Objectives
This is a protocol for a Cochrane Review (intervention). The objectives are as follows:
To evaluate the effectiveness of interventions aiming to improve coordination of emergency medical services on patient outcomes of emergency care systems.
Background
Description of the condition
Emergency care is time‐sensitive medical care that involves acute conditions, such as injuries/trauma, infections, stroke, acute cardiac events, acute complications of pregnancy, and asthma. Emergencies are generally life‐threatening or disabling, and thus require early recognition and life‐saving interventions. A delay in the initiation of care may result in avoidable death or disability, or reduce the effectiveness of the treatment (Calvello 2013; Reynolds 2017). According to the Emergency Care System Framework developed by the World Health Organization (WHO) (WHO 2018b), emergency care systems span multiple sectors, including the scene of the emergency, the transport of patients, and healthcare facilities managing patients presenting with an emergency condition. Emergency care systems typically entail at least two different services, with each service functioning in a different sector and provided by different stakeholders (e.g. bystanders, ambulance dispatchers, ambulance workers, and healthcare personnel at emergency units). Coordination of care is a crucial factor in emergency care systems, and should be done from the earliest onset of symptoms. The World Health Assembly of 2019 passed Resolution 72/31, which called on all state members to develop an emergency care system to ensure coordination of emergency care.
The disease burden caused by emergencies is noteworthy, and places individuals, caregivers, and the health system in an undesirable position. It is estimated that globally 90% of deaths and 84% of disability‐adjusted life‐years (DALYs) are caused by emergent conditions (Chang 2016). Previous studies have reported that the incidence of emergencies and acute diseases is six times higher in low‐middle income countries (LMICs) than in high‐income countries, accounting for 9 of the 10 leading causes of death, and approximately half of the total disease burden (Razzak 2019; Thind 2015; Werner 2020). Furthermore, it has been estimated that annually over 50% of deaths and up to 2.5 billion DALYs in LMICs could be addressed by the introduction of effective emergency care systems (Reynolds 2017; Thind 2015).
Timely transition of care, appropriate exchange of information, and the delivery of high‐quality care are all important elements of an emergency care system. However, the potential positive effects are nullified by fragmentation in emergency care, which results in a decrease in the potential number of DALYs that could have been prevented in an emergency care system (Hirshon 2013). Fragmented care is characterised by poor coordination of care, a lack of direct communication between role players, and the lack of collaboration across the various healthcare settings (Kripalani 2007). This can result in the loss of vital clinical information, duplicate diagnostic testing, clinical errors, and inadequate communication between the various stakeholders (Miller 2009; Zanello 2015). The delayed and ineffective transfer of clinical information can also result in adverse health outcomes, such as prolonged length of stay and increased morbidity and mortality (Pham 2008).
An effective emergency care system cannot be achieved without effective coordination of care. Understanding and implementing effective care coordination practices are thus imperative for an effective emergency care system to ensure the delivery of high‐quality care, and to reduce the evitable loss of life in the process of emergency care.
Description of the intervention
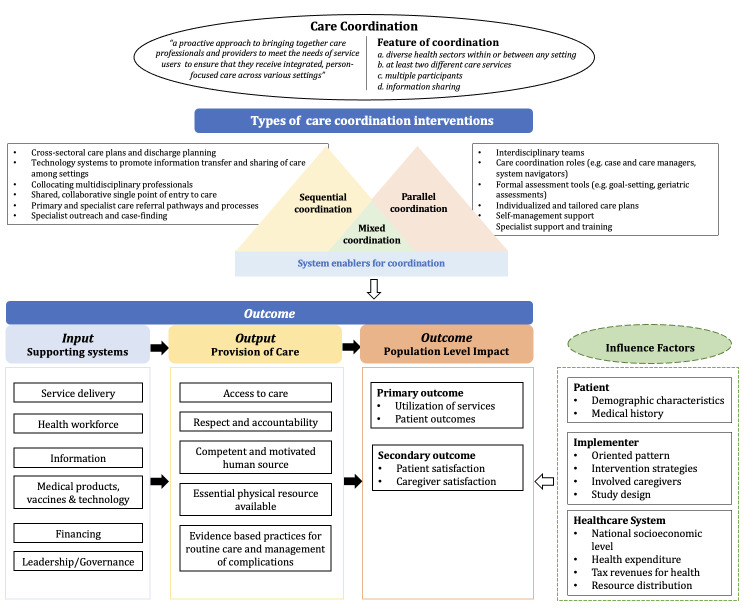
Coordination of care is a broad concept that can be related to other similar terms, such as service integration, collaborative practice, continuum of care, continuity of care, disease management, and care or care management (Strobel 2017). Before identifying the interventions intended to improve care coordination, it is essential to first define coordination of care. According to the WHO, coordination of care is "a proactive approach to bringing together care professionals and providers to meet the needs of service users to ensure that they receive integrated, person‐focused care across various settings" (WHO 2018a). We have adapted the WHO definition for this review, and our definition of coordination of care includes the following:
involves diverse health sectors within or between any setting;
requires at least two different care services, with each service performed by different providers;
involves multiple participants;
relies on information sharing.
This review will focus on system‐level coordination of care and interventions that include at least one of the first three features. The last feature, information sharing, is at the core of all interventions, and is indispensable for all efforts improving the emergency care coordination.
According to the WHO Continuity and Coordination of Care Framework (WHO 2018a), existing practice interventions can be grouped into three types: sequential coordination, parallel coordination, and system enablers for coordination. The interventions may be designed with diverse perspectives and directly target varied subjects, but they all aim to enhance continuity and coordination of care, improve the well‐being and behaviour of providers, and increase the satisfaction and health outcomes of people receiving care by providing seamless care across care system boundaries. Combining the features and categories of coordination of care, we plan to group coordination interventions for emergency services into three types according to whether the intervention involves different settings or not. These settings include the scene of an emergency, ambulances, emergency department (ED) centres, hospitals, nursing homes, and so on. The three types of coordination interventions are as follows.
