Abstract
Tumor-selective viruses are a novel therapeutic approach for treating cancer. Tumor-Specific Immuno Gene Therapy (T-SIGn) vectors are tumor-selective adenoviral vectors designed to express immunomodulatory transgenes. Prolonged activated partial thromboplastin time (aPTT), associated with the presence of antiphospholipid antibodies (aPL), has been observed in patients with viral infections, and following administration of adenovirus-based medicines. aPL may be detected as lupus anticoagulant (LA), anti-cardiolipin (aCL) and/or anti-beta 2 glycoprotein antibodies (aβ2GPI). No subtype alone is definitive for development of clinical sequalae, however, patients who are ‘triple positive’ have a greater thrombotic risk. Additionally, isolated aCL and aβ2GPI IgM do not appear to add value in thrombotic association to aPL positivity, rather IgG subtypes must also be present to confer an increased risk. Here we report induction of prolonged aPTT and aPL in patients from eight Phase 1 studies who were treated with adenoviral vectors (n = 204). Prolonged aPTT (≥ Grade 2) was observed in 42% of patients, with a peak at 2–3 weeks post-treatment and resolution within ~ 2 months. Among patients with aPTT prolongation, LA, but not aCL IgG nor aβ2GPI IgG, was observed. The transience of the prolongation and discordance between positive LA and negative aCL/aβ2GPI IgG assays is not typical of a prothrombotic state. Among the patients with prolonged aPTT there was no evidence of an increased rate of thrombosis. These findings elucidate the relationship between viral exposure and aPL in the context of clinical trials. They suggest a framework in which hematologic changes can be monitored in patients receiving similar treatments.
Clinical trial registration:
NCT02028442, NCT02636036, NCT02028117, NCT03852511, NCT04053283, NCT05165433, NCT04830592, NCT05043714.
Keywords: Activated partial thromboplastin time, Adenoviral vector, Antiphospholipid antibody, Clinical study, Safety
Introduction
Tumor-selective viruses are a novel therapeutic approach for treating cancer. These viruses have been shown to directly lyse infected tumor cells, produce an inflammatory microenvironment, and induce tumor-specific immune responses. Enadenotucirev is an investigational, chimeric, tumor-selective group B adenovirus (Ad11p/Ad3), with which over 170 patients have been treated to date [1–3]. Additionally, enadenotucirev has been modified to create variants, known as Tumor-Specific Immuno Gene Therapy (T-SIGn) vectors, which carry immunomodulatory transgenes designed to re-program immunosuppressive tumor microenvironments. Two T-SIGn vectors are in clinical development: NG-350A which encodes a full-length agonist anti-CD40 agonist antibody and NG-641 which encodes a bispecific human fibroblast activation protein and human CD3 epsilon bispecific T cell activator antibody, CXC motif chemokine ligands (CXCL) 9 and 10, and interferon α2. Structural and biophysical properties of such modified vectors are indistinguishable from those of enadenotucirev as the encoded transgenes in NG-350A and NG-641 are not components of the virus particle structure.
As adenoviral vectors are increasingly being used for a range of gene therapy applications, understanding their safety is critical. During clinical trials of enadenotucirev and T-SIGn vectors, we identified a signal of frequently prolonged activated partial thromboplastin time (aPTT; which is used as a measure of coagulability) without discernible clinical sequalae.
Antiphospholipid antibodies (aPL) are autoantibodies that are directed against phospholipid-binding proteins and can prolong aPTT [4, 5]. There is no single gold standard assay for detection of aPL. However, the standard approach is to rule out a coagulation factor deficiency and confirm the presence of an inhibitor by coagulation-based assays. Subsequently, more specific coagulation tests are performed to identify the presence of lupus anticoagulant (LA), the term used to describe the coagulation-based aPL, and enzyme-linked immunosorbent assays (ELISA) to detect specific aPL subtypes (typically anti-cardiolipin [aCL] and anti-β2-glycoprotein I antibodies [aβ2GPI]; IgG and IgM) [6]. Patients may be positive for one, two, or three of the aPL assays with no one assay definitive for the development of clinical manifestations. As such, test results must be considered together and in view of the clinical context. Patients with all three positive are referred to as triple positive [7].
