Abstract
Adrenal hemorrhage is an uncommon, underrecognized condition that can be encountered in several clinical contexts. Diagnosing adrenal hemorrhage is challenging due to its nonspecific clinical features. Therefore, it remains a diagnosis that is made serendipitously on imaging of acutely unwell patients rather than with prospective clinical suspicion. Adrenal hemorrhage can follow abdominal trauma or appear on a background of predisposing conditions such as adrenal tumors, sepsis, or coagulopathy. Adrenal hemorrhage is also increasingly reported in patients with COVID-19 infection and in the context of vaccine-induced immune thrombocytopenia and thrombosis. Unexplained abdominal pain with hemodynamic instability in a patient with a predisposing condition should alert the physician to the possibility of adrenal hemorrhage. Bilateral adrenal hemorrhage can lead to adrenal insufficiency and potentially fatal adrenal crisis without timely recognition and treatment. In this article, we highlight the clinical circumstances that are associated with higher risk of adrenal hemorrhage, encouraging clinicians to prospectively consider the diagnosis, and we share a diagnostic and management strategy.
Keywords: adrenal apoplexy, adrenal crisis, adrenal incidentaloma, adrenal insufficiency, antiphospholipid syndrome, COVID-19
Adrenal hemorrhage is bleeding within the capsule of the adrenal gland. It is challenging to diagnose because it is considered rare and therefore overlooked in the differential diagnosis, while no clinical features or routine laboratory tests can immediately alert to the diagnosis. Therefore, it may be an unidentified cause of acute clinical deterioration and mostly remains an incidental finding on imaging of acutely unwell patients. Adrenal hemorrhage can follow significant abdominal trauma or be atraumatic on a background of predisposing conditions. While unilateral adrenal hemorrhage is usually clinically silent, bilateral adrenal hemorrhage can cause primary adrenal insufficiency, which if unrecognized can lead to fatal adrenal crisis. High quality data on adrenal hemorrhage are lacking and management standards have not been precisely established.
Illustrative Cases
Case 1: Adrenal Hemorrhage
Initial findings and investigations
A 44-year-old woman presented with deep vein thrombosis which was treated with rivaroxaban. Two months earlier she underwent abdominal computed tomography (CT) scan for suspected renal colic (Fig. 1A), but she was otherwise well. A few days after staring anticoagulants, she presented with bilateral pulmonary emboli and incidental bilateral adrenal masses, 3.5 cm on the left and 2.5 cm on the right, with surrounding soft tissue stranding suggestive of bilateral hemorrhage (Fig. 1B). Thrombocytopenia and raised D-Dimer were noted, with evidence of lupus anticoagulant raising the possibility of catastrophic antiphospholipid syndrome. Random cortisol was undetectable with adrenocorticotropic hormone (ACTH) level of 135 ng/L (Table 1). Therefore, hydrocortisone was commenced at a high dose and fludrocortisone was later added in as renin was 422 µIU/mL with undetectable aldosterone.
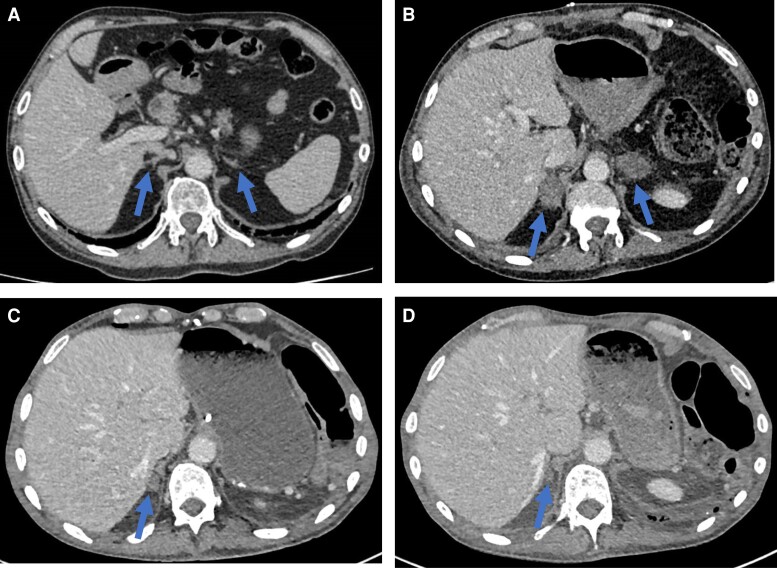
Figure 1.
Representative scans for Case 1. A, CT scan undertaken 2 months prior to the detection of adrenal hemorrhage. The arrows point to normal appearing adrenal glands. B, CT scan showing the acute development of bilateral adrenal masses with surrounding soft tissue stranding consistent with bilateral adrenal hemorrhage. C, 3-month interval CT scan showing marked reduction in the size of the adrenal hematomas. D, 9-month interval CT scan showing resolution of hematomas and atrophied adrenal glands.
Table 1.
Case 1 laboratory tests at time of presentation
| Test | Value | Reference range |
|---|---|---|
| Random cortisol | <28 nmol/L (1.01 µg/dL) | — |
| ACTH | 135 ng/L | 7.1-56.3 ng/L |
| Renin | 422 µIU/mL (252.6 pg/mL) | 6.1-62.7 µIU/mL (3.6-37.5 pg/mL) |
| Aldosterone | <30 pmol/L (1.08 ng/dL) | <750 pmol/L (27 ng/dL) |
Abbreviation: ACTH, adrenocorticotropin hormone.
Follow-up findings
A repeat CT scan 3 months later showed marked reduction of the bilateral hematomas (Fig. 1C), and further CT 6 months later demonstrated resolution of hematomas with the appearance of atrophied adrenal glands (Fig. 1D). As follow-up endocrine testing for 2 years continued to show glucocorticoid and mineralocorticoid deficiency, with early morning cortisol 61 nmol/L (2.21 µg/dL), ACTH 611 ng/L, and renin 235 µIU/mL (140.7 pg/mL), she remains on replacement doses of hydrocortisone and fludrocortisone.
Case 2: COVID-19-Related Adrenal Hemorrhage
Initial findings and investigations
During hospitalization for severe COVID-19 pneumonia, a 26-year-old woman was incidentally found to have a large 11.5 cm heterogeneous left adrenal mass that remained indeterminate at contrast-enhanced CT scan (Fig. 2A). The patient had no signs or symptoms of Cushing syndrome or hyperandrogenism. A full endocrine workup showed no biochemical features of adrenal hormonal excess or deficiency (Table 2).
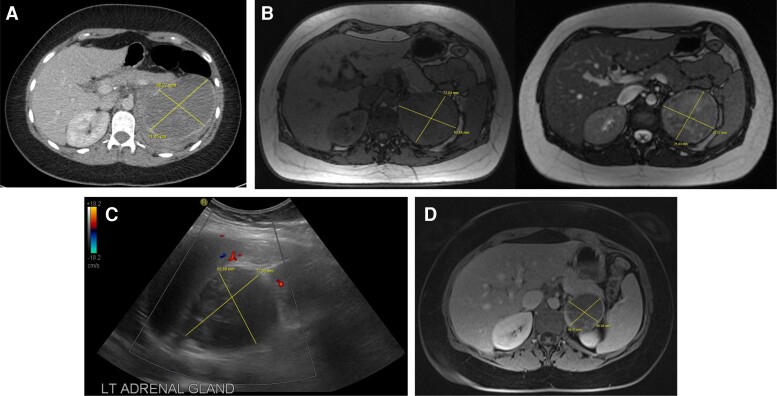
Figure 2.
