Abstract
Purpose of review
Endoscopic eradication therapy is an effective and durable treatment for Barrett’s esophagus (BE) related neoplasia, but even after achieving successful eradication, these patients remain at risk for recurrence and require ongoing routine examinations. The optimal surveillance protocol including endoscopic technique, sampling strategy, and timing are still being refined. The aim of this review is to discuss current management principles for the post ablation patient and emerging technologies to guide clinical practice.
Recent findings
There is increasing evidence to support less frequent surveillance exams in the first year after complete eradication of intestinal metaplasia and a move towards targeted biopsies of visible lesions and sampling high-risk locations such as the gastroesophageal junction. Promising technologies on the horizon that could impact management include novel biomarkers, personalized surveillance intervals, and non-endoscopic approaches.
Summary
Ongoing high-quality examinations after endoscopic eradication therapy are key to limiting recurrent BE. Surveillance intervals should be based on the pretreatment grade of dysplasia. Future research should focus on technologies and surveillance practices that are most efficient for patients and the healthcare system.
Keywords: Barrett’s esophagus (BE), Esophageal adenocarcinoma (EAC), Endoscopic eradication therapy (EET), Recurrence, Ablation, Complete eradication of intestinal metaplasia (CEIM)
Introduction
The incidence of esophageal adenocarcinoma (EAC) has been steadily rising in the USA and carries a poor prognosis since most patients present with late-stage disease [1, 2]. The only known premalignant lesion is Barrett’s esophagus (BE), making early identification and appropriate management of paramount importance to improve outcomes in EAC [3]. BE is defined as intestinal metaplasia (IM, the presence of >1 cm of columnar lined esophagus with corresponding histology demonstrating goblet cells) and progresses from non-dysplastic (NDBE) to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) to intramucosal carcinoma (IMC) and then invasive cancer. Given this sequence, gastroenterology societies worldwide recommend endoscopic eradication therapy (EET) for dysplastic BE with resection and ablation to restore squamous epithelium [4, 5]. Yet even after the Barrett’s segment has been successfully treated or seemingly “cured,” patients remain at risk for recurrent IM and dysplasia and require ongoing surveillance examinations. The management of a patient after BE therapy is nuanced and requires consideration of the original pathology, endoscopic techniques, resource utilization, and risk tolerance. The aim of this review is to discuss current management principles for the post ablation BE patient and emerging technologies to guide clinical practice.
Endpoints and outcomes of endoscopic eradication therapy
The main objectives of EET are the removal of any visible lesions which may harbor dysplasia followed by ablation of the remaining BE segment [6]. The immediate goal is complete eradication of intestinal metaplasia (CEIM) with an additional intermediate endpoint of complete eradication of dysplasia (CE-D). Rates of CEIM and CE-D for EET are high at 78% and 91%, respectively, on meta-analysis [7]. Furthermore, ablation for LGD decreases the risk of progression to HGD or IMC by 25% [8, 9]. Recent data emphasizes the long-term durability of EET in maintaining these key outcome measures [10, 11]. CEIM is achieved after 1–2 surveillance endoscopies confirm no visible BE and biopsies from the gastroesophageal junction (GEJ) and esophagus are negative for IM. The question of whether 1 versus 2 endoscopies are necessary to call CEIM is a topic of ongoing debate, and inconsistent definitions and reporting make it difficult to compare rates and timing across studies. Nonetheless, CEIM patients will need indefinite long-term surveillance for early detection of recurrent IM and neoplasia. The recent ACG guidelines provide a strong recommendation for ongoing endoscopic surveillance post CEIM based on moderate quality evidence which is consistent with prior ASGE and AGA guidelines [4, 5, 12].
