 years), 16 (41%) were male, 23 (59%) were female, 7 were Asian (17.9%), 3 were Black (7.7%), 1 was Native Hawaiian (2.6%), and 26 were White (66.7%); data were not available for 2 patients (5.1%). Patients’ characteristics are detailed in Table 1. Data were analyzed from May 9, 2022, to June 24, 2022. No DLTs were observed in the first 6 patients at the first dose level. However, 3 of the 6 patients were not DLT evaluable because their treatment was held for 7 days for grade 2 and higher skin toxic effects, which was not DLT defining. Three additional evaluable patients were enrolled for DLT evaluation at dose level 1. None of the first 6 DLT-evaluable patients experienced a DLT. Therefore, dose level 1 was defined as the RP2D.
Table 1. Basic Characteristics.
| Characteristic | Overall population (n = 39) | RP2D cohort (n = 29) | Dose optimization cohort (n = 10) |
|---|---|---|---|
| Age, median (range), y | 54 (25-75) | 55 (36-75) | 52 (25-73) |
| ECOG PS score (grade 0/grade 1) | 19/20 | 17/12 | 2/8 |
| Sex, No. (%) | |||
| Male | 16 (41.0) | 12 (41.4) | 4 (40) |
| Female | 23 (59.0) | 17 (58.6) | 6 (60) |
| Race and ethnicity, No. (%) | |||
| Asian | 7 (17.9) | 4 (13.8) | 3 (30) |
| Black | 3 (7.7) | 3 (10.3) | 0 |
| Native Hawaiian | 1 (2.6) | 0 | 1 (10) |
| White | 26 (66.7) | 21 (72.4) | 5 (50) |
| NA | 2 (5.1) | 1 (3.4) | 1 (10) |
| Side of primary tumor, No. (%) | |||
| Right | 9 (23.1) | 9 (31.0) | 0 |
| Left | 30 (76.9) | 20 (69.0) | 10 (100) |
| RAS variation, No. (%) | 24 (61.5) | 20 (69.0) | 4 (40) |
| BRAFV600E variation, No. (%) | 3 (7.7) | 3 (10.3) | 0 |
| TMB, median (range)a | 2 (1-11) | 3 (1-11) | 1 (1-4) |
| PD-L1 TPS, median (range)b | 0 (0-10) | 0 (0-10) | 1 (1-5) |
| Prior therapy, No. (%) | |||
| Lines of therapy | |||
| Median (range) | 3 (1-6) | 2 (1-6) | 3 (2-5) |
| 1 | 1 (2.6) | 1 (3.4) | 0 |
| 2 | 18 (46.2) | 14 (48.3) | 4 (40) |
| >2 | 20 (51.3) | 14 (48.3) | 6 (60) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status Scale; NA, not available; PD-L1, anti–programmed cell death protein ligand 1; RP2D, recommended phase 2 dose; TMB, tumor mutation burden; TPS, tumor proportion score.
Fifteen unavailable (9 from the RP2D).
Twenty-four unavailable (18 from the RP2D).
Safety
Among the 29 patients treated at the RP2D, common treatment-related, immune-related adverse events (irAEs) grade 3 or higher were rash (11 patients [37.9%]), elevation of aspartate aminotransferase or alanine aminotransferase level (4 patients [13.8%]), and myalgia (3 patients [10.3%]) (Table 2). Despite the high incidence of grade 3 rash, this toxic effect was manageable with a short regorafenib interruption and a short course of oral steroid.
