Abstract
Purpose
A previous meta-analysis examining the relationship between statin use and breast cancer reported that the inhibitory effect of statins on breast cancer may be more pronounced in early-stage cases. In this study, we aimed to investigate the effects of hyperlipidemia treatment at the time of breast cancer diagnosis and to examine its correlation with metastasis to axillary lymph nodes among patients with so-called cT1 breast cancer whose primary lesion was 2 cm or less and was pathologically evaluated by sentinel lymph node biopsy or axillary lymph node dissection. We also investigated the effects of hyperlipidemic drugs on the prognosis of patients with early-stage breast cancer.
Methods
After excluding cases that did not meet the criteria, we analyzed data from 719 patients who were diagnosed with breast cancer, with a primary lesion of 2 cm or less identified by preoperative imaging, and who underwent surgery without preoperative chemotherapy.
Results
Regarding hyperlipidemia drugs, no correlation was found between statin use and lymph node metastasis (p = 0.226), although a correlation was found between lipophilic statin use and lymph node metastasis (p = 0.042). Also, the disease-free survival periods were prolonged following treatment of hyperlipidemia (p = 0.047, hazard ratio: 0.399) and statin administration (p = 0.028, hazard ratio: 0.328).
Conclusion
In cT1 breast cancer, the results suggest that oral statin therapy may contribute to favorable outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10631-w.
Keywords: breast cancer, statin, hyperlipidemia, axillary lymph node, prognosis
Background
Although various orally administered drugs are clinically used in the treatment of a wide variety of diseases, it has been reported that some may have unexpected effects on cancer. For example, in a systematic review and meta-analysis, the diabetes drug metformin reduced the risk of colorectal cancer and prostate cancer [1]. However, the analysis in that report did not reveal a reduction in breast cancer risk, whereas some studies have reported that metformin reduces breast cancer risk and improves prognosis [1, 2]. Another meta-analysis of observational studies reported that long-term use of angiotensin-receptor blockers / angiotensin-converting enzyme inhibitors for the treatment of hypertension might reduce the risk of breast cancer [3]. Among these drugs, statins, which are typically used for the treatment of hyperlipidemia, have also been reported to suppress the development of cancer and reduce the rate of recurrence [4–12]. These outcomes may be explained by many preclinical studies that have reported antiproliferative and anti-apoptotic effects in breast cancer [13–17]. In addition, based on the anti-invasive properties [18–22] and metastasis-suppressing effects of statins that have been demonstrated in preclinical studies, some reports have clinically examined their progression-suppressing effects in breast cancer [23–29].
Another meta-analysis examining the relationship between statin use and breast cancer reported that the inhibitory effect of statins on breast cancer may be more pronounced in patients with early-stage breast cancer [30]. Therefore, we hypothesized that statins may affect metastasis to lymph nodes in breast cancer cases involving a small primary lesion. In recent years, axillary surgery for early-stage breast cancer has been reduced due to the increased effectiveness of multidisciplinary treatment before and after surgery, and evaluation of axillary lymph node metastasis before treatment has become even more important. If our hypothesis is correct, statin administration may affect the evaluation. In this study, we aimed to investigate the treatment of hyperlipidemia at the time of breast cancer diagnosis and to examine its correlation with the metastatic status in axillary lymph nodes among patients with so-called cT1 breast cancer involving a primary lesion of 2 cm or less who underwent pathological evaluations of metastasis in an axillary lymph node by sentinel lymph node biopsy or axillary lymph node dissection. We also aimed to investigate the effects of hyperlipidemic drugs on the prognosis of patients with early-stage breast cancer.
Methods
Patient background and classification
Seven hundred forty-two patients were diagnosed with breast cancer involving a primary lesion of 2 cm or less by preoperative imaging and underwent surgery without preoperative chemotherapy from April 2007 to March 2020 at Osaka City University Hospital. Pathological diagnosis of breast cancer was based on core needle biopsy (CNB) or vacuum-assisted biopsy (VAB). As an evaluation of their general condition before initiating treatment for breast cancer, the patients were confirmed to have a history of pre-treatment and oral medication use. We classified the drugs used to treat hyperlipidemia for further examination. The pharmacological classification of statins based on their hydrophilicity and lipophilicity was performed according to the classification system widely used in cardiovascular studies [31, 32]. Specifically, rosuvastatin and pravastatin are classified as hydrophilic statins, while atorvastatin, pitavastatin, simvastatin and fluvastatin are classified as lipophilic statins. Either mastectomy or breast-conserving surgery was performed because the preoperative imaging examinations such as ultrasonography (US), computed tomography (CT), and bone scintigraphy revealed that radical resection was possible. Axillary lymph node dissection was performed for cases in which axillary lymph node metastasis was suspected, and sentinel lymph node biopsy was performed for cases in which no metastasis was diagnosed. During surgery for breast cancer, the sentinel lymph node was identified using a combination of radioisotope and dye methods according to previous reports [33, 34]. Histopathological diagnosis of sentinel lymph node metastasis was conducted by slicing the entire sentinel lymph node into 2-mm-thick sections [35, 36]. Sentinel lymph node metastases were categorized by size according to previously reported parameters (macrometastasis: tumor diameter > 2 mm; micrometastasis: tumor diameter > 0.2 mm, ≤ 2 mm or < 200 tumor cells; for isolated tumor cells: tumor diameter < 0.2 mm or < 200 tumor cells) [37]. Axillary dissection was additionally performed in patients with macrometastasis that was confirmed via sentinel lymph node biopsy.
The expression levels of estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2), and Ki67, a marker of proliferation, were examined immunohistochemically in both the biopsy tissue used for breast cancer diagnosis and the surgically removed tissue. Based on the results of the immunohistological staining, breast cancer was classified into the following three subtypes: triple-negative breast cancer (TNBC; negative for ER, PgR, and HER2); hormone receptor (HR)-HER2+ breast cancer (HR-negative and HER2-positive breast cancer; ER-, PgR-, and HER2+); and HR+ breast cancer (hormone receptor-positive breast cancer; ER+ and/or PgR+).
There were 742 preoperatively diagnosed cases of cT1 breast cancer. However, 15 cases did not undergo axillary lymph node dissection or sentinel lymph node biopsy, and eight cases were being treated with unknown medications at the time of diagnosis. Therefore, these 23 cases were excluded from this study, and data was analyzed from the remaining 719 cases.
Statistical analysis
All statistical analyses were performed using the JMP software package (SAS, Tokyo, Japan). Each correlation was examined using Pearson’s chi-square test. The odds ratio (OR) and 95% confidence interval (CI) were calculated by logistic analysis, and multivariable analysis was performed using the multivariable logistic regression model. Prognostic analyses, such as the calculation of recurrence-free survival (RFS) or overall survival (OS), were conducted using the Kaplan–Meier method and the log-rank test. The hazard ratios (HR) and 95% CI were calculated using the Cox proportional hazards model. Multivariable analysis was performed using the Cox regression model. A p-value of < 0.05 was considered statistically significant.
