Abstract
Recent evidence suggests that physical activity may influence the functional connectivity of the aging brain. The purpose of this study was to examine the influence of physical activity on the association between executive function and functional connectivity of key brain networks and graph theory metrics in community-dwelling older adults. Participants were 47 older adults (M = 73 years; SD = 5.92) who participated in neuropsychological testing, physical activity measurements, and magnetic resonance imaging (MRI). Seed-to-voxel moderation analyses and graph theory analyses were conducted. Physical activity was significantly positively associated with default mode network functional connectivity (DMN FC; Posterior Cingulate Gyrus, p-FDR = 0.005; Frontal Pole (L), p-FDR = 0.005; Posterior Cingulate Gyrus, p-FDR = 0.006; Superior Frontal Gyrus (L), p-FDR = 0.016) and dorsal attention network functional connectivity (DAN FC; Inferior Frontal Gyrus Pars Opercularis (R), p-FDR = 0.044). The interaction between physical activity and executive function on the DMN FC and DAN FC was analyzed. The interaction between executive function and physical activity was significantly associated with DMN FC. When this significant interaction was probed, the association between physical activity and DMN FC differed between levels of high and low executive function such that the association was only significant at levels of high executive function. These results suggest that greater physical activity in later life is associated with greater DMN and DAN FC and provides evidence for the importance of physical activity in cognitively healthy older adults.
Keywords: Physical activity, Older adults, Functional connectivity, Graph theory
Introduction
As the number of older adults continues to grow at an unprecedented rate, there is a need to examine the neuroprotective effects of health and wellness factors that promote healthy aging. While long touted as good for physical health, several meta-analyses have found that exercise interventions positively impact cognitive function in older adulthood [13], [38], [46], [50]. Self-reported and objectively measured physical activity have also been shown to be positively associated with cognitive functioning in aging [64], [24], [35], [3], [39], [34]. Using objective devices to record the activity level of older adults has allowed for many different aspects of physical activity to be examined (e.g., intensity of activity, number of steps, distance traveled). Some studies have found that the intensity of physical activity has the greatest association to current cognitive ability for older adults [2], [7], [39], [68], while others have posited that simply moving around throughout the day (i.e., number of steps) is significantly related to cognition [3], [8]. The use of performance-based measures to examine physical activity in older adults allows for more accurate and specific information to be examined in its association with cognitive functional ability.
Exercise interventions have been shown to impact cognitive function in aging, particularly executive function [2], [13], [51]. Executive functions involve the integration of several cognitive processes including inhibitory control, working memory, set-shifting, and cognitive flexibility. These processes support higher order cognitive functions such as problem solving, decision-making, and planning and are one of the earliest domains effected by aging related cognitive decline [19], [12], [36], [55]. Executive function ability is crucial to maintaining functional independence in aging, and the preservation of this ability is important to researchers and clinicians [61]. A component of executive functions, set-shifting ability, is related to several outcomes in older adulthood such as risk of fall, subjective cognitive complaints, and everyday functioning [27], [31], [43], [44], [45]. Better executive function performance in older adulthood has specific neuronal underpinnings such as greater connectivity in both the default mode network (DMN) and in the dorsal attention network [1], [15], [41]. Therefore, there is a need to examine how physical activity might interact with executive function to impact the functioning of the aging brain.
The impact of physical activity on the functional connectivity (FC) of the aging brain is an emerging area. Recent reviews [54], [66] examined the current state of the literature concerning the associations between physical activity and FC for older adults and found relatively few studies on this topic. There are several cross-sectional studies which have examined aspects of these associations with a particular focus on the brain networks that are most susceptible to aging including the DMN and DAN. The DAN is based in the intraparietal sulcus and frontal eye fields and is active during tasks that require voluntary and sustained attention, while the DMN, housed in the ventromedial prefrontal cortex and posterior cingulate cortex, is active at rest [22], [60]. Boraxbekk and colleagues [6] showed that self-reported physical activity of the previous decade was related to DMN FC in older adults. Using objectively measured physical activity, Veldsman and colleagues [67] found that physical activity level was related to DAN FC in older adult stroke survivors. The current literature indicates an association between physical activity and DMN and DAN FC in aging, but less is known about how executive function might play into this association.