Sequential coordination
Interventions using sequential coordination are designed to facilitate the smooth handover of responsibilities and transfer of care between different settings. These interventions are implemented to improve the coordination of care throughout the patient pathway across various settings (i.e. from the scene of an emergency to hospital). An example of a sequential coordination intervention is Geographic Information Systems (GIS). These systems are used to estimate the time of arrival (ETA) for patients on their way to a healthcare facility (Raaber 2016). Real‐time GIS could display the ETA of ambulances to EDs, which would enable the assembly of a medical emergency team prior to the patient's arrival. Sequential coordination interventions may include:
cross‐sectoral care plans, tools, standard protocols and guidelines for the management of emergency presentations;
systems to promote information transfer and sharing of care among sectors;
shared, collaborative single point of entry to care;
primary and specialist care referral processes and pathways;
specialist outreach.
Parallel coordination
Parallel coordination interventions are aimed at enhancing collaboration among professionals, with agreed sharing of responsibility within the same care setting. These interventions are implemented to improve the coordination of care in relation to the different tasks delivered by diverse healthcare providers working in the same setting, such as an ambulance or hospital. For instance, after receiving education on the appropriate model of care transition according to the 'Identify, Situation, Background, Assessment, and Recommendation' (ISBAR) tool, ED workers were reported to be more likely to follow the relevant instructions, which subsequently resulted in more accurate and complete information (Fahim Yegane 2017). The following approaches are examples of parallel coordination interventions:
interdisciplinary teams;
care coordination roles (e.g. case and care managers, system navigators);
formal assessment tools (e.g. goal‐setting, assessments on skill mix);
individualised and tailored care plans;
self‐management support and specialist support and training.
Mixed coordination
There are inevitably some interventions with features of both the intervention groups described above, and these will be included in this category.
We have not defined system enablers as an independent category, as the system influencers or enablers of care coordination (e.g. protocols, pathways, financial incentives, technology, and education) are usually used in all interventions, both sequential and parallel, to enhance coordination between various settings and providers or services. For example, an e‐learning programme is an enabler, but it is also a tool to improve the handover from pre‐hospital notification to the ED and between different professionals (Ebben 2015).
How the intervention might work
Interventions of interest and expected pathways to outcomes are illustrated in the logic framework (Figure 1). The WHO Emergency Care System Framework points out that emergency care has three essential elements: i) the scene of the emergency, ii) the transport of patients, and iii) healthcare facilities (WHO 2018b). Each of the elements corresponds to a different component within the emergency care system. On the scene of the emergency, dispatchers play a major role in remotely instructing patients or bystanders while immediately dispatching an ambulance. During patient transport, ambulance workers are responsible for the initial assessment and care of the patient as well as to provide continuous monitoring en route to the healthcare facility. In the ED, a series of medical activities are performed, provided by different healthcare professions, such as the triage nurse, general practitioners, physicians, and physician assistants. A seamless transition of care between the different role players will result in high‐quality coordination of care.
1.
Logic framework (Moresky 2019; WHO 2007; WHO 2018a).
One mechanism of how coordination of care interventions work is to improve the seamless interaction among multiple healthcare providers during the transition of care. A crucial determinant of effective emergency care is the ability of multiple healthcare providers to accurately capture and transfer clinical information of patients. Coordination of care interventions emphasise enhancing participants' communication during service delivery, leadership/governance for teamwork, and problem‐solving skills. This could prevent the loss of important clinical information caused by omitting information or transmitting inaccurate information during the short and high‐stressed transition‐of‐care time periods. Ultimately, through accurate and timely information communication, coordination interventions could reduce a number of risk factors during patient handovers (e.g. interruptions, repetition, unnecessary waiting time) and may help to create a simple, well‐summarised, effective, and standardised report for the patient. Furthermore, smooth information exchange could also promote the more efficient utilisation of resources and a smooth referral process within diverse settings, both of which are direct contributors to accurate and effective emergency care. Studies suggest that optimal coordination of care within the emergency care system could contribute to better workflow and better work relationships (Raaber 2016). It may also result in reducing distraction and stress of healthcare providers (Fitzpatrick 2018; Raaber 2016), or result in more sufficient data collection (Bergrath 2013). In addition to improving care through smooth information exchange, another role of an intervention to improve coordination of care is to ensure that effective high‐quality interdisciplinary care is provided despite care being transitioned through various care periods and settings. As Figure 1 illustrates, it is also assumed that the coordination interventions can improve delivery of care through facilitating more appropriate utilisation of resources in the emergency care system (e.g. the healthcare workforce, medical products and financing). It is thus understandable that improvements to the coordination of care will not only benefit individual patients, but also healthcare professionals and the entire healthcare system. Previous studies have indicated that better integration results in improved accessibility and timeliness to care (Hudon 2015), better health outcomes (Norris 2002), fewer hospitalisations (Tricco 2014), improved satisfaction with care (Neumeyer‐Gromen 2004), and reduced family burden, both psychologically and financially (Farmer 2011).
Why it is important to do this review
Care coordination is a critical component for the delivery of qualified adequate emergency care. This will facilitate the timely recognition of emergencies, while also initiating and continuously providing high‐quality care to acutely ill patients at any level within the health system. To date, there are several reviews related to interventions aiming to improve emergency care systems. As for Cochrane Reviews, Khangura 2012 conducted a review evaluating the effectiveness of non‐urgent care in hospital emergency departments for patients with non‐urgent conditions, while Mwandri 2017 assessed the effects of organised trauma systems and designated trauma centres for improving outcomes in injured patients. Though the interventions related to coordination of care can be included in the above two reviews, their focus differs from the target of this review. Our review will focus on coordination interventions in emergency healthcare services at the health system level, so we will include more interventions than Khangura 2012, and more kinds of health problems in emergency compared with Mwandri 2017. As for non‐Cochrane Reviews, five reviews did not focus on coordination of care within the broader emergency care system, covering the full linkage between pre‐hospital care, emergency departments, and the rest of the healthcare facility (Aghajafari 2020; Cassarino 2019; Katz 2012; Luu 2016; Reay 2017).
