Abstract
Background
Though weight gain has been reported in some clinical trials of CFTR modulators, the effect of elexacaftor-tezacaftor-ivacaftor on body weight, body mass index (BMI), blood pressure, lipids and glycemic control in the real-world setting remains incompletely described.
Methods
We performed a single-center, retrospective, observational analysis of the effect of elexacaftor-tezacaftor-ivacaftor on body weight and cardiometabolic parameters in 134 adult CF patients of the Washington University Adult Cystic Fibrosis Center. Body weight, BMI, and blood pressure were extracted from outpatient clinic visits for the year preceding and the period following the initiation of elexacaftor-tezacaftor-ivacaftor. Other metabolic parameters were extracted at baseline and at latest available follow-up.
Results
A mean of 12.2 months of follow-up data was available for analysis. The mean rate of change in BMI was 1.47 kg/m2/yr (95% CI, 1.08 to 1.87) greater after initiation of elexacaftor-tezacaftor-ivacaftor. Significant increases in blood pressure were observed. In those without CFRD, random blood glucose and hemoglobin A1c were decreased after elexacaftor-tezacaftor-ivacaftor initiation. In those with CFRD, elexacaftor-tezacaftor-ivacaftor increased serum total cholesterol, HDL-cholesterol, and LDL-cholesterol.
Conclusions
In this single-center, retrospective, observational study of 134 adults with CF, initiation of elexacaftor-tezacaftor-ivacaftor was associated with increases in BMI at a mean follow up of 12.2 months. Changes in other cardiometabolic risk factors were also observed. Widespread use of elexacaftor-tezacaftor-ivacaftor may be expected to increase the incidence of overnutrition in the CF population.
Keywords: Elexacaftor-tezacaftor-ivacaftor, obesity, BMI, body weight, metabolism, CFRD
1. Introduction
The combination cystic fibrosis transmembrane regulator (CFTR) modulator therapy elexacaftor-tezacaftor-ivacaftor was approved by the United States Food and Drug Administration in October 2019 for patients with cystic fibrosis (CF) who are 12 years or older and who have at least one copy of the CFTR F508del allele; about 85% of patients with CF are eligible for this therapy (1). European Medicines Agency approval of elexacaftor-tezacaftor-ivacaftor followed in August 2020 for patients with CF aged 12 or older with either a homozygous F508del/F508del genotype or a heterozygous genotype with one F508del allele and one minimal function allele (2). In phase 3 randomized clinical trials, elexacaftor-tezacaftor-ivacaftor was found to increase percent predicted FEV1, improve respiratory symptoms, and decrease sweat chloride concentration (3, 4).
Patients with CF tend to have lower body weight and body mass index (BMI) than age-matched controls (5). Pancreatic exocrine insufficiency causing intestinal malabsorption, impaired taste and smell related to sinus disease, poor oral intake during exacerbations, and high energy expenditure from increased work of breathing all likely contribute to difficulty maintaining target body weight. Low BMI in CF is associated with impaired pulmonary function and increased mortality (6, 7). For this reason, guidelines from the Cystic Fibrosis Foundation recommend that adult women maintain BMI ≥ 22 kg/m2 and adult men maintain BMI ≥ 23 (8). Improved CF care has reduced the rate of malnutrition (BMI < 18.5) in American adults with CF from 18.2% in 1999 to 6.6% in 2019 (1). In parallel, the rate of overweight and obesity (BMI > 25) has increased from 12.8% in 1999 to 31.4% in 2019.
Studies of early CFTR modulators revealed variable effects on weight and cardiometabolic parameters (9). Ivacaftor was associated with a significant increase in BMI-for-age z-score compared to placebo in patients aged ≤ 20 with CF and G551D mutation treated for 48 weeks (10). In F508del homozygotes aged ≥ 12, lumacaftor-ivacaftor treatment for 24 weeks was associated with an increase in BMI of about 0.2 kg/m2 above placebo (11). In the extension of this trial, lumacaftor-ivacaftor treatment was associated with sustained increases in blood pressure, with mean increases from baseline of 4.6 mmHg in systolic blood pressure (SBP) and 4.1 mmHg in diastolic blood pressure (DBP) at 96 weeks of follow-up (12). Clinically significant hypertension, including hypertensive emergency, has been described with lumacaftor-ivacaftor (13). However, tezacaftor-ivacaftor therapy for 24 weeks in the same population was not associated with a significant change in BMI compared to placebo; effects on blood pressure were not reported (14).
The effect of elexacaftor-tezacaftor-ivacaftor on body weight and cardiometabolic parameters has not been comprehensively described. In the phase 3 trial of elexacaftor-tezacaftor-ivacaftor in F508del heterozygotes, the absolute change in body weight from baseline after 24 weeks of treatment was +3.4 kg, compared to +0.5 kg for placebo, with a between group difference of +2.9 kg (95% CI, 2.3 to 3.4) (3). The absolute increase in BMI from baseline after 24 weeks of treatment was 1.1 kg/m2 (95% CI, 1.0 to 1.3) (3). Increases in SBP (between-group difference, 3.2 mmHg) and DBP (between-group difference, +1.6 mmHg) were observed (3). In the phase 3 trial of elexacaftor-tezacaftor-ivacaftor in F508del homozygotes, the duration of treatment was only 4 weeks (4). Despite this short duration, treatment was associated with an absolute change in body weight from baseline of 1.6 kg (95% CI, 1.0 to 2.1) and an absolute increase in BMI from baseline of 0.6 kg/m2 (95% CI, 0.4 to 0.8) (4). Little is known regarding effects of elexacaftor-tezacaftor-ivacaftor on metabolic parameters such as plasma glucose, hemoglobin A1c, and plasma lipids.