Finally, the literature examining the associations between physical activity and graph theory metrics such as global and local efficiency is extremely sparse. Graph theory posits that the brain is made up of a complex series of nodes (e.g., anatomical elements) and edges (e.g., relationships between nodes) and this methodology can model the overall connectivity of the brain and thus characterizes this organization [9], [63]. This methodology can be used to determine both global and local efficiency of the brain. Kawagoe, Onoda, and Yamaguchi [37] examined the associations between executive function, global efficiency, local efficiency, and physical fitness in older adults. They found that global efficiency was positively associated with executive function and physical fitness, while local efficiency was negatively associated with executive function and physical fitness. The authors posited that while the findings for global efficiency were intuitive, their hypothesis for why local efficiency was negatively related to executive function and physical fitness was because of the tendency of high functioning older adults to have connections across a broader range of brain areas at the expense of local efficiency (i.e., a compensatory mechanism). In addition, a recent study by Yue and colleagues [67] examined the impact of Tai Chi practice on network efficiency in Chinese female older adults and found no difference in global and local efficiency between those that engaged in Tai Chi and those that walked, although local efficiency attributes expressed positive trends in favor of Tai Chi practice. While these studies give us some insight into the associations between physical activity, executive function, and measures of functional connectivity and efficiency, this area is ripe for a greater exploration of global and local efficiency of networks susceptible to the effects of aging.
The purpose of the current study was to expand upon the limited current state of the literature in this area by exploring the impact of physical activity on FC and executive function in older adults. This study is important because it contributes to a better understanding of how physical activity may modify the connections between cognition and key brain networks implicated in cognitive aging. This study had several aims. The first aim was to examine whether executive function performance was related to DMN and DAN FC in community-dwelling older adults and to examine whether physical activity moderated this association. We hypothesized that better executive function performance would be related to greater DMN and DAN connectivity. We also hypothesized that physical activity would moderate the association between both executive function and DMN/DAN FC such that higher levels of physical activity would buffer the association between lower executive function and DAN/DMN FC. The second aim was to evaluate whether physical activity was related to whole brain local and global efficiency for community-dwelling older adults. We hypothesized that greater physical activity would be significantly associated with both greater global efficiency and greater local efficiency, as evidence indicates that there is greater whole brain connectivity and lower local connectivity in aging [10], [53], [33].
Methods
Participants
Participants were a total of 47 community-dwelling older adults (aged 65 years and older) from the surrounding community of a southeastern college town recruited over a four-year period. Participants were recruited through community ads, presentations to community aging groups by study staff, and word of mouth. Some participants’ data were acquired as part of a separate intervention study, but for the purpose of the present study, only data from their baseline assessments (pre-intervention) was used. All participants completed sessions that included neuropsychological testing, physical activity and fitness measurements, and magnetic resonance imaging (MRI). Participants were eligible if they had no major neurological (e.g., Alzheimer’s, Parkinson’s) or psychiatric disorders, were right-handed, Native English speakers, and were compatible with the MRI environment (i.e., no metal implants, no recent surgeries, etc). Participants were excluded if their cognitive functioning was below mild cognitive impairment (MCI) as indicated by their performance on the MMSE (totals score < 24; [57]). This study was approved by the Institutional Review Board at the University of Georgia. Informed consent was obtained from all participants and the Declaration of Helsinki was followed.
Physical activity
Physical activity (PA) was measured using NL-1000 Accelerometers (New Lifestyles, Inc.; Lee’s Summit, Missouri, USA). This device measures the number of steps using a piezoelectric strain gauge. Participants in this study wore the NL-1000 on their waist for 7 days and completed a log including times that the accelerometer was removed (e.g., sleeping, showering) to ensure adequate wear time (e.g., at least 8 h per day [48]. The average number of steps taken per day over the span of seven days was calculated as follows: Average Steps = [(Total Steps Day 1 + Total Steps Day 2 + Total Steps Day 3 + Total Steps Day 4 + Total Steps Day 5 + Total Steps Day 6 + Total Steps Day 7)/7]. This device has been validated to capture steps in aging populations [4]. Daily step count is a readily accessible way to monitor physical activity [40].
Neuropsychological measures
Each participant received neuropsychological assessment by a trained assessor. All participants were administered the Delis-Kaplan Executive Function System (DKEFS) Color-Word Interference Test, the DKEFS Verbal Fluency Test, and the DKEFS Trail Making Test, which are subtests of the full DKEFS battery, measure non-verbal and verbal executive function, are validated in English speaking older adults, and have relatively brief administration times [17]. Scaled scores were determined based on the DKEFS normative sample, participant age, and performance on the task. For the Trail Making Test, a scaled score was calculated for Condition 4: Number-Letter Switching, which measures the ability to set-shift. For Verbal Fluency, a scaled score for Category Switching was calculated, which measures the ability to generate words that fall under categories while being able to set-shift. For the Color-Word Inference Test, a scaled score was calculated for Condition 4: Inhibition/Switching, which measures verbal inhibition and set-shifting (D-KEFS; [16]). All scaled scores were then averaged together for each participant to create an executive function composite specific to set-shifting. In order to determine whether these three subtests were correlated with each other and were thus measuring similar constructs, a Cronbach’s alpha based on standardized items [5] was calculated (α = 0.607) indicating an acceptable fit for a small number of items [32]. Our group has previously utilized this composite method to capture executive function in an older adult population [28].