Results
Clinicopathological features
Table 1 shows the clinicopathological features of the 719 patients with cT1 breast cancer who underwent surgery without receiving preoperative chemotherapy. The median age was 58 years (range, 29–79 years), and the median tumor diameter was 13 mm (range, 3.0–20.0 mm). A total of 612 patients (85.7%) were positive for ER, 398 patients (55.4%) were positive for PgR, and 621 patients (86.3%) were classified as having HR+ breast cancer, which represented the majority of cases. There were 66 patients (9.2%) with HER2-positive breast cancer, but only 27 patients (3.8%) were classified as having HR-HER2+ breast cancer. Seventy-one patients (9.9%) were classified as having TNBC. Ki67 was expressed at a level higher than 20% in 133 patients (18.5%).
Table 1.
Clinicopathological features of 719 cT1 breast cancer patients who underwent surgery without preoperative chemotherapy
| Parameters | Number of patients (n = 719) (%) |
|---|---|
| Age at operation (years old) | median 58 (range, 29–91) |
| Tumor size (mm) | median 13 (range, 3–20) |
| Estrogen receptor | |
| Negative / Positive | 107 (14.9%) / 612 (85.1%) |
| Progesterone receptor | |
| Negative / Positive | 321 (44.6%) / 398 (55.4%) |
| HER2 | |
| Negative / Positive | 653 (90.8%) / 66 (9.2%) |
| Ki67 | |
| ≤ 20% / > 20% | 586 (81.5%) / 133 (18.5%) |
| Intrinsic subtype | |
| HR + BC / HR-HER2 + BC / TNBC | 621 (86.3%) / 27 (3.8%) / 71 (9.9%) |
| Pathological axillary lymph node metastasis | |
| No metastasis / only isolated tumor cell / only micrometastasis / metastasis | 573 (79.7%) / 5 (0.7%) / 29 (4.0%) / 112 (15.6%) |
| Lymph vascular invasion | |
| No / Yes | 528 (73.4%) / 191 (26.6%) |
| Hyperlipidemia | |
| No / Yes | 572 (79.6%) / 147 (20.4%) |
| Number of medicine types for hyperlipidemia | |
| 0 / 1 / 2 | 572 (79.6%) / 139 (19.3%) / 8 (1.1%) |
| Statins | |
| Non-user / User | 587 (81.6%) / 132 (18.4%) |
| Lipophilic statins | |
| Non-user / User | 658 (91.5%) / 61 (8.5%) |
| Hydrophilic statins | |
| Non-user / User | 648 (90.1%) / 71 (9.9%) |
| Fibrate | |
| Non-user / User | 709 (98.6%) / 10 (1.4%) |
| Nicotinic acid (tocopherol acetate) | |
| Non-user / User | 712 (99.0%) / 7 (1.0%) |
| Sterol absorption inhibitors (ezetimibe) | |
| Non-user / User | 713 (99.2%) / 6 (0.8%) |
HER2 Human epidermal growth factor receptor 2, HR + BC Hormone receptor-positive breast cancer (ER+ and/or PgR+), HR-HER2 + BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+), TNBC Triple negative breast cancer (ER-, PgR-, and HER2-)
Postoperative pathological examinations revealed no axillary lymph node metastasis in 607 patients (84.4%), including five patients (0.7%) with isolated tumor cells and 29 patients (4.0%) with micrometastases based on sentinel lymph node biopsies. The median number of lymph node metastases in 112 patients (15.6%) with axillary lymph node metastases was two (range, 1–26). Lymphovascular invasion was detected in 191 patients (26.6%).
At the time of breast cancer diagnosis, 147 patients (20.4%) were undergoing treatment with orally administered drugs for hyperlipidemia. Among them, only eight patients (1.1%) were taking multiple drugs, whereas most were treated with single drugs. Among the 132 patients (18.4%) who were being treated with statins, 61 patients (8.5%) were taking lipophilic statins, and 71 patients (9.9%), about half, were taking hydrophilic statins. Specifically, rosuvastatin, one of the hydrophilic statins, users were 36 patients (5.0%) and pravastatin users were 35 patients (4.9%). On the other hand, the results for lipophilic statins were as follows: atorvastatin; 27 patients (3.8%), pitavastatin; 20 patients (2.8%), simvastatin; 13 patients (1.8%), and fluvastatin 1 patients (0.1%). There were 10 fibrate users (1.4%), seven nicotinic acid (tocopherol acetate) users (1.0%), and six sterol absorption inhibitors (ezetimibe) users (0.8%).
Correlations between clinicopathological features and axillary lymph node metastasis
The correlations between clinicopathological features and axillary lymph node metastasis are listed in Table 2. Metastasis occurred significantly more frequently when the breast cancer tumor diameter exceeded 10 mm (p < 0.001). Although the relationship was not statistically significant, metastases tended to be found in PgR-positive breast cancer cases (p = 0.063). Metastases occurred significantly more frequently in breast cancer cases involving lymphovascular invasion (p < 0.001). Regarding hyperlipidemia drugs, no correlation was found between statin use in general and lymph node metastasis (p = 0.226); however, a significant correlation was found between the use of lipophilic statins and lymph node metastasis (p = 0.042).
Table 2.
