Abstract
The mammalian Ror family of receptor tyrosine kinases consists of two structurally related proteins, Ror1 and Ror2. We have shown that mRor2-deficient mice exhibit widespread skeletal abnormalities, ventricular septal defects in the heart, and respiratory dysfunction, leading to neonatal lethality (S. Takeuchi, K. Takeda, I. Oishi, M. Nomi, M. Ikeya, K. Itoh, S. Tamura, T. Ueda, T. Hatta, H. Otani, T. Terashima, S. Takada, H. Yamamura, S. Akira, and Y. Minami, Genes Cells 5:71–78, 2000). Here we show that mRor1-deficient mice have no apparent skeletal or cardiac abnormalities, yet they also die soon after birth due to respiratory dysfunction. Interestingly, mRor1/mRor2 double mutant mice show markedly enhanced skeletal abnormalities compared with mRor2 mutant mice. Furthermore, double mutant mice also exhibit defects not observed in mRor2 mutant mice, including a sternal defect, dysplasia of the symphysis of the pubic bone, and complete transposition of the great arteries. These results indicate that mRor1 and mRor2 interact genetically in skeletal and cardiac development.
Receptor tyrosine kinases (RTKs) play several crucial roles in developmental morphogenesis, regulating cellular proliferation, differentiation, and migration, as well as survival and death (26, 30). The Ror family RTKs are a recently identified family of orphan RTKs, characterized by the presence of extracellular Frizzled-like cysteine-rich domains and membrane-proximal Kringle domains, both of which are assumed to mediate protein-protein interactions (15, 20, 24, 25, 29). The Ror family RTKs are evolutionarily conserved among Caenorhabditis elegans, Drosophila, mice, and humans (7, 14, 19, 20, 34). Pairs of structurally similar Ror family RTKs are found in Drosophila and mammals: Dror and Dnrk in Drosophila melanogaster, Ror1 and Ror2 in humans, and mRor1 and mRor2 in mice. Although it has been reported that CAM-1, a C. elegans ortholog of the Ror family RTKs, plays several important roles in regulating cellular migration, polarity of asymmetric cell divisions, and axonal outgrowth of neurons during nematode development (7), the functional and developmental roles of the mammalian Ror family RTKs remain largely elusive.
The spatial and temporal expression of mRor1 and mRor2 mostly overlap and are detected in the face, limbs, heart, and lungs during mouse embryogenesis (16). These expression patterns suggest that mRor1 and mRor2 may interact to play a role in the development of these organs. It has been shown that mice lacking mRor2 expression exhibit dwarfism, short limbs (with mesomelic dysplasia) and tail, facial anomalies, ventricular septal defect (VSD), and respiratory dysfunction, ultimately leading to neonatal lethality (5, 32). Histological analyses of the skeletal systems reveal that mRor2 plays a crucial role in the proliferation, differentiation, maturation, and motility of chondrocytes (5, 32). Interestingly, it has recently been reported that mutations within Ror2 can cause the autosomal recessive Robinow syndrome or autosomal dominant brachydactyly type B in humans (1, 21, 31, 33), further emphasizing essential roles of Ror2 in morphogenetic and developmental processes. However, little is known about the function of mRor1 during mouse development.
In order to elucidate the functional and developmental roles of mRor1, we generated mice lacking a functional mRor1 gene by targeted gene disruption. mRor1−/− mice died within 24 h after birth, presumably due to respiratory dysfunction. However, unlike the mRor2−/− mice, they did not exhibit any obvious morphological abnormalities of the skeleton or heart. Given that the spatiotemporal expression patterns of mRor1 and mRor2 mostly overlap during development (16), we investigated whether the loss of mRor1 function can be compensated for by mRor2 in mRor1−/− mice. To determine whether mRor1 interacts genetically with mRor2 during mouse development, we generated mRor1/mRor2 double mutants. Interestingly, the double mutants exhibited defects in the skeletal and cardiac systems similar to but more severe than those observed in the single mRor2 mutants. Furthermore, the double mutant mice exhibited several defects not found in either the mRor1 or mRor2 single mutants, namely, defects in the sternum, dysplasia of the symphysis of the public bone, and complete transposition of the great arteries. Analyses of these mutant mice indicate that mRor1 and mRor2 are functionally redundant and that mRor1 and mRor2 interact genetically in skeletal and cardiac development.