Neuroimaging
MRI Acquisition
Brain images were acquired using a General Electric (GE; Waukesha, WI) 3 T Signa HDx MRI system. A high-resolution 3D T1-weighted fast spoiled gradient recall echo sequence was used to collect structural scans (TR = 7.5 ms; TE = < 5 ms; FOV = 256 × 256 mm matrix; flip angle = 20°; slice thickness = 1.2 mm; 154 axial slices) with an acquisition time of 6 min and 20 s. This protocol collected 176 images. Resting state functional scans were aligned to each participant’s anterior commissure-posterior commissure (AC-PC) line, collected axially, and used a T2*-weighted single shot EPI sequence (TR = 5000 ms; TE = 25 ms; 90° RF pulse; acquisition matrix = 128 × 128; FOV = 220 × 220 mm; in-plane resolution = 220/128 mm; slice thickness = 2 mm; 60 interleaved axial slices). Total acquisition time was 9 min and 25 s. 108 volumes were acquired.
Resting state pre-processing
The resting state functional scans were pre-processed using the default pre-processing pipeline in the CONN toolbox from the Neuroimaging Tools & Resources Collaboratory (v.18.b; www.nitrc.org/projects/conn; [65]. The CONN Toolbox default pre-processing pipeline includes realigning and unwarping the data, centering the coordinates, applying a slice-time correction, outlier detection (using ART-based identification of outlier scans for scrubbing; www.nitric.org/projects/artifact_detect), direct functional and structural segmentation and normalization (using simultaneous Grey Matter/White Matter/CSF segmentation and MNI normalization), and functional smoothing. In addition, functional scans were denoised in CONN to remove physiological effect, subject movement, and other confounding effects from the BOLD signal. Within the CONN Toolbox, cortical and subcortical ROIs were defined by the Harvard-Oxford atlas [23], [18], [42], [29], Automated Anatomical Labelling (AAL) Atlas [59], and CONN default networks.
Seed-to-voxel analyses
Seed-to-voxel analyses were conducted a priori to examine the level of functional connectivity between the DMN and every voxel in the brain and the DAN and every voxel in the brain, which produces seed-based correlation (SBC) maps for each participant. ROIs/seeds were defined by coordinates provided by the CONN toolbox regarding the DMN and DAN. SBC maps contain Fisher r to z transformed bivariate correlation coefficients between each seed/ROI BOLD timeseries (averaged across all voxels within an ROI) and an individual voxel BOLD timeseries. Group summary maps of SBC maps for each participant were used in statistical analyses (voxel-wise FDR-corrected p < 0.05). The DMN consists of four ROIs (lateral parietal R & L, medial prefrontal cortex, posterior cingulate cortex) as does the DAN (frontal eye field R & L, intraparietal sulcus R & L). The connectivity in all ROIs in the network was averaged.
The associations between executive function and DMN/DAN connectivity and the associations between physical activity and DMN/DAN connectivity were examined. To test the moderating effect of physical activity and fitness on the association between executive function and functional connectivity of DMN/DAN using seed-to-voxel analyses, contrasts were run in CONN to determine whether significant executive function × physical activity interactions existed. When significant interactions existed, executive function and physical activity were discretized into “high” and “low” groups and between-subjects contrasts were conducted within CONN to interpret the directionality of the results.
Graph theory analyses
To examine the global and local properties of the whole brain and the impact of physical activity on these properties, graph theory analyses were applied in CONN. Network ROIs in CONN were defined by independent cluster analyses (ICA) by CONN software creators. Whole brain global efficiency was computed as the average inverse distance for all possible pairs of nodes and represents the efficiency of information transfer among all ROIs. The local efficiency was computed as the average of the inverse shortest path lengths among the ROIs in the immediately connected neighborhood of an ROI. At the whole brain network level, local efficiency represents the average sub-network efficiency across all ROIs and reflects the ability to effectively compensate for the localized failure of a single node [52]. Calculation of global efficiency and local efficiency cost was set at 0.15 and corrected for multiple comparisons (FDR-corrected p < 0.05). Adjacency matrix thresholding is typically implemented using a fixed network cost level (e.g. keeping the strongest 15% of connections) in order to allow sensitive between-network comparisons of other graph measures of interest [65], [30].
Power analysis
A post-hoc power analysis was conducted using GPower [20] with power (1 - β) set at 0.80 and α = 0.05 using the a priori setting Linear Multiple Regression: Fixed model, R2 deviation from zero. Based on the current sample size of 51 participants, this sample size would provide enough power to detect a medium to large effect with three predicters (i.e., physical activity, executive function, physical activity × executive function interaction; f2 = 0.23) and a medium effect with one predictor (i.e., physical activity predicting efficiency; f2 = 0.16).
