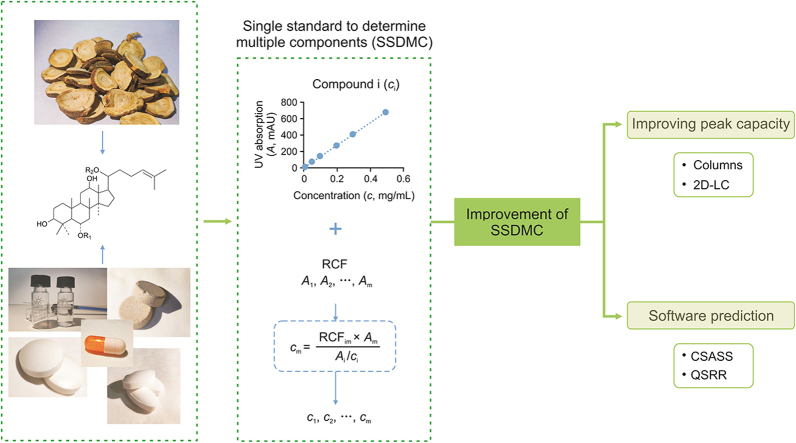
Abstract
Complex systems exist widely, including medicines from natural products, functional foods, and biological samples. The biological activity of complex systems is often the result of the synergistic effect of multiple components. In the quality evaluation of complex samples, multicomponent quantitative analysis (MCQA) is usually needed. To overcome the difficulty in obtaining standard products, scholars have proposed achieving MCQA through the “single standard to determine multiple components (SSDMC)” approach. This method has been used in the determination of multicomponent content in natural source drugs and the analysis of impurities in chemical drugs and has been included in the Chinese Pharmacopoeia. Depending on a convenient (ultra) high-performance liquid chromatography method, how can the repeatability and robustness of the MCQA method be improved? How can the chromatography conditions be optimized to improve the number of quantitative components? How can computer software technology be introduced to improve the efficiency of multicomponent analysis (MCA)? These are the key problems that remain to be solved in practical MCQA. First, this review article summarizes the calculation methods of relative correction factors in the SSDMC approach in the past five years, as well as the method robustness and accuracy evaluation. Second, it also summarizes methods to improve peak capacity and quantitative accuracy in MCA, including column selection and two-dimensional chromatographic analysis technology. Finally, computer software technologies for predicting chromatographic conditions and analytical parameters are introduced, which provides an idea for intelligent method development in MCA. This paper aims to provide methodological ideas for the improvement of complex system analysis, especially MCQA.
Keywords: Multicomponent quantification analysis, Single standard to determine multiple components, Predictive software
Graphical abstract
SSDMC: single standard to determine multiple components; RCF: relative correction factor; 2D-LC: Two dimensional liquid chromatography; CSASS: complex sample analysis software system; QSRR: quantitative structure-retention relationship.
Highlights
-
•
Reviewing development progress of SSDMC method in complex system analysis.
-
•
Reviewing chromatographic columns selection and 2D-LC analysis method construction.
-
•
Summarizing computer-aided techniques for predicting chromatographic analytical parameters.
-
•
Predicting future development direction of MCQA.
1. Introduction
A complex system is a kind of research object that often appears in analytical chemistry research and widely exists in different domains, such as food and pharmaceuticals [1]. Chemical substances are the basis for drugs to exert their therapeutic effects [2]. There are thousands of components with various structures in one complex sample, whether it is a synthetic complex sample or a sample from a natural source. Meanwhile, the complex sample may also contain many structural analogues (including isomers) with similar physical and chemical properties, and these analogues and/or their metabolites may have similar biological activities. As a result, for the quality evaluation of these complex systems, especially the quantitative evaluation of samples, the index components need to contain different types of components and different components of the same structural analogues. Therefore, multicomponent quantitative analysis (MCQA) is needed to comprehensively characterize the quality of complex samples [3,4].
Currently, “quantitative analysis of multiple components by a single marker (QAMS)” and “single standard to determine multiple components (SSDMC)” based on liquid chromatography (LC) analysis are important methods that meet the practical application needs in MCQA. Due to its low cost, ease of use and durability, (ultra) high-performance liquid chromatography (U/HPLC) is the most commonly used, modern, and universal separation and quantitative technology. The most commonly used detector for U/HPLC is an ultraviolet (UV) and/or photodiode array detector, and its quantitative principle is based on the Lambert‒Beer law [5]. In practical applications, generally, all the standards of the targeted components are purchased, and external standard methods are used for multicomponent analysis (MCA). However, difficulties in obtaining most standard products limit the development of the MCQA of complex systems, which are related to the difficult preparation, unstable structure, and high prices of some standard products. In 2006, Wang et al. [6] proposed the MCQA concept of QAMS, which quantified multiple components simultaneously with a standard product by establishing internal functional relations between the components. The key factor connecting a single component and other components was called the relative correction factor (RCF), whose accuracy and durability largely determined the quantitative accuracy and stability of the QAMS. Guo and co-workers [7] called it SSDMC, conducted a comprehensive study on the stability of RCFs and standardized the basic procedures of the SSDMC approach. The established SSDMC approach of Salvia Miltiorrhizae Radix et Rhizoma was written into the Chinese Pharmacopoeia (Ch.P.) and the United States Pharmacopoeia. On this basis, Wang et al. [8] studied the influence of various factors on the accuracy of SSDMC quantitative results. Scholars such as Dong et al. [9] and Yang et al. [10] studied RCF calculation methods and proposed relevant suggestions.
The efficient utilization and development of modern chromatographic separation technology is one of the important directions for the development of MCA methods. In complex system analysis with LC, maximizing the effective separation of different kinds of compounds is a key step in MCA, including MCQA and fingerprint research. This directly leads to inaccurate quantitative results and brings great difficulties to subsequent quantitative and related studies if the adjacent peaks fail to reach baseline separation or produce overlapping peaks [11,12]. In LC, the choice of the optimal separation conditions should consider a range of experimental parameters, such as the choice of LC mode, the stationary phase type, and the nature of the mobile phase (e.g., the type of mobile phase) [13]. In addition to column selection, two-dimensional LC (2D-LC) with columns that have different retention mechanisms may separate the adjacent peaks that cannot achieve baseline separation in one dimension to reach good peak capacity and high accuracy in MCA.
The intelligent development of analytical methods is another important direction for the efficient development of MCA methods in the future. With the continuous development of computer technology, traditional chemical analysis experiments are gradually combined with high-tech methods to save manpower, material resources, and time costs. Some prediction software has been gradually developed to assist experimental analyses [14,15]. The development of these techniques, to some extent, replaces many complicated experimental operations, whether in terms of chromatographic separation conditions or retention time (tR) prediction. At the same time, these computer-aided techniques provide the possibility for accurate analysis of multiple components of complex samples.
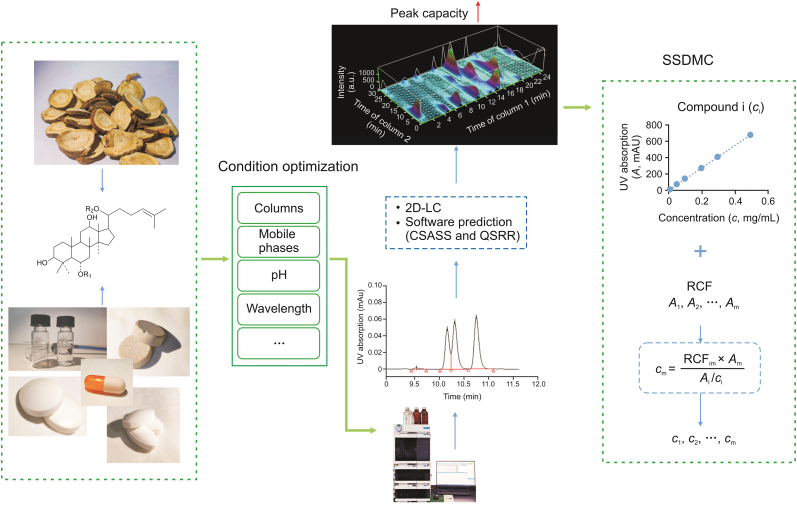
This paper summarizes the development process and application cases of the SSDMC approach in complex system analysis. From the perspective of improving the efficiency of MCA method establishment, the rational selection of chromatographic columns and the construction of 2D-LC analysis method, performed to improve the separation ability and peak capacity, are reviewed. Then, the computer-aided techniques for predicting chromatographic conditions and analytical parameters are summarized, which provides an idea for intelligent development in MCA. Finally, the existing problems and future development direction of MCA are predicted. In summary, this paper summarizes and analyzes the research on SSDMC methodology, chromatographic separation methods, and computer-aided techniques applied in MCA to provide insights into the improvement of complex system analysis, especially the MCQA method (Fig. 1).
Fig. 1.
Solutions to improve the multicomponent quantification analysis of complex systems. 2D-LC: two-dimensional liquid chromatography; CSASS: complex sample analysis software system; QSRR: quantitative structure-retention relationship; SSDMC: single standard to determine multiple components; RCF: relative correction factor.
2. Multicomponent quantification analysis by SSDMC
2.1. Theoretical foundation
The QAMS concept was proposed by Zhimin Wang in 2006 [6] as a MCQA method for traditional Chinese medicine (TCM). The basic idea is that there are internal functions or proportion relationships among the different active components of TCM; when one component (for which the standard product is available) is measured, other multiple components can be calculated simultaneously (for which the standard products are unavailable or difficult to supply) [6]. Later, Guo and co-workers [7] called this method SSDMC (Fig. 2). The SSDMC approach is mainly aimed at components with similar UV spectral and chromatographic behavior [16]. The SSDMC concept is based on the principle of LC quantification, which can be expressed as the amount (mass or concentration, c) of one component proportional to its peak area (A) in the detector in a certain range (linear range) (Formula 1, the “F” is proportionality coefficient).
| (1) |
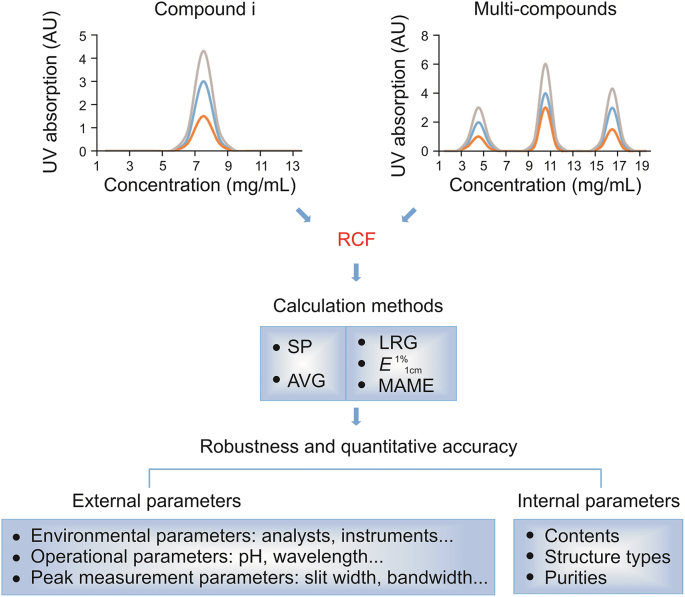
Fig. 2.
Single standard to determine multiple components (SSDMC) approach for the quality analysis of complex systems. UV: ultraviolet; RCF: relative correction factor; SP: slope; AVG: average; LRG: linear regression; E1%1cm: absorption coefficient; MAML: multimarkers assay by the monolinear.
In the MCQA, a typical component is used as the single reference standard (SRS) to establish the RCF between the SRS and other components. The contents of other components are calculated by the RCF. One of the components, i, is selected as the SRS. The RCF between component i and other components m is calculated by using Formula 2.
| (2) |
| (3) |
Formula 2, used for quantitative calculation, can be transformed into Formula 3, where Ai is the peak area of the SRS and ci is the concentration of the SRS. Am is the peak area of other components m, and cm is the concentration of other components m.
2.2. Different calculation methods of RCFs
2.2.1. Classic calculation method of RCFs
2.2.1.1. Slope (SP) method
The RCF is defined as the ratio of the responses in a unit concentration of the SRS and other components to be measured [17]. The RCF is a key factor determining the accuracy of the SSDMC approach. There are two main methods of calculating the RCF. The first method is the SP method. The RCF can be calculated by the ratio of the slopes of the SRS (ki) and analyte (km) when the intercept (b) is small enough to be neglected ((4), (5)).
| (4) |
| (5) |
The SP method can avoid the effects caused by concentration changes, but in most cases, the intercept of the standard curve cannot be ignored due to systematic and/or random errors.
