Abstract
A tetrahedral FeII4L4 cage assembled from the coordination of triangular chiral, face-capping ligands to iron(II). This cage exists as two diastereomers in solution, which differ in the stereochemistry of their metal vertices, but share the same point chirality of the ligand. The equilibrium between these cage diastereomers was subtly perturbed by guest binding. This perturbation from equilibrium correlated with the size and shape fit of the guest within the host; insight as to the interplay between stereochemistry and fit was provided by atomistic well-tempered metadynamics simulations. The understanding thus gained as to the stereochemical impact on guest binding enabled the design of a straightforward process for the resolution of the enantiomers of a racemic guest.
Introduction
Enzymes possess chirotopic cavities, enabling stereoselective recognition of target substrates and stereospecific chemical reactions.1−3 Enantiopure metal–organic cages with enclosed cavities,4 constructed by coordination-driven self-assembly, are able to mimic the functions of enzymes and have found uses across diverse areas, including stereoselective sensing, separation,5 and catalysis.6,7 Studies on the communication of stereochemistry within cages also help to elucidate the flow of stereochemical information in both artificial and living systems and may lead to the discovery of bioinspired applications.8
The stereochemistry of metal–organic cages can be influenced by enantiopure counterions and guests, through templation during cage formation or postassembly resolution of racemic cage mixtures.4,9 More frequently, enantiopure components, i.e., ligands and metal complexes, are used to control the stereochemistry of self-assembled structures, whereby the resulting metal–organic cages are enantiopure.4,10 In cases where the metal ions, particularly those from the d-block or f-block, have octahedral11 or pseudotricapped trigonal prismatic geometry,12 stereochemical information from the ligands can transfer to the metal vertices to produce either a preferred Δ or Λ handedness during higher-order self-assembly. Based upon this strategy, examples of the diastereoselective formation of homochiral cages, with precise control of the handedness of both metal vertices and the final assembled structure, have been reported.4,11,12
Metal–organic cages with electron-deficient walls have displayed extensive host–guest properties, binding electron-rich and even electron-poor guests with high affinities.13 We therefore envisioned that the incorporation of an electron-poor ligand into a chiral cage framework might optimize binding ability,14 resulting in the discovery of self-assembled cages with new potential applications.15 Herein, we describe the self-assembly of an electron-deficient enantiopure ligand with FeII to afford an FeII4L4 tetrahedron existing as a pair of distinct diastereomers, adopting either an all Δ or Λ configuration of metal centers, with moderate diastereocontrol. The ratio of the Δ4 to Λ4 configurations was then subtly modulated and even inverted by the encapsulation of guests. The diastereoenriched cage enabled the selective encapsulation of functionalized fullerenes from mixtures and enantioselective separation of racemic cryptophane-A (CRY-A).
Results and Discussion
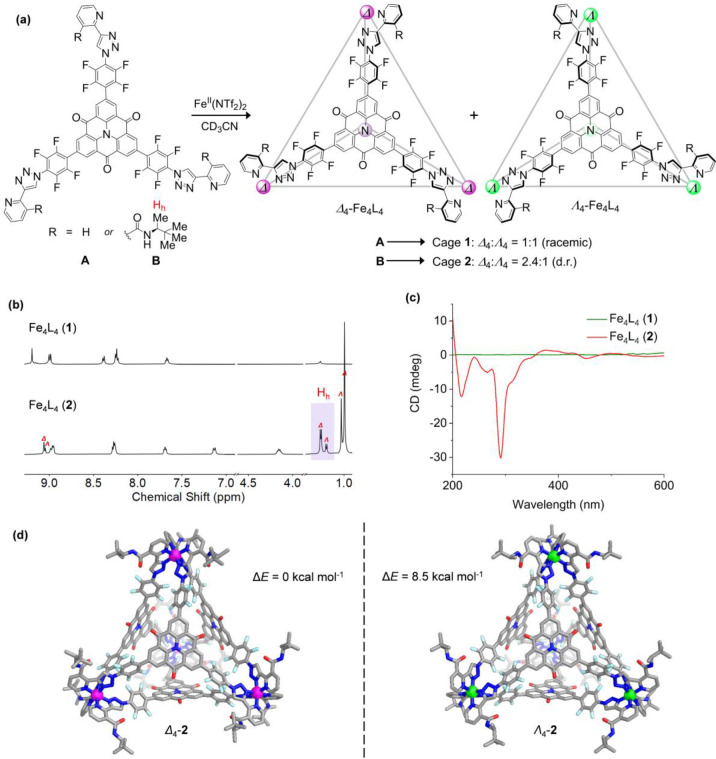
Tritopic ligand A with pyridyl-triazole “click” chelates14,16 was synthesized from iodinated N-heterotriangulene over three steps (Figure S1). The carbonyl groups and perfluorophenyl rings ensure its electron-deficient nature. The self-assembly of A (4 equiv) with iron(II) bis(trifluoromethanesulfonyl)imide (Fe(NTf2)2, 4 equiv) in acetonitrile at 343 K gave rise to cage 1 (Figure 1a). Its FeII4L4 composition, as anticipated following the foundational work of Lusby,16 was confirmed by electrospray ionization mass spectrometry (ESI-MS, Figure S21). One set of proton signals in the 1H NMR spectrum of 1 indicated the exclusive formation of a T-symmetric framework, with each octahedral tris-chelate metal vertex displaying the same handedness (Figure 1b). The absence of Cotton effects in the circular dichroism (CD) spectrum was consistent with the formation of a racemic mixture of Δ4-1 and Λ4-1 in solution (Figure 1c).
Figure 1.
(a) Self-assembly of cages 1 and 2 from ligands A and B, respectively. (b) Partial 1H NMR spectra of cages 1 and 2, with Hh used to gauge the d.r. of 2 (500 MHz, CD3CN, 298 K). (c) CD spectra of cages 1 and 2. (d) Front view of the DFT-optimized molecular models of Δ4-2 and Λ4-2, with ΔE representing the difference in total energy between the two diastereomers at 298 K as estimated by molecular dynamics.
We hypothesized that the stereochemistry of such FeII4L4 cages might be controlled by using an enantiopure ligand, which could dictate the configuration of the iron centers. Chiral ligand B, having the same ligand core as A, was therefore prepared, with each arm bearing an amide-containing chiral directing group (Figure S7). The stereocenter-containing side chain was incorporated at the 3-position of the pyridyl ring to secure its proximity to the metal vertex. Such a design should also avoid steric clash within the coordination environment around the metal centers that would be induced by substituents at the pyridyl 6-position, which might destabilize assembled structures.17
Ligand B underwent self-assembly with Fe(NTf2)2 to produce FeII4L4 cage 2 (Figure 1a), as confirmed by ESI-MS (Figure S30). The 1H NMR spectrum of cage 2 shows two sets of proton signals, consistent with the formation of Δ4-2 and Λ4-2 as diastereomeric complexes. The well-separated signals of Hh allowed determination of a diastereomeric ratio (d.r.) of 2.4:1 (Figure 1b). The same diffusion coefficient was observed for all peaks in the diffusion-ordered spectroscopy (DOSY) spectrum, indicating similar hydrodynamic radii for both diastereomers (Figure S25). Other N-heterotriangulene-based chiral ligands bearing modified chiral directing groups were also employed in the self-assembly process; however, lower diastereomeric ratios were observed in all cases compared to the present ratio of 2.4:1 (Figures S34 and S35).
