Abstract
Objective:
To assess the effectiveness and acceptability of antimicrobial stewardship-focused implementation strategies on inpatient fluoroquinolones.
Methods:
Stewardship champions at 15 hospitals were surveyed regarding use and acceptability of strategies to improve fluoroquinolone prescribing. Antibiotic days of therapy (DOT) per 1000 days-present (DP) for sites with and without prospective audit and feedback (PAF) and/or prior approval were compared.
Results:
60% of sites had PAF or prior approval implemented for fluoroquinolones. Compared to sites with neither strategies (64.2+34.4 DOT/DP), fluoroquinolone prescribing rates were lower for sites that employed PAF/prior approval (35.5+9.8; p=0.03) and decreased from 2017–2018 (p<0.001). This decrease occurred without an increase in advanced generation cephalosporins. Total antibiotic rates were 13% lower for sites with PAF/prior approval, but this did not reach statistical significance (p=0.2). Sites reporting that PAF/prior approval were “completely” accepted had lower fluoroquinolone rates than “moderately” accepted sites (34.2+5.7 vs 48.7+4.5; p<0.01). Sites reported clinical pathways/local guidelines (93%), prior approval (93%), and order forms (80%) “would” or “may” be effective in improving fluoroquinolone use. While most sites (73%) indicated that requiring infectious disease consults “would” or “may” be effective in improving fluoroquinolones, 87% perceived implementation to be difficult.
Conclusions:
PAF and prior approval implementation strategies focused on fluoroquinolones were associated with significantly lower fluoroquinolone prescribing rates and non-significant decreases in total antibiotic use, suggesting limited evidence for class substitution. The association of acceptability of strategies with lower rates highlights the importance of culture. This may indicate increased acceptability of implementation strategies and/or sensitivity to FDA warnings.
Keywords: audit and feedback, prior approval, antibiotic, fluoroquinolone, formulary, antibiotic stewardship, prescribing patterns, implementation strategies
INTRODUCTION
The U.S. Food and Drug Administration (FDA) has issued multiple safety communications on the fluoroquinolone class. These statements recommend use of alternative antibiotics for common infections because adverse events associated with the fluoroquinolone class outweigh potential benefits in some situations.1 FDA safety warnings for the fluoroquinolones include aortic dissection, central nervous system effects, glucose homeostasis disturbances, QT prolongation and disabling side effects involving tendons, muscles, joints, nerves and the central nervous system. These side effects can occur hours to weeks after exposure to fluoroquinolones and may potentially be permanent.1–7 An FDA advisory panel recommended avoidance of fluoroquinolones unless suitable alternatives were not available for acute bacterial sinusitis, acute chronic obstructive pulmonary disease exacerbations, and uncomplicated urinary tract infections.8 Despite these FDA warnings, fluoroquinolones continue to be prescribed and remain a high priority for stewardship.
Moreover, fluoroquinolone use has been associated with bacterial resistance and Clostridioides difficile (CDI).9–13 The most modifiable risk factor to decrease resistance and CDI is antibiotic use. A meta-analysis found that stewardship interventions directed at reducing the use of fluoroquinolones and cephalosporins had a protective effect against the development of CDI (pooled risk ratio: 0.48; 95% CI 0.38, 0.62).14 Prior approval and prospective audit with intervention and feedback are core antibiotic stewardship implementation strategies. These stewardship strategies have been shown to be effective in decreasing antibiotic prescribing and are recommended by the Centers for Disease Control and Prevention (CDC), the Infectious Diseases Society of America (IDSA), and the Society for Healthcare Epidemiology of America (SHEA).15–18 Implementation of such policies has been associated with decreased rates of CDI, Extended Spectrum Beta-Lactamase (ESBL) producing bacteria, Methicillin Resistant Staphylococcus aureus (MRSA), and antimicrobial costs without adversely affecting hospital length of stay and survival.18–24
Despite these successes, prior approval is not often implemented due to concerns about loss of prescriber autonomy, potential delays in treatment, adverse effects on professional culture, and the consequences of replacing the use of one antibiotic or antibiotic class with another.18,25,26 Furthermore, the effectiveness of prior approval depends on the manner of enforcement, expertise of the approver, enforcement policies during evenings and weekends, and permitting providers to self-approve the use of formulary restricted antimicrobials if specified criteria are satisfied (e.g, multi-drug resistance).20 These concerns have led many facilities to instead implement prospective audit and feedback (PAF) programs, under which antibiotics can be freely initiated but in which there is a post-prescriptive review within 24 – 72 hours of prescribing. During post-prescriptive review, alternative recommendations for antimicrobials are issued; compliance with such recommendations is voluntary.18 PAF programs have been shown to improve outcomes in general medical/surgical units and intensive care units without adversely affecting patient outcomes.18 Disadvantages of PAF include that implementation is labor intensive, providers may be reluctant to change therapy, specialized technology support may be needed, and success is dependent on the presence of dedicated personnel and the method used to communicate recommendations to prescribers.18,27
Few studies have explicitly assessed the impact of prior approval and PAF implementation strategies on antibiotic prescribing. In light of the FDA safety warnings and recommendations, evidence is needed for implementation strategies to guide prescribing of fluoroquinolones when suitable alternatives are available. Thus, we sought to evaluate the implementation, perception, and effectiveness of prior approval and PAF to impact fluoroquinolone and total antibiotic prescribing in a Practice-Based Research Network (PBRN) of Veterans Affairs (VA) acute care facilities.
METHODS
Study Design, Setting, and Study Population
A cross-sectional survey was conducted with antimicrobial stewardship champions at 15 acute care facilities in April 2018. Study data were collected and managed using REDCap electronic data capture tools. The sample included VA medical centers enrolled in a Practice-Based Research Network (PBRN) jointly supported by the CDC and the VA. The sampling strategy included all VA PBRN facilities to ensure that we capture the diversity of perceptions and implemented practices. The antimicrobial stewardship leader was defined as the facility personnel designated to direct antimicrobial stewardship. The survey focused on the local implementation and acceptability of different strategies to improve fluoroquinolone prescribing.