Correlation between axillary lymph node metastasis and clinicopathological features
| Parameters | Axillary lymph node metastasis | p value | |
|---|---|---|---|
| No metastasis, including even micrometasis (n = 607) | metastasis (n = 112) | ||
| Age at operation (years old) | 0.872 | ||
| ≤ 60 | 331 (54.5%) | 62 (55.4%) | |
| > 60 | 276 (45.5%) | 50 (44.6%) | |
| Tumor size (mm) | < 0.001 | ||
| ≤ 10.0 | 202 (33.3%) | 15 (13.4%) | |
| > 10.0 | 405 (66.7%) | 97 (86.6%) | |
| Estrogen receptor | 0.441 | ||
| Negative | 93 (15.3%) | 14 (12.5%) | |
| Positive | 514 (84.7%) | 98 (87.5%) | |
| Progesterone receptor | 0.063 | ||
| Negative | 280 (46.1%) | 41 (36.6%) | |
| Positive | 327 (53.9%) | 71 (63.4%) | |
| HER2 | 0.920 | ||
| Negative | 551 (90.8%) | 102 (91.1%) | |
| Positive | 56 (9.2%) | 10 (8.9%) | |
| Ki67 | 0.734 | ||
| ≤ 20% | 496 (81.7%) | 90 (80.4%) | |
| > 20% | 111 (18.3%) | 22 (19.6%) | |
| Intrinsic subtype HR + BC | 0.201 | ||
| No | 87 (14.3%) | 11 (9.8%) | |
| Yes | 520 (85.7%) | 101 (90.2%) | |
| Intrinsic subtype HR-HER2 + BC | 0.911 | ||
| No | 584 (96.2%) | 108 (96.4%) | |
| Yes | 23 (3.8%) | 4 (3.6%) | |
| Intrinsic subtype TNBC | 0.162 | ||
| No | 543 (89.5%) | 105 (93.8%) | |
| Yes | 64 (10.5%) | 7 (6.3%) | |
| Lymph vascular invasion | < 0.001 | ||
| No | 478 (78.7%) | 50 (44.6%) | |
| Yes | 129 (21.3%) | 62 (55.4%) | |
| Hyperlipidemia | 0.212 | ||
| No | 478 (78.7%) | 94 (83.9%) | |
| Yes | 129 (21.3%) | 18 (16.1%) | |
| Multiple medicine types for hyperlipidemia | 0.460 | ||
| No | 601 (99.0%) | 110 (98.2%) | |
| Yes | 6 (1.0%) | 2 (1.8%) | |
| Statins | 0.226 | ||
| Non-user | 491 (80.9%) | 96 (85.7%) | |
| User | 116 (19.1%) | 16 (14.3%) | |
| Lipophilic statins | 0.042 | ||
| Non-user | 550 (90.6%) | 108 (96.4%) | |
| User | 57 (9.4%) | 4 (3.6%) | |
| Hydrophilic statins | 0.746 | ||
| Non-user | 548 (90.3%) | 100 (89.3%) | |
| User | 59 (9.7%) | 12 (10.7%) | |
| Fibrate | 0.624 | ||
| Non-user | 598 (98.5%) | 111 (99.1%) | |
| User | 9 (1.5%) | 1 (0.9%) | |
| Nicotinic acid (tocopherol acetate) | 0.341 | ||
| Non-user | 602 (99.2%) | 110 (98.2%) | |
| User | 5 (0.8%) | 2 (1.8%) | |
| Sterol absorption inhibitors (ezetimibe) | 0.941 | ||
| Non-user | 602 (99.2%) | 111 (99.1%) | |
| User | 5 (0.8%) | 1 (0.9%) | |
HER2 Human epidermal growth factor receptor 2, HR + BC Hormone receptor-positive breast cancer (ER+ and/or PgR+), HR-HER2 + BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+), TNBC Triple negative breast cancer (ER-, PgR-, and HER2-)
Examination of the correlation between lipophilic statin use and clinicopathological factors revealed that the users were significantly older than the non-users (p < 0.001) (Table 3).
Table 3.
Correlation between lipophilic statins user and clinicopathological features
| Parameters | Lipophilic statins | p value | |
|---|---|---|---|
| Non-user (n = 658) | User (n = 61) | ||
| Age at operation (years old) | < 0.001 | ||
| ≤ 60 | 388 (59.0%) | 5 (8.2%) | |
| > 60 | 270 (41.0%) | 56 (91.8%) | |
| Tumor size (mm) | 0.450 | ||
| ≤ 10.0 | 196 (29.8%) | 21 (34.4%) | |
| > 10.0 | 462 (70.2%) | 40 (65.6%) | |
| Estrogen receptor | 0.247 | ||
| Negative | 101 (15.3%) | 6 (9.8%) | |
| Positive | 557 (84.7%) | 55 (90.2%) | |
| Progesterone receptor | 0.548 | ||
| Negative | 296 (45.0%) | 25 (41.0%) | |
| Positive | 362 (55.0%) | 36 (59.0%) | |
| HER2 | 0.228 | ||
| Negative | 595 (90.4%) | 58 (95.1%) | |
| Positive | 63 (9.6%) | 3 (4.9%) | |
| Ki67 | 0.554 | ||
| ≤ 20% | 538 (81.8%) | 48 (78.7%) | |
| > 20% | 120 (18.2%) | 13 (21.3%) | |
| Intrinsic subtype HR + BC | 0.367 | ||
| No | 92 (14.0%) | 6 (9.8%) | |
| Yes | 566 (86.0%) | 55 (90.2%) | |
| Intrinsic subtype HR-HER2 + BC | 0.107 | ||
| No | 631 (95.9%) | 61 (100.0%) | |
| Yes | 27 (4.1%) | 0 (0.0%) | |
| Intrinsic subtype TNBC | 0.992 | ||
| No | 593 (90.1%) | 55 (90.2%) | |
| Yes | 65 (9.9%) | 6 (9.8%) | |
| Lymph vascular invasion | 0.504 | ||
| No | 481 (73.1%) | 47 (77.0%) | |
| Yes | 177 (26.9%) | 14 (23.0%) | |
| Hyperlipidemia | < 0.001 | ||
| No | 572 (86.9%) | 0 (0.0%) | |
| Yes | 86 (13.1%) | 61 (100.0%) | |
| Multiple medicine types for hyperlipidemia | 0.003 | ||
| No | 653 (99.2%) | 58 (95.1%) | |
| Yes | 5 (0.8%) | 3 (4.9%) | |
| Hydrophilic statins | 0.007 | ||
| Non-user | 587 (89.2%) | 61 (100.0%) | |
| User | 71 (10.8%) | 0 (0.0%) | |
| Fibrate | 0.862 | ||
| Non-user | 649 (98.6%) | 60 (98.4%) | |
| User | 9 (1.4%) | 1 (1.6%) | |
| Nicotinic acid (tocopherol acetate) | 0.055 | ||
| Non-user | 653 (99.2%) | 59 (96.7%) | |
| User | 5 (0.8%) | 2 (3.3%) | |
| Sterol absorption inhibitors (ezetimibe) | 0.454 | ||
| Non-user | 652 (99.1%) | 61 (100.0%) | |
| User | 6 (0.9%) | 0 (0.0%) | |
HER2 Human epidermal growth factor receptor 2. HR + BC Hormone receptor-positive breast cancer (ER+ and/or PgR+). HR-HER2 + BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+), TNBC Triple negative breast cancer (ER-, PgR-, and HER2-)
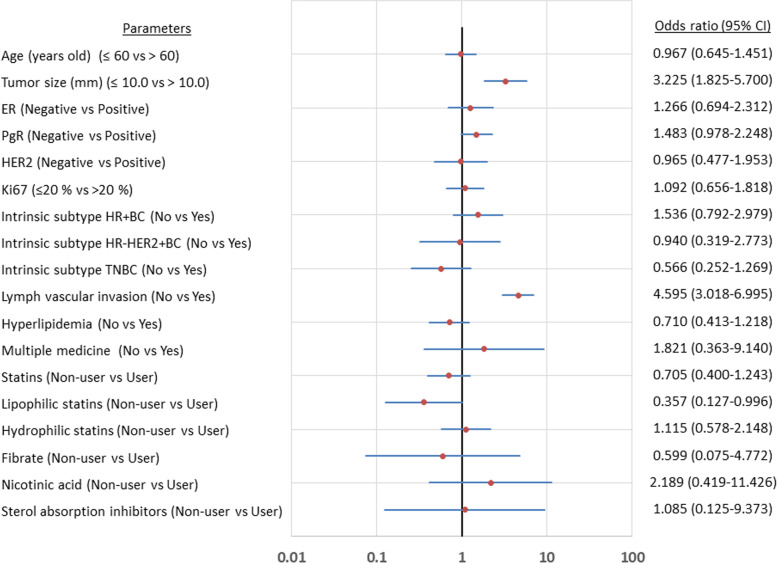
We examined the factors causing axillary lymph node metastasis in patients with cT1 breast cancer; tumor size (p < 0.001, OR = 3.225) and lymphovascular invasion (p < 0.001, OR = 4.595), as well as the use of lipophilic statins (p = 0.042, OR = 0.357) were the factors associated with axillary lymph node metastasis (Table 4) (Fig. 1). Even after performing the multivariate analysis, these remained independent factors (tumor size: p = 0.003, OR = 2.352; lymphovascular invasion: p < 0.001, OR = 3.891; lipophilic statin use: p = 0.048, OR = 0.384). Thus, lipophilic statin was the only factor that reduced axillary lymph node metastasis.
