Abstract
Background:
Previous studies have demonstrated that aerobic exercise (AE) and the Dietary Approaches to Stop Hypertension (DASH) diet can improve neurocognition. However, the mechanisms by which lifestyle improves neurocognition have not been widely studied. We examined the associations between changes in metabolic, neurotrophic, and inflammatory biomarkers with executive functioning among participants from the Exercise and Nutritional Interventions for Neurocognitive Health Enhancement (ENLIGHTEN) trial.
Objective:
To examine the association between changes in metabolic function and neurocognition among older adults with cognitive impairment, but without dementia (CIND) participating in a comprehensive lifestyle intervention.
Methods:
ENLIGHTEN participants were randomized using a 2 × 2 factorial design to receive AE, DASH, both AE+DASH, or a health education control condition (HE) for six months. Metabolic biomarkers included insulin resistance (homeostatic model assessment [HOMA-IR]), leptin, and insulin-like growth factor (IGF-1); neurotrophic biomarkers included brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF); and inflammatory biomarkers included interleukin-6 (IL-6) and C-Reactive Protein (CRP).
Results:
Participants included 132 sedentary older adults (mean age = 65 [SD = 7]) with CIND. Results demonstrated that both AE (d = 0.48, p = 0.015) and DASH improved metabolic function (d = 0.37, p = 0.039), without comparable improvements in neurotrophic or inflammatory biomarkers. Greater improvements in metabolic function, including reduced HOMA-IR (B = −2.3 [−4.3, −0.2], p = 0.033) and increased IGF-1 (B = 3.4 [1.2, 5.7], p = 0.004), associated with increases in Executive Function.
Conclusion:
Changes in neurocognition after lifestyle modification are associated with improved metabolic function.
Keywords: Aerobic exercise, CIND, DASH diet, executive function, lifestyle modification, metabolic function, vascular risk factors
INTRODUCTION
Emerging evidence suggests that lifestyle modification including aerobic exercise and dietary modification may improve neurocognition among individuals at risk for cognitive decline and Alzheimer’s disease and related dementias (ADRD) [1–8]. Studies examining multicomponent interventions, including the FINGER trial, have demonstrated that interventions combining exercise and dietary modification can improve executive functions among older adults with vascular risk factors [9, 10], independent of age, education, or severity of cardiovascular disease (CVD) risk [11]. CVD risk factors may impair neurocognition through their impact on vascular function and cardiometabolic function, which is worsened by CVD risk factors and may be improved through lifestyle modification. Indeed, evidence increasingly suggests that lifestyle modification may improve neurocognition, both directly through vascular risk reduction [12] and indirectly through overlapping improvements in inflammatory [13], metabolic [14, 15], and neurotrophic pathways [16–20].
In the recently completed ENLIGHTEN (Exercise and NutritionaL Interventions for neurocoGnitive HealTh EnhaNcement) randomized clinical trial, we demonstrated that a 6-month intervention for older adults with CIND and CVD risk factors utilizing aerobic exercise and the Dietary Approaches to Stop Hypertension (DASH) diet showed that executive function was better among exercisers compared to non-exercisers, with improved aerobic fitness, reduced CVD risk, and reduced salt intake associated with changes in neurocognition [21]. Further, results showed that combining exercise with the DASH diet produced better performance compared to education controls [21]. In addition to changes in neurocognitive performance, results also demonstrated that individuals in the aerobic exercise groups demonstrated improvements in aerobic fitness, walk distance, and actigraphy-assessed physical activity. Similarly, individuals in the DASH diet groups demonstrated improvements in overall DASH diet pattern, as well as dietary potassium, magnesium, calcium, and sodium. In addition, both aerobic exercise and the DASH diet intervention groups demonstrated improvements in CVD risk factors [21]. The present secondary analyses studied metabolic, inflammatory, and neurotrophic biomarkers among ENLIGHTEN participants in order to better understand possible mechanistic pathways by which exercise and the DASH diet may affect neurocognition in older sedentary adults with CIND and CVD risk factors. We therefore examined 1) intervention-related changes in metabolic, inflammatory, and neurotrophic biomarkers, and 2) the associations between changes in biomarkers and changes in neurocognition.
METHODS
Trial overview and primary results
The ENLIGHTEN trial was a randomized clinical trial of sedentary older adults at risk for dementia. Specific inclusion criteria included age ≥55 years, subjective cognitive complaints as indicated by a score of ≥0.5 on the Mail-In Cognitive Function Screening Instrument, sedentary lifestyle, at least one CVD risk factor (in addition to being sedentary), and objective evidence of cognitive impairment as indicated by a score of 19–25 on the Montreal Cognitive Assessment (MoCA) or a score of 12 on letter fluency or ≤15 on Animal fluency. Details of the study protocol and principal findings have been previously reported and included [21–23]. Briefly, a 2 × 2 factorial design was employed in which participants were randomized to one of 4 groups: Aerobic Exercise alone (AE), DASH diet alone (DASH), a combination of Aerobic Exercise and the DASH diet (AE+DASH), or Health Education (HE).
As previously reported, 160 participants completed a comprehensive neurocognitive test battery [24] as well as measures of aerobic fitness, dietary intake, and biomarker data at baseline and again following intervention. Participants in both AE conditions demonstrated changes in Executive Function (p = 0.046) with similar, albeit weaker, changes in both DASH conditions (p = 0.059), that persisted 1 year after completion of the intervention [23]. Further, changes in Executive Function were associated with changes in improved aerobic fitness, reduced CVD risk, and reduced dietary salt intake [21].
Assessment of biomarkers
Metabolic biomarkers
Insulin resistance:
Insulin resistance was defined using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) [25–27].
Leptin:
Plasma leptin levels were analyzed from EDTA plasma samples, assayed using solid phase quantitative ELISA according to a standard protocol [28–30]. Leptin is an adipocyte-secreted hormone thought to be involved in both regulation of appetite and cognitive function [31, 32].
Insulin like Growth Factor (IGF-1):
IGF-1 is an essential neurotrophic factor that is produced both peripherally and in the brain. Peripheral levels of IGF-1 have previously been associated with neurocognition [33–35], with lower IGF-1 levels associating with worse neurocognition and a greater likelihood of cognitive decline.