With more than a year elapsed since the regulatory approval of elexacaftor-tezacaftor-ivacaftor, we sought to describe the real-world effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in a single-center, retrospective, observational analysis. We evaluated the effect of elexacaftor-tezacaftor-ivacaftor treatment on body weight, BMI, blood pressure, random blood glucose, hemoglobin A1c, plasma lipids, plasma proteins, and percent predicted FEV1, and whether these effects varied by CFRD status or genotype.
2. Research design and methods
2.1. Study subjects
All adult patients of the Washington University Cystic Fibrosis Adult Care Center taking elexacaftor-tezacaftor-ivacaftor were eligible for inclusion. Of 200 total patients in the clinic, 179 patients had genotypes eligible for elexacaftor-tezacaftor-ivacaftor therapy at the time of analysis. Of these, 163 were taking elexacaftor-tezacaftor-ivacaftor during the study period. Exclusion criteria included pregnancy within the year preceding or since starting elexacaftor-tezacaftor-ivacaftor, lung transplantation within the year preceding or since starting elexacaftor-tezacaftor-ivacaftor, patients taking this medication as part of a clinical trial, documented nonadherence to elexacaftor-tezacaftor-ivacaftor, or availability of less than 3 months of follow-up body weight data by April 1, 2021. 29 patients were excluded by these criteria. 134 patients met criteria for analysis. The research protocol was approved by the Institutional Review Board of Washington University School of Medicine.
2.2. Methods
Clinical data from subjects who did not meet exclusion criteria were manually extracted from the electronic medical record and deidentified. The elexacaftor-tezacaftor-ivacaftor start date, CFTR genotype, and history of prior CFTR modulator use were available for all subjects. Only data obtained in the outpatient setting were included; data from inpatient hospitalizations were not extracted. The baseline time point was the outpatient visit date closest to, but not after, the elexacaftor-tezacaftor-ivacaftor start date. The time between baseline visit and initiation of elexacaftor-tezacaftor-ivacaftor was 26 ± 39 (mean ± SD) days. For body weight and BMI, data from 12 months, 6 months, and 3 months prior to baseline, at baseline, and 3 months, 6 months, and 12 months after baseline were extracted (with the visit closest to each time point used). Out of 7 possible visits, 6.3 ± 0.9 (mean ± SD) visits with body weight and BMI were available for the cohort. For blood pressure, data from 12 months and 6 months prior to baseline and 6 months and 12 months after baseline were extracted (visit closest to each time point was used). For laboratory data including random blood glucose, hemoglobin A1c, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, total protein, and albumin, a baseline (value closest to, but not after, the elexacaftor-tezacaftor-ivacaftor start date, and within 12 months of the elexacaftor-tezacaftor-ivacaftor start date) and follow-up (most recent available value) data point were extracted. The protein gap was calculated as the serum total protein less the serum albumin. The presence or absence of cystic fibrosis-related diabetes, exocrine pancreatic insufficiency, cystic fibrosis-related liver disease, and cystic fibrosis exacerbation requiring hospitalization or intravenous antibiotic administration in the time since starting elexacaftor-tezacaftor-ivacaftor were extracted. Percent predicted FEV1 data from baseline, 6 months, and 12 months after the elexacaftor-tezacaftor-ivacaftor start date were extracted from clinic-performed pulmonary function testing.
2.3. Statistics
Percent predicted FEV1, random blood glucose, hemoglobin A1c, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, and protein gap changes from baseline to follow up for each subject were calculated. To account for variations in precise duration of follow-up, changes were annualized by dividing the mean change by the number of intervening days and multiplying the result by 365. Mean change per unit time was compared to no change using one-sample t-tests. In sub-analyses for genotype, PS/PI, and CFRD, t-tests were stratified. For interactions of each of these factors with elexacaftor-tezacaftor-ivacaftor, differences in change were tested in analysis of variance (ANOVA). A two-stage approach was used to analyze BMI, body weight, SBP, and DBP change per year during periods without and with elexacaftor-tezacaftor-ivacaftor. First, regression slopes were estimated by subject and treatment period to represent annual change. Second, these slopes were compared in a mixed random effects factorial ANOVA with elexacaftor-tezacaftor-ivacaftor, CFRD, and elexacaftor-tezacaftor-ivacaftor *CFRD interaction as fixed effects and with subject as a random effect. For BMI and body weight, interactions with CF hospitalizations, genotype, and PS/PI were similarly estimated and tested. The absolute values for BMI, body weight, and blood pressure post-treatment for subjects without a 12-month follow-up visit were calculated as the last observation carried forward. The effect of elexacaftor-tezacaftor-ivacaftor treatment on the distribution of weight and blood pressure categories, and the effects of CFTR genotype on sex, race, prior CFTR modulator use, or CF-related comorbidities were assessed with the chi-square test. Effect of genotype on continuous-variable baseline characteristics was assessed with two-tailed t-tests. Chi-square tests and t-tests of baseline characteristics were performed using GraphPad Prism 9.2.0 (San Diego, CA, USA) and all other statistical analyses were conducted in SAS 9.4 (Cary, NC, USA).
3. Results
3.1. Study cohort characteristics
Table 1 describes characteristics of the study cohort at baseline, prior to starting elexacaftor-tezacaftor-ivacaftor. In all, 134 subjects met criteria for inclusion and analysis. The study cohort was 54% male, 46% female, and 97% white. F508del homozygotes made up 58% of the study cohort, and F508del heterozygotes 42%. A mean of 12.2 months of follow-up data was available for analysis. During the period of follow-up, 26 patients (19.4%) had a CF exacerbation requiring hospitalization or intravenous antibiotic course.