Results
Descriptive characteristics
Table 1 provides means and standard deviations for sociodemographic characteristics. The average age was 72.96 years (SD = 5.92). Slightly over half (61.7%) of the participants were female and a majority of the participants were White (91.5%). On average, participants had completed 16.96 years (SD = 2.45) of education. The average steps per day (M = 5360.91; SD = 3223.83) was below most recommended average steps per day for older adults [25], [58], [40]. Our sample fell in the average range on the composite measure of executive functioning (M = 11.65; SD = 2.44).
Table 1.
Sample Demographics and Key Study Variables.
| Variable | % or M (SD) |
|---|---|
| Demographics | |
| Age | 72.96 (5.92) |
| Sex (% female) | 61.7% |
| Race (% White) | 91.5% |
| Years of Education | 16.96 (2.45) |
| Average Steps (day) | 5360.91 (3223.83) |
| DKEFS Composite | 11.65 (2.44) |
DKEFS = Delis Kaplan Executive Function System
Seed-to-voxel analyses
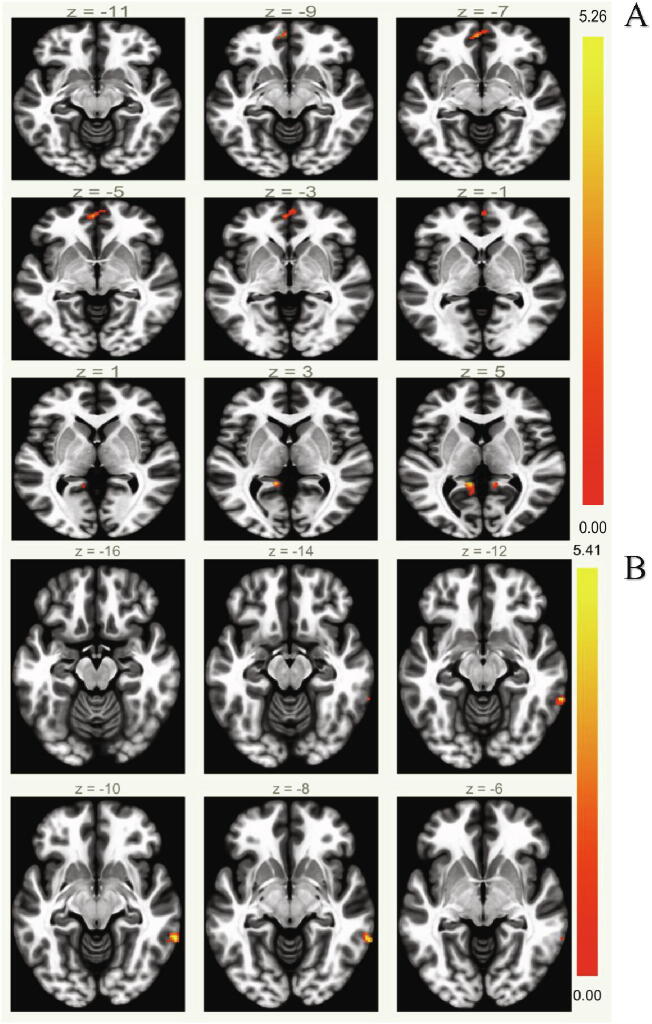
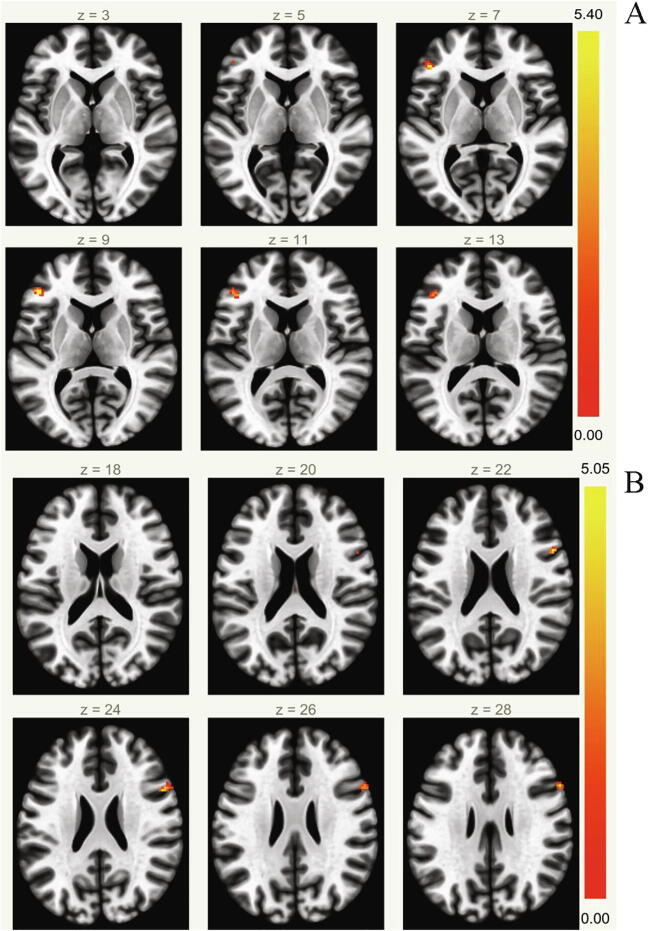
Executive function was not significantly associated with DMN FC, but it was significantly related to DAN FC in our sample (Inferior Frontal Gyrus, Pars Triangularis (L), p-FDR = 0.045). Physical activity was significantly positively associated with DMN FC (Posterior Cingulate Gyrus, p-FDR = 0.005; Frontal Pole (L), p-FDR = 0.005; Posterior Cingulate Gyrus, p-FDR = 0.006; Superior Frontal Gyrus (L), p-FDR = 0.016; Fig. 1) and DAN FC (Inferior Frontal Gyrus Pars Opercularis (R), p-FDR = 0.044; Fig. 2). The interaction between executive function and PA was significantly associated with DMN FC (see Table 2). When this significant interaction between executive function and physical activity was probed, the association between physical activity and DMN FC differed between levels of high and low executive function such that the association was only significant at levels of high executive function. The interaction between executive function and physical activity was not significantly associated with DAN FC.