MATERIALS AND METHODS
Preparation of mRor1 and mRor1/mRor2 mutants.
Genomic DNA containing the mRor1 locus was isolated from a genomic library of mouse strain 129 (Stratagene). The exon of the mRor1 gene, containing an immunoglobulin (Ig)-like domain, was replaced by the neo gene, and the herpes simplex virus thymidine kinase gene was fused to the 5′ end. The targeting vector was inserted into the E14 line of embryonic stem cells by electroporation, and homologous recombinants were selected by G418 and ganciclovir and identified by PCR and Southern blot analysis. Targeted embryonic stem cells were injected into blastocysts of C57BL/6 mice. The chimeras were mated with C57BL/6 mice, and the mRor1 mutation was transmitted to the germ line. The generation of mRor2 mutant mice has been described (32). mRor1/mRor2 double mutants were generated by intercrossing mRor1+/−; mRor2+/− mice, and the embryos were genotyped by PCR analysis of extraembryonic membranes.
Whole-mount in situ hybridization.
In situ hybridization analyses of whole-mount embryos were performed as previously described (35). The 0.7-kb PstI/KpnI fragment of mRor1 or the 0.86-kb KpnI/NotI fragment of mRor2 was utilized as a template to synthesize single-strand RNA probes.
Histological analysis.
Embryos and newborns were fixed with 4% paraformaldehyde, dehydrated, embedded in wax, sectioned, and processed for hematoxylin-and-eosin staining as described previously (17).
Skeletal preparation.
Skeletal specimens were prepared as described previously (12, 23) with minor modifications. In brief, newborn mice were eviscerated, fixed in 100% ethanol for 24 h, and transferred to acetone. After 24 h, they were rinsed with water and stained for 4 to 6 h at 37°C and for 24 h at room temperature in a staining solution consisting of 1 volume of 0.2% Alizarin red S (Sigma) in 95% ethanol, 1 volume of 0.3% Alcian blue 8GX (Sigma) in 70% ethanol, 1 volume of 100% acetic acid, and 17 volumes of ethanol. The specimens were treated with 1% trypsin in 30% saturated sodium borate solution at 37°C until ribs became clear and then in 1% KOH at room temperature. After several rinses with distilled water, the specimens were kept in 20% glycerol at room temperature.