Rates of recurrent intestinal metaplasia and dysplasia
Recurrence is defined as the presence of IM (with or without dysplasia) in the esophagus or GEJ after CEIM, recognizing that studies vary on whether 1 or 2 negative exams are necessary for the designation of CEIM. In most studies that include a mixed cohort of baseline histology followed for approximately 5 years, recurrence occurs in about 1/3 of the group. Several studies have demonstrated relatively low rates of incident IM ranging from 4.9 to 10.8%/person-year and even lower rates of incident HGD or EAC ranging from 0.3 to 1.6%/person-year (Table 1) [11, 13–18]. In a 2016 systematic review and meta-analysis of 21 studies and 3186 patients achieving CEIM after RFA, there were 603 cases of recurrent IM over 5741 patients-years of follow-up (annual incidence 9.5% per patient-year, 95% CI 6.7–12.3%). Recurrence of any dysplasia or HGD/EAC was much lower at 2% and 1.2%/person-year, respectively. Similar results were demonstrated in a subsequent meta-analysis and several observational studies since [14, 16]. In a cohort of 337 veterans achieving CEIM after RFA, the incidence of recurrent IM, dysplasia, and cancer was 10.8%, 2.2%, and 0.3%/patient-year, respectively [15]. Predictors of recurrent dysplasia included baseline neoplasia (dysplasia HR 1.71, IMC 2.32) and long-segment BE (> 3 cm HR 1.59). Several other studies similarly highlight this relationship between baseline histology and risk of recurrence. In follow-up data from the AIM dysplasia trial where 110 patients achieved CEIM, the incidence of recurrent IM was 8.3% compared to 13.5%/person-year for baseline LGD versus HGD, and corresponding dysplasia recurrence rates were 3.3% versus 7.3%/person-year, though these numerical differences were not statistically significant. The two most recent prospective studies by Wani et al. (US multicenter cohort of 807 patients) and van Munster et al. (Netherlands multicenter cohort of 1270 patients who achieved CEIM) both demonstrate a lower incidence of overall recurrence (4.9–5.2%) compared to earlier studies [11, 17]. This may reflect the high quality of care provided at large volume centers with specialized endoscopists and pathologists with expertise in BE management.
Table 1.
Recurrence rates of intestinal metaplasia and neoplasia after endoscopic eradication therapy.
| Study | Study type and setting | CEIM patients (N) | Incidence (IM per 100 PY) | Incidence (any dysplasia per 100 PY) | Incidence (HGD/EAC per 100 PY) |
|---|---|---|---|---|---|
| Krishnamoorthi 2016 [13] | Systematic review and meta-analysis: subset of study with data from 21 RFA studies | 3186 | 9.5 | 2.0 | 1.2 |
| Fujii-Lau 2017 [14] | Systematic review and meta-analysis: 39 studies (25 RFA, 13 stepwise complete EMR, 2 mixed) | 3213 | 8.6 | 1.9 | -- |
| Tan 2019 [15] | Veterans Affairs, 40 centers | 337 | 10.8 | 2.2 | 0.3 |
| Sami 2019 [16] | 3 US and 2 UK tertiary referral centers | 594 | 9.6 | 2.8 | 1.6 |
| Wani 2020 [17] | 4 US tertiary referral centers | 807 | 5.2 | 1.6 | -- |
| Munster 2021 [11] | Dutch Expert Barrett’s Centers | 1154 | 4.9 | 1.0 | 0.7 |
Timing of recurrent intestinal metaplasia and dysplasia
Although rates of IM seem to be consistent across the literature and lower in more recent reports, the timing of recurrence differs considerably between studies. This is important because it impacts the timing of surveillance endoscopy and informs interval recommendations. The long-term analysis from the AIM dysplasia trial showed the highest likelihood of recurrence in the first year post CEIM compared to the next 4 years combined, with decreasing rates over time [18]. To account for the possibility of declaring CEIM prematurely, they performed a sensitivity analysis requiring 2 negative endoscopies to designate CEIM and found no meaningful impact, supporting their conclusion for aggressive surveillance in the first year. Results from a systematic review and meta-analysis similarly demonstrated higher rates of early recurrence, though corresponding high rates of HGD/EAC in the first year suggest that recurrence rates may have been overestimated and the findings were not actually recurrence, but rather incompletely treated or missed prevalent disease [19]. Recently published long-term outcomes from the UK National HALO RFA registry also showed that most recurrences happen in the first 2 years (4.2% recurrence rate after CEIM at 1 year, 10.1% at 2 years, 18.7% at 8 years) [10]. Results