Abstract
Controlled exocytosis and endocytosis of integrin adhesion receptors is required for normal cell adhesion, migration and signaling. In this chapter we describe the design of functional β1 integrins carrying extracellular fluorescent or chemically traceable tags (ecto-tag) and methods for their use to image β1 integrin trafficking in cells. We provide approaches to generate cells in which endogenous β1 integrins are replaced by ecto-tagged integrins containing a pH-sensitive fluorophore pHluorin or a HaloTag and describe strategies using photobleaching, selective extracellular/intracellular labeling and chase, quenching and blocking to reveal β1 integrin exocytosis, endocytosis and recycling by Live Total Internal Reflection Fluorescence (TIRF) Microscopy.
Keywords: integrins, ecto-tagged, pHluorin, HaloTag, exocytosis, endocytosis, recycling, Total Internal Reflection Fluorescence microscopy
1. INTRODUCTION
The binding of integrin adhesion receptors to the complex web of extracellular matrix (ECM) that surrounds most mammalian cells permits bidirectional transmission of mechanical and biochemical signals required for cell adhesion, migration, differentiation, and survival [1–3]. Integrins are obligate heterodimers of α and β subunits, each type I transmembrane proteins with a large multi-domain extracellular portion, a single transmembrane domain and a generally short cytoplasmic tail. In humans the 18 α subunits and 8 β subunits combine to generate 24 different αβ heterodimers with specific ECM ligand binding activities; e.g., α5β1 binds fibronectin (FN) while α2β1 binds collagen. ECM engagement causes large structural rearrangements in integrins’ extracellular domains and results in clustering of integrins into nano- to micron-sized structures and the association of integrin cytoplasmic tails with cytoplasmic adaptor, cytoskeletal and signaling proteins [3,4]. Depending on their size, shape and composition, these sites of integrin clustering are termed focal complexes, focal adhesions (FA) or fibrillar adhesions [5–7], and are important sites connecting the intracellular actin cytoskeleton to the extracellular environment, permitting mechanotransduction and signaling. Notably, these adhesions are not fixed, rather, they continually remodel as cells spread and move; e.g., during cell migration new adhesions form at the cell front and are released from the rear.
The importance of tightly controlling integrin-mediated adhesion during development, wound healing, hemostasis, inflammation and the immune response is now well established and there have been extensive studies investigating the molecular bases for assembly, maturation and turnover of integrin-mediated adhesions [2,3,8]. Integrins are known to be regulated at multiple levels, including by control of integrin expression levels, through conformational regulation that impacts integrin affinity for extracellular ligands, via interactions with specific intracellular adaptors and, crucially, through trafficking of integrins to and from the cell surface [1,3,9–12].
Membrane traffic-mediated delivery of integrins to the cell surface (exocytosis), removal of integrins by internalization (endocytosis) and recycling of internalized integrins back to the cell surface have received considerable interest in recent years, especially as alterations in integrin trafficking promote cell invasion and cancer metastasis [9–11,13–22]. Newly synthesized integrins reach the cell surface after transiting the endoplasmic reticulum, the Golgi and the secretory machinery, they are subsequently internalized from the cell surface via various endocytic pathways and can be recycled back to the cell surface using several different trafficking routes. Integrins have been shown to traffic via retrograde flow and to signal from the endosome compartment. However, exactly how integrins are reorganized in space and time and how their trafficking is tuned to different cell and ECM environments, remain key questions.
A variety of approaches have been taken to investigate integrin trafficking. Genetic studies show a clear requirement for integrin exocytosis and endocytosis and biochemical studies (e.g., cell-surface biotinylation) have revealed bulk integrin internalization and recycling rates [17,23,24,14]. Fixed point endocytic assays, often using antibodies, have helped characterize molecular pathways of integrin endocytosis and many molecular adaptors involved in membrane trafficking have been found to regulate integrin surface levels and to affect integrin-mediated activities, with some adaptors shown to directly bind integrin subunits [13,14,19,23–25].
However, fundamental insights into the dynamic spatiotemporal control of integrin traffic in cell physiology and pathology requires sophisticated microscopy tools. To help provide the tools to address critical gaps in understanding integrin exocytosis, endocytosis and recycling, we recently generated functional ‘ecto-tagged’ β1 integrins that allowed us to follow integrin delivery to, and internalization from, the plasma membrane in live cells [26]. Specifically, we inserted GFP, the pH-sensitive fluorophore pHluorin, or a chemical genetic HaloTag into an exposed loop in the extracellular hybrid domain of β1 and established that these ecto-tagged integrins pair with endogenous α-subunits, express at normal levels, localize normally and support normal cell adhesion [26].
Extracellular tagging offers three major advantages to traditional probes: i) accessibility to the extracellular environment permits use of affinity tags for selective covalent surface labeling, ii) exposure of tags to the extracellular environment enables the use of pH-sensitive fluorophores to selectively detect integrins at the cell surface, and iii) ecto-tags avoid modification of the short (20–70 amino acids) cytoplasmic tails of integrins preventing potential interference with the binding of cytoplasmic partners [27–30]. Notably, using pHluorin as an ecto-tag we obtained the first live-cell studies of exocytosis of β1 integrin-rich vesicles with the plasma membrane and, using HaloTag as a self-labeling ecto-tag that covalently binds synthetic chemical ligands [31], we selectively labeled cell-surface and intracellular β1 integrins with different fluorophores allowing us to follow β1 integrin internalization and trafficking in cells [26]. The ability to image both integrin exocytosis and endocytosis opens the possibility of mapping the entire integrin endo-exo cycle in live cells, and investigating how sites and timing of delivery are determined, their effect on focal adhesion function and dynamics, and ultimately their impact on cell morphology and motility. In this chapter we describe the approaches we employ to generate ecto-tagged integrins and their use in experiments to assess various aspects of integrin trafficking in live cells.
2. MATERIALS
2.1. Cells
HEK293T cells (ATCC#CRL-3216)
HeLa cells (ATCC#CCL-2)
EAhy.926 cells (a gift from William Sessa, Yale University)
Immortalized β1itg-null murine fibroblasts (a gift from Antony Koleske, Yale University)
2.2. Plasmids:
pcDNA3-human β1itg (designed by the Calderwood lab)
pEGFP-C2 (Clontech)
sspH-mSmo [32]
C-Halo (Promega)
pDONR221 (Thermo Fisher Scientific)
pLENTI-CMV-Puro-ecto-PH-β1itg (designed by Calderwood lab)
pLENTI-CMV-Puro-ecto-Halo-β1itg (designed by Calderwood lab)
pLENTI-PGK-Blast-ecto-PH-β1itg (designed by Calderwood lab)
pLENTI-PGK-Blast-ecto-Halo-β1itg (designed by Calderwood lab)
pLENTI-CMV-Hygro-Paxillin-mCherry (designed by the Calderwood lab)
pLENTI-CMV-Hygro-Paxillin-GFP (designed by the Calderwood lab)
psPAX2 (viral proteins Gag and Rev under the SV40 promoter; Addgene plasmid #12260, a gift from D. Trono)
pMD2.G (viral protein VSV-G expressed under the CMV promoter, Addgene plasmid #12259, a gift from D. Trono)
pLKO.1-β1itg shRNA TRCN0000275133 (Sigma)
pLENTI-CMV-Puro (AddGene plasmid #17452, gift from Eric Campeau [33]
pLENTI-PGK-Blast (gift from Ben Turk, Yale University).