RESULTS AND DISCUSSION
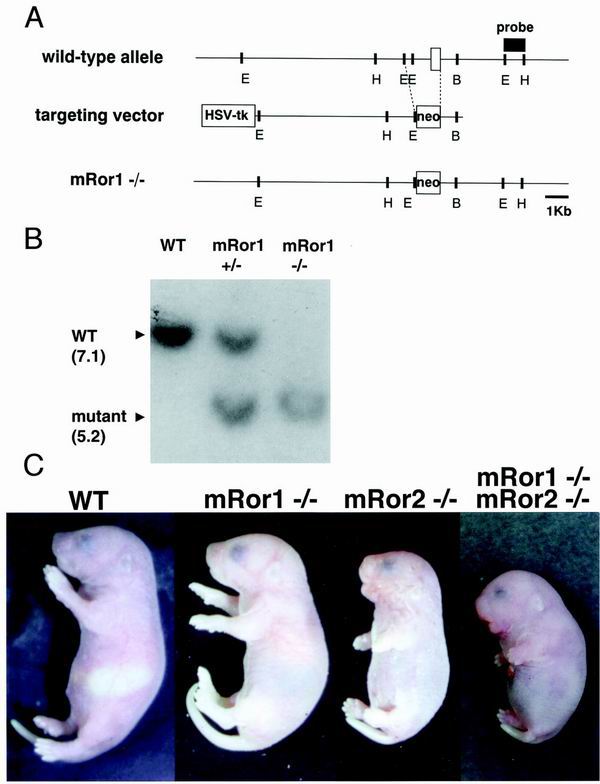
To elucidate the functions of mRor1, we generated mice lacking the exon of mRor1 containing the Ig-like domain (Fig. 1A). Heterozygous mRor1+/− mice were viable and fertile and appeared normal. Heterozygous mice were crossed to produce wild-type, heterozygous, and homozygous mutant mice, as assessed by genotyping using Southern blot and PCR analyses (Fig. 1B and data not shown). The mRor1−/− newborns were similar in size to the wild-type mice and showed no apparent gross abnormalities (Fig. 1C). However, after birth, mRor1−/− mice exhibited forced respiration and cyanosis and died within 24 h (data not shown). In contrast, mRor2−/− newborns exhibited dwarfism, short limbs and tail, and malformation of facial structures (Fig. 1C).
FIG. 1.
Targeted disruption of the mRor1 gene. (A) Targeting strategy. The wild-type mRor1 locus, targeting vector, and predicted mutant locus are shown. The exon (including the Ig-like domain), PGK-tk, and PGK-neo are depicted as open boxes. B, BamHI; E, EcoRI; H, HincII; HSV-tk, herpes simplex virus thymidine kinase. (B) Southern blot analysis of fetal DNA. Genomic DNA isolated from yolk sacs of embryos was digested with EcoRV and HincII and hybridized with the EcoRI-HincII probe shown in panel A. Sizes of bands are in kilobases. (C) Gross appearance of wild-type (WT) and mutant newborns. While the mRor1−/− newborn looks essentially identical to the WT newborn, the mRor2−/− newborn is small and cyanotic and has short limbs and tail. Enhancement of mRor2−/− phenotypes is observed in mRor1−/−; mRor2−/− newborns.
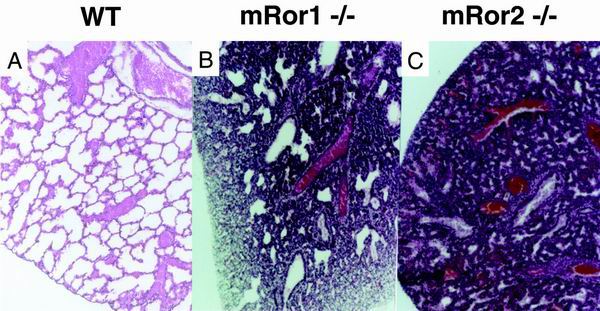
Since mRor1−/− newborns died apparently as a result of respiratory dysfunction, similar to mRor2−/− mice, we performed a histological examination of their lungs. Expansion of the alveoli in mRor1 and mRor2 mutant newborns was found to be incomplete, while the lungs of the wild-type newborns displayed normally expanded alveoli (Fig. 2), suggesting that mRor1 and mRor2 mutants die due to difficulty in breathing. Since both mRor1 and mRor2 are expressed in primitive alveoli in the developing lung (16), mRor1 and/or mRor2 may play important roles in the development and function of alveolar type II cells, which produce dipalmitoylphosphatidylcholine (DPPC) and surfactant proteins (9, 13). However, expression levels of the surfactant protein genes SP-A, -B, -C, and -D in the lungs and the amounts of DPPC in bronchoalveolar lavage fluid from mRor1 and mRor2 mutant newborns were comparable to those of their wild-type littermates, as assessed by Northern blot and gas chromatographic analyses, respectively (M. Nomi, Y. Kuroki, and Y. Minami, unpublished data). Thus, the development and function of alveolar type II cells is unaffected in the absence of mRor1 or mRor2. Further study is required to clarify the molecular or cellular basis underlying pulmonary dysfunction in these mutant mice and to understand the functional roles of mRor1 and mRor2 during lung development.