Table 4.
Univariate and multivariate analysis with axillary lymph node metastasis for cT1 breast cancer
| Parameters | Univarite analysis | Multivarite analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Age at operation (years old) | ||||||
| ≤ 60 vs > 60 | 0.967 | 0.645–1.451 | 0.872 | |||
| Tumor size (mm) | ||||||
| ≤ 10.0 vs > 10.0 | 3.225 | 1.825–5.700 | < 0.001 | 2.352 | 1.337–4.391 | 0.003 |
| Estrogen receptor | ||||||
| Negative vs Positive | 1.266 | 0.694–2.312 | 0.441 | |||
| Progesterone receptor | ||||||
| Negative vs Positive | 1.483 | 0.978–2.248 | 0.063 | 1.457 | 0.945–2.269 | 0.089 |
| HER2 | ||||||
| Negative vs Positive | 0.965 | 0.477–1.953 | 0.920 | |||
| Ki67 | ||||||
| ≤ 20% vs > 20% | 1.092 | 0.656–1.818 | 0.734 | |||
| Intrinsic subtype HR + BC | ||||||
| No vs Yes | 1.536 | 0.792–2.979 | 0.201 | |||
| Intrinsic subtype HR-HER2 + BC | ||||||
| No vs Yes | 0.940 | 0.319–2.773 | 0.911 | |||
| Intrinsic subtype TNBC | ||||||
| No vs Yes | 0.566 | 0.252–1.269 | 0.162 | |||
| Lymph vascular invasion | ||||||
| No vs Yes | 4.595 | 3.018–6.995 | < 0.001 | 3.891 | 2.529–6.016 | < 0.001 |
| Hyperlipidemia | ||||||
| No vs Yes | 0.710 | 0.413–1.218 | 0.212 | |||
| Multiple medicine types for hyperlipidemia | ||||||
| No vs Yes | 1.821 | 0.363–9.140 | 0.460 | |||
| Statins | ||||||
| Non-user vs User | 0.705 | 0.400–1.243 | 0.226 | |||
| Lipophilic statins | ||||||
| Non-user vs User | 0.357 | 0.127–0.996 | 0.042 | 0.384 | 0.113–0.987 | 0.048 |
| Hydrophilic statins | ||||||
| Non-user vs User | 1.115 | 0.578–2.148 | 0.746 | |||
| Fibrate | ||||||
| Non-user vs User | 0.599 | 0.075–4.772 | 0.624 | |||
| Nicotinic acid (tocopherol acetate) | ||||||
| Non-user vs User | 2.189 | 0.419–11.426 | 0.341 | |||
| Sterol absorption inhibitors (ezetimibe) | ||||||
| Non-user vs User | 1.085 | 0.125–9.373 | 0.941 | |||
HER2 Human epidermal growth factor receptor 2, HR + BC Hormone receptor-positive breast cancer (ER+ and/or PgR+), HR-HER2 + BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+), TNBC Triple negative breast cancer (ER-, PgR-, and HER2-), CI Confidence intervals
Fig. 1.
Forest plot showed odd ratios for the univariate association of the risk factors for axillary lymph node metastasis
Effects of lipophilic statins on prognosis
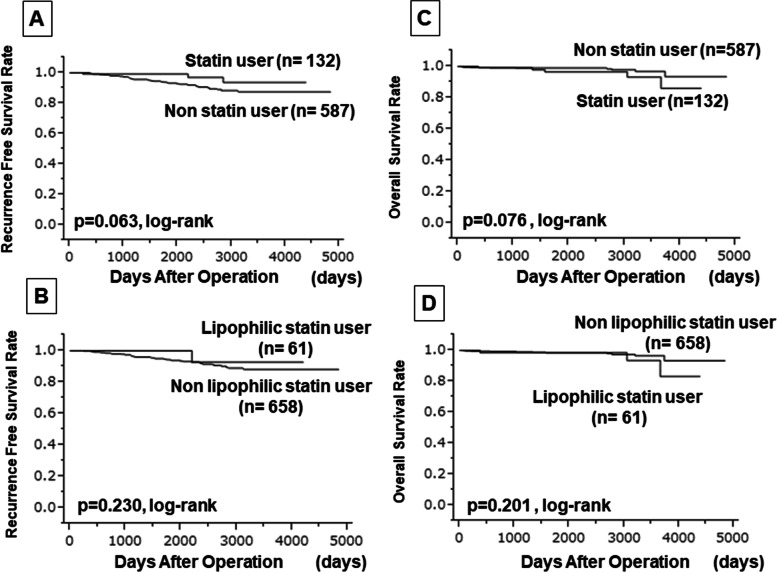
We examined the prognosis of 719 patients with cT1 breast cancer included in this study. The median follow-up period was 1838 days (range, 54–4841 days). During that period, 42 patients (5.8%) experienced recurrence, three patients (0.4%) died from breast cancer, and 11 patients (1.5%) died from other causes. Univariate analysis of disease-free survival (DFS) times showed that tumor size affected prognosis (p = 0.011, HR: 2.902) and that vascular infiltration tended to lead to a poor prognosis (p = 0.086, HR: 1.712) (Online Resource Supplementary Table 1). Among the factors, the treatment of hyperlipidemia (p = 0.047, HR: 0.399) and statin use (p = 0.028, HR: 0.328) were associated with prolonged DFS periods. In the multivariate analysis, only tumor size was an independent factor (p = 0.025, HR: 2.620). Similarly, in the univariate analysis for RFS, tumor size (p = 0.017, HR: 2.732) as well as statin use (p = 0.038, HR: 0.345) affected prognosis (Table 5) (Fig. 2). No clinicopathological factors significantly affected OS (Table 6).