Neurotrophic biomarkers
Brain derived neurotrophic factor (BDNF):
Total and free plasma BDNF levels were assayed. BDNF regulates neuronal development and function in both the central and peripheral nervous system and may mediate the improvements in cognition associated with exercise [36]. Total BDNF included both bound and unbound BDNF levels, whereas free BDNF assessed BDNF protein levels that were not bound to other proteins or receptors. In the present analyses, we focused on total BDNF as our primary outcome metric of interest.
Vascular endothelial growth factor (VEGF):
VEGF induces endothelial cell proliferation, promotes cell migration, inhibits apoptosis and induces permeabilization of blood vessels [36].
Inflammatory biomarkers
Interleukin-6 (IL-6) and High-Sensitivity C-Reactive Protein (hsCRP):
Both IL-6 and hsCRP were quantified by Lab Corp using commercial enzyme-linked immunosorbent assay kits (R&D Systems). Values exceeding 10 mg/dL were truncated, consistent with current guidelines for the analysis of inflammatory markers [37]. In addition, a small number of individuals had inflammation levels measured below the conventional lower levels of detection at either baseline (≤5%) or after treatment (≤11%).
Assessment of neurocognitive functioning
Neurocognitive functioning was assessed using a modified test battery recommended by the Neuropsy-chological Working Group for vascular cognitive disorders [24] designed to tap, a priori, 3 key domains of cognitive functioning: Executive Function, Memory, and Language/Verbal Fluency. Our primary outcome was the Executive Function, assessed by the Trail Making Test, the Stroop Test, Digit Span Forward and Backwards subtest from the Wechsler Adult Intelligence Scale (WAIS), the Digit Symbol Substitution Test from the WAIS, the Ruff 2 & 7 Test, and the Animal Naming Test [22].
Assessment of aerobic capacity and dietary habits
Aerobic fitness
Participants underwent a maximal graded exercise treadmill test in which workloads were increased at a rate of one metabolic equivalent per minute [38]. Expired air was collected by mouthpiece for quantification of minute ventilation, oxygen consumption, and carbon dioxide production with the Parvo Medics TrueOne measurement system (model 2400; Parvo Medics, Sandy, Utah).
Dietary habits
Diet was assessed by the Block Food Frequency questionnaire (FFQ) and a 4-day food diary. We quantified adherence to the DASH eating pattern, using a modified DASH scoring algorithm adopted from Folsom and colleagues [39, 40]. Dietary intake from a 4-day diary was utilized for all dietary components for which a score could be derived (fruits, vegetables, dairy, grains, fat calories, saturated fat calories, and sodium), with only those categories that could not be quantified by the diary being generated from the FFQ (meats, nuts/seeds/legumes, and sweets).
Interventions
Following baseline assessments, participants were randomized into one of four groups using a 2 × 2 factorial design. Participants were assigned equally between two intervention factors: 1) aerobic exercise and 2) the DASH diet, creating four separate groups:
Aerobic Exercise (AE) alone: Participants performed Aerobic Exercise for 6 months. For the initial 3 months, participants exercised 3 times a week at a level of 70–85% of their initial peak heart rate reserve (HRR) under supervision at a cardiac rehabilitation facility in central North Carolina. During months 4–6, participants exercised 3 times per week on their own at home at 70–85% HRR, which they documented in weekly exercise logs. Participants did not receive any DASH or dietary counseling.
DASH Diet (DASH): Education on the DASH diet and feedback on participants’ adherence were provided by a nutritionist in a series of half-hour sessions conducted weekly for the initial 12 weeks and then bi-weekly for weeks 13–24. Participants in the DASH condition were asked to maintain their current exercise habits for the 6-month trial duration.
Combined Exercise and DASH diet (AE+DASH): Participants in the AE+DASH condition received both the Aerobic exercise and DASH interventions as described above.
Health Education Control Group (HE): The HE control group received weekly 30 min educational phone calls for 3 months and then bi-weekly for 3 months and were asked to maintain their lifestyle habits. Phone calls were conducted by a health educator (a physician’s assistant) on relevant, health-related topics.
Data analysis
Analyses were conducted using general linear models within SAS 9.4. Due to strong correlations among biologically-related biomarkers, we combined outcomes within domains in order to focus on three sets of outcomes: neurotrophins (BDNF and VEGF), metabolic function (HOMA-IR, Leptin, and IGF-1), and inflammation (IL-6 and CRP). Within each set of analyses, pre- and post-treatment biomarker levels were combined using a within-sample, mean z-score; [41], for neurocognition we used a ‘gatekeeper’ analytic approach, which we have used in other studies including our primary publication [42–44]. This latter approach has been advocated for its parsimonious approach to minimizing type-I error without substantial loss of power [45, 46]. Analyses of intervention-related biomarker changes were conducted using pre-planned factorial comparisons of DASH factor groups (AE + DASH and DASH-A) versus non-DASH groups (AE and HE), and Aerobic Exercise factor groups (AE + DASH and AE) versus non-AE groups (DASH-A and HE). Consistent with our primary analyses [21], neurocognitive function was indexed a priori in domains of interest, which were examined using separate rank-based composite scores within each domain of function. Because Executive Function was the only neurocognitive domain improved in the primary trial, changes in Language and Memory performance were not examined in the present analyses. In order to improve the robustness of model estimates, overlapping covariates were clustered where possible [47]. All analyses controlled for age, education, MoCA score, sex, race, cardiometabolic risk factor severity (CRF) [48], cardiovascular/inflammatory medications [21], genetic/familiar risk of ADRD (APOE genotype [21] and family history of dementia) [21, 49], and baseline level (T1) of the respective outcome. Analyses of changes in biomarkers examined the composite outcome score (T2 – T1) within each domain (neurotrophins, inflammation, and metabolic function) separately with the two treatment factors as the predictors of interest (AE and DASH): BiomarkersT2–T1 = BiomarkersT1 + Age + Education +MoCA + Sex + Race + CRF + Medications + ADRD Risk + AE + DASH. Within each domain, scores were combined such that the direction of change was consistent across markers. For example, for metabolic function, change values represented reductions in HOMA-IR, reductions in leptin, and increases in IGF-1.