Table 1.
Baseline characteristics of study patients
| All | F508del homozygous | F508del heterozygous | P value | |
|---|---|---|---|---|
| N (%) | 134 | 78 (58%) | 56 (42%) | |
| Sex | 0.38 | |||
| Male (%) | 73 (54%) | 40 (51%) | 33 (59%) | |
| Female (%) | 61 (46%) | 38 (49%) | 23 (41%) | |
| Race | 0.73 | |||
| White (%) | 130 (97%) | 76 (97%) | 54 (96%) | |
| Nonwhite (%) | 4 (3%) | 2 (3%) | 2 (4%) | |
| Age (mean ± SD), years | 33.6 ± 10.6 | 32.9 ± 9.1 | 34.7 ± 12.3 | 0.31 |
| Duration of follow-up (mean ± SD), months | 12.2 ± 2.5 | 12.2 ± 2.1 | 12.3 ± 2.9 | 0.85 |
| Earlier-generation CFTR modulator use (%) | 81 (60%) | 70 (90%) | 11 (20%) | <0.0001 |
| BMI (mean ± SD), kg/m2 | 23.6 ± 5.2 | 22.7 ± 3.8 | 24.9 ± 6.5 | 0.02 |
| Body weight (mean ± SD), kg | 65.3 ± 18.5 | 62.8 ± 12.4 | 70.9 ± 20.5 | 0.006 |
| Systolic blood pressure (mean ± SD), mmHg | 120.0 ± 14.4 | 120.0 ± 15.1 | 119.9 ± 13.4 | 0.97 |
| Diastolic blood pressure (mean ± SD), mmHg | 75.6 ± 8.6 | 74.5 ± 9.1 | 77.1 ± 7.8 | 0.09 |
| % predicted FEV1 (mean ± SD) | 57.9 ± 24.1 | 57.3 ± 23.8 | 58.9 ± 24.7 | 0.71 |
| CF comorbidities | ||||
| Cystic fibrosis-related diabetes (%) | 46 (34%) | 34 (44%) | 12 (21%) | 0.008 |
| Exocrine pancreatic insufficiency (%) | 123 (92%) | 78 (100%) | 45 (80%) | <0.0001 |
| Cystic fibrosis-related liver disease (%) | 11 (8%) | 5 (6%) | 6 (11%) | 0.37 |
P values are for effect of genotype.
3.2. Effect of elexacaftor-tezacaftor-ivacaftor on BMI and body weight
Data describing the effect of elexacaftor-tezacaftor-ivacaftor on BMI and body weight are shown in Table 2. The mean BMI at baseline was 23.6 (95% CI: 22.7 to 24.4) and at latest available follow up was 25.2 (95% CI: 24.3 to 26.2). We used each subject as their own control by comparing the rate of change in BMI and body weight during the year before elexacaftor-tezacaftor-ivacaftor initiation to the rate of change after elexacaftor-tezacaftor-ivacaftor initiation. The annualized difference in BMI trajectory was 1.47 kg/m2/yr (95% CI: 1.08 to 1.87, P < 0.0001). The annualized difference in body weight trajectory was 4.43 kg/yr (95% CI: 3.14 to 5.36, P < 0.0001). There was no significant interaction between the BMI effect of elexacaftor-tezacaftor-ivacaftor and CFTR genotype, CFRD status, prior CFTR modulator exposure, or the presence or absence of CF-related hospitalizations during the study period. There was a significant interaction (P < 0.05) between the BMI effect of elexacaftor-tezacaftor-ivacaftor and pancreatic sufficiency status. Though only 11 patients in the study cohort had exocrine pancreatic sufficiency, this subgroup displayed similar increases in BMI during both the year prior to elexacaftor-tezacaftor-ivacaftor and the period following elexacaftor-tezacaftor-ivacaftor initiation, with no significant difference in BMI trajectory with treatment. By contrast, the subgroup with exocrine pancreatic insufficiency had a significant increase in BMI trajectory after starting elexacaftor-tezacaftor-ivacaftor.
Table 2.