The CD spectrum of cage 2 displayed intense negative signals around 240–340 nm, corresponding to high-energy π–π* transitions in the ligands, while metal-to-ligand charge transfer (MLCT) and d–d transitions produced weaker signals from 360 to 540 nm (Figure 1c). The Cotton effects observed in the CD spectrum are correlated with the handedness of the octahedral tris-chelate iron vertices. Comparison of the CD spectra of structurally similar Δ- and Λ-[Fe(bpy)3]2+ complexes, particularly peaks resulting from π–π* and MLCT transitions, allowed us to infer there is an excess of iron centers having Δ configuration within cage 2.18,19 The major diastereomer of 2 was thus determined to be Δ4-2, and the minor diastereomer was Λ4-2.
After many unsuccessful attempts to grow crystals suitable for X-ray diffraction, we undertook density functional theory (DFT) calculations to obtain the energy-minimized molecular models of Δ4-2 and Λ4-2 (Figure 1d; for details, see Supporting Information Section 10).20 In accordance with previous observations of face-capped M4L4 tetrahedral cages,5i,9h Δ4-2 adopts a clockwise orientation of its four ligand faces, while Λ4-2 is paired with ligands of anticlockwise orientation. The FeII···FeII distances in both diastereomers are similar (ca. 23 Å). The calculated cavity volumes are only slightly different: 1281 Å3 for Δ4-2 and 1266 Å3 for Λ4-2 (Figure S107).21
In control experiments, an FeIIL3 complex was formed by the reaction of Fe(NTf2)2 with a monomeric pyridyl-triazole ligand bearing the same chiral side chain (Figure S36). Very weak signals observed in the CD spectrum indicated a weaker chiral induction effect in this mononuclear complex relative to that of the tetranuclear cage (Figure S38). These results reflect that diastereoselectivity during the formation of 2 emerges as a result of higher-order assembly, in which the stereochemical information transfer from ligand to metal vertex and stereochemical communication between metal centers may cooperatively play a role in amplifying the energy differences between the two diastereomers.22
The relative energy differences (ΔE) between Δ4-2 and Λ4-2 was gauged to be 8.5 kcal mol–1 by performing molecular dynamics simulations on a model of cage 2 in explicit acetonitrile at 298 K performed using the GROMACS software package patched with plumed23 (model description and simulation setup in Supporting Information Section 10).24 These calculations supported the conclusion that Δ4-2 is more favored from an enthalpic point of view, as this difference mainly arises from the difference in potential energy.
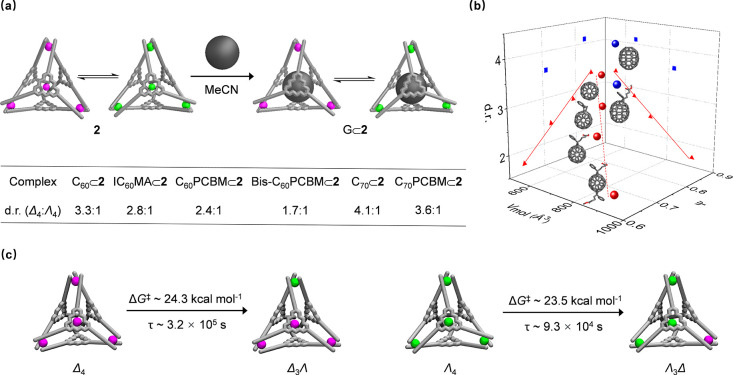
Both diastereomers of 2 have flat ligand cores and enclosed cavities, thus rendering 2 a good prospective host for large π-extended guests. We therefore began to investigate the host–guest properties of 2 with fullerenes and fullerene derivatives (Figure 2a). Heating an equimolar mixture of guest and 2 in acetonitrile at 343 K for 30 min resulted in the quantitative formation of the 1:1 host–guest complexes G⊂2, as confirmed by 1H NMR, 19F NMR, and ESI-MS spectra for all investigated guests (Supporting Information, Section 6.1). The major contributions to binding were inferred to be extensive stacking interactions between host and guest, as well as solvophobic effects in acetonitrile. The insolubility of these π-extended guests in acetonitrile prevented quantification of binding strength through 1H NMR titration experiments.
Figure 2.
(a) Schematic and table showing guest-binding-induced Δ4 ⇄ Λ4 interconversion, with d.r. determined by 1H NMR. (b) Diastereomeric ratio of the host–guest complex plotted against the molecular volume (Vmol) and sphericity (Ψ) of guests. PCBM = [6,6]-phenyl-Cn-butyric acid methyl ester (n = 61 or 71). IC60MA = indene-C60 monoadduct. Bis-C60PCBM exists as a mixture of regioisomers. C70PCBM exists as a mixture of regioisomers with about 85% α-C70PCBM. (c) Schematic showing conversion of one metal vertex within the framework of 2, with ΔG⧧ representing the calculated transition energy barrier and τ representing the characteristic transition timescale at 298 K.
1H NMR and CD spectra confirmed that both Δ4-2 and Λ4-2 were able to accommodate the guests, with G⊂Δ4-2 as the major species (Figures 2a and S69); Δ4⇄Λ4 interconversion was also observed during the binding process. We inferred that the size and shape fit between guest and cage change the energy differences between two configurations. To quantify this phenomenon, molecular volume (Vmol)21 was used to determine the size of the guest, while sphericity (Ψ)25 was employed to reflect the shape of the guest considering the near-spherical cavity of 2 (Table S2). The plot of d.r. against Vmol and Ψ revealed that binding a smaller and more spherical guest resulted in a stronger energetic preference for the Δ4 configuration, whereas binding larger and less spherical guests reduced energy differences between diastereomers (Figure 2b). Although the stereochemical effects of guests upon the host observed here were subtle, the model plotted in Figure 2b allowed quantification of guest-induced diastereomer interconversion for binding C60 and its adducts, according to the linear relationships between d.r. and Vmol and between d.r. and Ψ. However, two outlying points prevented good linear regression fits for binding C70 and C70PCBM ([6,6]-phenyl-C71-butyric acid methyl ester).