2.3. Culture media
Complete culture medium: DMEM high glucose with L-glutamine, 9% Fetal Bovine Serum, non-essential amino-acids and sodium pyruvate
Complete imaging medium: phenol red-free DMEM high glucose supplemented with GlutaMax, 9% Fetal Bovine Serum, non-essential amino-acids and sodium pyruvate
Complete imaging medium with Hepes: phenol red-free DMEM high glucose containing L-glutamine and 25 mM Hepes, 9% Fetal Bovine Serum, non-essential amino-acids and sodium pyruvate
0.05% Trypsin/EDTA solution
ProLong Live Antifade Reagent (Thermo Fisher Scientific)
2.4. Plasticware
10 cm diameter tissue culture treated Petri dishes (Falcon)
35 mm Glass-bottom dishes (No.1.5 coverslip 14 mm glass diameter from MatTek). MatTek dishes were coated with 10 μg/ml bovine plasma fibronectin for 1h at 37°C prior to use
Acrodisc filters (0.45um) (Pall Corporation)
2.5. Chloroalkane Halo ligands
Membrane impermeant AF488-CA (Alexa Fluor 488-HaloTag chloroalkane ligand, commercially available from Promega)
Membrane permeant SiR-CA (SiR-HaloTag Chloroalkane ligand, Gift from Promega)
Membrane impermeant CF568-SS-CA (reducible CF568 labeled HaloTag chloroalkane ligand, gift from Promega)
Membrane impermeant PEG-biotin-CA (PEG-biotin chloroalkane ligand, commercially available from Promega)
2.6. Buffers and Reagents
Bovine plasma fibronectin (Sigma Aldrich)
MycoAlert mycoplasma detection kit (Lonza)
Gateway BP and LR Clonase II Enzyme mix (Thermo Fisher Scientific)
PEI (Linear Polyethylenimine MW 25,000, Polysciences, Inc.). Make stock solution at 1 mg/ml in water
Polybrene (Hexadimethrine Bromide, Sigma, H9268)
TCEP-HCl (Gold Biotechnology)
Anti-Alexa Fluor 488 antibody (Life Technology, A11094)
Anti-CD29 antibody clone HMβ1–1 (Biolegend, 112203)
NT Buffer: 150 mM NaCl, 1.0 mM EDTA, 0.2% BSA, 20 mM Tris, pH 8.6
PBS 2+: Phosphate buffered saline (pH 7.4) supplemented with 1.5 mM MgCl2 and 0.2 mM CaCl2
Stripping solution: 50 mM TCEP in NT buffer, pH 7
Oligonucleotide primer sequences are provided in Table 1
Table 1.
Oligonucleotide primer sequences
| Primer name | Primer sequence (5’-3’) |
|---|---|
| β1 integrin EcoRI-XhoI For | GTAACCAACCGTAGCAAAGGAGAATTCCTCGAGACAGCAGAGAAGCTCAAGCCAGAG |
| β 1 integrin EcoRI-XhoI Rev | CTCTGGCTTGAGCTTCTCTGCTGTCTCGAGGAATTCTCCTTTGCTACGGTTGGTTAC |
| 4AA GFP EcoRI For | GAATTCGGAGGTATGGTGAGCAAGGGC |
| 4AA GFP XhoI Rev | CTCGAGACCTCCCTTGTACAGCTCGTC |
| 9AA GFP EcoRI For | GAATTCGGAGGTTCTGGAGGTTCTGGTATGGTGAGCAAGGGCAG |
| 9AA GFP XhoI Rev | CTCGAGACCAGAACCTCCAGAACCTCCCTTGTACAGCTCGTC |
| 4AA pHluorin EcoRI For | GAATTCGGAGGTATGAGTAAAGGAGAAG |
| 4AA pHluorin XhoI Rev | CTCGAGACCTCCACTAGTTTTGTATAGTTCATCC |
| 9AA pHluorin EcoRI For | GAATTCGGAGGTTCTGGAGGTTCTGGTATGAGTAAAGGAGAAGAAC |
| 9AA pHluorin XhoI Rev | CTCGAGACCAGAACCTCCAGAACCTCCACTAGTTTTGTATAGTTCATCC |
| 9AA Halo EcoRI For | GGGGAATTCGGAGGTTCTGGAGGTTCTGGTGAAATCGGTACTGGCTTTCCATTC |
| 9AA Halo XhoI Rev | GGGCTCGAGACCAGAACCTCCAGAACCTCCACCGGAAATCTCCAGAGTAGACAG |
| attB1 β1 integrin For | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGGTACCATGAATTTACAACCAATTTTCTGG |
| attB2 β1 integrin Rev | GGGGACCACTTTGTACAAGAAAGCTGGGTCTAACTATTTTCCCTCATACTTCGGATT |
2.7. Microscopy equipment
Image cells with a TIRF microscope in a 7°C/5% CO2-controled environment. We use an OkoLab chamber (OkoLab; Burlingame, CA, USA) mounted onto a Nikon Ti-2 Eclipse microscope (Nikon; Tokyo, Japan, USA) equipped with a motorized Ti-LA-HTIRF module with LUN4 488, 561nm and 640 lasers (15 mW), a CFI PlanApo Lambda 100x Oil TIRF objective (1.45 NA) and a 1100×1100 pixels Teledyne Photometrics Prime95B sCMOS Camera 110 nm/pixel, and controlled by NIS Elements AR Software.
For HILO experiments, we use a custom built axicon lens Ring-TIRF illumination system built around an Olympus XI71 inverted microscope, a Innova 70 laser coupled to single-mode fiber, equipped with a 2048 × 2048 pixels Andor Zyla sCMOS 65 nm/pixel scale and controlled by the Micromanager ImageJ Software. The position of the axicon lens is adjusted to switch the illumination between TIRF and HILO using a PlanApo 100X objectives (1.49 NA).
3. METHODS
3.1. Design and generation of ecto-tagged integrins
With the goal of generating integrins containing an extracellular tag (ecto-tag) that facilitates analysis of integrin trafficking we initially tagged the β1 integrin subunit [26]. The β1 subunit is broadly expressed and pairs with many different α subunits [34], potentially enabling analysis of integrin traffic in many different settings. The methods described in this chapter therefore focus on our validated ecto-tagged β1 integrins [26] but, in principle, similar strategies could be applied to generate and validate other ecto-tagged integrins (see Note 1).