Effect of elexacaftor-tezacaftor-ivacaftor on BMI and body weight
| Outcome | Subgroup | N | Pre-treatment (95% CI) | Post-treatment (95% CI) | Mean difference (95% CI) | P value |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | All | 134 | 23.6 (22.7 to 24.4) | 25.2 (24.3 to 26.2) | 1.65 (1.37 to 1.93) | <0.05 |
| Δ BMI (kg/m2/yr) | All | 134 | 0.16 (−0.12 to 0.45) | 1.64 (1.36 to 1.92) | 1.47 (1.08 to 1.87) | <0.0001 |
| Weight (kg) | All | 134 | 66.0 (63.2 to 68.9) | 70.6 (67.6 to 73.6) | 4.55 (3.79 to 5.31) | <0.05 |
| Δ Weight (kg/yr) | All | 134 | 0.11 (−0.81 to 1.03) | 4.60 (3.67 to 5.52) | 4.43 (3.14 to 5.36) | <0.0001 |
| Δ BMI (kg/m2/yr) | Male | 73 | 0.20 (−0.18 to 0.58) | 1.76 (1.38 to 2.14) | 1.56 (1.02 to 2.10) | <0.0001 |
| Δ BMI (kg/m2/yr) | Female | 61 | 0.12 (−0.29 to 0.54) | 1.49 (1.07 to 1.91) | 1.37 (0.77 to 1.96) | <0.0001 |
| Δ BMI (kg/m2/yr) | With CF hospitalization | 26 | 0.24 (−0.40 to 0.88) | 1.22 (0.58 to 1.86) | 0.98 (0.07 to 1.88) | <0.05 |
| Δ BMI (kg/m2/yr) | Without CF hospitalization | 108 | 0.15 (−0.17 to 0.46) | 1.74 (1.42 to 2.05) | 1.59 (1.15 to 2.04) | <0.0001 |
| Δ BMI (kg/m2/yr) | With CFRD | 46 | 0.05 (−0.44 to 0.53) | 1.56 (1.08 to 2.05) | 1.52 (0.84 to 2.20) | <0.0001 |
| Δ BMI (kg/m2/yr) | Without CFRD | 88 | 0.23 (−0.12 to 0.57) | 1.68 (1.33 to 2.02) | 1.45 (0.96 to 1.94) | <0.0001 |
| Δ BMI (kg/m2/yr) | F508del homozygous | 78 | 0.06 (−0.31 to 0.43) | 1.44 (1.07 to 1.81) | 1.38 (0.86 to 1.90) | <0.0001 |
| Δ BMI (kg/m2/yr) | F508del heterozygous | 56 | 0.31 (−0.13 to 0.74) | 1.91 (1.48 to 2.35) | 1.60 (0.99 to 2.22) | <0.0001 |
| Δ BMI (kg/m2/yr) | Pancreatic insufficient | 123 | 0.06 (−0.23 to 0.35) | 1.69 (1.40 to 1.98) | 1.63 (1.22 to 2.04) | <0.0001 |
| Δ BMI (kg/m2/yr) | Pancreatic sufficient | 11 | 1.33 (0.36 to 2.30) | 1.07 (0.10 to 2.04) | −0.26 (−1.64 to 1.11) | 0.71 |
| Δ BMI (kg/m2/yr) | Prior CFTR modulator use | 81 | 0.29 (−0.07 to 0.65) | 1.50 (1.13 to 1.86) | 1.21 (0.70 to 1.72) | <0.0001 |
| Δ BMI (kg/m2/yr) | No prior CFTR modulator use | 53 | −0.03 (−0.47 to 0.42) | 1.85 (1.41 to 2.30) | 1.88 (1.25 to 2.51) | <0.0001 |
Significant increases in BMI persisted when the cohort was stratified by sex, by the presence or absence of CF-related hospitalizations during the follow-up period, by the presence or absence of CF-related diabetes mellitus, by genotype, and by exposure to earlier-generation CFTR modulators (Table 2). The effect of elexacaftor-tezacaftor-ivacaftor on the rate of BMI increase was not modified by baseline weight (P = 0.53).
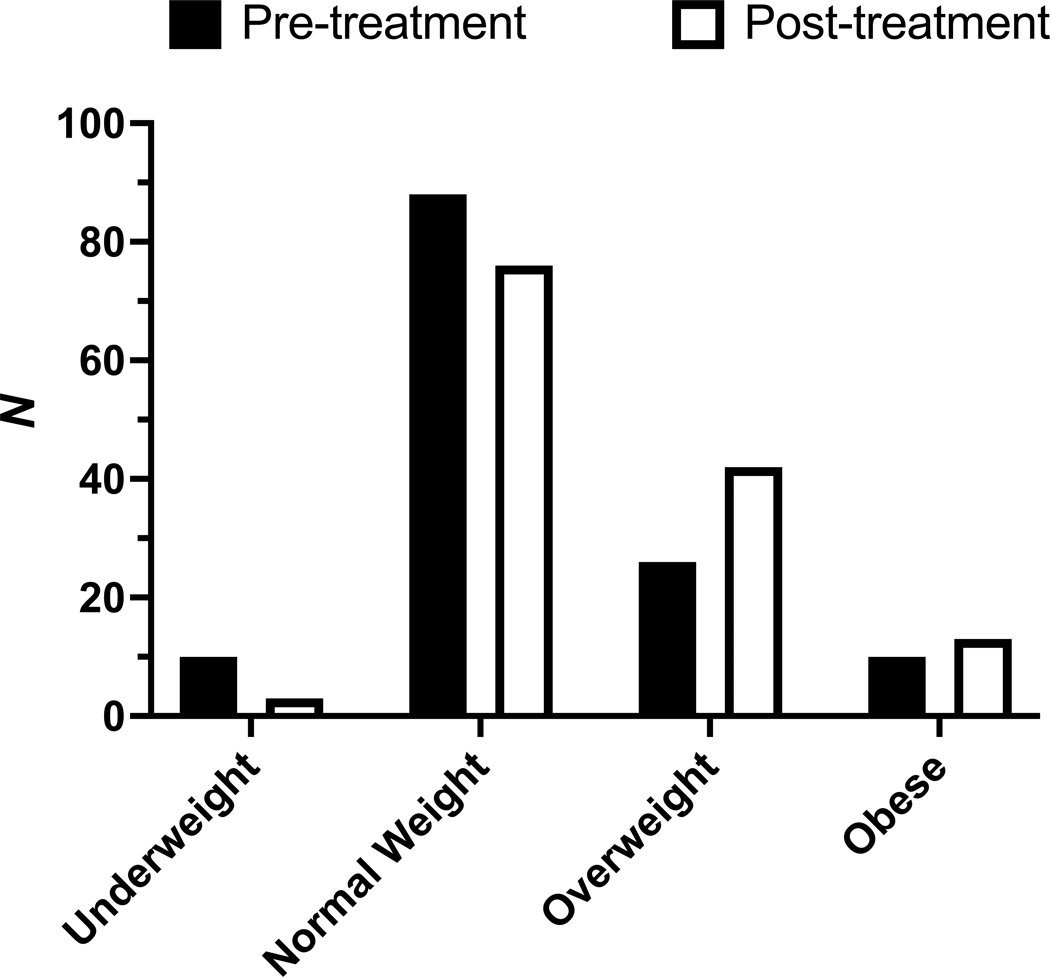
The weight category distribution of the study population significantly changed between baseline and latest available follow-up (P < 0.001). Decreases were observed in rates of underweight (7.5% to 2.2%) and normal weight (65.7% to 56.7%). Conversely, increases were observed in rates of overweight (19.4% to 31.3%) and obesity (7.5% to 9.7%) (Figure 1).