In order to examine the association between changes in biomarkers and neurocognition, we conducted a secondary analysis in which changes in biomarkers served as the predictor of interest with Executive Function as the outcome: Executive FunctionT2–T1 = Executive FunctionT1 + Age + Education + MoCA + Sex + Race,+ CRF + Medicatio ns + ADRD Risk + BiomarkersT1 + BiomarkersT2–T1. Because changes in our composite marker of Executive Function were based on mean-ranks and therefore cannot be interpreted as ‘improvements’, we hereafter refer to favorable changes in Executive Function as ‘increases’. Assumptions regarding linearity, independence and distribution of residuals were assessed in all analyses, with log-transformation of highly skewed outcome variables (BDNF, VEGF, and leptin). Multiple imputation using PROC MI was used to handle missing data. Continuous predictors were scaled using the interquartile range, which can be interpreted as showing differences in the outcome variable between individuals at a high (75th percentile) and low (25th percentile) level of the predictor. Because results did not differ substantively between analyses using the full cohort (n = 160) and the substudy with complete biomarker data (n = 132), we have restricted our analyses to the sub-sample only.
RESULTS
Intervention
One hundred sixty individuals were randomized to the trial, all of whom completed the program. Of these, 132 (83% of randomized participants from the full cohort) consented to provide biomarker specimens at both pre- and post-intervention samples for analysis (Table 1). Participants who did not agree to participate in the biomarker ancillary study had better baseline Memory performance (p = 0.002), had a lower CVD medication burden (p = 0.014), and were more likely to be male (p = 0.050) compared to those who agreed to participate. The cohort providing biomarker data was comparable in distribution to the primary trial cohort regarding intervention group assignment: 35 participants in AE + DASH (27%), 34 in DASH-A (26%), 32 in AE (24%), and 31 in HE (23%).
Table 1:
Background and metabolic characteristics across the study sample. P-values represent omnibus tests for overall group differences using analysis of variance for continuous outcomes and chi-square for categorical outcomes
| Variable | AE +DASH (n = 35) | DASH-A (n = 34) | AE (n = 32) | HE (n = 31) | Omnibus p |
|---|---|---|---|---|---|
| Age, y | 64.4 (6.4) | 66.1 (6.7) | 65.9 (7.3) | 65.2 (7.0) | 0.715 |
| Education, y | 15.9 (2.1) | 16.0 (2.0) | 15.2 (2.4) | 15.9 (2.7) | 0.411 |
| Gender, Male | 13 (37%) | 14 (41%) | 10 (31%) | 12 (39%) | 0.862 |
| Race/Ethnicity | |||||
| Caucasian, non-Hispanic | 15 (43%) | 21 (62%) | 12 (38%) | 17 (55%) | 0.183 |
| Caucasian, Hispanic | 1 (3%) | 0 (0%) | 1 (3%) | 0 (0%) | |
| African-American | 17 (49%) | 11 (32%) | 16 (50%) | 14 (45%) | |
| American Indian | 0 (0%) | 2 (6%) | 0 (0%) | 0 (0%) | |
| Other | 2 (6%) | 0 (0%) | 3 (9%) | 0 (0%) | |
| MoCA Score | 24.9 (2.5) | 24.6 (2.6) | 24.4 (2.6) | 24.5 (2.2) | 0.819 |
| ASCVD Risk Score | 14.7 (9.4) | 16.2 (12.6) | 16.1 (9.8) | 16.5 (13.2) | 0.922 |
| Body mass Index, kg/m2 | 32.9 (3.6) | 32.2 (4.7) | 32.3 (5.8) | 32.7 (5.8) | 0.897 |
| Diabetes, n (%) | 9 (26%) | 8 (24%) | 9 (28%) | 6 (19%) | 0.869 |
| Metabolic Risk Score, z-score | −2.15 (1.1) | −2.07 (1.1) | −2.2 (0.9) | −2.1 (1.0) | 0.942 |
| Systolic Blood Pressure, mm Hg | 130 (15) | 129 (15) | 132 (14) | 133 (12) | 0.734 |
| Diastolic Blood Pressure, mm Hg | 76 (7) | 75 (7) | 76 (8) | 77 (9) | 0.599 |
| Triglycerides | 129 (53) | 114 (41) | 119 (50) | 112 (49) | 0.479 |
| High Density Lipoprotein | 56 (14) | 59 (18) | 61 (17) | 60 (22) | 0.624 |
| Anti-Depressant Medication, n (%) | 8 (23%) | 7 (21%) | 7 (22%) | 8 (26%) | 0.966 |
| Biomarkers | |||||
| HOMA-IR, U (n = 132) | 4.3 (3.1) | 3.0 (2.1) | 3.5 (2.1) | 3.5 (2.2) | 0.159 |
| Leptin, pg/ml (n = 132) | 44,123 (44,857) | 39,474 (44,145) | 51,082 (53,700) | 43,022 (42,648) | 0.893 |
| IGF-1, pg/ml (n = 132) | 64.1 (20.2) | 60.0 (15.2) | 63.1 (19.5) | 59.2 (19.6) | 0.633 |
| Total BDNF, pg/ml (n = 132) | 5454 (4780) | 4708 (3051) | 4500 (3266) | 5595 (5154) | 0.748 |
| VEGF-A, pg/ml (n = 132) | 51.5 (52.2) | 55.5 (60.1) | 48.2 (34.0) | 48.6 (33.9) | 0.857 |
| IL-6, pg/ml (n = 132) | 3.0 (1.9) | 3.4 (2.4) | 3.7 (2.1) | 3.6 (2.8) | 0.655 |
| CRP, pg/ml (n = 132) | 2.9 (2.9) | 2.6 (2.9) | 3.5 (3.3) | 2.5 (2.9) | 0.534 |
Intervention-related changes in biomarkers
Intervention-related changes in biomarkers were examined in three separate domains: Metabolic (leptin, HOMA-IR, and IGF-1), Neurotrophic (BDNF and VEGF), and Inflammatory (IL-6 and CRP).Examination of intervention-related changes in metabolic biomarkers revealed that the AE groups showed improvements in metabolic function compared to the non-AE groups (AE: −0.11 [−0.26, 0.03] versus 0.17 [−0.03, 0.32]; p = 0.015). A similar effect was observed in the comparison of DASH factor groups to non-DASH groups (DASH: −0.08 [−0.22, 0.07] versus 0.13 [−0.28, 0.01]; p = 0.039) (Fig. 1). Changes in metabolic function corresponded to small-to-moderate effect size changes for both AE (d = 0.48) and DASH (d = 0.37). Follow-up characterization of group differences revealed that the AE + DASH group showed the largest metabolic function improvements (z-score change −0.26 [−0.5, −0.1]), followed by the DASH (z-score change −0.1 [−0.3, 0.1]), AE (z-score change −0.04 [−0.3, 0.2]), and HE demonstrating the worst metabolic changes (z-score change 0.23 [0.02, 0.40]) (Fig. 1).