Integrin β subunits contain 8 extracellular domains, with three N-terminal domains nested within one another (Fig. 1a). Ideally, effective ecto-tagged integrins should enable tracking of the integrin without altering the function of these complex multi-domain heterodimeric receptors. Suitable sites for inserting a large tag such as GFP or Halo are therefore limited by a number of factors, including: i) the need to retain correct folding of the nested N-terminal domains, ii) the requirement for retention of the N-terminal signal peptide, iii) the necessity of avoiding interference with integrin heterodimerization, and iv) the ability to accommodate the large-scale conformational rearrangements that occur during integrin activation. To identify suitable tagging sites, we examined the crystal structures of α5β1, αvβ3 and αIIbβ3 alone or in complex with inhibitors or ligands [35–42,11,43,44]. We sought a surface loop far from the ligand-binding site that is exposed in both full-length bent and active headpiece structures. We selected a long loop (β1 residues 92–114) between β strands X and A [36] (Fig. 1b) which we hypothesized could accommodate a tag with flexible linkers. A very similar strategy for ecto-tagging β1 integrin in this loop has now also been described by others [45], confirming the utility of this approach. Expression constructs for our ecto-tagged β1 integrins are available upon request, but the general strategy for generating these constructs is provided below.
Once a suitable insertion site has been identified, introduce unique restriction enzyme sites to allow cloning into that site. We used QuikChange Mutagenesis with primers “β1 integrin EcoRI-XhoI For” and “β1 integrin EcoRI-XhoI Rev” (Table 1) to insert a GAATTCCTCGAG sequence introducing unique EcoRI and XhoI sites into the ecto-domain coding region of a human β1 integrin pCDNA3 expression construct between codons encoding Gly101 and Tyr102.
PCR-amplify eGFP from the pEGFP-C2 vector, ecliptic pHluorin from the sspH-mSmo vector [32] or HaloTag from the C-Halo vector using primers that add suitable spacers regions and restrictions sites at the 5’ and 3’ linkers. We used a 5′ linker containing an EcoRI site and a 3′ linker containing a XhoI site along with a spacer sequence encoding an additional GlyGly or a GlyGlySerGlyGlySerGly flexible linker on each side (Table 1).
Subclone PCR products into the modified human β1 integrin expression construct generated in step 1. In our case this generates β1 containing tags flanked with 4 or 9 amino acid spacers (Fig 1c).
Figure 1:

Design of an ecto-tagged β1 integrin. A, Cartoon of the conformational changes in the integrin αβ heterodimer during integrin activation (α subunit depicted in red, β subunit in blue with polypeptide chain in back), B, Ribbon diagram of the crystal structures of the α5β1 integrin head piece (PDB: 3VI4) and GFP (PDB: 1GFL). The hybrid domain loop into which ecto-tags were inserted is indicated. C, Zoom-in on the amino acid sequence of human ecto-tagged β1 integrins at the tag insertion site. Each tag (pHluorin or Halo, in blue, N- and C-terminal sequences specified) was inserted into the hybrid domain of human β1 integrin between residues Gly101 and Tyr102 (in green). Linkers of 4 or 9 amino acids (in red) were added on each side of the tag to facilitate cloning and provide flexibility. This figure was adapted from [26].
3.2. Generation of stable cell lines expressing ecto-tagged β1 integrins
Although for simple imaging experiments it is possible to express ecto-tagged β1 integrin in wild type cells, to validate the functionality of ecto-tagged integrins and for detailed investigation of integrin trafficking, we prefer to stably express ecto-tagged β1 integrins in a β1-null background. This helps ensure an optimum level of ecto-tagged β1 at the cell surface as the exogenous integrin does not need to compete with endogenous untagged integrin. We have achieved this using lentiviral transduction of either immortalized β1-knockout mouse fibroblasts or of human HeLa cells and EA.hy926 cells in which endogenous β1 expression has been stably knocked down by shRNA.
3.2.1. Production of lentiviral particles driving expression of ecto-tagged β1 integrin
To generate lentiviral particles driving expression of ecto-tagged β1 the ecto-tagged expression construct is recombined into a lentiviral vector and viral particles are generated in HEK293T cells.
Generate final lentivirus expression vectors by Gateway LR recombination with pLENTI-CMV-Puro or pLENTI-PGK-Blast Destination vectors.
Verify all constructs by DNA sequencing.
PCR amplify the ecto-tagged β1 expression construct (generated as described in Section 3.1) with primers designed according to the Gateway Cloning manufacturer’s instructions to introduce flanking attB1 and attB2 recombination sites – primers “attB1 β1 integrin For” and “attB2 β1 integrin Rev” (Table 1).
Generate pENTRY vectors by Gateway BP recombination with the PCR product and pDONR221 and verify all constructs by DNA sequencing.
Generate final pLENTI-ecto-tagged β1 integrin lentivirus expression vectors by Gateway LR recombination of the pENTRY vector with pLENTI-CMV-Puro or pLENTI-PGK-Blast Destination vectors and verify all constructs by digestion with restriction enzymes.
Plate HEK293T cells in 10 cm diameter dishes in complete growth medium without antibiotics
When HEK293T cells confluence reaches 50–70%, transfect with 4.5 μg pLENTI-ecto-tagged β1 integrin plasmid DNA along with lentivirus packaging plasmid DNA (4.5 μg pcPAX2 + 0.45 μg pMD2.G) and 28 μl PEI stock solution (1 mg/ml) in 500 μl DMEM
24h after HEK293T cell transfection, replace medium with complete growth medium
Harvest lentivirus-containing supernatant at 48h and 72h, pass through a 0.45 μm pore size Acrodisc filter and store at −80 °C.
3.2.2. Replacing endogenous β1 integrins with ecto-tagged β1 integrins in murine fibroblasts
For studies in murine fibroblasts, we use a clonal line of β1 integrin knockout fibroblasts (KO6) (see Note 2). Use of a clonal line ensures that all subsequent lines start from the same original population and minimize the chances of differences arising due to clonal variation. Prior to infection with lentivirus, puromycin and hygromycin kill curves were performed on KO6 cells to identify optimum concentrations needed for selection of transduced cells [46].
Culture β1 null fibroblast clone KO6 in complete growth medium.
Infect cells with lentivirus generated from pLENTI-CMV-puro-ecto-tagged β1 plasmids (Section 3.2.1) and 8 μg/ml polybrene. The dilution of the virus is adjusted according to the viral titer in order to achieve 50% surviving cells after selection with puromomycin.
24h after infection, select cells with 2 μg/ml puromycin and maintain cells in selection medium for 5 days.
When an independent label for focal adhesions is required, KO6 fibroblasts reconstituted with ecto-tagged β1 can be infected with lentiviral particles generated with pLENTI-CMV-Hygro-paxillin-mCherry. These particles are prepared using protocols similar to those in Section 3.2.1.
3.2.3. Replacing endogenous β1 integrins with ecto-tagged β1 integrins in human cell lines
To allow investigation of integrin traffic in cells other than mouse fibroblasts, we used stable lentiviral-mediated delivery of a previously validated shRNA targeting human β1 integrins to knock β1 down in various human cell lines. Similar approaches using CRISPR/Cas9-mediated knockout of β1 integrins could also be applied. The use of a puromycin-resistant pLKO.1 knockdown plasmid requires the use of a different selection marker on the ecto-tagged integrin construct – we have used pLENTI-PGK-Blast for these studies.
