Abstract
Background
Artemisia subg. Seriphidium, one of the most species-diverse groups within Artemisia, grows mainly in arid or semi-arid regions in temperate climates. Some members have considerable medicinal, ecological, and economic value. Previous studies on this subgenus have been limited by a dearth of genetic information and inadequate sampling, hampering our understanding of their phylogenetics and evolutionary history. We therefore sequenced and compared the chloroplast genomes of this subgenus, and evaluated their phylogenetic relationships.
Results
We newly sequenced 18 chloroplast genomes of 16 subg. Seriphidium species and compared them with one previously published taxon. The chloroplast genomes, at 150,586–151,256 bp in length, comprised 133 genes, including 87 protein-coding genes, 37 tRNA genes, 8 rRNA genes, and one pseudogene, with GC content of 37.40–37.46%. Comparative analysis showed that genomic structures and gene order were relatively conserved, with only some variation in IR borders. A total of 2203 repeats (1385 SSRs and 818 LDRs) and 8 highly variable loci (trnK – rps16, trnE – ropB, trnT, ndhC – trnV, ndhF, rpl32 – trnL, ndhG – ndhI and ycf1) were detected in subg. Seriphidium chloroplast genomes. Phylogenetic analysis of the whole chloroplast genomes based on maximum likelihood and Bayesian inference analyses resolved subg. Seriphidium as polyphyletic, and segregated into two main clades, with the monospecific sect. Minchunensa embedded within sect. Seriphidium, suggesting that the whole chloroplast genomes can be used as molecular markers to infer the interspecific relationship of subg. Seriphidium taxa.
Conclusion
Our findings reveal inconsistencies between the molecular phylogeny and traditional taxonomy of the subg. Seriphidium and provide new insights into the evolutionary development of this complex taxon. Meanwhile, the whole chloroplast genomes with sufficiently polymorphic can be used as superbarcodes to resolve interspecific relationships in subg. Seriphidium.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-023-04113-1.
Keywords: Compositae, Comparative genomics, Molecular markers, Phylogenomics
Background
The genus Artemisia L., comprising ca. 500 herb and shrub species, is one of the largest in the Asteraceae [1–5]. Members of this genus are distributed mainly in temperate regions of the northern hemisphere [1, 6], with the current centers of species diversity located in China and surrounding areas followed by Russia and adjacent states, Europe, Americas and North Africa [7–9]. Artemisia typically attracts extensive scientific interest because of its antimalarial properties, and other pharmacological and economic value [1, 10, 11]. Although Artemisia is currently divided into the generally accepted five subgenera [subg. Artemisia, subg. Absinthium (Miller) Less., subg. Dracunculus (Besser) Rydb., subg. Tridentatae (Rydb.) McArthur. and subg. Seriphidium Besser ex Less] based on morphological and molecular data [12–18], there has been some controversy about its definition and infrageneric delimitation.
The subg. Seriphidium, one of the most diverse taxa in Artemisia [18], comprises ca. 130 species and 30 infraspecific taxa worldwide [3, 9, 19]. This subgenus grows mainly in arid and semi-arid regions of Central Asia and Northwest China, with a few species spreading to the Middle East, North Africa and Europe [19]. Its species are usually drought-, cold- and salinity-tolerant, and can become dominant in arid and semi-arid areas, playing an important ecological role in terms of wind and sand control [20]. In addition, some are rich in essential oils and terpenes, having anti-malarial, anticancer and antidiabetic properties [7, 20–22]. However, the gaps that remain in our knowledge of the subg. Seriphidium and of its taxonomic complexity still call for further research. Since Besser grouped all homogamous species of Artemisia in sect. Seriphidium Besser [23, 24], the first comprehensive revision of Seriphidium taxa was not published until 1961, Poljakov separated the homogamous species from Artemisia in Eurasia and established the new genus Seriphidium (Besser) Poljakov [25]. However, the same author did not follow his own proposal in Flora of the USSR published the same year and still treated Seriphidium as a subgenus within Artemisia [7], and divided the subg. Seriphidium into two sections: (i) sect. Seriphidium with pinnate-lobed leaves; and (ii) sect. Junceum with mostly 3-lobed lower stem leaves. After studying Seriphidium in Eurasia and North Africa, Filatova in 1986 proposed a different proposal from Poljakov’s on the two sections within subg. Seriphidium, dividing the subgenus into six sections [sect. Calciphilum, sect. Junceum, sect. Leucophyton, sect. Sclerophyllum, sect. Halophilum and sect. Pycnanthum] based on traits such as leaf type, leaf segments, involucre and florets [26].
When Ling studied the entire genus Artemisia and its allies [9, 19, 20, 27], he supported the taxonomic view of establishing Seriphidium as an independent genus based on homogamous flowers, involucral bracts multilayered and flowering pattern, and divided the 130 Seriphidium taxa (containing species and infraspecific taxa) into three sections: (i) sect. Seriphidium with pinnate-lobed leaves; (ii) sect. Junceum with mostly 3-lobed lower stem leaves; and (iii) sect. Minchunensa with pectinate or narrowly serrate pinnatisect leaves. The first two sections are similar in species composition to the two sections within subg. Seriphidium established by Poljakov. Moreover, sect. Junceum (A. juncea) and sect. Minchunensa (A. minchunensis) are both monospecific groups. However, the rationality of the classification of subg. Seriphidium based on morphological traits remains to be further explored.
In the past two decades, the emergence of molecular systematics has provided new methods for studying the systematic relationships between complex taxa [28]. Some molecular markers from both the nuclear and plastid genomes, including nuclear ribosomal DNA internal and external transcribed spacers (ITS and ETS) and chloroplast fragments (matK, rbcL, rpl32 – trnL, ndhC – trnV and psbA – trnH) have been used to estimate phylogenetic relationships within Artemisia [4, 5, 12–18, 29–32]. Unfortunately, the subg. Seriphidium has received less attention in comparison to other subgenera of Artemisia [18]. Furthermore, many of the prior phylogenetic studies of subg. Seriphidium [4, 18, 31], based on plastid or nuclear gene fragments, have achieved low resolution at major clade nodes, owing to the high sequence similarity between its closely related taxa arising from its rapid evolutionary radiation and hybridization. Recent molecular phylogenies did not support the traditional morphology-based subg. Seriphidium classifications, have revealed that it is not monophyletic [18]. At present, phylogenetic relationships among the major lineages of the subg. Seriphidium remain uncertain, such as owing to limited sampling, the systematic position of the Chinese endemic species A. minchunensis which constitutes the monospecific group (sect. Minchunensa) has not been clarified. Further investigations, based on a combination of representative sampling and sequences with rich genetic information, is therefore necessary to reconstruct these phylogenetic relationships.
The chloroplast, a multifunctional plant organelle, plays an important role in photosynthesis as well as various metabolic processes [33–35]. In most angiosperms, the complete chloroplast genome is usually a double-stranded, circular and quadripartite structure, consisting of four evolutionarily relatively conserved regions: a large single copy region (LSC), a small single copy region (SSC) and a pair of inverted repeat regions (IRa and IRb) [36–38]. Compared to plant mitochondrial and nuclear genomes, the chloroplast genomes of most land plants exhibit slow evolution and uniparental inheritance, and are appropriately sized and relatively conservative in structure [21, 39, 40]. Unlike gene fragments, complete chloroplast genome contains much genetic information and many mutation sites, contributing to resolving the complex evolutionary relationships in land plants [41]. The complete chloroplast genome is therefore widely used for phylogenetic inference and species delimitation, such as Ligularia (Asteraceae) [42], Amomum (Zingiberaceae) [43], Calligonum (Polygonaceae) [44], Ilex (Aquifoliaceae) [45] and Rhododendron (Ericaceae) [46]. It is worth noting that a recent study analyzed 18 Artemisia species from East Asia using the whole chloroplast genome, and the results showed that whole chloroplast genomes with sufficient polymorphic genetic information loci could be used to resolve interspecific relationships within Artemisia [47]. Unfortunately, this study did not include any subg. Seriphidium species. Nevertheless, this provides a reference for exploring the use of whole chloroplast genomes for resolving the systematic position and interspecific relationships of taxa in subg. Seriphidium.
To date, GenBank (National Center for Biotechnology Information; accessed 1 April 2022) contains the complete chloroplast genome for only one species (A. maritima) of subg. Seriphidium, accounting for ca. 1% of its species diversity. Based on the above problems of subg. Seriphidium, here we newly sequenced 18 complete chloroplast genomes from 16 subg. Seriphidium species, collected in arid and semi-arid regions of northwestern China and adjacent countries (Russia and Tajikistan). It is noteworthy that these samples have included representative species from three sections within subg. Seriphidium with reference to Ling (1991) [19], particularly A. minchunensis which constitutes the monospecific group (sect. Minchunensa). The main objectives of the present study were: (1) to examine variation in the structure and composition of subg. Seriphidium chloroplast genomes; (2) to assess the ability of the complete chloroplast genome to resolve interspecific relationships within this subgenus, and (3) to explore the systematic position of the main subg. Seriphidium taxa, especially A. minchunensis. This study provides guidance for the taxonomic revision of the entire subg. Seriphidium, and facilitates the development and utilization of its genetic resources.