from Sami et al. (3 US and 2 UK prospective databases, recurrent IM in 151/594 patients) demonstrated that recurrence of IM and/or dysplasia remained constant over time or even increased progressively over time, including beyond the 4-year mark [16]. Therefore, contrary to the conclusions in the AIM dysplasia follow-up paper, these authors maintain that surveillance should continue beyond 5 years. In a larger multicenter US study by Wani et al., recurrence was most common between 1 and 2 years and peaked at 18 months, suggesting that surveillance might not be necessary in the first year [17]. Consistent findings from the Dutch RFA data demonstrating no difference between an aggressive surveillance strategy during the first year (every 3 months) versus a more relaxed approach (only at the 1-year point) similarly suggest that lengthening the short-term surveillance intervals may be appropriate [11]. In the VA cohort described earlier, 29.1% of the 337 patients had recurrent BE at a median of 1.9 years. The risk of BE recurrence started around 12 months for NDBE and 6 months for dysplastic BE/IMC, and both short- and long-segment BE had parallel curves in the first year. Interestingly, they found a shorter time to BE recurrence among individuals ablated at low-volume facilities, whereas treatment at high-volume RFA centers reduced the risk of BE recurrence (HR 0.19 for quartile 4 vs 1, 95% CI 0.05–0.68). This concept of better outcomes among patients with BE/EAC at high-volume centers has been previously demonstrated and further emphasizes the importance of centralized care at experienced facilities [20].
Surveillance intervals after achieving CEIM
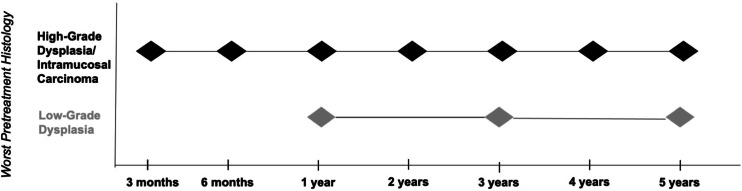
Until recently, recommendations for surveillance intervals for BE patients post CEIM were largely based on data from cohort studies and expert opinion and tended to recommend frequent exams [21, 22]. As summarized, newer studies indicating lower rates of early recurrence in the first year seem to support a strategy of less frequent surveillance. A modeling study that used data from the US Radiofrequency Ablation Registry and the UK National HALO Registry recommended surveillance exams at year 1 and year 3 for LGD and at 3 months, 6 months, 1 year then annually for HGD/IMC [23]. These findings provided evidence-based support for the post CEIM recommendations in both the 2020 AGA Clinical Practice Update and 2022 ACG guidelines, which continue to be based on the worst pretreatment histology grade [4, 5]. According to the most recent ACG document, surveillance is recommended at year 1, year 3, and then every 2 years after for LGD. Surveillance is recommended at 3 months, 6 months, then annually for HGD/early EAC [5]. The AGA guidelines are similar with the exception that they do not recommend further surveillance after year 3 for LGD (Fig. 1). Results from the long-term outcomes of the EET Dutch study where dysplasia recurrence was similar with a surveillance exam at year 1 versus every 3 months during the first year suggest that less aggressive surveillance intervals could be appropriate regardless of baseline histology [11].
Fig. 1.
Recommended surveillance intervals after achieving complete eradication of intestinal metaplasia.
Surveillance post CEIM: cost and societal implications
Another important consideration when determining surveillance intervals is cost and resource utilization, though data on this topic is lacking. A recent modeling study compared three different post CEIM surveillance strategies: (1) the Cotton approach—surveillance at years 1 and 3 for LGD/IND and every 3 months for the first year followed by annually for HGD/IMC, (2) the 2016 ACG guidelines—surveillance every 6 months then annually for LGD/IND, and every 3 months in the first year followed by every 6 months in the second year then annually for HGD/IMC [21], and (3) the UK approach—every 3 months in the first year followed by every 6 months in the second year then annually regardless of pretreatment histology [11, 24]. The 2016 ACG approach was the most cost-effective, and the Cotton approach performed the worst. The authors suggest that the poor performance of the Cotton approach may be a result of less frequent surveillance leading to increased development of advanced EAC, although EET durability studies suggest against this [11]. In some ways, the results of this study are moot given that the ACG guidelines referenced in the model have since been updated.
