Abstract
Background
The outcome of COVID-19 in allogeneic hematopoietic stem cell transplantation (HSCT) recipients is almost uniformely considered poor. The aim of present study was to retrospectively analyse the outcome and risk factors for mortality in a large series of patients who developed COVID-19 infection after an allogeneic HSCT.
Methods
This multicenter retrospective study promoted by the European Hematology Association – Infections in Hematology Study Working Group, included 326 adult HSCT patients who had COVID-19 between January 2020 and March 2022.
Results
The median time from HSCT to the diagnosis of COVID-19 was 268 days (IQR 86-713; range 0-185 days). COVID-19 severity was mild in 21% of the patients, severe in 39% and critical in 16% of the patients. In multivariable analysis factors associated with a higher risk of mortality were, age above 50 years, presence of 3 or more comorbidities, active hematologic disease at time of COVID-19 infection, development of COVID-19 within 12 months of HSCT, and severe/critical infections. Overall mortality rate was 21% (n=68): COVID-19 was the main or secondary cause of death in 16% of the patients (n=53).
Conclusions
Mortality in HSCT recipients who develop COVID-19 is high and largely dependent on age, comorbidities, active hematologic disease, timing from transplant and severity of the infection.
Keywords: allogeneic HSCT, COVID-19 infection, immunocompromised patients, SARS-CoV-2, hematological malignances
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was recognized in late 2019 and developed into a pandemic with life-threatening disease documented in high-risk groups (1). Allogeneic hematopoietic stem cell transplantation (HSCT) has been increasingly adopted as a curative treatment option for a great variety of hematologic malignancies, however HSCT recipients are vulnerable to viral infections due to neutropenia, immunosuppressive treatments, graft-versus-host disease (GVHD) and incomplete immune reconstitution occurring in the post-transplant period. In this respect, efforts have been made not to postpone transplantation during the pandemic (2). To date, scattered case series of HSCT recipients with coronavirus disease 2019 (COVID-19) have been reported (3–11). Overall, the prognosis of HSCT recipients has been uniformly reported dismal with COVID-19-related mortality ranging between 20 and 39% (5, 6, 12–16), much higher than in the general population. Advanced age, presence of active GVHD and a short time interval from HSCT to COVID-19 were identified as predictors of adverse outcome (3, 5, 14). These findings may be discouraging the treating physicians, fearing for the high fatality rate of HSCT recipients. On the other hand, the use of immunosuppressive agents may potentially mitigate the deleterious systemic inflammatory syndrome secondary to the cytokine storm unleashed by SARS-Cov-2 leading to multiorgan dysfunction and eventually death. According to this observation some studies have reported a lower mortality rate of allogeneic HSCT recipients as compared to non-stem cell transplant patients (18% vs 31%) (3). Hence, aim of our retrospective study was to address the outcome and risk factors for mortality in a large series of patients who developed COVID-19 infection after an allogeneic HSCT.
Patients and methods
Study population
This is retrospective multicenter cohort study promoted by the European Hematology Association – Infections in Hematology Study Working Group (EHA-IDWP; EPICOVIDEHA survey, https://pubmed.ncbi.nlm.nih.gov/34235404/). Data have been collected on all consecutive adult patients who received an allogeneic HSCT and had COVID-19 in more than 150 European centers between January 2020 and March 2022. Only patients for whom allogeneic HSCT represented the last treatment performed were included into the study. Each institutional review board independently approved the study.
Data collection
Researchers at each center collected data using an online questionnaire hosted at www.clinicalsurveys.net, EPICOVIDEHA is registered at http://www.clinicaltrials.gov, with the identifier NCT 04733729. Only de-identified data have been analyzed.
Data collected included: age at transplantation (dichotomized as <50 years and ≥50 years), sex (male vs female), time from HSCT to the diagnosis of COVID-19, immunosuppression within 6 months of COVID-19 diagnosis, conditioning intensity (myeloablative vs reduced intensity), GVHD prophylaxis, time to engraftment, development of acute or chronic GVHD before COVID-19 diagnosis and immunodeficiency scoring index (ISI) at the time of COVID-19 infection. Clinically significant outcomes (hospital admission and intensive care unit [ICU] admission, vital status) were also evaluated. We did not collect information on treatment strategies of COVID-19.
Definitions
Confirmed cases of COVID-19 were defined by a positive reverse transcription polymerase chain reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab.
The severity of COVID-19 at admission is graded according to the China Center for Disease Control and Prevention definitions (17).
Disease status at the time of SARS-CoV-2 detection was defined according to each specific disease’s revised criteria for leukemia, myeloproliferative neoplasm, multiple myeloma, and lymphoma. The ISI was calculated as previously described (4).
Endpoints and statistical analysis
The primary outcome of this analysis was overall survival 30 days after COVID-19 diagnosis.