Results
Subg. Seriphidium chloroplast genome structural variation
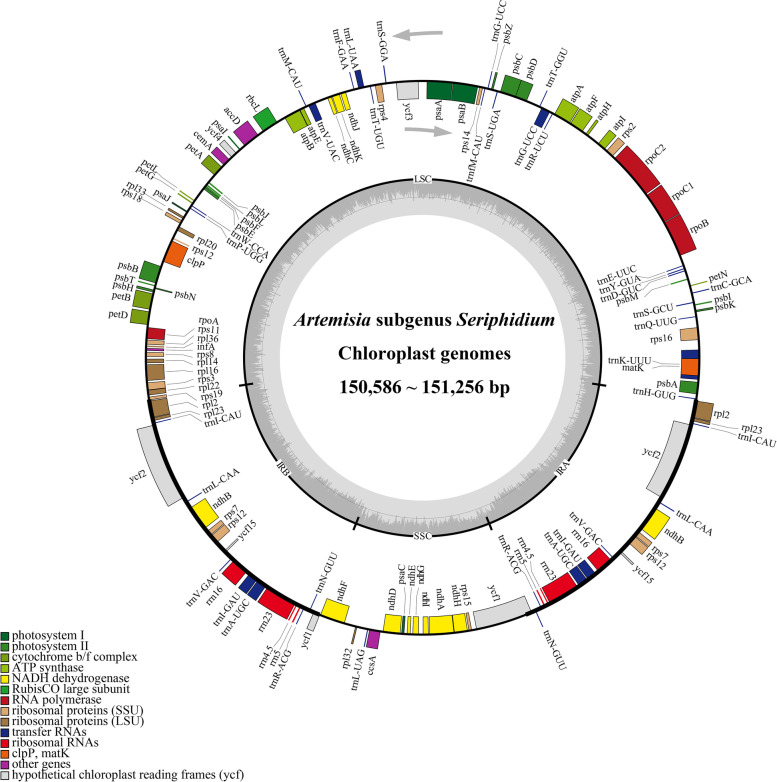
All of the 18 newly sequenced subg. Seriphidium chloroplast genomes possessed the typical vascular plant quadripartite structure, comprising LSC, SSC, IRa and IRb regions (Fig. 1). Genome length ranged from 150,586 bp (A. ferganensis) to 151,256 bp (A. santonicum). LSC region length ranged from 82,313 bp (A. ferganensis) to 82,976 bp (A. santonicum). SSC region length ranged from 18,329 bp (A. ferganensis) to 18,379 bp (A. santolina). IR region length ranged from 24,959 bp (A. sawanensis and A. schrenkiana) to 24,972 bp (A. ferganensis) (Table 1). Interestingly, while A. ferganensis had the shortest total chloroplast genome, and shortest LSC and SSC regions, it had the longest inverted repeat regions. There was slight variation in guanine-cytosine contents, at 37.40 to 37.46% (Table 1). All 18 plastomes contained 87 protein-coding genes, 37 transfer RNA (tRNA) genes, 8 ribosomal RNA (rRNA) genes, and one pseudogene, and exhibited the same order and orientation of syntenic blocks (Table 1; Additional file 1: Table S2; Additional file 2: Fig. S1), indicating that these chloroplast genomes are highly conserved and collinear.
Fig. 1.
Gene circle map of 16 newly sequenced Artemisia subg. Seriphidium species. Arrows indicate transcription direction. Genes located outside the outer circle were transcribed counter-clockwise, and those inside were transcribed clockwise. Colored bars indicate different functional groups. Thick lines of the large circle indicate the extent of inverted repeat regions (IRa and IRb) that separate the genome into large single and small copy regions (LSC and SSC, respectively). Darker gray columns in the inner circle correspond to guanine-cytosine content, and light gray to adenosine-thymine content
Table 1.
Summary of complete chloroplast genomes of 16 newly sequenced Artemisia subg. Seriphidium species
| Taxon | Sample ID | Herbarium /Voucher No. | Localities | Locations | Accession number | Gene number | Length (bp) | GC(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | E | CDS | tRNA | rRNA | Total | LSC | IRs | SSC | ||||||
| A. ferganensis | J-3 | XJBI/jgz-3 | China: Xinjiang | 39.535 | 75.614 | ON871797 | 87 | 37 | 8 | 150,586 | 82,313 | 24,972 | 18,329 | 37.46 |
| A. finita | J-23 | PE/8554 | China: Neimenggu | – | – | ON871798 | 87 | 37 | 8 | 151,157 | 82,868 | 24,969 | 18,351 | 37.46 |
| A. juncea | J-5-1 | XJBI/jgz-18-1 | China: Xinjiang | 43.867 | 87.564 | ON871799 | 87 | 37 | 8 | 151,004 | 82,749 | 24,962 | 18,331 | 37.46 |
| A. juncea | J-5-2 | XJBI/jgz-18-2 | China: Xinjiang | 43.867 | 87.564 | ON871800 | 87 | 37 | 8 | 151,004 | 82,749 | 24,962 | 18,331 | 37.46 |
| A. karatavica | J-25 | PE/1343386 | Russia | – | – | ON871801 | 87 | 37 | 8 | 151,114 | 82,806 | 24,969 | 18,370 | 37.44 |
| A. kaschgarica | J-4 | XJBI/jgz-045–2 | China: Xinjiang | 44.326 | 85.518 | OL890688 | 87 | 37 | 8 | 151,091 | 82,808 | 24,969 | 18,345 | 37.44 |
| A. lercheana | J-40 | PE/1341747 | Russia | – | – | ON871802 | 87 | 37 | 8 | 151,089 | 82,801 | 24,969 | 18,350 | 37.45 |
| A. leucotricha | J-42 | PE/1342944 | Tadzhikistan | – | – | ON871803 | 87 | 37 | 8 | 151,056 | 82,774 | 24,968 | 18,346 | 37.46 |
| A. minchunensis | J-2-1 | XJBI/jgz-103 | China: Gansu | 38.981 | 103.548 | ON871804 | 87 | 37 | 8 | 151,099 | 82,810 | 24,970 | 18,349 | 37.45 |
| A. minchunensis | J-2-1 | XJBI/jgz-104 | China: Gansu | 38.981 | 103.548 | ON871805 | 87 | 37 | 8 | 151,099 | 82,810 | 24,970 | 18,349 | 37.45 |
| A. santolina | J-6 | XJBI/jgz-052 | China: Xinjiang | 44.483 | 82.911 | ON871806 | 87 | 37 | 8 | 151,112 | 82,795 | 24,969 | 18,379 | 37.44 |
| A. santonicum | J-33 | PE/1339064 | Russia | – | – | ON871807 | 87 | 37 | 8 | 151,256 | 82,976 | 24,969 | 18,342 | 37.40 |
| A. sawanensis | J-7-5 | XJBI/jgz-086-3 | China: Xinjiang | 47.499 | 87.736 | ON871808 | 87 | 37 | 8 | 151,066 | 82,799 | 24,959 | 18,349 | 37.45 |
| A. schrenkiana | J-7-4 | XJBI/jgz-086-1 | China: Xinjiang | 47.499 | 87.736 | ON871809 | 87 | 37 | 8 | 151,073 | 82,806 | 24,959 | 18,349 | 37.45 |
| A. scopaeformis | J-26 | XJBI/jgz-43-1 | China: Xinjiang | 42.996 | 88.749 | ON871810 | 87 | 37 | 8 | 151,017 | 82,727 | 24,970 | 18,350 | 37.46 |
| A. sublessingiana | J-9 | XJBI/jgz-080-1 | China: Xinjiang | 47.559 | 86.909 | ON871811 | 87 | 37 | 8 | 151,065 | 82,787 | 24,969 | 18,340 | 37.45 |
| A. terrae-albae | J-10 | XJBI/jgz-079-1 | China: Xinjiang | 47.451 | 86.815 | ON871812 | 87 | 37 | 8 | 151,107 | 82,824 | 24,969 | 18,345 | 37.45 |
| A. transiliensis | J-13 | XJBI/jgz-012-3 | China: Xinjiang | 43.534 | 87.167 | ON871813 | 87 | 37 | 8 | 151,112 | 82,832 | 24,970 | 18,350 | 37.44 |
CDS coding sequence, IRs inverted repeat regions, tRNA transfer RNA, SSRs Simple sequence repeats, LSC large single copy, SSC small single copy, GC guanine-cytosine, N north latitude, E east longitude
IR expansion and contraction