There are also important unmeasurable factors and potentially overlooked aspects related to surveillance timing. Endoscopy has associated cost for the patient and requires time off work. The psychological impact is also relevant and can work on both sides, as more frequent surveillance may both contribute to as well as alleviate patient anxiety and fear of cancer depending on the individual. There is no data on patient preferences related to surveillance endoscopy specifically after CEIM; however, among a group of patients at risk for BE/EAC, 2/3 indicated they wanted to prioritize BE screening and that getting an upper endoscopy would reduce their concern [25]. The frequency of surveillance exams also stresses the healthcare system. Fewer exams may help alleviate the growing burden on gastroenterologists to perform other diagnostic and therapeutic procedures, especially given the backlogs created by the COVID-19 pandemic and the new colorectal cancer screening guidelines that include adults age 45–49. Nevertheless, these factors should not deter physicians or patients from remaining diligent with surveillance. Future studies should focus not only on whether lengthening surveillance intervals post CEIM leads to similar clinical outcomes but how these impact patient satisfaction and resource utilization.
High-quality endoscopy for surveillance after achieving CEIM
Surveillance endoscopy to follow patients after therapy and CEIM rely on the same principles of a high-quality BE screening or surveillance exam with the goal in this population to detect new dysplasia [26]. BE exams should be performed with high-definition white light endoscopy (HD-WLE), and a distal attachment cap can help with stabilization and up-close mucosal visualization. Routine use of virtual or dye-based chromoendoscopy during surveillance is recommended by major GI society guidelines due to its ability to increase dysplasia detection compared to HD-WLE alone [4, 5, 27, 28]. A thorough and comprehensive examination means spending at least 1 min inspecting each cm of the Barrett’s segment as well as a retroflexed view of the cardia [29]. Documentation of esophageal landmarks including the presence of a hiatal hernia, use of the Prague classification to report the circumference and maximal length of the segment, and application of the Paris classification to describe visible lesions are key components of standardized reporting [6, 30].
Location of recurrence and sampling strategies after achieving CEIM
The typical location of recurrent BE is critically important and influences our sampling protocol. In the study by Sami et al., 74% of recurrences occurred at the GEJ, 25% of these were dysplastic, and only 40% of these dysplastic recurrences were visible to the endoscopist [16]. Most of the 26% of recurrences that occurred in the tubular esophagus were visible. These results emphasize the importance of a high-quality exam using image-enhanced endoscopy to perform a detailed inspection of the distal esophagus and GEJ in forward view as well as retroflexion to examine the cardia. Additional studies indicating high rates of recurrence in the distal esophagus and GEJ have led to a shift in the biopsy protocol for post-ablative surveillance exams [31, 32]. Previously, guidelines recommended 4 quadrant biopsies every 2 cm along the neosquamous epithelium, similar to the Seattle protocol [33]. However, random biopsies throughout the entirety of the neosquamous epithelium have proven to be extremely low yield for detecting IM or dysplasia during post CEIM surveillance [11, 16]. In fact, a modification in the sampling protocol post CEIM midway through the Dutch EET study, from random 4 quadrant biopsies every 2 cm to targeted biopsies for visible abnormalities, had no impact on outcomes [11]. The standard approach has therefore evolved to a more effective strategy that incorporates targeted biopsies of visible lesions with random biopsies of specific areas at high risk of recurrence such as the GEJ. Accordingly, ACG guidelines recommend taking surveillance biopsies post CEIM from the GEJ and the distal 2–5 cm of the tubular esophagus and placing them in separate pathology jars [5, 16].