Representative scans for Case 2. A, CT scan at time of diagnosis showing a large, indeterminate left adrenal mass. B, 3-month interval MRI scan showing a reduction in size of the adrenal mass suggestive of hemorrhage. Adrenal mass is hypointense on T1 (left) with mixed intensity on T2 (right) weighted images. C, Color flow Doppler ultrasound, undertaken due to the MRI T2 mixed intensity, showing a nonvascular well-defined round lesion, consistent with adrenal hematoma. D, 6-month interval MRI showing a further reduction in size of the T1 hypointense adrenal mass with peripheral contrast enhancement suggestive of an underlying adrenal tumor with resolution of the hemorrhagic component.
Table 2.
Case 2 laboratory tests at the time of presentation
| Test | Value | Reference range |
|---|---|---|
| Early morning cortisol | 517 nmol/L (18.73 µg/dL) | — |
| Cortisol on overnight dexamethasone suppression test | <50 nmol/L (<1.5 µg/dL) | <50 nmol/L (<1.5 µg/dL) |
| DHEAS | 3.75 µmol/L (138 µg/dL) | 2.6-13.9 µmol/L (95.8-512 µg/dL) |
| Androstenedione | 5.5 nmol/L (157 ng/dL) | 0.9-7.5 nmol/L (25.7-215 ng/dL) |
| Testosterone | 1.5 nmol/L (43 ng/dL) | <1.9 nmol/L (<55 ng/dL) |
| ACTH | 19 ng/L | 0-50 ng/L |
| Aldosterone | 172 pmol/L (6.2 ng/dL) | <750 pmol/L (27 ng/dL) |
| Renin | 50.7 µIU/mL (30.35 pg/mL) | 6.1-62.7 µIU/mL (3.6-37.5 pg/mL) |
| Plasma metanephrines and normetanephrines | Normal | — |
Abbreviations: ACTH, adrenocorticotropin hormone; DHEAS, dehydroepiandrosterone sulfate.
Follow-up findings
Magnetic resonance imaging (MRI) performed 3 months later showed a size reduction of the adrenal lesion to 7.9 cm (Fig. 2B), which argued against malignancy and favored adrenal hemorrhage; however, the mass showed mixed intensity on T2-weighted images which was atypical of chronic hematoma and suspicious for underlting tumor (Fig. 2B, right). Therefore, a color flow Doppler ultrasound was performed, which was consistent with a nonvascular, well-defined round lesion typical of an adrenal hematoma (Fig. 2C), likely a complication of her recent COVID-19 infection. Follow-up imaging was planned.
Epidemiology
Prior to the development of CT, adrenal hemorrhage was almost exclusively an autopsy finding. Large consecutive unselected hospital postmortem series reported an incidence of unilateral or bilateral adrenal hemorrhage in 0.14% to 1.8% of autopsies (1, 2). In most autopsies, there was a history of sepsis, recent abdominal surgery, anticoagulants therapy, burns, or pregnancy. Suggesting that unselected autopsy studies underestimate its true prevalence, adrenal hemorrhage was reported in as high as 15% of autopsies of patients who died in shock (3). With the advent of CT scanning, adrenal hemorrhage is reported in ill patients undergoing abdominal imaging and therefore remains an unanticipated diagnosis rather than with prospective suspicion (4).
Pathophysiology
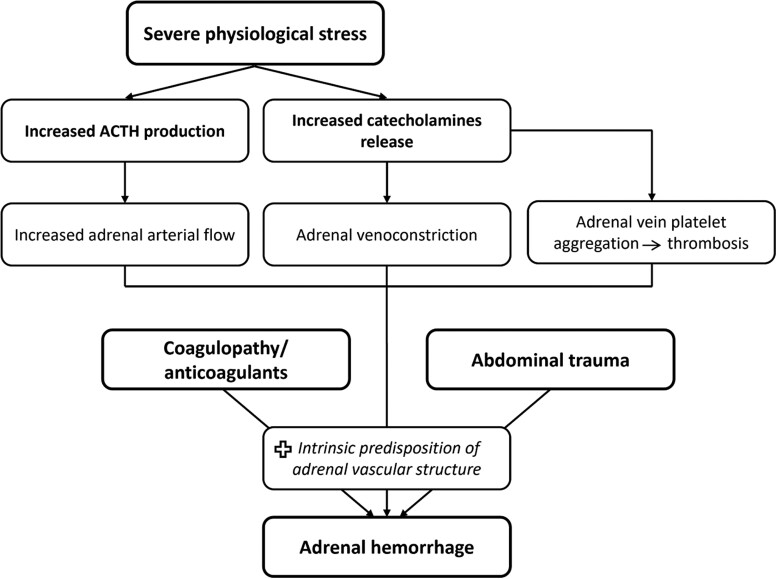
The adrenal glands have a unique vascular structure, with abundant blood supply via 50 to 60 small arterial branches derived from 3 main arteries, but venous drainage is via relatively few venous channels to a single vein, resulting in an arrangement described as a “vascular dam” with an increased propensity to bleeding (5). In severe physiological stress, the dramatic ACTH production increases the adrenal vascularity and blood flow while the catecholamine surge results in vasoconstriction of the draining venules. The excessive catecholamines in the venules stimulate platelet aggregation and predispose to adrenal vein thrombosis (6). Eventually, the venous stasis and the increased adrenal venous pressure lead to rupture of the thin-walled adrenal venules (Fig. 3).
Figure 3.
Depiction of the pathophysiological mechanism that precipitate adrenal hemorrhage. Abbreviation: ACTH, adrenocorticotropic hormone.
Several factors have been associated with an increased risk of atraumatic adrenal hemorrhage (Table 3), all of which share the same pathophysiological mechanism depicted in Fig. 3.
Table 3.
Predisposing factors for adrenal hemorrhage
|
| ȃȃPheochromocytoma |
| ȃȃAdrenocortical carcinoma |
| ȃȃMetastasis |
| ȃȃMyelolipoma |
| ȃȃAdrenocortical adenoma |
|
|
|
| ȃȃAntiphospholipid syndrome |
| ȃȃHeparin-induced thrombocytopenia |
| ȃȃAnticoagulants |
| ȃȃVaccine-induced immune thrombocytopenia and thrombosis |
|
|
|
|
Underlying Adrenal Tumor
In all patients with adrenal hemorrhage, an underlying tumor must be considered. In a systematic review of hemorrhagic adrenal masses, about 50% were pheochromocytomas, 20% were malignant (primary adrenocortical carcinoma or adrenal metastasis, mostly from lung cancer), and hemorrhage also occurred in adenomas (7). Pheochromocytomas are highly vascular tumors (8) that produce excess catecholamines and this combination increases the propensity to hemorrhage. Rapid adrenal tumor growth increases the intracapsular pressure and predisposes to capsular tear and hemorrhage, which explains why hemorrhage is less likely in benign adrenal tumors compared with malignant tumors.