FIG. 2.
Histological analysis of the lung. Respiratory malfunction was identified in mRor1−/− and mRor2−/− newborns. Postmortem histological analysis of the lung demonstrates that the alveolar air sacs in the mutant newborns are not fully expanded as they are in the wild-type (WT) control littermate. In addition, pulmonary bleeding was observed in the mutant mice.
Although severe skeletal and cardiac phenotypes were found in mRor2−/− mice, mRor1−/− mice did not exhibit any apparent abnormalities of the skeleton or heart (see below). Since mRor1 and mRor2 exhibited similar expression patterns in the developing face, limbs, heart, and lungs (16), the lack of apparent abnormalities in the mRor1−/− mice may be attributable to the functional redundancy between mRor1 and mRor2. Given the more severe phenotypes in mRor2−/− mice than in mRor1−/− mice, we also considered that expression of mRor1 may be down-regulated in mRor2−/− mice. However, our in situ hybridization analyses of mRor2−/− and mRor1−/− embryos at day 10.5 of development (E10.5) revealed that the spatial expression pattern and level of mRor1 are unaffected by disruption of mRor2 and vice versa (data not shown).
To determine whether mRor1 interacts genetically with mRor2 during morphogenesis, we generated mRor1/mRor2 double mutants by intercrossing mRor1+/−; mRor2+/− mice. The mRor1/mRor2 mice exhibited perinatal lethality, and, indeed, most if not all newborns were dead upon birth. Although mRor1−/− mice appeared to be essentially identical to wild-type mice, mRor1/mRor2 mice exhibited enhanced mRor2−/− phenotypes (Fig. 1C). The shortening of limb and tail length in proportion to body length and malformation of the facial structures observed in mRor2−/− mice were more profound in the double mutant mice, indicating that mRor1 and mRor2 interact genetically during embryonic morphogenesis.
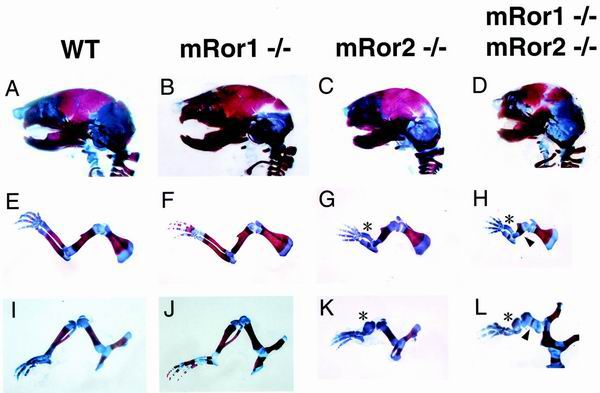
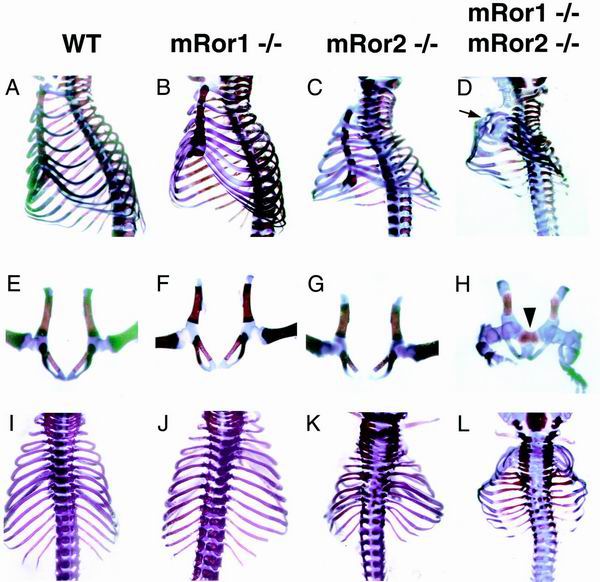
To examine more precisely the defects in limbs and body length of mRor1/mRor2 double mutants, we next compared skeletons from wild-type, mRor1−/−, and mRor2−/− newborns and double mutant embryos (E19.5) and newborns by staining with Alizarin red and Alcian blue. It has been shown that mRor2−/− mice have abnormally short limbs and tails and abnormal vertebrae and facial structures and that these defects are more severe in the more distal portions (5, 32) (Fig. 3 and 4). mRor2−/− mice also possess a unique anomaly characterized by mesomelic dysplasia (significant