Culture HeLa cells or EA.hy926 in complete growth medium.
Infect cells with lentivirus generated from shRNA-encoding pLKO.1 plasmid and 8 μg/ml polybrene. These lentiviral particles are generated as in Section 3.2.1. The dilution of the virus is adjusted according to the viral titer in order to achieve less than 50% surviving cells after selection with puromycin.
24h after infection, select cells with 1 μg/ml puromycin and maintain cells in selection medium for 3 days.
Validate effective integrin knockdown by flow cytometry
Infect β1 integrin knockdown cells with lentivirus generated from pLENTI-PGK-Blast-ecto-tagged β1-itg plasmids (Section 3.2.1) and 8 μg/ml polybrene. The dilution of the virus is adjusted according to the viral titer in order to achieve less than 50% surviving cells after selection with blasticidin.
24h after infection, select cells with blasticidin (5 μg/ml for EA.hy926 and 4 μg/ml for HeLa) and maintain selection for 10 days.
3.3. Validation of ecto-tagged integrin function
As introduction of ecto-tags into integrin subunits has the potential to interfere with their expression, folding, heterodimerization, traffic, activation and ligand binding, it is important to validate the functionality of ecto-tagged integrins expressed in cells. The ecto-tagged β1 integrins described in this chapter have been extensively characterized [26] for their expression level and molecular mass, ability to pair with appropriate α subunits, express at the cell surface, localize to focal adhesions, bind soluble FN-9–10 fragments in an EDTA and manganese-sensitive manner, and, importantly, rescue the adhesion defect of β1-null fibroblasts. We note that the differing lengths of spacers used (Fig. 1) had no consistent impact on integrin function and both spacers are effective. Similar validation of any newly generated ecto-tagged integrins, including potential optimization of linker length, will be required prior to their use in trafficking assays. Detailed protocols for classical assays of integrin function are not provided here but are available elsewhere [47–51].
3.4. Imaging exocytosis of ecto-tagged integrins
The availability of cells expressing ecto-tagged integrins enables a variety of experiments to examine integrin exocytosis. However, challenges to imaging exocytosis include visualization of the relatively small changes in total local surface levels that occur in response to exocytic vesicle fusion, especially when in the context of relatively high integrin surface levels. The rapid dynamics of the pH sensitivity of pHluorin fluorescence [52] or the selective, irreversible and environment specific labeling of surface-exposed Halo-tagged integrins provided by cell impermeant Halo-tags [31,53] can be used to overcome these problems as described below.
3.4.1. Imaging exocytosis of pHluorin-β1 integrins by live TIRFM
Imaging exocytosis of ecto-pHluorin tagged β1 integrins takes advantage of the pHluorin fluorescence pH-sensitivity [52]. The pHluorin fluoresces poorly in intracellular vesicles due to their low internal pH but brightly after exposure to higher pH once these vesicles fuse with the plasma membrane. Thus, fusion events generate rapid localized increases in fluorescence that exhibit characteristic kinetics as the contents diffuse in the plasma membrane or the extracellular medium (Fig 2), and this phenomenon has allowed analysis of exocytosis of a range of pHluorin-tagged proteins [52,54,55]. To apply this approach to integrins we use stable lines expressing β1 integrin carrying an ecto-pHluorin (Section 3.2). Cells co-expressing the focal adhesion marker paxillin-mCherry can also be used as this enables ready identification of adhesions. Cells are imaged by live cell Total Internal Reflection Fluorescence Microscopy (TIRFM) after photobleaching of the surface β1 integrin pHluorin fluorescence to allow visualization of new vesicle fusion. Images are collected at high temporal resolution to reveal the transient fusion events and diffusion of the β1 integrin pHluorin cargo. Further analysis of recordings using custom MatLab algorithms [26] allows localization of fusion events on images (Fig 2) and shows that, at least in these murine fibroblasts, exocytosis occurs in proximity to focal adhesions, in agreement with our previously published study [26].
Plate cells expressing ecto-PH-β1itg and paxillin-mCherry in a glass bottomed FN-coated MatTek dish in complete imaging medium and culture until cells are fully spread.
Transfer dish to Okolab chamber-fitted Nikon Ti2 microscope for imaging at 37 °C and 5% CO2 atmosphere.
Image ecto-PH-β1itg and paxillin-mCherry by 2 color TIRFM with 488 nm and 568 nm lasers
Photobleach the ecto-PH-β1itg signal with the 488 nm TIRF laser set at 100% intensity for 10–30 s.
Immediately image ecto-PH-β1itg with the 488 nm TIRF laser set at 25% laser intensity at 5 frames/s for 3–5 min. Exocytosis events are visible as fluorescent spots appearing suddenly, reaching maximum intensity within 0.2 s and decaying more slowly (within 1–3 s) with a spreading of the fluorescence as the cargo diffuses into the plasma membrane. We used custom MatLab algorithms to detect and validate fusion events and for mapping their coordinates onto a focal adhesion map [26].
Figure 2:

Imaging exocytosis of ecto-PH-β1itg
HeLa cells knocked down for endogenous β1 integrin and reconstituted with ecto-PH-β1 were imaged by TIRFM at 5 frames/sec. A. TIRFM image of ecto-PH-β1itg prior to photobleaching overlaid with MatLab-validated fusion events marked as red spots. Bar = 10μm. B. Gallery of a single ecto-PH-β1itg fusion event over time, Bar = 1μm. C. Temporal alignment of the 50 MatLab validated fusion events recorded in this HeLa cell in 5 minutes. Data is shown as mean +/− 95% confidence interval.
3.4.2. Imaging newly exocytosed ecto-Halo-β1 integrins
While direct imaging of ecto-pHluorin-β1 integrin exocytosis in individual cells is a powerful technique, it requires high-frequency imaging of cells and consequently individual cells cannot be imaged for extended periods. An alternative method that uses the inter-changeable chemical-genetic labeling technology of the HaloTag in ecto-Halo-β1 integrins allows assessment of the localization of β1 integrins exocytosed over a longer period of time. The HaloTag is a modified haloalkane dehalogenase that covalently binds synthetic small-molecule ligands which can be coupled to fluorophores or other affinity tags [31,53,56]. Membrane-permeant and -impermeant chloroalkane ligands tagged with fluorescent markers or functional groups (e.g., biotin) can be purchased from Promega. To identify newly exocytosed integrins first block all surface-exposed ecto-Halo-β1 integrins by incubating cells with saturating concentrations of the membrane impermeant PEG-biotin-Halo ligand (1 μM using protocol below). Then culture cells for various times (30 min in Fig 3) and reveal the localization of newly exocytosed, unblocked, ecto-Halo-β1 integrins with a fluorescent membrane impermeant HaloTag ligand (see Note 3 for discussion of optimization of signal over background). As shown in Fig 3B, staining with fluorescent membrane-impermeant Halo-tag immediately after blocking shows minimal cell surface labelling (although focal adhesions are intact) but after a 30 min incubation at 37°C newly exocytosed ecto-Halo-β1 integrins mostly colocalize with the focal adhesion marker paxillin (Fig 3C). These data are consistent with the idea that exocytosis of recycled β1 integrins preferentially occurs in the vicinity of focal adhesions (as suggested from our analysis of ecto-pHluorin-β1 integrin, Fig 2 [26]), or that exocytosed integrins are rapidly incorporated into focal adhesions.