Survey Development
Questions were closed-ended in the form of a survey (See supplemental Table 1). Development of the survey was focused on topic areas in the Consolidated Framework for Implementation Research (CFIR) domains including: inner setting (organizational context) including current practices and procedures (ie. existing guidelines/formularies), characteristics of individuals (ie. knowledge, attitudes, beliefs) and outer setting (ie. awareness of external policies and incentives). The purpose of the survey and accompanying questions were to 1) Determine which antimicrobial stewardship strategies were implemented, 2) Assess the perception of the potential effectiveness of antimicrobial stewardship strategies, and 3) Identify the perceived acceptability of antimicrobial stewardship strategies. Questions in the survey were developed based on stakeholder input, current work (AHRQ R01HS025175 (PI: Samore); NIA P30AG022849 (PI: Hughes)), and the American Society for Health-Systems Pharmacists best practice statement on formulary management.28–31 Study team members included operational partners, clinicians, and researchers with expertise in the design of surveys to capture provider behavior and strategies to improve prescribing. All study team members reviewed and provided feedback at each stage of questionnaire development. One antimicrobial stewardship physician champion and one antimicrobial stewardship pharmacist piloted the survey and provided feedback.
Antibiotic Prescribing and Facility Characteristics data collection
Facility characteristics and antibiotic administration data were collected from national VA datasets, including the Veterans’ Health Administration (VHA) Corporate Data Warehouse (CDW) for 2017–2018. These datasets were also used to gather information on mean bed days of care, facility complexity, specialty services available, and antibiotic exposure. Facility complexity is a standardized classification system across VHA. Complexity is based on patient characteristics, clinical programs, and teaching programs. Facilities with complexity levels of 1a-c were classified as high complexity facilities and levels 2–3 were classified as low complexity facilities. Specialty services were categorized into presence of bone marrow or stem cell transplant unit, spinal cord injury center, and long-term care facility. Antibiotic exposure was reported overall (all systemic antibacterial classes combined), for fluoroquinolones, and advanced generation cephalosporins. Advanced generation cephalosporins were defined as the third and four generation cephalosporins. For each facility, antibiotic exposure was reported as mean days of therapy and total days of therapy per 1000 acute care days present.
Statistical Analysis
Facilities with audit and feedback (PAF) and/or prior approval were compared with sites without these strategies implemented. PAF was defined as a one-on-one interaction between an antimicrobial steward and a prescriber regarding antibiotic use in a specific case that is conducted within one business day after a restricted antibiotic is prescribed. Prior authorization was defined as the medication cannot be used without review of the specific patient case and indication (includes instances where first dose is allowed but subsequent doses require approval). Independent t-tests and contingency tables were used to determine the differences in continuous and nominal data, respectively. Simple linear regression was applied to test differences antibiotic prescribing over time. All data and statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC). A p value <0.05 was considered statistically significant. The Institutional Review Board (IRB) at the VA Greater Los Angeles Healthcare System approved this study through expedited review.
RESULTS
All PBRN acute care facilities (N=15; 87% antimicrobial stewardship physician; 13% antimicrobial stewardship pharmacist responders) completed the survey for a 100% response rate. Overall, nine (60%) of antimicrobial stewardship champions identified their site as having fluoroquinolone implementation strategies, 2/9 (22%) PAF, 4/9 (44%) prior approval, and 3/9 (33%) both PAF and prior approval. At sites without PAF or prior approval implementation strategies, 20% (N=3) had criteria for use and 13% (N=2) had specific prescriber or care area restrictions in place for fluoroquinolones. The majority of the facilities were high complexity (10/15, 67%) and all were urban sites. Most of the facilities were regionally located in the Midwestern (6/15, 40%) and Southern (5/15, 33%) U.S. geographic regions. Table 1 describes characteristics of facilities with either PAF or prior approval and without these strategies implemented. There was no significant difference in facility characteristics by group, including presence of specialty services and bed days of care.
Table 1.
Facility characteristics and fluoroquinolone PAF and/or prior approval implementation strategies.
| Facility Characteristics | With PAF/prior approval | Without PAF/prior approval | p-value |
|---|---|---|---|
|
Number of facilities |
9 |
6 |
- |
|
Bone marrow or stem cell transplant unit |
3 (33.3%) | 2 (33.3%) | 1 |
|
Long term care |
6 (66.7%) | 4 (66.7%) | 1 |
|
Spinal Cord Injury (SCI) Specialty Care Center |
9 (100.0%) | 6 (100.0%) | 1 |
|
Region | |||
|
Northeast |
1 (11.1%) | 0 | 1 |
|
Midwest |
4 (44.4%) | 2 (33.3%) | 1 |
|
South |
2 (22.2%) | 3 (50.0%) | 0.3287 |
|
West |
2 (22.2%) | 1 (16.7%) | 1 |
|
Facility Complexity Based on Levels of Patient Volume and Risk, Teaching, and Research | |||
|
1a |
5 (55.6%) | 5 (83.3%) | 0.5804 |
|
1b |
3 (33.3%) | 1 (16.7%) | 0.6044 |
|
1c |
1 (11.1%) | 0 | 1 |
|
Other Facility Characteristics (mean±stdev) | |||
| Authorized Beds | 157.67 (±69.5) | 196.33 (±76.4) | 0.3283 |
| Daily Census 2017 | 104.98 (±51.4) | 118.74 (±45.0) | 0.6033 |
| Admissions 2017 | 6,685.56 (±1,867.8) | 8,154.00 (±2,172.4) | 0.185 |
| Admissions 2018 | 6,758.56 (±1,957.9) | 8,264.50 (±2,021.3) | 0.1732 |
| Bed days of care 2017 | 36,333.33 (±14,440.8) | 47,938.83 (±22,742.3) | 0.2452 |
| Bed days of care 2018 | 34,744.33 (±13,148.8) | 43,105.83 (±15,590.0) | 0.2821 |
Advanced generation cephalosporin = 3rd and 4th generation cephalosporins combined
There were no differences in total days of therapy at the facility-level for 2017 or 2018 by the presence or absence of implementation strategies. Figure 1 illustrates the total antibiotic (Figure 1a), fluoroquinolone (Figure 1b), and advanced generation cephalosporin (Figure 1c) prescribing rates for facilities with PAF/prior approval implemented for fluoroquinolones as compared to facilities without PAF/prior approval in place for 2017 and 2018. While the total antibiotic rates (Figure 1a) did not differ by group, fluoroquinolone days of therapy rates (Figure 1b) were significantly lower in facilities with PAF/prior approval. Interestingly, the decrease in fluoroquinolones was achieved in the absence of increased prescribing of advanced generation cephalosporins (Figure 1c).