Fig. 1.
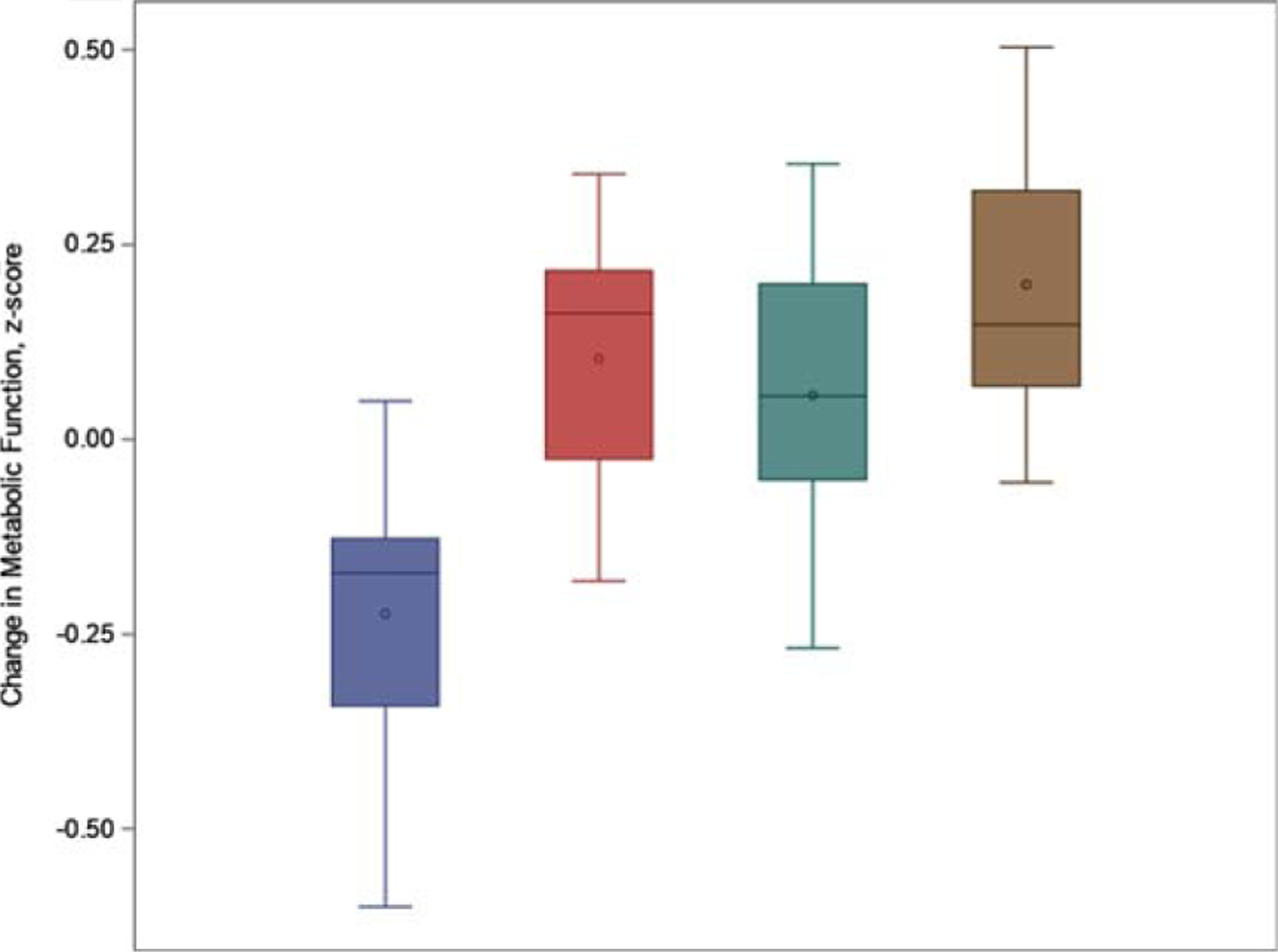
Pre-to-post intervention changes in metabolic biomarker levels (mean z-score change) across intervention groups. Values are adjusted for baseline metabolic function, age, education, sex, cardiometabolic risk factors, genetic risk, race, and MoCA score. Blue = AE + DASH, Red = DASH, Green = AE, Gold = HE. Changes following intervention were noted for both the AE factor groups (AE + DASH and AE; d = 0.48, p = 0.015) and the DASH groups (AE + DASH and DASH; d = 0.37, p = 0.039).
In contrast, no consistent intervention factor differences were noted for any of our inflammation (AE: −0.01 [−0.16, 0.14] versus 0.00 [−0.16, 0.15], p = 0.508; DASH: −0.03 [−0.18, 0.12] versus 0.02 [−0.13, 0.17], p = 0.689) or neurotrophins (AE: −0.02 [−0.23, 0.18] versus 0.03 [−0.18, 0.23], p = 0.501; DASH: 0.01[−0.19, 0.20] versus −0.01 [−0.21, 0.20], p = 0.448).
Associations with neurocognitive change
In order to further characterize the relationships between biomarkers and Executive Function, we examined the associations between changes in individual metabolic biomarkers and Executive Function. Both reductions in HOMA-IR (B = −2.3 [−4.3, −0.2], p = 0.033) and increases in IGF-1 (B = 3.4 [1.2, 5.7], p = 0.004) associated with increases in Executive Function, whereas leptin changes were not associated.