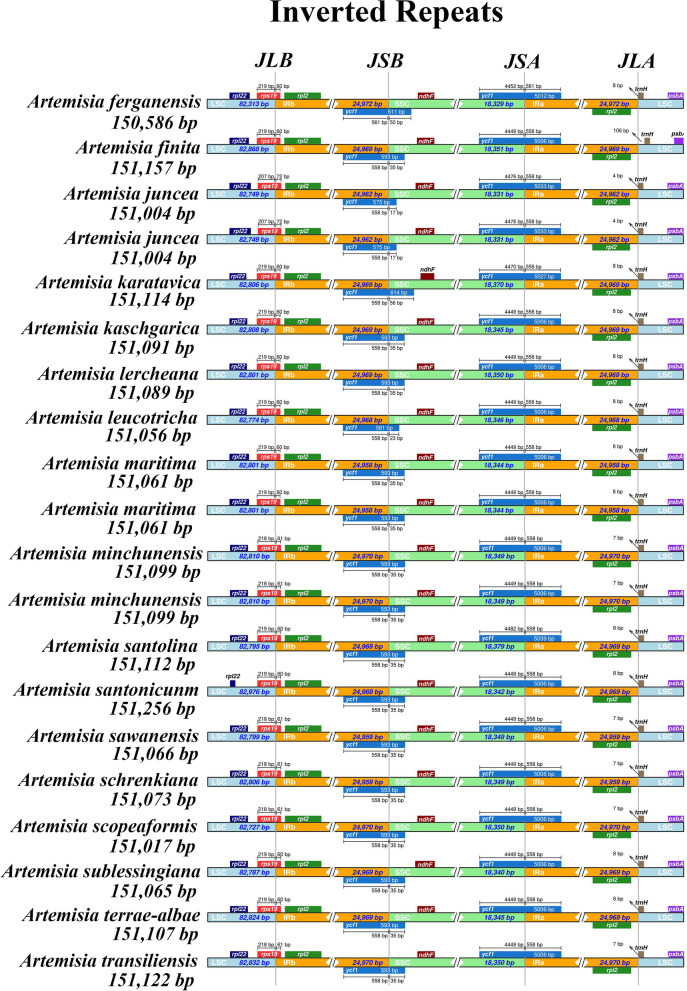
Comparative sequence analysis of 17 subg. Seriphidium species (16 newly sequenced and one published [21]) revealed that chloroplast genome structure and gene order were highly conserved, although with slight variations at the IR boundaries (Fig. 2). The length of IR was relatively consistent among all subg. Seriphidium species. A. sawanensis and A. schrenkiana had the shortest IR length (24,959 bp), while A. ferganensis had the longest (24,972 bp). All of the subg. Seriphidium chloroplast genomes had LSC/IRb junctions in gene rps19, with 60 to 72 bp crossing into the IRb region, indicating an expansion of the IR in these species (Fig. 2). Similarly, in all of subg. Seriphidium chloroplast genomes, the IRb/SSC junctions were located in gene ycf1, extending 17–35 bp into the SSC region, away from the ndhF gene. All of the subg. Seriphidium chloroplast genomes had SSC/IRa junctions located in gene ycf1, extending 561–558 bp into the IRa region. Most of the IRa/LSC junctions were located between genes rpl2 and trnH, with 4–8 bp far from the gene trnH, although in A. finite, the IRa/LSC junction was located 106 bp far from gene trnH (Fig. 2).
Fig. 2.
Comparison of the single copy-inverted repeat junctions among the 17 subg. Seriphidium species (16 newly sequenced and one published [21]). JLB, JSB, JSA and JLA: LSC/IRb, SSC/IRb, SSC/IRa and LSC/IRa, respectively. IRa, IRb: two IR regions that are identical but in opposite orientations; LSC: large single copy; SSC: small single copy
Analysis of repeats
Simple sequence repeats (SSRs) are shorter tandem repeats consisting of 1–6 bp repeat units and are also known as microsatellite repeats. In total, 1385 SSRs were detected in the 20 subg. Seriphidium chloroplast genomes (17 species), including 777 mononucleotides (mono-), 216 dinucleotides (di-), 78 trinucleotides (tri-), 275 tetranucleotides (tetra-), 38 pentanucleotides (penta-), and one hexanucleotide (hex-) (Fig. S2a; Additional file 1: Table S3). Most of the SSRs were located in LSC regions (1088), followed by SSCs (181) and IR (116) regions (Fig. S2b; Additional file 1: Table S3). Moreover, these SSRs were mainly distributed in intergenic spacer regions (IGS) (1017), with some in CDS (227) and intron regions (141) (Fig. S2c; Additional file 1: Table S3). Among the mononucleotide repeats, A/T repeats were most frequent; C repeats were present in all but two taxa (A. ferganensis and A. maritima); and no G repeats were detected (Fig. S2d; Additional file 1: Table S3). Dinucleotide repeats were represented by only the AT/TA motif. Trinucleotide repeats (ATT/TTC) were present in all 20 subg. Seriphidium chloroplast genomes analyzed, however only one trinucleotide repeat (AAT) was detected in A. finite. Tetra- and pentanucleotide contained motifs AATA/AATC, AAAT/AATT, ATTG/CAAT, ATTT/TAAT, TATT/TTTC and TTAA/TTTA, as well as AAATT/ACGAC, ATAAA/ATATT, ATTTA/TATAT, and TTAAT repeats, respectively. Furthermore, only one hexanucleotide (AATATA) was detected distributed in LSC region of A. finita (Fig. S2d; Additional file 1: Table S3).
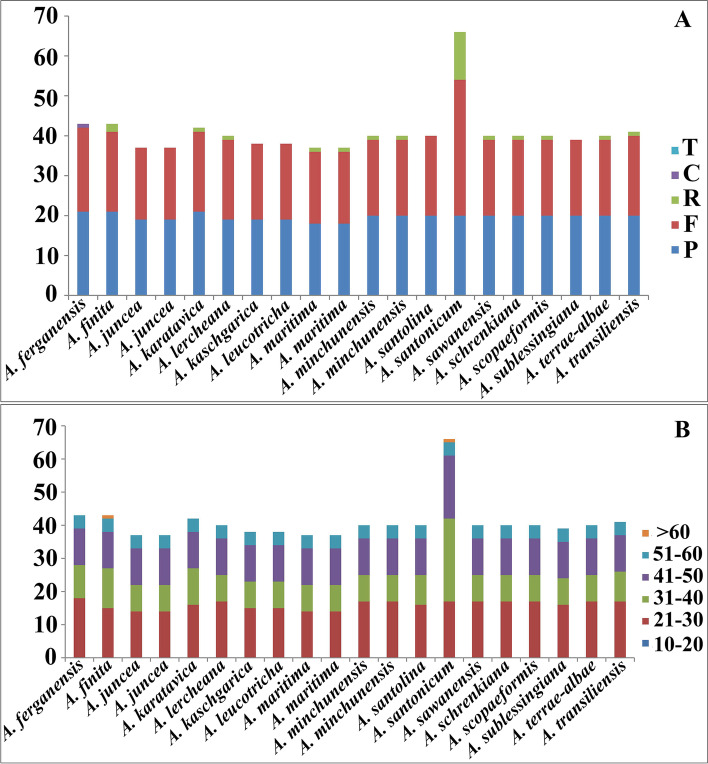
The forward (F), palindromic (P), reverse (R), and complement (C) repeat sequences in the 20 subg. Seriphidium chloroplast genomes (17 species) were detected using REPuter. In total, 818 long dispersed repeats were detected, including 398 forward, 394 palindromic, 25 reverse and one complement repeats (Additional file 1: Table S4). All species had forward and palindromic repeats, only one complement repeat was detected, in A. ferganensis. Approximately half (12/20) of the species had 1 or 2 reverses. Interestingly A. santonicum had 12 reverses, far more than the other species (Fig. 3A; Additional file 1: Table S4). Long dispersed repeat length was variable, at 30–86 bp, most commonly 30–50 bp. However, there were only two repeat regions were > 60 bp long [A. finita (86 bp) and A. santonicum (85 bp)] (Fig. 3B; Additional file 1: Table S4).
Fig. 3.
Long dispersed repeats of 20 Artemisia subg. Seriphidium chloroplast genomes. A Numbers of the five long repeat types; B Long dispersed repeat size
Hypervariable regions and genomic divergence
Nucleotide variability (Pi) was 0.000–0.00557 (average, 0.00115) for the 18 newly assembled plastomes and two A. maritima plastomes from GenBank (MK532038 and NC_045093). At the cutoff value of Pi > 0.0045, eight highly variable regions were identified: trnK-UUU – rps16, trnE-UUC – ropB, 35 bp + trnT-GGU + 508 bp, ndhC – trnV-UAC, 123 bp + ndhF, rpl32 – trnL-UAG, ndhG – ndhI and ycf1(1010–4275 bp) (Fig. S3; Additional file 1: Table S5). Four of these (trnK-UUU – rps16, trnE-UUC – ropB, 35 bp + trnT-GGU + 508 bp and ndhC – trnV-UAC) are located in the LSC region; while the other four are in the SSC region (Fig. S3). For these hypervariable loci, Pi ranges from 0.00451 (ndhC – trnV-UAC) to 0.00557 (ndhG – ndhI) (Additional file 1: Table S5).