or complete loss of the radius, ulna, tibia, and fibula). Consistent with their gross appearance, mRor1−/− newborns did not show any skeletal abnormalities (Fig. 3 and 4). Interestingly, the mRor1/mRor2 double mutant mice exhibited a drastic enhancement of the mRor2−/− skeletal phenotypes (Fig. 3 and 4). Compared with mRor2−/− mice, more severe hypoplasia of the maxilla and mandible was found in the double mutant mice (Fig. 3C and D). Importantly, dysplasia of the proximal long bones (the humerus and femur), in addition to the distal long bones (mesomelic bones), was observed in the double mutant mice (Fig. 3H and I). Significantly, the mRor1/mRor2 mice exhibited a sternal defect (sternal agenesis) and dysplasia of the symphysis of the pubic bone, skeletal abnormalities that were not observed in mRor2−/− mice (Fig. 4D and H). These results indicate that mRor1 and mRor2 are functionally redundant in the development of the skeletal system and that mRor2 can compensate for the lack of mRor1 function in mRor1−/− mice. Our observation that mutation of mRor1 in an mRor2−/− background caused enhanced skeletal defects as well as additional new phenotypes further indicates the genetic interaction of mRor1 and mRor2 during development.
FIG. 3.
Examination of craniofacial bones and appendicular skeletons. The craniofacial bones and appendicular skeletons from wild-type (WT), mRor1−/−, and mRor2−/− newborns and double mutant embryos (E19.5) were double stained with Alizarin red and Alcian blue as described in Materials and Methods. Lateral views of the skeleton of the head (A to D) and extremities (forelimb [E to H] and hind limb [I to L]) from wild-type and mutant newborns and a double mutant embryo are shown. Asterisks and arrowheads, dysplasia of the distal and proximal parts of limb bones in mRor2−/− and double mutant mice, respectively. In some cases, apparently delayed ossification of the cranial suture was also observed (data not shown).
FIG. 4.
Examination of ribs, sternal bone, vertebrae, and pelvic bones. The skeletons in wild-type (WT)and mutant newborns and double mutant embryos were double stained with Alizarin red and Alcian blue. Lateral (A to D), ventral (E to H), and dorsal (I to L) views are shown. Arrow and arrowhead, sternal defect (D) and dysplasia of the symphysis of the pubic bone (H) in mRor1/mRor2 mice, respectively. The sternal defect and dysplasia of the symphysis of the pubic bone were observed in 100% (four of four) and 25% (one of four) of the double mutant mice.