Figure 1.
Effect of elexacaftor-tezacaftor-ivacaftor on rates of underweight (BMI < 18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9), and obesity (BMI ≥ 30). N = 134. P < 0.001 for difference in weight category distribution.
3.3. Effect of elexacaftor-tezacaftor-ivacaftor on cardiometabolic parameters
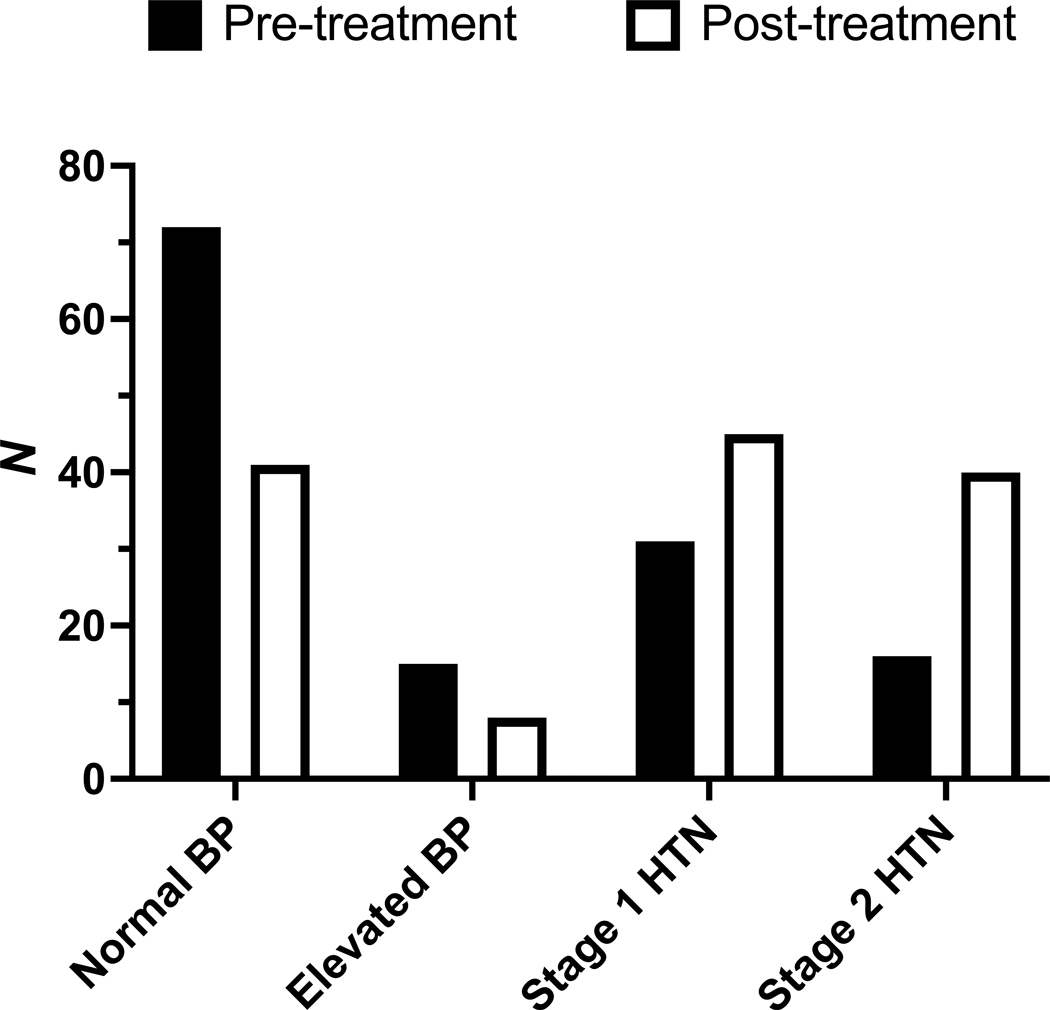
The effect of elexacaftor-tezacaftor-ivacaftor on blood pressure was assessed in the same manner as body weight and BMI, as a difference in annualized rate of change between the period following elexacaftor-tezacaftor-ivacaftor initiation and the year preceding elexacaftor-tezacaftor-ivacaftor initiation. The mean blood pressure at baseline was 120/75 mmHg (95% CI, 117–122/74–77) and at latest available follow-up was 127/81 mmHg (95% CI, 124–130/80–82, P < 0.0005). The difference in annualized rate of change in systolic blood pressure (SBP) was 4.94 mmHg (95% CI, 0.31 to 9.57, P < 0.05). The difference in annualized rate of change in diastolic blood pressure (DBP) was 3.49 mmHg (95%, 0.65 to 6.34, P < 0.05). The number of patients meeting diagnostic criteria for either stage 1 hypertension or stage 2 hypertension (15) was 47 (35%) at baseline and 85 (63%) at latest available follow-up. The distribution of blood pressure categories in the study cohort was significantly altered after treatment (P < 0.0001) (Figure 2). CFRD status did not modify the effect of elexacaftor-tezacaftor-ivacaftor on SBP and DBP (P = 0.65).