Table 2. Immune-Related Adverse Events.
| irAEs | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| RP2D cohort (n = 29)a | Dose optimization cohort (n = 10) | |||||
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Rash maculopapular | 4 (13.8) | 11 (37.9) | 0 | 0 | 1 (10) | 0 |
| ALT elevation | 1 (3.4) | 3 (10.3) | 1 (3.4) | 0 | 0 | 0 |
| AST elevation | 1 (3.4) | 4 (13.8) | 0 | 0 | 0 | 0 |
| Lipase elevation | 3 (10.3) | 2 (6.9) | 0 | 1 (10) | 0 | 0 |
| Amylase elevation | 0 | 2 (6.9) | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 1 (3.4) | 1 (3.4) | 0 | 1 (10) | 0 | 0 |
| Pruritus | 1 (3.4) | 1 (3.4) | 0 | 0 | 0 | 0 |
| Colitis | 1 (3.4) | 1 (3.4) | 0 | 0 | 0 | 0 |
| Meningitis | 0 | 1 (3.4) | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 1 (10) | 0 |
| Myalgia | 0 | 3 (10.3) | 0 | 0 | 0 | 0 |
| Arthritis | 3 (10.3) | 0 | 0 | 0 | 0 | 0 |
| Xerostomia | 1 (3.4) | 0 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 4 (13.8) | 0 | 0 | 0 | 0 | 0 |
| Hypoadrenalism | 1 (3.4) | 0 | 0 | 0 | 0 | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; irAEs, immune-related adverse events; RP2D, recommended phase 2 dose.
One patient died during the study.
Among the 10 patients in the dose optimization cohort, irAEs grade 3 or higher were rash (1 patient [10%]) and pneumonitis (1 patient [10%]). In comparison with the RP2D cohort, the dose optimization cohort had a diminished rate of grade 2 or higher skin toxic effects (15 of 29 [51.7%] vs 1 of 10 [10%]) or any other grade 2 or higher irAEs (19 of 29 [65.5%] vs 3 of 10 [30%]). Grade 2 or higher irAEs at the RP2D and dose optimization cohort are summarized in Table 2. Non-irAE grade 2 or higher adverse events are summarized in eTable 1 in Supplement 2.
At the RP2D, dose interruptions in regorafenib occurred mostly on cycle 1 and were due to skin toxic effects in 9 of 29 patients; 7 events led to a dose reduction to 40 mg. During any cycle, 8 patients required interruption of nivolumab and ipilimumab owing to irAEs (4 instances of hepatitis, 2 colitis, 1 meningitis, and 1 squamous cell carcinoma of the skin). Treatment was reintroduced with nivolumab after resolution of toxic effects in 2 patients with colitis. One patient had a sudden death due to cardiopulmonary arrest on cycle 8, with a possible attribution to immunotherapy, although an acute thrombotic event associated with malignancy or regorafenib was more likely.
In the dose optimization cohort, 8 of 10 patients were able to escalate to 80 mg of regorafenib at cycle 2. Regorafenib was not escalated for 1 patient with grade 3 rash on cycle 1 and for 1 patient who discontinued therapy early owing to clinical progression.
Activity Analysis
RP2D Cohort
Among the 29 patients in the RP2D effectiveness cohort, the ORR and the disease control rate were 27.6% and 62.1%, respectively. The median PFS and OS were 4 months (95% CI, 3-9 months) and 20 months (95% CI, 9 months to not reached), respectively (Figure, A and B). In a post hoc unplanned analysis, we interrogated the effectiveness of RIN based on the presence or absence of liver metastases. For the 7 patients with liver metastases who were receiving the RP2D, the ORR, disease control rate, median PFS, and median OS were 0%, 42.9%, 2 months (range, 2-9 months), and 7 months (range, 4-23 months), respectively (Table 3). For the 22 patients without liver metastases who were receiving the RP2D, the ORR, disease control rate, median PFS, and median OS were 36.4%, 68.2%, 5 months (range, 1-28 months), and greater than 22 months (range, 2 months to not estimable), respectively. By iRECIST, the ORR, disease control rate, median PFS, and median OS in the RP2D cohort without liver metastases were 40.9%, 81.8%, 6 months (range, 1-28 months), and greater than 22 months (range, 2-28 months), respectively (Table 3). Tumor response and associated tumor characteristics are detailed in the eFigure and eTable 2 in Supplement 2. At data cutoff (September 30, 2022), 5 patients with partial response (PR) or stable disease by RECIST and 1 patient with PR by iRECIST (6 patients total) were still receiving treatment or surveillance with PFS of at least 22 months. One patient with pseudoprogression and BRAFV600E variation who developed progressive disease with more than 10 new lung metastases and progressive pelvic disease at 2 months and who kept receiving treatment beyond progression went on to have complete remission of lung metastases and near-complete disappearance of pelvic disease along with normalization of tumor markers and resolution of circulating tumor DNA (ctDNA).