Dysplasia of the distal long bones in mRor2−/− mice and of both the distal and proximal long bones in mRor1/mRor2 double mutant mice suggests that mRor2 alone or in collaboration with mRor1 may contribute to the compartmentalization of the appendicular skeletons. It has been shown that the radius and ulna are almost completely missing in hoxa-11, hoxd-11 double mutant mice (4). In this respect, it will be of interest to examine the possible relationships of mRor1 and mRor2 with hoxa-11, hoxd-11, and other hox family genes. As described above, several unique skeletal abnormalities were observed in mRor1/mRor2 mice, including a sternal defect and dysplasia of the symphysis of the pubic bone. Since it has been reported that a subset of transgenic mice with aberrant expression of hoxd-12 exhibit similar sternal defects as well as abnormalities in the pelvis (11), we should also consider the possible relationship of mRor1 and mRor2 with hoxd-12. Previous histological analyses of the long bones from mRor2−/− mice have shown that they have fewer small flattened chondrocytes and exhibit disarranged and short longitudinal columns of proliferative chondrocytes in the zones of proliferation and maturation, suggesting that mRor2 is required for the proper proliferation, differentiation, maturation, and motility of chondrocytes (5, 32). Our histological analyses of the long bones from double mutant mice revealed essentially identical results (data not shown), although further study will be required to understand the roles of mRor1 and mRor2 in growth plate expansion.
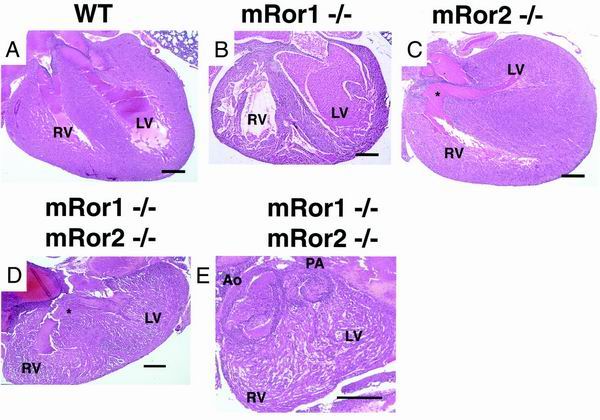
mRor2 mutant newborns exhibited VSD (Fig. 5C) but no other abnormalities of the heart, i.e., malformation of valves, aortic arch, and great vessels (32) (data not shown). Our histological survey did not reveal any apparent abnormalities in the hearts of mRor1−/− mice (Fig. 5B). Intriguingly, in addition to VSD, mRor1/mRor2 double mutant embryos exhibited complete transposition of the great arteries (Fig. 5D and E), a phenotype not observed in mRor2−/− mice, indicating that mRor1 and mRor2 interact genetically in regulating the development of the cardiovascular system. Transposition of the great vessels occurs when the conotruncal septum fails to follow its normal spiral course and runs straight down (28). This condition is frequently associated with a defect in the membranous part of the interventricular septum, as observed in mRor1/mRor2 double mutant mice. It has also been reported that both transposition of the great arteries and VSD are found in mice deficient in the type IIB activin receptor or endothelin A receptor (2, 18). It will be of interest to test whether mRor1 and/or mRor2 interact functionally with these receptors. It has been shown that neural crest cells play two major roles in cardiovascular patterning: they participate in the patterning of the pharyngeal arches and their derivatives, including the aortic arch arteries, and they migrate into the cardiac outflow tract and participate in the formation of the outflow septum (10, 22, 27). It should be noted that both the mRor1 and mRor2 genes are expressed in neural crest cells (16). Therefore, we envisage that mRor2 and mRor1 play important roles in the migration and function of the neural crest cells, which in turn are required for proper formation of the cardiovascular system. Genetic analyses have revealed that many regulatory molecules are involved in cardiac development, including looping effectors and septation effectors (8, 22). It will be important to examine the possible relationships between these regulatory molecules and the mRor proteins.
FIG. 5.
Histological analysis of hematoxylin-and-eosin-stained longitudinal sections through hearts from wild-type (WT), mRor1−/−, and mRor2−/− newborns and a double mutant embryo (E19.5). The hearts of mRor1−/− newborns exhibit no apparent abnormalities. Asterisks indicate cardiac VSD in the mRor2−/− newborn and the mRor1−/−; mRor2−/− embryo (D) and the complete transposition of the great arteries in the mRor1−/−; mRor2−/− embryo (E). The complete transposition of the great arteries with a situs solitus was observed and was characterized by a discordant arterial connection, while the atrioventricular connection was concordant (E) (3, 6). Serial sections revealed that the pulmonary artery (PA) arising from the left ventricle (LV) ran into the lung directly and that the aorta (Ao) ran out from the right ventricle (RV) in the double mutant embryo (data not shown). The spleen was found on the left side of the abdominal cavity of the double mutant embryo, and neither situs inversus nor asplenia was observed (data not shown). Bar, 300 μm.