Additional tools for imaging post RFA epithelium such as volumetric laser endomicroscopy require further study. Wide-area transepithelial sampling with computer-assisted three-dimensional (WATS-3D) uses an abrasive cytologic brush to sample a large region followed by computer modeling that recreates glandular structures. Although the 2019 ASGE guidelines recommended the use of WATS-3D in BE examinations based on higher rates of dysplasia detection, the 2022 ACG guidelines do not make a recommendation regarding WATS-3D in routine surveillance given the lack of comparative studies [5, 28]. Whether this adjunctive sampling strategy will have a role in surveillance exams specifically in the post CEIM patient remains to be seen. Artificial intelligence and machine learning for BE show promising initial results. A meta-analysis of twelve studies indicated high sensitivity and specificity for identifying BE-related neoplasia (90% and 84%, respectively) [34]. While most of the current literature focuses on patients with untreated disease, application of these technologies to post CEIM patients has great potential to increase the yield of surveillance exams.
Management of recurrent intestinal metaplasia and dysplasia
Most recurrences after CEIM are non-dysplastic and tend to occur at a lower grade than the baseline histology [13, 17]. Recurrent disease is typically amenable to endoscopic resection, and therapy follows the same principle of resection for any visible abnormalities followed by ablation of residual flat IM [4, 5]. In the Dutch RFA durability cohort, 87% of patients who had recurrence were successfully managed endoscopically [11]. In the longitudinal study from the UK National Halo RFA Registry, treatment of recurrent BE was successful in over 50% of cases [10]. Even after CEIM, once daily low-dose proton pump inhibitors should be continued in all patients. While anti-reflux surgery may have a role in patients with treatment-refractory BE, its use after CEIM to prevent recurrence has not been well studied.
Non-endoscopic detection of Barrett’s esophagus
In addition to optimizing surveillance examinations through endoscopy and adjunctive technologies, investigation into complementary non-endoscopic modalities is underway. Most of these consist of a swallowable cell collection device that are tethered to a string and sample the distal esophagus while being removed through the mouth. Cytology can then be combined with biomarker evaluation. Additional tests include the Aenose, which detects volatile organic compounds from exhaled breaths [35] or unsedated transnasal endoscopy. These testing modalities are attractive from a resource utilization standpoint given their ability to be used by non-physicians in an office setting. Although they have mostly been studied in the screening setting, they may eventually have a role in BE surveillance including surveillance post CEIM.
Personalizing surveillance
The crux of optimizing surveillance recommendations for patients post CEIM is the identification of risk factors and molecular markers to predict recurrence. This paradigm shift will facilitate individualized treatment plans for each patient based on their unique features. A prognostic model was developed using data from the Dutch Barrett Expert Center Registry and determined the following predictors of visible recurrence from strongest to weakest: new “incident” lesion during treatment, higher number of EET sessions, male sex, increasing BE length, HGD/cancer at baseline, and younger age. The model was validated externally in patients treated in Switzerland and Belgium with good discrimination and calibration (C-statistic 0.91, 95% CI 0.86–0.94) [36]. This concept of personalizing follow-up based on individual risk offers tremendous opportunity to improve the quality and value of clinical care and warrants further studies in other practice settings and populations.
Biomarkers to predict progression
The issues related to predicting recurrent IM and dysplasia post CEIM are similar to the challenges in predicting which NDBE patients or dysplastic BE will progress. At the present time, the degree of dysplasia is the best predictor of progression, though smoking, increasing age, male sex, and longer BE segments are also relevant risk factors [37]. Prediction models that use a combination of demographic and clinical factors coupled with molecular markers are gaining traction. The finding that mutations in the tumor suppressor gene p53 are associated with increased progression of BE led to interest in using it as a predictive tool [38]. Multiple meta-analyses have shown the utility of p53 staining to predict progression versus no progression to HGD/EAC (OR ranges 4–17) [39–41]. TissueCypher is an assay that provides a risk score for 5-year progression to HGD/EAC and has demonstrated 68% sensitivity and 79% specificity [42–44]. The 2022 ACG guidelines were unable to recommend routine use of p53 immunohistochemical staining or TissueCypher given the overall low sensitivity [5]. Ongoing investigations will hopefully identify subsets of patients who would benefit most from the adjunctive use of biomarkers for risk stratification. It is possible that these tools will eventually also play a role in delineating a patient-tailored surveillance interval post CEIM.