Table 5.
Univariate and multivariate analysis with recurrence-free survival for cT1 breast cancer
| Parameters | Univarite analysis | Multivarite analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Age at operation (years old) | ||||||
| ≤ 60 vs > 60 | 0.607 | 0.310–1.133 | 0.119 | |||
| Tumor size (mm) | ||||||
| ≤ 10.0 vs > 10.0 | 2.732 | 1.177–7.946 | 0.017 | 2.658 | 1.14–7.739 | 0.021 |
| Estrogen receptor | ||||||
| Negative vs Positive | 1.166 | 0.549–2.870 | 0.707 | |||
| Progesterone receptor | ||||||
| Negative vs Positive | 1.509 | 0.808–2.950 | 0.200 | |||
| HER2 | ||||||
| Negative vs Positive | 0.887 | 0.214–2.449 | 0.839 | |||
| Ki67 | ||||||
| ≤ 20% vs > 20% | 0.798 | 0.274–1.854 | 0.626 | |||
| Intrinsic subtype HRBC | ||||||
| No vs Yes | 1.216 | 0.551–3.213 | 0.651 | |||
| Intrinsic subtype HER2BC | ||||||
| No vs Yes | 0.536 | 0.030–2.460 | 0.494 | |||
| Intrinsic subtype TNBC | ||||||
| No vs Yes | 0.947 | 0.325–2.201 | 0.909 | |||
| Pathological axillary lymph node metastasis | ||||||
| No metastasis vs Metastasis | 0.945 | 0.385–2.002 | 0.891 | |||
| Lymph vascular invasion | ||||||
| No vs Yes | 1.673 | 0.888–3.076 | 0.109 | |||
| Hyperlipidemia | ||||||
| No vs Yes | 0.421 | 0.126–1.047 | 0.064 | 1.014 | 0.057–4.693 | 0.989 |
| Multiple medicine types for hyperlipidemia | ||||||
| No vs Yes | – | – | 0.294 | |||
| Statins | ||||||
| Non-user vs User | 0.345 | 0.083–0.951 | 0.038 | 0.353 | 0.045–7.151 | 0.413 |
| Lipophilic statins | ||||||
| Non-user vs User | 0.316 | 0.018–1.451 | 0.166 | |||
| Hydrophilic statins | ||||||
| Non-user vs User | 0.402 | 0.065–1.307 | 0.147 | |||
| Fibrate | ||||||
| Non-user vs User | 1.9701 | 0.111–9.059 | 0.545 | |||
| Nicotinic acid (tocopherol acetate) | ||||||
| Non-user vs User | – | – | 0.345 | |||
| Sterol absorption inhibitors (ezetimibe) | ||||||
| Non-user vs User | – | – | 0.409 | |||
HER2 Human epidermal growth factor receptor 2, HRBC Hormone receptor-positive breast cancer (ER+ and/or PgR+), HER2BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+), TNBC Triple negative breast cancer (ER-, PgR-, and HER2-), CI Confidence intervals
Fig. 2.
Kaplan–Meier method comparing recurrence-free survival (RFS) and overall survival (OS) by statin or lipophilic statin. There was no significant difference in RFS due to statin (A) and lipophilic statin (B). No significant difference was found in OS due to statin (C) and lipophilic statin (D)
Table 6.
Univariate and multivariate analysis with overall survival for cT1 breast cancer
| Parameters | Univarite analysis | Multivarite analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Age at operation (years old) | ||||||
| ≤ 60 vs > 60 | 2.351 | 0.809–7.677 | 0.117 | |||
| Tumor size (mm) | ||||||
| ≤ 10.0 vs > 10.0 | 1.323 | 0.412–5.861 | 0.660 | |||
| Estrogen receptor | ||||||
| Negative vs Positive | 3.092 | 0.612–56.249 | 0.202 | |||
| Progesterone receptor | ||||||
| Negative vs Positive | 2.809 | 0.876–12.425 | 0.085 | 2.654 | 0.824–11.770 | 0.106 |
| HER2 | ||||||
| Negative vs Positive | 0.870 | 0.048–4.383 | 0.892 | |||
| Ki67 | ||||||
| ≤ 20% vs > 20% | 2.381 | 0.649–7.178 | 0.174 | |||
| Intrinsic subtype HRBC | ||||||
| No vs Yes | 2.596 | 0.515–47.194 | 0.292 | |||
| Intrinsic subtype HER2BC | ||||||
| No vs Yes | – | – | 0.243 | |||
| Intrinsic subtype TNBC | ||||||
| No vs Yes | 0.571 | 0.031–2.879 | 0.559 | |||
| Pathological axillary lymph node metastasis | ||||||
| No metastasis vs Metastasis | 0.858 | 0.133–3.150 | 0.838 | |||
| Lymph vascular invasion | ||||||
| No vs Yes | 1.324 | 0.406–3.839 | 0.621 | |||
| Hyperlipidemia | ||||||
| No vs Yes | 2.373 | 0.727–6.910 | 0.143 | |||
| Multiple medicine types for hyperlipidemia | ||||||
| No vs Yes | 6.109 | 0.335–31.184 | 0.169 | |||
| Statins | ||||||
| Non-user vs User | 2.605 | 0.798–7.578 | 0.107 | 2.425 | 0.742–7.057 | 0.134 |
| Lipophilic statins | ||||||
| Non-user vs User | 2.287 | 0.355–8.449 | 0.328 | |||
| Hydrophilic statins | ||||||
| Non-user vs User | 2.253 | 0.508–7.247 | 0.251 | |||
| Fibrate | ||||||
| Non-user vs User | 7.765 | 0.424–40.367 | 0.131 | |||
| Nicotinic acid (tocopherol acetate) | ||||||
| Non-user vs User | – | – | 0.591 | |||
| Sterol absorption inhibitors (ezetimibe) | ||||||
| Non-user vs User | – | – | 0.672 | |||
HER2 Human epidermal growth factor receptor 2, HRBC Hormone receptor-positive breast cancer (ER+ and/or PgR+), HER2BC Human epidermal growth factor receptor 2-enriched breast cancer (ER-, PgR-, and HER2+). TNBC Triple negative breast cancer (ER-, PgR-, and HER2-), CI Confidence intervals
The prognoses were examined among the 607 patients who did not have macrometastases, and similar results were obtained. The median follow-up period was 1825 days (range, 54–4841 days). During that period, 35 patients (5.8%) experienced recurrence, two patients (0.3%) died from breast cancer, and 10 patients (1.6%) died from other causes. In the univariate analysis for DFS, tumor size (p = 0.084, HR: 1.888) and PgR status (p = 0.032, HR: 1.977) affected prognosis, whereas the use of hyperlipidemic drugs did not (Online Resource Supplementary Table 2). On the other hand, in the univariate analysis for RFS, tumor size (p = 0.036, HR: 2.493) and PgR status (p = 0.043, HR: 2.064) affected the prognosis (Online Resource Supplementary Table 3). The analysis revealed that statin use (p = 0.096, HR: 0.411) tended to affect prognosis, but this did not reach statistical significance (Online Resource Supplementary Fig. 1). In the univariate analysis for OS, statin use (p = 0.047, HR: 3.460) was poor prognostic factor; in the multivariate analysis, no independent factors were found (Online Resource Supplementary Table 4).