Figure 1a. Comparison of overall antibiotic prescribing rates stratified by implementation strategies.
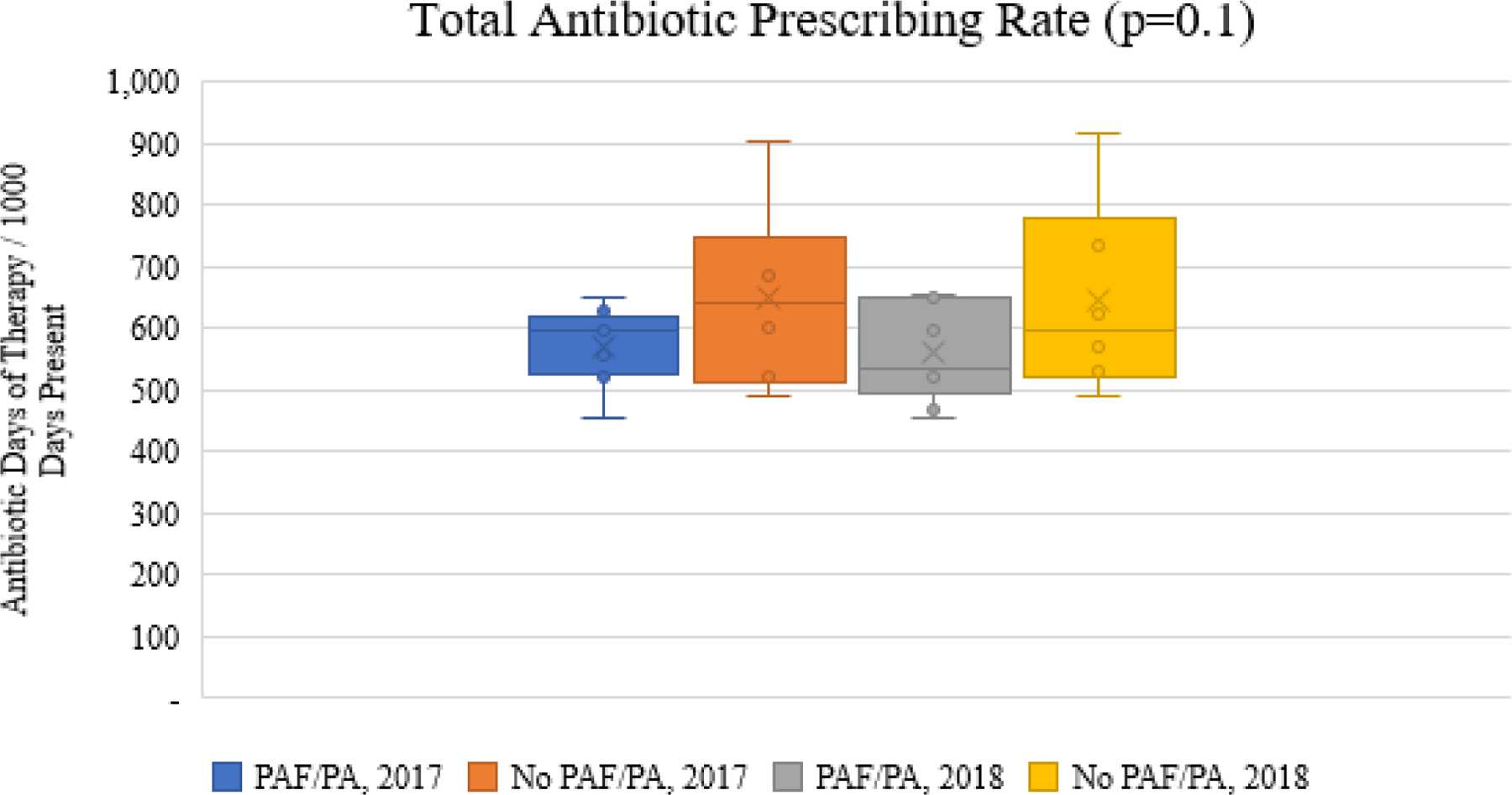
(footnote for the bottom of the figure: *PAF=audit and feedback; PA=prior approval, the bar above and below each box represents the range)
Figure 1b.