Categorical variables were summarised in frequencies and percentages and continuous variables with median, interquartile range (IQR) and absolute range. Additionally, to determine which factors were associated to mortality in our sample, we performed a Cox regression, with the backwards Wald method. Those variables with a p value <0.1 in the univariable model were included into the multivariable analysis. A p<0.05 was considered significant. Overall survival probability has been plotted in a Kaplan-Meier survival curve. SPSSv25.0 was employed for statistical analyses (SPSS, IBM Corp., Chicago, IL, United States).
Results
Demographics
Between January 2020 and March 2022, 326 patients receiving an allogeneic HSCT were diagnosed with COVID-19 infection and registered in EPICOVIDEHA.
Baseline transplant characteristics are shown in Table 1 . The median age at the time of COVID-19 diagnosis was 51 years (IQR 38-61; range 18-75), 132 patients (41%) were female, and 194 patients (59%) were male. Acute leukemia and myelodysplastic syndromes (MDS) (n=257, 79%) were the most common indications for allogeneic HSCT. Overall, 232 patients (71%) received grafts from alternative donors, including matched unrelated donors in 158 cases (48%) and haploidentical donors in 74 cases (23%). For GVHD prophylaxis, 114 patients (35%) received calcineurin inhibitors-based immunosuppression and 71 patients (22%) received post-transplantation cyclophosphamide (PT/Cy). The majority of the patients (297 out of 326 patients, 91%) were not vaccinated at the time of HSCT.
Table 1.
Characteristics of HSCT patients with COVID-19 diagnosis.
| No. patients | 326 |
| Age, median (range), years | 51 (18-75) |
| Sex male/female | 194 (60%) / 132 (40%) |
| Diagnosis | |
| AML/MDS ALL Lymphomas Multiple myeloma Chronic myeloproliferative malignancies other |
196 (60%) 61 (19%) 36 (11%) 5 (2%) 17 (5%) 11 (3%) |
| Disease status at HSCT | |
| CR/partial remission stable disease R/R disease unknown |
289 (89%) 14 (4%) 15 (5%) 11 (2%) |
| Conditioning intensity | |
| myeloablative RIC unknown |
224 (69%) 30 (9%) 72 (22%) |
| Donor | |
| matched sibling donor MUD Haploidentical unknown |
88 (27%) 158 (48%) 74 (23%) 6 (2%) |
| Graft source BM PBSC CB unknown |
28 (9%) 287 (88%) 4 (1%) 7 (2%) |
| GVHD prophylaxis | |
| CI plus other PT/Cy other unknown |
114 (35%) 71 (22%) 86 (26%) 55 (17%) |
| Vaccination before HSCT | |
| no 1 dose 2 doses 3 doses |
297 (91%) 3 (1%) 25 (8%) 1 (0.3% |
| Comorbidities at HSCT* | |
| 1 comorbidity 2 comorbidities 3 comorbidities |
95 (29%) 41 (12%) 21 (6%) |
HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; MDS myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; CR, complete remission; R/R relapse/refractory; RIC, reduced intensity conditioning; MUD, matched unrelated donor; BM, bone marrow; PBSC, peripheral blood stem cell; CB, cord blood; CI, calcineurin inhibitors; PT/Cy, post-transplant cyclophosphamide.
*Comorbidities included diabetes, liver disease, renal impairment, smoking history.
Clinical characteristics
The median time from HSCT to the diagnosis of COVID-19 was 268 days (IQR 86-713; range 0.185 days). Collectively, 45 patients (14%) received post-HSCT vaccination before COVID-19 diagnosis: 6 patients received one dose, 18 patients two doses, 19 patients three doses and 2 patients four doses.
At the time of COVID-19 diagnosis, active grade II-IV acute GVHD (aGVHD) was present in 15 patients (5%), and 32 patients (10%) had evidence of moderate to severe chronic GVHD (cGVHD). Overall, 199 patients (61%) were on systemic immunosuppressive treatments during the last 6 months before COVID-19 diagnosis. Only a minority of patients was neutropenic (ANC ≤500/mm3) or lymphocytopenic (ALC ≤200/mm3) at the time of COVID-19 diagnosis (6% and 10% respectively).
COVID-19 severity was mild in 21% of the patients, severe in 39% and critical in 16% of the patients; 79 patients (24%) had asymptomatic infection.
Among the 184 patients who were hospitalized, 51 patients (28%) were admitted to ICU, and 34 patients required mechanical ventilation.
Patient characteristics at the time of COVID-19 diagnosis are summarized in Table 2 .
Table 2.