This review therefore aims to summarise the available evidence on the effectiveness of care coordination interventions on outcomes of emergency care systems. The interventions to be evaluated should be designed to improve care coordination by organising different providers and services to ensure the timely and efficient delivery of emergency care for patients. The identification of effective interventions might be beneficial to maximise the utilisation of medical resources, improve the health outcomes of patients, and reduce the overall disease burden.
Objectives
To evaluate the effectiveness of interventions aiming to improve coordination of emergency medical services on patient outcomes of emergency care systems.
Methods
Criteria for considering studies for this review
Types of studies
We will include individual and cluster‐randomised trials involving pairwise comparisons. We will include studies irrespective of their publication status and language.
Types of participants
Multiple participants may be included in interventions to improve coordination of emergency care, including the following.
Patients of all ages who require emergency care that includes pre‐hospital and/or in‐hospital services, e.g. patients with injuries/trauma, stroke, acute cardiac events, acute complications of pregnancy, and asthma.
Healthcare professionals providing emergency care, which could refer to individuals or teams of professionals including physicians, general practitioners, nurses, midwives, nurse practitioners, physician assistants, allied health professionals, care managers, supporting staff, social workers, liaison workers, health visitors, dispatchers, or ambulance workers.
Health system representatives for coordinating emergency care systems, including system administrators, and representatives of social systems, education, or healthcare facilities. These healthcare facilities might include hospitals, emergency departments, emergency medical services centres, centres for pre‐hospital care, ambulance stations, day care centres, or primary healthcare centres.
Types of interventions
We will include interventions aiming to improve coordination of care in emergency settings. Coordination interventions may be implemented before and/or when performing emergency care and/or following emergency care. The interventions to be included in this review must have at least one of the three features mentioned above, and must involve information sharing or other enablers to support the coordination. From this perspective, we developed three criteria that correspond to the features, as follows.
Need to occur across diverse sectors. 'Sectors' may refer to scene of emergency, emergency medical services (EMS) facilities, ambulance stations, emergency departments of hospitals, other departments involving emergency care of hospitals, and healthcare institutions that patients visit after discharge.
If occurring in the same sector, the interventions need to involve multiple participants, including patients, healthcare professionals/teams with different disciplines, and health system representatives.
If only involving the same participants, e.g. healthcare professionals, the interventions require inclusion of at least two different healthcare services. Relevant services may include ambulance care, emergency department care, hospital care, care after discharge intended to reduce relapse or further emergency care visits.
Inverventions evaluated in the experimental group should fulfil at least one of the criteria described above. We will then classify and group the included interventions as predominantly sequential coordination, parallel coordination, or mixed coordination, based on the judgement of the review authors.
As comparators, we will include:
usual care, i.e. no any changes in existing delivery of emergency services;
any other type of coordination intervention that meets the inclusion criteria described above but for which the content differs from the other arm of the trial.
Types of outcome measures
Outcomes maybe prioritised differently by different stakeholders, including healthcare system representatives, patients, and healthcare professionals, and we have tried to reflect this in our selection of primary and secondary outcomes.
Primary outcomes
Primary outcomes for this review focus on key outcomes from the healthcare system perspective, as follows.
-
Utilisation and access to health services:
utilisation of services, such as emergency department (ED) length of stay (continuous; measured in hours), hospital length of stay for admitted patients (continuous; measured in days);
access to services, such as time to: start of the ED assessment, laboratory tests sent and results received, diagnosis, consultation (continuous; measured in minutes);
quality of care, including adherence to clinical guidelines or recommended practice (dichotomous; measured in numbers);
occurrence of adverse or unanticipated negative effects (dichotomous; measured in numbers), such as increased waiting time for an ambulance; clinical, monitor, or medication errors; delays in standards of treatment; surgical complications.
-
Patient outcomes, including:
patient physical health and treatment outcomes, such as mortality (in‐hospital mortality or 30‐day mortality or one‐year mortality), incidence of complications;
treatment after discharge, such as rate of ED reattendance, rate of hospital readmission.
Secondary outcomes
Secondary outcomes for this review focus on key outcomes from the perspectives of patients and healthcare professionals, as follows.
Patient/family report of satisfaction with healthcare service, which might be measured by the researchers using a specific tool.
Healthcare professional report of satisfaction with coordination of care, such as staff workload, staff turnover, time spent in coordinating referrals, which might be measured by the researchers using a specific tool.
Search methods for identification of studies
Electronic searches
We will use an iterative approach to search for primary studies:
search CENTRAL, Cochrane Library for trials;
screen all records;
depending on the results of the screening, decide to only search for randomised trials in additional databases or broaden the search to also include Cochrane Effective Practice and Organisation of Care (EPOC) relevant non‐randomised trials (e.g. interrupted time series).
We will develop the strategies in consultation with the EPOC Information Specialist. We will search Epistemonikos, Epistemonikos Foundation (www.epistemonikos.org), for primary studies included in related systematic reviews.
We will search the following databases (from inception):
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library;
MEDLINE, Ovid;
Embase, Ovid;
Web of Science, Core Collection (for a cited reference search using all included studies);
PubMed, National Library of Medicine;
ProQuest Dissertations and Theses (about.proquest.com/en/dissertations/);
Chinese Medicine Premier (Wanfang Database);
Chinese National Knowledge Infrastructure (CNKD‐CNKI).
Search strategies will consist of keywords and controlled vocabulary terms. There will be no restriction on publication status, language, or country of publication. To limit retrieval to appropriate study designs, we will employ the Cochrane Highly Sensitive Search Strategy (sensitivity‐ and precision‐maximising version ‐ 2008 revision; Lefebvre 2011) to identify randomised trials (Higgins 2011), and if including designs other than randomised controlled trials, an EPOC methodology filter to identify non‐randomised trial designs.
See Appendix 1 for the CENTRAL, Cochrane Library search strategy.