2.2.1.2. Average (AVG) method
The second method is to calculate the average value of the ratios of A/c, called the AVG method, which uses Formula 2 to calculate the RCF at each concentration point at different concentrations (x = 1, 2, …, n) and then calculate the average RCF value (NRCF) ((6), (7)).
| (6) |
| (7) |
For the AVG, the background absorption and smaller peak areas may cause a large A/c difference [18]. Experimental instruments, structural features, detection wavelengths, and purity of standards may cause fluctuations in the RCF at low concentrations [16]. For example, in the study of triterpene acids in Ganoderma lucidum by the SSDMC approach [18], the standard curve of compound 7 included seven points, namely, S1–S7, with increasing concentration. The A/c ratios of S1 and S2 (concentrations < 0.015 mg/mL) were significantly different from those of S3–S7 (0.15–0.30 mg/mL). The relative standard deviation (RSD) of compound 7 calculated by the AVG and SP was 7.80%. If the concentration points of S3–S7 were selected, the RSD calculated by the two methods was 1.89%. However, the slope values obtained from S1–S7 and S3–S7 were similar (RSD of 0.83%).
The SP method and AVG method are the two most commonly used methods for RCF calculations in the SSDMC approach. Some researchers have further studied RCF calculation methods to reduce the inaccuracy of quantitative results caused by RCF errors.
2.2.2. Development of RCF calculation methods
2.2.2.1. Linear regression (LRG) method
To solve the above problems, Wang et al. [8] established a new RCF calculation method based on Formula 3, called the LRG method, which calculated the RCF by linear regression using the linear relationship between cm and (Am × ci/Ai). That is, the linear regression equation of cm and (Am × ci/Ai) was established, and the slope of the equation was regarded as the RCF.
| (8) |
The stability of the RCF was affected by SRS species and the concentrations of other components. The results showed that the LRG method was more consistent than the AVG method when different SRSs were used. The RCFs calculated by the AVG method fluctuated significantly at different concentrations of analytes. When the SRS content was low (<0.1 mg/mL), the difference between the RCFs calculated using these two methods increased, and a better calculation method should be selected [8]. Wang et al. [8] used the SMD (calculated according to Formula 8) to represent the difference between the results obtained by the SSDMC approach and the external standard method to evaluate the accuracy of the LRG-SSDMC and AVG-SSDMC methods. The results showed that the SMDs of the LRG-SSDMC method were mostly lower than those of the AVG-SSDMC method, which means that the determination result of the LRG-SSDMC method was closer to that obtained by the external standard method and that LRG-SSDMC had higher accuracy than the AVG-SSDMC method. However, the results of Yi et al. [19] are slightly different from those of Wang et al. [8]. Yi et al. [19] used the SSDMC approach to study the phenolic acids, triterpenoids, and flavonoids of Ilex Kudingcha C.J. Tseng. The results showed that the deviation between the results of the LRG-SSDMC method and the external standard method was relatively larger when the analytical content level was in parts per million. However, such deviation at low concentration levels would be dramatically reduced with decreasing intercept values. The content calculated by the LRG-SSDMC method would be closer to that calculated by the external standard method only if the intercept value was negligible. Therefore, Yi et al. [19] proposed that the AVG-SSDMC method was more suitable and general in most cases and suggested that the LRG-SSDMC method could replace the AVG-SSDMC method to achieve higher accuracy for quantitative determination only when intercept influence could be neglected.
2.2.2.2. Absorption coefficient (E1%1cm) method
Dong et al. [9] proposed that the RCF calculated by the A/c between the SRS and other components could be replaced by the ratio of the E1%1cm of the SRS and other components based on the absorption intensity being positively correlated with the concentration and absorption coefficient according to the Lambert‒Beer law. The RCF was calculated by the ratio of the E1%1cm values of the SRS and other components (Formula 9).
| (9) |
The RCF calculated by E1%1cm was compared with the results obtained by two classical calculation methods (SP and AVG). The results showed that the RCFs calculated by the E1%1cm method were similar to the RCFs determined by the AVG method but slightly different from those determined by the SP method. It was presumed that the linear regression equation usually had an intercept that could not be ignored. The SMDs between E1%1cm-SSDMC (using E1%1cm to obtain the RCF) and the external standard method were calculated, as well as the SMDs between AVG-SSDMC and the external standard method. The results showed that SMDs ranged from 0% to 7.1% in E1%1cm-SSDMC method and 0%–12.5% in AVG-SSDMC method. E1%1cm-SSDMC method could avoid bias when the sample concentration was close to the limit of quantification (LOQ).
2.2.2.3. Multimarker assay by the monolinear (MAML) method
Yang et al. [10] also proposed a multimarker assay by using the MAML method, which was based on the SSDMC principle to establish standard curves of multiple compounds. The MAML method uses the standard curve of the SRS to calculate the RCF, errors, and reliability of the standard curve parameters of other compounds. The calculation formula ((10), (11)) is as follows:
| (10) |
| (11) |
where ki and km represent the curve slope of the SRS and other components, respectively, b is the curve intercept, and bi/ci and bm/cm reflect the influence of the intercept and concentration, respectively. When b/c is smaller, b/c ≤ 1% b (error less than 1.0%), the slope formula (Formula 5) can be directly obtained, that is, the SP method.
This calculation method is an algorithm of the SP method improved by adding an intercept into the calculation formula, which is often ignored in the SP method, after simple mathematical operation of the standard curve of the SRS and other components. The error problem caused by the neglected intercept in the SP method has been overcome, and the final calculation results also prove that the accuracy of this calculation method is higher than that of the AVG method.
2.2.2.4. Calculation of relative correction factors in silico
Yin et al. [20] proposed an in silico-based strategy for the large-scale production of RCFs that consisted of four data processing steps, namely, raw data collection, calibration curve screening, RCF generation based on computer algorithms, and, if necessary, the introduction of a modifying factor of RCFs based on experimental validation. Calibration curves were screened from the collected data by the criterion b/(k × cLOQ) < 1, where b is the curve intercept, k is the curve slope, and cLOQ is the lower LOQ. Then, an algorithm was developed and could randomly and automatically pick up a large number of different concentration points and corresponding peak areas (up to 1000 concentration points) from the linear gradients of the measured compounds and SRS to generate the NRCF based on the principle of the AVG method. In the corresponding published papers, the contents of the corresponding components were calculated by using the NRCF in silico, the results of which were compared with those generated by the external standard method to evaluate accuracy.
This research enables interdisciplinary SSDMC research, using modern high-tech technology to simplify experimental operations and improve accuracy. However, there are few studies on the practical application of the algorithm in this study, and its convenience and accuracy in the practical application process remain to be investigated.
The strengths, weaknesses, and application range of different RCF calculation methods are summarized in Table 1.
Table 1.
Strengths and limitations of different calculation methods of relative correction factors (RCFs).
| Methods | Strengths | Weaknesses | Application range |
|---|---|---|---|
| SP | Avoid effects caused by concentration changes; Simple calculation process. | The ignored intercept may lead to large errors in most cases. | Wide range of analyte content; highly accurate results when intercept can be ignored. |
| AVG | Wide application; simple calculation process. | RCF fluctuates significantly at different wavelengths and concentrations. | The analyte is not a trace component; stable wavelength. |
| LRG | High accuracy and stability when the intercept tends to be zero. | The intercept significantly affects the accuracy of the results. | Intercept is almost zero; the analyte is not a trace component; high accuracy calculation. |
| E1%1cm | When E1%1cm is known, it can be calculated directly to avoid experiment. When the sample concentration is close to LOQ, deviation can be avoided. | The E1%1cm of compound is unknown, and additional measurement of E1%1cm will increase experimental error. | Known E1%1cm. The content of analyte is close to LOQ. |
| MAML | Improved algorithm of SP method; overcome error caused by ignored intercept in SP method; higher accuracy. | Relatively complicated calculation process. | The non-negligible intercept; wide range of analyte content; high accuracy calculation. |
SP: slope; AVG: average; RCF: relative correction factor; LRG: linear regression method; E1%1cm: absorption coefficient; LOQ: limit of quantification; MAML: multimarkers assay by the monolinear method.
2.3. Robustness and quantitative accuracy of the method
2.3.1. Factors for the repeatability of RCF quantitative accuracy
The RCF is the key parameter of the SSDMC approach, and its stability determines the quantitative accuracy of the SSDMC approach. Therefore, researchers are investigating various factors that may affect the stability of RCFs, such as wavelength, column, and concentration. Hou et al. [7] comprehensively investigated the influence of various external factors on RCFs, which were divided into 1) operational parameters, including the pH value, proportion and gradient of mobile phase, flow rate, detection wavelength, column length, column temperature, injection volume, and concentration of the reference standard; 2) peak measurement parameters, including different slit width, bandwidth, and integration parameters; and 3) environmental parameters, including different analysts, instruments, and columns. The results showed that the UV absorption spectrum and content of the analyte had the greatest influence on RCFs. If the UV absorption curve of the analyte was sharp at the measured wavelength, the influence of wavelength change on peak area was more obvious, which led to the influence of the instrument, wavelength, slit, and bandwidth related to UV absorption on RCFs. The wavelength factor was the most significant factor. Furthermore, the variation of the peak shape of an analyte with a lower content was more likely to affect the peak area value, so the factors related to the peak shape and the peak area, such as the column, injection volume, column temperature, and integration parameters, would also lead to the instability of RCFs.
The quantitative accuracy of the SSDMC approach is evaluated by calculating the SMD value between the quantitative results of the SSDMC approach and those of the external standard method. The external standard method stipulated in Ch.P. is usually taken as the reference standard for quantitative accuracy comparison. The two quantitative results are closer, with a smaller SMD; that is, the SSDMC approach is more accurate. Wang et al. [8] studied the influence of internal factors on the accuracy of the SSDMC approach, including the contents, structure type, and purity of other components and SRS. Panax notoginseng was taken as the research object, and the influencing factors were grouped according to the following criteria: 1) structure types of the SRS, including panaxadiol-type and panaxatriol-type; 2) structural types of other components, including panaxadiol-type and panaxatriol-type; 3) content of the SRS, including high, middle, and trace contents; 4) content of other components, including high, middle, and trace contents; and 5) purities of standard products, including high (>98%) and low (<98%) purity. The results showed that the degree of influence of each parameter was contents of other components > purity of standard product > chemical structure type > SRS content, indicating that the content of other components was the most important factor. The accuracy of the SSDMC approach increased as the content of the other components increased. This result was later confirmed in the study of the SSDMC approach of Ilex Kudingcha C.J. Tseng by Yi et al. [19]. As the three types of dominant constituents (phenolic acids, triterpenes, and flavonoids) in Ilex Kudingcha C.J. Tseng had quite different structural features and properties, Yi et al. [19] selected the corresponding wavelength and SRS for each type of component. Eighteen components of Ilex Kudingcha C.J. Tseng were quantified by LRG-SSDMC and AVG-SSDMC. The content of other components was the most important factor, with increasing accuracy as the content increased. Compared with the study of Wang et al. [8], the study of Yi et al. [19] involved a wider range of compound types, but the results were similar to those of Wang et al. [8].
2.3.2. Recommendations for SSDMC approach establishment
Through the above analysis, we find that the SSDMC approach requires a certain minimum concentration limit to ensure quantitative accuracy. Hou et al. [7] also proved that the RCF was not constant over a broad concentration range. Therefore, it is necessary to investigate the appropriate concentration range for accurate quantification when establishing the SSDMC approach. Wang et al. [8] found a linear relationship between the SMD and the reciprocal of the concentration of the analyte determined using an external standard method, which could be used to calculate concentration limits for SSDMC approaches with specific accuracy requirements. A linear regression model could be obtained when taking the SMD and 1/c as dependent and independent variables, respectively [8]. The upper and lower limits of c could be calculated according to the upper and lower limits of the SMD by establishing regression equations, which was the applicable concentration range of the SSDMC approach.