Antiphospholipid syndrome (APS) is characterized by recurrent thrombotic or obstetrical events that occur in patients with persistent (> 12 weeks) aPL [4, 5]. However, the presence of aPL may or may not be associated with APS and an increased risk of thrombosis or obstetric complications. While the association of thrombosis and single aPL positivity is debated and results from investigations in to this matter differ, a strong correlation between triple positivity and thrombosis has been seen [7]. Notably, research has shown that isolated aCL and aβ2GPI IgM does not add value in thrombotic association to aPL positivity, rather IgG subtypes must also be present to confer an increased thrombotic risk [7–9].
Prolonged aPTT, associated with aPL, has occasionally been observed in patients with viral infections (most frequently with hepatitis C virus and human immunodeficiency virus [HIV]), [10, 11] and following the administration of adenovirus-based vector therapeutics [12] and vaccines [13–15]. These aPL are generally transient in nature, but an acquired prolonged aPTT associated with aPL in patients without a bleeding history could nevertheless indicate an increased thrombotic risk. This is particularly of concern in patients with cancer, as cancer itself is associated with an increased risk of thrombosis [16, 17]. Here we report induction of aPL in a prospective cohort of clinical trial patients treated with a novel adenoviral immuno-oncology therapy that appears to be transient and not to be associated with APS nor a thrombotic tendency. In this report, we further characterize this signal to understand its kinetics and clinical significance and to assess the potential need for monitoring in patients, including in future studies.
Methods
We analyzed data from eight multicenter, open-label, non-randomized Phase 1 studies of enadenotucirev, NG-350A or NG-641, with a data lock point of 8 July 2022 (Table 1). This included four completed studies of enadenotucirev or a T-SIGn vector and four ongoing studies of T-SIGn vectors. All eight studies involve patients with metastatic or advanced epithelial cancer, except for MOAT, which is enrolling patients with surgically resectable squamous cell carcinoma of the head and neck. To be eligible for inclusion, patients were required to have a baseline coagulation profile within the normal range or ≤ 1.5 times the upper limit of normal, dependent on the study.
Table 1.
Enadenotucirev and T-SIGn studies included
| Study name | Cancer type | Treatments | Number of patients at data lock |
|---|---|---|---|
|
EVOLVE [1] (NCT02028442) |
Metastatic or advanced epithelial tumors | Enadenotucirev (IV) | 61 |
|
(NCT02636036) |
Metastatic or advanced epithelial tumors | Enadenotucirev (IV) in combination with a PD-1 inhibitor | 51 |
| OCTAVE [2] (NCT02028117) | Platinum-resistant epithelial ovarian cancer | Enadenotucirev (IP or IV) with/without paclitaxel | 38 |
| FORTITUDE [20] (NCT03852511) | Metastatic or advanced epithelial tumors | NG-350A (IT or IV) with/without pembrolizumab | 28 |
| FORTIFY [21] (NCT05165433) | Metastatic or advanced epithelial tumors | NG-350A (IV) with pembrolizumab | 2 |
|
STAR [22] (NCT04053283) |
Metastatic or advanced epithelial tumors | NG-641 (IV) | 20 |
|
MOAT [23] (NCT04830592) |
Surgically resectable squamous cell carcinoma of the head and neck | NG-641 (IV)a | 3 |
|
NEBULA [24] (NCT05043714) |
Metastatic or advanced epithelial tumors | NG-641 (IV) with nivolumab | 1 |
IP = intraperitoneal; IT = intratumoral; IV = intravenous
aMOAT includes both monotherapy and pembrolizumab combination-therapy arms; however, at the time of this analysis patients had only been treated with monotherapy
Coagulation parameters, including aPTT, were measured at multiple timepoints. Degree of aPTT prolongation was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Information on incidence and degree of aPTT prolongation was obtained from laboratory reports.
If aPTT was shown to be prolonged, further testing was conducted. To distinguish whether aPTT prolongation was due to a coagulation factor deficiency versus an assay inhibitor, we further characterized this prolonged aPTT using 1:1 mixing studies. A lack of correction of coagulation time in these assays suggests the presence of an inhibitor, the most common of which is aPL. If the presence of an inhibitor was indicated on mixing studies, aPL confirmatory assays (hexagonal phase phospholipid neutralization [Staclot-LA] or Dilute Russell Viper Venom Time [dRVVT] tests) were conducted. Staclot-LA tests utilize an excess of phospholipid to neutralize aPL whereas dRVVT tests utilize enzymes that directly activate coagulation factors V and X, bypassing the activation of factors VII, VIII, IX, XI, and XII, and therefore, the effect of deficiencies or inhibitors of these factors. dRVVT testing is comprised of three tests conducted in the presence of dRVV: (1) a screening test for prolongation (screen), (2) a mixing test in the presence of normal plasma (mix), and (3) a confirmatory test in the presence of excess phospholipids (confirm).