Fig. 1.
Default Mode Network Functional Connectivity Results. Physical activity was significantly positively associated with Default Mode Network functional connectivity (A). The interaction between executive function and physical activity was significantly associated with Default Mode Network Functional Connectivity (B). (color print).
Fig. 2.
Dorsal Attention Network Functional Connectivity Results. Executive function was significantly positively associated with Dorsal Attention Network Functional Connectivity (A). Physical activity was significantly positively associated with Dorsal Attention Network Functional Connectivity (B). (color print).
Table 2.
Moderation Analyses.
| Clusters (x,y,z) | Size (Voxels) | p-FDR | Area | |
|---|---|---|---|---|
| DMN FC | ||||
| PA | −12, −44, +04 | 56 | 0.005 | Posterior Cingulate Gyrus |
| −04, +54, −04 | 55 | 0.005 | Frontal Pole (L) | |
| +10, −44, +08 | 51 | 0.006 | Posterior Cingulate Gyrus | |
| −20, +18, +52 | 40 | 0.016 | Superior Frontal Gyrus (L) | |
| PA × EF | +64, −44, −10 | 42 | 0.007 | Middle Temporal Gyrus (R) |
| DAN FC | ||||
| EF | −46, +36, +10 | 47 | 0.024 | Inferior Frontal Gyrus, Pars Triangularis (L) |
| PA | +52, +14, +22 | 45 | 0.044 | Inferior Frontal Gyrus, Pars Opercularis (R) |
EF = Executive Function; PA = Physical Activity; DMN = Default Mode Network; DAN = Dorsal Attention Network; FC = Functional Connectivity.
Whole brain graph theory analyses
Contrary to our hypothesis, physical activity was not significantly associated with whole brain global efficiency (t (45) = -0.77, p = 0.45) or whole brain local efficiency (t (45) = 0.29, p = 0.77).
Discussion
The protective effects of physical activity on the brain have been well-documented in aging, but the mechanisms by which physical activity is neuroprotective is less understood. The current study sought to examine how physical activity impacts the association between executive function and resting state brain connectivity. Importantly, we utilized an objective measure of physical activity as well as seed-to-voxel and graph theory analyses. Consistent with the existing literature, we hypothesized that better executive function performance would be related to greater DMN and DAN connectivity. We also hypothesized that physical activity would moderate the association between both executive function and DMN/DAN FC such that higher levels of physical activity would buffer the association between lower executive function and DAN/DMN FC.
Our first hypothesis was partially supported. Executive function was not significantly associated with DMN FC, but it was significantly related to DAN FC with frontal areas. This significant finding is in line with research that has demonstrated stronger positive connectivity between dorsal attention and frontoparietal networks on an executive functioning task [56]. However, this is not universally seen in the literature, as some studies have failed to find any association between common resting state networks (i.e., including the DMN) and cognitive performance in older adulthood [47], [21], [26]. This indicates the need for future replication and additional exploration. Interestingly, physical activity was significantly positively associated with DMN FC in frontal and parietal areas associated with reasoning and emotional regulation. Physical activity was only significantly positively related to DAN FC with the inferior frontal gyrus pars opercularis, an area in the frontal lobe involved in language production and semantic processing. This finding adds to the growing literature demonstrating an association between physical activity and increased functional connectivity in aging [6], [62].