For the establishment of the SSDMC approach, Guo and co-workers [7] proposed four suggestions. 1) A stable measurement wavelength should be selected. A common wavelength in the flat range of the absorptive peak on the UV spectrum should be selected as the optimal measurement wavelength. 2) More detailed parameters about the detector and peak measurement, including integration parameters, should be mentioned in the analysis program to ensure RCF stability and quantitative accuracy. 3) A second reference standard could be introduced to calculate the RCFs between different analysis systems. The second standard could be one of the analytes or other compounds that were not contained in the sample. 4) The standard concentration used to calculate the RCF should be within an appropriate range. This group also proved the feasibility of external reference standards in the above suggestions [16]. In this study, ethyl benzoate, as the external reference standard, was used for the simultaneous determination of 13 components in the roots of Kansui Radix, and the results were compared with those of the external standard method. The RSDs were less than 1.7%, which proved that the external reference standard was feasible [16]. A second reference standard was introduced in the study of Yin et al. [20], which introduced another available component q in the analyte that was different from the SRS as a correction indicator. The mathematical expression (Formula 12) was constructed to find the intermediate value of the RCF to solve the cases in which different RCFs were obtained by different laboratories.
| (12) |
where i is the SRS, m is the other component, q is another component. That is, the correction indicator, and RCFim′ and RCFiq′ are results calculated in different laboratories.
In summary, appropriate SRS and RCF calculation methods need to be selected according to the properties of analytes when establishing SSDMC approaches. Then, the stability of the RCF should be investigated, and the accuracy of quantitative results should be investigated by calculating the SMD. Finally, the best applicable concentration range of this method should be determined. When examining the stability of the RCF and the accuracy of quantitative results, we should focus on the factors related to UV absorption and the concentration of components to be measured.
2.4. Application examples
It has been more than ten years since the SSDMC approach was proposed, during which researchers continuously established corresponding SSDMC approaches for different TCM. The representative SSDMC approaches established from 2018 to 2022 are summarized in Table S1 [10,19,[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]], including a total of 28 experimental studies. Most research objects of SSDMC are single TCM, but there are also studies on mixtures of herbal slices [24,32] and synthetic drugs [37]. There are a wide range of components studied, including flavonoids, alkaloids, phenolic acids, and other components with strong UV absorption, as well as saponins, amino acids, triterpenes, coumarins, monosaccharides, and other components. Each study involves a class of components with similar structures and UV absorption. At present, the SSDMC approaches of Salvia Miltiorrhiza Radix et Rhizoma, Zingiberis Rhizoma Recens, Andrographis Herba, Epimedii Folium, Bufonis Venenum, Gongxuening capsules, Zhennaoning capsules, Keteling capsules, and related preparations of Ginkgo Folium are recorded in Ch.P. (2020 edition). The components include flavonoids, saponins, lactones, quinones, and alkaloids. The targeted quantitative components with similar structures are selected.
In the RCF calculation, most of the studies adopted the AVG method, one study adopted the SP method [22], and two studies [10,24] from the same research group adopted the MAML method. One paper [19] adopted the LRG method proposed by Wang et al. [8]. In a study [23], the quantitative components were isomers, of which the structures and UV absorptions were very similar, i.e., RCF = 1. This is a special type of RCF mentioned by Hou et al. [7], i.e., the value of the RCF is considered to be 1 when the molar absorptivity and molecular weights of all analytes possess high similarities.
3. Method for improving separation capacity in MCA
Good separation and peak capacity are important prerequisites for the MCQA of complex systems. In the analysis of a complex system with SSDMC approaches by LC, if the target chromatographic peak fails to achieve baseline separation, the quantitative accuracy will be affected. In addition, the index component is sometimes a trace component in samples from natural sources, and its quantitative accuracy is more easily affected if the chromatography peak shape is not good. The first step in establishing the LC analysis method is to select an appropriate column according to the research object and purpose and then maximize separation by adjusting the chromatographic conditions. For complex systems, however, it is often difficult to achieve MCQA by changing columns and adjusting chromatographic conditions. Therefore, 2D-LC has become a compelling technique for the analysis of complex samples due to its higher peak capacity. In this part, we introduce how to increase the separation ability and the peak capacity in MCQA from both the column selection and 2D separation techniques and introduce some application examples.
3.1. Selection of a chromatographic column
The porous stationary phase materials in LC are mostly silica gel due to its good physical and chemical properties, high mechanical strength, thermal stability, adjustable pore size, and large surface area [11]. Porous particles can be divided into fully porous particles, the most commonly used column packing type in research, and superficially porous particles, also known as core-shell columns. Fully porous particles of 1.5, 1.7, 1.8, 1.9, and 2 μm are now commercially available and have been successfully applied in pharmaceutical, biomedical, and environmental analyses [47].
The separation principle in LC is based on the adsorption/retention and desorption between the target molecules in the mobile phase and stationary phase, and the increase in stationary phase surface area can increase the number of adsorption/desorption sites and improve the separation efficiency [48]. Fully porous particles have a larger surface area for very fine particles (<2 and <1 μm). However, sensitivity and separation are improved at the expense of pressure due to the narrow peak [47]. High operational pressure can give rise to detrimental effects of frictional heating generated by percolation of the mobile phase flowing through the packed particles, which creates the temperature gradient along the axial direction (the flowing direction of the mobile phase) and the radial direction (due to the high temperature in the central part and heat loss through the wall), leading to serious band broadening and poor reproducibility [48]. Therefore, how to reduce the back pressure while ensuring the sensitivity and separation degree is the main direction of chromatographic column research. The use of core-shell silica particles with a solid core and porous shell as a column filler is considered to represent great progress in HPLC column technology. Ahmed et al. [48] and Ali et al. [49] comprehensively reviewed the structure, chemical properties, separation, optimization, comparison with other fillers, and application of surface porous silica particle columns.
In addition to the silica gel carrier, different types of groups are also embedded in the silica gel to separate the different types of compounds. The commonly used column structures in MCA are summarized in Fig. S1. The chimeric alkyl group is the most common, where the alkyl chains on the stationary phase surface mainly provide hydrophobic interactions and can effectively regulate the chromatographic retention behavior and selectivity of the alkyl-bonded stationary phase by changing the alkyl chain length, type of ligand, bonding density, and bonding mode (monomer or polymer) [11]. The classical C18-bonded stationary phase tends to collapse in a highly aqueous mobile phase; as a result, the separation performance for polar compounds is relatively poor. The introduction of a polar functional group on the alkyl chain can solve this problem [11]. The most common polar functional groups are amide and carbamate groups, as well as urea and amino acid surfactants. Ionic liquids or carbonaceous nanomaterials can also be introduced into a silica gel matrix for separation [11]. Wu et al. [11] conducted a comprehensive review of the study of different groups embedded in stationary phases and introduced the characteristics and applications of embedded groups in silica gel chimers, such as ionic liquids, carbonaceous nanomaterials, and alkyl bonded stationary phases with a polar functional group. Then, they summarized examples of hydrophilic interaction liquid chromatography (HILIC) and chiral separation applications.
The separation of different types of compounds can be achieved by combining different types of stationary phase materials and embedded groups. The most common separation modes are reversed phase (RP), normal phase (NP), and HILIC. RPLC is the most commonly used separation mode, with a nonpolar stationary phase and polar mobile phase. The disadvantage of RPLC is the lack of sufficient retention for polar compounds [13]. NPLC uses both polar stationary and nonpolar mobile phases. HILIC mode is a valid alternative to both RPLC and NPLC, and it uses a polar stationary phase and an organic solvent-rich hydro-organic mobile phase for the good separation of polar analytes [13]. The columns used in the separation experiments of different types of components in the past 5 years are summarized in Table S2 [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77]], including 28 references on the separation of TCM and chemical drugs.
Superficially porous particles and positively charged columns are applied for strongly polar analytes such as strongly polar glycosides, amino acids, and nucleosides. There is usually a trailing phenomenon when using an ordinary C18 column to separate basic compounds. This phenomenon can be avoided by using a positively charged RP column, such as the CSH series and XCharge series columns [78]. Wang et al. [79] assessed the surface charge properties of an XCharge C18 column at different pH values and buffer concentrations. Wang et al. [63] established a chromatographic fingerprint analysis method with an XCharge C18 column, which provided a better peak shape and resolution for both the alkaline compounds and flavonoid components in Nelumbinis folium at lower pH with formic acid in the mobile phase. CORTECS is a core-shell silica gel chromatography column that provides a higher column efficiency for multicomponent separation while reducing the back pressure associated with particle size reduction. Quaternary ammonium hydroxides are mostly separated by HILIC, which can provide a high polar selectivity for the retention and isolation of hydrophilic compounds [51,59]. Moderate-polarity, weak-polarity, and other analytes, such as flavonoids and chemical drugs, are mostly separated using C18 columns. Partial C18 columns embedded with bridged ethylene hybrids in silica gels, such as the bridged-ethylene-hybrid column, have higher mechanical strength and are suitable for a wide pH range [60,65,76]. Better retention, separation, and loading are achieved by using high-pH conditions when analyzing acidic and alkaline compounds. The fluorine atoms in the pentafluorophenyl (PFP) of the Hypersil PFP column (Fig. S1E) can be closely linked to the aromatic ring, so the Hypersil PFP column is more suitable for the separation of aromatic compounds, such as saponin [50].
3.2. 2D separation technique
2D-LC is one of most effective approaches to improving peak capacity in MCA. When dealing with complex samples, 2D-LC can achieve several thousand peak capacities, with a peak-production rate (peak capacity divided by the analysis time) of approximately 1 peak/s, compared to 1 peak/min for typical high-resolution one-dimensional LC (1D-LC) [80]. 2D-LC can be divided into offline mode and online mode according to the existence of the interface between the two dimensions [81]. Offline 2D-LC mode can achieve high peak capacities, and it does not require high-speed separation in the second dimension, unlike online 2D-LC. Offline 2D-LC does not consider the solvent incompatibility issues of the two dimensions, but it suffers from a long analysis time, a loss of sample, low degree of automation, and possible contamination [81,82]. A special interface, such as a switching valve, loop, and capture column, is introduced in online 2D-LC analysis to automatically transfer the fraction to the second dimension [81]. The capture column lies between the first and the second dimensions, trapping the sample eluted from the first dimension. The samples trapped by capture columns are eventually eluted by the second dimension, which can avoid incompatibility between the mobile phases [83]. Online 2D-LC can be divided into comprehensive 2D-LC, heart-cutting 2D-LC, and multiple heart-cutting (MHC) 2D-LC according to whether all first dimensional effluents are analyzed in the second dimension. In comprehensive 2D-LC, the sample is first separated in the first dimensional column, and then, its effluent is divided into many fractions by a modulator. These fractions are then sequentially transferred to the second-dimensional column for further separation, during which the first dimensional separation remains intact, and all the first dimensional chromatography peaks can be separated again using a fast second dimensional system [82,84]. Heart-cutting 2D-LC selects specific components (such as a single peak, specific time period, and part of peaks) for second dimensional separation through the switching valve. If multiple parts are selected for second dimensional separation, it is called MHC 2D-LC or selective-comprehensive 2D-LC [84]. Heart-cutting (including MHC) 2D-LC is expressed as LC-LC in this paper. LC-LC is suited for the selective separation of a limited number of target components from the first dimension without influence from other components [82]. Comprehensive 2D-LC, which is expressed as LC × LC in this paper, is more suitable for untargeted screening. LC × LC separations typically take longer than 1D-LC separations (analysis time of 30 min to several hours is common) and require dedicated data analysis and visualization software [80]. Pirok [80] summarized the strengths and weaknesses of LC-LC and LC × LC, as well as their respective development directions. LC-LC allows target peaks to be rigorously purified and the purity of target analytes to be assessed. LC × LC is of general interest for analysing complex samples.
The key for 2D-LC techniques to maximize separation is the selection of 2D column binding modes. The 2D mode can combine many different retention mechanisms, and the combination process needs to consider two key issues: the orthogonality of the two dimensions and the solubility of the solvent in the two dimensions. Orthogonality between the two dimensions determines the retention space coverage, but the combination of high orthogonality, such as NPLC and RPLC, generates an enhanced solvent effect or immiscibility issue, causing peak broadening or peak distortion to affect the second dimensional separation [85]. Four combined modes, namely, NP × RP, HILIC × RP, RP × RP, and other × RP, are summarized in Table S3 [[86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125]], with RP columns usually as the second dimensional columns [81,85].