If aPL was shown to be present, ELISA assays (IgG and IgM) for aCL and aβ2GPI were performed, as recommended by Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis (ISTH).
To further assess this signal and determine whether these laboratory findings resulted in clinical sequalae, the incidence of bleeding and clotting events by aPTT grade were assessed.
Results
Patient population
A total of 204 patients are included in this report. Across the studies included, median age was ~ 60 years. The cancer types reported in the highest proportions of patients were colorectal cancer, ovarian cancer and squamous cell carcinoma of the head and neck. Most patients had received at least 3 prior systemic therapies, including chemotherapy.
Incidence and timing of aPTT prolongation
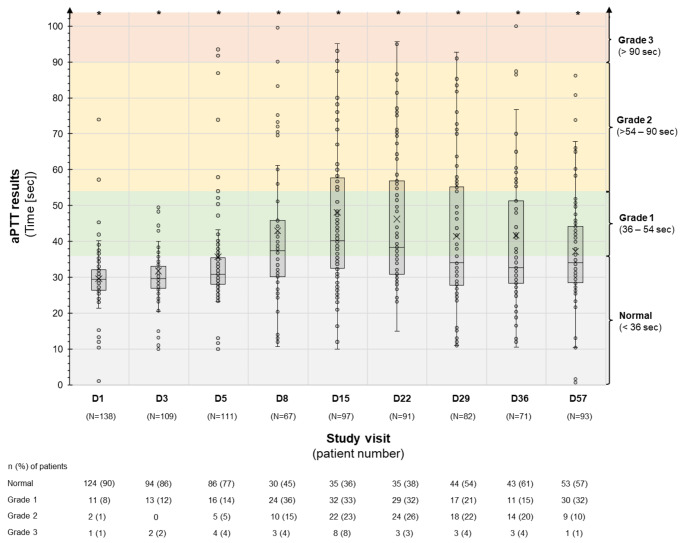
Data on aPTT were not available for 12 patients in the EVOLVE study. Among the 192 patients with aPTT data available, maximum grade of prolongation per patient was: normal, 21%; Grade 1, 37%, Grade 2, 27%; Grade 3, 15%. There was no appreciable association between incidence or severity of aPTT prolongation with the dose or type of vector received. As shown in Fig. 1, aPTT prolongation typically began by Day 8, and peaked between Days 15 and 22, following first dose of virus. Most cases resolved or improved to Grade 1 within 2 months.
Fig. 1.
aPTT prolongation over time
aPTT = activated partial thromboplastin time; D = day; sec = seconds.
aPTT tests were performed in the following study visits: day (D) 1, D3, D5, D8, D15, D29, D36 and D57 (or end of treatment). Patients from the EVOLVE study excluded from this graph due to infrequent assessments of aPTT. The data is presented in a ‘box and whisker’ graph. The box displays the interquartile range (which contains the middle 50% of values) and includes the mean marker (X) and the median marker (-). The whiskers indicate the range of the non-outlier data. Dots represent individual patients; due to the number of patients, a dot is not included for every patient, rather these have been included to provide a representation of the spread of the data, including outlier data.
*Nine patients had an aPTT results of > 100 s at one or more time point (D1 n = 1; D3 n = 2; D5 n = 2; D8 n = 1; D15 n = 4; D22 n = 1; D29 n = 1; D36 n = 2; D57 n = 1).
Characterization of aPTT prolongation
While both liver dysfunction and elevated C-reactive protein have been associated with prolonged aPTT, [25, 26] neither liver toxicity nor consistent patterns of C-reactive protein elevation have been observed with enadenotucirev or T-SIGn vectors. Alternatively, an acquired prolonged aPTT in patients without a bleeding history may be due to aPL.
Among patients with an aPTT prolongation, mixing studies suggested the presence of an inhibitor (Table 2). A positive result on at least one aPL test was seen in all cases when testing was performed (23/23 patients; Table 2). By contrast, aCL and aβ2GPI IgG were negative in all tested patients (17/17). aCL and aβ2GPI IgM were negative in all but 3 patients (aCL IgM n = 2; aβ2GPI IgM n = 2 [one patient positive for both]).