Searching other resources
Grey literature
To identify studies that are not indexed in the databases listed above, we will carry out a grey literature search using the following resources:
OpenGrey (easy.dans.knaw.nl/ui/datasets/id/easy-dataset:200362);
Grey Literature Report (New York Academy of Medicine) (www.greylit.org);
World Health Organization (WHO) (www.who.int/).
Trial registries
We will search the following trial registries for completed but not published, ongoing, and planned trials:
WHO International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
We will also:
contact authors of relevant papers regarding any additional published or unpublished information;
review the reference lists of all included studies, relevant reviews/studies;
conduct cited reference searches for all included studies in Web of Science Core collection, KCI‐Korean Journal Database, Russian Science Citation Index, SciELO Citation Index, and Clarivate Analytics for papers that cited any of the studies included in this review;
search PubMed using the 'Similar articles' function to any included studies;
contact researchers with expertise relevant to the review topic/EPOC interventions.
Data collection and analysis
Selection of studies
We will download all titles and abstracts retrieved by the electronic searches to a reference management database and remove duplicates. Two review authors (MM and JL) will independently screen the titles and abstracts for potential relevance. We will obtain the full‐text reports of studies deemed potentially relevant, and two review authors (MM and JL) will independently screen the full‐text articles and identify studies for inclusion. We will record the excluded studies along with the reasons for their exclusion in 'Characteristics of excluded studies' tables. Any disagreements will be resolved through discussion or by consulting a third review author (DJvH) if required.
We will collate multiple reports of the same study so that each study, rather than each report, is the unit of interest. In addition, we will provide information on any ongoing studies. We will record the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We will use a standard data collection form adapted from the Cochrane EPOC Group for extracting study characteristics and outcome data (EPOC 2017). Before applying the data extraction tool, we will pilot it on at least two to three studies to evaluate its suitability. Two review authors (MM and JL) will independently extract the following study characteristics from the included studies and transfer the information into Review Manager 5 (Review Manager 2020).
Methods: study design, number of study centres and location, study setting, withdrawals, date of study, duration of follow‐up.
Participants: number, mean age, age range, gender, race, ethnicity, education, severity of condition, inclusion criteria, exclusion criteria, other relevant characteristics.
Intervention: classified according to the EPOC taxonomy of health system interventions and described using the TIDieR checklist (Hoffmann 2014), including the nature of primary or specialist health care provided.
Outcomes: as listed in Types of outcome measures, with time points.
Notes: funding source for trial, conflicts of interest of trial authors, ethical approval.
We will note in the 'Characteristics of included studies' table if outcome data were reported in an unusable way. Any disagreements will be resolved by consensus or by consulting a third review author (DJvH).
Assessment of risk of bias in included studies
Two review authors (MM and JL) will independently assess the risk of bias for each included study using the Cochrane risk of bias tool, Higgins 2011, and additional criteria specified by Cochrane EPOC (EPOC 2017). Any disagreements will be resolved by discussion or by consulting a third review author (DJvH). We will assess risk of bias based on the following domains.
For randomised trials:
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Baseline outcomes measurement
Baseline characteristics
Other bias (bias due to problems not covered by sources of bias specified above; for cluster‐randomised trials, we will assess the following specific issues: recruitment bias, baseline imbalance, protection against contamination, incorrect analysis).
We will judge each study to be at low, high, or unclear risk of bias for each domain listed above, and provide justifications for our judgement in the risk of bias table. Where information on risk of bias is related to correspondence with trialists or unpublished data, we will note this in the risk of bias table. We will summarise the risk of bias judgements across different studies for each of the domains listed and include the summary figure generated by Review Manager 5 software (Review Manager 2020). We will not exclude studies on the grounds of their risk of bias, but will clearly report the risk of bias in our presentation of results.
When considering intervention effects, we will take into account the risk of bias for the studies that contribute to that outcome, and incorporate this into our judgements of the certainty of the evidence. We will conduct a summary assessment of the risk of bias of each study using three key domains: sequence generation and allocation concealment (selection bias), and blinding of outcome assessors (detection bias). We will consider studies to be at low risk of bias if all three key domains were at low risk of bias; unclear risk of bias if at least one of the key domains was at unclear risk of bias and none of the key domains was at high risk of bias; and high risk of bias if at least one of the key domains was at high risk of bias. The methods of assessing risk of bias mentioned above apply to all types of studies included in this review.
Measures of treatment effect
We will assess the effect of the intervention using the following:
risk ratios (RRs), adjusting for baseline differences for dichotomous data, with the appropriate associated 95% confidence interval (CI);
mean difference (MD) or standardised mean difference (SMD) for continuous data, with 95% CI.
Ratios greater than 1 and differences greater than 0 between the control and intervention groups will represent benefit for the intervention group. We will ensure that an increase in scores for continuous outcomes can be interpreted in the same way for each outcome, explain the direction to the reader, and report where the directions were reversed, if this was necessary.
For measurement of intervention effect for randomised trials, we will extract the intervention effect estimate reported for outcomes in the included studies along with the P value, 95% CI, and the method used in their calculation. We will use the described methods for dichotomous outcomes and continuous outcomes.
These estimates are calculated from regression models adjusting for autocorrelation. It is not appropriate to present means and standard deviations of pre‐intervention versus postintervention time points. If analysis or reporting is not correct, which is very common due to the inappropriate use of t‐tests (Ramsay 2003), we will reanalyse according to the recommendations in the Cochrane EPOC Group guideline (EPOC 2017; Ramsay 2003).
Unit of analysis issues
We will perform analysis at the same level as the allocation for the included studies to prevent a unit of analysis error. For clustered designs, such as cluster‐randomised trials, we will report the data after adjusting for clustering. If the data from clustering trials have not been adjusted correctly, we will reanalyse the results based on guidance provided in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). Adjusting for clustering requires dividing the original sample size (and number of events in the case of dichotomous data) by the design effect, which is calculated from the average cluster size and the intracluster correlation coefficient (ICC). Where the ICC is not reported, we will impute the most commonly reported value from studies in where it is reported.
If there is a unit of analysis error, and if insufficient information precludes reanalysis of the study data, we will contact the authors of the original study to obtain the necessary information. If the information is not available, we will not report the CI and P value, and will note that the study has a unit of analysis error.