Notably, there was a significant interaction between executive function and physical activity on DMN FC. When this interaction was probed, the association between physical activity and DMN FC differed between levels of high and low executive function such that the association was only significant at high levels of executive functioning. This suggests that older adults may see the greatest benefit of physical activity on DMN FC if they are already performing at high levels of executive function and provides evidence for the importance of physical activity in cognitively healthy older adults. Increasing physical activity in high executive functioning older adults may act as buffer against age-related DMN FC decline and supports lifestyle factors such as physical activity as enhancing compensatory scaffolding in the aging brain [49]. In addition, this finding suggests that interventions may consider boosting both physical activity and executive functioning in tandem to achieve preserve or improve the functional connectivity of the aging brain.
Our second hypothesis was not supported. Physical activity was not significantly associated with whole brain global or local efficiency in our sample. It is possible that physical activity does have an impact on efficiency in more localized and specific ways that was missed by examining efficiency using whole brain metrics. In addition, there may have been a small effect and we did not have enough power based on our sample size to detect it. Future studies should consider examining efficiency in regions of interest. Given that this sample was engaging in far less physical activity than is recommended by CDC guidelines [11], it would be important to examine this relationship in a group of older adults with a wider range of physical activity levels.
There are several limitations that should be considered. First, this study was cross-sectional and thus limits causal conclusions that can be drawn. While it was hypothesized that physical activity impacted the association between cognition and brain function, it is possible that these relationships are also bidirectional [14] and should be explored in future longitudinal studies. Additionally, our measurement of physical activity was a time-limited (i.e., one week) sample of current activity and may not represent historical physical activity. Future studies might consider tracking physical activity over time. In addition, our sample was relatively sedentary. It is possible that the impact of physical activity on these associations might be greater in a wider range of physical activity levels. Our sample was highly educated, racially homogenous, cognitively intact, and relatively sedentary, which may have implications for the generalizability of the results. While a limit to generalizability, the ability to find effects in a highly educated and cognitively unimpaired sample indicates that effects may be greater for those with lower levels of education and thus lower cognitive reserve. In addition, this sample was modestly sized, and thus power was limited to conduct additional analyses. Despite these limitations, the current study contributes to our understanding of how physical activity may impact cognition and the functional connectivity of the aging brain. These analyses provide insight into the neuroprotective effects of physical activity and may inform future research in this important area.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Marissa A Gogniat, Email: mag53440@uga.edu.
Talia L Robinson, Email: talia.robinson25@uga.edu.
Kharine R Jean, Email: kjean@uga.edu.
L Stephen Miller, Email: lsmiller@uga.edu.
References
- 1.Andrews-Hanna J.R., Snyder A.Z., Vincent J.L., Lustig C., Head D., Raichle M., et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angevaren M., Vanhees L., Wendel-Vos W., Verhaar H.J.J., Aufdemkampe G., Aleman A., et al. Intensity, but not duration, of physical activities is related to cognitive function. Eur J Cardiovasc Prev Rehabil. 2007;14(6):825–830. doi: 10.1097/HJR.0b013e3282ef995b. [DOI] [PubMed] [Google Scholar]
- 3.Barnes D.E., Blackwell T., Stone K.L., Goldman S.E., Hillier T., Yaffe K. Cognition in older women: The importance of daytime movement. J Am Geriatr Soc. 2008;56(9):1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreira T.V., Brouillette R.M., Foil H.C., Keller J.N., Tudor-Locke C. Comparison of older adults' steps per day using NL-1000 pedometer and two GT3X+ accelerometer filters. J Aging Phys Act. 2013;21(4):402–416. doi: 10.1123/japa.21.4.402. [DOI] [PubMed] [Google Scholar]
- 5.Bland J.M., Altman D.G. Statistics notes: Cronbach’s alpha. BMJ. 1997;314(7080):572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boraxbekk C.-J., Salami A., Wåhlin A., Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network—A multimodal approach. NeuroImage. 2016;131:133–141. doi: 10.1016/j.neuroimage.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Brown B.M., Peiffer J.J., Sohrabi H.R., Mondal A., Gupta V.B., Rainey-Smith S.R., et al. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry. 2012;2(11):e191. doi: 10.1038/tp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman A.S., Wilson R.S., Bennett D.A. Total daily activity is associated with cognition in older persons. The American Journal of Geriatric Psychiatry. 2008;16(8):697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- 9.Bullmore E.d., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 10.Cao M., Wang J.-H., Dai Z.-J., Cao X.-Y., Jiang L.-L., Fan F.-M., et al. Topological organization of the human brain functional connectome across the lifespan. Dev. Cogn. Neurosci. 2014;7:76–93. doi: 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control How much physical activity do older adults need? 2021. https://www.cdc.gov/physicalactivity/basics/older_adults/index.htm Retrieved from.