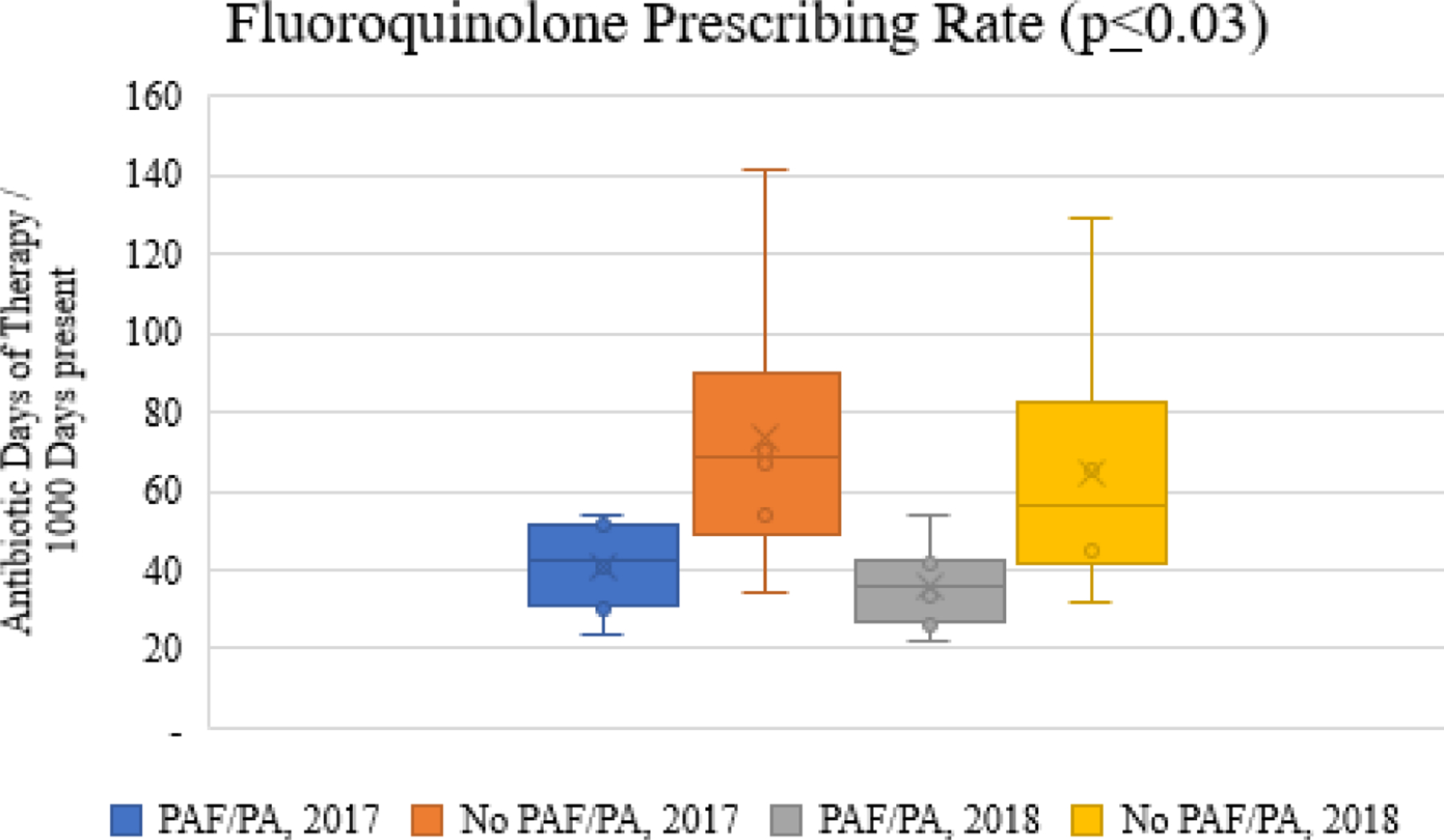
Comparison of fluoroquinolone prescribing rates stratified by implementation strategies
Figure 1c.
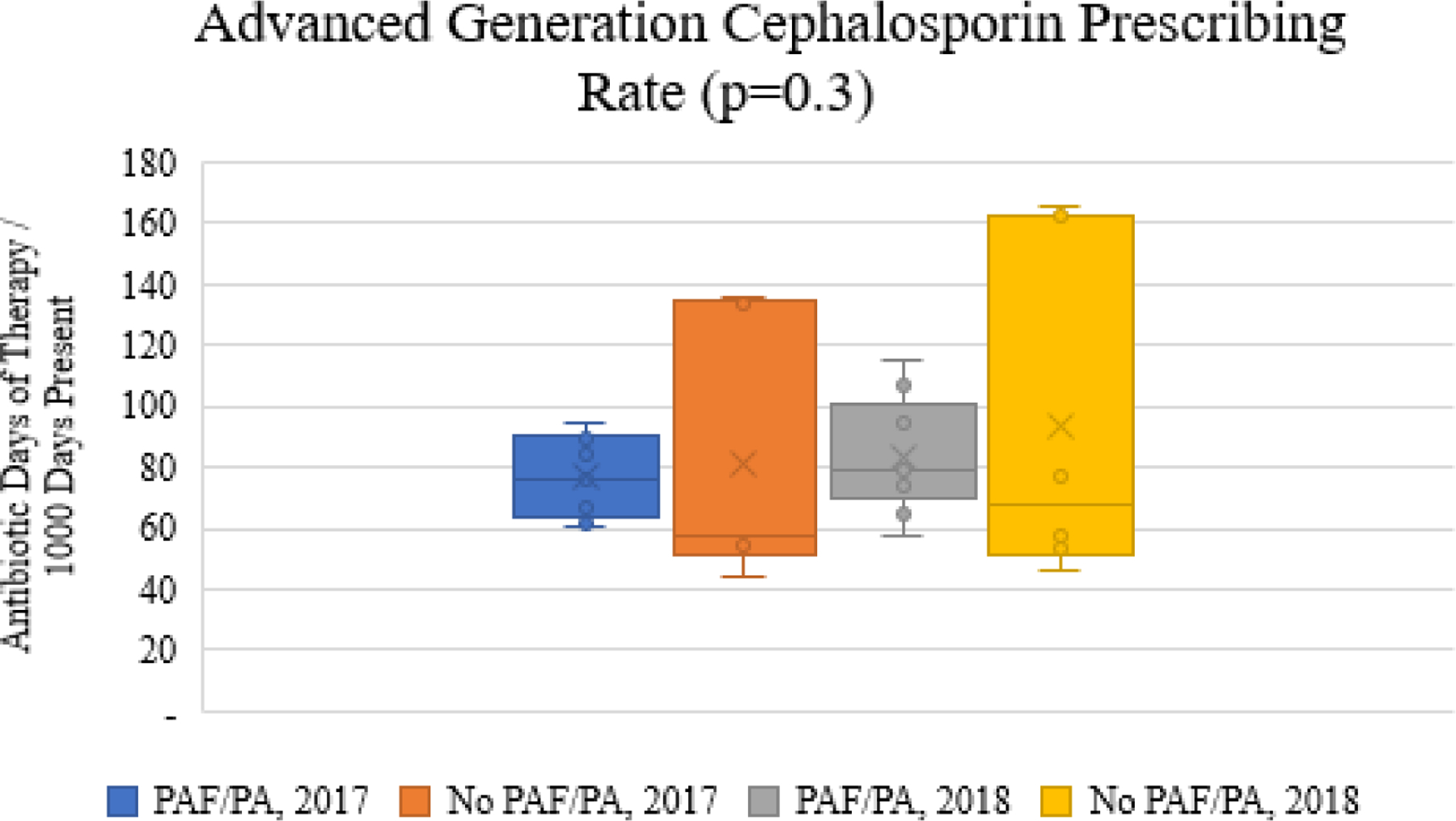
Comparison of advanced generation cephalosporin prescribing rates stratified by implementation strategies
Figures 2a and 2b illustrate fluoroquinolone prescribing rates over time for facilities. The fluoroquinolone days of therapy rate were much lower in facilities with fluoroquinolone PAF/prior approval implementation strategies compared to sites without fluoroquinolone implementation strategies. However, both groups experienced significant decreases in the fluoroquinolone days of therapy rate over the two-year period (p=0.0001; Figures 2a and 2b). Interestingly, in facilities with fluoroquinolone PAF/prior approval implementation strategies there was no change in advanced generation cephalosporin days of therapy rate (p=0.1; Figure 2a) while there was a significant increase (p=0.001; Figure 2b) in cephalosporin rates in the facilities without fluoroquinolone PAF/prior approval. There was no temporal decrease in total antibiotic days of therapy rate for either group (p>0.2; Figures 2a and 2b).