To obtain information related to the energy barriers for the interconversion between Δ4-2 and Λ4-2, we employed multiple infrequent well-tempered metadynamics (WT-MetaD) simulations.26 These biased simulations allowed us to activate the escape from the two local Δ and Λ minima and to obtain information on the associated barriers and characteristic timescales expected for these transitions in unbiased conditions. In particular, we activated the transition of one of the four metal vertices, exploring the Δ4 → Δ3Λ and Λ4 → Λ3Δ transitions (Figure 2c), which are first necessary steps in the Δ4 ⇄ Λ4 isomerization. Fifty infrequent WT-MetaD simulations were run for both transitions.
For the Δ4 → Δ3Λ transition, the infrequent WT-MetaD simulations provided a transition energy barrier ΔG⧧ ∼ 24.3 kcal mol–1 and a characteristic transition timescale τ ∼ 3.2 × 105 s, while the corresponding energy barrier and timescale for Λ4 → Λ3Δ were calculated to be ΔG⧧ ∼ 23.5 kcal mol–1 and τ ∼ 9.3 × 104 s, respectively. These results suggested that the dynamics of interconversion between Δ4 and Λ4 diastereomers are slow at room temperature. Similar energy barriers were obtained for the diastereomer transformations of C70⊂2. However, by rescaling the obtained transition timescales at 343 K (mixing condition), we could estimate that these transition events can occur within a timescale of minutes. This suggests that the Δ4 and Λ4 configurations dynamically equilibrate during the self-assembly process and reequilibrate during mixing with guest molecules.
We then investigated the ability of 2 to purify high-value fullerenes, starting with preparing a mixture consisting of equimolar amounts of C60, C60PCBM, bis-C60PCBM, and 2 in acetonitrile (Figure 3). Notably, after being kept at 343 K for 30 min, the host–guest complex bis-C60PCBM⊂2 was observed to form exclusively, as confirmed by ESI-MS and 1H NMR (Figures S89 and S90). Likewise, cage 2 was also able to selectively extract C70PCBM from a mixture with C70 (Figures S91 and S92). The efficient and selective encapsulation of bis-C60PCBM and C70PCBM by 2 may provide an alternative method for the purification of fullerene covalent adducts contaminated with numerous side-products from reaction mixtures.27 We attribute the excellent selectivity observed here to the higher solubility of these alkyl chain-substituted fullerenes in organic solvents.28
Figure 3.
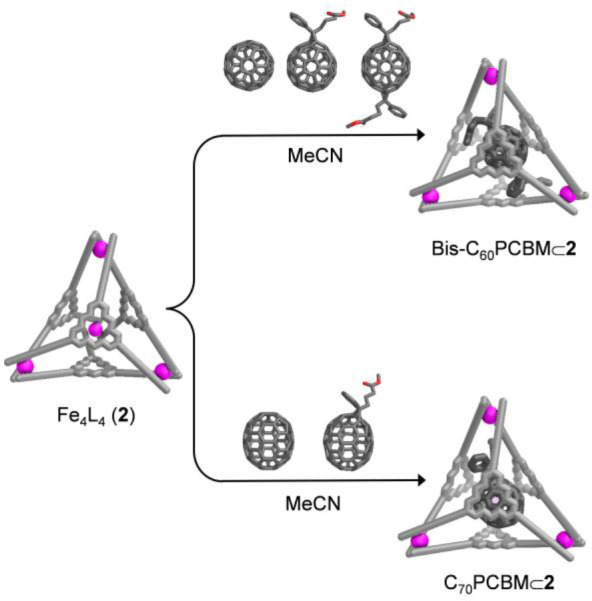
Schematic showing the selective encapsulation of bis-C60PCBM and C70PCBM.
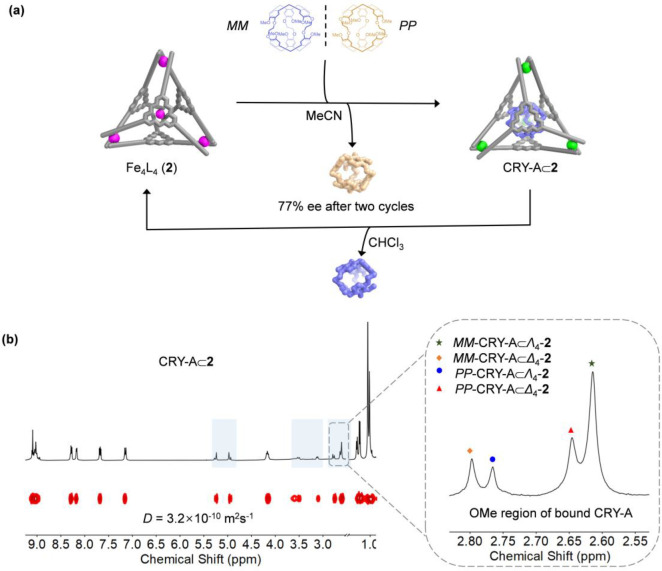
Electron-deficient 2 was also observed to bind electron-rich enantiopure cryptophane-A, which is an example of an important class of organic supramolecular host.29 The host–guest adduct CRY-A⊂2 was formed upon heating equimolar amounts of CRY-A and 2 in acetonitrile at 343 K for 30 min (Figure 4). PP-CRY-A⊂2 (Δ4:Λ4 = 2.4:1) retained the stereochemical configuration of the parent cage 2 (Δ4:Λ4 = 2.4:1), whereas the encapsulation of MM-CRY-A occurred with inversion of host stereochemistry, providing MM-CRY-A⊂2 in a d.r. of Δ4:Λ4 = 1:2.6. Opposite Cotton effects observed in the CD spectra of both host–guest complexes also confirmed such stereochemical outcomes upon encapsulation of enantiopure CRY-A (Figure S94). The Δ4 configuration was thus favored by PP-CRY-A, whereas the Λ4 configuration was preferred by MM-CRY-A. The inversion of the stereochemistry of 2 induced by MM-CRY-A reflected that host 2 can dynamically adapt its stereochemistry and chiral inner void to maximize binding affinity for a chiral guest.
Figure 4.
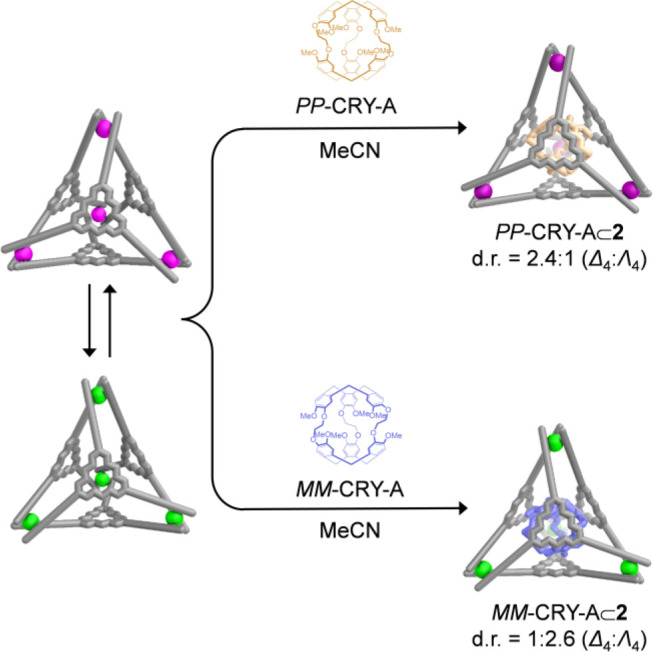
Schematic showing the stereochemical communication between 2 and CRY-A.