The results of the sequence identity analysis of the 20 subg. Seriphidium chloroplast genomes (17 species), with A. ferganensis chloroplast genome as reference (Additional file 2: Fig. S4), are consistent with those of the nucleotide diversity analysis: IR regions were more conserved than SC regions, and non-coding regions were more divergent than coding regions. For the 20 chloroplast genomes, the divergent regions were in IGS, such as trnE-UUC – ropB, trnS-GGA – ycf3, trnV-UAC – ndhC, psbE – petL, rbcL – accD, petA – psbJ and rpl32 – trnL-UAG. One distinct gap was observed, in the psbM region of the A. sawanensis chloroplast genome (Additional file 2: Fig. S4). In total, 931 polymorphic sites, 273 singleton variable sites, and 658 parsimony informative sites were detected among the 20 chloroplast genome sequences.
Molecular markers for subg. Seriphidium species
To explore subg. Seriphidium molecular markers with increased resolution of phylogeny reconstruction, we tested eight screened highly variable regions and their combinations. Comparative sequence analysis revealed that ndhF is highly polymorphic in the subg. Seriphidium plastomes (Table 2). We constructed phylogenetic trees for each of the eight highly variable regions screened from whole chloroplast genes using 17 subg. Seriphidium species (16 newly sequenced and one published [21]) and assessed their potential potency. Our results revealed that the resolution of phylogenetic trees constructed based on each highly variable region was low (Additional file 2: Fig. S5–12). Moreover, the resolution of phylogenetic tree constructed using tandem sequences from eight highly variable regions was improved for the major clades compared to each highly variable region, but there are still deficiencies in discriminating at interspecific relationship (Additional file 2: Fig. S13). To further explore the resolution of phylogenetic tree, we made a first attempt to use whole chloroplast genome for 17 subg. Seriphidium species (16 newly sequenced and one published). We found that the resolution of phylogenetic tree was extremely high, both in the major clades and among species (Additional file 2: Fig. S14).
Table 2.
DNA polymorphisms identified in 17 Artemisia subg. Seriphidium species (16 newly sequenced and one published)
| Loci | Length (bp) | Number of sequence | Polymorphic site | Singleton variable sites | Parsimony informative sites |
|---|---|---|---|---|---|
| ndhC – trnV-UAC | 1180 | 20 | 17 | 3 | 14 |
| trnE-UUC – ropB | 877 | 20 | 12 | 3 | 9 |
| trnK-UUU – rps16 | 855 | 20 | 25 | 9 | 16 |
| rpl32 – trnL-UAG | 923 | 20 | 13 | 2 | 11 |
| ndhG – ndhI | 376 | 20 | 16 | 8 | 8 |
| trnT-GGU | 68 | 20 | 2 | 1 | 1 |
| ndhF | 2234 | 20 | 443 | 437 | 6 |
| ycf1 | 5073 | 20 | 72 | 21 | 51 |
Phylogenetic analysis
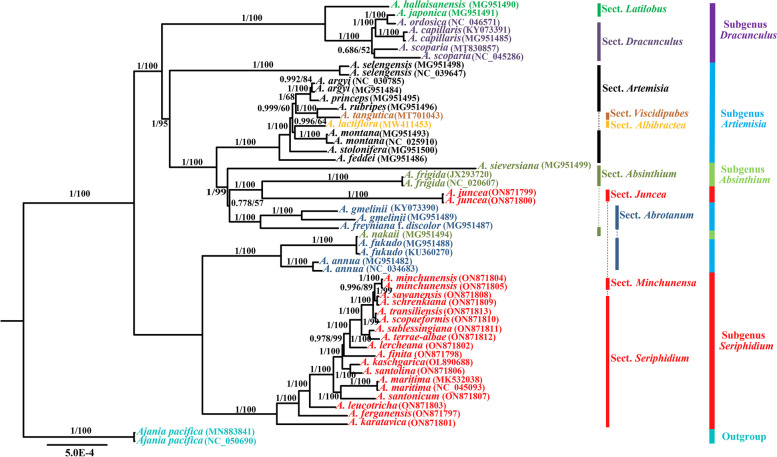
To evaluate the monophyly of subg. Seriphidium and its phylogenetic relationship with other subgenus in Artemisia, we reconstructed phylogenetic relationships based on 52 complete chloroplast genomes and 80 protein-coding genes from 38 Artemisia species, using Bayesian inference (BI) and maximum likelihood (ML), with the closely related species Ajania pacifica (NC_050690 and MN883841) as outgroup. The total alignment length (after removing one inverted repeat) was 125,171 bp, with 849 singleton variable sites and 1707 parsimony informative sites. The backbones of the BI and ML trees were nearly identical, whether based on complete chloroplast genomes or protein-coding genes, hence we present only the tree (branch lengths were estimated by BI analysis) for the whole chloroplast genome with posterior probability (PP) and bootstrap support (BS) values shown (Fig. 4; BI PP: 1.00; ML BS: 100%).
Fig. 4.
Phylogenetic tree inferred from Bayesian inference (BI) and maximum likelihood (ML) analyses, using the complete chloroplast genomes of 38 Artemisia species. Branch lengths were estimated using Bayesian inference. Numbers near the nodes are Bayesian posterior probabilities (to the left) and maximum likelihood bootstrap support values (to the right). Colored lines and braces at the right indicate the traditional section and subgenus classification of Artemisia. The sections of Artemisia subg. Seriphidium are divided according to Ling (1991) [19]
Based on these phylogenetic analyses, Artemisia is monophyletic; most of the clades have high support, with all samples of the same species clustered together (Fig. 4). All individuals of subg. Dracunculus are clustered together in a monophyletic clade (BI PP: 1.00; ML BS: 100%), but neither sect. Latilobus nor sect. Dracunculus within subg. Dracunculus are monophyletic. With the exception of sect. Viscidipubes and sect. Albibractea, the subg. Artemisia and its two other sections (sect. Artemsia and sect. Abrotanum) were recovered as polyphyletic (Fig. 4). Subg. Absinthium, with only one sect. Absinthium, was resolved as polyphyletic as well. Subg. Seriphidium is fully nested within genus Artemisia, forming two highly supported clades (Fig. 4; BI PP: 1.00; ML BS: 100%). Within subg. Seriphidium, a small clade containing A. juncea (sect. Juncea) forms a sister group to A. frigida (sect. Absinthium), and is located far from the other large monophyletic clade consisting of sect. Seriphidium and sect. Minchunensa. However, the inclusion of sect. Minchunensa within sect. Seriphidium is unexpected.
Discussion
Comparison of subg. Seriphidium chloroplast genomes
As in most angiosperms [36], we found that subg. Seriphidium has highly conserved structure, gene content and gene order, with little variation between species, based on complete chloroplast genome analysis (20 samples of 17 subg. Seriphidium species). Chloroplast genome size varied between the species, while there was sequence uniformity within species (Table 1). However, sequence variation has been reported within other species, such as Ilex viridis [45], Calligonum junceum [44] and Calanthe davidii [48]. Furthermore, this phenomenon was present in other subgenera of Artemisia [47, 49], such as Artemisia selengensis, Artemisia argyi, and Artemisai annua, however it is not found in subg. Seriphidium, probably due to the small sample size of the same species in the subgenus.
IR expansion and contraction is a common evolutionary phenomenon and often generates variation of chloroplast genome length [50]. Although the IR junctions of these subg. Seriphidium chloroplast genomes exhibited modest expansion or contraction (Fig. 2), the IR regions, which varied by 13 bp, were more conserved than the SC regions, which varied by 663 bp (for LSC regions) and 50 bp (for SSC regions) (Table 1). Moreover, IR expansion and contraction also play important roles in plastome rearrangements and gene content variations [50]. Although genome rearrangement has been reported for Compositae [51], Plantaginaceae [52] and Hypericaceae [53], this has not been observed in subg. Seriphidium (Additional file 2: Fig. S1, S4) and in other subgenera of Artemisia [21, 47, 49, 54].
Repeated sequence analysis
As a result of their high rate of polymorphism and abundant variation at the species level, SSRs are commonly employed in genetic diversity, population structure and species classifications [55–57]. SSR distributions can be used to infer highly polymorphic regions, contributing to the development of molecular markers for inferring phylogenetic relationships [58]. Among the 1385 SSR loci identified in the 20 subg. Seriphidium chloroplast genomes (Additional file 1: Table S3), A/T motif mononucleotide repeats were abundant (Fig. S2d). This finding, which is consistent with similar pattern of SSRs distribution in chloroplast genomes of other subgenera in Artemisia and other genera in Asteraceae [21, 47, 49, 54, 58, 59], may be because polyA and polyT have more stable structures than polyC and polyG [60].
In closely related species, the abundant variation in long dispersed repeats longer than 30 bp provides some evolutionary flexibility [45]; further, it results in insertion/deletion mismatches and genome rearrangement [58]. Among the 818 long dispersed repeats in the 20 subg. Seriphidium chloroplast genomes (Additional file 1: Table S4), forward and palindromic repeats accounted for 398 (48.66%) and 394 (48.17%) of all repeats, respectively, while reverse and complementary repeats were quite rare, accounting for just 25 (3.05%) and 1 (0.12%), respectively. This pattern of long dispersed repeats is similar to other subgenera of Artemisia and other angiosperms [21, 40, 45, 47, 61–63].