Figure 2a and 2b.
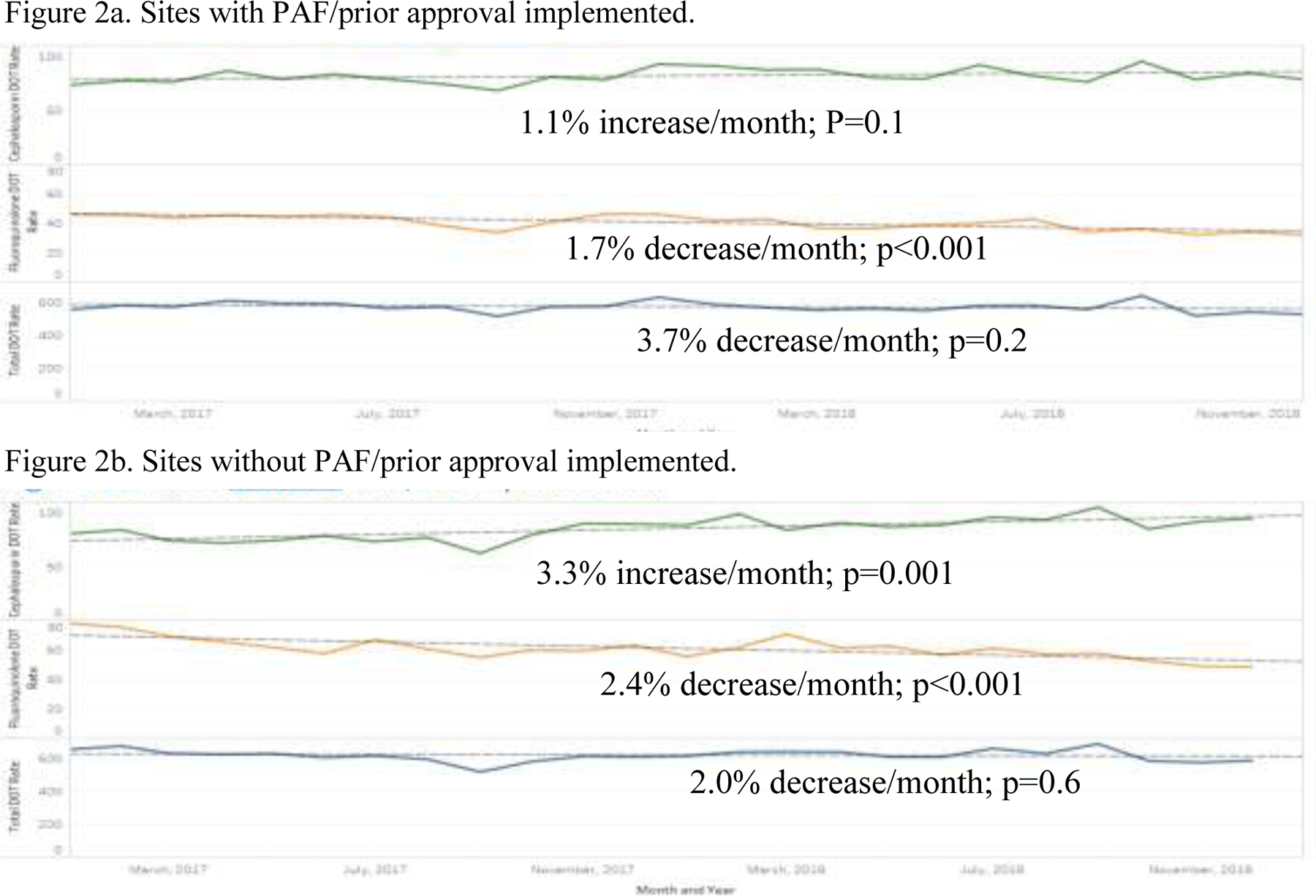
Trends in advanced generation cephalosporin (green), fluoroquinolone (orange) and total (blue) prescribing, 2017–2018.
Table 2 illustrates antimicrobial stewardship leaders’ perception of the level of provider acceptance for current fluoroquinolone PAF/prior approval strategies implemented at their facility (N=8). (One additional site with implementation strategies did not complete this section of the survey and was excluded from the acceptability analysis.) Of the sites with either PAF or prior approval implemented, 5/8 (62.5%) perceived that PAF/prior approval were completely accepted and 3/8 (37.5%) were moderately accepted, respectively. Facilities that perceived fluoroquinolone PAF/prior approval strategies were “completely” accepted by providers had lower fluoroquinolone days of therapy rates than sites where formulary restrictions were “moderately” accepted (p<0.04, Table 2).
Table 2.