DISCUSSION
Results from the present analyses suggest that changes in neurocognitive function following lifestyle modification may be partially attributable to improved metabolic function and insulin sensitivity, in particular. In explanatory analyses of individual metabolic biomarkers, changes in insulin sensitivity showed the strongest association with changes in neurocognition after carefully controlling for known confounders. In contrast, we did not observe any consistent associations between neurotrophic or inflammatory biomarkers and neurocognitive function.
Previous studies have linked metabolic risk factors, including obesity and diabetes, to a greater risk of cognitive impairment [50, 51], Alzheimer’s disease [52–54], and dementia [55–58]. There are several plausible mechanistic pathways linking changes in peripheral metabolic function to neurocognition. Impaired peripheral metabolism is thought to dysregulate CNS metabolic function [59], with numerous studies demonstrating that metabolic risk factors associated with CNS hypometabolism [60, 61]. Changes in peripheral markers of metabolism have been hypothesized to improve neurocognition by augmenting ‘cross talk’ between peripheral and central metabolic function, in which improved peripheral metabolism increases the efficiency and recruitment of central glucose resources and transport across the blood-brain barrier [62]. Because metabolic dys-function impairs both glucose and lipid homeostasis, causing central glucotoxicity and impaired brain insulin signaling, it has been suggested that exercise and diet may ‘recalibrate’ central homeostatic function by altering brain metabolic function [63, 64]. It has also been suggested that improved metabolic function may reduce microvascular burden [65] and stabilize cortical atrophy [15, 58], slowing the progression of neurocognitive impairment.
Several studies have shown that changes in metabolic risk factors are associated with improved cognitive outcomes, including the FINGER [66], Pre-DIVA [67], LOOK-AHEAD [68], and PREDIMED trials [69], among others [70, 71]. For example, the FINGER trial [66] recently demonstrated that a combined lifestyle and cognitive intervention focusing on vascular risk reduction may confer cognitive benefits among older adults with vascular risk factors in processing speed, executive functions, and on a composite measure of neuropsychological functioning comprised of 14 subtests. While ENLIGHTEN differs from previously published trials in several important ways (e.g., lack of cognitive training and vascular risk reduction), the present findings nevertheless support the potential utility of reducing metabolic risk factors as a potential means of reducing the risk of late-life neurocognitive impairment. In addition, examination of individual components demonstrates that greater dietary changes conferred larger cognitive benefits [10], even among individuals with varying degrees of neuropathological burden [72]. Alternative intervention strategies to augment metabolic function, including weight loss and intranasal insulin administration, also suggest a potential role for targeted metabolic interventions to improve neurocognition. Intentional weight loss, which has robust effects on metabolic function [73], also has been suggested to improve neurocognition across varying intervention modalities [74]. Similarly, increasing evidence suggests that intranasal insulin administration may improve neurocognition [75, 76] and brain biomarkers [77–79] in both healthy and clinical samples. Additional studies should examine alterations in CNS metabolic function among individuals with metabolic risk factors to further delineate possible benefits of improved systemic metabolism on neurocognition.
Limitations
The present study has several limitations. First, the ENLIGHTEN trial examined changes over a 6-month time period, whereas many of the putative mechanisms of change may require a longer period of exposure to result in changes in behavioral markers of brain function or conversion to dementia. Future studies should attempt to integrate biomarker assessments over a longer period of time in order to better delineate the unique and overlapping influences of these important mechanistic pathways. In addition, some of our methodological approaches may have been limited by the short time period of intervention and follow-up, such as our assessments of dietary intake that used both diary measures and the FFQ. Second, our study was relatively small and the present findings must therefore be replicated in order to ensure the observed associations are robust. Specifically, we collected numerous neurocognitive outcomes and biomarkers of interest, increasing the possibility of type-I error. Although we attempted to mitigate this by carefully aggregating both neurocognitive outcome data and our biomarkers within biologically-related domains, it is possible the present findings were influenced by our small sample size and replication is therefore critical. Third, we did not collect neuroimaging markers of brain health in the present study and it is therefore possible that additional, subtle changes in brain function occurred that could only be detected using more sensitive neuroimaging modalities (e.g., functional connectivity or magnetic resonance spectroscopy) [80–82]. Finally, future studies would benefit from more careful collection of other metabolic biomarkers to more comprehensively examine the relationship between augmented metabolic function and changes inneurocognition. Collection of cerebrospinal biomarkers may also be considered in order to increase the specificity of biomarker data for CNS markers such as BDNF.
Conclusions
In the present study, lifestyle modification through aerobic exercise and the DASH diet resulted in small changes in insulin sensitivity, which were associated with changes in Executive Function. Future studies should examine changes in both peripheral and central markers of metabolic function in order to better delineate mechanisms linking improved peripheral metabolism to neurocognitive outcomes. Findings could provide important insight to guide prevention strategies among individuals at risk for neurocognitive decline and ADRD.
Table 2:
Biomarker changes from pre to post aerobic exercise (AE) and dietary approaches to stop hypertension (DASH) intervention factors
| Biomarker Changes | AE + DASH | DASH | AE | HE | AE p | DASH p |
|---|---|---|---|---|---|---|
| Neurotrophic Biomarkers | ||||||
| Neurotrophins (n = 132), z-score | −0.12 (−0.4, 0.16) | 0.19 (−0.1, 0.5) | 0.12 (−0.2, 0.4) | −0.03 (−0.3, 0.3) | 0.763 | 0.843 |
| Inflammatory Biomarkers | ||||||
| Inflammation (n = 132), z-score | −0.02 (−0.2, 0.2) | −0.05 (−0.3, 0.2) | −0.02 (−0.3, 0.2) | −0.04 (−0.3, 0.2) | 0.820 | 0.891 |
| Metabolic Biomarkers | ||||||
| Metabolic Function (n - 132), z-score | −0.26 (−0.5, −0.1) | −0.04 (−0.3, 0.2) | −0.11 (−0.3, 0.1) | 0.23 (0.4, −0.02) | 0.015 | 0.039 |
| IGF-1, pg/ml (n = 132) | 0.5 (−2.9, 3.9) | 1.1 (−2.3, 4.5) | 2.2 (−1.4, 5.7) | 1.1 (−2.5, 4.7) | 0.763 | 0.555 |
| Leptin, pg/ml (n = 132) | −10,633 (−15952, −5314) | −1,192 (−6579, 4194) | −3,245 (−8857, 2367) | −2133 (−7714, 3448) | 0.062 | 0.246 |
| HOMA-IR, U (n = 159) | −0.6 (−1.1, −0.1) | −0.1 (−0.6, 0.5) | −10.1 (−0.7, 0.5) | 0.7 (0.2, 1.3) | 0.018 | 0.018 |
ACKNOWLEDGMENTS
The study was supported by a grant from the National Institutes of Health (HL109219); Clinical Trials Identifier: NCT02342808.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0374r3).