We next explored the enantioselective separation of racemic guests by 2. We observed that 2 displayed no enantioselectivity in binding racemic C70PCBM, as confirmed by 1H NMR and CD spectra (Figures S65 and S105). Diastereoenriched 2 was nonetheless capable of enantioselectively separating racemic CRY-A (Figure 5a).
Figure 5.
(a) Schematic showing the enantioselective resolution of CRY-A by cage 2 in acetonitrile and the recycling of 2 in chloroform. (b) Partial 1H NMR spectrum of CRY-A⊂2 obtained in the first round of the resolution procedure, with the peaks for the encapsulated guest highlighted with a light blue background, showing an expansion of the OMe region of bound CRY-A (400 MHz, CD3CN, 298 K).
Two equivalents of racemic CRY-A were added to an acetonitrile solution of 2, and the reaction mixture was maintained at 343 K for 30 min. The host–guest complex CRY-A⊂2 was isolated by precipitation with diethyl ether; evaporating the excess diethyl ether subsequently afforded unbound CRY-A. All signals in the 1H NMR spectrum of CRY-A⊂2 had the same diffusion coefficient, with proton signals corresponding to bound CRY-A shifted upfield due to host shielding effects (Figure 5b). Four sets of signals from the methoxy groups of CRY-A were observed in the 2.55–2.83 ppm region, indicating that CRY-A⊂2 consists of four diastereomers, PP-CRY-A⊂Δ4-2, PP-CRY-A⊂Λ4-2, MM-CRY-A⊂Δ4-2, and MM-CRY-A⊂Λ4-2. Comparison with the 1H NMR spectra for PP-CRY-A⊂2 and MM-CRY-A⊂2 allowed us to identify each diastereomer in solution (Figure S93).
The 1H NMR spectrum clearly showed that more MM-CRY-A was encapsulated when two equivalents of racemic guest were used. Cotton effects assigned to MM-CRY-A were also observed in the CD spectrum of CRY-A⊂2 (Figure S94). The enantiomeric excess (ee) of the unbound CRY-A was determined to be 32% by chiral HPLC, with PP-CRY-A being enriched (Figure S96). The bound CRY-A was released by sonicating a suspension of the host–guest complex in chloroform (Figure S102), and the solid 2 was then recovered by centrifugation (Figures S103 and S104).
In control experiments, PP-CRY-A was observed to be encapsulated kinetically faster than MM-CRY-A at the initial stage of the binding process, as PP-CRY-A is bound more strongly by the Δ4 configuration of 2 (Δ4:Λ4 = 2.4:1 for this guest). Heating the reaction mixture resulted in re-equilibration of the cage framework, eventually giving MM-CRY-A⊂Λ4-2 as the major host–guest complex, indicating that MM-CRY-A⊂Λ4-2 is the thermodynamically favored diastereomer within the four-diastereomer CRY-A⊂2 system (Figure S101). These results indicated that the enantioselectivity in binding racemic CRY-A by 2 is driven by the formation of the thermodynamically stable host–guest diastereomer, MM-CRY-A⊂Λ4-2.
Encouraged by the chiral resolution observed, we ran a second round of separation experiments, through addition of the CRY-A (32% ee) obtained from the first round to a fresh acetonitrile solution of 2. After precipitation of the host–guest adduct and removal of the solvent, the unbound CRY-A was obtained in 77% ee, as determined by chiral HPLC (Figure S98).
Conclusions
The moderately stereoselective self-assembly of FeII4L4 tetrahedron 2 thus can serve as the basis of an enantioseparation process, based upon a nuanced understanding of how stereochemistry influences guest fit within this host. Cage 2 was also capable of selectively extracting bis-C60PCBM and C70PCBM from mixtures of their derivatives. The strategy outlined herein may thus become applicable to the design of new cage-based purification methods, particularly stereoselective ones. Future work will focus on the immobilization of such N-heterotriangulene-based cages on solid supports, such as alumina, for the development of efficient large-scale separation and purification processes.30
Acknowledgments
This study was supported by the European Research Council (695009) and the UK Engineering and Physical Sciences Research Council (EPSRC, EP/T031603/1 and EP/P027067/1). W.X. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship. G.M.P. acknowledges funding received from the Swiss National Science Foundation (IZLIZ2_183336) and the European Research Council (818776). G.M.P. and L.P. also acknowledge the computational resources provided by the Swiss National Supercomputing Center (CSCS). We acknowledge the Department of Chemistry NMR facility, University of Cambridge, for performing some NMR experiments.
Data Availability Statement
Structure and parameters of the computational models used in atomistic molecular dynamics are available at 10.5281/zenodo.7350671 (ref (24)).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c00294.