Conclusions
Improvement in endoscopic techniques for removal of BE-related neoplasia, refinement of therapeutic algorithms, and expansion of the toolbox for ablative devices beyond RFA all contribute to growing efficacy and durability data for EET. The goal of therapy is CEIM, but even once this outcome is achieved, patients must remain in a surveillance program to detect early recurrent dysplasia. Multiple studies over the past 5 years have provided relatively consistent quantitative estimates of recurrence rates for IM and dysplasia, though the timing of recurrence varies considerably across studies. In some cases, a finding of early recurrence may in fact represent prevalent or missed lesions after a label of CEIM was incorrectly assigned. Taken together, recurrence is probably more likely to occur beyond the first year, and a more lenient surveillance strategy in the initial 12 months following CEIM may be appropriate. Recent updates from multiple GI societies provide consistent recommendations that continue to separate surveillance intervals based on pretreatment histology. Personalized post CEIM surveillance strategies that use risk prediction models to incorporate patient characteristics, results from non-endoscopic testing modalities, biomarkers, and that harness the power of AI and machine learning may seem aspirational but appear to be on the horizon.
Author Contributions
All authors contributed to the preparation, writing, and review of the manuscript. The authors have all read and approve of the submitted manuscript.
Data Availability
The data in this paper are publicly available and can be provided by the corresponding author upon request.
Compliance with Ethical Standards
Competing Interests
Christian Davis declares that he has no conflict of interest. Jennifer M. Kolb declares that she has no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
- 1.Cook MB, Thrift AP. Epidemiology of Barrett’s esophagus and esophageal adenocarcinoma: implications for screening and surveillance. Gastrointest. Endosc. Clin. 2021;31(1):1–26. doi: 10.1016/j.giec.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb JM, Han S, Scott FI, Murphy CC, Hosokawa P, Wani S. Early-onset esophageal adenocarcinoma presents with advanced-stage disease but has improved survival compared with older individuals. Gastroenterology. 2020;159(6):2238–40.e4. doi: 10.1053/j.gastro.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtius K, Rubenstein JH, Chak A, Inadomi JM. Computational modelling suggests that Barrett’s oesophagus may be the precursor of all oesophageal adenocarcinomas. Gut. 2020;70(8):1435–1440. doi: 10.1136/gutjnl-2020-321598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Shaheen NJ, Katzka D, Bergman J. AGA Clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: expert review. Gastroenterology. 2020;158(3):760–769. doi: 10.1053/j.gastro.2019.09.051. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, et al. Diagnosis and management of Barrett’s esophagus: an updated ACG guideline. Am. J. Gastroenterol. 2022;117(4):559–587. doi: 10.14309/ajg.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb JM, Wani S. Endoscopic eradication therapy for Barrett’s oesophagus: state of the art. Curr. Opin. Gastroenterol. 2020;36(4):351–358. doi: 10.1097/MOG.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013;11(10):1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 2009;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 9.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 10.Wolfson P, Ho KMA, Wilson A, McBain H, Hogan A, Lipman G, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: a final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest. Endosc. 2022;96(2):223–233. doi: 10.1016/j.gie.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 11.van Munster S, Nieuwenhuis E, Weusten B, Alvarez Herrero L, Bogte A, Alkhalaf A, et al. Long-term outcomes after endoscopic treatment for Barrett’s neoplasia with radiofrequency ablation ± endoscopic resection: results from the national Dutch database in a 10-year period. Gut. 2021;71(2):265–276. doi: 10.1136/gutjnl-2020-322615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wani S, Qumseya B, Sultan S, Agrawal D, Chandrasekhara V, Harnke B, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest. Endosc. 