REFERENCES
- [1].Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C (2011) Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med 269, 107–117. [DOI] [PubMed] [Google Scholar]
- [2].Hussenoeder FS, Riedel-Heller SG (2018) Primary prevention of dementia: From modifiable risk factors to a public brain health agenda? Soc Psychiatry Psychiatr Epidemiol 53, 1289–1301. [DOI] [PubMed] [Google Scholar]
- [3].Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA (2018) Effect of the Mediterranean diet on cognition and brain morphology and function: A systematic review of randomized controlled trials. Am J Clin Nutr 107, 389–404. [DOI] [PubMed] [Google Scholar]
- [4].Wu L, Sun D, Tan Y (2017) Intake of fruit and vegetables and the incident risk of cognitive disorders: A systematic review and meta-analysis of cohort studies. J Nutr Health Aging 21, 1284–1290. [DOI] [PubMed] [Google Scholar]
- [5].Aarsland D, Sardahaee FS, Anderssen S, Ballard C (2010) Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health 14, 386–395. [DOI] [PubMed] [Google Scholar]
- [6].Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L (2008) Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev, CD005381. [DOI] [PubMed] [Google Scholar]
- [7].Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A (2010) Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med 72, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 14, 125–130. [DOI] [PubMed] [Google Scholar]
- [9].Pentikainen H, Savonen K, Ngandu T, Solomon A, Komulainen P, Paajanen T, Antikainen R, Kivipelto M, Soininen H, Rauramaa R (2019) Cardiorespiratory fitness and cognition: Longitudinal associations in the FINGER Study. J Alzheimers Dis 68, 961–968. [DOI] [PubMed] [Google Scholar]
- [10].Lehtisalo J, Levalahti E, Lindstrom J, Hanninen T, Paajanen T, Peltonen M, Antikainen R, Laatikainen T, Strandberg T, Soininen H, Tuomilehto J, Kivipelto M, Ngandu T (2019)Dietary changes and cognition over 2 years within a multidomain intervention trial-The Finnish Geriatric Intervention Study to prevent cognitive impairment and disability (FINGER). Alzheimers Dement 15, 410–417. [DOI] [PubMed] [Google Scholar]
- [11].Solomon A, Turunen H, Ngandu T, Peltonen M, Levalahti E, Helisalmi S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lehtisalo J, Lindstrom J, Paajanen T, Pajala S, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M (2018) Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: A subgroup analysis of a randomized clinical trial. JAMA Neurol 75, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stephen R, Liu Y, Ngandu T, Rinne JO, Kemppainen N, Parkkola R, Laatikainen T, Paajanen T, Hanninen T, Strandberg T, Antikainen R, Tuomilehto J, Keinanen Kiukaanniemi S, Vanninen R, Helisalmi S, Levalahti E, Kivipelto M, Soininen H, Solomon A (2017) Associations of CAIDE Dementia Risk Score with MRI, PIB-PET measures, and cognition. J Alzheimers Dis 59, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Witte AV, Fobker M, Gellner R, Knecht S, Floel A (2009) Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A 106, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A (2015) A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: The Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628]. Nutr J 14, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S (2010) Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis 22, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scarmeas N, Stern Y, Mayeux R, Luchsinger JA (2006) Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol 63, 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abbatecola AM, Russo M, Barbieri M (2018) Dietary patterns and cognition in older persons. Curr Opin Clin Nutr Metab Care 21, 10–13. [DOI] [PubMed] [Google Scholar]
- [18].Smith PJ, Blumenthal JA (2016) Dietary factors and cognitive decline. J Prev Alzheimers Dis 3, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith PJ, Blumenthal JA (2010) Diet and neurocognition: Review of evidence and methodological considerations. Curr Aging Sci 3, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, Welsh-Bohmer KA, Browndyke JN, Kraus WE, Doraiswamy PM, Burke JR, Sherwood A (2019) Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 92, e212–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blumenthal JA, Smith PJ, Welsh-Bohmer K, Babyak MA, Browndyke J, Lin PH, Doraiswamy PM, Burke J, Kraus W, Hinderliter A, Sherwood A (2013) Can lifestyle modification improve neurocognition? Rationale and design of the ENLIGHTEN clinical trial. Contemp Clin Trials 34, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blumenthal JA, Smith PJ, Mabe SM, Hinderliter A, Welsh-Bohmer KA, Browndyke JN, Doraiswamy PM, Lin PH, Kraus WE, Burke JR, Sherwood A (2020) Longer term effects of diet and exercise on neurocognition: 1-year follow-up of the ENLIGHTEN trial. J Am Geriatr Soc 68, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG (2006) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241. [DOI] [PubMed] [Google Scholar]
- [25].Macesic M, Lalic NM, Kostic VS, Jotic A, Lalic K, Stefanova E, Milicic T, Lukic L, Gajovic JS, Krako N (2017) Impaired insulin sensitivity and secretion in patients with Alzheimer’s disease: The relationship with other atherosclerosis risk factors. Curr Vasc Pharmacol 15, 158–166. [DOI] [PubMed] [Google Scholar]
- [26].Benedict C, Brooks SJ, Kullberg J, Burgos J, Kempton MJ, Nordenskjold R, Nylander R, Kilander L, Craft S, Larsson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB (2012) Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care 35, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geroldi C, Frisoni GB, Paolisso G, Bandinelli S, Lamponi M, Abbatecola AM, Zanetti O, Guralnik JM, Ferrucci L (2005) Insulin resistance in cognitive impairment: The InCHIANTI study. Arch Neurol 62, 1067–1072. [DOI] [PubMed] [Google Scholar]
- [28].Horie NC, Serrao VT, Simon SS, Gascon MR, Dos Santos AX, Zambone MA, Del Bigio de Freitas MM, Cunha-Neto E, Marques EL, Halpern A de Melo ME, Mancini MC, Cercato C(2016) Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab 101, 1104–1112. [DOI] [PubMed] [Google Scholar]