Plate ecto-Halo-β1itg KO6 fibroblasts on FN-coated MatTek dishes in complete growth medium, culture until cells are fully spread
Block all surface Halo binding sites with 1 μM PEG-Biotin-CA (membrane impermeant) in serum free phenol red-free DMEM for 5 min at room temperature
Wash cells 3 times with cold PBS2+
Add Complete imaging medium and incubate at 37 °C for defined time period to allow for exocytosis to occur
Wash once with cold PBS2+
Chill cells on ice for 5 min
Label with a membrane impermeant fluorophore tagged HaloTag ligand (250 nM-1μM final) in complete imaging medium for 5 min at room temperature
Wash with complete imaging medium with HEPES for 10 min on ice, then with cold PBS2+ for 10 min on ice
Fix with 4% PFA for 15 min at room temperature
Wash once with PBS and image by TIRFM
Figure 3:
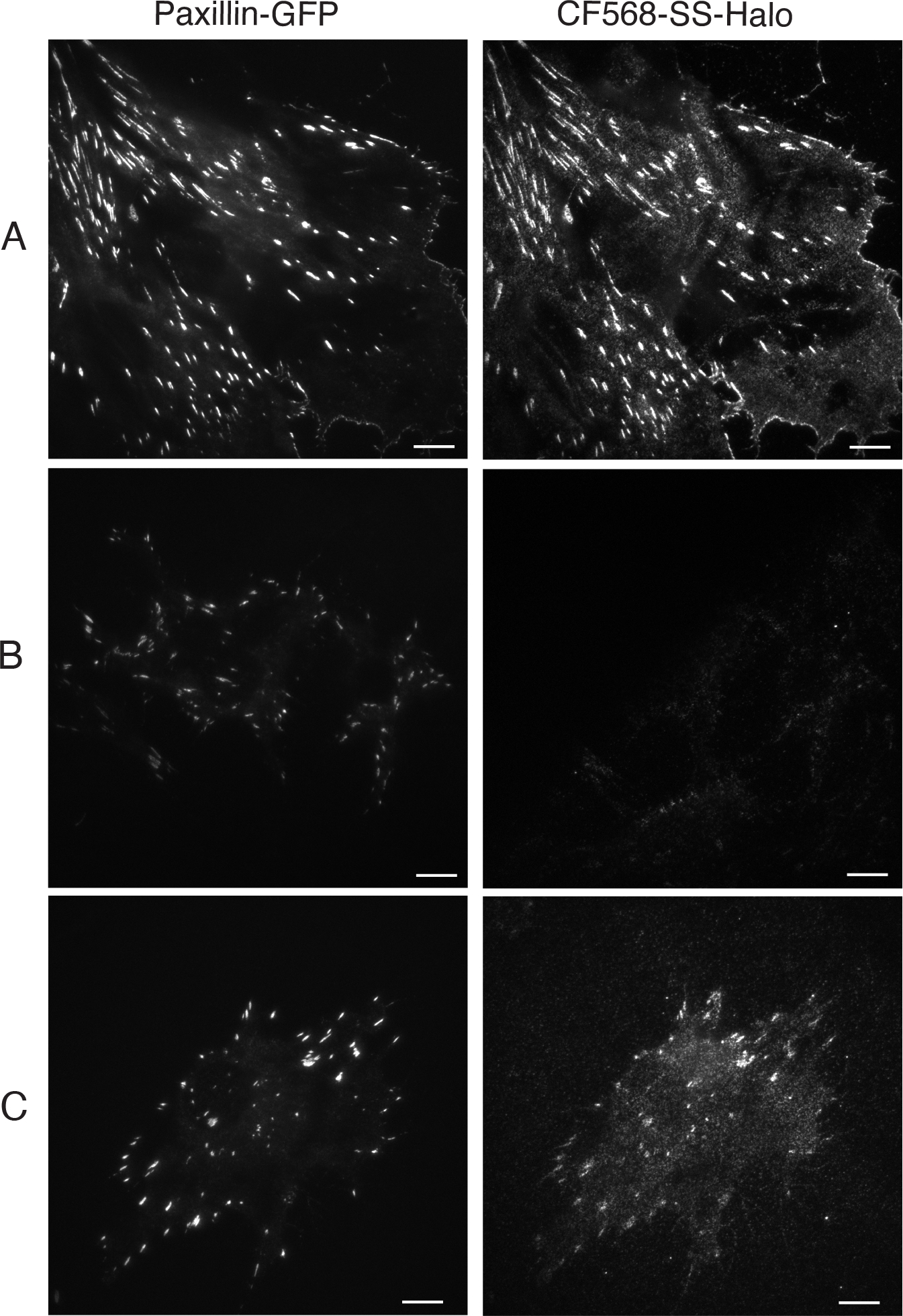
Imaging recent exocytosis using ecto-Halo-β1itg
TIRFM images of ecto-Halo-β1itg labeled with a membrane impermeant HaloTag ligand (here we used CF568-SS-CA, left) and paxillin-GFP (right) in reconstituted fibroblasts. A. Positive control: cells were labeled with CF568-SS-CA without prior block with PEG-biotin-CA. B. Negative control: cells were blocked with PEG-biotin-CA and immediately labeled with CF568-SS-CA. C. Cells were blocked with PEG-biotin-CA, incubated for 30 min at 37°C to allow for exocytosis to a occur and then labeled with CF568-SS-CA. Different cells are shown in two channels. Bar = 10 μm.
3.5. Using ecto-Halo-β1 integrins to investigate integrin endocytosis and recycling
Combining cell lines expressing ecto-Halo-β1 integrins with the diversity of HaloTag ligands (both commercially available and custom generated), sequential labeling protocols, fluorophore quenching and ligand cleavage, and advanced microscopy techniques allows for detailed investigation of integrin traffic. The exact protocol employed will depend on the experimental questions being asked but here we highlight three related methodologies to investigate the trafficking of β1 integrins.
3.5.1. Labeling surface and intracellular pools of ecto-Halo-β1 integrins with distinct Halo ligands and following exchange
Using a combination of membrane permeable and non-permeable HaloTag ligands, we have developed a straightforward method to selectively label intracellular and extracellular pools of β1 integrins with two distinct fluorophores and to then follow the exchange of these pools over time by live cell microscopy [26]. By first saturating labelling of the cell surface pool, we ensure that subsequent incubation with the membrane-permeant HaloTag ligand labels only intracellular ecto-Halo-β1 integrins as the cell surface population is already fully labeled with the original HaloTag ligand. Using this method, we have observed that, by 1 hour after labeling and for more than 20 hours thereafter, β1 integrins from both the originally intracellular labeled pool and the cell surface labeled pool are co-localized in focal adhesions, indicating that β1 integrins are long-lived receptors that undergo highly dynamic trafficking and recycling within the cell.