Table 2.
Coagulation study results in patients with prolonged (≥ Grade 2) aPTT
| Coagulation test | Number of patients with an abnormal result per local or central testing | Normal ranges | Median (min, max)a |
|---|---|---|---|
| aPL (n = 23) | |||
| dRVVT (confirmatory result; n = 8b) | 8 | ratio < 1.21 |
1.63 (1.24, 2.01) n = 6 |
| Hexagonal phase phospholipids (n = 15) | 15 | 0–11 s |
48 (18, 129) n = 15 |
| aCL IgG (n = 17) | 0 |
< 15 GPL (indeterminate 15–20 GPL) |
10 (10, 10) n = 14 |
| aCL IgM (n = 17) | 2 |
< 13 MPL (indeterminate 13–20 MPL) |
10 (10, 36) n = 14 |
| aβ2GPI IgG (n = 17) | 0 | < 21 SGU |
10 (10, 12) n = 14 |
| aβ2GPI IgM (n = 17) | 2 | < 33 SMU |
10 (10, 94) n = 14 |
aPL testing was not conducted for patients without prolonged aPTT. Testing for aCL and aβ2GPI was only conducted in patients with a positive aPL result. For aCL and aβ2GPI most values were reported as ‘<[ numeric value]’. Where available, central laboratory results were used in this table. For the median, numeric values after the symbol have been used. In the cases of a range, the higher value has been selected for the median calculation
aβ2GPI = anti-β2-glycoprotein I antibodies; aCL = anticardiolipin; aPL = antiphospholipid antibody; aPTT = activated partial thromboplastin time; dRVVT = Dilute Russell Viper Venom Time; GPL/MPL = IgG/IgM phospholipid unit; SGU/SMU = standard IgG/IgM aβ2GPI unit
aResults only included in median (min, max) calculations if normal ranges match that of the central laboratory.
bTwo patient had both dRVVT and hexagonal phase phospholipid tests conducted; the dRVVT results were used in this analysis.
Clinical sequalae
Across the platform, there were 9 clinically significant (≥ Grade 2; CTCAE) treatment-emergent bleeding (n = 3) or thrombotic events (n = 6) in 8 patients (Table 3). This represents 4% (8/204) of the patients included in this report, which does not exceed what would be expected in a population of patients with advanced cancer who have received multiple cytotoxic treatments [27]. Three patients experienced ≥ Grade 2 CTCAE treatment-emergent bleeding events, one of whom also experienced a thrombotic event. No association between clinical sequala and aPTT prolongation was observed. One episode of Grade 2 embolism (type unspecified) was classified as possibly related to enadenotucirev-treatment and one event of disseminated intravascular coagulation (not associated with bleeding) was classified as related. All other ≥ Grade 2 bleeding/clotting events were deemed not related or unlikely related to study treatment.
Table 3.
Clinically significant (≥ Grade 2) treatment-emergent bleeding or clotting events
| Across the platform (n = 204) | Total | No aPTT data during event | aPTT normal/Grade 1 during event | aPTT ≥ Grade 2 during event |
|---|---|---|---|---|
| Bleeding events | 3 | 2 | 1 | 0 |
| EVOLVE: Grade 2 hemorrhoidal hemorrhage. aPTT: No data collected. | STAR: Grade 3 perihepatic hemorrhage (post-procedural). aPTT: Grade 1 (36.6 s) 1 day after event start | |||
| SPICE: Grade 2 hematuria.a aPTT: Grade 1 (42.6 s) 5 days before event. | ||||
| Thrombotic events | 6 | 2 | 2 | 2 |
| SPICE: Grade 3 deep vein thrombosis.a aPTT: Grade 1 (42.6 s) 38 days before event | OCTAVE: Grade 3 thromboembolic pulmonary event. aPTT: Grade 1 (44.4 s) 13 days after event start |
OCTAVE: Grade 2 embolism.b aPTT: • Grade 3 (120 s) 21 days after event start • Grade 3 (201.6 s) 25 days after event start • Grade 2 (69.1 s) 42 days after event start |
||
| STAR: Grade 3 pulmonary embolism. aPTT Grade 2 (81.8 s) 28 days before event |
SPICE: Grade 3 (SAE) pulmonary embolism. aPTT: • Grade 1 (42.9 s) 8 days after event start • Grade 2 (86.6 s) 22 days after event start • Grade 1 (39.2 s) 29 days after event start |
SPICE: Grade 2 disseminated intravascular coagulation. aPTT: • Grade 2 (73.3 s) on event start • Grade 3 (95.7 s) 15 days after event start • Grade 2 (83.5 s) 23 days after event start • Grade 1 (44.7 s) 107 days after event start |
aPTT = activated partial thromboplastin time; ISTH = International Society on Thrombosis and Haemostasis; SAE = serious adverse event