- [29].Bove RM, Brick DJ, Healy BC, Mancuso SM, Gerweck AV, Bredella MA, Sherman JC, Miller KK (2013) Metabolic and endocrine correlates of cognitive function in healthy young women. Obesity (Silver Spring) 21, 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zeki Al Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J (2012) Central obesity, leptin and cognitive decline: The Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disord 33, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kalra SP, Kalra PS (2010) Neuroendocrine control of energy homeostasis: Update on new insights. Prog Brain Res 181, 17–33. [DOI] [PubMed] [Google Scholar]
- [32].Fadel JR, Jolivalt CG, Reagan LP (2013) Food for thought: The role of appetitive peptides in age-related cognitive decline. Ageing Res Rev 12, 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morley JE, Kaiser F, Raum WJ, Perry HM 3rd, Flood JF, Jensen J, Silver AJ, Roberts E (1997) Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: Progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A 94, 7537–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Landi F, Capoluongo E, Russo A, Onder G, Cesari M, Lulli P, Minucci A, Pahor M, Zuppi C, Bernabei R (2007) Free insulin-like growth factor-I and cognitive function in older persons living in community. Growth Horm IGF Res 17, 58–66. [DOI] [PubMed] [Google Scholar]
- [35].Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P (2003) Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol Aging 24, 573–581. [DOI] [PubMed] [Google Scholar]
- [36].Maass A, Duzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lovden M, Lindenberger U, Backman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Muller NG, Lessmann V, Sendtner M, Duzel E (2016) Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 131, 142–154. [DOI] [PubMed] [Google Scholar]
- [37].Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP; CDC; AHA (2004) CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice: Report from the laboratory science discussion group. Circulation 110, e545–e549. [DOI] [PubMed] [Google Scholar]
- [38].Blumenthal JA, Rejeski WJ, Walsh-Riddle M, Emery CF, Miller H, Roark S, Ribisl PM, Morris PB, Brubaker P, Williams RS (1988) Comparison of high- and low-intensity exercise training early after acute myocardial infarction. Am J Cardiol 61, 26–30. [DOI] [PubMed] [Google Scholar]
- [39].Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia C, Lin PH, Babyak MA, Johnson JJ, Hinderliter A, Blumenthal JA (2012) Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and White adults with high blood pressure: Results from the ENCORE Trial. J Acad Nutr Diet 112, 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Folsom AR, Parker ED, Harnack LJ (2007) Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens 20, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Brien PC (1984) Procedures for comparing samples with multiple endpoints. Biometrics 40, 1079–1087. [PubMed] [Google Scholar]
- [42].Smith PJ, Blumenthal JA, Babyak MA, Hinderliter A, Sherwood A (2011) Association of vascular health and neurocognitive performance in overweight adults with high blood pressure. J Clin Exp Neuropsychol 33, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A (2010) Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 55, 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A (2007) Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med 69, 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Koch GG, Gansky SA (1996) Statistical considerations for multiplicity in confirmatory protocols. Drug Inf J 30, 523–533. [Google Scholar]
- [46].Koch GG, Schwartz TA (2014) An overview of statistical planning to address subgroups in confirmatory clinical trials. J Biopharm Stat 24, 72–93. [DOI] [PubMed] [Google Scholar]
- [47].Harrell FE (2015) Regression Modeling Strategies: With applications to linear modeling, logistic regression, and survival analysis, Springer, New York. [Google Scholar]
- [48].Lai MMY, Ames DJ, Cox KL, Ellis KA, Sharman MJR, Hepworth G, Desmond P, Cyarto EV, Szoeke C, Martins R, Masters CL, Lautenschlager NT (2020) Association between cognitive function and clustered cardiovascular risk of metabolic syndrome in older adults at risk of cognitive decline. J Nutr Health Aging 24, 300–304. [DOI] [PubMed] [Google Scholar]
- [49].Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Welsh-Bohmer K, Browndyke JN, Lin PH, Kraus W, Doraiswamy PM, Burke J, Sherwood A (2017) Lifestyle and neurocognition in older adults with cardiovascular risk factors and cognitive impairment. Psychosom Med 79, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang M, Norman JE, Srinivasan VJ, Rutledge JC (2016) Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am J Neurodegener Dis 5, 171–177. [PMC free article] [PubMed] [Google Scholar]
- [51].Bischof GN, Park DC (2015) Obesity and aging: Consequences for cognition, brain structure, and brain function. Psychosom Med 77, 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Clark LR, Koscik RL, Allison SL, Berman SE, Norton D, Carlsson CM, Betthauser TJ, Bendlin BB, Christian BT, Chin NA, Asthana S, Johnson SC (2019) Hypertension and obesity moderate the relationship between beta-amyloid and cognitive decline in midlife. Alzheimers Dement 15, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, Kivimaki M (2018) Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 14, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, Egan K (2017) Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 8, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Anstey KJ, Sargent-Cox K, Eramudugolla R, Magliano DJ, Shaw JE (2015) Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res Ther 7, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC, Tan L (2014) Midlife vascular risk factors and the risk of Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis 42, 1295–1310. [DOI] [PubMed] [Google Scholar]
- [57].Bruce DG, Davis WA, Starkstein SE, Davis TM (2014) Mid-life predictors of cognitive impairment and dementia in type 2 diabetes mellitus: The Fremantle Diabetes Study. J Alzheimers Dis 42, S63–S70. [DOI] [PubMed] [Google Scholar]