Plate ecto-Halo-β1itg KO6 fibroblasts on FN-coated MatTek in complete growth medium
Label extracellular ecto-Halo-β1itg with 250 nM AF488-CA (membrane impermeant Halo ligand) in complete imaging medium for 5 min at room temperature
Wash 3 times with PBS
Label intracellular ecto-Halo- β1itg with 250 nM SiR-CA (membrane permeant Halo ligand) in complete imaging medium for 5min at room temperature
Wash 3 times with PBS
Add complete imaging medium
Image exchange between the 2 pools of fluorescently labeled β1itg by live cell microscopy. This labelling method may be used with TIRFM or other approaches such as confocal microscopy.
3.5.2. Imaging endocytosis of ecto-Halo-β1 integrins using HILO-TIRF
In principle, the differential labelling approach from Section 3.5.1 could be used to visualize internalization of cell-surface β1 integrins. However, a major challenge is that the low level of internalization is easily obscured by the strong surface labelling, particularly if internalization takes place close to bright focal adhesions. To overcome this problem, we have combined highly inclined and laminated optical sheet (HILO) illumination with antibody quenching of surface fluorophores. Surface ecto-Halo-β1 integrins are first labeled with a membrane impermeant fluorescent HaloTag ligand and cells are cultured for various times to allow internalization. Labeled integrins at the surface and below can then be monitored by low angle HILO-TIRFM (see Note 4). Furthermore, upon addition of anti-Alexa Fluor 488 antibody to the imaging medium, the surface fluorescence is immediately quenched while labeled internalized ecto-Halo-β1 integrins are protected. Cells can then be fixed and immunolabeled with makers of trafficking compartments to identify the exact localization of internalized ecto-Halo-β1itg.
Plate ecto-Halo-β1itg KO6 fibroblasts on FN-coated MatTek in complete growth medium until they are fully spread
Label surface ecto-Halo-β1itg with 250 nM AF488-CA (membrane impermeant) for 5 min at room temperature
Wash 3 times with PBS
Add Complete imaging medium and image in Live TIRFM with HILO illumination every 30 s for 40 min
Add anti-Alexa Fluor 488 antibody (5 μg/ml final) to the culture medium to quench the surface fluorescence and keep imaging by Live TIRFM or fix samples if immunostaining is required. Ecto-Halo-β1integrins internalized during the 40 min incubation at 37°C are protected from the quenching by the antibody and can therefore be visualized by HILO TIRFM
3.5.3. Imaging the recycling of ecto-Halo-β1 integrins using reducible Halo ligands
A further development of the ecto-Halo-β1 integrin assays that does not rely on the quenching antibody involves the use of cleavable HaloTag ligands. With support from Promega, we obtained a novel HaloTag ligand containing a reducible disulfide bound connecting the CF568 fluorophore to the chloroalkane moiety. This allows us to remove surface labelling with a reducing agent such as TCEP, enabling development of methods to image the recycling of ecto-Halo-β1itg to the plasma membrane after internalization (Fig 4). To do so, ecto-Halo-β1itg fibroblasts are labelled with a membrane impermeant reducible fluorescent Halo ligand, cells are allowed to internalize labeled ectoHalo-β1itg for 30 min to 2h at 37°C (chase), the fluorophore is stripped from non-internalized ecto-Halo-β1itg with the reducing agent TCEP, and labeled ecto-Halo-β1itg recycling back to the plasma membrane is imaged by TIRFM. TIRF and Epifluorescence images taken at various stages of the procedure (Fig 4) show the effectiveness of the TCEP treatment at eliminating surface fluorescence, the internalization of labeled ecto-Halo-β1itg during the 30 min incubation at 37°C prior to stripping, and the recycling of labeled ecto-Halo-β1 back to the cell surface and to focal adhesions in particular.
Plate ecto-Halo-β1itg expressing cells in a FN-coated MatTek dish in complete medium until they are fully spread
Label surface ecto-Halo-β1itg with 2 μM CF568-SS-CA (membrane- impermeant Halo ligand) in complete imaging medium with Hepes for 5 min at room temperature (see Note 5).
Wash cells 4 times with PBS2+, add complete imaging medium and return cells to tissue culture incubator to allow for integrin internalization for 30 min to 2 hours (chase).
Freshly prepare stripping solution
Aspirate medium and incubate cells with stripping solution for 5 min at room temperature
Wash 4 times with PBS2+
Add Complete imaging medium and image the recycling of labeled ecto-Halo-β1itg back to the cell surface either by Live TIRM in presence of ProLong Live Reagent to reduce photobleaching, or after fixation with 4% PFA in PBS for 15 min at room temperature.
Figure 4:

Imaging the recycling of Ecto-Halo-β1itg
A. Structure of CF568-SS-CA, a membrane impermeant reducible fluorescent Halo ligand. B. TIRFM images of ecto-Halo-β1itg labeled with CF568-SS-CA and paxillin-mCherry in reconstituted fibroblasts at various steps of the labeling/stripping/chasing process. Different cells are shown in two channels. Bar = 10 μm.
4. NOTES
We anticipate that tagging other β integrin subunits at similar sites to those used for β1 integrins will also allow generation and imaging of functional integrin heterodimers using the approaches described in this chapter. Our preliminary unpublished data support this for β3 integrins. We also note that αv and αL integrins with GFP inserted into a loop in their extracellular β propeller domain have been reported [57,58]. These proteins are surface expressed, target to adhesions and support ligand binding, therefore insertion of Halo or pHluorin at these sites would presumably enable analysis of ecto-tagged α subunit trafficking using the approaches described here for β1 integrins.
We obtained immortalized β1 integrin floxed (β1 integrin fl/fl) and Cre-induced β1 integrin null (β1 integrin KO) fibroblasts from Antony Koleske (Yale University) [59] and confirmed them to be mycoplasma free using MycoAlert. β1 integrin KO cells were cloned by limited dilution and the absence of surface β1 integrin in the resulting clonal lines was verified by flow cytometry using a biotinylated anti-CD29 antibody. The line designated KO6 was chosen for our studies.
Obtaining a good signal/background ratio requires extensive washes and limiting labeling time. Extensive washes on ice after labeling are essential to reduce non-specific fluorescence. Limiting the duration of the Halo labeling steps to 5 min at room temperature is also very important to obtain a low signal in negative control cells (labeled straight after block) and to avoid blocking recycling integrins during the blocking step.
For HILO TIRFM, the collimated laser beam exits the aqueous solution above coverslip dish at an acute angle instead of forming an evanescent wave due to total internal reflection. This method allows deeper imaging than with TIRFM (e.g. 500 nm) yet provides a better signal/noise ratio than spinning disk confocal. The caveat is that it works best on the lower cell surface (i.e. ventral side of the cell).