Collectively, our findings help shed light on the roles of mRor1 and mRor2 in developmental processes. mRor1 and mRor2 are functionally redundant during cardiac and skeletal development, with mRor2 being able to compensate for the functions of mRor1 in mRor1−/− mice. On the other hand, both mRor1 and mRor2 are required for the development and function of the lung, since the absence of either mRor1 or mRor2 results in pulmonary dysfunction. Interestingly, the expression patterns of mRor1 and mRor2 are essentially identical in the developing lung (16). Furthermore, preliminary results indicate that mRor1 and mRor2 interact physically when both are expressed in cultured cells (A. Yoda, I. Oishi, and Y. Minami, unpublished data). Hence, the possible physical association of mRor1 and mRor2 may be important for normal lung development. Another important conclusion drawn from this study is that mRor1 interacts genetically with mRor2 in the regulation of cardiac and skeletal development, since both enhancement of the mRor2−/− cardiovascular and skeletal phenotypes and the severe phenotypes not observed in mRor2−/− mice are found in mRor1/mRor2 mice. Thus far, neither the ligands of mRor1 and mRor2 nor the cytoplasmic signaling molecules that associate with mRor1 and/or mRor2 have been identified. Identification and characterization of such molecules will facilitate our understanding of the functional roles of mRor1 and mRor2 during development.
ACKNOWLEDGMENTS
We thank M. Lamphier for a critical reading of the manuscript.
This work was supported by a Research Grant for Cardiovascular Diseases and a Research Grant for Comprehensive Research on Aging and Health from the Ministry of Health and Welfare of Japan (Y.M.) and by the Uehara Memorial Foundation (Y.M.), the Kowa Life Science Foundation (Y.M.), the Yamanouchi Foundation for Research on Metabolic Disorders (Y.M.), the Hyogo Science and Technology Association (I.O.), Nippon Boehringer Ingelheim Co., Ltd., Kawanishi Pharma Research Institute (Y.M.), and Daiichi Pharmaceutical Co., Ltd. (Y.M.).
REFERENCES
- 1.Afzal A R, Rajab A, Fenske C D, Oldridge M, Elanko N, Ternes-Pereira E, Tüysüz B, Murday V A, Patton M A, Wilkie A O M, Jeffery S. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of Ror2. Nat Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 2.Clouthier D E, Hosoda K, Richardson J A, Williams S C, Yanagisawa H, Kuwaki T, Kumada M, Hammer R E, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 3.Colin L B. Cardiac pathology. In: Becker A E, Anderson R H, editors. Paediatric pathology. New York, N.Y: Springer-Verlag; 1981. pp. 87–145. [Google Scholar]
- 4.Davis A P, Witte D P, Hsieh-Li H M, Potter S S, Capecchi M R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375:791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- 5.DeChiara T M, Kimble R B, Poueymirou W T, Rojas J, Masiakowski P, Valenzuela D M, Yancopoulos G D. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff A A, Martin R J. The cardiovascular system. In: Lees M H, King D H, editors. Neonatal-perinatal medicine. St. Louis, Mo: The C. V. Mosby Company; 1987. pp. 639–743. [Google Scholar]
- 7.Forrester W C, Dell M, Perens E, Garriga G. C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400:881–885. doi: 10.1038/23722. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert S F. Lateral plate mesoderm and endoderm. In: Gilbert S F, editor. Developmental biology. 6th ed. Sunderland, Mass: Sinauer Associates, Inc.; 2000. pp. 471–501. [Google Scholar]