Figure 2.
Effect of elexacaftor-tezacaftor-ivacaftor on rates of normal blood pressure (SBP < 120 mmHg and DBP < 80 mmHg), elevated blood pressure (SBP 120–129 mmHg and DBP < 80 mmHg), stage 1 hypertension (HTN) (SBP 130–139 mmHg or DBP 80–89 mmHg), and stage 2 hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). P < 0.0001 for difference in blood pressure category distribution.
Data for random blood glucose, hemoglobin A1c, plasma lipids, and plasma protein gap were extracted at baseline (prior to elexacaftor-tezacaftor-ivacaftor initiation) and at the latest available follow-up time point. The effect of elexacaftor-tezacaftor-ivacaftor therapy on these parameters, calculated as a rate of change owing to the variable time between baseline and follow-up data points, is shown in Table 3.
Table 3.
Effect of elexacaftor-tezacaftor-ivacaftor on other metabolic parameters
| Outcome | Subgroup | N | Mean (95% CI) | P |
|---|---|---|---|---|
| Δ Random blood glucose (mΜ/yr) | Without CFRD | 83 | −0.78 (−0.23 to −1.33) | <0.01 |
| Δ Random blood glucose (mΜ/yr) | With CFRD | 46 | −0.40 (−1.93 to 1.12) | 0.60 |
| Δ Hemoglobin A1c (%/yr) | Without CFRD | 57 | −0.16 (−0.07 to −0.26) | <0.005 |
| Δ Hemoglobin A1c (%/yr) | With CFRD | 46 | −0.17 (−0.47 to 0.12) | 0.25 |
| Δ Total cholesterol (mM/yr) | With CFRD | 22 | 0.67 (0.37 to 0.97) | <0.0005 |
| Δ LDL cholesterol (mM/yr) | With CFRD | 21 | 0.47 (0.25 to 0.69) | <0.0005 |
| Δ HDL cholesterol (mM/yr) | With CFRD | 22 | 0.23 (0.04 to 0.42) | <0.05 |
| Δ Plasma triglyceride (mM/yr) | With CFRD | 21 | 0.01 (−0.19 to 0.21) | 0.92 |
| Δ Protein gap (g/L/yr) | All | 125 | −5.84 (−4.67 to −7.04) | <0.0001 |
CFRD, cystic fibrosis-related diabetes. Data on these parameters were not available for all subjects.
The effect of elexacaftor-tezacaftor-ivacaftor on percent predicted FEV1 in the study cohort was a mean annualized rate of increase of 7.81% per year (95% CI: 6.39 to 9.23, P < 0.0001). This effect was not modified by genotype (F508del homozygous vs. heterozygous, P = 0.93) or CFRD status (P = 0.25).
4. Conclusions
A fundamental nutritional support strategy for patients with CF is maintaining adequate BMI to help optimize pulmonary function. Yet overweight status and obesity are now more than four times as prevalent as underweight status in American adults with CF (1, 16). Data from clinical trials suggest that CFTR modulators, including elexacaftor-tezacaftor-ivacaftor, may increase weight in CF patients (3, 4, 9).
This single-center, observational, retrospective analysis of 134 adults with CF after a mean of 12 months of elexacaftor-tezacaftor-ivacaftor therapy reveals that elexacaftor-tezacaftor-ivacaftor was indeed associated with increased body weight and BMI in a real-world setting. The rate of BMI and weight increase was corrected for each individual’s pre-treatment weight trajectory by subtracting the rate of change from the previous year from the rate of change following elexacaftor-tezacaftor-ivacaftor initiation. Significant increases in BMI were observed regardless of sex, CFTR genotype, prior exposure to earlier-generation CFTR modulators, and CFRD status.
The effects of elexacaftor-tezacaftor-ivacaftor on other metabolic parameters included some changes conventionally considered favorable to cardiometabolic health and others conventionally considered unfavorable. Favorable changes include a decrease in random blood glucose and hemoglobin A1c (in patients without CFRD) and an increase in HDL-c (in patients with CFRD). Unfavorable changes include increases in systolic and diastolic blood pressure (in the full cohort), and increases in total cholesterol and LDL-c (in patients with CFRD). Though the increases in blood pressure may be partially accounted for by increases in body weight, it is also possible that reduced salt losses in the setting of CFTR modulator therapy contribute to the modest increase in blood pressure observed. The mechanism of the observed effects on glycemia and plasma lipid profiles is also uncertain but may relate to reduced systemic inflammation with elexacaftor-tezacaftor-ivacaftor therapy. This hypothesis is supported by the significant decrease in protein gap (which likely reflects decreased immunoglobulin production) and may account for the lack of change in plasma triglycerides (which are an acute phase reactant) despite increased weight. Oxidative stress is a well described mechanism of inflammation in CF that has been linked to impaired glucose homeostasis (17); though preclinical data suggest that elexacaftor-tezacaftor-ivacaftor may reduce oxidative stress (18), markers of oxidative stress such as 8-isoprostane were not available for analysis in this study. Increased first phase insulin secretion, which has been described with ivacaftor therapy but found not to occur with lumacaftor-ivacaftor therapy (19, 20), is another possible mechanism for the reduced glycemia observed in patients without CFRD. The effect of elexacaftor-tezacaftor-ivacaftor on beta-cell function is unknown, and assessment of beta-cell function was not performed in this study.