Perceived acceptability of fluoroquinolone PAF and prior approval strategies implemented and facility demographics.
| Facility Characteristics | Moderately | Completely | p-value |
|---|---|---|---|
|
Number of facilities |
3 |
5 |
- |
|
Bone marrow or stem cell transplant unit |
1 (33.3%) | 2 (40.0%) | 1 |
|
Long term care |
3 (100.0%) | 4 (80.0%) | 1 |
|
Spinal Cord Injury (SCI) Specialty Care Center |
3 (100.0%) | 5 (100.0%) | 1 |
|
Region |
|
|
|
|
Northeast |
0 | 1 (20.0%) | 1 |
|
Midwest |
1 (33.3%) | 2 (40.0%) | 1 |
|
South |
1 (33.3%) | 1 (20.0%) | 1 |
|
West |
1 (33.3%) | 1 (20.0%) | 1 |
|
Complexity |
|
|
|
|
1a |
3 (100.0%) | 3 (60.0%) | 0.4643 |
|
1b |
0 | 2 (40.0%) | 0.4643 |
|
1c |
0 | 0 | 0.4643 |
|
Other Facility Characteristics (mean±stdev) |
|
|
|
| Authorized Beds | 195.33 (±46.7) | 202.40 (±85.6) | 0.9014 |
| Daily Census 2017 | 109.51 (±69.4) | 97.59 (±25.9) | 0.7309 |
| Admissions 2017 | 8,793.67 (±1,544.6) | 7,712.20 (±1,821.0) | 0.4258 |
| Admissions 2018 | 8,765.33 (±1,157.6) | 7,655.40 (±1,787.2) | 0.3802 |
| Bed days of care 2017 | 49,011.33 (±6,995.7) | 51,682.20 (±22,864.2) | 0.8545 |
| Bed days of care 2018 | 48,472.33 (±8,170.4) | 44,470.00 (±14,817.0) | 0.6877 |
| Days of therapy 2017 | 28,344.00 (±1,535.5) | 28,912.20 (±9,997.5) | 0.9276 |
| Antibiotic prescribing (mean±stdev) | |||
| Total Antibiotic Days, 2017 | 28,344.0 (±1,535.5) | 28,912.2 (±9,997.5) | 0.9276 |
| Total Antibiotic Days, 2018 | 26,545.7 (±1,242.7) | 25,209.4 (±7,444.7) | 0.7751 |
| Fluoroquinolone days of therapy / 1000 days present, 2017 | 57.4 (±8.3) | 40.1 (±9.0) | 0.0363 |
| Fluoroquinolone days of therapy / 1000 days present, 2018 | 48.7 (±4.5) | 34.2 (±5.7) | 0.0097 |
| Advanced generation cephalosporin days of therapy / 1000 days present, 2017 | 64.28 (±27.3) | 74.20 (±14.1) | 0.5128 |
| Advanced generation cephalosporin days of therapy / 1000 days present, 2018 | 64.53 (±26.3) | 80.29 (±17.6) | 0.3416 |
Advanced generation cephalosporin = 3rd and 4th generation cephalosporins combined
Antimicrobial stewardship leaders at all study sites (N=15 sites) were asked on their perceived effectiveness of potential stewardship strategies. These included mandatory infectious disease (ID) consults for certain conditions, clinical pathways / local guidelines, antimicrobial order forms, and prior approval on fluoroquinolone prescribing. With the exception of mandatory ID consults, the majority of stewards perceived clinical pathways / local guidelines, antimicrobial order forms, and prior approval would be effective or has already been implemented to improve fluoroquinolone prescribing (Figure 3). For prior approval and clinical pathways, only one site either did not already have this strategy implemented for fluoroquinolones or did not believe that it would be effective in reducing fluoroquinolone use. Most antimicrobial stewardship leaders (13/15; 87%) perceived implementation of mandatory ID consults would be difficult.
Figure 3.
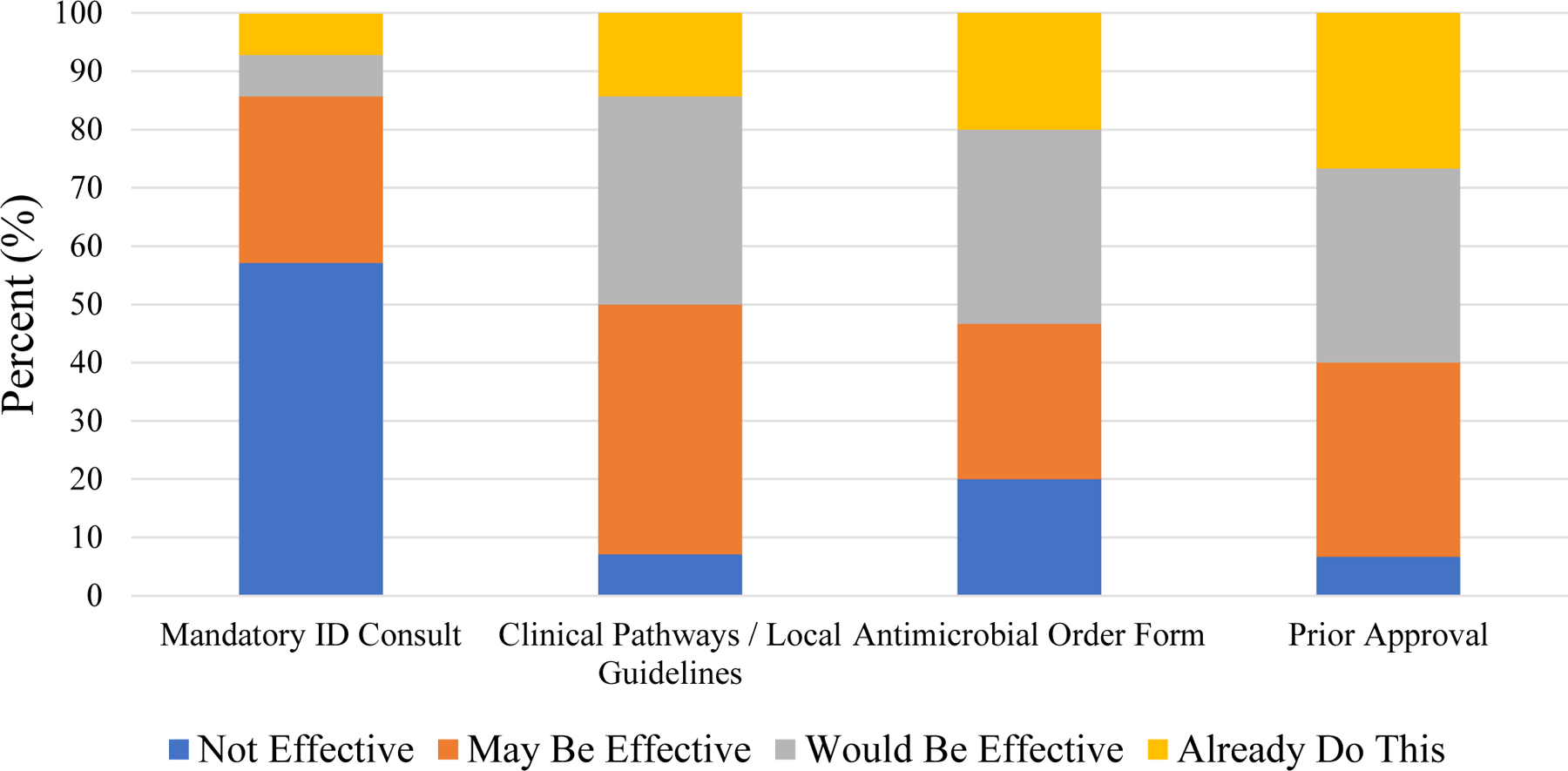
Perceived effectiveness of implementation strategies on fluoroquinolone prescribing.