Sepsis
Adrenal hemorrhage was typically described in fulminant septicemia (Waterhouse-Friedrichsen syndrome) (9). While Neisseria meningitides is the most frequent infection associated with adrenal hemorrhage, other organisms have also been implicated (10, 11). In one study, sepsis was associated with a 6-fold increased risk of bilateral adrenal hemorrhage (12). Endotoxin-mediated coagulopathy and septic shock predispose to hemorrhage which is therefore simply a marker of severe, physiologic stress rather than a disease entity per se and has been linked to poor clinical outcomes (4, 6).
Coagulopathy/Anticoagulants
The presence of antiphospholipid antibodies is a major identifiable risk factor for adrenal hemorrhage, most commonly bilateral (13). Adrenal vein thrombosis is the likely primary event but hemorrhage without thrombosis may also occur in the context of surgery or anticoagulant use (14). Bilateral adrenal hemorrhage is a rare manifestation of antiphospholipid syndrome (APS), reported in less than 1% of patients in large series (15), however, the frequency of adrenal gland involvement in catastrophic APS ranges between 10% and 16% (16).
Adrenal hemorrhage as a complication of anticoagulant therapy is rare. Heparin can predispose to adrenal hemorrhage in 2 ways. As an anticoagulant, heparin potentiates the bleeding risk, particularly in the setting of acute illness. Adrenal hemorrhage may also occur as part of heparin-induced thrombocytopenia (HIT). In HIT, antibodies against platelet factor 4/heparin complex are stimulated, which aggregates and activates platelets leading to thrombocytopenia and a paradoxical high risk of thromboembolism, which may involve the adrenal veins and precipitate hemorrhage (17).
Pregnancy
Pregnancy-related adrenal hemorrhage is rare, reported in 0.14% to 1.1% of pregnancies, and mostly unilateral (18). The increased vascular blood flow to the adrenal gland and the hypercoagulability state during pregnancy are the likely underlying predisposing factors for spontaneous hemorrhage.
Endovascular Procedures
Adrenal hemorrhage may also occur as a complication of endovascular interventions such as adrenal vein sampling due to adrenal vein rupture or, less frequently, dissection, infarction, or thrombosis (19). The rate of adrenal vein rupture following adrenal vein sampling was reported as 0.6% (20). Adrenal hemorrhage was usually, but not always, unilateral, and it was more commonly in the right adrenal gland, reflecting the less favorable anatomy for cannulation (19).
Trauma
Traumatic adrenal hemorrhage usually arises in the context of multi-organ injuries (21). Adrenal hematomas were detected in 51 of 2692 (1.9%) abdominal trauma CT scans (22). Traumatic adrenal hemorrhage most commonly affects the right adrenal gland and can be bilateral in 5% of cases (21). The right adrenal predilection to hemorrhage may be due to the risk of the gland being compressed between the liver and vertebral column leading to deceleration forces that can tear the adrenal arterioles, and to the inferior vena cava compression during trauma that raises intra-adrenal venous pressure acutely in the right adrenal gland (23).
It is essential to consider that more than one predisposing factor may coexist in the same patient (24). Commonly, however, an underlying predisposing risk factor may not be identified (25).
COVID-19 and Adrenal Hemorrhage
COVID-19 and the Adrenal Glands
The coronovirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, was declared as pandemic in March 2020. The pathophysiology of SARS-CoV-2 infection involves predominance of severe inflammation and cytokine release, predisposing patients to thrombotic complications due to endothelial cell activation and injury, platelet activation, and hypercoagulability (26). COVID-19 infection primarily involves the respiratory system but also has extrapulmonary multi-system manifestations, including endocrine sequalae. These widespread manifestations are attributed to the tissue expression of the angiotensin converting enzyme 2 (ACE2) receptors which bind the SARS-CoV-2 coronavirus spike protein and the S protein priming by the cellular protease transmembrane serine protease 2 (TMPRSS2) (27). Immunohistochemical studies demonstrated the co-localization of ACE2 and TMPRSS2 in human adrenocortical cells (28), and other endocrine tissues (29). We take this opportunity to highlight adrenal hemorrhage as a COVID-19-related endocrine complication, which may be an unrecognized cause of clinical deterioration during the COVID-19 pandemic.
COVID-19 Infection-Related Adrenal Hemorrhage
Several cases of unilateral or bilateral adrenal hemorrhage and/or infarction in hospitalized patients with COVID-19 infection were reported (30–35). In all cases, imaging was undertaken to evaluate clinical deterioration or to exclude thromboembolic disease. COVID-19 infection is associated with an overall thrombotic events rate of 10% (36) but as high as 30% in critically ill patients (37) and therefore adrenal vein thrombosis is likely to be central to the pathogenesis of hemorrhage. SARS-CoV-2-mediated direct endothelial damage may also be implicated (26). Underlying comorbidities such as APS may have been contributory in some cases (31). Zinserling et al examined the adrenal morphology in autopsy of patients deceased from severe COVID-19 infection (38). While the adrenal glands were macroscopically unremarkable, immunohistochemistry showed a remarkable CD3 and CD8 mononuclear cell infiltration and other changes akin to those observed in the lungs that are considered to result from direct action of SARS-CoV-2 (38). A recent study also detected SARS-CoV-2 spike protein in human adrenocortical cells (39). Therefore, virus entry into adrenocortical cells may have a direct cytopathic effect.
COVID-19 Vaccine-Related Adrenal Hemorrhage
The first COVID-19 vaccine was approved in December 2020, and several are now available worldwide. Increasing incidences of vaccine-induced immune thrombocytopenia and thrombosis (VITT) (40) have been encountered with the adenoviral vector-based COVID-19 vaccines, namely ChAdOx1 (AstraZeneca, University of Oxford) and Ad26.COV2.S (Janssen; Johnson & Johnson) (41). A rising number of case reports documented the occurrence of usually bilateral adrenal hemorrhage in the context of VITT after receiving the Oxford-AstraZeneca vaccine (42–50). In all cases, more than one organ was affected by thrombosis. Adrenal vein thrombosis was detected in 3% of VITT cases (40). The pathophysiology of VITT involves platelet-activating antibodies against platelet factor 4, therefore closely resembling HIT (51). Guidance from The Expert Haematology Panel on COVID-19 VITT set case definition criteria including onset of symptoms 5 to 30 days post-COVID-19 vaccination, presence of thrombosis, thrombocytopenia (platelet count < 150 × 109/L), D-Dimer > 4000 mcg/mL, and positive anti-platelet factor 4 antibodies (40). The presence of all 5 criteria makes VITT “definite”, with other set definitions for VITT “probable”, “possible”, and “unlikely” (40). As the COVID-19 vaccines roll out and booster dosing continues, clinicians should have a high index of suspicion for adrenal hemorrhage and the risk of adrenal crisis in patients with VITT.
Clinical Relevance
Adrenal insufficiency will develop in most cases of bilateral adrenal hemorrhage. The frequency of adrenal insufficiency in 16 patients with APS-related bilateral adrenal hemorrhage was 100% (15). Primary adrenal insufficiency usually occurs once 90% of the adrenal cortex is affected and therefore, there may be a latent period between the acute hemorrhagic event and the onset of acute adrenal insufficiency or adrenal crisis, indicating that the early identification and management can significantly improve the outcome of these patients (12). Adrenal hemorrhage is associated with significant morbidity and mortality due to adrenal crisis or secondary to the underlying predisposing condition. Mortality can even increase up to 55% to 60% in patients with Waterhouse-Friedrichsen syndrome (52).