Due to impact of CF568-SS-CA on pH, we use medium containing 25mM Hepes to prepare the labeling mixture.
Acknowledgments
We acknowledge Mark McDougall at Promega for providing the HaloTag ligands CF568-SS-CA and SiR-CA. We thank Emil B. Kromann (Technical University of Denmark) for writing the MatLab algorithms used to detect and validate fusion events. This work was supported by the National Institute of General Medical Sciences award R01 GM134148 to DAC and DT and Imaging Core DK045735.
References
- 1.Iwamoto DV, Calderwood DA (2015) Regulation of integrin-mediated adhesions. Curr Opin Cell Biol 36:41–47. doi: 10.1016/j.ceb.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chastney MR, Conway JRW, Ivaska J (2021) Integrin adhesion complexes. Current biology : CB 31 (10):R536–R542. doi: 10.1016/j.cub.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 3.Kadry YA, Calderwood DA (2020) Chapter 22: Structural and signaling functions of integrins. Biochim Biophys Acta Biomembr 1862 (5):183206. doi: 10.1016/j.bbamem.2020.183206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastney MR, Lawless C, Humphries JD, Warwood S, Jones MC, Knight D, Jorgensen C, Humphries MJ (2020) Topological features of integrin adhesion complexes revealed by multiplexed proximity biotinylation. J Cell Biol 219 (8). doi: 10.1083/jcb.202003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehrle-Haller B (2012) Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol 24 (5):569–581. doi: 10.1016/j.ceb.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 6.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468 (7323):580–584. doi:nature09621 [pii]; 10.1038/nature09621 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamir E, Geiger B (2001) Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci 114 (Pt 20):3583–3590 [DOI] [PubMed] [Google Scholar]
- 8.Bachmann M, Kukkurainen S, Hytonen VP, Wehrle-Haller B (2019) Cell Adhesion by Integrins. Physiol Rev 99 (4):1655–1699. doi: 10.1152/physrev.00036.2018 [DOI] [PubMed] [Google Scholar]
- 9.Shattil SJ, Kim C, Ginsberg MH (2010) The final steps of integrin activation: the end game. NatRevMolCell Biol 11 (4):288–300. doi:nrm2871 [pii]; 10.1038/nrm2871 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu G, Wang W, Luo BH (2012) Overview: structural biology of integrins. Methods Mol Biol 757:81–99. doi: 10.1007/978-1-61779-166-6_7 [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Zhu J, Springer TA (2013) Complete integrin headpiece opening in eight steps. J Cell Biol 201 (7):1053–1068. doi: 10.1083/jcb.201212037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolte MA, Nolte-’t Hoen ENM, Margadant C (2021) Integrins Control Vesicular Trafficking; New Tricks for Old Dogs. Trends Biochem Sci 46 (2):124–137. doi: 10.1016/j.tibs.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margadant C, Monsuur HN, Norman JC, Sonnenberg A (2011) Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 23 (5):607–614. doi: 10.1016/j.ceb.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 14.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J (2015) Integrin traffic - the update. J Cell Sci 128 (5):839–852. doi: 10.1242/jcs.161653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PV, von Thun A, Morton JP, Gourley C, Timpson P, Nixon C, McKay CJ, Carter R, Strachan D, Anderson K, Sansom OJ, Caswell PT, Norman JC (2012) Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell 22 (1):131–145. doi: 10.1016/j.devcel.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH (2009) Mutant p53 drives invasion by promoting integrin recycling. Cell 139 (7):1327–1341. doi: 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 17.Rainero E, Norman JC (2013) Late endosomal and lysosomal trafficking during integrin-mediated cell migration and invasion: cell matrix receptors are trafficked through the late endosomal pathway in a way that dictates how cells migrate. Bioessays 35 (6):523–532. doi: 10.1002/bies.201200160 [DOI] [PubMed] [Google Scholar]
- 18.Calderwood DA, Campbell ID, Critchley DR (2013) Talin and kindlins: partners in integrin-mediated adhesion. Nat RevMol Cell Biol In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG (2009) Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 187 (5):733–747. doi: 10.1083/jcb.200904054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezratty EJ, Partridge MA, Gundersen GG (2005) Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7 (6):581–590. doi: 10.1038/ncb1262 [DOI] [PubMed] [Google Scholar]
- 21.Caswell PT, Vadrevu S, Norman JC (2009) Integrins: masters and slaves of endocytic transport. NatRevMolCell Biol 10 (12):843–853. doi:nrm2799 [pii]; 10.1038/nrm2799 [doi] [DOI] [PubMed] [Google Scholar]
- 22.Kharitidi D, Apaja PM, Manteghi S, Suzuki K, Malitskaya E, Roldan A, Gingras MC, Takagi J, Lukacs GL, Pause A (2015) Interplay of Endosomal pH and Ligand Occupancy in Integrin alpha5beta1 Ubiquitination, Endocytic Sorting, and Cell Migration. Cell Rep 13 (3):599–609. doi: 10.1016/j.celrep.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 23.Bridgewater RE, Norman JC, Caswell PT (2012) Integrin trafficking at a glance. J Cell Sci 125 (Pt 16):3695–3701. doi: 10.1242/jcs.095810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Franceschi N, Arjonen A, Elkhatib N, Denessiouk K, Wrobel AG, Wilson TA, Pouwels J, Montagnac G, Owen DJ, Ivaska J (2016) Selective integrin endocytosis is driven by interactions between the integrin alpha-chain and AP2. Nat Struct Mol Biol 23 (2):172–179. doi: 10.1038/nsmb.3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouvard D, Pouwels J, De Franceschi N, Ivaska J (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nature reviews Molecular cell biology 14 (7):430–442. doi: 10.1038/nrm3599 [DOI] [PubMed] [Google Scholar]
- 26.Huet-Calderwood C, Rivera-Molina F, Iwamoto DV, Kromann EB, Toomre D, Calderwood DA (2017) Novel ecto-tagged integrins reveal their trafficking in live cells. Nat Commun 8 (1):570. doi: 10.1038/s41467-017-00646-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laukaitis CM, Webb DJ, Donais K, Horwitz AF (2001) Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol 153 (7):1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons M, Messent AJ, Humphries JD, Deakin NO, Humphries MJ (2008) Quantification of integrin receptor agonism by fluorescence lifetime imaging. J Cell Sci 121 (Pt 3):265–271. doi: 10.1242/jcs.018440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plancon S, Morel-Kopp MC, Schaffner-Reckinger E, Chen P, Kieffer N (2001) Green fluorescent protein (GFP) tagged to the cytoplasmic tail of alphaIIb or beta3 allows the expression of a fully functional integrin alphaIIb(beta3): effect of beta3GFP on alphaIIb(beta3) ligand binding. Biochem J 357 (Pt 2):529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrle-Haller B (2007) Analysis of integrin dynamics by fluorescence recovery after photobleaching. Methods Mol Biol 370:173–202. doi: 10.1007/978-1-59745-353-0_13 [DOI] [PubMed] [Google Scholar]