DISCUSSION
Implementation strategies to improve fluoroquinolone prescribing varied across the PBRN acute care sites. Those facilities that implemented fluoroquinolone PAF/prior approval implementation strategies had decreased quinolone prescribing over the study period without a corresponding increase in advanced generation cephalosporin prescribing, a common alternative. Conversely, in facilities without implementation strategies decreased fluoroquinolone use was accompanied by increased use of advanced generation cephalosporins. Neither group had decreases in overall antibiotic prescribing by year or overall. While stewards are frequently concerned that implementing strategies focused on the fluoroquinolones will lead to increases in other antibiotics with a high risk of CDI (i.e., advanced generation cephalosporins), we found that facilities without fluoroquinolone PAF or prior approval experienced increases in advanced generation cephalosporins. The literature on the impact of stewardship strategies to shift use from fluoroquinolones to advanced generation cephalosporins is scarce. However, many of the facilities with PAF and prior approval implemented for quinolones also had strategies in place for cephalosporins.
PAF and prior approval implementation strategies have been associated with a 50% reduction in acute care fluoroquinolone prescribing.14 A quasi-experimental, crossover trial found that PAF had a greater effect on decreasing antimicrobial use than prior approval.33 In contrast, a 2013 Cochrane review of 52 studies found that although outcomes at 12–24 months were similar, restrictive prior approval had a more rapid, salutary effect on antimicrobial use than did persuasive, PAF programs.17 Similarly, a meta-analysis found prior approval to be more effective than persuasive PAF strategies in reducing CDI14; this difference was not found in a second analysis that compared a broader range of restrictive and persuasive policies.25 A study in Scotland found that prior approval of amoxicillin/clavulanate, clindamycin, fluoroquinolones and 3rd generation cephalosporins were associated with reductions in CDI and methicillin resistant S. aureus (MRSA) infections despite a reciprocal doubling of alternative antibiotic use.22,24 A systematic review found that prior approval strategies targeting the fluoroquinolone class were associated with decreases in MRSA, CDI and quinolone-resistant P. aeruginosa, A. baumannii, and E. coli.34 PAF strategies focused on fluoroquinolones similarly observed decreases in MRSA, CDI, extended spectrum B-lactamase-producing organisms and quinolone-resistant P. aeruginosa.34
This study is not without limitations. The sample of acute care hospitals participated in a Practice-Based Research Network and, thus may have been more supportive of evidence-based stewardship practices than other VA or non-VA facilities. Also, whereas 8 of the 9 facilities that implemented PAF and/or prior approval for fluoroquinolones implemented similar strategies for cefepime, ceftazidime, or ceftriaxone/cefotaxime only one of the 5 facilities without such fluroquinolone policies had similar policies for the use of these cephalosporins. Pharmacist time directly engaged in antimicrobial stewardship may also impact our results. At our PBRN sites, pharmacists spent 100% of their time on antimicrobial stewardship activities at 46.7% of facilities, 75% time at 20% of facilities, and 50% time at 33.3% of facilities. The survey was composed of closed-ended questions and allowed for little depth in material obtained. Only one person responded per facility to the survey. Thus, the responses may not reflect overall attitudes at their facility and may have introduced ecologic fallacy. The survey was administered in April 2018 and may not reflect current practices or perceptions after the multiple warnings issued on the fluoroquinolone class by the Food and Drug Administration in 2018.
Regardless, our study informs the effectiveness and acceptability of implementation strategies to improve fluoroquinolone prescribing. Furthermore, we demonstrate the feasibility of decreasing fluroquinolone use without increasing the use of other drugs associated with increased rates of C. difficile infection, namely third and fourth generation cephalosporins. Facilities are motivated and perceive an opportunity to reduce fluoroquinolone use with a variety of implementation strategies. While clinical pathways / local guidelines, antimicrobial order forms, and prior approval were perceived to be effective in improving fluoroquinolone prescribing, mandatory ID consults were perceived to be difficult to implement. Therefore, external facilitation should be provided to sites to encourage interventions targeting fluoroquinolones. While sites may perceive a high workload with the fluoroquinolone class, data on all new acute care orders should be assessed for appropriateness and potential for alternative treatment. Our work has demonstrated that the volume of new orders is lower than that perceived. Therefore, antimicrobial stewardship programs should be involved in all implementation strategies that use persuasive or restrictive strategies on antimicrobials to assess acceptability and feasible implementation. The antimicrobial stewardship program should also determine effectiveness, especially class substitution (a reduction in prescribing of a particular class with a corresponding increase in another class). For the fluoroquinolone class, a common substitution are the advanced generation cephalosporins. This is concerning due to similar concerns of CDI as the fluoroquinolone class.35 Our results demonstrate that facilities with PAF/prior approval implemented were effective in decreasing fluoroquinolone prescribing without a corresponding increase in advanced generation cephalosporins. While there was a decrease in fluoroquinolone prescribing over time in facilities without PAF/prior approval implemented, this was achieved with an increase in advanced generation cephalosporins.
Additional research should be conducted to provide further insight as to facilitators and barriers to implementing persuasive and restrictive strategies on the use of fluoroquinolones. In addition, appropriate antibiotic substitutes for these agents should be identified based on safety, local sensitivities, and as recommended in treatment guidelines. Finally, factors impacting the decision making of antimicrobial selection and feasibility and acceptability of implementation of PAF and prior approval by non-stewards should be assessed.