Adrenal hemorrhage can remain undiagnosed for a prolonged period, resulting in high mortality; this highlights the importance of a high index of clinical suspicion to identify subtle features supportive of acute adrenal insufficiency or adrenal crisis. Prompt clinical diagnosis, supported by biochemical parameters and imaging findings, evaluation with serum cortisol and pre-emptive initiation of corticosteroid replacement without delay is the key to successful management which can be lifesaving. Endocrine advice should be sought urgently.
Clinical Presentation
No signs or symptoms are specific to adrenal hemorrhage and most clinical features present at the time of diagnosis can be ascribed to other ongoing clinical problems. Therefore, currently with the advent of modern imaging techniques, adrenal hemorrhage is increasingly becoming an incidental finding on scanning for other purposes such as acute abdominal pain and trauma (4).
Although adrenal insufficiency more typically occurs in bilateral adrenal hemorrhage. Rare cases were reported with unilateral involvement (4, 53), presumably due to microinfarction or microhemorrhage in the contralateral gland, and thus adrenal hemorrhage should be strongly considered if the contralateral gland appears infarcted/atrophic on imaging. When symptomatic, patients can present with features of acute adrenal insufficiency or adrenal crisis. The most commonly documented symptom is hypotension or shock, in 83%, followed by confusion (79%), and then anorexia, nausea, and vomiting (55%), all attributed to adrenal crisis (5, 54). In patients who present with acute adrenal insufficiency, the diagnosis of adrenal hemorrhage is usually made through imaging carried out as a part of evaluation for an underlying etiology.
The hemorrhage itself can cause abdominal pain, often central and radiating to the flank, which was reported in half to two-thirds of patients with bilateral adrenal hemorrhage (6, 14). Patients with acute abdominal pain can present with guarding, rigidity, and rebound tenderness (5, 6). The presence of unexplained abdominal pain in an acutely unwell patient should raise the suspicion for underlying adrenal hemorrhage, particularly when an underlying predisposing factor is identified. Low-grade fever is common and was detected in around of 50% of patients (4, 6), more commonly seen with bilateral than unilateral adrenal hemorrhage. Despite the elevated ACTH, the acute onset may not allow enough time for the hyperpigmentation of primary adrenal insufficiency to occur.
Although typically seen in the pediatric population, adrenal hemorrhage associated with infections such as meningococcal septicemia (Waterhouse-Friedrichsen syndrome or purpura fulminans) can present with abrupt onset petechial rash in 50% to 60% of patients in addition to the above-described clinical features (55).
Radiological Characterization
Computed Tomography
CT usually represents the first clue to the diagnosis of adrenal hemorrhage, as it is the most widely used imaging modality in trauma and acutely unwell patients. Acute adrenal hemorrhage is characterized by the development of a non-contrast-enhancing high-or mixed-attenuation adrenal lesion within hours to few days. In early hemorrhage, the adrenal gland maintains its configuration and preserves its contrast enhancement peripherally but with hemorrhagic central hypodensity (tram-track appearance). Surrounding ill-defined soft tissue stranding and retroperitoneal hematoma can be an important clue to the diagnosis (21, 56). With the progression of acute bleeding, the adrenal gland swells and CT scanning shows an oval or round adrenal with high attenuation values (50-90 Hounsfield units) (57) and can vary in size from few millimeters within otherwise normal-looking adrenal gland to well over 10 cm. A characteristic feature of adrenal hemorrhage is gradual reduction in size and CT attenuation over time and usually resolve completely or persist as a chronic organized hematoma lesion with hypoattenuating center (pseudocyst) which may show calcification at 1 year (58). At a mean interval of 19 days (range, < 24 hours to 70 days) after injury, most of 35 traumatic adrenal hematomas in a single study decreased in size and attenuation (22).
Magnetic Resonance Imaging
MRI is the most sensitive and specific modality for diagnosing adrenal hemorrhage and therefore plays a key role if hemorrhage is suspected and further characterization of the adrenal mass is required (59). If CT scanning shows acute development of adrenal mass (over hours to few days) and/or non-contrast-enhancing hyperattenuating adrenal lesion with surrounding soft tissue stranding, then MRI is not necessary as these are features typical for hemorrhage. MRI has the advantage of avoiding ionizing radiation and is therefore preferred for radiological surveillance of hematoma in young patients and during pregnancy. It may even determine the age of the hematoma with good precision (60). In the acute phase (less than 7 days; blood is in the form of deoxyhemoglobin), MRI shows an adrenal lesion with high T1 signal intensity with hypointensity in T2-weighted images. From approximately 1 week to 2 months (subacute phase; blood is in the form of methemoglobin) after the onset of hemorrhage, a hematoma appears hyperintense on both T1- and T2-weighted images. In a chronic hematoma, the hemorrhage becomes hypointense on both T1- and T2-weighted images because of the presence of hemosiderin. Graded echo sequences can magnify the susceptibility effects of decreased signal intensity seen with hemosiderin and deoxyhemoglobin, thereby increasing their conspicuity (blooming artifact), a feature that is helpful in the characterization of the adrenal lesion (61). Contrast-enhanced MRI with subtraction imaging may identify an adrenal tumor underlying the hemorrhage (60). As adrenal hemorrhage shows a bright signal on unenhanced T1-weighted MRI, subtraction imaging will digitally subtract the unenhanced T1-weighted sequence and remove any signal from blood products. Therefore, a nontumorous hematoma will appear as a signal void as it does not contain enhancing elements, while a tumor underlying hemorrhage will contrast enhance (62).
Ultrasound
Ultrasound is the modality of choice for initial evaluation and follow-up of adrenal hemorrhage in pediatric patients due to their relatively large adrenal glands and as it avoids ionizing radiation exposure. In adults, it is challenging to evaluate a smaller adrenal mass with ultrasound, particularly in the left adrenal gland. Acute hemorrhage appears as an adrenal lesion that is heterogeneously hyperechoic due to the presence of blood clots. As the hematoma matures, lysis of the blood clots liquefies the contents, which appear hypoechoic and may assume a cystic appearance with or without peripheral calcification (60, 63, 64). Color flow Doppler can confirm the avascular nature of the adrenal mass (65), which is useful when more certainty is needed, such as in Case 2 when MRI was also inconclusive.
A summary of the radiological characteristics of adrenal hemorrhage is presented in Table 4.
Table 4.