Burlet-Parendel [85] and Hou [81] discussed the advantages and disadvantages of the different column binding modes. The NP × RP mode uses two different columns with strong orthogonality in retention mechanisms. However, there is an important eluting strength mismatch in these two mobile phases, which may result in severe injection effects. As a result, a part of the analyte population elutes at the dead time, while the other part elutes at the normal retention time, hence reducing the sensitivity of the method [85]. Furthermore, the solvents used in NP mode may have environmental contamination problems [81]. RP × RP mode, which utilizes pH orthogonality, changes the analyte ionization state by applying different pH values in two dimensions [81,85]. However, when dealing with neutral species, pH cannot be used to modulate selectivity. Partial selectivity can be brought through some nonconventional stationary phases, such as porous graphitic carbon and zirconium-based or polymeric organic stationary phases, but the retention rate of compounds between the two dimensions is usually strongly correlated, which leads to the poor applicability of RP × RP in isolated neutral analytes [85]. HILIC shows separation complementary with conventional RP separation. The mobile phases of HILIC × RP are more soluble than those of NP × RP, and this mode is suitable for moderately polar compounds such as oligosaccharides, steroids, and phenolic acids, but also has poor separation ability for neutral compounds [81]. In addition, RP can also directly screen the active components when combined with cell membrane chromatography and size exclusion chromatography (SEC) for neutral compound analysis [85]. Therefore, different combinations of columns can be selected according to the object and the analysis purpose.
2D-LC technology is still evolving, and scientists are focused on improving the modulation interface between the two dimensions to solve the undersampling effect, nonideal transfer between the two dimensions, and incomplete retention space coverage [80,85]. Abdulhussain [84] summarized recent advances in three-dimensional chromatographic separation techniques. Zhou [126] generalized the progress of 2D-LC in TCM applications from 2008 to 2018, and Pirok [80] summarized the applications of 2D-LC in the environment and pharmaceutical fields from 2016 to 2018; thus, this paper summarizes the applications of 2D-LC in pharmaceuticals from 2018 to the present (Table S3).
In Table S3, 40 examples are included for multicomponent 2D isolation in complex systems such as TCM, chemical drugs, food, nutrients, and their metabolites, 27 of which use HILIC × RP and RP × RP. The offline HILIC × RP model is often used in the analysis of TCM due to the presence of both polar and nonpolar components in most TCM. The separation effect of the analyte can be improved by mobile phases with different pH values in RP × RP mode, which is suitable for the separation of medium-polarity and weak-polarity components [126]. In addition, different stationary phases can also be selected to achieve 2D separation [102]. Chiral separation columns can be used for chiral isomerism [108,110]. Depending on the analyte nature, RP is combined with ion exchange chromatography (IEX), supercritical fluid chromatography (SFC), SEC, or other modes. For example, Xu et al. [127] developed a column selection guide for 2D-LC analysis of alkaloids, and IEX × RP mode was proven to be a high-orthogonality method [116]. Based on the unique properties of SFC, including the low viscosity, good diffusivity, high density, and excellent solvating power of supercritical CO2, RP × SFC mode was used for the analysis of bufadienolides in Venenum bufonis [103]. In the analysis of organic acids in honey, the organic acids were first separated from carbohydrates by SEC and then separated from each other by RP columns [65]. Carthamus tinctorius L. contains a large number of strong polar components, so Wang et al. [104] used HILIC × HILIC to analyze flavonoids and alkaloids in Carthamus tinctorius L. Therefore, different 2D-LC modes can be selected for the MCA of complex systems.
3.3. Application examples
Most complex systems contain structural analogues, especially natural products (including functional foods, botanical medicine, and TCM). For synthetic drugs, structural analogues also exist in the form of impurities. The separation of structural analogues in complex systems is difficult. If the separation is incomplete, the quantification of index components will be inaccurate or incomplete, which will bring great trouble to subsequent related research. Optimum column selection or 2D-LC separation is vital to the MCA of these compounds.
During the production of chemical drugs, due to the purity of raw materials, preparation process, degradation, and other factors, there may be impurities in the drug, which will affect the purity of the drug and may also affect the actual efficacy of the drug and even cause adverse reactions in serious cases [114,121,123]. Most of these impurities are structural analogues that are difficult to separate. Table 2 shows four examples of separating chemical drug impurities in one or two dimensions [50,55,[64], [65], [66],75,76,89,93,98,104,105,114,[119], [120], [121],124,128]. Among them, MHC separation mode was adopted for metformin in the binding mode of strong cation exchange and RP columns [121]. Psychoactive substances contain relatively more components with similar structures. The separation mode of LC × LC was used to separate psychoactive substances on RP × RP (biphenyl column), and the orthogonality of the two separation systems was fully utilized to enhance the separation capacity [114]. The Symbicort® inhaler is one of the most widely used drugs for treating asthma, and it contains two major active components, budesonide and formoterol fumarate, which have complementary effects in dealing with different symptoms of asthma. Budesonide is a mixture of two epimers at a ratio of approximately 1:1, and budesonide also has several known degradation products (related compounds E, G, and L, Fig. S2 [76]). The degree of separation is critical in assessing product quality and ensuring efficacy due to their similar structures shown in Fig. S2 [76]. Alkhateeb et al. [76] separated the three major active components and the four degradation products in Symbicort by screening the columns for different fillers and gradient procedures, as shown in Fig. S2.
Table 2.
Application examples of system separation methods in multicomponent analysis.
| Category | Analyte (compounds) | Column, mobile phase A/B | Refs. |
|---|---|---|---|
| Chemical medicine | Nexium® capsule (omeprazole/esomeprazole and impurities) | K, Poroshell EC/HPH C18, H2O/ACN | [75] |
| Symbicort (formoterol, budesonide, and its related compounds E, G, and L) | A, BEH C18, H2O/ACN | [76] | |
| Novel psychoactive substance (synthetic cannabinoids) | 1D: M, Poroshell 120 Bonus-RP, 0.1% TFA/95% ACN 2D: J, biphenyl, 0.1% TFA/MeOH:H2O (95:5, V/V) |
[114] | |
| Metformin (major degradation products in metformin) | 1D: CAPCEL PAK SCX UG80, 17 g/L ADP pH 3.0 2D: A, XBridge C18, 0.1% FA/ACN |
[121] | |
| Flavone | Urceola rosea (seven flavonoids) | B, Synergi MAX-RP C12, 0.1% FA/ACN | [64] |
| Chrysanthemi Flos (six flavonoids) | G, BEH Shield RP C18, 1% FA/MeOH:ACN (2.5:1, V/V) with 1% FA | [65] | |
| Buddleja lindleyana Fort (six flavonoids) | A, Symmetry C18, 0.1% FA/ACN | [66] | |
| Ginkgo biloba L. leave extract (metabolites of quercetin, genistein, kaempferol and isorhamnetin) | 1D: D, XBridge Amide, 0.1% FA/ACN 2D: A, ZORBAX XDB-C18, 0.1% FA/MeOH |
[93] | |
| Cannabis (four flavonoids) | 1D: L, Kinetex PFP, 0.1% FA/MeOH with 0.1% FA 2D: K, Kinetex C18, 0.1% FA/ACN with 0.1% FA |
[98] | |
| Safflower (four flavonoids) | 1D: D, XBridge Amide, 0.1% FA/ACN 2D: D, Ultimate Amide, 0.2 μM AF/ACN |
[104] | |
| Fenugreek seed (14 flavonoids) | 1D: K, Kinetex RP C18, 0.1% TFA/ACN:H2O:TFA (50:50:0.1, V/V/V) 2D: K, Kinetex C18, 0.1% TFA/ACN: H2O: TFA (50:50:0.1, V/V/V) |
[119] | |
| Rutin tablet (five flavonoids) | 1D: A, Acclaim 120 C18, 0.1 M SDPA (pH 4.4)/ACN 2D: A, Shim-pack GISS C18, AF (10 mM)/MeOH |
[120] | |
| Saponin | American ginseng (pseudoginsenosides RT5, F11; sixteen ginsenosides) | E, Hypersil Gold PFP, 0.1% FA/ACN | [50] |
| Liliium (steroidal saponins) | 1D: O, CORTECS HILIC, 0.1% FA/ACN 2D: A, HSS T3 C18 0.1% FA/ACN |
[89] | |
| Danshen dripping pill (tanshinones, salvianolic acids, and saponins) | 1D: D, XBridge Amide, 5 mM AA/5 mM AA in ACN 2D: A, HSS T3 C18, 0.1% FA/ACN |
[105] | |
| Panax notoginseng leaves (seven notoginsenosides; eight ginsenosides; gypenoside XVII) | 1D: C, BEH C8, 0.1% FA/ACN 2D: D, XBridge Amide, 0.1% FA/ACN |
[124] | |
| White ginseng and red ginseng (ginsenosides) | 1D: D, Acchrom XAmide, 0.2% FA and 10 mM AF/ACN with 0.2% FA 2D: A, HSS T3, 0.2% FA/ACN |
[128] | |
| Others | Honey (free amino acids) | A, Hypersil ODS C18, 10% ACN (adjusted pH to 6.0 by 20 mM acetate and 5 mM AA)/60% ACN (adjusted pH to 5.4 by 20 mM acetate)/60% ACN (adjusted pH to 4.4 by 20 mM acetate)/pure ACN. | [55] |
EC: ethyl cellulose; ACN: acetonitrile; BEH: bridged-ethylene-hybrid; RP: reversed phase; TFA: trifluoroacetic acid; MeOH: methanol; UG: ultra grade; SCX: strong cation exchange; ADP: ammonium dihydrogen phosphate; FA: formic acid; AF: ammonium formate; SDPA: sodium dihydrogen phosphate aqueous; PFP: pentafluorophenyl; HILIC: hydrophilic interaction liquid chromatography; AA: ammonium acetate; HSS: high strength silica; ODS: octadecyl silane; A−O in the "Column, Mobile phase A/B" represents the column packing structures corresponding to A−O in Fig. S1.
Food or drugs of plant origin may be more difficult to separate than chemical drugs. Here, we take flavonoids and saponins as examples. The biosynthesis of flavonoids is shown at Fig. S3. Flavonoids are medium-polarity compounds, so RP columns are commonly used for flavonoid separation. Most flavonoids have hydroxyl substituents based on the parent nucleus or form flavonoid glycosides with carbohydrates. The polarity of the flavonoids increases when these polar groups occupy a large proportion of the structure, so retention on the RP column weakens. A column with a certain hydroxyl group on the silica gel carrier can be selected to increase polarity retention. The subtle changes in the structure of flavonoids, including different substituents and positions, make flavonoids highly diverse. The similar biosynthesis pathway and corresponding structures make a large number of flavonoid isomers exist in plants, such as Ginkgo biloba leaves, as the main components [93]. Due to the complexity and similarity of flavonoid structures, it is difficult to achieve complete separation in LC analysis, thus affecting the quantitative analysis results of each flavonoid. Table 2 shows that silica gel-bonded C18 is the main column used in the current analysis of flavonoids. Due to the acidity caused by phenolic hydroxyl groups in flavonoids, buffering agents or buffer salts are often used in the mobile phase, which adjust the mobile phase in an acidic environment to ensure that the analyte is in a nonionized state and to enhance chromatographic retention. Moreover, 2D separation can improve the separation of flavonoids with very similar structures, connecting HILIC for polar compound separation [93,104] or PFP columns for aromatic analysis [98] with RP columns to increase the separation capacity.
Saponins are a class of glycosides whose aglycons are triterpenoids or steroids. The main biosynthetic pathway of terpenes and steroids is the isopentene pathway. The same type of saponins often has the same framework. In saponins, aglycon has different degrees of lipophilicity, and the carbohydrate chain has strong hydrophilicity; moreover, the UV absorption of saponins is not sensitive. All these structural characteristics result in difficulties in the LC analysis of saponins, and different separation modes, such as the PFP or HILIC mode, are often used for separation. For samples mainly composed of saponins, such as Panax ginseng and Panax notoginseng, 2D-LC methods are also used to improve the separation efficiency [89,105,124,128]. For example, in the quality evaluation of saponin components in Panax notoginseng leaves, RP × HILIC mode was used to realize the accurate quantification of 17 saponin components, which could realize the orthogonality of the separation mechanism and prevent the incompatibility of the two sets of mobile phase systems (Fig. S4 [124] and Table S4).