CONCLUSION
PAF and prior approval implementation strategies focused on fluoroquinolones were associated with lower fluoroquinolone prescribing rates in acute care. With a trend toward lower total antibiotic use there was also no evidence of significant class substitution. Fluoroquinolone prescribing rates decreased in the PAF/prior approval sites without a corresponding increase in advanced generation cephalosporins. This may indicate increased acceptability of implementation strategies and/or sensitivity to the FDA warnings. The acute care PBRN sites perceived most formulary restrictions to be effective in improving fluoroquinolone use. Acceptability of antibiotic stewardship strategies which focus on medication restrictions may lower antibiotic prescribing rates and improve implementation.
Supplementary Material
ACKNOWLEDGEMENTS
Financial Support:
This material is based upon work supported by Centers for Disease Control and Prevention, Practice-Based Research Network (PBRN).
Footnotes
Previous presentation: This work was presented in part at the 2019 IDWeek in Washington, D.C. as a poster presentation.
Conflicts of Interest: None
Disclosures: The opinions expressed are those of the authors and do not represent those of the Department of Veterans Affairs, the Centers for Disease Control or Prevention or the U.S. government.
REFERENCES
- 1.US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects FDA website. www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Published 2016. Updated 2018. Accessed August 30, 2020. [Google Scholar]
- 2.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004;38:S341–S345. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients FDA website. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics. Published 2018. Updated 2019. Accessed August 30, 2020. [Google Scholar]
- 4.US Food and Drug Administration. FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes FDA website. www.fda.gov/Drugs/DrugSafety/ucm611032.htm. Published 2019. Updated 2018. Accessed August 30, 2020. [Google Scholar]
- 5.Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ 2018;360:k678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park-Wyllie L, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006;354:1352–1361. [DOI] [PubMed] [Google Scholar]
- 7.Chou HW, Wang JL, Chang CH, Lee JJ, Shau WY, Lai MS. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin Infect Dis 2013;57:971–980. [DOI] [PubMed] [Google Scholar]
- 8.Summary Minutes of the Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Risk and Safety Management Advisory Committee. Food and Drug Administration Center for Drug Evaluation and Research; November 5, 2015; Silver Spring, MD. [Google Scholar]
- 9.Low M, Neuberger A, Hooton TM, et al. Association between urinary community-acquired fluoroquinolone-resistant Escherichia coli and neighborhood antibiotic consumption: a population-based case-control study. Lancet Infect Dis 2019;19:419–428. [DOI] [PubMed] [Google Scholar]
- 10.Brown KA, Khanafer N, Daneman N, et al. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013;57:2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014;69:881–891. [DOI] [PubMed] [Google Scholar]
- 12.Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents 2016;48:1–10. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013;68:1951–1961. [DOI] [PubMed] [Google Scholar]
- 14.Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014;69:1748–1754. [DOI] [PubMed] [Google Scholar]
- 15.Dellit TH, Owens RC, McGowan JE Jr., et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 16.Filice G, Drekonja D, Greer N, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014;35:1209–1228. [DOI] [PubMed] [Google Scholar]
- 17.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013;4:CD003543. [DOI] [PubMed] [Google Scholar]
- 18.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62:e51–e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White AC Jr., Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis 1997;25:230–239. [DOI] [PubMed] [Google Scholar]
- 20.Buising KL, Thursky KA, Robertson MB, et al. Electronic antibiotic stewardship--reduced consumption of broad-spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J Antimicrob Chemother 2008;62:608–616. [DOI] [PubMed] [Google Scholar]
- 21.Dancer SJ, Kirkpatrick P, Corcoran DS, Christison F, Farmer D, Robertson C. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum beta-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 2013;41:137–142. [DOI] [PubMed] [Google Scholar]
- 22.Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis 2015;15:1438–1449. [DOI] [PubMed] [Google Scholar]
- 23.Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017;990–1001. [DOI] [PubMed]
- 24.Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: a non-linear time-series analysis. Lancet Infect Dis 2017;17:194–206. [DOI] [PubMed] [Google Scholar]
- 25.Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szymczak J, Muller B, Shakamuri N, et al. Prescriber perceptions of fluoroquinolones, extended-spectrum cephalosporins, and Clostridioides difficile infection. Infect Control Hosp Epidemiol 2020;41:914–920. [DOI] [PubMed] [Google Scholar]
- 27.Cosgrove SE, Seo SK, Bolon MK, et al. Evaluation of postprescription review and feedback as a method of promoting rational antimicrobial use: a multicenter intervention. Infect Control Hosp Epidemiol 2012;33:374–380. [DOI] [PubMed] [Google Scholar]
- 28.American Society of Health-System Pharmacists. ASHP statement on the pharmacy and therapeutics committee and the formulary system. Am J Health-Syst Pharm 2008;65:2384–2386. [Google Scholar]
- 29.American Society of Health-System Pharmacists. ASHP guidelines on the pharmacy and therapeutics committee and the formulary system. Am J Health-Syst Pharm 2008;65:1272–1283. [DOI] [PubMed] [Google Scholar]
- 30.American Society of Health-System Pharmacists. Principles of a Sound Drug Formulary System Available at: https://www.ashp.org/-/media/assets/policy-guidelines/docs/endorsed-documents/endorsed-documents-principles-sound-drug-formulary-system.ashx. Published 2000. Updated 2011. Accessed August 30, 2020.
- 31.American Society of Health-System Pharmacists. Formulary Management Available at: https://www.ashp.org/-/media/assets/policy-guidelines/docs/policy-positions/policy-positions-formulary-management.ashx?la=en&hash=70B0D7D96BD1B3FF03A06FBE5F7757EEFF5BFDE7. Published 2000. Updated 2015. Accessed August 30, 2020. [PMC free article] [PubMed]
- 32.US Department of Veterans Affairs. VHA Directive 1108.08, VHA Formulary management Process November 2, 2016.
- 33.Tamma PD, Avdic E, Keenan JF, et al. What Is the More Effective Antibiotic Stewardship Intervention: Preprescription Authorization or Postprescription Review With Feedback? Clin Infect Dis 2017;64:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitiriga V, Vrioni G, Saroglou G, Tsakris A. The Impact of Antibiotic Stewardship Programs in Combating Quinolone Resistance: A Systematic Review and Recommendations for More Efficient Interventions. Adv. Ther 2017;34:854–865. [DOI] [PubMed] [Google Scholar]
- 35.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