Dealing with missing data
We will contact the original study authors to verify key study characteristics and to obtain missing data. If necessary, we will attempt to calculate missing summary statistics from other reported data (Higgins 2011). We plan to use standard Cochrane methods for imputing missing values standard deviations from P values (Higgins 2020). If data cannot be obtained, we will report which data are missing and consider how this might impact the certainty of the evidence.
Assessment of heterogeneity
We will use visual inspection of forest plots and the Chi2 test to assess heterogeneity. We plan to perform meta‐analyses irrespective of the value of measures of statistical heterogeneity (I2 statistic), but we will interpret the degree of heterogeneity observed through evaluating the estimated value of the variance component that characterises the spread of likely treatment effects.
If we find substantial heterogeneity (I2 ≥ 50%) across studies, we will explore possible reasons for the variability by conducting subgroup analyses of potential risk of bias, population, and intervention characteristics that are considered to have potentially influenced the results.
Assessment of reporting biases
We will try to minimise reporting bias by:
including both published and unpublished studies;
in the case of studies with multiple publications, extracting data on outcomes from the publication with the most mature data;
not excluding studies solely on the basis of language of publication;
contacting study authors to ask for missing outcome data. If the information cannot be obtained, and the missing data would cause serious bias, we will explore the influence of including such studies in the overall assessment of results.
If we are able to pool more than 10 trials, we will generate a funnel plot employing Review Manager 5 software, Review Manager 2020, to examine possible publication biases, interpreting the results with caution (Higgins 2020; Sterne 2011).
Data synthesis
We intend to analyse the data on an intention‐to‐treat basis for all outcomes, that is we will include all participants randomised to each group in the analyses, and analyse data according to initial group allocation irrespective of whether or not participants received, or complied with, the planned intervention. If intention‐to‐treat analyses are not possible due to missing data, we will conduct an available‐case analysis, that is we will only include the number of participants on whom the outcome was measured in both the intervention and control groups. If we are unsuccessful in obtaining the missing information, we will perform a sensitivity analysis to detect the impact of missing data (Higgins 2011).
If the types of interventions and the outcome measures in the included studies fall into the same category, according to our classifications described above, we will carry out meta‐analysis using Review Manager 5 software (Review Manager 2020). Due to anticipated variability in the intervention and populations of included studies, we will use a random‐effects model for meta‐analysis.
Where trialists report medians and interquartile ranges, we will estimate the sample mean, standard deviation, and estimates of uncertainty from the sample size, median, range and/or interquartile range (Wan 2014). If a study has multiple trial arms, we will extract and analyse data from the relevant arms. If two comparisons (e.g. intervention A versus usual care and intervention B versus usual care) must be entered into the same meta analysis, we will halve the control group to avoid double‐counting.
We will follow the Synthesis Without Meta‐analysis (SWiM) guidance on reporting findings for outcomes where meta‐analysis is inappropriate (Campbell 2020). We will report a structured table of results across studies and use vote counting based on the direction of effect to summarise the results (Higgins 2020).
We do not plan to undertake a full economic analysis given the marked variation in studies we expect to uncover and the inherent risk of significant heterogeneity. Consequently, in accordance with Cochrane guidelines, we will provide a narrative summary of economic results instead of performing pooled analyses (Higgins 2020).
Subgroup analysis and investigation of heterogeneity
If there is significant heterogeneity and sufficient studies, we will perform subgroup analyses according to the characteristics of targeted patient, interventions implementer, and healthcare system supporting the intervention. These explanatory factors include:
targeted patient: demographic characteristics (e.g. age, race, ethnicity, education), medical history (e.g. type of illness, complications);
intervention: oriented pattern (e.g. government‐oriented, hospital‐oriented), intervention strategies (e.g. types, settings);
healthcare system: national socioeconomic level (e.g. high‐income countries, middle‐income countries, low‐income countries), resource distribution.
Table 1: Planned subgroup analysis
| Dimension | Factors | Hypothesis on how the factors may impact outcomes |
| Targeted patient | Demographic characteristics (e.g. age, race, ethnicity, education) | Patients with higher level of socioeconomic status (e.g. well‐educated, high income, less vulnerable) might benefit more from the intervention. |
| Medical history (e.g. different diseases and/or complications) | Intervention might be optimally performed in patients with more serious and acute conditions. | |
| Intervention | Oriented pattern (e.g. government‐oriented vs hospital‐oriented) | Government‐oriented intervention might be more effective in coordination of care than the hospital‐oriented intervention, as the former is policy‐driven and has larger coverage. |
| Intervention strategies (e.g. different types, different settings) | Interventions that are more problem‐centred, easier to operate, and target participants who play key role in care coordination, such as EMS dispatchers, may have better effect. | |
| Healthcare system | National socioeconomic level (e.g. high‐income countries, middle‐income countries, low‐income countries) | Intervention might be more effective when it is implemented in countries or regions with a higher socioeconomic level. |
| Resource distribution | Intervention might be more effective when it is implemented in countries or regions where medical resources are distributed less unequally. |
For all subgroup analyses, we will examine the primary and secondary outcomes described in the Types of outcome measures section. We will employ a test of interaction to evaluate statistically significant differences between subgroups. If there are too few included studies to conduct statistical subgroup analyses, we will investigate relationships in the data narratively, such as by presenting a narrative form of subgroup analyses.
Sensitivity analysis
We will perform sensitivity analyses by employing multiple imputation methods that produce complete data sets from incomplete data by imputing the missing data several times (Higgins 2011), when the following occur:
we are unable to obtain important missing data from study authors;
studies with high risk of bias are included;
we have performed reanalysis (e.g. in a cluster‐randomised trial where the ICC was not considered initially) to check the stability of our result.