Hypervariable regions and molecular markers
Given that genes are not all equally important in the development of barcoding, or in population genetic and phylogenetic studies [21], screening of hypervariable regions can provide a wealth of phylogenetic information for such research [64–66]. We identified eight hypervariable regions, all within SC regions, with IR regions exhibiting lower variation (Fig. S3), consistent with our genomic divergence analysis (Additional file 2: Fig. S4). Phylogenetic analyses of Artemisia have often been based on plastid markers (mainly matK, rbcL, trnL – trnF, psbA – trnH, rpl32 – trnL and ndhC – trnV), this has left many interspecific relationships poorly resolved, particularly in subg. Seriphidium [16, 17, 31]. When comparing these markers with the highly variable regions identified here, only two (ndhC – trnV and rpl32 – trnL) have been used for phylogenetic inference in subg. Seriphidium, with weak resolution power [18]. Furthermore, the presence of rapid radiation differentiation in subg. Seriphidium has led to phylogenetic trees reconstructed based on either each highly variable regions screened or their tandem sequences being poorly resolved in terms of interspecific relationships (Additional file 2: Fig. S5–13). However, phylogenetic reconstructions of evolutionarily complex taxa using complete chloroplast genomes, such as those for Calligonum [44], Hoya [67] and Ilex [45], typically provide higher resolution and more stable backbones than those based on multiple gene fragments. Our results also confirmed that the whole chloroplast genome resolves interspecific relationships well in subg. Seriphidium (Additional file 2: Fig. S14), and the same effect was found in other subgenera of genus Artemisia [47, 61, 62]. This provides a good reference for using the whole chloroplast genome as superbarcodes to analysis the phylogenetic relationship of Artemisia and its allies.
Phylogenetic inference
We have reconstructed the phylogenetic relationships of Artemisia via Bayesian inference and maximum likelihood, using 38 Artemisia species representing the most extensive chloroplast genome sample to date (Fig. 4). This work provides a solid and high-resolution phylogenetic backbone of Artemisia, revealing inconsistencies between molecular systematics and traditional taxonomic studies. Most of the morphologically derived subgenera and sections within Artemisia are revealed to be polyphyletic, suggesting that the morphologically derived classifications are inaccurate. To resolve the relationships within subg. Seriphidium, we sampled three major clades in this subgenus (Fig. 4). Our results validate the earlier molecular findings that merge the subg. Seriphidium into the genus Artemisia [4, 13, 15, 16, 18]. While some authors still consider Seriphidium to be an independent genus [17, 68], this view not supported by the current knowledge.
Here, subg. Seriphidium was revealed to be polyphyletic, divided into two clades separated by a large genetic distance, reaffirming previous molecular phylogenetic findings on subg. Seriphidium [18]. While various taxonomists have divided A. juncea into different sections or series within subg. Seriphidium based on morphology, none has been aware of its evolutionary differentiation extended beyond this subgenus boundaries [7, 19, 20, 26, 68]. According to our results of molecular systematics, the proposal of removing A. juncea from subg. Seriphidium to obtain a monophyletic subgenus [18] is supported. However, our observations on the morphological traits of A. juncea revealed that its bracts layer (4–5), homogamous bisexual florets (4–7) and leaf indumentum are consistent with the morphological characters of subg. Seriphidium taxa, but its palmately ternate leaf pattern is uncommon (Fig. 5A) in this subgenus. In view of this, the systematic position of A. juncea remains to be further explored by combining the evidence of morphology and molecular systematics.
Fig. 5.
Leaves of four Artemisia subg. Seriphidium species. A Artemisia juncea (sect. Juncea); B Artemisia minchunensis (sect. Minchunensa); C Artemisia sawanensis (sect. Seriphidium); D Artemisia schrenkiana (sect. Seriphidium). The sections of Artemisia subg. Seriphidium are divided according to Ling (1991) [19]
Ling established A. minchunensis as a special group (sect. Minchunensa) within subg. Seriphidium mainly based on its leaves pectinately 2(or 3)-pinnatisect; lobules serrate or subserrate, arachnoid pubescent or glabrescent [19, 68]. The phylogenetic position of A. minchunensis has been unclear, owing to limited sampling in earlier molecular phylogenetic studies [18]. Here, our focused sampling revealed that A. minchunensis formed a highly supported (PP = 0.997; BS = 89) sister group to A. sawanensis and A. schrenkiana in sect. Seriphidium. Apparently our molecular phylogenetic results did not support the establishment of sect. Minchunensa. Actually, after careful observation of the leaf morphological characteristics of the above three species, we found that a high similarity in leaf morphology and indumentum, such as pinnatisect (bipartite or ternate) ovate or broadly ovate and densely pilose, with pinnately divided pseudo-stipules (Fig. 5B – D). Based on our molecular phylogenetic studies and morphological observations, it is considered inappropriate to establish morphologically-based sect. Minchunensa, which should be abolished and placed within sect. Seriphidium.
Conclusions
We newly sequenced 18 chloroplast genomes of 16 subg. Seriphidium species and compared them with one previously published taxon. Comparative analysis showed that genomic structures and gene order were relatively conserved, with only some variation in IR borders. Phylogenetic analysis revealed inconsistencies between the molecular phylogeny and traditional taxonomy of the subg. Seriphidium and the whole chloroplast genomes can be used as superbarcodes to resolve interspecific relationships in this subgenus. In future, combining complete chloroplast genomes and morphological data, based on detailed sampling, could enhance our understanding of the complex phylogenetic relationships in this group, providing the basis for a worldwide taxonomic revision of Artemisia subg. Seriphidium.
Materials and methods
Taxon sampling, DNA extraction, and sequencing
In total, 18 samples of 16 Artemisia subg. Seriphidium species were collected from northwestern China and adjacent countries (Russia and Tajikistan). For most of the species in the subgenus, we sampled one individuals, except for A. minchunensis and A. juncea, for which we sampled two individuals each (Table 1). No specific permissions were required for our locations/activities. Additional file 1 (Table S1) provides GenBank information for the remaining species used in the phylogenetic analysis. Nomenclature follows the accepted World Flora Online (http://www.worldfloraonline.org/) species names for the subg. Seriphidium. Voucher specimens were deposited in the Herbarium of the Xinjiang Institute of Ecology and Geography Chinese Academy of Sciences (XJBI) and the Herbarium of the Institute of Botany, Chinese Academy of Sciences (PE).
Total genomic DNA was extracted from ca. 100 mg of silica-dried leaves and isolated according to the cetyltrimethyl ammonium bromide (CTAB) method [69]. Extracted DNA samples were randomly fragmented to construct a 300 bp short-insert library and − 2 × 150 bp paired-end (PE) reads were performed on DNBSEQ™ technology platforms at the Beijing Genomics Institute (BGI, Shenzhen, China). The raw reads were evaluated using fastQC 0.11.5 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and edited using Trimmomatic 0.35 [70] to remove adapters and low-quality bases. Finally ca. 2.5 G bp paired-end clean read was obtained for each sample.
Chloroplast genome assembly and annotation
The clean data were assembled using GetOrganelle v. 1.7.1 [71], The complete circular assembly graph was checked and further extracted using Bandage v. 0.8.1 [72]. The finished plastid genomes were annotated by DOGMA [73], and GeSeq [74], and then manually adjusted by Geneious v. 9.1.7 [75]. Gene start and stop codons were determined via comparison with the A. maritima (NC_045093) and A. annua (NC_034683) genomes. The annotated plastid genomes were submitted to GenBank (Table 1) and Organellar Genome Draw (OGDRAW) [76] was used to illustrate a circular genome map.
Genome comparison and divergence analysis
Sequence alignment of the 20 subg. Seriphidium samples complete chloroplast genomes was conducted using MAFFT v. 7 [77]. The Mauve v. 2.3.1 [78], with default parameters, was used to identify locally collinear blocks among the chloroplast genomes. The genome variability across the 20 subg. Seriphidium samples was assessed using mVISTA [79] in Shuffle-LAGAN mode. Expansions and contractions of inverted repeat regions were visualized at the junctions of the four main (LSC/IRb/SSC/IRa) of the chloroplast genome, via IRScope [80]. Nucleotide diversity (Pi) was estimated by sliding window analysis conducted in DnaSP v. 6 [81] (window length, 600 bp; step size, 200 bp).
Repetitive sequences analysis
Simple sequence repeats (SSRs) across the 20 plastomes were identified using web-MISA [82] with the following parameters: ten repetitions for mononucleotide motifs, five for dinucleotide motifs, four for trinucleotide motifs and three for tetranucleotide, pentanucleotide and hexanucleotide motifs. The long dispersed repeats (LDRs): including forward (F), palindromic (P), reverse (R), and complement (C) repeats were identified using the online tool REPuter [83], with a Hamming distance of 3 and a minimum repeat size of 30 bp.