The mechanism of weight gain with elexacaftor-tezacaftor-ivacaftor therapy is uncertain but is likely multifactorial. Patient survey data suggest that elexacaftor-tezacaftor-ivacaftor improves appetite, which leads to increased food intake (21). This may plausibly relate to improvements in chronic rhinosinusitis and olfaction, though this has not yet been well studied (22, 23). The requirement that elexacaftor-tezacaftor-ivacaftor be taken with dietary fat may also increase caloric intake. The reduction in CF pulmonary exacerbations observed with elexacaftor-tezacaftor-ivacaftor therapy may reduce energy expenditure related to respiratory muscle work and mitigate the poor oral intake that traditionally accompanies exacerbations; this has been investigated previously for ivacaftor but not yet for elexacaftor-tezacaftor-ivacaftor (10, 24). Finally, some evidence suggests that CFTR modulators such as ivacaftor may improve pancreatic exocrine function (25–27), for example via enhanced CFTR-mediated bicarbonate secretion (28), but this has not yet been well studied for elexacaftor-tezacaftor-ivacaftor. Interestingly, in our study cohort, only patients with exocrine pancreatic insufficiency had their weight trajectory significantly altered by elexacaftor-tezacaftor-ivacaftor therapy. The 11 subjects with pancreatic sufficiency had significant weight gain in the year prior to starting elexacaftor-tezacaftor-ivacaftor, which continued at a similar rate after starting the drug. The small number of patients with exocrine pancreatic sufficiency in the study cohort limits our ability to draw conclusions, but does generate the hypothesis that elexacaftor-tezacaftor-ivacaftor may promote weight gain by partially restoring exocrine pancreatic function. Data directly addressing this hypothesis (such as measurements of fecal elastase) are not available from this cohort.
Strengths of this study include a moderately large sample size with sizable cohorts of both F508del homozygotes and heterozygotes. Despite the study period coinciding with the COVID-19 pandemic, multiple in-person CF clinic visits with weight and vital sign data after elexacaftor-tezacaftor-ivacaftor treatment initiation were available for most patients.
There are several limitations to this retrospective observational study, which also draw attention to potential areas of improvement in clinical practice and suggest future research directions. As a single-center experience at an academic medical center with a multidisciplinary CF clinic, the data may not be generalizable to different practice settings. The cohort was 97% white, which is slightly higher than the percentage of white adults in the Cystic Fibrosis Foundation Patient Registry (1). We did not have the statistical power to assess whether the effect of elexacaftor-tezacaftor-ivacaftor was modified by patient race. Statistical hypothesis testing was not adjusted for multiple comparisons, increasing the probability of introducing type I error. Not all cardiometabolic parameters (random blood glucose, hemoglobin A1c, and plasma lipids) were measured in all subjects, which could introduce a selection bias to the analysis. The small number of patients with exocrine pancreatic sufficiency limits our ability to draw conclusions regarding that subgroup. Changes in body composition in patients exhibiting changes in weight were not assessed, though body composition may be a better predictor of pulmonary function in CF than BMI (29). Assessment of energy requirements by indirect calorimetry was not performed but could have yielded insights into the mechanism of weight gain with elexacaftor-tezacaftor-ivacaftor treatment. Though more detailed assessment of glucose homeostasis by oral glucose tolerance testing would have been of interest in CF patients with and without CFRD, only a small number of patients in the study cohort underwent oral glucose tolerance testing during the study period. Plasma lipid panels were collected primarily in patients with CFRD, so the effect on plasma lipids in CF patients without CFRD was unable to be assessed. Finally, detailed dietary intake data, including quantification of calories from fat, total calories, and sodium intake, were not available for analysis but would help clarify mechanisms of weight gain and increased blood pressure with elexacaftor-tezacaftor-ivacaftor therapy. Patients of this CF clinic have traditionally been encouraged to consume high calorie diets to maintain goal body weight, including items high in added sugars, fats, and sodium. Exercise was traditionally infrequent due to cough, decreased pulmonary function, and frequent pulmonary exacerbations. However, in the era of elexacaftor-tezacaftor-ivacaftor, the increased prevalence of overweight has changed dietary recommendations. Nutritional goals are individualized, but in general, well-balanced diets of fresh produce, lean meats, whole grains, and healthy fats are encouraged, and salt, sugar, and processed foods are discouraged. Patients are encouraged to exercise regularly to increase lean body mass and to improve cardiometabolic parameters. It is unknown to what extent these recommendations affect our results. These limitations highlight the potential utility of monitoring body composition by DEXA, measuring energy requirements by indirect calorimetry, screening for abnormal glucose homeostasis with oral glucose tolerance testing, and individualized nutritional counseling, all of which are recommended by professional society guidelines (16, 30).
Overall, our data suggest that the approval and widespread use of elexacaftor-tezacaftor-ivacaftor should be expected to accelerate the trend toward increased weight and BMI in adults with CF that has emerged and progressed over the last 20 years. As we demonstrated in our patient population, patients with CF on elexacaftor-tezacaftor-ivacaftor are at risk of developing hyperlipidemia and hypertension. Given the increasing median age of patients living with CF and the increased likelihood of developing CFRD with age, the rising prevalence of overnutrition, hypertension, and hyperlipidemia in the CF population is likely to increase the incidence of cardiovascular and cerebrovascular disease, conditions for which patients with CF have been at low risk previously. Acknowledging this changing paradigm, recent guidelines from the Academy of Nutrition and Dietetics highlight the increased prevalence of overweight and obesity in the CF population and recommend use of dietary patterns associated with cardiovascular health in the general population, rather than traditional high-fat, high-energy diets (16). In addition, as elexacaftor-tezacaftor-ivacaftor use becomes the standard of care in pediatric patients with CF, obesity and its metabolic consequences will be of paramount importance.