- 12.Clark L.R., Schiehser D.M., Weissberger G.H., Salmon D.P., Delis D.C., Bondi M.W. Specific measures of executive function predict cognitive decline in older adults. J Int Neuropsychol Soc. 2012;18(1):118–127. doi: 10.1017/S1355617711001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colcombe S., Kramer A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 14.Daly M., McMinn D., Allan J.L. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci. 2015;8:1044. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damoiseaux J.S., Beckmann C.F., Arigita E.J.S., Barkhof F., Scheltens P.h., Stam C.J., et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 16.Delis D.C., Kaplan E., Kramer J.H. The Psychological Corporation; San Antonio, TX: 2001. Delis-Kaplan Executive Function System (D-KEFS) [Google Scholar]
- 17.Delis D.C., Kramer J.H., Kaplan E., Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. J Int Neuropsychol Soc. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- 18.Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Diamond A. Executive functions. Annu Rev Psychol. 2013;64(1):135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdfelder E., Faul F., Buchner A. GPOWER: A general power analysis program. Behav Res Meth Instrum Comput. 1996;28(1):1–11. doi: 10.3758/BF03203630. [DOI] [Google Scholar]
- 21.Ferreira L.K., Busatto G.F. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37(3):384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frazier J.A., Chiu S., Breeze J.L., Makris N., Lange N., Kennedy D.N., et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 24.Frith E., Loprinzi P.D. The association between physical activity and cognitive function with considerations by social risk status. Eur J Psychol. 2017;13:767–775. doi: 10.5964/ejop.v13i4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and Science in Sports and Exercise, 43(7), 1334–1359. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed]
- 26.Geerligs L., Renken R.J., Saliasi E., Maurits N.M., Lorist M.M. A brain-wide study of age-related changes in functional connectivity. Cereb. Cortex. 2015;25:1987–1999. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- 27.Geiger P.J., Reed R.G., Combs H.L., Boggero I.A., Segerstrom S.C. Longitudinal associations among older adults’ neurocognitive performance, psychological distress, and self-reported cognitive function. Psychol Neurosci. 2019;12(2):224. doi: 10.1037/pne0000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogniat M.A., Mewborn C.M., Robinson T.L., Jean K.R., Miller L.S. The Relations between physical activity level, executive function, and white matter microstructure in older adults. J Phys Act Health. 2021;18(10):1286–1298. doi: 10.1123/jpah.2021-0012. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein J.M., Seidman L.J., Makris N., Ahern T., O’Brien L.M., Caviness V.S., et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61(8):935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Goparaju B., Rana K.D., Calabro F.J., Vaina L.M. A computational study of whole-brain connectivity in resting state and task fMRI. Medical science monitor: international medical journal of experimental and clinical research. 2014;20:1024–1042. doi: 10.12659/MSM.891142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higby E., Cahana-Amitay D., Vogel-Eyny A., Spiro A., III, Albert M.L., Obler L.K. The role of executive functions in object-and action-naming among older adults. Exp Aging Res. 2019;45(4):306–330. doi: 10.1080/0361073X.2019.1627492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinton P.R., Brownlow C., McMurray I., Cozens B. Routledge; East Sussex, UK: 2004. SPSS explained. [Google Scholar]
- 33.Iordan A.D., Cooke K.A., Moored K.D., Katz B., Buschkuehl M., Jaeggi S.M., et al. Aging and network properties: Stability over time and links with learning during working memory training. Frontiers in Aging. Neuroscience. 2018;9 doi: 10.3389/fnagi.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iso‐Markku P., Waller K., Vuoksimaa E., Vähä‐Ypyä H., Lindgren N., Heikkilä K., et al. Objectively measured physical activity profile and cognition in Finnish elderly twins. Alzheimer's & Dementia. 2018;4(1):263–271. doi: 10.1016/j.trci.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong S., Jang J.Y. Association between physical activity and cognitive dysfunction in the Korean: a cross-sectional study. Exercise Medicine. 2017;1 doi: 10.26644/em.2017.003. [DOI] [Google Scholar]
- 36.Johnson J.K., Lui L.Y., Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawagoe T., Onoda K., Yamaguchi S. Associations among executive function, cardiorespiratory fitness, and brain network properties in older adults. Sci Rep. 2017;7:40107. doi: 10.1038/srep40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly M.E., Loughrey D., Lawlor B.A., Robertson I.H., Walsh C., Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a Systematic review and meta-analysis. Ageing research reviews. 2014;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Kerr J., Marshall S.J., Patterson R.E., Marinac C.R., Natarajan L., Rosenberg D., et al. Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61(11):1927–1931. doi: 10.1111/jgs.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraus W.E., Janz K.F., Powell K.E., Campbell W.W., Jakicic J.M., Troiano R.P., et al. Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc. 2019;51(6):1206–1212. doi: 10.1249/MSS.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majerus S., Péters F., Bouffier M., Cowan N., Phillips C. The dorsal attention network reflects both encoding load and top–down control during working memory. J Cognit Neurosci. 2018;30(2):144–159. doi: 10.1162/jocn_a_01195. [DOI] [PubMed] [Google Scholar]