2018;87(4):907–31.e9. doi: 10.1016/j.gie.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamoorthi R, Singh S, Ragunathan K, Ak D, Kw K, Gi P. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest. Endosc. 2016;83(6):1090–106.e3. doi: 10.1016/j.gie.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii-Lau LL, Cinnor B, Shaheen N, Gaddam S, Komanduri S, Muthusamy VR, et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett’s esophagus: a systematic review and meta-analysis. Endosc. Int. Open. 2017;5(6):E430–EE49. doi: 10.1055/s-0043-106578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan MC, Kanthasamy KA, Yeh AG, Kil D, Pompeii L, Yu X, et al. Factors associated with recurrence of Barrett’s esophagus after radiofrequency ablation. Clin. Gastroenterol. Hepatol. 2019;17(1):65–72.e5. doi: 10.1016/j.cgh.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Sami SS, Ravindran A, Kahn A, Snyder D, Santiago J, Ortiz-Fernandez-Sordo J, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicentre study. Gut. 2019;68(8):1379–1385. doi: 10.1136/gutjnl-2018-317513. [DOI] [PubMed] [Google Scholar]
- 17.Wani S, Han S, Kushnir V, Early D, Mullady D, Hammad H, et al. Recurrence is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin. Gastroenterol. Hepatol. 2020;18(11):2609–17.e2. doi: 10.1016/j.cgh.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Cotton CC, Wolf WA, Overholt BF, Li N, Lightdale CJ, Wolfsen HC, et al. Late Recurrence of Barrett’s esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in intestinal metaplasia containing dysplasia trial. Gastroenterology. 2017;153(3):681–8.e2. doi: 10.1053/j.gastro.2017.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawas T, Iyer PG, Alsawas M, Cotton CC, Leggett CL, Murad MH, et al. Higher rate of Barrett’s detection in the first year after successful endoscopic therapy: meta-analysis. Am. J. Gastroenterol. 2018;113(7):959–971. doi: 10.1038/s41395-018-0090-z. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Kolb JM, Hosokawa P, Friedman C, Fox C, Scott FI, et al. The volume-outcome effect calls for centralization of care in esophageal adenocarcinoma: results from a large national cancer registry. Am. J. Gastroenterol. 2021;116(4):811–815. doi: 10.14309/ajg.0000000000001046. [DOI] [PubMed] [Google Scholar]
- 21.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am. J. Gastroenterol. 2008;103(3):788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 23.Cotton CC, Haidry R, Thrift AP, Lovat L, Shaheen NJ. Development of evidence-based surveillance intervals after radiofrequency ablation of Barrett’s esophagus. Gastroenterology. 2018;155(2):316–26.e6. doi: 10.1053/j.gastro.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon S, Norman R, Mannath J, Iyer PG, Ragunath K. Comparative cost-effectiveness of three post-radiofrequency ablation surveillance intervals for Barrett’s esophagus. Endosc Int Open. 2022;10(8):E1053–E1e64. doi: 10.1055/a-1858-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb JM, Chen M, Tavakkoli A, Gallegos J, O'Hara J, Tarter W, et al. Patient knowledge, risk perception and barriers to Barrett’s esophagus screening. Am. J. Gastroenterol. 2022. 10.14309/ajg.0000000000002054. [DOI] [PMC free article] [PubMed]
- 26.Kolb JM, Wani S. Endoscopic Management of Barrett's Esophagus. Dig. Dis. Sci. 2022;67(5):1469–1479. doi: 10.1007/s10620-022-07395-x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62(1):15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 28.Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019;90(3):335–59.e2. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Gupta N, Gaddam S, Wani SB, Bansal A, Rastogi A, Sharma P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest. Endosc. 2012;76(3):531–538. doi: 10.1016/j.gie.2012.04.470. [DOI] [PubMed] [Google Scholar]