aIn the SPICE study, hematuria and deep vein thrombosis occurred in the same patient
baPTT was Grade 3 (132.9 s) one week before the event started (Day 3). Patient also experienced a Grade 2 hepatic hemorrhage prior to initiation of study treatment (aPTT normal at the time of event [28.9 s])
Discussion
In this study of patients receiving adenoviral vectors, prolonged aPTT (≥ Grade 2) laboratory abnormalities were observed in 42% of patients, with a peak at 2–3 weeks after treatment and resolving over approximately 2 months. The prolonged aPTT was associated with a LA-type inhibitor. Additionally, among patients with aPTT prolongation, presence of aCL or aβ2GPI IgG, were not observed. This discordance between the presence of positive LA assays and negative ELISA assays for aCL or aβ2GPI is not a typical pattern of clinically relevant APS [4]. Likewise the transient nature of the aPTT prolongation is also inconsistent with a diagnosis of APS; International guidelines stipulate that aPL should persist for > 12 weeks as a pre-requisite for diagnosis of APS [5]. Importantly, in the patients receiving enadenotucirev/T-SIGn vectors with aPTT prolongation, including those with positive aPL assays, there was no evidence of an increased rate of thrombosis. Given the limited number of patients with aPL data in this study, future studies will be needed to definitively rule out a thrombotic risk and confirm that the prolonged aPTT is caused by aPL. It is also important to assess if other viral vectors may exhibit a similar pattern of coagulation changes.
The causes of infection-induced aPL remain unknown, however, molecular mimicry due to shared genetic epitopes between infectious agents and phospholipids has been proposed as a potential mechanism [28, 29]. Notably, pre-clinical studies and clinical experience have shown that not all aPL produced during infections are pathogenic or associated with clinical symptoms [30]. Research has shown that aPL are directed against plasma proteins that bind to phospholipids, rather than phospholipids themselves [31]. As such, the different mechanisms of inducing aPL may lead to the production of different subsets of aPL with different clinical impact. Indeed, the presence of LA-inducing anti-aβ2GPI with an affinity for domain I appears to correlate almost uniformly with the occurrence of thrombosis [31]. In this study we saw prolonged aPTT and induction of aPL with both enadenotucirev and the modified T-SIGn vectors. The structural and biophysical properties of T-SIGn vectors are indistinguishable from those of enadenotucirev. Therefore, we hypothesize that a shared genetic epitope in the viral capsid is causing cross-reactivity that leads to the generation of a non-pathogenic aPL. Further development of assays with the specificity to distinguish between different aPL subsets is needed.
The data presented here provide additional support for the safety of the T-SIGn vector platform. Although the risk of bleeding/clotting is believed to be low, several measures have been implemented in ongoing and future enadenotucirev and T-SIGn studies to monitor and further characterize any possible toxicity related to aPTT and aPL, including delaying biopsies in patients with Grade 2 or 3 prolongation. As adenoviral vectors are being increasingly used, this is an important phenomenon for the wider medical and research communities to recognize, and we recommend testing and characterization of such phenomena early in the development of these therapies. Furthermore, these findings may have implications for the care of patients who have had adenoviral vector vaccination against COVID-19 and other infectious diseases.
Acknowledgements
This study was funded by Akamis Bio Limited.
Author Contribution
All authors were involved in drafting of manuscript and revising it critically for important intellectual content and approved the final version to be published. Conception and design of the study: I.P.G-A., T.L. Study supervision and collection of data: D.N.K., T.L., L.R. Analysis and interpretation of data: I.P.G-A., D.N.K., T.L., L.R., A.S., L.P. G.A.S.
Funding
This study was funded by Akamis Bio Ltd.