Experimental procedures; NMR characterizations; mass spectrometry data; CD spectra; volume calculations; HPLC data; computational model parametrization; model simulation protocol (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Koshland D. E. Jr. The Key–Lock Theory and the Induced Fit Theory. Angew. Chem., Int. Ed. 1995, 33, 2375–2378. 10.1002/anie.199423751. [DOI] [Google Scholar]
- Bell E. L.; Finnigan W.; France S. P.; Green A. P.; Hayes M. A.; Hepworth L. J.; Lovelock S. L.; Niikura H.; Osuna S.; Romero E.; Ryan K. S.; Turner N. J.; Flitsch S. L. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. 10.1038/s43586-021-00044-z. [DOI] [Google Scholar]
- Mislow K.; Siegel J. Stereoisomerism and Local Chirality. J. Am. Chem. Soc. 1984, 106, 3319–3328. 10.1021/ja00323a043. [DOI] [Google Scholar]
- For comprehensive reviews on metal–organic cages, see:; a Seeber G.; Tiedemann B. E. F.; Raymond K. N. Supramolecular Chirality in Coordination Chemistry. Top Curr. Chem. 2006, 265, 147–183. 10.1007/128_033. [DOI] [Google Scholar]; b Chen L.-J.; Yang H.-B.; Shionoya M. Chiral Metallosupramolecular Architectures. Chem. Soc. Rev. 2017, 46, 2555–2576. 10.1039/C7CS00173H. [DOI] [PubMed] [Google Scholar]; c Pan M.; Wu K.; Zhang J.-H.; Su C.-Y. Chiral Metal–Organic Cages/Containers (MOCs): From Structural and Stereochemical Design to Applications. Coord. Chem. Rev. 2019, 378, 333–349. 10.1016/j.ccr.2017.10.031. [DOI] [Google Scholar]; d Gosselin A. J.; Rowland C. A.; Bloch E. D. Permanently Microporous Metal–Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. 10.1021/acs.chemrev.9b00803. [DOI] [PubMed] [Google Scholar]
- For selected examples on chiral sensing, recognition, and separation by cages, see:; a Fiedler D.; Leung D. H.; Bergman R. G.; Raymond K. N. Enantioselective Guest Binding and Dynamic Resolution of Cationic Ruthenium Complexes by a Chiral Metal–Ligand Assembly. J. Am. Chem. Soc. 2004, 126, 3674–3675. 10.1021/ja039225a. [DOI] [PubMed] [Google Scholar]; b Liu T.; Liu Y.; Xuan W.; Cui Y. Chiral Nanoscale Metal-Organic Tetrahedral Cages: Diastereoselective Self-Assembly and Enantioselective Separation. Angew. Chem., Int. Ed. 2010, 49, 4121–4124. 10.1002/anie.201000416. [DOI] [PubMed] [Google Scholar]; c Xuan W.; Zhang M.; Liu Y.; Chen Z.; Cui Y. A Chiral Quadruple-Stranded Helicate Cage for Enantioselective Recognition and Separation. J. Am. Chem. Soc. 2012, 134, 6904–6907. 10.1021/ja212132r. [DOI] [PubMed] [Google Scholar]; d Dong J.; Zhou Y.; Zhang F.; Cui Y. A Highly Fluorescent Metallosalalen-Based Chiral Cage for Enantioselective Recognition and Sensing. Chem.—Eur. J. 2014, 20, 6455–6461. 10.1002/chem.201304606. [DOI] [PubMed] [Google Scholar]; e Bolliger J. L.; Belenguer A. M.; Nitschke J. R. Enantiopure Water-Soluble [Fe4L6] Cages: Host–Guest Chemistry and Catalytic Activity. Angew. Chem., Int. Ed. 2013, 52, 7958–7962. 10.1002/anie.201302136. [DOI] [PubMed] [Google Scholar]; f Wu K.; Li K.; Hou Y.-J.; Pan M.; Zhang L.-Y.; Chen L.; Su C.-Y. Homochiral D4-Symmetric Metal–Organic Cages from Stereogenic Ru(II) Metalloligands for Effective Enantioseparation of Atropisomeric Molecules. Nat. Commun. 2016, 7, 10487. 10.1038/ncomms10487. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Schulte T. R.; Holstein J. J.; Clever G. H. Chiral Self-Discrimination and Guest Recognition in Helicene-Based Coordination Cages. Angew. Chem., Int. Ed. 2019, 58, 5562–5566. 10.1002/anie.201812926. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Howlader P.; Zangrando E.; Mukherjee P. S. Self-Assembly of Enantiopure Pd12 Tetrahedral Homochiral Nanocages with Tetrazole Linkers and Chiral Recognition. J. Am. Chem. Soc. 2020, 142, 9070–9078. 10.1021/jacs.0c03551. [DOI] [PubMed] [Google Scholar]; i Yang Y.; Ronson T. K.; Lu Z.; Zheng J.; Vanthuyne N.; Martinez A.; Nitschke J. R. A Curved Host and Second Guest Cooperatively Inhibit the Dynamic Motion of Corannulene. Nat. Commun. 2021, 12, 4079. 10.1038/s41467-021-24344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhu C.; Tang H.; Yang K.; Fang Y.; Wang K.-Y.; Xiao Z.; Wu X.; Li Y.; Powell J. A.; Zhou H.-C. Homochiral Dodecanuclear Lanthanide “Cage in Cage” for Enantioselective Separation. J. Am. Chem. Soc. 2021, 143, 12560–12566. 10.1021/jacs.1c03652. [DOI] [PubMed] [Google Scholar]
- For reviews on cage catalysis, see:; a Brown C. J.; Toste F. D.; Bergman R. G.; Raymond K. N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]; b Tan C.; Chu D.; Tang X.; Liu Y.; Xuan W.; Cui Y. Supramolecular Coordination Cages for Asymmetric Catalysis. Chem.—Eur. J. 2019, 25, 662–672. 10.1002/chem.201802817. [DOI] [PubMed] [Google Scholar]; c Wang K.; Jordan J. H.; Hu X.-Y.; Wang L. Supramolecular Strategies for Controlling Reactivity within Confined Nanospaces. Angew. Chem., Int. Ed. 2020, 59, 13712–13721. 10.1002/anie.202000045. [DOI] [PubMed] [Google Scholar]; d Morimoto M.; Bierschenk S. M.; Xia K. T.; Bergman R. G.; Raymond K. N.; Toste F. D. Advances in Supramolecular Host-Mediated Reactivity. Nat. Catal. 2020, 3, 969–984. 10.1038/s41929-020-00528-3. [DOI] [Google Scholar]; e Olivo G.; Capocasa G.; Del Giudice D.; Lanzalunga O.; Di Stefano S. New Horizons for Catalysis Disclosed by Supramolecular Chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. 10.1039/D1CS00175B. [DOI] [PubMed] [Google Scholar]