Phylogenetic analyses
Phylogenetic analyses were conducted using 80 protein-coding genes and 52 complete chloroplast genomes (after removing one inverted repeat). In total 38 Artemisia species from four subgenera and 10 sections, including 17 subg. Seriphidium species from three sections, were used for phylogenetic analysis (Fig. 4). Ajania pacifica (Accessions NC_050690 and MN883841) was used as the outgroup. Genome alignment was performed by MAFFT v. 7 [77] and trimmed using the “-gappyout” setting in trimAI v. 1.2, a PhyloSuite [84] plugin. According to the Bayesian information criterion (BIC), the most appropriate substitution models, estimated using jModelTest2 [85], were TVM + I + G for the complete chloroplast genome sequences and the protein-coding genes. Maximum likelihood (ML) analyses were conducted using RaxML-HPC v.8 [86], with 1000 bootstrap iterations. Based on the eight hypervariable regions screened and their tandem sequences, using ML method to reconstruct phylogenetic tree respectively in accordance with the above method. Only first the eight hypervariable regions screened were manually extracted and concatenated from the whole chloroplast genomes of 17 subg. Seriphidium species (16 newly sequenced and one published) by Geneious v. 9.1.7 [75]. Bayesian inference (BI) analysis was carried out using MrBayes v.3.2 [87], with Markov chain Monte Carlo simulations algorithm (MCMC) for 2,000,000,000 generations, using four incrementally-heated chains. This was conducted on the CIPRES Science Gateway portal [88]. The final trees were visualized and edited using FigTree v. 1.4.2 [89].
Supplementary Information
Additional file 1: Table S1. GenBank information for species derived from the NCBI database used in the phylogenetic analysis. Table S2. List of annotated genes in the subg. Seriphidium chloroplast genomes. Table S3. Raw data from the analysis of simple sequence repeats in the subg. Seriphidium. Table S4. Raw data from the analysis of long dispersed repeats in the subg. Seriphidium. Table S5. Raw values for each variant region of the subg. Seriphidium chloroplast genome used for hypervariable regions analysis.
Additional file 2: Figure S1. Intraspecific synteny analyses of 20 subg. Seriphidium chloroplast genomes. The A. ferganensis chloroplast genome appears at the top as the reference sequence. Within each of the Mauve alignments, locally collinear blocks are indicated the same color and are connected by lines. Figure S2. Analysis of simple sequence repeats (SSRs) of the 20 Artemisia subg. Seriphidium chloroplast genomes. a. Numbers of the six SSR types; b. Numbers of SSRs distributed in the various copy regions; c. NumberS of SSRs distributed in various gene regions; d. Numbers of SSR repeat unit types. Figure S3. Sliding-window analysis of nucleotide diversity (Pi) of the aligned Artemisia subg. Seriphidium chloroplast genomes (window length 800 bp; step size 200 bp). Figure S4. Variation in subg. Seriphidium chloroplast genome sequences. Y axis: variation (50–100%). X axis: coordinate in the chloroplast genome. Figure S5. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhC – trnV-UAC) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S6. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhF) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S7. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhG – ndhI) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S8. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (rpl32 – trnL-UAG) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S9. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnE-UUC – ropB) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S10. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnK-UUU – rps16) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S11. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnT-GGU) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S12. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ycf1) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S13. Phylogenetic tree constructed using the maximum likelihood method based on tandem sequences from eight highly variable regions selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S14. Phylogenetic tree constructed using the maximum likelihood method based on the whole chloroplast genomes of 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values.
Acknowledgments
We thank Meng Wei and Jiye Zheng of the Institute of Botany, Chinese Academy of Sciences, and Sheng Zhang of the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, for their assistance in sample collection; Yuan Jiang (Beijing University of Chinese Medicine); and Ning Fu (South China Botanical Garden, Chinese Academy of Sciences) for assistance with chloroplast genome analysis; and an anonymous person for assistance with English language and grammatical editing.
Abbreviations
- cp
Chloroplast
- CDS
Coding sequence
- IRs
Inverted repeat regions
- IRa, IRb
Two IR regions that are identical but in opposite orientations
- LSC
Large single copy
- SSC
Small single copy
- GC
Guanine-cytosine
- tRNA
Transfer RNA
- rRNA
Ribosomal RNA
- SSRs
Simple sequence repeats
- LDRs
Long dispersed repeats
- IGS
Intergenic regions
- ITS
Nuclear ribosomal internal transcribed space
- ETS
Nuclear ribosomal external transcribed space
- Pi
Nucleotide diversity
- CTAB
Cetyltrimethyl ammonium bromide method
- DnaSP
DNA Sequences Polymorphism
- DOGMA
Dual Organellar Genome Annotator
- MCMC
Markov Chain Monte Carlo
- BIC
Bayesian information criterion
- ML
Maximum Likelihood
- PP
Posterior probability
- BI
Bayesian Inference
- BS
Bootstrap
- NCBI
National Center for Biotechnology Information
Authors’ contributions
ZB and YF designed the research. GZ conducted sample collection and data analysis, and drafted the manuscript. WJ provided guidance on taxonomy. FS and LY conducted some of the data processing. ZB and YF revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Science Foundation of China (NSF-42271072), the Science and Technology Basic Resources Survey special (2018FY100704) and Project of National Plant Specimen Resource Center (NPSRC), E0117G1001.
Availability of data and materials
All the newly sequenced sequences in this study are available from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/; accession numbers: ON871797 – ON871813 and OL890688; see Table 1). Information for other samples used for phylogenetic analysis download from GenBank can be found in Additional file 1: Table S1.
Declarations
Ethics approval and consent to participate
Not applicable. No specific permits were required for voucher specimens for this study. All materials used in the study were collected in public areas of China in compliance with the relevant laws of China. Voucher specimens were prepared and deposited at the Herbarium of Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences (XJBI) and the Herbarium of the Institute of Botany, Chinese Academy of Sciences (PE).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhibin Wen, Email: zhibinwen@ms.xjb.ac.cn.
Ying Feng, Email: luckfy@ms.xjb.ac.cn.
References
- 1.Vallès J, Garcia S, Hidalgo O, Martín J, Pellicer J, Sanz M, et al. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae) In: Kader J, Delseny M, et al., editors. Advances in botanical research Vol 60. London: Academic Press; 2011. pp. 349–419. [Google Scholar]
- 2.Oberprieler C, Himmelreich S, Källersjö M, Vallès J, Watson LE, Vogt R. Anthemideae. In: Funk VA, Susanna A, editors. Systematics, evolution, biogeography of Compositae. Vienna: International Association for Plant Taxonomy; 2009. [Google Scholar]
- 3.Bremer K, Humphries C. Generic monograph of the Asteraceae-anthemideae. Bull Nat His Mus. 1993;23:71–177. [Google Scholar]
- 4.Valles J, Garnatje T. Artemisia and its allies: genome organization and evolution and their biosystematic, taxonomic, and phylogenetic implications in the artemisiinae and related subtribes (Asteraceae, anthemideae) In: Sharma AK, Sharma A, editors. Plant genome: biodiversity and evolution, vol. 1B, Phanerogams (higher groups) Enfield: M/S Science Publishers; 2005. pp. 255–285. [Google Scholar]
- 5.Martin I, Torrell M, Korobkov AA, Valles J. Palynological features as a systematic marker in Artemisia L. and related genera (Asteraceae, anthemideae) - II: implications for subtribe artemisiinae delimitation. Plant Biol. 2003;5(1):85–93. doi: 10.1055/s-2003-37979. [DOI] [Google Scholar]
- 6.Vallès J, McArthur ED. Artemisia systematics and phylogeny: cytogenetic and molecular in sights. In: McArthur ED, Fairbanks DJ, editors. Shrubland ecosystem genetics and biodiversity: proceedings. Utah: USDA Forest Service; 2001. pp. 67–74. [Google Scholar]
- 7.Poljakov PP. Artemisia L. In: Shishkin BK, Bobrov EG, editors. Flora of the USSR. Leningrad: Akademia Nauk; 1961. pp. 425–631. [Google Scholar]
- 8.Ling YR. The Old World Artemisia (Compositae) Bull Bot Res, Harbin. 1991;12:1–108. [Google Scholar]
- 9.Ling YR. The genera Artemisia L. and Seriphidium (Bess.) Poljak. In the world. Compositae Newslett. 1994;25:39–45. [Google Scholar]
- 10.Wright CW. Medicinal and aromatic plants-industrial profiles. London: Taylor and Francis; 2002. Artemisia; pp. 10–22. [Google Scholar]
- 11.Duffy PE, Mutabingwa TK. Artemisinin combination therapies. Lancet. 2006;367(9528):2037–2039. doi: 10.1016/S0140-6736(06)68900-9. [DOI] [PubMed] [Google Scholar]