Figure. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival.
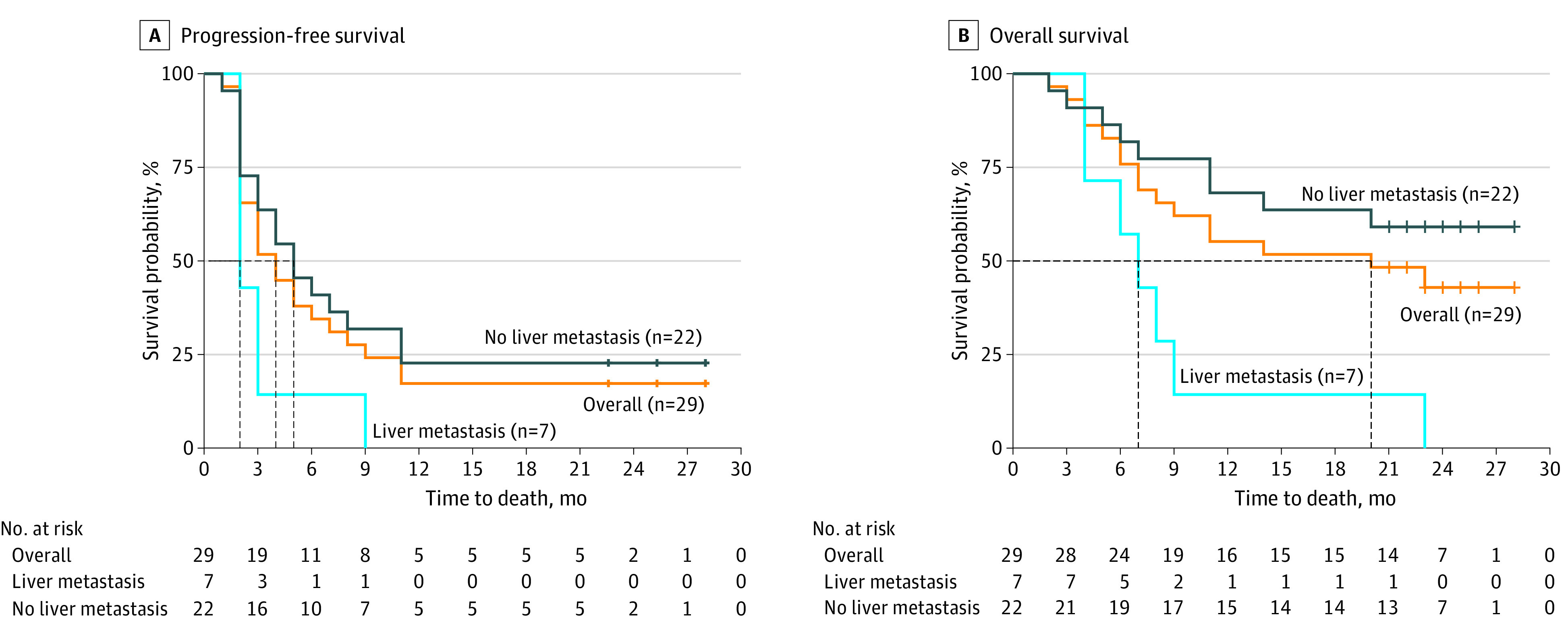
Table 3. Clinical Effectiveness of Regorafenib, Ipilimumab, and Nivolumab at Recommended Phase 2 Dose for Patients With Metastatic Colorectal Cancer.