Data Availability
Data are available on reasonable request. The datasets generated and/or analyzed during the current study are not publicly available, however, any reasonable requests for access to available data underlying the results reported in this article will be considered. Such proposals should be submitted to the corresponding author.
Declarations
Ethics approval statement
This study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Approval from the appropriate institutional review board and independent ethics committee was received for the protocol, amendments, and consent forms before initiating the study at each site.
Competing interests
D.N.K.: Merck Sharp & Dohme: Intellectual Property Rights. AbbVie and Akamis Bio Ltd: Provision of Services.
I.P.G-A.: Akamis Bio Ltd: Employee (at the time of analysis) and Stock Options.
T.L. AS, LP: Akamis Bio Ltd: Employees and Stock Options.
L.R.: Akamis Bio Ltd: Research Funding to Institution.
G.A.S.: Amgen, Janssen Scientific Affairs and Dova/Sobi Pharmaceuticals: Research Support. Amgen, Janssen Scientific Affairs and Dova Pharmaceuticals, Novartis, Anthos Therapeutics, Hengrui (USA) Ltd: Advisory Boards/Consulting.
Footnotes
The original online version of this article was revised: The article was amended to clearly define the affiliation of an author at the time the study was performed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/11/2023
A Correction to this paper has been published: 10.1007/s10637-023-01369-0
References
- 1.Machiels JP, Salazar R, Rottey S, Duran I, Dirix L, Geboes K, et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE) J Immunother Cancer. 2019;7(1):20. doi: 10.1186/s40425-019-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno V, Barretina-Ginesta M-P, García-Donas J, Jayson GC, Roxburgh P, Vázquez RM, et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: a phase 1 clinical trial. J Immunother Cancer. 2021;9(12):e003645. doi: 10.1136/jitc-2021-003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer. 2017;5(1):71. doi: 10.1186/s40425-017-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia D, Erkan D. Diagnosis and management of the Antiphospholipid Syndrome. N Engl J Med. 2018;378(21):2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 5.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, CERVERA R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 6.Devreese KM. Antiphospholipid antibody testing and standardization. Int J Lab Hematol. 2014;36(3):352–363. doi: 10.1111/ijlh.12234. [DOI] [PubMed] [Google Scholar]
- 7.Chayoua W, Kelchtermans H, Moore GW, Musiał J, Wahl D, de Laat B, et al. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J Thromb Haemost. 2018;16(10):2016–2023. doi: 10.1111/jth.14261. [DOI] [PubMed] [Google Scholar]
- 8.Chayoua W, Kelchtermans H, Gris JC, Moore GW, Musiał J, Wahl D, et al. The (non-)sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J Thromb Haemost. 2020;18(1):169–179. doi: 10.1111/jth.14633. [DOI] [PubMed] [Google Scholar]
- 9.Vandevelde A, Chayoua W, de Laat B, Gris J-C, Moore GW, Musiał J, et al. Semiquantitative interpretation of anticardiolipin and antiβ2glycoprotein I antibodies measured with various analytical platforms: communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid antibodies. J Thromb Haemost. 2022;20(2):508–524. doi: 10.1111/jth.15585. [DOI] [PubMed] [Google Scholar]
- 10.Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62(5):388–393. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Wahab N, Talathi S, Lopez-Olivo MA, Suarez-Almazor ME. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27(4):572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 12.Malaeb BS, Gardner TA, Margulis V, Yang L, Gillenwater JY, Chung LWK, et al. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology. 2005;66(4):830–834. doi: 10.1016/j.urology.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 13.Crank MC, Wilson EM, Novik L, Enama ME, Hendel CS, Gu W, et al. Safety and Immunogenicity of a rAd35-EnvA prototype HIV-1 vaccine in combination with rAd5-EnvA in healthy adults (VRC 012) PLoS ONE. 2016;11(11):e0166393. doi: 10.1371/journal.pone.0166393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29(2):304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Sheets RL, Stein J, Bailer RT, Koup RA, Andrews C, Nason M, et al. Biodistribution and toxicological safety of adenovirus type 5 and type 35 vectored vaccines against human immunodeficiency virus-1 (HIV-1), Ebola, or Marburg are similar despite differing adenovirus serotype vector, manufacturer’s construct, or gene inserts. J Immunotoxicol. 