In conclusion, elexacaftor-tezacaftor-ivacaftor use is contributing to the changing landscape of nutrition in CF. In addition to providing nutritional support services to identify and treat patients who are underweight, clinicians should monitor CF patients for evidence of overnutrition and its complications. Caloric, nutrition and fitness goals should be reassessed and individualized at every visit, and blood pressure and lipids should be closely monitored.
Acknowledgments
We would like to thank Dr. Daniel Rosenbluth for comments on an earlier draft of the manuscript and thank Dr. Clay Semenkovich, Dr. Daniel Rosenbluth, and Dr. Jeffrey Atkinson for their continued enthusiastic support.
Financial support
M.C.P. is supported by NIH grant T32-DK007120. M.L. is supported by Division of Endocrinology, Washington University School of Medicine. The funding source had no role in study design, data collection/analysis/interpretation, or manuscript preparation.
Footnotes
Supplementary materials
None
Conflict of interest statement
The authors have no relevant disclosures.
References
- 1.Registry CFFP. 2019 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2020. [Google Scholar]
- 2.Assessment Report: Kaftrio. Amsterdam, The Netherlands: European Medicines Agency; 2020 10 July 2020. [Google Scholar]
- 3.Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381(19):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litvin M, Yoon JC, Leey Casella J, Blackman SM, Brennan AL. Energy balance and obesity in individuals with cystic fibrosis. J Cyst Fibros. 2019;18 Suppl 2:S38–S47. [DOI] [PubMed] [Google Scholar]
- 6.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137(3):374–80. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJ, et al. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax. 2001;56(10):746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H, Clinical Practice Guidelines on G, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. [DOI] [PubMed] [Google Scholar]
- 9.Bailey J, Rozga M, McDonald CM, Bowser EK, Farnham K, Mangus M, et al. Effect of CFTR Modulators on Anthropometric Parameters in Individuals with Cystic Fibrosis: An Evidence Analysis Center Systematic Review. J Acad Nutr Diet. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Borowitz D, Lubarsky B, Wilschanski M, Munck A, Gelfond D, Bodewes F, et al. Nutritional Status Improved in Cystic Fibrosis Patients with the G551D Mutation After Treatment with Ivacaftor. Dig Dis Sci. 2016;61(1):198–207. [DOI] [PubMed] [Google Scholar]
- 11.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5(2):107–18. [DOI] [PubMed] [Google Scholar]
- 13.Dagenais RVE, Su VCH, Quon BS. Real-World Safety of CFTR Modulators in the Treatment of Cystic Fibrosis: A Systematic Review. J Clin Med. 2020;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–23. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 16.McDonald CM, Alvarez JA, Bailey J, Bowser EK, Farnham K, Mangus M, et al. Academy of Nutrition and Dietetics: 2020 Cystic Fibrosis Evidence Analysis Center Evidence-Based Nutrition Practice Guideline. J Acad Nutr Diet. 2021;121(8):1591–636 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Causer AJ, Shute JK, Cummings MH, Shepherd AI, Gruet M, Costello JT, et al. Circulating biomarkers of antioxidant status and oxidative stress in people with cystic fibrosis: A systematic review and meta-analysis. Redox Biol. 2020;32:101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veltman M, De Sanctis JB, Stolarczyk M, Klymiuk N, Bahr A, Brouwer RW, et al. CFTR Correctors and Antioxidants Partially Normalize Lipid Imbalance but not Abnormal Basal Inflammatory Cytokine Profile in CF Bronchial Epithelial Cells. Front Physiol. 2021;12:619442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, et al. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14(6):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moheet A, Beisang D, Zhang L, Sagel SD, VanDalfsen JM, Heltshe SL, et al. Lumacaftor/ivacaftor therapy fails to increase insulin secretion in F508del/F508del CF patients. J Cyst Fibros. 2021;20(2):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin C, Burnet E, Ronayette-Preira A, de Carli P, Martin J, Delmas L, et al. Patient perspectives following initiation of elexacaftor-tezacaftor-ivacaftor in people with cystic fibrosis and advanced lung disease. Respir Med Res. 2021;80:100829. [DOI] [PubMed] [Google Scholar]
- 22.Johnson BJ, Choby GW, O’Brien EK. Chronic rhinosinusitis in patients with cystic fibrosis-Current management and new treatments. Laryngoscope Investig Otolaryngol. 2020;5(3):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor-Cousar JL. Impact of Triple Combination CFTR Therapy on Sinus Disease. 2019. [Available from: https://clinicaltrials.gov/ct2/show/NCT04056702.
- 24.Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy Balance and Mechanisms of Weight Gain with Ivacaftor Treatment of Cystic Fibrosis Gating Mutations. J Pediatr. 2018;201:229–37 e4. [DOI] [PubMed] [Google Scholar]
- 25.Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4(2):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med. 2018;6(7):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelfond D, Heltshe S, Ma C, Rowe SM, Frederick C, Uluer A, et al. Impact of CFTR Modulation on Intestinal pH, Motility, and Clinical Outcomes in Patients With Cystic Fibrosis and the G551D Mutation. Clin Transl Gastroenterol. 2017;8(3):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JA, Ziegler TR, Millson EC, Stecenko AA. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition. 2016;32(4):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]