- 12.Torrell M, Garcia-Jacas N, Susanna A, Valles J. Phylogeny in Artemisia (Asteraceae, anthemideae) inferred from nuclear ribosomal DNA (ITS) sequences. Taxon. 1999;48(4):721–736. doi: 10.2307/1223643. [DOI] [Google Scholar]
- 13.Watson LE, Bates PL, Evans TM, Unwin MM, Estes JR. Molecular phylogeny of subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol Biol. 2002;2:12. doi: 10.1186/1471-2148-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valles J, Torrell M, Garnatje T, Garcia-Jacas N, Vilatersana R, Susanna A. The genus Artemisia and ITS allies: phylogeny of the subtribe Artemisiinae (Asteraceae, anthemideae) based on nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers (ITS) Plant Biol. 2003;5(3):274–284. doi: 10.1055/s-2003-40790. [DOI] [Google Scholar]
- 15.Sanz M, Vilatersana R, Hidalgo O, Garcia-Jacas N, Susanna A, SchneeweiSs GM, et al. Molecular phylogeny and evolution of floral characters of Artemisia and allies (anthemideae, Asteraceae): evidence from nrDNA ETS and ITS sequences. Taxon. 2008;57(1):66–78. [Google Scholar]
- 16.Riggins CW, Seigler DS. The genus Artemisia (Asteraceae: Anthemideae) at a continental crossroads: molecular insights into migrations, disjunctions, and reticulations among old and New World species from a Beringian perspective. Mol Phylogenet Evol. 2012;64(3):471–490. doi: 10.1016/j.ympev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Haghighi AR, Belduz AO, Vahed MM, Coskuncelebi K, Terzioglu S. Phylogenetic relationships among Artemisia species based on nuclear ITS and chloroplast psbA-trnH DNA markers. Biologia. 2014;69(7):834–839. doi: 10.2478/s11756-014-0379-3. [DOI] [Google Scholar]
- 18.Malik S, Vitales D, Hayat MQ, Korobkov AA, Garnatje T, Vallès J. Phylogeny and biogeography of Artemisia subgenus Seriphidium (Asteraceae: Anthemideae) Taxon. 2017;66(4):934–952. doi: 10.12705/664.8. [DOI] [Google Scholar]
- 19.Ling YR. The Old World Seriphidium (Compositae) Bull Bot Res, Harbin. 1991;11:1–40. [Google Scholar]
- 20.Ling YR. The chinense Seriphidium (Bess.) Poljak. The classification, distribution and application of Seriphidium (Bess.) Poljak. Bull Bot Res, Harbin. 1988;8:111–123. [Google Scholar]
- 21.Shahzadi I, Abdullah MF, Ali Z, Ahmed I, Mirza B. Chloroplast genome sequences of Artemisia maritima and Artemisia absinthium: comparative analyses, mutational hotspots in genus Artemisia and phylogeny in family Asteraceae. Genomics. 2020;112(2):1454–1463. doi: 10.1016/j.ygeno.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Bhat ZA, Kumar V, Zargar MI. A short review on Artemisia maritima Linn. Int J Res Phytochem Pharmacol. 2011;1(4):201–206. [Google Scholar]
- 23.Besser WS, de Mr L, le Dr. Besser au Directeur Monsieur le Directeur. Bull Soc Imp Naturalistes Moscou. 1829;1:219–265. [Google Scholar]
- 24.Besser WS. De Seriphidiis seu de sectione IIIa Artemisiarum Linnaei. Bull Soc Imp Naturalistes Moscou. 1834;7:1–46. [Google Scholar]
- 25.Poljakov PP. Materialy k sistematike roda polyn-Artemisia L. Trudy Inst Bot Akad Nauk Kazakhsk SSR. 1961;11:134–177. [Google Scholar]
- 26.Filatova NS. Sistema polynej podroda Seriphidium (Bess.) Peterm. (Artemisia L., Asteraceae) Evrazii i Severnoj Afriki. Evrazii i Severnoj Afriki. Novosti Sist Vyssh Rast. 1986;23:217–239. [Google Scholar]
- 27.Ling YR. On the system of the genus Artemisia L. and the relationship with its allies. Bull Bot Res, Harbin. 1982;2:1–60. [Google Scholar]
- 28.Zhang L, Huang YW, Huang JL, Ya JD, Zhe MQ, Zeng CX, Zhang ZR, Zhang SB, Li DZ, Li HT, et al. DNA barcoding of Cymbidium by genome skimming: call for next-generation nuclear barcodes. Mol Ecol Resour. 2022;00:1–16. doi: 10.1111/1755-0998.13719. [DOI] [PubMed] [Google Scholar]
- 29.Kornkven AB, Watson LE, Estes JR. Phylogenetic analysis of Artemisia section Tridentatae (Asteraceae) based on sequences from the internal transcribed spacers (ITS) of nuclear ribosomal DNA. Am J Bot. 1998;85(12):1787–1795. doi: 10.2307/2446513. [DOI] [PubMed] [Google Scholar]
- 30.Kornkven AB, Watson LE, Estes JR. Molecular phylogeny of Artemisia section Tridentatae (Asteraceae) based on chloroplast DNA restriction site variation. Syst Bot. 1999;24(1):69–84. doi: 10.2307/2419387. [DOI] [Google Scholar]
- 31.Garcia S, McArthur ED, Pellicer J, Sanderson SC, Valles J, Garnatje T. A molecular phylogenetic approach to western North America endemic Artemisia and allies (Asteraceae) untangling the sagebrushes. Am J Bot. 2011;98(4):638–653. doi: 10.3732/ajb.1000386. [DOI] [PubMed] [Google Scholar]
- 32.Hussain A, Potter D, Kim S, Hayat MQ, Bokhari SAI. Molecular phylogeny of Artemisia (Asteraceae-anthemideae) with emphasis on undescribed taxa from Gilgit-Baltistan (Pakistan) based on nrDNA (ITS and ETS) and cpDNA (psbA-trnH) sequences. Plant Ecol Evol. 2019;152(3):507–520. doi: 10.5091/plecevo.2019.1583. [DOI] [Google Scholar]
- 33.Jansen RK, Ruhlman TA. Plastid genomes of seed plants. In: Bock R, Knoop V, editors. Genomics of chloroplasts and mitochondria. Advances in photosynthesis and respiration. Springer; 2012. pp. 103–126. [Google Scholar]
- 34.Ruhlman TA, Jansen RK. The plastid genomes of flowering plants. In: Maliga P, editor. Chloroplast biotechnology. Methods in molecular biology. Totowa, NJ: Humana Press; 2014. pp. 3–38. [DOI] [PubMed] [Google Scholar]
- 35.Jensen PE, Leister D. Chloroplast evolution, structure and functions. F1000prime reports. 2014;6:40. [DOI] [PMC free article] [PubMed]
- 36.Mower JP, Vickrey TL. Structural diversity among plastid genomes of land plants. In: Chaw SM, Jansen RK, editors. Plastid genome evolution. London: Academic Press Ltd-Elsevier Science Ltd; 2018. pp. 263–292. [Google Scholar]
- 37.Palmer JD. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- 38.Bendich AJ. Circular chloroplast chromosomes: the grand illusion. Plant Cell. 2004;16(7):1661–1666. doi: 10.1105/tpc.160771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke SV, Grennan CP, Duvall MR. Plastome sequences of two New World bamboos- Arundinaria gigantea and Crytpochloa strictiflora (Poaceae)-extend phylogenomic understanding of Bambusoideae. Am J Bot. 2012;99(12):1951–1961. doi: 10.3732/ajb.1200365. [DOI] [PubMed] [Google Scholar]
- 40.Ren J, Tian J, Jiang H, Zhu XX, Mutie FM, Wanga VO, et al. Comparative and phylogenetic analysis based on the chloroplast genome of Coleanthus subtilis (Tratt.) Seidel, a protected rare species of monotypic genus. Front Plant Sci. 2022;13:828467. doi: 10.3389/fpls.2022.828467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang SD, Jin JJ, Chen SY, Chase MW, Soltis DE, Li HT, et al. Diversification of Rosaceae since the late cretaceous based on plastid phylogenomics. New Phytol. 2017;214(3):1355–1367. doi: 10.1111/nph.14461. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Zhou J, Cui Y, Wang Y, Duan B, Yao H. Identification of Ligularia herbs using the complete chloroplast genome as a super-barcode. Front Pharmacol. 2018;9:695. doi: 10.3389/fphar.2018.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong L, Ding X, Guan W, Zhang D, Zhang J, Bai J, Xu W, Huang J, Qiu X, Zheng X, et al. Comparative chloroplast genome analyses of Amomum: insights into evolutionary history and species identification. BMC Plant Biol. 2022;22(1):520. doi: 10.1186/s12870-022-03898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song F, Li T, Burgess KS, Feng Y, Ge XJ. Complete plastome sequencing resolves taxonomic relationships among species of Calligonum L. (Polygonaceae) in China. BMC Plant Biol. 2020;20(1):261. doi: 10.1186/s12870-020-02466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu K, Lin C, Lee SY, Mao L, Meng K. Comparative analysis of complete Ilex (Aquifoliaceae) chloroplast genomes: insights into evolutionary dynamics and phylogenetic relationships. BMC Genomics. 2022;23(1):203. doi: 10.1186/s12864-022-08397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mo ZQ, Fu CN, Zhu MS, Milne RI, Yang JB, Cai J, Qin HT, Zheng W, Hollingsworth PM, Li DZ, et al. Resolution, conflict and rate shifts: insights from a densely sampled plastome phylogeny for Rhododendron (Ericaceae) Ann Bot. 2022;130(5):687–701. doi: 10.1093/aob/mcac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim GB, Lim CE, Kim JS, Kim K, Lee JH, Yu HJ, et al. Comparative chloroplast genome analysis of Artemisia (Asteraceae) in East Asia: insights into evolutionary divergence and phylogenomic implications. BMC Genomics. 2020;21(1):415. doi: 10.1186/s12864-020-06812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YQ, Zhong H, Zhu YT, Huang YZ, Wu SS, Liu ZJ, et al. Plastome structure and adaptive evolution of Calanthe s.l. species. PeerJ. 2020;8:24. doi: 10.7717/peerj.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Miao Y, Luo D, Li J, Wang Z, Luo M, et al. Sequence characteristics and phylogenetic analysis of the Artemisia argyi chloroplast genome. Front Plant Sci. 2022;13:906725. doi: 10.3389/fpls.2022.906725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wicke S, Schneeweiss GM, dePamphilis CW, Muller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant MolBiol. 2011;76(3–5):273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KJ, Choi KS, Jansen RK. Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae) Mol Biol Evol. 2005;22(9):1783–1792. doi: 10.1093/molbev/msi174. [DOI] [PubMed] [Google Scholar]