| Measure | Patients assessed by Response Evaluation Criteria in Solid Tumours | Patients assessed by Response Evaluation Criteria in Solid Tumours modified for immunotherapy | ||||
|---|---|---|---|---|---|---|
| No liver metastases (n = 22) | Liver metastases (n = 7) | All (n = 29) | No liver metastases (n = 22) | Liver metastases (n = 7) | All (n = 29) | |
| ORR, No. (%) | 8 (36.4) | 0 | 8 (27.6) | 9 (40.9) | 0 | 9 (31.0) |
| PR, No. (%) | 8 (36.4) | 0 | 8 (27.6) | 9 (40.9) | 0 | 9 (31.0) |
| SD, No. (%) | 7 (31.8) | 3 (42.9) | 10 (34.5) | 9 (40.9) | 3 (42.9) | 12 (41.4) |
| DCR, No. (%) | 15 (68.2) | 3 (42.9) | 18 (62.1) | 18 (81.8) | 3 (42.9) | 21 (72.4) |
| PD, No. (%) | 7 (31.8) | 4 (57.1) | 11 (37.9) | 4 (18.2) | 4 (57.1) | 8 (27.6) |
| PFS, median (range), mo | 5 (1-28) | 2 (2-9) | 4 (1-28) | 6 (1- 28) | 2 (2-9) | 5 (1- 28) |
| OS, median (range), mo | >22 (2 to NE) | 7 (4-23) | 20 (2-28) | >22 (2 to NE) | 7 (4-23) | 20 (2-28) |
Abbreviations: DCR, disease control rates; NE, not estimable; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Among the 8 RECIST responders, 2 did not have confirmed responses. One older patient with extensive lung metastatic disease experienced a fall resulting in a hip fracture after 2 months of treatment. This led to discontinuation of study treatment. Only 1 restaging study was performed at 4 months from study initiation and showed major regression in this patient with lung and mediastinal metastatic disease. This patient subsequently succumbed to infectious complications associated with hip surgery and without evidence of progression. The second patient had metastatic BRAFV600E colon cancer to the lungs and distant lymph nodes. She had diffuse regression of disease at 2 months, followed by a mixed response with the development of new metastatic lymph nodes at 4 months. The other 6 patients had confirmed responses. The median duration of response was not reached and exceeded 17 months (range, 4 months to not estimable).
Of 20 patients with RAS variation, 6 achieved a PR. Of 3 patients with BRAFV600E variation, 2 achieved a PR, and 1 had progressive disease followed by a PR by iRECIST (described earlier). Tumor mutation burden was available for 7 responders; all had 9 or fewer (1, 1, 2, 2, 3, 4, and 9). Among 29 patients in the RP2D cohort, prior PD-L1 testing by a Clinical Laboratory Improvement Amendments–certified assay (anti–PD-L1, clone 22C3) was available for 11 patients. Seven patients had a negative PD-L1 expression (tumor proportion score [TPS] of 0), 1 of whom had a PR (PFS >26 months), whereas 4 had stable disease (PFS of 4, 5, 8, and 9 months) and 1 had progressive disease. A PD-L1 positivity was noted in 4 patients; 1 had progressive disease (TPS of 1), and 3 had stable disease (PFS: 3 months [TPS of 10], 3 months [TPS of 5], and >22 months [TPS of 1]) (eTable 2 in Supplement 2).