4. Applications of predictive software in MCA
In the MCA of complex systems, the optimization of chromatography conditions is the most time-consuming part in analysis method establishment. Researchers need to repeatedly select, optimize, and adjust analysis conditions. With the development of technology, computer-aided prediction technologies are applied to replace a large number of repetitive experimental works or combine big data to simulate and predict some parameters, thus greatly improving the efficiency of method development in MCA. In this part, we introduce computer-aided prediction technologies that have been used to optimize chromatography conditions and predict partial parameters to provide ideas and methods for the intelligent development of MCA in complex systems.
4.1. Chromatographic condition prediction
There are some common problems in MCA by LC. LC method development is performed by trial and error, during which chromatographic conditions such as mobile phase gradients need to be continuously optimized. At the same time, the separation ability of the chromatographic system is limited, and the overlapping peak in the chromatogram is always unavoidable. Most chromatographic workstations still use the integral data processing method. For acromion without serious overlap, it is difficult to obtain the accurate peak area of overlapping components by integrating the classical middle cut method or tangent line. Therefore, it is necessary to develop a method to automatically fit chromatographic curves, providing rapid and accurate determination of peak shape parameters, as well as the ability to calculate overlapping peak areas [14].
Based on the above problems existing in chromatographic analysis, Jin et al. [14] developed a complex sample analysis software system (CSASS) for chromatographic peak-assisted qualitative analysis, quantitative fitting, and analysis condition optimization in complex unknown samples according to the chromatographic outflow curve. The method can obtain the retention parameters of the analyte with 3–5 linear gradients and output the simulated chromatogram and predicted tR according to the retention parameters and the input chromatographic conditions. Moreover, this method describes the chromatographic peaks using a Gaussian model and an exponential modified Gaussian model, achieving a fit to the actual chromatographic outflow curve by a nonlinear least squares method, thus obtaining accurate peak shape parameters and overlapping peak areas, which can be used for accurate determination of overlapping peak areas.
CSASS software has been used in many studies of chromatographic condition optimization and retention parameters in MCA. Zhou et al. [129] obtained the retention parameters of O-diglycosyl flavanones in the extract of Fructus aurantii to study the structure-retention relationship with CSASS software. When establishing the analysis method for iridoid glucosides from Hedyotis diffusa Willd., CSASS software was used to simulate the optimized separation conditions [130]. In the study of quality control analysis of Shuang-huang-lian oral liquid, the CSASS system was used to quickly and accurately calculate the retention parameters and peak shape parameters of each component to study the chromatographic conditions and optimize the gradient. Finally, a quantitative fingerprint method was developed [131]. In addition, when developing an offline method to evaluate and optimize the capture ability of capture columns in 2D-LC, CSASS software was used with five isocratic conditions to calculate analyte retention parameters on different capture columns to predict the retention behavior. Therefore, it was more convenient to predict the capture ability of the capture column under different conditions [85]. The above studies show that CSASS software has been used in many studies of chromatographic condition optimization and retention parameter analysis, which provides a more convenient and fast optimization method for the development of complex sample analysis methods.
4.2. Quantitative structure-retention relationship
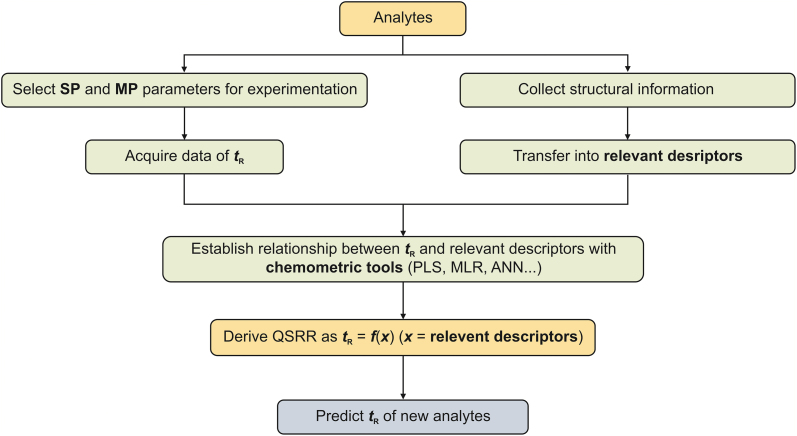
There has been an effort to develop methods for predicting the tR of analytes to reduce the number of experiments. There are two approaches that are used to predict retention. The first is a mechanistic understanding of the analyte retention process to derive a mathematical model that relates tR with physical and chemical parameters of the analyte's interaction with the stationary phase and mobile phase. When the elution mechanism for analyte retention is clear, tR can be predicted by inserting the parameters into the model [15]. The second approach to the prediction of tR is to provide a mathematical description of the observed retention behavior. Using statistical techniques applied to large retention databases, an observational relationship is determined between the tR of the analyte and easily measured properties of the components in the chromatographic system [15]. Most methods of retention factor prediction are designed based on the above two theories. This paper mainly introduces quantitative structure-retention relationships (QSRRs) based on the second idea. QSRR theory studies the relationship between chromatographic retention and solute structure to predict the tR of new analytes, with the process implemented in Fig. 3. QSRRs use some form of relevant numerical descriptor representing the structure of the analyte and then use statistical methods to build the mathematical relationship between tR and the relevant descriptor. For the new analyte, if its relevant descriptor is known, the resulting statistical retention model can be used to predict the tR of the new analyte [15].
Fig. 3.
Schematic representation of the quantitative structure-retention relationship (QSRR) approach process. SP: sationary phase; MP: mobile phase; tR: retention time; PLS: partial least-squares regression; MLR: multiple linear regression; ANN: artificial neural network.
The QSRR model has been used to predict the tR of some impurities. For impurities of chemical drugs analyzed with chromatographic peaks, if the tR of one impurity is longer than the analytical time, it suggests that the elution strength should be increased. If tR is very close to the dead time, it may indicate that other methods of orthogonal elution mechanisms should be developed. In the study of impurities in macrolides, Zhang et al. [132] constructed a QSRR model to predict the tR of some undetected known impurities. Macrolides are complex mixtures typically composed of a couple of main components accompanied by minor components and impurities of structural similarity. However, few standard products are available for impurities in multicomponent macrolides, so it is not feasible to determine tR by their standard products. Researchers have used QSRRs to study the tR of compounds with similar impurity structures. Twenty-four major components in common leucomycin-related 16-membered ring macrolides, including josamycin, midecamycin, meleumycin, kitasamycin, josamycin propionate, and midecamycin acetate, were used as solutes to build the QSRR model. The tR values of the other six impurities of josamycin and two impurities of josamycin propionate in their respective chromatographic systems were predicted. This study provides a reference for the development of macrolide analysis methods and improves the efficiency and accuracy of method development.
TCM has the characteristics of multiple components, large content differences, and many isomers, which increase the difficulty of separating and identifying TCM components. Phthalide compounds are the main active ingredients of Chuanxiong Rhizoma, and many isomers exist. Zhang et al. [133] developed a QSRR model using 23 phthalide standards in Chuanxiong Rhizoma to predict phthaloid monomers and dimers. Flavonoids have the same common core, which show the diversity of the chemical structure in terms of molecular weight, shape, size, and geometric configuration, and exhibit a series of hydrophobic properties and the ability to form hydrogen bonds. This study applied molecular descriptors in the QSRR approach to correctly evaluate the interactions between flavonoids and the stationary phase, characterize the analyte retention mechanism, analyse the relationship between the retention of flavonoids and structure, and consequently determine their lipophilicity. It can also be used as a good supportive tool in the selection of a suitable column [134]. In the identification of echinacoside metabolites, Song et al. [135] built a QSRR model to predict the tR of a given compound based on three different LC mechanisms, RPLC, HPLC, and coupled RPLC-HILIC. Hu et al. [136] developed a method for detecting hydrophilic metabolites. As isobars and isomers in the absence of tR were very difficult to resolve, they constructed a QSRR model with the measured tR, which could predict the tR of metabolites and offer an efficient method for large-scale quantitative analysis for hydrophilic metabolite profiling, even when metabolite standards were unavailable.
In summary, QSRRs can be used to gain a greater understanding of the mechanisms of retention, predict the tR of analytes, significantly reduce the time for method development, and increase the accuracy and robustness. It can also exclude the ‘trial-and-error’ approach for many applications and reduce the amounts of solvents routinely used for method development experiments [137,138]. At present, this method has been widely used in column characterization and selection, chromatographic method development, metabolomics analysis [136], retention mechanism analysis [134], and bioactivity determination. Its applications have been extended to many systems, including thin-layer chromatography, RP [135], ion-pair RP [136], and HILIC [135].
5. Conclusion and future perspectives
Complex systems exist widely, and their quality control is very important for public health. Enterprises must realize quality control of these products with effective and practical methods. Holistic quality evaluation of complex samples, particularly MCA, is an effective method to ensure product quality and is also an important research direction in analytical chemistry. Although MS analysis can improve the precision and detection range of the analytical method, it is not suitable for general industries. MCQA based on LC is a scientific, economical, and stable quality evaluation method for the routine quality analysis of complex systems in industry.
Scholars proposed the SSDMC approach for MCQA. This method overcomes the difficulty in obtaining multiple standard references and has been applied in the quantitative analysis of TCM and impurity analysis of chemical drugs. The theoretical basis, methodological evaluation, and application examples of SSDMC are systematically summarized in this paper. The key step in SSDMC approach development is to select the proper RCF calculation method, and then, the robustness and accuracy of the established SSDMC approach should be comprehensively investigated to determine the applicable scope of the method. There are still many problems in the application of the SSDMC approach: the repeatability of most existing methods is greatly affected by the concentration of analytes and detection wavelength, and large errors are observed for low-content components; the RCFs of the SSDMC approach in different laboratories are different for the same study subjects; and most studies are limited to chemical component analysis of one kind of TCM slice, and few studies are based on mixtures of herbal medicines.
The MCQA of complex systems can be improved and developed from the following four aspects. 1) Improving the accuracy of MCA methods is an important problem of SSDMC approaches. Recent studies have confirmed that when establishing SSDMC approaches, appropriate standard compounds and RCF calculation methods should be selected according to the properties of analytes, and the optimal concentration range of this method should be determined. 2) More attention should be paid to improving the efficiency of MCQA method establishment by introducing computer software such as stoichiometry and applying artificial intelligence for data mining. CSASS software is combined to investigate the appropriate analysis program for choosing the appropriate chromatography conditions. The QSRR model can be applied to predict tR for the analysis of complex systems. 3) The separation modes of LC × LC or LC-LC should be considered to improve the peak capacity in MCQA. The orthogonal modes of columns should be selected according to the characteristics of the components in the research object. 4) In addition to the SSDMC approach, the exploration of new quantitative methods might result in a revolution in MCQA.
CRediT author statement
Xi Chen: Writing - Original draft preparation, Revieiwng and Editing, Visualization; Zhao Yang: Writing - Original draft preparation, Visualization; Yang Xu, Zhe Liu, and Yanfang Liu: Writing - Reviewing and Editing; Yuntao Dai: Supervision, Writing - Reviewing and Editing; Shilin Chen: Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank the support by the National Natural Science Foundation of China (Grant No.: 81803734) and National S&T Major Special Project for New Innovative Drugs Sponsored (Grant No.: 2019ZX09201005).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.11.011.
Contributor Information
Yuntao Dai, Email: ytdai@icmm.ac.cn.