We will also conduct the following sensitivity analyses to assess the robustness of our conclusions and explore their impact on effect sizes:
restricting the analysis to published studies to evaluate whether publication status has an impact on effect size;
restricting the analysis to studies with a low risk of bias on each of the three key risk of bias domains to evaluate whether risk of bias in studies has an impact on effect size;
investigating the effect of imputing missing data, by restricting the analysis to studies where data were not imputed. If summary statistics were imputed by estimating correlation coefficients from other studies, the analysis will be repeated with different values of correlation coefficient to evaluate whether the overall result is sensitive to changes in assumptions used to impute data.
We will conduct the review according to this published protocol and report any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Summary of findings and assessment of the certainty of the evidence
Two review authors (MM and JL) will independently assess the certainty of the evidence (high, moderate, low, or very low) using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) (Guyatt 2008). We will use the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011, and the EPOC worksheets (EPOC 2013), employing GRADEpro GDT software (GRADEpro GDT).
We will create summary of findings tables for the main intervention comparisons, which will include the primary outcomes, adverse effects, and the certainty of the evidence. Any disagreements will be resolved by discussion, and we will provide justification for our decisions to down‐ or upgrade the ratings using footnotes in the table and make comments to aid the reader's understanding of the review where necessary. We will use plain language statements to report these findings in the review (EPOC 2013). If, during the review process, we become aware of an important outcome that we failed to list in our planned summary of findings table(s), we will include the relevant outcome and explain the reasons for this is the 'Differences between protocol and review' section of the systematic review.
Acknowledgements
Cochrane Effective Practice and Organisation of Care (EPOC) supported the authors in the development of this protocol. The Methods section of this protocol is based on a standard template used by Cochrane EPOC.
The following people conducted the editorial process for this protocol:
Sign‐off Editor (final editorial decision): Simon Lewin, Norwegian University of Science and Technology;
Contact Editor (methods review): Tomas Pantoja, Pontificia Universidad Católica de Chile;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Elizabeth Paulsen, Norwegian Institute of Public Health;
Copy Editor (copy editing and production): Lisa Winer;
Peer reviewers (provided comments and recommended an editorial decision): Benjamin Wachira, Accident & Emergency Department, The Aga Khan University, Nairobi (clinical/content review); Hans Clausdorff, Pontificia Universidad Católica de Chile (clinical/content review); Peter Hodkinson, Division of Emergency Medicine, University of Cape Town (clinical/content review); and Chris Rose, Norwegian Institute of Public Health (statistical review).
Appendices
Appendix 1. Search strategy
CENTRAL, Cochrane Library
| #1 | MeSH descriptor: [Patient Handoff] this term only | 34 |
| #2 | MeSH descriptor: [Patient Transfer] this term only | 161 |
| #3 | MeSH descriptor: [Referral and Consultation] this term only | 2020 |
| #4 | MeSH descriptor: [Critical Pathways] this term only | 205 |
| #5 | MeSH descriptor: [Geographic Information Systems] this term only | 42 |
| #6 | MeSH descriptor: [Information Dissemination] this term only | 241 |
| #7 | MeSH descriptor: [Patient Care Team] this term only | 1755 |
| #8 | MeSH descriptor: [Hospital Rapid Response Team] this term only | 16 |
| #9 | MeSH descriptor: [Patient Care Planning] this term only | 613 |
| #10 | MeSH descriptor: [Cooperative Behavior] this term only | 967 |
| #11 | MeSH descriptor: [Intersectoral Collaboration] this term only | 51 |
| #12 | MeSH descriptor: [Interdisciplinary Communication] this term only | 268 |
| #13 | MeSH descriptor: [Delivery of Health Care, Integrated] this term only | 421 |
| #14 | MeSH descriptor: [Continuity of Patient Care] this term only | 639 |
| #15 | MeSH descriptor: [Comprehensive Health Care] this term only | 79 |
| #16 | MeSH descriptor: [Patient Care Management] this term only | 154 |
| #17 | MeSH descriptor: [Case Management] this term only | 718 |
| #18 | MeSH descriptor: [Case Managers] this term only | 15 |
| #19 | MeSH descriptor: [Crew Resource Management, Healthcare] this term only | 4 |
| #20 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 | 7239 |
| #21 | MeSH descriptor: [Emergency Medical Services] explode all trees | 4298 |
| #22 | MeSH descriptor: [Emergency Medicine] this term only and with qualifier(s): [organization & administration ‐ OG] | 11 |
| #23 | MeSH descriptor: [Critical Care] this term only and with qualifier(s): [organization & administration ‐ OG] | 33 |
| #24 | MeSH descriptor: [Emergency Responders] this term only | 29 |
| #25 | MeSH descriptor: [Emergency Medical Technicians] this term only | 180 |
| #26 | MeSH descriptor: [Emergency Nursing] this term only | 80 |
| #27 | MeSH descriptor: [Trauma Nursing] this term only | 0 |
| #28 | #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 | 4480 |
| #29 | #20 AND #28 | 454 |
| #30 | MeSH descriptor: [Emergency Medical Service Communication Systems] this term only | 53 |
| #31 | #29 OR #30 | 505 |
| #32 | ("emergency service" or "emergency services" or "emergency care" or "emergency medical care" or "emergency health care" or "emergency healthcare") NEXT system*:ti,ab,kw | 9 |
| #33 | ((integrat* or coordinat* or co‐ordinat* or collaborat* or cooperat* or co‐operat*) and (emergency or emergencies)):ti,kw | 261 |