- 42.Makris N., Goldstein J.M., Kennedy D., Hodge S.M., Caviness V.S., Faraone S.V., et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83(2-3):155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 43.McAlister C., Schmitter-Edgecombe M. Everyday functioning and cognitive correlates in healthy older adults with subjective cognitive concerns. The Clinical neuropsychologist. 2016;30(7):1087–1103. doi: 10.1080/13854046.2016.1190404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKay J., Lang K.C., Ting L., Hackney M. A cross-sectional study of set shifting impairments and falling in individuals with and without Parkinson's disease. BioRxiv. 2017;146332 doi: 10.1101/146332. [DOI] [Google Scholar]
- 45.Mitchell M., Miller L.S. Prediction of functional status in older adults: the ecological validity of four Delis-Kaplan Executive Function System tests. J Clin Exp Neuropsychol. 2008;30(6):683–690. doi: 10.1080/13803390701679893. [DOI] [PubMed] [Google Scholar]
- 46.Northey J.M., Cherbuin N., Pumpa K.L., Smee D.J., Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 47.Onoda K., Ishihara M., Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- 48.Prescott S., Traynor J.P., Shilliday I., Zanotto T., Rush R., Mercer T.H. Minimum accelerometer wear-time for reliable estimates of physical activity and sedentary behaviour of people receiving haemodialysis. BMC nephrology. 2020;21:230. doi: 10.1186/s12882-020-01877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reuter-Lorenz P.A., Park D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014;24(3):355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders L.M.J., Hortobágyi T., la Bastide-van Gemert S., van der Zee E.A., van Heuvelen M.J.G., Regnaux J.-P. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE. 2019;14(1):e0210036. doi: 10.1371/journal.pone.0210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh-Bohmer K., et al. Aerobic exercise and neurocognitive performance: A meta- analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith R., Sanova A., Alkozei A., Lane R.D., Killgore W. Higher levels of trait emotional awareness are associated with more efficient global information integration throughout the brain: a graph-theoretic analysis of resting state functional connectivity. Soc Cogn Affect Neurosci. 2018;13(7):665–675. doi: 10.1093/scan/nsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song J., Birn R.M., Boly M., Meier T.B., Nair V.A., Meyerand M.E., et al. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 2014;4(9):662–676. doi: 10.1089/brain.2014.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stillman C.M., Donofry S.D., Erickson K.I. Exercise, fitness and the aging brain: A Review of functional connectivity in aging. Archives of. Psychology. 2019;3(4) doi: 10.31296/aop.v3i4.98. [DOI] [Google Scholar]
- 55.Thibeau S., McFall G.P., Wiebe S.A., Anstey K.J., Dixon R.A. Genetic factors moderate everyday physical activity effects on executive functions in aging: Evidence from the Victoria Longitudinal Study. Neuropsychology. 2016;30(1):6. doi: 10.1037/neu0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian L., Kong Y., Ren J., Varoquaux G., Zang Y., Smith S.M. Spatial vs. temporal features in ICA of resting-state fMRI–A quantitative and qualitative investigation in the context of response inhibition. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tombaugh T.N., McIntyre N.J. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 58.Tudor-Locke C., Craig C.L., Aoyagi Y., Bell R.C., Croteau K.A., De Bourdeaudhuij I., et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Activity. 2011;8(1):80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 60.Uddin L.Q., Clare Kelly A.M., Biswal B.B., Xavier Castellanos F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughan L., Giovanello K. Executive function in daily life: age-related influences of executive processes on instrumental activities of daily living. Psychol Aging. 2010;25(2):343. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- 62.Veldsman M., Churilov L., Werden E., Li Q., Cumming T., Brodtmann A. Physical activity after stroke is associated with increased interhemispheric connectivity of the dorsal attention network. Neurorehabilitation and Neural Repair. 2017;31(2):157–167. doi: 10.1177/1545968316666958. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Zuo X., He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weuve J., Kang J.H., Manson J.E., Breteler M.M.B., Ware J.H., Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 65.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 66.Won J., Callow D.D., Pena G.S., Gogniat M.A., Kommula Y., Arnold-Nedimala N.A., et al. Evidence for exercise-related plasticity in functional and structural neural network connectivity. Neurosci Biobehav Rev. 2021;131:923–940. doi: 10.1016/j.neubiorev.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue C., Zou L., Mei J., Moore D., Herold F., Müller P., et al. Tai Chi training evokes significant changes in brain white matter network in older women. Healthcare (Basel, Switzerland) 2020;8(1):57. doi: 10.3390/healthcare8010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu W., Howard V.J., Wadley V.G., Hutto B., Blair S.N., Vena J.E., et al. Association between objectively measured physical activity and cognitive function in older adults—The Reasons for geographic and racial differences in stroke study. J Am Geriatr Soc. 2015;63(12):2447–2454. doi: 10.1111/jgs.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]