Shilin Chen, Email: slchen@icmm.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fidan O., Ren J., Zhan J. Engineered production of bioactive natural products from medicinal plants. World J. Tradit. Chin. Med. 2022;8:59–76. [Google Scholar]
- 2.Chen S., Li Z., Zhang S., et al. Emerging biotechnology applications in natural product and synthetic pharmaceutical analyses. Acta Pharm. Sin. B. 2022;12:4075–4097. doi: 10.1016/j.apsb.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junker A., Bergmann G. Auswahl, Vergleich und Bewertung optimaler Arbeitsbedingungen für die quantitative Mehrkomponenten-Analyse - 3. Mitteilung. Genauigkeit von Eichung und Analyse, Festlegung der Zahl der Überbestimmungen. Z. Anal. Chem. 1976;278:273–281. [Google Scholar]
- 4.Junker A., Bergmann G. Auswahl, Vergleich und Bewertung optimaler Arbeitsbedingungen für die quantitative Mehrkomponenten-Analyse - 2. Mitteilung. Auswahl optimaler Meßstellen mit Hilfe der Distension der Eichmatrix. Z. Anal. Chem. 1976;278:191–198. [Google Scholar]
- 5.Nahar L., Onder A., Sarker S.D. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019) Phytochem. Anal. 2020;31:413–457. doi: 10.1002/pca.2906. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Gao H., Fu X., et al. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi. 2006;31:1925–1928. [PubMed] [Google Scholar]
- 7.Hou J.-J., Wu W.-Y., Da J., et al. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J. Chromatogr. A. 2011;1218:5618–5627. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 8.Wang C.-Q., Jia X.-H., Zhu S., et al. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta. 2015;134:587–595. doi: 10.1016/j.talanta.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y., Guo Q., Liu J., et al. Simultaneous determination of seven phenylethanoid glycosides in Cistanches Herba by a single marker using a new calculation of relative correction factor. J. Sep. Sci. 2018;41:1913–1922. doi: 10.1002/jssc.201701219. [DOI] [PubMed] [Google Scholar]
- 10.Yang H., Yang T., Gong D., et al. A trinity fingerprint evaluation system of traditional Chinese medicine. J. Chromatogr. A. 2022;1673 doi: 10.1016/j.chroma.2022.463118. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y., Zhang N., Luo K., et al. Recent advances of innovative and high-efficiency stationary phases for chromatographic separations. Trends Analyt. Chem. 2022;153 [Google Scholar]
- 12.Sahu P.K., Ramisetti N.R., Cecchi T., et al. An overview of experimental designs in HPLC method development and validation. J. Pharm. Biomed. Anal. 2018;147:590–611. doi: 10.1016/j.jpba.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Taraji M., Haddad P.R., Amos R.I.J., et al. Chemometric-assisted method development in hydrophilic interaction liquid chromatography: A review. Anal. Chim. Acta. 2018;1000:20–40. doi: 10.1016/j.aca.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y., Xue X., Liu Y., et al. A novel method of prediction and optimization for preparative high-performance liquid chromatography separation. J. Chromatogr. A. 2008;1183:76–86. doi: 10.1016/j.chroma.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Haddad P.R., Taraji M., Szücs R. Prediction of analyte retention time in liquid chromatography. Anal. Chem. 2021;93:228–256. doi: 10.1021/acs.analchem.0c04190. [DOI] [PubMed] [Google Scholar]
- 16.Hou J.-J., Wu W.-Y., Liang J., et al. A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. J. Pharm. Biomed. Anal. 2014;88:321–330. doi: 10.1016/j.jpba.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Stavrianidi A., Stekolshchikova E., Porotova A., et al. Combination of HPLC-MS and QAMS as a new analytical approach for determination of saponins in ginseng containing products. J. Pharm. Biomed. Anal. 2017;132:87–92. doi: 10.1016/j.jpba.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Da J., Cheng C.-R., Yao S., et al. A reproducible analytical system based on the multi-component analysis of triterpene acids in Ganoderma lucidum. Phytochemistry. 2015;114:146–154. doi: 10.1016/j.phytochem.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Yi H., Zhou J., Shang X., et al. Multi-component analysis of Ilex Kudingcha C.J. Tseng by a single marker quantification method and chemometric discrimination of HPLC fingerprints. Molecules. 2018;23:854. doi: 10.3390/molecules23040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y.-H., Zheng J.-Y., Wang X.-N., et al. In silico production of relative correction factor for the quantitative analysis of multi-components by single marker method. Phytochem. Anal. 2021;32:124–128. doi: 10.1002/pca.2881. [DOI] [PubMed] [Google Scholar]
- 21.Su C., Li C., Sun K., et al. Quantitative analysis of bioactive components in walnut leaves by UHPLC-Q-Orbitrap HRMS combined with QAMS. Food Chem. 2020;331 doi: 10.1016/j.foodchem.2020.127180. [DOI] [PubMed] [Google Scholar]
- 22.Liu W., Zhang J., Han W., et al. One single standard substance for the simultaneous determination of 17 triterpenes in Ganoderma lingzhi and its related species using high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1068–1069:49–55. doi: 10.1016/j.jchromb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Fan Y., Gong Y., et al. Simultaneous determination of nine kinds of dominating bile acids in various snake bile by ultrahigh-performance liquid chromatography with triple quadrupole linear iontrap mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1068–1069:245–252. doi: 10.1016/j.jchromb.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Yan H., Sun G., Zhang J., et al. Overall control herbal medicine in best consistency. J. Pharm. Biomed. Anal. 2021;195 doi: 10.1016/j.jpba.2020.113867. [DOI] [PubMed] [Google Scholar]
- 25.Xu R., Mao F., Zhao Y., et al. UPLC quantitative analysis of multi-components by single marker and quality evaluation of Polygala tenuifolia Wild. extracts. Molecules. 2017;22:2276. doi: 10.3390/molecules22122276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T.-J., Lu J., Ni H., et al. Construction of an optimized method for quality evaluation and species discrimination of Coptidis Rhizoma by ion-pair high performance liquid chromatography combined with response surface methodology. J. Pharm. Biomed. Anal. 2018;153:152–157. doi: 10.1016/j.jpba.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Zeng S.-L., Li S.-Z., Lai C.-J.-S., et al. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time. Phytomedicine. 2018;43:103–109. doi: 10.1016/j.phymed.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z., Li N., Zhang H.-F., et al. Simultaneous quantitative analysis of 11 flavonoid derivatives with a single marker in persimmon leaf extraction and evaluation of their myocardium protection activity. J. Nat. Med. 2019;73:404–418. doi: 10.1007/s11418-018-1274-y. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Zhang Y., Zhang Z., et al. Quality evaluation of Gastrodia elata tubers based on HPLC fingerprint analyses and quantitative analysis of multi-components by single marker. Molecules. 2019;24:1521. doi: 10.3390/molecules24081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Liang J., Shen Y., et al. A new application of acetylation for analysis of acidic heteropolysaccharides by liquid chromatography-electrospray mass spectrometry. Carbohydr. Polym. 2020;245 doi: 10.1016/j.carbpol.2020.116439. [DOI] [PubMed] [Google Scholar]
- 31.Wang S., Gan Y., Kan H., et al. Exploitation of HPLC analytical method for simultaneous determination of six principal unsaturated fatty acids in Oviductus Ranae based on quantitative analysis of multi-components by single-marker (QAMS) Molecules. 2021;26:479. doi: 10.3390/molecules26020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Liu X., Wang J., et al. Study on multiple fingerprint profiles control and quantitative analysis of multi-components by single marker method combined with chemometrics based on Yankening tablets. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;253 doi: 10.1016/j.saa.2021.119554. [DOI] [PubMed] [Google Scholar]
- 33.Yu P., Li Q., Feng Y., et al. Extraction and analysis of six effective components in Glycyrrhiza uralensis Fisch by deep eutectic solvents (DES) combined with quantitative analysis of multi-components by single marker (QAMS) method. Molecules. 2021;26:1310. doi: 10.3390/molecules26051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J., Zhang Q., Ma C., et al. An effective workflow for differentiating the same genus herbs of Chrysanthemum morifolium flower and Chrysanthemum indicum flower. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.575726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C., Cheng J., Li C., et al. Quality evaluation of Acanthopanax senticosus via quantitative analysis of multiple components by single marker and multivariate data analysis. J. Pharm. Biomed. Anal. 2021;201 doi: 10.1016/j.jpba.2021.114090. [DOI] [PubMed] [Google Scholar]
- 36.Gao M., Han X., Huang J., et al. Simultaneous determination of multiple active 2-(2-phenylethyl)chromone analogues in agarwood by HPLC, QAMS, and UPLC-MS. Phytochem. Anal. 2021;32:412–422. doi: 10.1002/pca.2989. [DOI] [PubMed] [Google Scholar]
- 37.Chen F., Fang B., Li P., et al. Simultaneous determination of five diuretic drugs using quantitative analysis of multiple components by a single marker. BMC Chem. 2021;15:39. doi: 10.1186/s13065-021-00764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Xu X., Qi H.-W., et al. Comprehensive quality evaluation of ShuXueNing injection employing quantitative high-performance liquid chromatography fingerprint and chemometrics. World J. Tradit. Chin. Med. 2021;7:54–62. [Google Scholar]
- 39.Lyu M., Liu Y., Qiu Y., et al. Differentiation between Chaenomelis Fructus and its common adulterant, Guangpi Mugua. J. AOAC Int. 2021;104:1652–1660. doi: 10.1093/jaoacint/qsab107. [DOI] [PubMed] [Google Scholar]
- 40.Ren R., Li Y., Chen H., et al. Carotenoid contents of Lycium barbarum: A novel QAMS analyses, geographical origins discriminant evaluation, and storage stability assessment. Molecules. 2021;26:5374. doi: 10.3390/molecules26175374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C., Tian Y., Zhao C., et al. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum) R. Soc. Open Sci. 2021;8 doi: 10.1098/rsos.210614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao J.-W., Zhu A.-L., Chen N.-D., et al. Simultaneous analysis of 20 free amino acids by a single marker combined with an HPLC fingerprint evaluation of Dendrobium huoshanense. J. Food Sci. 2021;86:4828–4839. doi: 10.1111/1750-3841.15931. [DOI] [PubMed] [Google Scholar]
- 43.Shi H., Chang Y.-Q., Feng X., et al. Chemical comparison and discrimination of two plant sources of Angelicae dahuricae Radix, Angelica dahurica and Angelica dahurica var. formosana, by HPLC-Q/TOF-MS and quantitative analysis of multiple components by a single marker. Phytochem. Anal. 2022;33:776–791. doi: 10.1002/pca.3129. [DOI] [PubMed] [Google Scholar]
- 44.Cao X., Li M., Ma L., et al. Quality assessment of Artemisia rupestris L. using quantitative analysis of multi-components by single marker and fingerprint analysis. Molecules. 2022;27:2634. doi: 10.3390/molecules27092634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo S., Ren X., Shi X., et al. Study on enhanced extraction and seasonal variation of secondary metabolites in Eucommia ulmoides leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2022;209 doi: 10.1016/j.jpba.2021.114514. [DOI] [PubMed] [Google Scholar]
- 46.Shi L., Wang R., Liu T., et al. A rapid protocol to distinguish between Citri Exocarpium Rubrum and Citri Reticulatae Pericarpium based on the characteristic fingerprint and UHPLC-Q-TOF MS methods. Food Funct. 2020;11:3719–3729. doi: 10.1039/d0fo00082e. [DOI] [PubMed] [Google Scholar]
- 47.Fekete S., Oláh E., Fekete J. Fast liquid chromatography: The domination of core-shell and very fine particles. J. Chromatogr. A. 2012;1228:57–71. doi: 10.1016/j.chroma.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed A., Skinley K., Herodotou S., et al. Core-shell microspheres with porous nanostructured shells for liquid chromatography. J. Sep. Sci. 2018;41:99–124. doi: 10.1002/jssc.201700850. [DOI] [PubMed] [Google Scholar]
- 49.Ali I., Al-Othman Z.A., Nagae N., et al. Recent trends in ultra-fast HPLC: New generation superficially porous silica columns. J. Sep. Sci. 2012;35:3235–3249. doi: 10.1002/jssc.201200454. [DOI] [PubMed] [Google Scholar]