- For selected examples on enantioselective cage catalysis, see:; a Nishioka Y.; Yamaguchi T.; Kawano M.; Fujita M. Asymmetric [2 + 2] Olefin Cross Photoaddition in a Self-Assembled Host with Remote Chiral Auxiliaries. J. Am. Chem. Soc. 2008, 130, 8160–8161. 10.1021/ja802818t. [DOI] [PubMed] [Google Scholar]; b Brown C. J.; Bergman R. G.; Raymond K. N. Enantioselective Catalysis of the Aza-Cope Rearrangement by a Chiral Supramolecular Assembly. J. Am. Chem. Soc. 2009, 131, 17530–17531. 10.1021/ja906386w. [DOI] [PubMed] [Google Scholar]; c Gadzikwa T.; Bellini R.; Dekker H. L.; Reek J. N. H. Self-Assembly of a Confined Rhodium Catalyst for Asymmetric Hydroformylation of Unfunctionalized Internal Alkenes. J. Am. Chem. Soc. 2012, 134, 2860–2863. 10.1021/ja211455j. [DOI] [PubMed] [Google Scholar]; d Wang Z. J.; Clary K. N.; Bergman R. G.; Raymond K. N.; Toste F. D. A Supramolecular Approach to Combining Enzymatic and Transition Metal Catalysis. Nat. Chem. 2013, 5, 100–103. 10.1038/nchem.1531. [DOI] [PubMed] [Google Scholar]; e Zhao C.; Sun Q.-F.; Hart-Cooper W. M.; DiPasquale A. G.; Toste F. D.; Bergman R. G.; Raymond K. N. Chiral Amide Directed Assembly of a Diastereo- and Enantiopure Supramolecular Host and its Application to Enantioselective Catalysis of Neutral Substrates. J. Am. Chem. Soc. 2013, 135, 18802–18805. 10.1021/ja411631v. [DOI] [PubMed] [Google Scholar]; f García-Simón C.; Gramage-Doria R.; Raoufmoghaddam S.; Parella T.; Costas M.; Ribas X.; Reek J. N. H. Enantioselective Hydroformylation by a Rh-Catalyst Entrapped in a Supramolecular Metallocage. J. Am. Chem. Soc. 2015, 137, 2680–2687. 10.1021/ja512637k. [DOI] [PubMed] [Google Scholar]; g Ueda Y.; Ito H.; Fujita D.; Fujita M. Permeable Self-Assembled Molecular Containers for Catalyst Isolation Enabling Two-Step Cascade Reactions. J. Am. Chem. Soc. 2017, 139, 6090–6093. 10.1021/jacs.7b02745. [DOI] [PubMed] [Google Scholar]; h Guo J.; Xu Y.-W.; Li K.; Xiao L.-M.; Chen S.; Wu K.; Chen X.-D.; Fan Y.-Z.; Liu J.-M.; Su C.-Y. Regio- and Enantioselective Photodimerization within the Confined Space of a Homochiral Ruthenium/Palladium Heterometallic Coordination Cage. Angew. Chem., Int. Ed. 2017, 56, 3852–3856. 10.1002/anie.201611875. [DOI] [PubMed] [Google Scholar]; i Tan C.; Jiao J.; Li Z.; Liu Y.; Han X.; Cui Y. Design and Assembly of a Chiral Metallosalen-Based Octahedral Coordination Cage for Supramolecular Asymmetric Catalysis. Angew. Chem., Int. Ed. 2018, 57, 2085–2090. 10.1002/anie.201711310. [DOI] [PubMed] [Google Scholar]; j Jiao J.; Tan C.; Li Z.; Liu Y.; Han X.; Cui Y. Design and Assembly of Chiral Coordination Cages for Asymmetric Sequential Reactions. J. Am. Chem. Soc. 2018, 140, 2251–2259. 10.1021/jacs.7b11679. [DOI] [PubMed] [Google Scholar]; k Guo J.; Fan Y.-Z.; Lu Y.-L.; Zheng S.-P.; Su C.-Y. Visible-Light Photocatalysis of Asymmetric [2 + 2] Cycloaddition in Cage-Confined Nanospace Merging Chirality with Triplet-State Photosensitization. Angew. Chem., Int. Ed. 2020, 59, 8661–8669. 10.1002/anie.201916722. [DOI] [PubMed] [Google Scholar]
- a Zhou J.; Rao L.; Yu G.; Cook T. R.; Chen X.; Huang F. Supramolecular Cancer Nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. 10.1039/D0CS00011F. [DOI] [PubMed] [Google Scholar]; b Zhu H.; Li Q.; Zhu W.; Huang F. Pillararenes as Versatile Building Blocks for Fluorescent Materials. Acc. Mater. Res. 2022, 3, 658–668. 10.1021/accountsmr.2c00063. [DOI] [Google Scholar]
- a Terpin A. J.; Ziegler M.; Johnson D. W.; Raymond K. N. Resolution and Kinetic Stability of a Chiral Supramolecular Assembly Made of Labile Components. Angew. Chem., Int. Ed. 2001, 40, 157–160. . [DOI] [PubMed] [Google Scholar]; b Ikeda A.; Udzu H.; Zhong Z.; Shinkai S.; Sakamoto S.; Yamaguchi K. A Self-Assembled Homooxacalix[3]arene-based Dimeric Capsule Constructed by a PdII–Pyridine Interaction Which Shows a Novel Chiral Twisting Motion in Response to Guest Inclusion. J. Am. Chem. Soc. 2001, 123, 3872–3877. 10.1021/ja003269r. [DOI] [PubMed] [Google Scholar]; c Davis A. V.; Fiedler D.; Ziegler M.; Terpin A.; Raymond K. N. Resolution of Chiral, Tetrahedral M4L6 Metal–Ligand Hosts. J. Am. Chem. Soc. 2007, 129, 15354–15363. 10.1021/ja0764815. [DOI] [PubMed] [Google Scholar]; d Wan S.; Lin L.-R.; Zeng L.; Lin Y.; Zhang H. Efficient Optical Resolution of Water-Soluble Self-Assembled Tetrahedral M4L6 Cages with 1,1′-Bi-2-naphthol. Chem. Commun. 2014, 50, 15301–15304. 10.1039/C4CC04145C. [DOI] [PubMed] [Google Scholar]; e Bonakdarzadeh P.; Pan F.; Kalenius E.; Jurček O.; Rissanen K. Spontaneous Resolution of an Electron-Deficient Tetrahedral Fe4L4 Cage. Angew. Chem., Int. Ed. 2015, 54, 14890–14893. 10.1002/anie.201507295. [DOI] [PubMed] [Google Scholar]; f Luo D.; Wang X.-Z.; Yang C.; Zhou X.-P.; Li D. Self-Assembly of Chiral Metal–Organic Tetartoid. J. Am. Chem. Soc. 2018, 140, 118–121. 10.1021/jacs.7b11285. [DOI] [PubMed] [Google Scholar]; g Hou Y.-J.; Wu K.; Wei Z.-W.; Li K.; Lu Y.-L.; Zhu C.-Y.; Wang J.-S.; Pan M.; Jiang J.-J.; Li G.-Q.; Su C.-Y. Design and Enantioresolution of Homochiral Fe(II)–Pd(II) Coordination Cages from Stereolabile Metalloligands: Stereochemical Stability and Enantioselective Separation. J. Am. Chem. Soc. 2018, 140, 18183–18191. 10.1021/jacs.8b11152. [DOI] [PubMed] [Google Scholar]; h Zhang D.; Ronson T. K.; Greenfield J. L.; Brotin T.; Berthault P.; Léonce E.; Zhu J.-L.; Xu L.; Nitschke J. R. Enantiopure [Cs+/Xe⊂Cryptophane]⊂FeII4L4 Hierarchical Superstructures. J. Am. Chem. Soc. 2019, 141, 8339–8345. 10.1021/jacs.9b02866. [DOI] [PubMed] [Google Scholar]; i Howlader P.; Mondal S.; Ahmed S.; Mukherjee P. S. Guest-Induced Enantioselective Self-Assembly of a Pd6 Homochiral Octahedral Cage with a C3-Symmetric Pyridyl Donor. J. Am. Chem. Soc. 2020, 142, 20968–20972. 10.1021/jacs.0c11011. [DOI] [PubMed] [Google Scholar]