Patient Characteristics at diagnosis of COVID-19.
| No. patients according to the time of COVID-19 diagnosis | |
| 1st wave (February-June 2020) 2nd wave (September-December 2020) 3rd wave (January-March 2022) |
65 (20%) 138 (42%) 123 (38%) |
| Median time form HSCT to COVID-19, days | 268 |
| Disease status at COVID-19 diagnosis | |
| CR/partial remission | 289 (89%) |
| stable disease | 14 (4%) |
| active disease | 18 (5%) |
| unknown | 5 (2%) |
| Acute GVHD grades II-IV | 15 (5%) |
| Chronic GVHD, moderate to severe | 32 (10%) |
| Patients on systemic immunosuppressive agents | 199 (61%) |
| Symptoms at COVID-19 onset | |
| Pulmonary | 110 (34%) |
| Pulmonary and extrapulmonary | 59 (18%) |
| Extrapulmonary | 74 (23%) |
| Screening, no symptoms | 83 (25%) |
| Patients requiring hospital admission | 184 (56%) |
| Median duration, days | 14 |
| Patients requiring ICU admission | 51 (16%) |
| Median duration, days | 15 |
| Immunity subset analysis | |
| Neutrophils | 20 (6%) |
| ≤ 500/mm3 | 20 (6%) |
| 501-999/mm3 | 232 (71%) |
| ≥ 1000/mm3 | 54 (17%) |
| Unknown | |
| Lymphocytes | 34 (10%) |
| ≤200/mm3 | 43 (13%) |
| 201-499/mm3 | 189 (58%) |
| ≥500/mm3 | 60 (19%) |
| Unknown |
HSCT, hematopoietic stem cell transplantation; CR, complete remission; GVHD, graft-versus-host disease; ICU, intensive care unit.
Factors associated with mortality and outcome of COVID-19 infection
The results of Cox regression analysis for factors associated with mortality after COVID-19 diagnosis in allogeneic HSCT recipients are shown in Table 3 .
Table 3.
Univariate and multivariate analysis for risk factors associated with COVID-19 mortality.
| Evaluable n | Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | ||||
| Lower | Upper | Lower | Upper | ||||||
| Age at HSCT | |||||||||
| <50 years | 157 | – | – | – | – | – | – | – | |
| ≥50 years | 169 | <0.001 | 3.880 | 2.185 | 6.890 | <0.001 | 3.206 | 1.756 | 5.853 |
| Time from HSCT to COVID-19 | |||||||||
| <12 months ≥12 months |
194 | – | – | – | – | – | – | – | – |
| 132 | 0.049 | 0.596 | 0.356 | 0.998 | 0.031 | 0.538 | 0.306 | 0.946 | |
| Underlying disease | |||||||||
| Acute leukemia (AML,ALL,MDS) Lymphomas (NHL,HD) Other |
257 | – | – | – | – | ||||
| 36 | 0.622 | 0.794 | 0.317 | 1.987 | |||||
| 37 | 0.317 | 1.435 | 0.708 | 2.910 | |||||
| Type of HSCT | |||||||||
| Matched sibling donor Alternative (MUD, Haploidentical, Cord) Unknown |
88 | – | – | – | – | ||||
| 232 | 0.759 | 0.921 | 0.547 | 1.553 | |||||
| 6 | 0.961 | 0.000 | 0.000 | . | |||||
| Preparative regimen | |||||||||
| Non-myeloablative RIC Myeloablative Unknown |
54 | – | – | – | – | ||||
| 30 | 0.819 | 1.107 | 0.464 | 2.644 | |||||
| 224 | 0.186 | 0.663 | 0.360 | 1.219 | |||||
| 18 | 0.250 | 1.756 | 0.673 | 4.577 | |||||
| GVHD prophylaxis | |||||||||
| No CI+MTX/MMF PT/Cy Other Unknown |
17 | – | – | – | – | ||||
| 114 | 0.858 | 1.116 | 0.336 | 3.703 | |||||
| 71 | 0.516 | 0.648 | 0.175 | 2.401 | |||||
| 86 | 0.519 | 1.487 | 0.445 | 4.974 | |||||
| 38 | 0.828 | 1.156 | 0.312 | 4.274 | |||||
| Comorbidities before COVID-19 | |||||||||
| No comorbidities 1-2 comorbidities 3 or more comorbidities |
169 | – | – | – | – | – | – | – | – |
| 136 | 0.039 | 1.720 | 1.028 | 2.877 | 0.164 | 1.476 | 0.853 | 2.553 | |
| 21 | 0.006 | 2.915 | 1.365 | 6.225 | 0.002 | 3.718 | 1.596 | 8.662 | |
| Disease status at COVID-19 diagnosis | |||||||||