- 50.Stekolshchikova E., Turova P., Shpigun O., et al. Application of quantitative analysis of multi-component system approach for determination of ginsenosides in different mass-spectrometric conditions. J. Chromatogr. A. 2018;1574:82–90. doi: 10.1016/j.chroma.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Hefni M.E., Bergström M., Lennqvist T., et al. Simultaneous quantification of trimethylamine N-oxide, trimethylamine, choline, betaine, creatinine, and propionyl-, acetyl-, and l-carnitine in clinical and food samples using HILIC-LC-MS. Anal. Bioanal. Chem. 2021;413:5349–5360. doi: 10.1007/s00216-021-03509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moras B., Loffredo L., Rey S. Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis) Food Chem. 2018;257:325–332. doi: 10.1016/j.foodchem.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Wang T.-L., Li Y.-C., Lin C.S., et al. Comprehensive analysis of natural polysaccharides from TCMs: A generic approach based on UPLC-MS/MS. Carbohydr. Polym. 2022;277 doi: 10.1016/j.carbpol.2021.118877. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Liang J., Gao J.-N., et al. A novel LC-MS/MS method for complete composition analysis of polysaccharides by aldononitrile acetate and multiple reaction monitoring. Carbohydr. Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118478. [DOI] [PubMed] [Google Scholar]
- 55.Gao Y., Tan J., Lu J., et al. A novel fluorescent labeling reagent, 2-(9-acridone)-ethyl chloroformate, and its application to the analysis of free amino acids in honey samples by HPLC with fluorescence detection and identification with online ESI-MS. Anal. Bioanal. Chem. 2020;412:8339–8350. doi: 10.1007/s00216-020-02969-y. [DOI] [PubMed] [Google Scholar]
- 56.Yao C.-L., Qian Z.-M., Tian W.-S., et al. Profiling and identification of aqueous extract of Cordyceps sinensis by ultra-high performance liquid chromatography tandem quadrupole-orbitrap mass spectrometry. Chin. J. Nat. Med. 2019;17:631–640. doi: 10.1016/S1875-5364(19)30066-4. [DOI] [PubMed] [Google Scholar]
- 57.Temova Rakuša Ž., Grobin A., Roškar R. A comprehensive approach for the simultaneous analysis of all main water-soluble vitamins in multivitamin preparations by a stability-indicating HPLC-DAD method. Food Chem. 2021;337 doi: 10.1016/j.foodchem.2020.127768. [DOI] [PubMed] [Google Scholar]
- 58.Qian Z.-M., Wu Z., Huang Q., et al. Development of an eco-friendly and fast HPLC method for quantitative analysis of four nucleosides in Cordyceps and related products. Chin. J. Nat. Med. 2021;19:954–960. doi: 10.1016/S1875-5364(22)60162-6. [DOI] [PubMed] [Google Scholar]
- 59.Rivoira L., Studzińska S., Szultka-Młyńska M., et al. New approaches for extraction and determination of betaine from Beta vulgaris samples by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017;409:5133–5141. doi: 10.1007/s00216-017-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A., Kamble S.H., León F., et al. Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2019;11:1162–1171. doi: 10.1002/dta.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarkowská D. A fast and reliable UHPLC-MS/MS-based method for screening selected pharmacologically significant natural plant indole alkaloids. Molecules. 2020;25:3274. doi: 10.3390/molecules25143274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei H., Zhang Y., Ye J., et al. A comprehensive quality evaluation of Fuzi and its processed product through integration of UPLC-QTOF/MS combined MS/MS-based mass spectral molecular networking with multivariate statistical analysis and HPLC-MS/MS. J. Ethnopharmacol. 2021;266 doi: 10.1016/j.jep.2020.113455. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Jiao Q., Wang C., et al. Establishment of holistic quality control methods for Nelumbinis Folium containing alkaloids and flavonoids with simple HPLC conditions. J. Chromatogr. Sci. 2022;60:871–879. doi: 10.1093/chromsci/bmab138. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen Ngoc H., Nghiem D.T., Pham T.L.G., et al. Phytochemical and analytical characterization of constituents in Urceola rosea (Hook. & Arn.) D.J. Middleton leaves. J. Pharm. Biomed. Anal. 2018;149:66–69. doi: 10.1016/j.jpba.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 65.Nie J., Xiao L., Zheng L., et al. An integration of UPLC-DAD/ESI-Q-TOF MS, GC-MS, and PCA analysis for quality evaluation and identification of cultivars of Chrysanthemi Flos (Juhua) Phytomedicine. 2019;59 doi: 10.1016/j.phymed.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X., Zhang Z.-Q., Zhang L.-C., et al. The development and validation of a sensitive HPLC-MS/MS method for the quantitative and pharmacokinetic study of the seven components of Buddleja lindleyana Fort. RSC Adv. 2021;11:26016–26028. doi: 10.1039/d1ra04154a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S., Singh A., Kumar B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017;7:214–222. doi: 10.1016/j.jpha.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang H., Yang L., Xing X., et al. Chemometrics coupled with UPLC-MS/MS for simultaneous analysis of markers in the raw and processed Fructus Xanthii, and application to optimization of processing method by BBD design. Phytomedicine. 2019;57:191–202. doi: 10.1016/j.phymed.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 69.Yu X., Gao Y., Xu Y., et al. Study of the contents of analogues of aristolochic acid in Houttuynia cordata by ultra-high performance liquid chromatography tandem mass spectrometry. Foods. 2022;11:302. doi: 10.3390/foods11030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C., Zhang X., Yan X., et al. Chemical profiling of Euphorbia fischeriana Steud. by UHPLC-Q/TOF-MS. J. Pharm. Biomed. Anal. 2018;151:126–132. doi: 10.1016/j.jpba.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y.-Y., Wu Z.-Y., Zhang H., et al. LC-MS-based multivariate statistical analysis for the screening of potential thrombin/factor Xa inhibitors from Radix Salvia Miltiorrhiza, Chin. Med. 2020;15:38. doi: 10.1186/s13020-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C., Zhao Y., Yang R., et al. Simultaneous analysis of five triterpenes in Centella asiatica by high performance liquid chromatography with cyclodextrins as the mobile phase additives. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasileiadou A., Karapanagiotis I., Zotou A. Development and validation of a liquid chromatographic method with diode array detection for the determination of anthraquinones, flavonoids and other natural dyes in aged silk. J. Chromatogr. A. 2021;1651 doi: 10.1016/j.chroma.2021.462312. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y., Yu X., Gui J., et al. Ultrasonic solvent extraction followed by dispersive solid phase extraction (d-SPE) cleanup for the simultaneous determination of five anthraquinones in Polygonum multiflorum by UHPLC-PDA. Foods. 2022;11:386. doi: 10.3390/foods11030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson E., Karlsson A., Ludvigsson J.W. Ultra high performance liquid chromatography method development for separation of omeprazole and related substances on core-shell columns using a Quality by Design approach. J. Sep. Sci. 2020;43:696–707. doi: 10.1002/jssc.201900726. [DOI] [PubMed] [Google Scholar]
- 76.Alkhateeb F.L., Wilson I., Maziarz M., et al. Ultra high-performance liquid chromatography method development for separation of formoterol, budesonide, and related substances using an analytical quality by design approach. J. Pharm. Biomed. Anal. 2021;193 doi: 10.1016/j.jpba.2020.113729. [DOI] [PubMed] [Google Scholar]
- 77.Gbylik-Sikorska M., Gajda A., Nowacka-Kozak E., et al. Simultaneous determination of 45 antibacterial compounds in mushrooms - Agaricus bisporus by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2019;1587:111–118. doi: 10.1016/j.chroma.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 78.Guo Z., Wang C., Liang T., et al. Polar-copolymerized approach based on horizontal polymerization on silica surface for preparation of polar-modified stationary phases. J. Chromatogr. A. 2010;1217:4555–4560. doi: 10.1016/j.chroma.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 79.Wang C., Guo Z., Long Z., et al. Overloading study of basic compounds with a positively charged C18 column in liquid chromatography. J. Chromatogr. A. 2013;1281:60–66. doi: 10.1016/j.chroma.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 80.Pirok B.W.J., Stoll D.R., Schoenmakers P.J. Recent developments in two-dimensional liquid chromatography: Fundamental improvements for practical applications. Anal. Chem. 2019;91:240–263. doi: 10.1021/acs.analchem.8b04841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou J.-J., Zhang J.-Q., Yao C.-L., et al. Deeper chemical perceptions for better traditional Chinese medicine standards. Engineering. 2019;5:83–97. [Google Scholar]
- 82.Brandão P.F., Duarte A.C., Duarte R.M.B.O. Comprehensive multidimensional liquid chromatography for advancing environmental and natural products research. Trends Analyt. Chem. 2019;116:186–197. [Google Scholar]
- 83.Cao L., Yu D., Wang X., et al. The development of an evaluation method for capture columns used in two-dimensional liquid chromatography. Anal. Chim. Acta. 2011;706:184–190. doi: 10.1016/j.aca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Abdulhussain N., Nawada S., Schoenmakers P. Latest trends on the future of three-dimensional separations in chromatography. Chem. Rev. 2021;121:12016–12034. doi: 10.1021/acs.chemrev.0c01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burlet-Parendel M., Faure K. Opportunities and challenges of liquid chromatography coupled to supercritical fluid chromatography. Trends Analyt. Chem. 2021;144 [Google Scholar]
- 86.Zhu H., Wu X., Huo J., et al. A five-dimensional data collection strategy for multicomponent discovery and characterization in traditional Chinese medicine: Gastrodia Rhizoma as a case study. J. Chromatogr. A. 2021;1653 doi: 10.1016/j.chroma.2021.462405. [DOI] [PubMed] [Google Scholar]
- 87.Pan H., Zhou H., Miao S., et al. An integrated approach for global profiling of multi-type constituents: Comprehensive chemical characterization of Lonicerae Japonicae Flos as a case study. J. Chromatogr. A. 2020;1613 doi: 10.1016/j.chroma.2019.460674. [DOI] [PubMed] [Google Scholar]
- 88.Fu L.-L., Ding H., Han L.-F., et al. Simultaneously targeted and untargeted multicomponent characterization of Erzhi Pill by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry. J. Chromatogr. A. 2019;1584:87–96. doi: 10.1016/j.chroma.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 89.Wu X., Hou J., Zhang Z., et al. In-depth exploration and comparison of chemical constituents from two Lilium species through offline two-dimensional liquid chromatography combined with multimode acquisition of high-resolution mass spectrometry. J. Chromatogr. A. 2022;1670 doi: 10.1016/j.chroma.2022.462980. [DOI] [PubMed] [Google Scholar]
- 90.Li X., Yao C., Li Y., et al. Systematic screening and structural characterization of dipeptides using offline 2D LC-LTQ-Orbitrap MS: A case study of Cordyceps sinensis. J. Pharm. Anal. 2022;12:263–269. doi: 10.1016/j.jpha.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dang J., Zhang L., Wang Q., et al. Target separation of flavonoids from Saxifraga tangutica using two-dimensional hydrophilic interaction chromatography/reversed-phase liquid chromatography. J. Sep. Sci. 2018;41:4419–4429. doi: 10.1002/jssc.201800534. [DOI] [PubMed] [Google Scholar]
- 92.Wang J., Chen L., Qu L., et al. Isolation and bioactive evaluation of flavonoid glycosides from Lobelia chinensis Lour using two-dimensional liquid chromatography combined with label-free cell phenotypic assays. J. Chromatogr. A. 2019;1601:224–231. doi: 10.1016/j.chroma.2019.04.073. [DOI] [PubMed] [Google Scholar]
- 93.Zheng X.-X., Du Y., Xu B.-J., et al. Off-line two-dimensional liquid chromatography coupled with diode array detection and quadrupole-time of flight mass spectrometry for the biotransformation kinetics of Ginkgo biloba leaves extract by diabetic rat liver microsomes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1109:1–9. doi: 10.1016/j.jchromb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Zuo T., Zhang C., Li W., et al. Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling four-dimensional separation and characterization of the multicomponents from white ginseng and red ginseng. J. Pharm. Anal. 2020;10:597–609. doi: 10.1016/j.jpha.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M., Xu X.-Y., Wang H.-D., et al. A multi-dimensional liquid chromatography/high-resolution mass spectrometry approach combined with computational data processing for the comprehensive characterization of the multicomponents from Cuscuta chinensis. J. Chromatogr. A. 2022;1675 doi: 10.1016/j.chroma.2022.463162. [DOI] [PubMed] [Google Scholar]
- 96.Wang C., Feng K., Fu Z., et al. Systematic quality evaluation of Peiyuan Tongnao capsule by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry and adjusted parallel reaction monitoring of quality markers. Anal. Bioanal. Chem. 2019;411:7747–7760. doi: 10.1007/s00216-019-02119-z. [DOI] [PubMed] [Google Scholar]