Radiological features suggestive of adrenal hemorrhage
| Computed tomography |
|
| |
| |
| |
| Magnetic resonance imaging |
|
| |
| |
| |
| |
| Ultrasound |
|
| |
|
Importantly, in patients with no predisposing factors for hemorrhage, the presence of underlying adrenal tumor must be explored. The distinction between tumoral and nontumoral adrenal hemorrhage may be straightforward if very recent imaging prior to the development of hematoma is available (such as in Case 1), or if there is a clear adrenal tumor adjacent to the hematoma. However, determining an underlying tumor is usually difficult in the acute phase and therefore, in the absence of high suspicion of adrenal malignancy (Table 5) that necessitate urgent intervention, follow-up imaging must be pursued (63, 66). Radiological features that argue for the presence of an underlying adrenal tumor are presence of calcification (but note that a chronic hematoma can contain calcification), contrast enhancement on CT or subtraction MRI, and hypermetabolic avidity on positron emission tomography (PET) (67). Although nontumorous adrenal hemorrhage may show fludeoxyglucose (FDG) PET avidity resulting from an inflammatory reaction due to fat necrosis, there is no contrast enhancement (68).
Table 5.
Comparison of the clinical, biochemical, and radiological characteristics of adrenal hemorrhage and adrenal malignancy
| Adrenal hemorrhage | Adrenal malignancy | |
|---|---|---|
| Clinical presentation | ||
| ȃAbdominal pain | Acute abdominal pain is common | Chronic abdominal pain may be present with large masses |
| ȃFever | Frequently observed | Unusual |
| ȃWeight loss | Unusual | Common |
| ȃHypotension | Common in bilateral hemorrhage | Unusual |
| Adrenocortical hormone testing | Adrenal insufficiency is common | Glucocorticoid and/or androgen excess are common. Adrenal insufficiency is unusual but possible in bilateral adrenal metastases |
| Radiology | ||
| ȃAdrenal mass growth | Rapid development of adrenal mass within hours to few days | Adrenal mass growth over months to few years |
| ȃCalcification | Unusual unless hematoma is chronic | Common |
| ȃContrast enhancement | Non-contrast-enhancing mass | Enhancing mass |
| ȃSubtraction MRI images | Signal void | Contrast enhancement |
| ȃFollow-up imaging | Reduce in size | Increase in size |
| ȃFDG PET | Usually non-FDG-avid but can occur from an inflammatory reaction due to fat necrosis | Usually FDG-avid |
Abbreviations: FDG, fludeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography.
A comparison of the clinical and radiological characteristics of adrenal hemorrhage and adrenal malignancy is shown in Table 5.
Laboratory Investigations
When adrenal crisis is suspected, treatment should be commenced without delay and investigations can be deferred until the patient is stabilized. If possible, blood samples for cortisol and ACTH can be drawn before commencement of glucocorticoids. Conditions that affect the cortisol binding globulin must be considered. The diagnosis of adrenal insufficiency is based on low early morning serum cortisol and confirmed by low stimulated cortisol (69). Primary adrenal insufficiency should be suspected if the early morning serum cortisol is <140 nmol/L (5 µg/dL) with a plasma ACTH over two-fold above the upper reference range. Basal cortisol level >500 nmol/L (18 µg/dL) in an unwell patient makes adrenal insufficiency unlikely. Synthetic ACTH-stimulation test when the patient is clinically stable is the standard confirmatory test for nonconclusive basal cortisol levels, and the peak cortisol should be interpreted with the assay-specific cutoffs (usually set at 420-550 nmol/L; 15-20 µg/dL). Plasma renin and serum aldosterone should also be measured. High renin with concomitantly low aldosterone confirms mineralocorticoid deficiency.
Electrolyte imbalances were present in less than 20% of patients with adrenal hemorrhage and may include hyponatremia, hyperkalemia, hypoglycemia, and hypercalcemia (4).
Low platelet count and anemia were reported in 62% and 48%, respectively, of patients with APS-related adrenal hemorrhage/infarction (14). Prolonged international normalized ratio and activated partial thromboplastin time are also common in patients with adrenal hemorrhage associated with anticoagulants, sepsis, APS, and heparin-induced thrombocytopenia (4).
Discovery of unexplained adrenal hemorrhage should lead to a search for predisposing conditions. Indeed, adrenal insufficiency can be the first clinical manifestation of APS in 35% of patients (14, 15). If no predisposing factor for adrenal hemorrhage can be identified, an APS screen should be undertaken, including lupus anticoagulant (functional coagulation assay) and anticardiolipin and β2-glycoprotein I (β2GPI) antibodies (ELISA) (70).
In patients with APS, adrenal insufficiency should be ruled out in the presence of abdominal pain, excessive fatigue, weight loss, or electrolyte disturbance (hyponatremia and/or hyperkalemia).
If an underlying adrenal tumor is suspected, hormonal assessment for a functional tumor can be challenging in hospitalized patients as the overnight dexamethasone suppression test can be falsely positive and the androgens may be low. Importantly, the levels of metanephrines and normetanephrines in hospitalized patients without pheochromocytoma may be indistinguishable from those seen in pheochromocytoma (71). Therefore, more emphasis should be placed on the clinical picture, such as presence of Cushing syndrome, hirsutism, or features of pheochromocytoma. Unless the clinical course indicates urgent adrenalectomy, the hormonal workup is best undertaken in an outpatient setting (72).
Differential Diagnosis
The differentiation between adrenal hemorrhage and heterogeneous adrenal tumors can represent a diagnostic dilemma. Large unilateral adrenal hemorrhages can be confused with lesions suspicious for malignancy. The absence of clinical and biochemical evidence of adrenal hormone excess together with the presence of predisposing risk factors for adrenal hemorrhage may guide the clinician to adopt a conservative approach for short-term radiological surveillance and therefore avoid unnecessary surgery.
In one case series, 30% of patients with adrenal hemorrhage had raised metanephrines and/or normetanephrines, up to twice the upper limit of normal (25), which had previously been confused with pheochromocytoma (73). High levels of metanephrines and normetanephrines in the presence of preexisting adrenergic symptoms and/or resistant hypertension should alert to the possibility of underlying pheochromocytoma.
Management
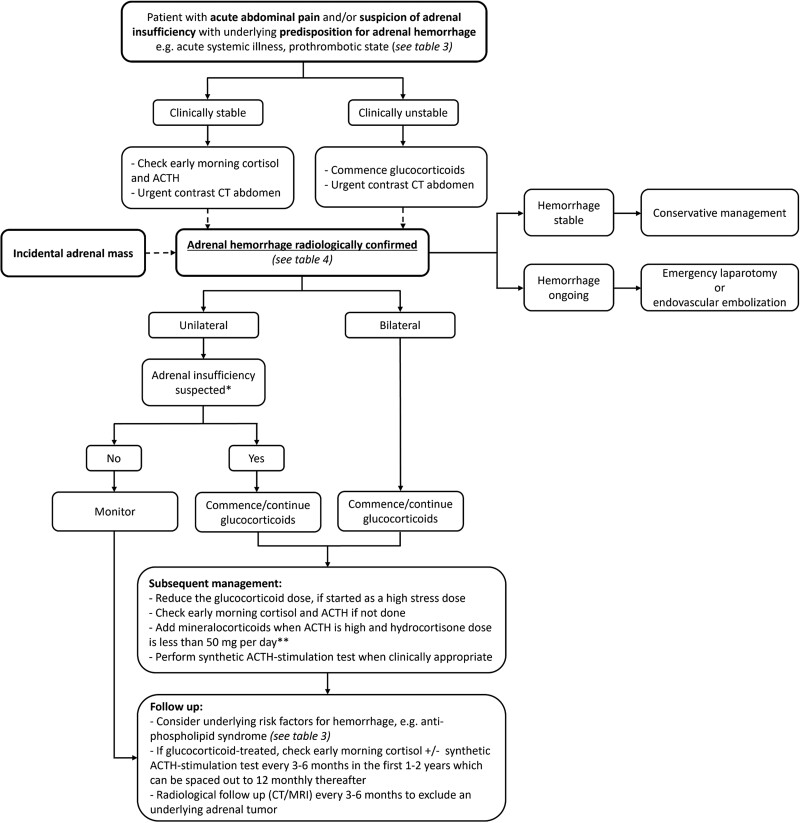
Appropriate and timely intervention can avoid adrenal crisis-related mortality. High level of clinical suspicion for adrenal hemorrhage is needed in patients with nonspecific symptoms such as abdominal pain, asthenia, hypotension, and low-grade fever, particularly in the presence of predisposing risk factor for hemorrhage, and imaging of the adrenal glands should be pursued. If bilateral adrenal hemorrhage is demonstrated on imaging, glucocorticoid replacement should be commenced without delay. Figure 4 shows a flow diagram for the management of adrenal hemorrhage, whether diagnosed incidentally or with prospective suspicion.