- a Chepelin O.; Ujma J.; Wu X.; Slawin A. M. Z.; Pitak M. B.; Coles S. J.; Michel J.; Jones A. C.; Barran P. E.; Lusby P. J. Luminescent, Enantiopure, Phenylatopyridine Iridium-Based Coordination Capsules. J. Am. Chem. Soc. 2012, 134, 19334–19337. 10.1021/ja309031h. [DOI] [PubMed] [Google Scholar]; b Ren D.-H.; Qiu D.; Pang C.-Y.; Li Z.; Gu Z.-G. Chiral Tetrahedral Iron (II) Cages: Diastereoselective Subcomponent Self-Assembly, Structure Interconversion and Spin-Crossover Properties. Chem. Commun. 2015, 51, 788–791. 10.1039/C4CC08041F. [DOI] [PubMed] [Google Scholar]; c Yang Y.; Jia J.-H.; Pei X.-L.; Zheng H.; Nan Z.-A.; Wang Q.-M. Diastereoselective Synthesis of O Symmetric Heterometallic Cubic Cages. Chem. Commun. 2015, 51, 3804–3807. 10.1039/C5CC00087D. [DOI] [PubMed] [Google Scholar]; d Martir D. R.; Escudero D.; Jacquemin D.; Cordes D. B.; Slawin A. M. Z.; Fruchtl H. A.; Warriner S. L.; Zysman-Colman E. Homochiral Emissive Λ8- and Δ8-[Ir8Pd4]16+ Supramolecular Cages. Chem.—Eur. J. 2017, 23, 14358–14366. 10.1002/chem.201703273. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Sun B.; Nurttila S. S.; Reek J. N. H. Synthesis and Characterization of Self-Assembled Chiral FeII2L3 Cages. Chem.—Eur. J. 2018, 24, 14693–14700. 10.1002/chem.201801077. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhu H.; Li Q.; Shi B.; Xing H.; Sun Y.; Lu S.; Shangguan L.; Li X.; Huang F.; Stang P. J. Formation of Planar Chiral Platinum Triangles via Pillar[5]arene for Circularly Polarized Luminescence. J. Am. Chem. Soc. 2020, 142, 17340–17345. 10.1021/jacs.0c09598. [DOI] [PubMed] [Google Scholar]; g Jiao J.; Dong J.; Li Y.; Cui Y. Fine-Tuning of Chiral Microenvironments within Triple-Stranded Helicates for Enhanced Enantioselectivity. Angew. Chem., Int. Ed. 2021, 60, 16568–16575. 10.1002/anie.202104111. [DOI] [PubMed] [Google Scholar]
- a Castilla A. M.; Ramsay W. J.; Nitschke J. R. Stereochemistry in Subcomponent Self-Assembly. Acc. Chem. Res. 2014, 47, 2063–2073. 10.1021/ar5000924. [DOI] [PubMed] [Google Scholar]; b Martir D. R.; Zysman-Colman E. Photoactive Supramolecular Cages Incorporating Ru(II) and Ir(III) Metal Complexes. Chem. Commun. 2019, 55, 139–158. 10.1039/C8CC08327D. [DOI] [PubMed] [Google Scholar]
- a Yan L.-L.; Tan C.-H.; Zhang G.-L.; Zhou L.-P.; Bünzli J.-C.; Sun Q.-F. Stereocontrolled Self-Assembly and Self-Sorting of Luminescent Europium Tetrahedral Cages. J. Am. Chem. Soc. 2015, 137, 8550–8555. 10.1021/jacs.5b03972. [DOI] [PubMed] [Google Scholar]; b Yeung C.-T.; Yim K.-H.; Wong H.-Y.; Pal R.; Lo W.-S.; Yan S.-C.; Yee-Man Wong M.; Yufit D.; Smiles D. E.; McCormick L. J.; Teat S. J.; Shuh D. K.; Wong W.-T.; Law G.-L. Chiral Transcription in Self-Assembled Tetrahedral Eu4L6 Chiral Cages Displaying Sizable Circularly Polarized Luminescence. Nat. Commun. 2017, 8, 1128. 10.1038/s41467-017-01025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhou Y.; Li H.; Zhu T.; Gao T.; Yan P. A Highly Luminescent Chiral Tetrahedral Eu4L4(L′)4 Cage: Chirality Induction, Chirality Memory, and Circularly Polarized Luminescence. J. Am. Chem. Soc. 2019, 141, 19634–19643. 10.1021/jacs.9b07178. [DOI] [PubMed] [Google Scholar]; d Hu S.-J.; Guo X.-Q.; Zhou L.-P.; Yan D.-N.; Cheng P.-M.; Cai L.-X.; Li X.-Z.; Sun Q.-F. Guest-Driven Self-Assembly and Chiral Induction of Photofunctional Lanthanide Tetrahedral Cages. J. Am. Chem. Soc. 2022, 144, 4244–4253. 10.1021/jacs.2c00760. [DOI] [PubMed] [Google Scholar]
- Takezawa H.; Murase T.; Resnati G.; Metrangolo P.; Fujita M. Recognition of Polyfluorinated Compounds Through Self-Aggregation in a Cavity. J. Am. Chem. Soc. 2014, 136, 1786–1788. 10.1021/ja412893c. [DOI] [PubMed] [Google Scholar]
- Xue W.; Ronson T. K.; Lu Z.; Nitschke J. R. Solvent Drives Switching between Λ and Δ Metal Center Stereochemistry of M8L6 Cubic Cages. J. Am. Chem. Soc. 2022, 144, 6136–6142. 10.1021/jacs.2c00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Crowley J. D.; Lisboa L. S.; van Hilst Q. V. C.. Supramolecular Systems: Metallo-Molecular Machines and Stimuli Responsive Metallo-Macrocycles and Cages. In Comprehensive Coordination Chemistry III,; Constable E. C.; Parkin G.; Que L. Jr., Eds.; Elsevier: Oxford, 2021; pp 174–205. [Google Scholar]; b Hauke C. E.; Cook T. R.. Self-Assembly Processes for the Construction of Supramolecular Coordination Compounds. In Comprehensive Coordination Chemistry III; Constable E. C.; Parkin G.; Que L. Jr., Eds.; Elsevier: Oxford, 2021; pp 1074–1085. [Google Scholar]; c Lewis J. E. M. Molecular Engineering of Confined Space in Metal–Organic Cages. Chem. Commun. 2022, 58, 13873–13886. 10.1039/D2CC05560K. [DOI] [PubMed] [Google Scholar]
- Symmers P. R.; Burke M. J.; August D. P.; Thomson P. I. T.; Nichol G. S.; Warren M. R.; Campbell C. J.; Lusby P. J. Non-Equilibrium Cobalt(III) “Click” Capsules. Chem. Sci. 2015, 6, 756–760. 10.1039/C4SC03036B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A. J.; Aitchison C. M.; Grommet A. B.; Nitschke J. R. Subcomponent Exchange Transforms an FeII4L4 Cage from High- to Low-Spin, Switching Guest Release in a Two-Cage System. J. Am. Chem. Soc. 2017, 139, 6294–6297. 10.1021/jacs.7b01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M.; von Zelewsky A. Charge-Transfer Excited State Properties of Chiral Transition Metal Coordination Compounds Studied by Chiroptical Spectroscopy. Coord. Chem. Rev. 1998, 177, 257–300. 10.1016/S0010-8545(98)00186-6. [DOI] [Google Scholar]
- a Hidaka J.; Douglas B. E. Circular Dichroism of Coordination Compounds. II. Some Metal Complexes of 2,2′-Dipyridyl and 1,10-Phenanthroline. Inorg. Chem. 1964, 3, 1180–1184. 10.1021/ic50018a026. [DOI] [Google Scholar]; b Mason S. F.; Peart B. J. Optical Rotatory Power of Coordination Compounds. Part XVII. The Circular Dichroism of Trisbipyridyl and Trisphenanthroline Complexes. J. Chem. Soc., Dalton Trans. 1973, 949–955. 10.1039/dt9730000949. [DOI] [Google Scholar]
- a Becke A. D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]; b Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, 2016, https://gaussian.com/gaussian16.