- 97.Walters N.A., de Beer D., de Villiers A., et al. Comprehensive off-line CCC × LC-DAD-MS separation of Cyclopia pubescens Eckl. & Zeyh. phenolic compounds and structural elucidation of isolated compounds. Phytochem. Anal. 2021;32:347–361. doi: 10.1002/pca.2981. [DOI] [PubMed] [Google Scholar]
- 98.Montero L., Meckelmann S.W., Kim H., et al. Differentiation of industrial hemp strains by their cannabinoid and phenolic compounds using LC × LC-HRMS. Anal. Bioanal. Chem. 2022;414:5445–5459. doi: 10.1007/s00216-022-03925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo X., Gao Z., Wang J., et al. Purification of tertiary and quaternary alkaloids from Rhizoma Corydalis using reversed-phase/weak cation-exchange mixed-mode class separation combined with preparative C18 and silica based strong cation-exchange chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1126–1127 doi: 10.1016/j.jchromb.2019.121742. 121742. [DOI] [PubMed] [Google Scholar]
- 100.Du N., Liu Y., Zhang X., et al. Discovery of new muscarinic acetylcholine receptor antagonists from Scopolia tangutica. Sci. Rep. 2017;7 doi: 10.1038/srep46067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu D., Jin H., Wang J., et al. Offline preparative 2-D polar-copolymerized reversed-phase chromatography × zwitterionic hydrophilic interaction chromatography for effective purification of polar compounds from Caulis Polygoni Multiflori. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1118–1119:70–77. doi: 10.1016/j.jchromb.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y., Yao C., Wang M., et al. Systematical characterization and comparison of saponins in Achyranthes bidentata Blume and its three analogous species. Phytochem. Anal. 2022;33:766–775. doi: 10.1002/pca.3128. [DOI] [PubMed] [Google Scholar]
- 103.Wei W., Hou J., Yao C., et al. A high-efficiency strategy integrating offline two-dimensional separation and data post-processing with dereplication: Characterization of bufadienolides in Venenum Bufonis as a case study. J. Chromatogr. A. 2019;1603:179–189. doi: 10.1016/j.chroma.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 104.Wang S., Cao J., Deng J., et al. Chemical characterization of flavonoids and alkaloids in safflower (Carthamus tinctorius L.) by comprehensive two-dimensional hydrophilic interaction chromatography coupled with hybrid linear ion trap Orbitrap mass spectrometry. Food Chem. X. 2021;12 doi: 10.1016/j.fochx.2021.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H.-D., Wang H.-M., Wang X.-Y., et al. A novel hybrid scan approach enabling the ion-mobility separation and the alternate data-dependent and data-independent acquisitions (HDDIDDA): Its combination with off-line two-dimensional liquid chromatography for comprehensively characterizing the multicomponents from Compound Danshen Dripping Pill. Anal. Chim. Acta. 2022;1193 doi: 10.1016/j.aca.2021.339320. [DOI] [PubMed] [Google Scholar]
- 106.Martín-Ortiz A., Ruiz-Matute A.I., Sanz M.L., et al. Separation of di- and trisaccharide mixtures by comprehensive two-dimensional liquid chromatography. Application to prebiotic oligosaccharides. Anal. Chim. Acta. 2019;1060:125–132. doi: 10.1016/j.aca.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 107.Roca L.S., Gargano A.F.G., Schoenmakers P.J. Development of comprehensive two-dimensional low-flow liquid-chromatography setup coupled to high-resolution mass spectrometry for shotgun proteomics. Anal. Chim. Acta. 2021;1156 doi: 10.1016/j.aca.2021.338349. [DOI] [PubMed] [Google Scholar]
- 108.Bäurer S., Ferri M., Carotti A., et al. Mixed-mode chromatography characteristics of chiralpak ZWIX(+) and ZWIX(−) and elucidation of their chromatographic orthogonality for LC × LC application. Anal. Chim. Acta. 2020;1093:168–179. doi: 10.1016/j.aca.2019.09.068. [DOI] [PubMed] [Google Scholar]
- 109.Karongo R., Ikegami T., Stoll D.R., et al. A selective comprehensive reversed-phase × reversed-phase 2D-liquid chromatography approach with multiple complementary detectors as advanced generic method for the quality control of synthetic and therapeutic peptides. J. Chromatogr. A. 2020;1627 doi: 10.1016/j.chroma.2020.461430. [DOI] [PubMed] [Google Scholar]
- 110.Acquaviva A., Siano G., Quintas P., et al. Chiral × achiral multidimensional liquid chromatography. Application to the enantioseparation of dintitrophenyl amino acids in honey samples and their fingerprint classification. J. Chromatogr. A. 2020;1614 doi: 10.1016/j.chroma.2019.460729. [DOI] [PubMed] [Google Scholar]
- 111.Zhou W., Guo Z., Yu L., et al. On-line comprehensive two-dimensional liquid chromatography tandem mass spectrometry for the analysis of Curcuma kwangsiensis. Talanta. 2018;186:73–79. doi: 10.1016/j.talanta.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 112.Olfert M., Bäurer S., Wolter M., et al. Comprehensive profiling of conjugated fatty acid isomers and their lipid oxidation products by two-dimensional chiral RP × RP liquid chromatography hyphenated to UV- and SWATH-MS-detection. Anal. Chim. Acta. 2022;1202 doi: 10.1016/j.aca.2022.339667. [DOI] [PubMed] [Google Scholar]
- 113.Pickens C.J., Haidar Ahmad I.A., Makarov A.A., et al. Comprehensive online multicolumn two-dimensional liquid chromatography-diode array detection-mass spectrometry workflow as a framework for chromatographic screening and analysis of new drug substances. Anal. Bioanal. Chem. 2020;412:2655–2663. doi: 10.1007/s00216-020-02498-8. [DOI] [PubMed] [Google Scholar]
- 114.Eckberg M.N., Arroyo-Mora L.E., Stoll D.R., et al. Separation and identification of isomeric and structurally related synthetic cannabinoids using 2D liquid chromatography and high resolution mass spectrometry. J. Anal. Toxicol. 2019;43:170–178. doi: 10.1093/jat/bky081. [DOI] [PubMed] [Google Scholar]
- 115.Long Z., Zhang Y., Gamache P., et al. Determination of tropane alkaloids by heart cutting reversed phase - Strong cation exchange two dimensional liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018;1072:70–77. doi: 10.1016/j.jchromb.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 116.Liu S.-S., Yang K., Sun Z.-L., et al. A novel two-dimensional liquid chromatography system for the simultaneous determination of three monoterpene indole alkaloids in biological matrices. Anal. Bioanal. Chem. 2019;411:3857–3870. doi: 10.1007/s00216-019-01859-2. [DOI] [PubMed] [Google Scholar]
- 117.Feng J., Zhong Q., Kuang J., et al. Simultaneous analysis of the metabolome and lipidome using polarity partition two-dimensional liquid chromatography-mass spectrometry. Anal. Chem. 2021;93:15192–15199. doi: 10.1021/acs.analchem.1c03905. [DOI] [PubMed] [Google Scholar]
- 118.Xu F., Xu Y., Liu G., et al. Separation of twelve posaconazole related stereoisomers by multiple heart-cutting chiral-chiral two-dimensional liquid chromatography. J. Chromatogr. A. 2020;1618 doi: 10.1016/j.chroma.2019.460845. [DOI] [PubMed] [Google Scholar]
- 119.Król-Kogus B., Głód D., Hałasa R., et al. 2D LC as a tool for standardization of Foenugraeci semen extracts containing compounds with anti-Helicobacter pylori activity. Food Funct. 2021;12:2686–2692. doi: 10.1039/d1fo00226k. [DOI] [PubMed] [Google Scholar]
- 120.Wang J., Ren X., Wen C., et al. Separation and characterization of unknown impurities in rutin tablets using trap-free two-dimensional liquid chromatography coupled with ion trap/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020;34 doi: 10.1002/rcm.8739. [DOI] [PubMed] [Google Scholar]
- 121.Luo M., Zheng L., Wang S., et al. Characterization of four major degradation products in metformin by 2D LC-QTOF/MS/MS. J. Pharm. Biomed. Anal. 2021;192 doi: 10.1016/j.jpba.2020.113662. [DOI] [PubMed] [Google Scholar]
- 122.Suto M., Kawashima H., Suto N. Heart-cutting two-dimensional liquid chromatography combined with isotope ratio mass spectrometry for the determination of stable carbon isotope ratios of gluconic acid in honey. J. Chromatogr. A. 2019;1608 doi: 10.1016/j.chroma.2019.460421. [DOI] [PubMed] [Google Scholar]
- 123.Móricz Á.M., Lapat V., Morlock G.E., et al. High-performance thin-layer chromatography hyphenated to high-performance liquid chromatography-diode array detection-mass spectrometry for characterization of coeluting isomers. Talanta. 2020;219 doi: 10.1016/j.talanta.2020.121306. [DOI] [PubMed] [Google Scholar]
- 124.Ma L.-J., Ma N., Cao J.-L., et al. Characterizing the influence of different drying methods on chemical components of Panax notoginseng leaves by heart-cutting two-dimensional liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130965. [DOI] [PubMed] [Google Scholar]
- 125.Jennings M.E., 2nd, Silveira J.R., Treier K.M., et al. Total retention liquid chromatography-mass spectrometry to achieve maximum protein sequence coverage. Anal. Chem. 2021;93:5054–5060. doi: 10.1021/acs.analchem.0c04292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou W., Liu Y., Wang J., et al. Application of two-dimensional liquid chromatography in the separation of traditional Chinese medicine. J. Sep. Sci. 2020;43:87–104. doi: 10.1002/jssc.201900765. [DOI] [PubMed] [Google Scholar]
- 127.Xu Y., Liu Y., Zhou H., et al. A guide of column selection for two-dimensional liquid chromatography method development of natural alkaloids. Talanta. 2023;251 doi: 10.1016/j.talanta.2022.123738. [DOI] [PubMed] [Google Scholar]
- 128.Zhang H., Jiang J.-M., Zheng D., et al. A multidimensional analytical approach based on time-decoupled online comprehensive two-dimensional liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry for the analysis of ginsenosides from white and red ginsengs. J. Pharm. Biomed. Anal. 2019;163:24–33. doi: 10.1016/j.jpba.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 129.Zhou D.-Y., Xu Q., Xue X.-Y., et al. Identification of O-diglycosyl flavanones in Fructus aurantii by liquid chromatography with electrospray ionization and collision-induced dissociation mass spectrometry. J. Pharm. Biomed. Anal. 2006;42:441–448. doi: 10.1016/j.jpba.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 130.Li C., Xue X., Zhou D., et al. Analysis of iridoid glucosides in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008;48:205–211. doi: 10.1016/j.jpba.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 131.Si W., Qiao Y., Liu Z., et al. Combination of multi-model statistical analysis and quantitative fingerprinting in quality evaluation of Shuang-huang-lian oral liquid. Anal. Bioanal. Chem. 2020;412:7073–7083. doi: 10.1007/s00216-020-02841-z. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X., Li J., Wang C., et al. Identification of impurities in macrolides by liquid chromatography-mass spectrometric detection and prediction of retention times of impurities by constructing quantitative structure-retention relationship (QSRR) J. Pharm. Biomed. Anal. 2017;145:262–272. doi: 10.1016/j.jpba.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Q., Huo M., Zhang Y., et al. A strategy to improve the identification reliability of the chemical constituents by high-resolution mass spectrometry-based isomer structure prediction combined with a quantitative structure retention relationship analysis: Phthalide compounds in Chuanxiong as a test case. J. Chromatogr. A. 2018;1552:17–28. doi: 10.1016/j.chroma.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 134.Zapadka M., Kaczmarek M., Kupcewicz B., et al. An application of QSRR approach and multiple linear regression method for lipophilicity assessment of flavonoids. J. Pharm. Biomed. Anal. 2019;164:681–689. doi: 10.1016/j.jpba.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 135.Song Q., Li J., Huo H., et al. Retention time and optimal collision energy advance structural annotation relied on LC-MS/MS: An application in metabolite identification of an antidementia agent namely echinacoside. Anal. Chem. 2019;91:15040–15048. doi: 10.1021/acs.analchem.9b03720. [DOI] [PubMed] [Google Scholar]
- 136.Hu Q., Sun Y., Yuan P., et al. Quantitative structure-retention relationship for reliable metabolite identification and quantification in metabolomics using ion-pair reversed-phase chromatography coupled with tandem mass spectrometry. Talanta. 2022;238 doi: 10.1016/j.talanta.2021.123059. [DOI] [PubMed] [Google Scholar]
- 137.Amos R.I.J., Haddad P.R., Szucs R., et al. Molecular modeling and prediction accuracy in quantitative structure-retention relationship calculations for chromatography. Trends Analyt. Chem. 2018;105:352–359. [Google Scholar]
- 138.Sagandykova G., Buszewski B. Perspectives and recent advances in quantitative structure-retention relationships for high performance liquid chromatography. How far are we? Trends Analyt. Chem. 2021;141 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.