| #34 | ((integrat* or coordinat* or co‐ordinat* or collaborat* or cooperat* or co‐operat*) NEAR/6 (emergency or emergencies)):ab | 105 |
| #35 | ((interdisciplin* or inter‐disciplin* or multidisciplin* or multi‐disciplin* or cross‐disciplin* or cross‐sectoral) and (emergency or emergencies)):ti,kw | 105 |
| #36 | ((interdisciplin* or inter‐disciplin* or multidisciplin* or multi‐disciplin* or cross‐disciplin* or cross‐sectoral) NEAR/6 adj6 (emergency or emergencies)):ab | 0 |
| #37 | (("seamless care" or "shared care") and (emergency or emergencies)):ti,kw | 1 |
| #38 | (("seamless care" or "shared care") NEAR/6 (emergency or emergencies)):ab | 1 |
| #39 | (("care plan" or "care plans" or "critical pathway" or "critical pathways" or clinical "pathway" or "clinical pathways" or guideline*) and (emergency or emergencies)):ti,kw | 583 |
| #40 | (("care plan" or "care plans" or "critical pathway" or "critical pathways" or clinical "pathway" or "clinical pathways" or guideline*) NEAR/6 (emergency or emergencies)):ab | 910 |
| #41 | ((case‐manag* or care‐manag*) and (emergency or emergencies)):ti,kw | 168 |
| #42 | ((case‐manage* or care‐manag*) NEAR/6 (emergency or emergencies)):ab | 41 |
| #43 | ((patient‐team* or care‐team*) and (emergency or emergencies)):ti,kw | 147 |
| #44 | ((patient‐team* or care‐team*) NEAR/6 (emergency or emergencies)):ab | 13 |
| #45 | ((patient or clinical) and (handoff* or hand‐off* or handover* or hand‐over*) and (emergency or emergencies)):ti,kw | 19 |
| #46 | (((patient or clinical) NEAR/6 (handoff* or hand‐off* or handover* or hand‐over*)) and (emergency or emergencies)):ab | 20 |
| #47 | ((patient*) and (transfer*) and (emergency or emergencies)):ti,kw | 79 |
| #48 | ("patient transfer" NEAR/6 (emergency or emergencies)):ab | 2 |
| #49 | (("information transfer" or information‐transmi* or "information exchange" or "information sharing" or "data transfer" or data‐transmi* or "data exchange" or "data sharing") and (emergency or emergencies)):ti,kw | 9 |
| #50 | (("information transfer" or information‐transmi* or "information exchange" or "information sharing" or "data transfer" or data‐transmi* or "data exchange" or "data sharing") NEAR/6 (emergency or emergencies)):ab | 4 |
| #51 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 | 2177 |
| #52 | #31 or #51 in Trials | 2441 |
Contributions of authors
Conceiving the protocol: Yinzi Jin, Beibei Yuan.
Designing the protocol: Yinzi Jin, Beibei Yuan.
Coordinating the protocol: Beibei Yuan.
Writing the protocol: Mailikezhati Maimaitiming, Yinzi Jin, Jia Li, Beibei Yuan.
Providing general advice on the protocol: Daniël Jacobus van Hoving.
Performing previous work that was the foundation of the current study: Mailikezhati Maimaitiming, Yinzi Jin.
Sources of support
Internal sources
-
National Natural Science Foundation of China (No. 71904004), China
This study will be funded by the National Natural Science Foundation of China (No. 71904004). The study sponsor will have no role in study design, data analysis and interpretation of data, the writing of manuscript, or the decision to submit the paper for publication.
-
2020 China Medical Board (CMB) Competition Program (No. #20‐376), China
This study will be funded by the 2020 China Medical Board (CMB) Competition Program (No. #20‐376). The study sponsor will have no role in study design, data analysis and interpretation of data, the writing of manuscript, or the decision to submit the paper for publication.
External sources
No sources of support provided
Declarations of interest
Yinzi Jin: none.
Mailikezhati Maimaitiming: none.
Jia Li: none.
Daniël Jacobus van Hoving: none.
Beibei Yuan: none.
New
References
Additional references
Aghajafari 2020
- Aghajafari F, Sayed S, Emami N, Lang E, Abraham J. Optimizing emergency department care transitions to outpatient settings: a systematic review and meta-analysis. American Journal of Emergency Medicine 2020;38(12):2667-80. [DOI] [PubMed] [Google Scholar]
Bergrath 2013
- Bergrath S, Rossaint R, Lenssen N, Fitzner C, Skorning M. Prehospital digital photography and automated image transmission in an emergency medical service - an ancillary retrospective analysis of a prospective controlled trial. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2013;21:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvello 2013
- Calvello EJ, Broccoli M, Risko N, Theodosis C, Totten VY, Radeos MS, et al. Emergency care and health systems: consensus-based recommendations and future research priorities. Academic Emergency Medicine 2013;20(13):1278-88. [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:1-6. [DOI: 10.1136/bmj.l6890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassarino 2019
- Cassarino M, Robinson K, Quinn R, Naddy B, O'Regan A, Ryan D, et al. Impact of early assessment and intervention by teams involving health and social care professionals in the emergency department: a systematic review. PLOS ONE 2019;14(7):e0220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2016
- Chang CY, Abujaber S, Reynolds TA, Camargo CA Jr, Obermeyer Z. Burden of emergency conditions and emergency care usage: new estimates from 40 countries. Emergency Medicine Journal 2016;33(11):794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebben 2015
- Ebben RH, Grunsven PM, Moors ML, Aldenhoven P, Vaan J, Hout R, et al. A tailored e-learning program to improve handover in the chain of emergency care: a pre-test post-test study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2015;23:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2013
- EPOC. Suggested risk of bias criteria for EPOC reviews. Good practice data extraction form, EPOC Resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 27 April 2015).
EPOC 2017
- EPOC. EPOC resources for review authors. epoc.cochrane.org/ resources/epoc-resources-review-authors (accessed 1 August 2019).