Dose Optimization Cohort
The 10-patient dose optimization RIN cohort was not powered for effectiveness signals. However, no responses were noted in this cohort of patients, 5 of 10 patients had stable disease as the best response, and the median PFS was 2 months (range, 1-2 months; 4 months [range, 2-7 months] for nonliver metastases); OS was not reached (>10 months; range, 1 month to not estimable).
ctDNA and Outcome
The ctDNA levels at baseline and 4 to 6 weeks after therapy were available for 23 of 39 patients. Twelve patients had a greater than 50% decrease in ctDNA, 4 achieved a response, and 8 achieved stable disease. The median PFS in ctDNA responders was 9.5 months (range, 3-23 months). Nine patients had an increase in ctDNA (range, 6%-300%), 8 had progressive disease, and 1 had a short stable disease. Two patients had a minor decrease in ctDNA (−14% and −30%), and both experienced progressive disease.
Discussion
Regorafenib, 80 mg, once daily for 21 days every 28-day cycle, along with ipilimumab, 1 mg/kg, intravenously every 6 weeks and nivolumab, 240 mg, intravenously every 2 weeks was associated with an ORR of 36.4% and a median PFS of 5 months (range, 1-28 months) among patients with MSS metastatic colorectal cancer without liver involvement. The responses were durable, and the median duration of response has still been unreached at 17 months (range, 6 months to not estimable). The limited benefits to patients with nonliver metastases are in line with results of our prior REGONIVO trial, in which a 21% ORR was observed in patients with nonliver metastatic MSS and no responses were noted in patients with liver metastatic disease.11 Use of REGONIVO was also investigated in 2 additional prospective clinical trials, which showed diverging response rates of 33% and 10% among patients with MSS cancer.10,17 In the Japanese EPOC1603 trial, 8 responses were noted in 24 patients with MSS cancer, only 1 of whom had target lesions in the liver.10 In the second trial, 3 of 4 responders were without liver metastases.17 Resistance in the setting of liver metastases was also noted in the setting of regorafenib in combination with other anti–PD-1 antibodies. A study of pembrolizumab and regorafenib failed to show responses in 70 patients with MSS metastatic colorectal cancer.18 However, a significant difference in PFS was noted in favor of patients without liver metastases vs with them (4.3 vs 1.8 months; P = .02).18 The combination of regorafenib plus toripalimab showed comparable differences in outcome.19 In our RIN trial, the ORR and PFS exceeded the responses and PFS in other US studies with regorafenib plus anti–PD-1.11,17,18 These findings support the value of adding a CTLA-4 inhibitor to anti–PD-1 therapy for patients with MSS colorectal cancer without liver metastases. A recent clinical trial of the Fc-engineered CTLA-4 inhibitor botensilimab combined with the PD-1 antibody balstilimab reported an ORR of 42% in patients with MSS metastatic colorectal cancer without liver metastatic disease and no responses in patients with liver metastases.20 This study, again, supports a role for CTLA-4 inhibitors in MSS metastatic colorectal cancer, especially with the known lack of responses with anti–PD-1 alone.
Persistent questions remain regarding the role of regorafenib in the setting of RIN. Given the enhanced activity of REGONIVO vs the historic lack of activity of anti–PD-1 monotherapy in patients with MSS metastatic colorectal cancer, we do believe that regorafenib accentuates the immune responses to checkpoint inhibitors. The significant clinical activity at the RP2D vs the dose optimization level in our study suggests that a minimum starting dose of regorafenib may be needed to induce a clinical response. Indeed, a starting regorafenib dose level of 80 mg was associated with a higher rate of rash in the first 2 to 4 weeks of treatment and a higher rate of immune-related toxic effects, indicating an enhanced biological activity of the higher dose level when combined with PD-1 or CTLA-4 antibodies. In contrast, a relatively low rate of rash and lower immune-related toxic effects at the dose optimization level was noted both at 40 mg/d during cycle 1 and at 80 mg/d during cycle 2 and subsequent cycles. All in all, our data support the selection of the RP2D of RIN for further investigation in patients with nonliver metastatic MSS colorectal cancer.