- The the internal cavity volumes of Δ4-2 and Λ4-2 and volumes of guests were calculated using the MoloVol program; see:; Maglic J. B.; Lavendomme R. MoloVol: An Easy-to-Use Program for Analyzing Cavities, Volumes and Surface Areas of Chemical Structures. J. Appl. Crystallogr. 2022, 55, 1033–1044. 10.1107/S1600576722004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ousaka N.; Clegg J. K.; Nitschke J. R. Nonlinear Enhancement of Chiroptical Response through Subcomponent Substitution in M4L6 Cages. Angew. Chem., Int. Ed. 2012, 51, 1464–1468. 10.1002/anie.201107532. [DOI] [PubMed] [Google Scholar]; b Castilla A. M.; Miller M. A.; Nitschke J. R.; Smulders M. M. J. Quantification of Stereochemical Communication in Metal–Organic Assemblies. Angew. Chem., Int. Ed. 2016, 55, 10616–10620. 10.1002/anie.201602968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hess B.; Kutzner C.; van der Spoel D.; Lindahl E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]; b GROMACS Release 2020.2; GROMACS development team: Uppsala, Sweden, 2020, https://manual.gromacs.org/2020.2. [Google Scholar]; c Tribello G. A.; Bonomi M.; Branduardi D.; Camilloni C.; Bussi G. PLUMED 2: New Feathers for an Old Bird. Comput. Phys. Commun. 2014, 185, 604–613. 10.1016/j.cpc.2013.09.018. [DOI] [Google Scholar]
- Pesce L.; Pavan G. M.. Computational data supporting: “Subtle Stereochemical Effects Influence Binding and Purification Abilities of an FeII4L4 Cage”, v1.0; Zenodo 2021, https://zenodo.org/record/7350671. [DOI] [PMC free article] [PubMed]
- Sabirov D. S.; Garipova R. R. The Increase in the Fullerene Cage Volume upon its Chemical Functionalization. Fuller. Nanotub. Carbon Nanostructures 2019, 27, 702–709. 10.1080/1536383X.2019.1633522. [DOI] [Google Scholar]
- a Tiwary P.; Parrinello M. From Metadynamics to Dynamics. Phys. Rev. Lett. 2013, 111, 230602. 10.1103/PhysRevLett.111.230602. [DOI] [PubMed] [Google Scholar]; b Salvalaglio M.; Tiwary P.; Parrinello M. Assessing the Reliability of the Dynamics Reconstructed from Metadynamics. J. Chem. Theory Comput. 2014, 10, 1420–1425. 10.1021/ct500040r. [DOI] [PubMed] [Google Scholar]; c Barducci A.; Bussi G.; Parrinello M. Well-Tempered Metadynamics: A Smoothly Converging and Tunable Free-Energy Method. Phys. Rev. Lett. 2008, 100, 020603. 10.1103/PhysRevLett.100.020603. [DOI] [PubMed] [Google Scholar]; d Pesce L.; Perego C.; Grommet A. B.; Klajn R.; Pavan G. M. Molecular Factors Controlling the Isomerization of Azobenzenes in the Cavity of a Flexible Coordination Cage. J. Am. Chem. Soc. 2020, 142, 9792–9802. 10.1021/jacs.0c03444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a García-Simón C.; Costas M.; Ribas X. Metallosupramolecular Receptors for Fullerene Binding and Release. Chem. Soc. Rev. 2016, 45, 40–62. 10.1039/C5CS00315F. [DOI] [PubMed] [Google Scholar]; b Fuertes-Espinosa C.; Pujals M.; Ribas X. Supramolecular Purification and Regioselective Functionalization of Fullerenes and Endohedral Metallofullerenes. Chem. 2020, 6, 3219–3262. 10.1016/j.chempr.2020.11.003. [DOI] [Google Scholar]
- Wang C. I.; Hua C. C. Solubility of C60 and PCBM in Organic Solvents. J. Phys. Chem. B 2015, 119, 14496–14504. 10.1021/acs.jpcb.5b07399. [DOI] [PubMed] [Google Scholar]
- a Brotin T.; Vanthuyne N.; Cavagnat D.; Ducasse L.; Buffeteau T. Chiroptical Properties of Nona- and Dodecamethoxy Cryptophanes. J. Org. Chem. 2014, 79, 6028–6036. 10.1021/jo500621g. [DOI] [PubMed] [Google Scholar]; b Zhang D.; Martinez A.; Dutasta J.-P. Emergence of Hemicryptophanes: From Synthesis to Applications for Recognition, Molecular Machines, and Supramolecular Catalysis. Chem. Rev. 2017, 117, 4900–4942. 10.1021/acs.chemrev.6b00847. [DOI] [PubMed] [Google Scholar]
- Ryan H. P.; Haynes C. J. E.; Smith A.; Grommet A. B.; Nitschke J. R. Guest Encapsulation within Surface-Adsorbed Self-Assembled Cages. Adv. Mater. 2021, 33, 2004192. 10.1002/adma.202004192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pesce L.; Pavan G. M.. Computational data supporting: “Subtle Stereochemical Effects Influence Binding and Purification Abilities of an FeII4L4 Cage”, v1.0; Zenodo 2021, https://zenodo.org/record/7350671. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Structure and parameters of the computational models used in atomistic molecular dynamics are available at 10.5281/zenodo.7350671 (ref (24)).