- 52.Mower JP, Guo WH, Partha R, Fan WS, Levsen N, Wolff K, et al. Plastomes from tribe Plantagineae (Plantaginaceae) reveal infrageneric structural synapormorphies and localized hypermutation for Plantago and functional loss of ndh genes from Littorella. Mol Phylogenet Evol. 2021;162:11. doi: 10.1016/j.ympev.2021.107217. [DOI] [PubMed] [Google Scholar]
- 53.Claude SJ, Park S, Park S. Gene loss, genome rearrangement, and accelerated substitution rates in plastid genome of Hypericum ascyron (Hypericaceae) BMC Plant Biol. 2022;22(1):135. doi: 10.1186/s12870-022-03515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan Z, Shi Y, Yin Q, Gao R, Liu C, Wang W, et al. Comparative and phylogenetic analysis of complete chloroplast genomes from five Artemisia species. Front Plant Sci. 2022;13:1049209. doi: 10.3389/fpls.2022.1049209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebert D, Peakall R. Chloroplast simple sequence repeats (cpSSRs): technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Mol Ecol Resour. 2009;9(3):673–690. doi: 10.1111/j.1755-0998.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 56.Singh RB, Mahenderakar MD, Jugran AK, Singh RK, Srivastava RK. Assessing genetic diversity and population structure of sugarcane cultivars, progenitor species and genera using microsatellite (SSR) markers. Gene. 2020;753:13. doi: 10.1016/j.gene.2020.144800. [DOI] [PubMed] [Google Scholar]
- 57.Duan N, Deng L, Zhang Y, Shi Y, Liu B. Comparative and phylogenetic analysis based on chloroplast genome of Heteroplexis (Compositae), a protected rare genus. BMC Plant Biol. 2022;22(1):605. doi: 10.1186/s12870-022-04000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng JY, Zhang XS, Zhang DG, Wang Y, Deng T, Huang XH, et al. Newly reported chloroplast genome of Sinosenecio albonervius Y. Liu & Q. E. Yang and comparative analyses with other Sinosenecio species. BMC Genomics. 2022;23(1):639. doi: 10.1186/s12864-022-08872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Qu D, Ma Y. Characterization of the chloroplast genome of Argyranthemum frutescens and a comparison with other species in anthemideae. Genes (Basel) 2022;13(10):1720. doi: 10.3390/genes13101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gragg H, Harfe BD, Jinks-Robertson S. Base composition of mononucleotide runs affects DNA polymerase slippage and removal of frameshift intermediates by mismatch repair in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(24):8756–8762. doi: 10.1128/MCB.22.24.8756-8762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J, Xia M, Wang Y, Chi X, Xu H, Chen S, Zhang F. Short and long reads chloroplast genome assemblies and phylogenomics of Artemisia tangutica (Asteraceae) Biologia. 2022;77(4):915–930. doi: 10.1007/s11756-021-00951-2. [DOI] [Google Scholar]
- 62.Jiang H, Tian J, Yang J, Dong X, Zhong Z, Mwachala G, et al. Comparative and phylogenetic analyses of six Kenya Polystachya (Orchidaceae) species based on the complete chloroplast genome sequences. BMC Plant Biol. 2022;22(1):177. doi: 10.1186/s12870-022-03529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghaddam M, Ohta A, Shimizu M, Terauchi R, Kazempour-Osaloo S. The complete chloroplast genome of Onobrychis gaubae (Fabaceae-Papilionoideae): comparative analysis with related IR-lacking clade species. BMC Plant Biol. 2022;22(1):75. doi: 10.1186/s12870-022-03465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed I, Matthews PJ, Biggs PJ, Naeem M, McLenachan PA, Lockhart PJ. Identification of chloroplast genome loci suitable for high-resolution phylogeographic studies of Colocasia esculenta (L.) Schott (Araceae) and closely related taxa. Mol Ecol Resour. 2013;13(5):929–937. doi: 10.1111/1755-0998.12128. [DOI] [PubMed] [Google Scholar]
- 65.Menezes APA, Resende-Moreira LC, Buzatti RSO, Nazareno AG, Carlsen M, Lobo FP, et al. Chloroplast genomes of Byrsonima species (Malpighiaceae): comparative analysis and screening of high divergence sequences. Sci Rep. 2018;8:12. doi: 10.1038/s41598-018-20189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong WP, Xu C, Li CH, Sun JH, Zuo YJ, Shi S, et al. ycf1, the most promising plastid DNA barcode of land plants. Sci Rep. 2015;5:5. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odago WO, Waswa EN, Nanjala C, Mutinda ES, Wanga VO, Mkala EM, et al. Analysis of the complete Plastomes of 31 species of Hoya group: insights into their comparative genomics and phylogenetic relationships. Front Plant Sci. 2021;12:814833. doi: 10.3389/fpls.2021.814833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling YR, Humphries CJ, Gilbert MG. Seriphidium (Besser ex Lessing) Fourreau. In: Wu ZY, Raven PH, editors. Flora of China, vol. 20–21. Beijing: Science Press; Saint Louis: Missouri Botanical Garden Press; 2011. pp. 737–747. [Google Scholar]
- 69.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 70.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, et al. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21(1):31. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics (Oxford, England) 2015;31(20):3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 74.Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, et al. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45(W1):W6–W11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007;52(5–6):267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 77.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amiryousefi A, Hyvonen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34(17):3030–3031. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 81.Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 82.Thiel T, Michalek W, Varshney RK, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;106(3):411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 83.Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29(22):4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller MA, Pfeiffer W, Schwartz T. Proceedings of the gateway computing environments workshop (GCE), 14 November 2010. New Orleans, LA: Creating the CIPRES science gateway for inference of large phylogenetic trees; 2010. pp. 1–8. [Google Scholar]
- 89.Rambaut A. FigTree-v1. 4.2. 2012. http://tree.bio.ed.ac.uk/software/figtree/. Accessed May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. GenBank information for species derived from the NCBI database used in the phylogenetic analysis. Table S2. List of annotated genes in the subg. Seriphidium chloroplast genomes. Table S3. Raw data from the analysis of simple sequence repeats in the subg. Seriphidium. Table S4. Raw data from the analysis of long dispersed repeats in the subg. Seriphidium. Table S5. Raw values for each variant region of the subg. Seriphidium chloroplast genome used for hypervariable regions analysis.
Additional file 2: Figure S1. Intraspecific synteny analyses of 20 subg. Seriphidium chloroplast genomes. The A. ferganensis chloroplast genome appears at the top as the reference sequence. Within each of the Mauve alignments, locally collinear blocks are indicated the same color and are connected by lines. Figure S2. Analysis of simple sequence repeats (SSRs) of the 20 Artemisia subg. Seriphidium chloroplast genomes. a. Numbers of the six SSR types; b. Numbers of SSRs distributed in the various copy regions; c. NumberS of SSRs distributed in various gene regions; d. Numbers of SSR repeat unit types. Figure S3. Sliding-window analysis of nucleotide diversity (Pi) of the aligned Artemisia subg. Seriphidium chloroplast genomes (window length 800 bp; step size 200 bp). Figure S4. Variation in subg. Seriphidium chloroplast genome sequences. Y axis: variation (50–100%). X axis: coordinate in the chloroplast genome. Figure S5. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhC – trnV-UAC) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S6. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhF) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S7. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ndhG – ndhI) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S8. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (rpl32 – trnL-UAG) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S9. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnE-UUC – ropB) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S10. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnK-UUU – rps16) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S11. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (trnT-GGU) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S12. Phylogenetic tree constructed using the maximum likelihood method based on highly variable sequences (ycf1) selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S13. Phylogenetic tree constructed using the maximum likelihood method based on tandem sequences from eight highly variable regions selected from 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values. Figure S14. Phylogenetic tree constructed using the maximum likelihood method based on the whole chloroplast genomes of 17 subg. Seriphidium species (16 newly sequenced and one published). Numbers near the nodes is maximum likelihood bootstrap support values.
Data Availability Statement
All the newly sequenced sequences in this study are available from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/; accession numbers: ON871797 – ON871813 and OL890688; see Table 1). Information for other samples used for phylogenetic analysis download from GenBank can be found in Additional file 1: Table S1.