Discussion
In a study examining the correlation between lymph node metastasis and clinicopathological features among 91,364 patients with T1 breast cancer using information from the “Surveillance, Epidemiology, and End Results Program (SEER)” study, age, race, primary site, tumor size, and ER, PgR, and HER2 status were influencing factors [38]. Tumor size and lymphovascular invasion are cited as risk factors for lymph node metastasis in most studies involving sentinel lymph node biopsy [39–46]. This result also shows that tumor size and lymphovascular invasion were strongly correlated with lymph node metastasis, which is consistent with previously reported results. Among the investigated factors, this study showed that the use of lipophilic statins may suppress lymph node metastasis. In preclinical studies, statins have been shown to exhibit anti-proliferative on cancer by being associated with mechanisms that drive cell cycle disruption in cancer cells [13–17]. Many studies have investigated the effects of factors capable of suppressing the risk of breast cancer and its recurrence, and there have also been some reports examining the effects of statins on suppressing the progression of breast cancer. For example, when examining the correlation between statin use and clinicopathological factors at the time of diagnosis in about 2000 and 3000 breast cancer patients, respectively, the rates of diagnosis for breast cancer with high pathological malignancy and for highly advanced breast cancer were significantly lower in statin users than in non-users [27, 28]. In addition, a study of approximately 130,000 postmenopausal women conducted by the Women’s Health Initiative reported that the use of lipophilic statins reduced the rate of diagnosis of highly advanced breast cancer [29]. However, the opportunity for patient consultation is likely to strongly influence these results. On the other hand, in this study, the tumor size based on the TNM classification was used as a condition for examination; this methodology is different from that of previous reports. In preclinical studies, anti-invasive properties have also been reported [18–22], as have metastasis-suppressing effects [23–26]. This study demonstrates the possibility of suppressing lymph node metastasis in clinical practice, which could improve prognosis.
Based on many results from preclinical studies, it is expected that statins should suppress the risk of breast cancer and its recurrence. However, in clinical practice, contradictory results have been reported regarding the suppressing effect of statins on breast cancer risk [6, 47, 48]. One reports have discussed why prospective studies with statins have not yielded the expected results [49]. On the other hand, many studies have reported that statins reduce the risk of breast cancer recurrence, and some groups have reported that only lipophilic statins are effective, not hydrophilic statins [4, 5, 7–10, 12, 42]. A report indicated that effects may vary considerably among lipophilic statins [49]. The classification of statins in this study was the same as that used in a meta-analysis that examined the correlation between statin type and breast cancer prognosis [50]. In this study, statins reduced OS in patients without lymph node metastases. However, this result is likely due to the fact that only two patients (0.3%) died from breast cancer and 10 patients (1.6%) died from other causes. Breast-cancer-specific survival could not be examined due to the low numbers of breast cancer-related deaths; therefore, the results pertaining to OS in this study should be considered for reference. However, statin use tended to prolong the RFS period, instead of the DFS period. Regarding this result, the event point was narrowed down to the day of recurrence / death from breast cancer, suggesting that statins may have a positive effect on the treatment of early-stage breast cancer.
This study has some limitations that should be considered. First, patients receiving preoperative chemotherapy were excluded, as the evaluation of axillary lymph node metastasis is uncertain based on diagnostic imaging alone. Since it is known that the therapeutic effect of preoperative chemotherapy is a predictor of prognosis in HER2-positive breast cancer and TNBC [51–54], preoperative chemotherapy is actively performed for those types of breast cancer. The number of patients with HER2-positive breast cancer and TNBC was low, which could have been a source of bias in this study. In addition, statin was correlated with age, although age itself had no clear effect on axillary lymph node metastasis or prognosis in this study, it may have a significant effect. Moreover, one of the limitations was the exclusion of cases involving a primary lesion of 20 mm or less, accompanied by advanced regional lymph node metastasis or distant metastasis. Another limitation was that the duration of oral treatment for hyperlipidemia was unknown for each patient. However, clinical data, rather than in vivo or in vitro data, suggest that lipophilic statins may suppress breast cancer metastasis to lymph nodes. Furthermore, it was suggested that statins may suppress postoperative recurrence. Regarding the examination and treatment of axillary lymph nodes, in recent years, even sentinel lymph node biopsy has been deemed an overly invasive procedure for early-stage breast cancer cases, so clinical trials are underway to omit sentinel lymph node biopsies from the protocols for cN0 breast cancer cases assessed using US [55, 56]. It is also possible that lipophilic statins may have affected the results of these clinical trials. Regarding the prognosis, some studies have reported that even if statins are administered after the diagnosis of breast cancer, they may suppress the recurrence of breast cancer [4, 5, 7, 9, 10, 30]. Especially in ER-positive breast cancer, the effects driving the suppression of the risk of recurrence are well-recognized [5, 30]. The fact that the prognosis was affected in this study may have been due to the fact that ER-positive breast cancer patients accounted for the majority of the cases. This study suggests the possibility of improving the prognosis of breast cancer patients through treatment with statins.
Conclusions
In patients with cT1 breast cancer, the results suggest that oral statin therapy may contribute to favorable outcomes.
Supplementary Information
Additional file 1: Supplementary Fig. 1. Kaplan–Meier method comparing recurrence-free survival (RFS) and overall survival (OS) by statin or lipophilic statin in patients without lymph node metastasis. There was no significant difference in RFS due to statin (A) and lipophilic statin (B). However, statin user had poor OS (p = 0.025, log-rank) (C). No impact on OS was found in lipophilic statin(D).
Additional file 2: Supplementary Table 1. Univariate and multivariate analysis with disease-free survival for cT1 breast cancer. Supplementary Table 2. Univariate and multivariate analysis with disease-free survival for cT1 breast cancer with no axillary lymph node metastasis pathologically. Supplementary Table 3. Univariate and multivariate analysis with recurrence-free survival for cT1 breast cancer with no axillary lymph node metastasis pathologically. Supplementary Table 4. Univariate and multivariate analysis with overall survival for cT1 breast cancer with no axillary lymph node metastasis pathologically.