Figure 4.
A flow diagram outlining a recommended approach to the patient with adrenal hemorrhage. *Adrenal insufficiency is unusual in unilateral adrenal hemorrhage but has very rarely been reported and it should be strongly considered if the contralateral adrenal gland appears infarcted/atrophic on imaging. **Hydrocortisone also provides mineralocorticoid activity, with 40 mg of hydrocortisone equivalent to 100 μg of fludrocortisone. Prednisolone has some mineralocorticoid activity, while dexamethasone has none (79).
Acute Management
If adrenal crisis is suspected, hydrocortisone 100 to 200 mg intravenously should immediately be administered, followed by 200 mg per 24 hours, either as a continuous infusion or 50 mg every 6 hours. Intravenous fluid resuscitation must be simultaneously commenced. At high doses, hydrocortisone treatment also provides adequate mineralocorticoid replacement. Dosing can subsequently be gradually tapered as the severity of the stress diminishes and hydrocortisone dose of 15 to 25 mg per day given in 2 to 3 divided doses (two-thirds of the dose to be taken in the morning) is recommended. Mineralocorticoid replacement can be added once the dose of hydrocortisone is less than 50 mg daily. Fludrocortisone 100 to 150 µg daily is a recommended starting dose. Other supportive therapy, such as blood transfusion, may be needed.
Adrenal hemorrhage is usually managed conservatively. Endovascular embolization of the adrenal arteries is a minimally invasive procedure and may be lifesaving in cases of ongoing adrenal hemorrhage and hemodynamic instability (74–76). It is the preferred approach to control a ruptured pheochromocytoma, as emergency surgery carries a mortality risk close to 50% (7). Due to the rich blood supply of the adrenal glands, it is crucial to identify the source of bleeding beforehand, which can be challenging. If interventional radiology capabilities are lacking or the bleeding vessel is difficult to delineate, exploratory laparotomy should be considered.
Subsequent Management
The dosing of glucocorticoid and mineralocorticoid replacement should be guided by clinical assessment of symptoms and signs of over- and underreplacement.
In most cases, the adrenal failure is irreversible, as most patients with bilateral hemorrhage resolve to severely atrophic adrenal glands and therefore remain dependent on lifelong glucocorticoid replacement (4, 15, 77). Recovery of glucocorticoid production is infrequent and may occur when areas of normal adrenal morphology can be seen on imaging (4, 15). Ramon et al and Jahangir-Hekmat et al reported recovery of glucocorticoid production in patients with bilateral adrenal hemorrhage at 2 to 2.5 years after the diagnosis of adrenal insufficiency (15, 77). Therefore, 3- to 6-monthly early morning serum cortisol checks are worthwhile at least in the first 1 to 2 years after the diagnosis of adrenal hemorrhage, which can be spaced out to 12-monthly thereafter. At any point, the development of features of glucocorticoid overreplacement while on conventional replacement doses should trigger testing for adrenal function recovery. If the morning cortisol is <140 nmol/L (5 µg/dL) then the glucocorticoids should be continued. If morning cortisol is between 140 nmol/L (5 µg/dL) and 350 nmol/L (12 µg/dL) then a stimulation test is warranted. If the morning cortisol is >350 nmol/L (12 µg/dL), this suggests adrenal function recovery and glucocorticoids can be discontinued.
On the other hand, the need for long-term mineralocorticoid replacement is more variable (77).
Radiological surveillance should continue until the clinician is satisfied that no underlying concerning adrenal tumor is present. Treatment of the underlying predisposing factor should also be pursued eg, surgery for pheochromocytoma or adrenocortical carcinoma.
Unresolved Questions
There is paucity of data detailing the natural history of adrenal insufficiency following bilateral adrenal hemorrhage. The available case series suggest that recovery of adrenal function is infrequent. It is also not possible to describe a time course for adrenal recovery, should it occur.
It is also not clear whether all patients with atraumatic adrenal hemorrhage should be screened for APS. The prevalence of antiphospholipid antibodies in the general population remains unknown due to the lack of population-based studies. Therefore, we suggest screening for APS when there is no alternative predisposing condition for adrenal hemorrhage and whenever there is another reason to clinically suspect APS, such as the presence of multiple thrombotic events.
Several reports and our own clinical experience suggest that the mineralocorticoid activity is not invariably involved in the adrenal insufficiency of bilateral adrenal hemorrhage and may be relatively spared (5, 13, 15, 77, 78). One may hypothesize that zona glomerulosa may, at least in the beginning, be less severely damaged, being the outermost adrenocortical layer while the vascular dam and onset of hemorrhage is at the innermost layer, zona reticularis, responsible for androgen production. Indeed, DHEAS was invariably low in patients with bilateral adrenal hemorrhage associated with APS (15).
Back to the Patients
Case 1: Progress and Outcome
Underlying adrenal tumor has already been excluded. Four years later, early morning serum cortisol was still low at 133 nmol/L (4.8 µg/dL) with renin 254 µIU/mL (152 pg/mL) and the patient remains on replacement doses of hydrocortisone and fludrocortisone. She also takes warfarin for the underlying anti-phospholipid syndrome.
Case 2: Progress and Outcome
Subsequent radiological surveillance with MRI scan performed 6 months after the initial scan showed a further size reduction of the left adrenal lesion to 5.5 cm, with resolution of the hemorrhagic component suggestive of an underlying adrenal tumor, as a second predisposing factor for hemorrhage in this case, beside the COVID-19 infection. Repeated clinical assessment and laboratory testing showed no evidence of adrenal hormone excess. Given the patient's young age and the large size of the underlying indeterminate adrenal mass radiological surveillance will continue.
Conclusion
Adrenal hemorrhage is a heterogenous clinical condition that is underrecognized in acutely unwell patients and has recently been observed as part of VITT following COVID-19 vaccination. A high index of suspicion is required to make a timely diagnosis to circumvent fatal adrenal crisis. Unexplained abdominal pain in an acutely unwell patient with an underlying predisposing factor for hemorrhage should alert to the possibility of the diagnosis. Adrenal hemorrhage can be the first presentation of an underlying hematologic disorder or adrenal tumor and therefore appropriate workup by an experienced adrenal team is recommended.