In this study, we interrogated in an exploratory fashion the relevance of molecular biomarkers and ctDNA dynamics in response to RIN. Contrary to recent reports suggesting resistance of RAS variant MSS colorectal cancer to combinations of tyrosine kinase inhibitor and PD-L1 inhibitors,21,22 6 of 20 patients with RAS variations had RECIST responses, whereas none of 6 patients with RAS/BRAF wild-type variations responded. Given the limited sample size in this study, it is not possible to make definitive conclusions about the predictive value of RAS variations. Three patients with BRAFV600E variations without liver metastatic disease were enrolled, and all responded to RIN (duration of response, 2 months, 7 months, and ≥17 months). BRAFV600E variations are enriched in consensus molecular subtype 1 (CMS1) colorectal cancers and may be more amenable to immunotherapy responses even in an MSS setting.23
In addition to RAS/BRAF, we investigated the association between tumor mutation burden and response to RIN. As expected, all enrolled patients with available results had a low tumor mutation burden, including all RECIST responders. A PD-L1 assay was not available for most patients, and the sole patient with an available PD-L1 level measurement among responders had a PD-L1 assay result of 0.
We also interrogated ctDNA dynamics in 23 patients who had this assay performed as part of their routine care. A decrease of greater than 50% in ctDNA clearance was associated with clinical benefits, consistent with other reports.24,25 Circulating tumor DNA can be considered in future validation studies as a biomarker of early termination of treatment for patients without a decrease in ctDNA by 6 weeks. In addition, a major decrease in ctDNA in the setting of early disease progression should be investigated as a biomarker for continuation of therapy beyond progression because such events may constitute pseudoprogression.
This study lends further support to the significance of metastatic disease site in dictating responses to immunotherapy. Consistent with our prior reports, our results suggest that liver metastatic disease is a strong biomarker of resistance to immunotherapy in patients with MSS colorectal cancer.26,27 Similar results have been capitulated in colorectal liver metastases preclinical models.28 Although we observed a high ORR in patients with nonliver metastatic disease in MSS, this does not imply that all nonliver metastatic sites are as likely to respond to RIN or other combinations of checkpoint inhibitors. Four of the 8 responding patients had lung-only metastatic disease, suggesting that this group may have the most favorable tumor microenvironment to predispose them to respond to therapy. More investigations are needed to determine the predictive value of additional extrahepatic metastatic disease sites, such as peritoneal disease, soft tissue metastases, and distant lymph nodes metastases, in response to immunotherapy.
Limitations
Some of the limitations of this study are the small sample size, which may limit the accurate estimation of the response rate and PFS of MSS metastatic colorectal cancer treated with RIN. In addition, the modest sample size of 22 patients with nonliver metastatic cancer treated at the RP2D of RIN limits the ability of assessing the association between other sites of metastatic disease, such as in peritoneal metastases, and outcome; therefore, such potential associations cannot be adequately evaluated based on this study. Finally, the lack of serial biopsies at baseline, during treatment, and at progression limits the understanding of the contributing factors to response or resistance in this patient population.
Conclusions
The results of this nonrandomized clinical trial of the RIN combination at the RP2D of regorafenib, 80 mg/day, ipilimumab, 1 mg/kg every 6 weeks, and nivolumab, 240 mg every 2 weeks, showed encouraging activity in patients with nonliver metastatic MSS colorectal cancer. Additional investigations will be needed to confirm the value of each of these components and to further define additional clinical and molecular biomarkers of response.