Acknowledgements
We thank Tomomi Okawa (Department of Breast Surgical Oncology, Osaka Metropolitan University Graduate School of Medicine) for helpful advice regarding data management.
Abbreviations
- CI
Confidence intervals
- CNB
Core needle biopsy
- CT
Computed tomography
- DFS
Disease-free survival
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- OR
Odds ratio
- OS
Overall survival
- PgR
Progesterone receptor
- RFS
Recurrence-free survival
- TNBC
Triple-negative breast cancer
- US
Ultrasonography
Authors’ contributions
KT participated in the design of the study and drafted the manuscript. SK participated in the design of the study and manuscript editing. NI, RK, AY, WG, YA, YT, TM and KO helped with study data collection and manuscript preparation. MS, HT and KM conceived the study, participated in its design and coordination and helped draft the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported in part by Grants-in-Aid for Scientific Research (KAKENHI, Nos. 17 K10559, 19 K18067 and 20 K08938) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Consent to publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Osaka Metropolitan University and abided with the ethical standards of the Helsinki Declaration on good clinical practice (Registration No.: 926, Date of Registration: 1/4/2018). Written informed consents was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koji Takada, Email: taka.cl22.kou.sp@gmail.com.
Shinichiro Kashiwagi, Email: spqv9ke9@view.ocn.ne.jp.
Nozomi Iimori, Email: nozomi_0422_fujita@yahoo.co.jp.
Rika Kouhashi, Email: kouhashi.r92@gmail.com.
Akimichi Yabumoto, Email: akimichi0924@icloud.com.
Wataru Goto, Email: saraikazemaru@gmail.com.
Yuka Asano, Email: asnyk0325@yahoo.co.jp.
Yukie Tauchi, Email: yukie_05_g@yahoo.co.jp.
Tamami Morisaki, Email: spitz4_5@yahoo.co.jp.
Kana Ogisawa, Email: knogswnico@gmail.com.
Masatsune Shibutani, Email: fbxbj429@ybb.ne.jp.
Hiroaki Tanaka, Email: hiroakitan@med.osaka-cu.ac.jp.
Kiyoshi Maeda, Email: kmaeda@omu.ac.jp.
References
- 1.Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–2195. doi: 10.1093/annonc/mdw410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park YM, Bookwalter DB, O'Brien KM, Jackson CL, Weinberg CR, Sandler DP. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann Oncol. 2021;32(3):351–359. doi: 10.1016/j.annonc.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni H, Rui Q, Zhu X, Yu Z, Gao R, Liu H. Antihypertensive drug use and breast cancer risk: a meta-analysis of observational studies. Oncotarget. 2017;8(37):62545–62560. doi: 10.18632/oncotarget.19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Investig. 2011;29(9):585–593. doi: 10.3109/07357907.2011.616252. [DOI] [PubMed] [Google Scholar]
- 5.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, Paskett ED, Vitolins MZ, Furberg CD, Chlebowski RT, Women's Health Initiative Research G Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst. 2006;98(10):700–707. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 7.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109(3):573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int J Cancer. 2016;139(6):1281–1288. doi: 10.1002/ijc.30185. [DOI] [PubMed] [Google Scholar]
- 9.Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology. 2015;26(1):68–78. doi: 10.1097/EDE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 10.Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS One. 2014;9(10):e110231. doi: 10.1371/journal.pone.0110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Harborg S, Heide-Jorgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat. 2020;183(1):153–160. doi: 10.1007/s10549-020-05749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 14.Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22(7):1061–1067. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal B, Halmos B, Feoktistov AS, Protiva P, Ramey WG, Chen M, Pothoulakis C, Lamont JT, Holt PR. Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis. 2002;23(3):521–528. doi: 10.1093/carcin/23.3.521. [DOI] [PubMed] [Google Scholar]
- 16.Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, Carteni M, Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40(4):935–941. doi: 10.3892/ijo.2011.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC, Song IS. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int J Cancer. 2008;123(4):951–957. doi: 10.1002/ijc.23593. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 19.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110(3):285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holstein SA, Wohlford-Lenane CL, Hohl RJ. Isoprenoids influence expression of Ras and Ras-related proteins. Biochemistry. 2002;41(46):13698–13704. doi: 10.1021/bi026251x. [DOI] [PubMed] [Google Scholar]
- 21.Duncan RE, El-Sohemy A, Archer MC. Statins and cancer development. Cancer Epidemiol Biomark Prev. 2005;14(8):1897–1898. doi: 10.1158/1055-9965.EPI-05-0027. [DOI] [PubMed] [Google Scholar]
- 22.Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol. 1999;55(6):949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- 23.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, Soria J, Soria C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22(8):1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 24.Beckwitt CH, Clark AM, Ma B, Whaley D, Oltvai ZN, Wells A. Statins attenuate outgrowth of breast cancer metastases. Br J Cancer. 2018;119(9):1094–1105. doi: 10.1038/s41416-018-0267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018;20(1):144. doi: 10.1186/s13058-018-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal CC, Ghosh-Choudhury N, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011;286(13):11314–11327. doi: 10.1074/jbc.M110.193714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sendur MA, Aksoy S, Yazici O, Ozdemir NY, Zengin N, Altundag K. Statin use may improve clinicopathological characteristics and recurrence risk of invasive breast cancer. Med Oncol. 2014;31(2):835. doi: 10.1007/s12032-013-0835-x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar AS, Benz CC, Shim V, Minami CA, Moore DH, Esserman LJ. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomark Prev. 2008;17(5):1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- 29.Desai P, Lehman A, Chlebowski RT, Kwan ML, Arun M, Manson JE, Lavasani S, Wasswertheil-Smoller S, Sarto GE, LeBoff M, Cauley J, Cote M, Beebe-Dimmer J, Jay A, Simon MS. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control. 2015;26(4):529–539. doi: 10.1007/s10552-015-0530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu WH, Zhou YH. The relationship between post-diagnostic statin usage and breast cancer prognosis varies by hormone receptor phenotype: a systemic review and meta-analysis. Arch Gynecol Obstet. 2021;304(5):1315–1321. doi: 10.1007/s00404-021-06065-z. [DOI] [PubMed] [Google Scholar]
- 31.White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42(9):963–970. doi: 10.1177/009127000204200902. [DOI] [PubMed] [Google Scholar]