- 9.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 10.Kirby M L, Waldo K L. Neural crest and cardiovascular patterning. Circ Res. 1995;77:211–215. doi: 10.1161/01.res.77.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Knezevic V, De Santo R, Schughart K, Huffstadt U, Chiang C, Mahon K A, Mackem S. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- 12.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y-H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 13.Kuroki Y, Voelker D R. Pulmonary surfactant proteins. J Biol Chem. 1994;269:25943–25946. [PubMed] [Google Scholar]
- 14.Masiakowski P, Carroll R D. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–26190. [PubMed] [Google Scholar]
- 15.Masiakowski P, Yancopoulos G D. The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Curr Biol. 1998;8:R407. doi: 10.1016/s0960-9822(98)70263-5. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, Takada S, Minami Y. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105:153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 17.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 18.Oh S P, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- 19.Oishi I, Sugiyama S, Liu Z-J, Yamamura H, Nishida Y, Minami Y. A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system: unique structural features and implication in developmental signaling. J Biol Chem. 1997;272:11916–11923. doi: 10.1074/jbc.272.18.11916. [DOI] [PubMed] [Google Scholar]
- 20.Oishi I, Takeuchi S, Hashimoto R, Nagabukuro A, Ueda T, Liu Z-J, Hatta T, Akira S, Matsuda Y, Yamamura H, Otani H, Minami Y. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells. 1999;4:41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 21.Oldridge M, Fortuna A M, Maringa M, Propping P, Mansour S, Pollitt C, DeChiara T M, Kimble R B, Valenzuela D M, Yancopoulos G D, Wilkie A O M. Dominant mutations in Ror2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet. 2000;24:275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- 22.Olson E N, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- 23.Otto F, Thornell A P, Crompton T, Denzel A, Gilmour K C, Rosewell I R, Stamp G W H, Beddington R S P, Mundlos S, Olsen B R, Selby P B, Owen M J. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 24.Patthy L, Trexler M, Vali Z, Banyai L, Varadi A. Kringles: modules specialized for protein binding. FEBS Lett. 1984;171:131–136. doi: 10.1016/0014-5793(84)80473-1. [DOI] [PubMed] [Google Scholar]
- 25.Rehn M, Pihlajaniemi T, Hofmann K, Bucher P. The frizzled motif: in how many different protein families does it occur? Trends Biochem Sci. 1998;23:415–417. doi: 10.1016/s0968-0004(98)01290-0. [DOI] [PubMed] [Google Scholar]
- 26.Robinson D R, Wu Y-M, Lin S-F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 27.Rossant J. Mouse mutants and cardiac development. Circ Res. 1996;78:349–353. doi: 10.1161/01.res.78.3.349. [DOI] [PubMed] [Google Scholar]
- 28.Sadler T W. Cardiovascular system. In: Sadler T W, editor. Langman's medical embryology. Baltimore, Md: Lippincott Williams & Wilkins Co.; 2000. pp. 208–259. [Google Scholar]
- 29.Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci. 1998;7:1632–1635. doi: 10.1002/pro.5560070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 31.Schwabe G C, Tinschert S, Buschow C, Meinecke P, Wolff G, Gillessen-Kaesbach G, Oldridge M, Wilkie A O M, Kömec R, Mundlos S. Distinct mutations in the receptor tyrosine kinase gene Ror2 cause brachydactyly type B. Am J Hum Genet. 2000;67:822–831. doi: 10.1086/303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 33.van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, Skovby F, Kerr B, Percin E F, Akarsu N, Brunner H G. Mutation of the gene encoding the Ror2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 34.Wilson C, Goberdhan D C I, Steller H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor kinases. Proc Natl Acad Sci USA. 1993;90:7109–7113. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshikawa Y, Fujimori T, McMahon A P, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]