Trial Protocol
eTable 1. Nonimmune-Related Adverse Events
eTable 2. Detailed Characteristics of Patients
eFigure. Waterfall Plot of Tumor Responses and Molecular Characteristics
Data Sharing Statement
References
- 1.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 2.O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti–PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. doi: 10.1371/journal.pone.0189848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol. 2016;34(15)(suppl):3501. doi: 10.1200/JCO.2016.34.15_suppl.3501 [DOI] [Google Scholar]
- 4.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 6.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375-386. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 8.Hoff S, Grünewald S, Röse L, Zopf D. Immunomodulation by regorafenib alone and in combination with anti PD1 antibody on murine models of colorectal cancer. Ann Oncol. 2017;28:v423. doi: 10.1093/annonc/mdx376.060 [DOI] [Google Scholar]
- 9.Wu RY, Kong PF, Xia LP, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530-4541. doi: 10.1158/1078-0432.CCR-18-2840 [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053-2061. doi: 10.1200/JCO.19.03296 [DOI] [PubMed] [Google Scholar]
- 11.Fakih M, Raghav KPS, Chang DZ, et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair–proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC). J Clin Oncol. 2021;39(15)(suppl):3560. doi: 10.1200/JCO.2021.39.15_suppl.3560 [DOI] [Google Scholar]
- 12.Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability–high/mismatch repair–deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161-170. doi: 10.1200/JCO.21.01015 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz LH, Seymour L, Litière S, et al. RECIST 1.1 - Standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer. 2016;62:138-145. doi: 10.1016/j.ejca.2016.03.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour L, Bogaerts J, Perrone A, et al. ; RECIST Working Group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grothey A, Van Cutsem E, Sobrero A, et al. ; CORRECT Study Group . Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi: 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 16.Chen EX, Jonker DJ, Loree JM, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian Cancer Trials Group CO.26 study. JAMA Oncol. 2020;6(6):831-838. doi: 10.1001/jamaoncol.2020.0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim RD, Kovari BP, Martinez M, et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer. 2022;169:93-102. doi: 10.1016/j.ejca.2022.03.026 [DOI] [PubMed] [Google Scholar]
- 18.Barzi A, Azad NS, Yang Y, et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC). J Clin Oncol. 2022;40(4)(suppl):15-15. doi: 10.1200/JCO.2022.40.4_suppl.015 [DOI] [Google Scholar]
- 19.Wang F, He MM, Yao YC, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2(9):100383. doi: 10.1016/j.xcrm.2021.100383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullock A, Grossman J, Fakih M, et al. LBA O-9 botensilimab, a novel innate/adaptive immune activator, plus balstilimab (anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer. Ann Oncol. 2022;33:S376. doi: 10.1016/j.annonc.2022.04.453 [DOI] [Google Scholar]
- 21.Saeed A, Park R, Dai J, et al. Phase II trial of cabozantinib (Cabo) plus durvalumab (Durva) in chemotherapy refractory patients with advanced mismatch repair proficient/microsatellite stable (pMMR/MSS) colorectal cancer (CRC): CAMILLA CRC cohort results. J Clin Oncol. 2022;40(4)(suppl):135. doi: 10.1200/JCO.2022.40.4_suppl.135 [DOI] [Google Scholar]
- 22.Abrams TA, Kazmi SMA, Winer IS, et al. A phase 1b multitumor cohort study of cabozantinib plus atezolizumab in advanced solid tumors (COSMIC-021): results of the colorectal cancer cohort. J Clin Oncol. 2022;40(4)(suppl):121. doi: 10.1200/JCO.2022.40.4_suppl.121 [DOI] [Google Scholar]
- 23.Smeby J, Sveen A, Merok MA, et al. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol. 2018;29(5):1227-1234. doi: 10.1093/annonc/mdy085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873-881. doi: 10.1038/s43018-020-0096-5 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti–programmed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717-721. doi: 10.1001/jamaoncol.2017.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncologist. 2020;25(8):e1188-e1194. doi: 10.1634/theoncologist.2020-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Sandhu J, Ouyang C, Ye J, Lee PP, Fakih M. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw Open. 2021;4(8):e2118416. doi: 10.1001/jamanetworkopen.2021.18416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiegle E, Doleschel D, Koletnik S, et al. Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia. 2019;21(9):932-944. doi: 10.1016/j.neo.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Nonimmune-Related Adverse Events
eTable 2. Detailed Characteristics of Patients
eFigure. Waterfall Plot of Tumor Responses and Molecular Characteristics
Data Sharing Statement