- 32.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84(3):413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 33.McMasters KM, Tuttle TM, Carlson DJ, Brown CM, Noyes RD, Glaser RL, Vennekotter DJ, Turk PS, Tate PS, Sardi A, Cerrito PB, Edwards MJ. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18(13):2560–2566. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwagi S, Onoda N, Asano Y, Kurata K, Noda S, Kawajiri H, Takashima T, Ohsawa M, Kitagawa S, Hirakawa K. Ambulatory sentinel lymph node biopsy preceding neoadjuvant therapy in patients with operable breast cancer: a preliminary study. World J Surg Oncol. 2015;13:53. doi: 10.1186/s12957-015-0471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee A, Krishnamurthy S, Sahin A, Symmans WF, Hunt K, Sneige N. Intraoperative touch imprint of sentinel lymph nodes in breast carcinoma patients. Cancer. 2002;96(4):225–231. doi: 10.1002/cncr.10721. [DOI] [PubMed] [Google Scholar]
- 36.Khanna R, Bhadani S, Khanna S, Pandey M, Kumar M. Touch imprint cytology evaluation of sentinel lymph node in breast cancer. World J Surg. 2011;35(6):1254–1259. doi: 10.1007/s00268-011-1094-7. [DOI] [PubMed] [Google Scholar]
- 37.Houvenaeghel G, Nos C, Mignotte H, Classe JM, Giard S, Rouanet P, Lorca FP, Jacquemier J, Bardou VJ, Groupe des Chirurgiens de la Federation des Centres de Lutte Contre le C Micrometastases in sentinel lymph node in a multicentric study: predictive factors of nonsentinel lymph node involvement--Groupe des Chirurgiens de la Federation des Centres de Lutte Contre le Cancer. J Clin Oncol. 2006;24(12):1814–1822. doi: 10.1200/JCO.2005.03.3225. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YX, Liu YR, Xie S, Jiang YZ, Shao ZM. A Nomogram Predicting Lymph Node Metastasis in T1 Breast Cancer based on the Surveillance, Epidemiology, and End Results Program. J Cancer. 2019;10(11):2443–2449. doi: 10.7150/jca.30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capdet J, Martel P, Charitansky H, Lim YK, Ferron G, Battle L, Landier A, Mery E, Zerdoub S, Roche H, Querleu D. Factors predicting the sentinel node metastases in T1 breast cancer tumor: an analysis of 1416 cases. Eur J Surg Oncol. 2009;35(12):1245–1249. doi: 10.1016/j.ejso.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Viale G, Zurrida S, Maiorano E, Mazzarol G, Pruneri G, Paganelli G, Maisonneuve P, Veronesi U. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005;103(3):492–500. doi: 10.1002/cncr.20809. [DOI] [PubMed] [Google Scholar]
- 41.Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga JY, Alran S, Salmon RJ, Fourchotte V, Vincent-Salomon A, Sastre-Garau X, Antoine M, Uzan S, Sigal-Zafrani B, De Rycke Y. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6(5):e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Li J, Fan Y, Li X, Qiu J, Zhu M, Li H. Risk factors for axillary lymph node metastases in clinical stage T1-2N0M0 breast cancer patients. Medicine (Baltimore) 2019;98(40):e17481. doi: 10.1097/MD.0000000000017481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding J, Jiang L, Wu W. Predictive Value of Clinicopathological Characteristics for Sentinel Lymph Node Metastasis in Early Breast Cancer. Med Sci Monit. 2017;23:4102–4108. doi: 10.12659/msm.902795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshihara E, Smeets A, Laenen A, Reynders A, Soens J, Van Ongeval C, Moerman P, Paridaens R, Wildiers H, Neven P, Christiaens MR. Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast. 2013;22(3):357–361. doi: 10.1016/j.breast.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Klar M, Foeldi M, Markert S, Gitsch G, Stickeler E, Watermann D. Good prediction of the likelihood for sentinel lymph node metastasis by using the MSKCC nomogram in a German breast cancer population. Ann Surg Oncol. 2009;16(5):1136–1142. doi: 10.1245/s10434-009-0399-3. [DOI] [PubMed] [Google Scholar]
- 46.Qiu PF, Liu JJ, Wang YS, Yang GR, Liu YB, Sun X, Wang CJ, Zhang ZP. Risk factors for sentinel lymph node metastasis and validation study of the MSKCC nomogram in breast cancer patients. Jpn J Clin Oncol. 2012;42(11):1002–1007. doi: 10.1093/jjco/hys150. [DOI] [PubMed] [Google Scholar]
- 47.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005;165(19):2264–2271. doi: 10.1001/archinte.165.19.2264. [DOI] [PubMed] [Google Scholar]
- 48.Boudreau DM, Yu O, Miglioretti DL, Buist DS, Heckbert SR, Daling JR. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol Biomark Prev. 2007;16(3):416–421. doi: 10.1158/1055-9965.EPI-06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdullah MI, de Wolf E, Jawad MJ, Richardson A. The poor design of clinical trials of statins in oncology may explain their failure - Lessons for drug repurposing. Cancer Treat Rev. 2018;69:84–89. doi: 10.1016/j.ctrv.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Yi Z, Guan X, Zeng YX, Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat. 2017;164(1):1–11. doi: 10.1007/s10549-017-4246-0. [DOI] [PubMed] [Google Scholar]
- 51.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 52.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 53.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 54.Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J, Moldovan C, Bodmer A, Zaman K, Cufer T, Campone M, Luporsi E, Malmstrom P, Werutsky G, Bogaerts J, Bergh J, Cameron DA, investigators EBS (2014) Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol 25 (6):1128–1136. 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed]
- 55.Gentilini O, Botteri E, Dadda P, Sangalli C, Boccardo C, Peradze N, Ghisini R, Galimberti V, Veronesi P, Luini A, Cassano E, Viale G, Veronesi U. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur J Surg Oncol. 2016;42(5):685–689. doi: 10.1016/j.ejso.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Reimer T, Stachs A, Nekljudova V, Loibl S, Hartmann S, Wolter K, Hildebrandt G, Gerber B. Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1-2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial. Geburtshilfe Frauenheilkd. 2017;77(2):149–157. doi: 10.1055/s-0042-122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Fig. 1. Kaplan–Meier method comparing recurrence-free survival (RFS) and overall survival (OS) by statin or lipophilic statin in patients without lymph node metastasis. There was no significant difference in RFS due to statin (A) and lipophilic statin (B). However, statin user had poor OS (p = 0.025, log-rank) (C). No impact on OS was found in lipophilic statin(D).
Additional file 2: Supplementary Table 1. Univariate and multivariate analysis with disease-free survival for cT1 breast cancer. Supplementary Table 2. Univariate and multivariate analysis with disease-free survival for cT1 breast cancer with no axillary lymph node metastasis pathologically. Supplementary Table 3. Univariate and multivariate analysis with recurrence-free survival for cT1 breast cancer with no axillary lymph node metastasis pathologically. Supplementary Table 4. Univariate and multivariate analysis with overall survival for cT1 breast cancer with no axillary lymph node metastasis pathologically.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.