Fahim Yegane 2017
- Fahim Yegane SA, Shahrami A, Hatamabadi HR, Hosseini-Zijoud SM. Clinical information transfer between EMS staff and emergency medicine assistants during handover of trauma patients. Prehospital and Disaster Medicine 2017;32(5):541-7. [DOI] [PubMed] [Google Scholar]
Farmer 2011
- Farmer JE, Clark MJ, Drewel EH, Swenson TM, Ge B. Consultative care coordination through the medical home for CSHCN: a randomized controlled trial. Maternal and Child Health Journal 2011;15(7):1110-8. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2018
- Fitzpatrick D, Maxwell D, Craigie A. The feasibility, acceptability and preliminary testing of a novel, low-tech intervention to improve pre-hospital data recording for pre-alert and handover to the Emergency Department. BMC Emergency Medicine 2018;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 1 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is 'quality of evidence' and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Hirshon 2013
- Hirshon JM, Risko N, Calvello EJ, Stewart de Ramirez S, Narayan M, Theodosis C, et al. Health systems and services: the role of acute care. Bulletin of the World Health Organization 2013;91(5):386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:1687. [DOI] [PubMed] [Google Scholar]
Hudon 2015
- Hudon C, Chouinard MC, Diadiou F, Lambert M, Bouliane D. Case management in primary care for frequent users of health care services with chronic diseases: a qualitative study of patient and family experience. Annals of Family Medicine 2015;13(6):523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2012
- Katz EB, Carrier ER, Umscheid CA, Pines JM. Comparative effectiveness of care coordination interventions in the emergency department: a systematic review. Annals of Emergency Medicine 2012;60(1):12-23 e1. [DOI] [PubMed] [Google Scholar]
Khangura 2012
- Khangura JK, Flodgren G, Perera R, Rowe BH, Shepperd S. Primary care professionals providing non-urgent care in hospital emergency departments. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD002097. [DOI: 10.1002/14651858.CD002097.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kripalani 2007
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007;297(8):831-41. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luu 2016
- Luu NP, Pitts S, Petty B, Sawyer MD, Dennison-Himmelfarb C, Boonyasai RT, et al. Provider-to-provider communication during transitions of care from outpatient to acute care: a systematic review. Journal of General Internal Medicine 2016;31(4):417-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2009
- Miller AR, Condin CJ, McKellin WH, Shaw N, Klassen AF, Sheps S. Continuity of care for children with complex chronic health conditions: parents' perspectives. BMC Health Services Research 2009;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moresky 2019
- Moresky RT, Razzak J, Reynolds T, Wallis LA, Wachira BW, Nyirenda M, et al. Advancing research on emergency care systems in low-income and middle-income countries: ensuring high-quality care delivery systems. BMJ Global Health 2019;4(Suppl 6):e001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwandri 2017
- Mwandri M, Stewart B, Hardcastle TC, Rubiano AM, Gruen RL. Organised trauma systems and designated trauma centres for improving outcomes in injured patients. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD012500. [DOI: 10.1002/14651858.CD012500] [DOI] [Google Scholar]
Neumeyer‐Gromen 2004
- Neumeyer-Gromen A, Lampert T, Stark K, Kallischnigg G. Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Medical Care 2004;42(12):1211-21. [DOI] [PubMed] [Google Scholar]
Norris 2002
- Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. American Journal of Preventive Medicine 2002;22(Suppl 4):15-38. [DOI] [PubMed] [Google Scholar]
Pham 2008
- Pham HH, Grossman JM, Cohen G, Bodenheimer T. Hospitalists and care transitions: the divorce of inpatient and outpatient care. Health Affairs (Millwood) 2008;27(5):1315-27. [DOI] [PubMed] [Google Scholar]
Raaber 2016
- Raaber N, Duvald I, Riddervold I, Christensen EF, Kirkegaard H. Geographic information system data from ambulances applied in the emergency department: effects on patient reception. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2016;24:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series design in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):612-23. [DOI] [PubMed] [Google Scholar]
Razzak 2019
- Razzak J, Beecroft B, Brown J, Hargarten S, Anand N. Emergency care research as a global health priority: key scientific opportunities and challenges. BMJ Global Health 2019;4(Suppl 6):e001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reay 2017
- Reay G, Norris JM, Alix Hayden K, Abraham J, Yokom K, Nowell L, et al. Transition in care from paramedics to emergency department nurses: a systematic review protocol. Systematic Reviews 2017;6(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Reynolds 2017
- Reynolds TA, Sawe H, Rubiano AM, Do Shin S, Wallis L, Mock CN. Chapter 13: Strengthening health systems to provide emergency care. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, et al, editors(s). Disease Control Priorities: Improving Health and Reducing Poverty. 3rd edition. Washington, DC: World Bank, 2017:247-65. [PMID: 30212151]30212151 [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Strobel 2017
- Strobel NA, Arabena K, East CE, Schultz EM, Kelaher M, Edmond KM, et al. Care co-ordination interventions to improve outcomes during pregnancy and early childhood (up to 5 years). Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD012500. [DOI: 10.1002/14651858.CD012500] [DOI] [Google Scholar]
Thind 2015
- Thind A, Hsia R, Mabweijano J, Hicks ER, Zakariah A, Mock CN. Chapter 14: Prehospital and emergency care. In: Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN, editors(s). Essential Surgery: Disease Control Priorities. 3rd edition. Vol. 1. Washington, DC: World Bank, 2015:245-62. [PMID: 26741008]26741008 [Google Scholar]
Tricco 2014
- Tricco AC, Antony J, Ivers NM, Ashoor HM, Khan PA, Blondal E, et al. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis. Canadian Medical Association Journal 2014;186(15):E568-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2020
- Werner K, Risko N, Burkholder T, Munge K, Wallis L, Reynolds T. Cost-effectiveness of emergency care interventions in low and middle-income countries: a systematic review. Bulletin of the World Health Organization 2020;98(5):341-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Everybody's business - strengthening health systems to improve health outcomes: WHO's framework for action. www.who.int/publications/i/item/everybody-s-business----strengthening-health-systems-to-improve-health-outcomes (accessed prior to 13 January 2023).
WHO 2018a
- World Health Organization. Continuity and coordination of care: a practice brief to support implementation of the WHO Framework on integrated people-centred health services. www.who.int/publications/i/item/9789241514033 (accessed prior to 13 January 2023).
WHO 2018b
- World Health Organization. WHO Emergency care system framework. www.who.int/publications/i/item/who-emergency-care-system-framework (accessed prior to 13 January 2023).
Zanello 2015
- Zanello E, Calugi S, Rucci P, Pieri G, Vandini S, Faldella G, et al. Continuity of care in children with special healthcare needs: a qualitative study of family's perspectives. Italian Journal of Pediatrics 2015;41:7. [DOI] [PMC free article] [PubMed] [Google Scholar]