Abbreviations
- ACE2
angiotensin converting enzyme 2
- ACTH
adrenocorticotropin hormone
- APS
antiphospholipid syndrome
- CT
computed tomography
- FDG
fludeoxyglucose
- HIT
heparin-induced thrombocytopenia
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- TMPRSS2
transmembrane serine protease 2
- VITT
vaccine-induced immune thrombocytopenia and thrombosis
Contributor Information
Yasir S Elhassan, Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham B15 2TT, UK; Department of Endocrinology, Queen Elizabeth Hospital Birmingham, Birmingham B15 2WB, UK.
Cristina L Ronchi, Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham B15 2TT, UK; Department of Endocrinology, Queen Elizabeth Hospital Birmingham, Birmingham B15 2WB, UK; Division of Endocrinology and Diabetes, University Hospital University Würzburg, Würzburg 97080, Germany.
Piyumi Wijewickrama, Department of Diabetes and Endocrinology, University College London Hospital NHS Foundation Trust, London NW1 2BU, UK.
Stephanie E Baldeweg, Department of Diabetes and Endocrinology, University College London Hospital NHS Foundation Trust, London NW1 2BU, UK; Centre for Obesity and Metabolism, Department of Experimental and Translational Medicine, Division of Medicine, University College London, London WC1E 6BT, UK.
Funding
This work received no dedicated funding support.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Botteri A, Orell SR. Adrenal hemorrhage and necrosis in the adult. Acta Med Scand. 1964;175(4):409–419. [DOI] [PubMed] [Google Scholar]
- 2. Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore). 1978;57(3):211–221. [DOI] [PubMed] [Google Scholar]
- 3. Russell P. The adrenal glands in shock. Pathology. 1972;4(1):5–8. [DOI] [PubMed] [Google Scholar]
- 4. Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161–168. [DOI] [PubMed] [Google Scholar]
- 5. Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110(3):227–235. [DOI] [PubMed] [Google Scholar]
- 6. Rao RH. Bilateral massive adrenal hemorrhage. Med Clin North Am. 1995;79(1):107–129. [DOI] [PubMed] [Google Scholar]
- 7. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75–82. [DOI] [PubMed] [Google Scholar]
- 8. Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas : distinctive traits in malignant tumors. Am J Pathol. 2002;161(4):1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waterhouse R. A case of suprarenal apoplexy. Lancet. 1911;177(4566):577–578. [Google Scholar]
- 10. Guarner J, Paddock CD, Bartlett J, Zaki SR. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod Pathol. 2008;21(9):1113–1120. [DOI] [PubMed] [Google Scholar]
- 11. Hamilton D, Harris MD, Foweraker J, Gresham GA. Waterhouse-Friderichsen syndrome as a result of non-meningococcal infection. J Clin Pathol. 2004;57(2):208–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacs KA, Lam YM, Pater JL. Bilateral massive adrenal hemorrhage: assessment of putative risk factors by the case–control method. Medicine (Baltimore). 2001;80(1):45–53. [DOI] [PubMed] [Google Scholar]
- 13. Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol. 2005;153(4):507–514. [DOI] [PubMed] [Google Scholar]
- 14. Espinosa G, Santos E, Cervera R, et al. Adrenal involvement in the antiphospholipid syndrome clinical and immunologic characteristics of 86 patients. Medicine (Baltimore). 2003;82(2):106–118. [DOI] [PubMed] [Google Scholar]
- 15. Ramon I, Mathian A, Bachelot A, et al. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013;98(8):3179–3189. [DOI] [PubMed] [Google Scholar]
- 16. Pazzola G, Zuily S, Erkan D. The challenge of bleeding in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2015;17(2):7. [DOI] [PubMed] [Google Scholar]
- 17. Kurtz LE, Yang S. Bilateral adrenal hemorrhage associated with heparin induced thrombocytopenia. Am J Hematol. 2007;82(6):493–494. [DOI] [PubMed] [Google Scholar]
- 18. Gavrilova-Jordan LP, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: a review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191–195. [DOI] [PubMed] [Google Scholar]
- 19. Monticone S, Satoh F, Dietz AS, et al. Clinical management and outcomes of adrenal hemorrhage following adrenal vein sampling in primary aldosteronism. Hypertension. 2016;67(1):146–152. [DOI] [PubMed] [Google Scholar]
- 20. Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97(5):1606–1614. [DOI] [PubMed] [Google Scholar]
- 21. Mehrazin R, Derweesh IH, Kincade MC, Thomas AC, Gold R, Wake RW. Adrenal trauma: elvis presley memorial trauma center experience. Urology. 2007;70(5):851–855. [DOI] [PubMed] [Google Scholar]
- 22. Rana AI, Kenney PJ, Lockhart ME, et al. Adrenal gland hematomas in trauma patients. Radiology. 2004;230(3):669–675. [DOI] [PubMed] [Google Scholar]
- 23. Lehrberg A, Kharbutli B. Isolated unilateral adrenal gland hemorrhage following motor vehicle collision: a case report and review of the literature. J Med Case Rep. 2017;11(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ochi K, Abe I, Yamazaki Y, et al. Adrenal hemorrhage in a cortisol-secreting adenoma caused by antiphospholipid syndrome revealed by clinical and pathological investigations: a case report. Front Endocrinol (Lausanne). 2022. Feb 3;12:769450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karwacka IM, Obolonczyk L, Sworczak K. Adrenal hemorrhage: a single center experience and literature review. Adv Clin Exp Med. 2018;27(5):681–687. [DOI] [PubMed] [Google Scholar]
- 26. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao Y, Xu B, Guan W, et al. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol (Lausanne). 2021. Jan 8;11:593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's Reliance on ACE2. Endocrinology. 2020;161(9):bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvarez-troncoso J, Larrauri MZ, Vega MDM, et al. Case report: COVID-19 with bilateral adrenal hemorrhage. Am J Trop Med Hyg. 2020;103(3):1156–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frankel M, Feldman I, Levine M, et al. Bilateral adrenal hemorrhage in coronavirus disease 2019 patient: a case report. J Clin Endocrinol Metab. 2020;105(12):3745–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar R, Guruparan T, Siddiqi S, et al. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep. 2020;6(3):20200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharrack N, Baxter CT, Paddock M, Uchegbu E. Adrenal haemorrhage as a complication of COVID-19 infection. BMJ Case Rep. 2020;13(11):e239643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elkhouly MMN, Elazzab AA, Moghul SS. Bilateral adrenal hemorrhage in a man with severe COVID-19 pneumonia. Radiol Case Rep. 2021;16(6):1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Machado IFR, Menezes IQ, Figueiredo SR, et al. Primary adrenal insufficiency due to bilateral adrenal infarction in COVID-19. J Clin Endocrinol Metab. 2022;107(1):e394–e400. [DOI] [PubMed] [Google Scholar]