- 30.Gorrepati VS, Sharma P. How should we report endoscopic results in patient's with Barrett’s esophagus? Dig. Dis. Sci. 2018;63(8):2115–2121. doi: 10.1007/s10620-018-5067-7. [DOI] [PubMed] [Google Scholar]
- 31.Omar M, Thaker AM, Wani S, Simon V, Ezekwe E, Boniface M, et al. Anatomic location of Barrett’s esophagus recurrence after endoscopic eradication therapy: development of a simplified surveillance biopsy strategy. Gastrointest. Endosc. 2019;90(3):395–403. doi: 10.1016/j.gie.2019.04.216. [DOI] [PubMed] [Google Scholar]
- 32.Cotton CC, Wolf WA, Pasricha S, Li N, Madanick RD, Spacek MB, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett’s esophagus: endoscopic findings and anatomic location. Gastrointest. Endosc. 2015;81(6):1362–1369. doi: 10.1016/j.gie.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald RC, Saeed IT, Khoo D, Farthing MJ, Burnham WR. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett’s esophagus. Dig. Dis. Sci. 2001;46(9):1892–1898. doi: 10.1023/A:1010678913481. [DOI] [PubMed] [Google Scholar]
- 34.Tan JL, Chinnaratha MA, Woodman R, Martin R, Chen HT, Carneiro G, et al. Diagnostic accuracy of artificial intelligence (AI) to detect early neoplasia in Barrett’s esophagus: a non-comparative systematic review and meta-analysis. Front. Med. (Lausanne). 2022;9:890720. doi: 10.3389/fmed.2022.890720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters Y, Schrauwen RWM, Tan AC, Bogers SK, de Jong B, Siersema PD. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut. 2020;69(7):1169–1172. doi: 10.1136/gutjnl-2019-320273. [DOI] [PubMed] [Google Scholar]
- 36.van Munster SN, Nieuwenhuis E, Bisschops R, Willekens H, Weusten B, Herrero LA, et al. Development and external validation of a model to predict complex treatment after radiofrequency ablation for Barrett’s esophagus with early neoplasia. Clin. Gastroenterol. Hepatol. 2022;20(11):2495–2504. doi: 10.1016/j.cgh.2022.02.057. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamoorthi R, Singh S, Ragunathan K, Visrodia K, Wang KK, Katzka DA, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018;16(7):1046–55.e8. doi: 10.1016/j.cgh.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Stachler MD, Camarda ND, Deitrick C, Kim A, Agoston AT, Odze RD, et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology. 2018;155(1):156–167. doi: 10.1053/j.gastro.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janmaat VT, van Olphen SH, Biermann KE, Looijenga LHJ, Bruno MB, Spaander MCW. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: a systematic review and meta-analysis. PLoS One. 2017;12(10):e0186305. doi: 10.1371/journal.pone.0186305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altaf K, Xiong JJ, la Iglesia D, Hickey L, Kaul A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br. J. Surg. 2017;104(5):493–502. doi: 10.1002/bjs.10484. [DOI] [PubMed] [Google Scholar]
- 41.Snyder P, Dunbar K, Cipher DJ, Souza RF, Spechler SJ, Konda VJA. Aberrant p53 immunostaining in Barrett’s esophagus predicts neoplastic progression: systematic review and meta-analyses. Dig. Dis. Sci. 2019;64(5):1089–1097. doi: 10.1007/s10620-019-05586-7. [DOI] [PubMed] [Google Scholar]
- 42.Prichard JW, Davison JM, Campbell BB, Repa KA, Reese LM, Nguyen XM, et al. TissueCypher(™): a systems biology approach to anatomic pathology. J. Pathol. Inform. 2015;6:48. doi: 10.4103/2153-3539.163987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Critchley-Thorne RJ, Duits LC, Prichard JW, Davison JM, Jobe BA, Campbell BB, et al. A tissue systems pathology assay for high-risk Barrett’s esophagus. Cancer. Epidemiol. Biomarkers. Prev. 2016;25(6):958–968. doi: 10.1158/1055-9965.EPI-15-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frei NF, Khoshiwal AM, Konte K, Bossart EA, Stebbins K, Zhang Y, et al. Tissue systems pathology test objectively risk stratifies Barrett’s esophagus patients with low-grade dysplasia. Am. J. Gastroenterol. 2021;116(4):675–682. doi: 10.14309/ajg.0000000000001037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this paper are publicly available and can be provided by the corresponding author upon request.