- [58].Watts AS, Loskutova N, Burns JM, Johnson DK (2013) Metabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adults. J Alzheimers Dis 35, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tumminia A, Vinciguerra F, Parisi M, Frittitta L (2018) Type 2 diabetes mellitus and Alzheimer’s disease: Role of insulin signalling and therapeutic implications. Int J Mol Sci 19, 3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Marchitelli R, Aiello M, Cachia A, Quarantelli M, Cavaliere C, Postiglione A, Tedeschi G, Montella P, Milan G, Salvatore M, Salvatore E, Baron JC, Pappata S (2018) Simultaneous resting-state FDG-PET/fMRI in Alzheimer disease: Relationship between glucose metabolism and intrinsic activity. Neuroimage 176, 246–258. [DOI] [PubMed] [Google Scholar]
- [61].Herholz K, Haense C, Gerhard A, Jones M, Anton-Rodriguez J, Segobin S, Snowden JS, Thompson JC, Kobylecki C (2018) Metabolic regional and network changes in Alzheimer’s disease subtypes. J Cereb Blood Flow Metab 38, 1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Heni M, Kullmann S, Preissl H, Fritsche A, Haring HU (2015) Impaired insulin action in the human brain: Causes and metabolic consequences. Nat Rev Endocrinol 11, 701–711. [DOI] [PubMed] [Google Scholar]
- [63].Sartorius T, Heni M, Tschritter O, Preissl H, Hopp S, Fritsche A, Lievertz PS, Gertler A, Berthou F, Taouis M, Staiger H, Haring HU, Hennige AM (2012) Leptin affects insulin action in astrocytes and impairs insulin-mediated physical activity. Cell Physiol Biochem 30, 238–246. [DOI] [PubMed] [Google Scholar]
- [64].Pugazhenthi S (2017) Metabolic syndrome and the cellular phase of Alzheimer’s disease. Prog Mol Biol Transl Sci 146, 243–258. [DOI] [PubMed] [Google Scholar]
- [65].Espeland MA, Erickson K, Neiberg RH, Jakicic JM, Wadden TA, Wing RR, Desiderio L, Erus G, Hsieh MK, Davatzikos C, Maschak-Carey BJ, Laurienti PJ, Demos-McDermott K, Bryan RN; Action for Health in Diabetes Brain Magnetic Resonance Imaging (Look AHEAD Brain) Ancillary Study Research Group (2016) Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care 39, 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385, 2255–2263. [DOI] [PubMed] [Google Scholar]
- [67].Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, Hoevenaar-Blom MP, Vermeulen M, van Gool WA (2016) Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet 388, 797–805. [DOI] [PubMed] [Google Scholar]
- [68].Espeland MA, Carmichael O, Hayden K, Neiberg RH, Newman AB, Keller JN, Wadden TA, Rapp SR, Hill JO, Horton ES, Johnson KC, Wagenknecht L, Wing RR; Action for Health In Diabetes Brain Magnetic Resonance Imaging (Look AHEAD Brain) and Action for Health Movement and Memory Ancillary Study Research Group (2018) Long-term impact of weight loss intervention on changes in cognitive function: Exploratory analyses from the action for health in diabetes randomized controlled clinical trial. J Gerontol A Biol Sci Med Sci 73, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA (2013) Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 84, 1318–1325. [DOI] [PubMed] [Google Scholar]
- [70].Smith PJ (2019) Pathways of prevention: A scoping review of dietary and exercise interventions for neurocognition. Brain Plast 5, 3–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kivipelto M, Mangialasche F, Ngandu T (2018) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14, 653–666. [DOI] [PubMed] [Google Scholar]
- [72].Stephen R, Liu Y, Ngandu T, Antikainen R, Hulkkonen J, Koikkalainen J, Kemppainen N, Lotjonen J, Levalahti E, Parkkola R, Pippola P, Rinne J, Strandberg T, Tuomilehto J, Vanninen R, Kivipelto M, Soininen H, Solomon A; FINGER Study Group (2019) Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to prevent cognitive impairment and disability (FINGER). Alzheimers Res Ther 11, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Blumenthal JA, Babyak MA, Sherwood A, Craighead L, Lin PH, Johnson J, Watkins LL, Wang JT, Kuhn C, Feinglos M, Hinderliter A (2010) Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension 55, 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Veronese N, Facchini S, Stubbs B, Luchini C, Solmi M, Manzato E, Sergi G, Maggi S, Cosco T, Fontana L (2017) Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci Biobehav Rev 72, 87–94. [DOI] [PubMed] [Google Scholar]
- [75].Ritze Y, Kern W, Ebner EM, Jahn S, Benedict C, Hallschmid M (2018) Metabolic and cognitive outcomes of subchronic once-daily intranasal insulin administration in healthy men. Front Endocrinol (Lausanne) 9, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S (2015) Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 45, 1269–1270. [DOI] [PubMed] [Google Scholar]
- [77].Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S (2017) Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J Alzheimers Dis 57, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, Blalock EM, Porter NM, Thibault O (2016) Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci 71, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V (2015) Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Voss MW, Sutterer M, Weng TB, Burzynska AZ, Fanning J, Salerno E, Gothe NP, Ehlers DK, McAuley E, Kramer AF (2018) Nutritional supplementation boosts aerobic exercise effects on functional brain systems. J Appl Physiol (1985) 126, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Garcia-Casares N, Bernal-Lopez MR, Roe-Vellve N, Gutierrez-Bedmar M, Fernandez-Garcia JC, Garcia-Arnes JA, Ramos-Rodriguez JR, Alfaro F, Santamaria-Fernandez S, Steward T, Jimenez-Murcia S, Garcia-Garcia I, Valdivielso P, Fernandez-Aranda F, Tinahones FJ, Gomez-Huelgas R (2017) Brain functional connectivity is modified by a hypocaloric mediterranean diet and physical activity in obese women. Nutrients 9, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Prehn K, Lesemann A, Krey G, Witte AV, Köbe T, Grittner U, Flöel A (2019) Using resting-state fMRI to assess the effect of aerobic exercise on functional connectivity of the DLPFC in older overweight adults. Brain Cogn 131, 34–44. [DOI] [PubMed] [Google Scholar]