- 31.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV (2008) HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3 (6):373–382. doi: 10.1021/cb800025k [DOI] [PubMed] [Google Scholar]
- 32.Kukic I, Rivera-Molina F, Toomre D (2016) The IN/OUT assay: a new tool to study ciliogenesis. Cilia 5:23. doi: 10.1186/s13630-016-0044-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD (2009) A versatile viral system for expression and depletion of proteins in mammalian cells. PloS one 4 (8):e6529. doi: 10.1371/journal.pone.0006529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kechagia JZ, Ivaska J, Roca-Cusachs P (2019) Integrins as biomechanical sensors of the microenvironment. Nature reviews Molecular cell biology 20 (8):457–473. doi: 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 35.Nagae M, Re S, Mihara E, Nogi T, Sugita Y, Takagi J (2012) Crystal structure of alpha5beta1 integrin ectodomain: atomic details of the fibronectin receptor. J Cell Biol 197 (1):131–140. doi: 10.1083/jcb.201111077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA (2001) Crystal structure of the extracellular segment of integrin aVb3. Science 294 (5541):339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA (2002) Crystal structure of the extracellular segment of integrin aVb3 in complex with an Arg-Gly-Asp ligand. Science 296 (5565):151–155 [DOI] [PubMed] [Google Scholar]
- 38.Xiong JP, Mahalingham B, Alonso JL, Borrelli LA, Rui X, Anand S, Hyman BT, Rysiok T, Muller-Pompalla D, Goodman SL, Arnaout MA (2009) Crystal structure of the complete integrin alphaVbeta3 ectodomain plus an alpha/beta transmembrane fragment. J Cell Biol 186 (4):589–600. doi: 10.1083/jcb.200905085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong X, Mi LZ, Zhu J, Wang W, Hu P, Luo BH, Springer TA (2012) alpha(V)beta(3) integrin crystal structures and their functional implications. Biochemistry 51 (44):8814–8828. doi: 10.1021/bi300734n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA (2004) Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432 (7013):59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Springer TA, Zhu J, Xiao T (2008) Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J Cell Biol 182 (4):791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32 (6):849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Choi WS, McCoy JG, Negri A, Zhu J, Naini S, Li J, Shen M, Huang W, Bougie D, Rasmussen M, Aster R, Thomas CJ, Filizola M, Springer TA, Coller BS (2012) Structure-guided design of a high-affinity platelet integrin alphaIIbbeta3 receptor antagonist that disrupts Mg(2)(+) binding to the MIDAS. Sci Transl Med 4 (125):125ra132. doi: 10.1126/scitranslmed.3003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Zhu J, Negri A, Provasi D, Filizola M, Coller BS, Springer TA (2010) Closed headpiece of integrin alphaIIbbeta3 and its complex with an alphaIIbbeta3-specific antagonist that does not induce opening. Blood 116 (23):5050–5059. doi: 10.1182/blood-2010-04-281154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soto-Ribeiro M, Kastberger B, Bachmann M, Azizi L, Fouad K, Jacquier MC, Boettiger D, Bouvard D, Bastmeyer M, Hytonen VP, Wehrle-Haller B (2019) beta1D integrin splice variant stabilizes integrin dynamics and reduces integrin signaling by limiting paxillin recruitment. J Cell Sci 132 (8). doi: 10.1242/jcs.224493 [DOI] [PubMed] [Google Scholar]
- 46.Delrue I, Pan Q, Baczmanska AK, Callens BW, Verdoodt LLM (2018) Determination of the Selection Capacity of Antibiotics for Gene Selection. Biotechnol J 13 (8):e1700747. doi: 10.1002/biot.201700747 [DOI] [PubMed] [Google Scholar]
- 47.Humphries MJ (2009) Cell adhesion assays. Methods Mol Biol 522:203–210. doi: 10.1007/978-1-59745-413-1_14 [DOI] [PubMed] [Google Scholar]
- 48.Bouaouina M, Harburger DS, Calderwood DA (2012) Talin and signaling through integrins. Methods MolBiol 757:325–347. doi: 10.1007/978-1-61779-166-6_20 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von der Mark K, Schober S, Goodman SL (1999) Integrins in cell migration. Methods Mol Biol 129:219–230. doi: 10.1385/1-59259-249-X:219 [DOI] [PubMed] [Google Scholar]
- 50.Schneller M, Arap W, Pasqualini R (1999) Immunoblotting of integrins. Methods Mol Biol 129:63–78. doi: 10.1385/1-59259-249-X:63 [DOI] [PubMed] [Google Scholar]
- 51.Rosfjord EC, Dickson RB (1999) Application of flow cytometry in the analysis and sterile sorting of cell populations based on integrin expression. Methods Mol Biol 129:79–90. doi: 10.1385/1-59259-249-X:79 [DOI] [PubMed] [Google Scholar]
- 52.Miesenböck G, De Angelis D, Rothman J (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394 (6689):192–195. doi: 10.1038/28190 [DOI] [PubMed] [Google Scholar]
- 53.Los GV, Wood K (2007) The HaloTag: a novel technology for cell imaging and protein analysis. Methods Mol Biol 356:195–208 [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Rubin BR, Orme CM, Karpikov A, Yu C, Bogan JS, Toomre DK (2011) Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J Cell Biol 193 (4):643–653. doi:jcb.201008135 [pii] 10.1083/jcb.201008135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivera-Molina F, Toomre D (2013) Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J Cell Biol 201 (5):673–680. doi: 10.1083/jcb.201212103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bottanelli F, Kromann EB, Allgeyer ES, Erdmann RS, Wood Baguley S, Sirinakis G, Schepartz A, Baddeley D, Toomre DK, Rothman JE, Bewersdorf J (2016) Two-colour live-cell nanoscale imaging of intracellular targets. Nat Commun 7:10778. doi: 10.1038/ncomms10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordenfelt P, Moore TI, Mehta SB, Kalappurakkal JM, Swaminathan V, Koga N, Lambert TJ, Baker D, Waters JC, Oldenbourg R, Tani T, Mayor S, Waterman CM, Springer TA (2017) Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat Commun 8 (1):2047. doi: 10.1038/s41467-017-01848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swaminathan V, Kalappurakkal JM, Mehta SB, Nordenfelt P, Moore TI, Koga N, Baker DA, Oldenbourg R, Tani T, Mayor S, Springer TA, Waterman CM (2017) Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc Natl Acad Sci U S A 114 (40):10648–10653. doi: 10.1073/pnas.1701136114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson MA, Bradley WD, Harburger D, Parsons M, Calderwood DA, Koleske AJ (2015) Direct interactions with the integrin beta1 cytoplasmic tail activate the Abl2/Arg kinase. J Biol Chem 290 (13):8360–8372. doi: 10.1074/jbc.M115.638874 [DOI] [PMC free article] [PubMed] [Google Scholar]