2008;5(3):315–335. doi: 10.1080/15376510802312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 17.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 18.Fakih M, Wang D, Harb W, Rosen L, Mahadevan D, Berlin J et al (2019) A phase I multicenter study of enadenotucirev in combination with nivolumab in tumors of epithelial origin: an analysis of the metastatic colorectal cancer patients in the dose escalation phase. Ann Oncol 30(Suppl 5):V198–V252
- 19.Krige D, Fakih M, Rosen L, Wang D, Harb W, Babiker H et al (2021) 342 Combining enadenotucirev and nivolumab increased tumour immune cell infiltration/activation in patients with microsatellite-stable/instability-low metastatic colorectal cancer in a phase 1 study. J Immunother Cancer 9(Suppl 2):A368–A9
- 20.Naing A, Rosen L, Camidge RD, Khalil D, Davies J, Miles D, et al. 1011P FORTITUDE phase I study of NG-350A, a novel tumour-selective adenoviral vector expressing an anti-CD40 agonist antibody: Monotherapy dose escalation results. Ann Oncol. 2021;32:S853–S4. doi: 10.1016/j.annonc.2021.08.1395. [DOI] [Google Scholar]
- 21.Lillie T, O’Hara M, Ottensmeier C, Parkes E, Rosen L, Krige D, et al. Abstract CT213: a multicenter phase 1a/b study of NG-350A, a tumor-selective anti-CD40-antibody expressing adenoviral vector, and pembrolizumab in patients with metastatic or advanced epithelial tumors (FORTIFY) Cancer Res. 2022;82(12Supplement):CT213–CT. doi: 10.1158/1538-7445.AM2022-CT213. [DOI] [Google Scholar]
- 22.Simon G, Subbiah V, Rosen L, Lenz H-J, Park H, Patel M, et al. 762 First-in-human phase 1a study of NG-641, a tumour-selective vector expressing a FAP-TAc bispecific antibody and immune enhancer module, in patients with metastatic/advanced epithelial tumours (STAR) J Immunother Cancer. 2022;10(Suppl 2):A793–A. [Google Scholar]
- 23.Ottensmeier C, Evans M, King E, Karydis I, Lillie T, Krige D, et al. 437 A multicentre phase 1b study of NG-641, a novel transgene-armed and tumour-selective adenoviral vector, and pembrolizumab as neoadjuvant treatment for squamous cell carcinoma of the head and neck. J Immunother Cancer. 2021;9(Suppl 2):A467–A. doi: 10.1136/jitc-2021-SITC2021.437. [DOI] [Google Scholar]
- 24.Lillie T, Parkes E, Ottensmeier C, Krige D, Ravanfar B, Evilevitch V, et al. Abstract CT214: a multicenter phase 1a/b study of NG-641, a tumor-selective transgene-expressing adenoviral vector, and nivolumab in patients with metastatic or advanced epithelial tumors (NEBULA) Cancer Res. 2022;82(12Supplement):CT214–CT. doi: 10.1158/1538-7445.AM2022-CT214. [DOI] [Google Scholar]
- 25.Wiwanitkit V. Activated partial Thromboplastin Time abnormality in patients with Cholangiocarcinoma. Clin Appl Thromb Hemost. 2004;10(1):69–71. doi: 10.1177/107602960401000112. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Li F, Shu K, Chen T, Wang X, Xie Y, et al. The analysis of false prolongation of the activated partial thromboplastin time (activator: silica): interference of C-reactive protein. J Clin Lab Anal. 2018;32(8):e22571. doi: 10.1002/jcla.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219–230. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016;25(14):1520–1531. doi: 10.1177/0961203316640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoenfeld Y, Blank M, Cervera R, Font J, Raschi E, Meroni P-L. Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis. 2006;65(1):2–6. doi: 10.1136/ard.2005.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gharavi AE, Pierangeli SS, Harris EN. Origin of antiphospholipid antibodies. Rheum Dis Clin North Am. 2001;27(3):551–563. doi: 10.1016/S0889-857X(05)70219-2. [DOI] [PubMed] [Google Scholar]
- 31.de Laat B, Mertens K, de Groot PG. Mechanisms of Disease: antiphospholipid antibodies—from clinical association to pathologic mechanism. Nat Clin Pract Rheumatol. 2008;4(4):192–199. doi: 10.1038/ncprheum0740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. The datasets generated and/or analyzed during the current study are not publicly available, however, any reasonable requests for access to available data underlying the results reported in this article will be considered. Such proposals should be submitted to the corresponding author.