- 36. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9(1):e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zinserling VA, Semenova NY, Markov AG, et al. Inflammatory cell infiltration of adrenals in COVID-19. Horm Metab Res. 2020;52(9):639–641. [DOI] [PubMed] [Google Scholar]
- 39. Kanczkowski W, Evert K, Stadtmüller M, et al. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2022;10(1):13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abrignani MG, Murrone A, De Luca L, et al. COVID-19, vaccines, and thrombotic events: a narrative review. J Clin Med. 2022;11(4):948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D’agostino V, Caranci F, Negro A, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Douxfils J, Vayne C, Pouplard C, et al. Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic thrombocytopenia syndrome after initial successful therapy with intravenous immunoglobulins—a rational for monitoring immunoglobulin G levels. Haematologica. 2021;106(12):3249–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varona JF, García-Isidro M, Moeinvaziri M, Ramos-López M, Fernández-Domínguez M. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur J Intern Med. 2021 Sep;91:90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Graf A, Armeni E, Dickinson L, et al. Adrenal haemorrhage and infarction in the setting of vaccine-induced immune thrombocytopenia and thrombosis after SARS-CoV-2 (Oxford–AstraZeneca) vaccination. Endocrinol Diabetes Metab Case Reports. 2022;1:21-0144. [Google Scholar]
- 47. Taylor P, Allen L, Shrikrishnapalasuriyar N, Stechman M, Rees A. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage. Clin Endocrinol (Oxf). 2022;97(1):26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tews HC, Driendl SM, Kandulski M, et al. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia with venous thrombosis, pulmonary embolism, and adrenal haemorrhage: a case report with literature review. Vaccines (Basel). 2022;10(4):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tha T, Martini I, Stefan E, Redla S. Bilateral adrenal haemorrhage with renal infarction after ChAdOx1 nCoV-19 AstraZeneca vaccination. BJR Case Rep. 2022;8(2):20210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Efthymiadis A, Khan D, Pavord S, Pal A. A case of ChAdOx1 vaccine-induced thrombocytopenia and thrombosis syndrome leading to bilateral adrenal haemorrhage and adrenal insufficiency. Endocrinol Diabetes Metab Case Rep. 2022;2022(1):22-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tormos LM, Schandl CA. The significance of adrenal hemorrhage: undiagnosed Waterhouse-Friderichsen syndrome, a case series. J Forensic Sci. 2013;58(4):1071–1074. [DOI] [PubMed] [Google Scholar]
- 53. Knight B. Sudden unexpected death from adrenal haemorrhage. Forensic Sci Int. 1980;16(3):227–229. [DOI] [PubMed] [Google Scholar]
- 54. Anderson KC, Kuhajda FP, Bell WR. Diagnosis and treatment of anticoagulant-related adrenal hemorrhage. Am J Hematol. 1981;11(4):379–385. [DOI] [PubMed] [Google Scholar]
- 55. Varon J, Chen K, Sternbach GL. Rupert Waterhouse and Carl Friderichsen: adrenal apoplexy. J Emerg Med. 1998;16(4):643–647. [DOI] [PubMed] [Google Scholar]
- 56. Huelsen-Katz AM, Schouten BJ, Jardine DL, Soule SG, Liu H. Pictorial evolution of bilateral adrenal haemorrhage. Intern Med J. 2010;40(1):87–88. [DOI] [PubMed] [Google Scholar]
- 57. Kawashima A, Sandler CM, Fishman EK, et al. Spectrum of CT findings in nonmalignant disease of the adrenal gland. Radiographics. 1998;18(2):393–412. [DOI] [PubMed] [Google Scholar]
- 58. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949–963. [DOI] [PubMed] [Google Scholar]
- 59. Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics. 2004;24(Suppl_1):S73–S86. [DOI] [PubMed] [Google Scholar]
- 60. Badawy M, Gaballah AH, Ganeshan D, et al. Adrenal hemorrhage and hemorrhagic masses; diagnostic workup and imaging findings. Br J Radiol. 2021;94(1127):20210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elsayes KM, Emad-Eldin S, Morani AC, Jensen CT. Practical approach to adrenal imaging. Urol Clin North Am. 2018;45(3):365–387. [DOI] [PubMed] [Google Scholar]
- 62. Newatia A, Khatri G, Friedman B, Hines J. Subtraction imaging: applications for nonvascular abdominal MRI. Am J Roentgenol. 2007;188(4):1018–1025. [DOI] [PubMed] [Google Scholar]
- 63. Lattin GE, Sturgill ED, Tujo CA, et al. From the radiologic pathology archives: adrenal tumors and tumor-like conditions in the adult: radiologic–pathologic correlation. Radiographics. 2014;34(3):805–829. [DOI] [PubMed] [Google Scholar]
- 64. Albano D, Agnello F, Midiri F, et al. Imaging features of adrenal masses. Insights Imaging. 2019;10(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simon DR, Palese MA. Clinical update on the management of adrenal hemorrhage. Curr Urol Rep. 2009;10(1):78–83. [DOI] [PubMed] [Google Scholar]
- 66. Diolombi ML, Khani F, Epstein JI. Diagnostic dilemmas in enlarged and diffusely hemorrhagic adrenal glands. Hum Pathol. 2016. Jul;53:63–72. [DOI] [PubMed] [Google Scholar]
- 67. Jordan E, Poder L, Courtier J, Sai V, Jung A, Coakley FV. Imaging of nontraumatic adrenal hemorrhage. Am J Roentgenol. 2012;199(1):W91–W98. [DOI] [PubMed] [Google Scholar]
- 68. Dong A, Cui Y, Wang Y, Zuo C, Bai Y. 18F-FDG PET/CT of adrenal lesions. Am J Roentgenol. 2014;203(2):245–252. [DOI] [PubMed] [Google Scholar]
- 69. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010–2021. [DOI] [PubMed] [Google Scholar]
- 71. Kline GA, Boyd J, Sadrzadeh HSM, Leung AA. Inpatient measurements of urine metanephrines are indistinguishable from pheochromocytoma: retrospective cohort study. Am J Med. 2021;134(8):1039–1046.e3. [DOI] [PubMed] [Google Scholar]
- 72. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 73. Rees GJ, Davies JS, Scott-Coombes DM. Pseudopheochromocytoma following adrenal hemorrhage. World J Endocr Surg. 2010;2(2):93–95. [Google Scholar]
- 74. Fowler AM, Burda JF, Kim SK. Adrenal artery embolization: anatomy, indications, and technical considerations. Am J Roentgenol. 2013;201(1):190–201. [DOI] [PubMed] [Google Scholar]
- 75. Velmahos GC, Chahwan S, Falabella A, Hanks SE, Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24(5):539–545. [DOI] [PubMed] [Google Scholar]
- 76. Giurazza F, Corvino F, Silvestre M, et al. Adrenal glands hemorrhages: embolization in acute setting. Gland Surg. 2019;8(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jahangir-Hekmat M, Taylor HC, Levin H, Wilbur M. Adrenal insufficiency attributable to adrenal hemorrhage: long-term follow-up with reference to glucocorticoid and mineralocorticoid function and replacement. Endocr Pract. 2004;10(1):55–61. [DOI] [PubMed] [Google Scholar]
- 78. Rosenberger LH, Smith PW, Sawyer RG, Hanks JB, Adams RB, Hedrick TL. Bilateral adrenal hemorrhage: the unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Crit Care Med. 2011;39(4):833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison's disease: rapid adenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.