| CR/Partial remission Stable disease Active disease Unknown |
289 | – | – | – | – | – | – | – | – |
| 14 | 0.534 | 1.382 | 0.499 | 3.821 | 0.475 | 1.474 | 0.508 | 4.275 | |
| 18 | <0.001 | 4.255 | 2.092 | 8.652 | <0.001 | 3.859 | 1.810 | 8.226 | |
| 5 | 0.056 | 3.968 | 0.963 | 16.353 | 0.282 | 2.239 | 0.516 | 9.713 | |
| ISI group | |||||||||
| Low risk Moderate/High risk |
149 | – | – | – | – | ||||
| 177 | 0.250 | 1.332 | 0.817 | 2.173 | |||||
| Acute GVHD before COVID-19 diagnosis | |||||||||
|
0-I II-IV Unknown |
237 | – | – | – | – | ||||
| 74 | 0.766 | 1.092 | 0.611 | 1.951 | |||||
| 15 | 0.203 | 1.820 | 0.724 | 4.576 | |||||
| Chronic GVHD before COVID-19 diagnosis | |||||||||
| Absent-Mild Moderate-Severe Unknown |
261 | – | – | – | – | ||||
| 48 | 0.797 | 1.089 | 0.570 | 2.080 | |||||
| 17 | 0.141 | 0.227 | 0.031 | 1.639 | |||||
| COVID-19 severity | |||||||||
| Asymptomatic Mild infection Severe infection Critical infection |
79 | – | – | – | – | – | – | – | – |
| 68 | 0.253 | 1.953 | 0.620 | 6.156 | 0.192 | 2.160 | 0.680 | 6.857 | |
| 128 | 0.014 | 3.363 | 1.273 | 8.887 | 0.012 | 3.628 | 1.333 | 9.879 | |
| 51 | <0.001 | 15.27 | 5.954 | 39.157 | <0.001 | 12.91 | 4.892 | 34.069 | |
| Drugs within 6 months of COVID-19 diagnosis | |||||||||
| None Immunosuppressive/Corticosteroids Unknown |
66 | – | – | – | – | ||||
| 221 | 0.198 | 1.596 | 0.784 | 3.250 | |||||
| 39 | 0.226 | 1.744 | 0.708 | 4.292 | |||||
HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; MDS myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; NHL, Non-Hodgkin lymphoma; HD, Hodgkin lymphoma; MUD, matched unrelated donor; RIC, reduced intensity conditioning; GVHD, graft-versus-host disease; CI, calcineurin inhibitors; MTX, methotrexate; MMF, mycophenolate mofetil; PT/Cy, post-transplant cyclophosphamide; CR, complete remission; ISI, immunodeficiency scoring index.
In multivariable analysis factors associated with a higher risk of mortality were, age 50 years or older, presence of 3 or more comorbidities, active hematologic disease at time of COVID-19 infection, development of COVID-19 within 12 months of HSCT, and severe/critical infections. The type of HSCT, the intensity of preparative regimen, the presence of aGVHD and cGVHD before COVID-19 infection and ISI group were not associated with increased mortality.
Vaccination and the number of doses (one, two or more doses) administered did not have an impact on the outcome of the patients.
At the time of last follow-up, 258 patients (79%) are alive: from the diagnosis of COVID-19, the median follow of survivors was 126 days (IQR 32-339; range 0-643 days). The Kaplan-Meier overall survival estimate is shown in Figure 1 . Overall, 68 patients (21%) had died after a median follow-up post COVID-19 of 26 days (IQR 12-56; range 0-379 days). Causes of death were COVID-19 infection in 42 cases (13%), COVID-19 in parallel to recurrence of the underlying hematologic malignancy in 11 cases (3%), and hematologic malignancy +/- other reasons in 15 cases (5%).
Figure 1.
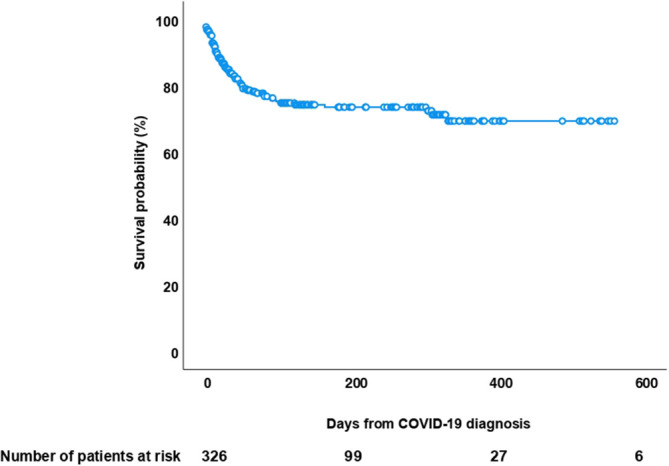
Kaplan-Meier overall survival curve after diagnosis of COVID-19 infection in 326 allogeneic HSCT recipients.
Discussion
To our knowledge, the present study includes the largest series of allogeneic HSCT recipients with COVID-19. The aim of our analysis was to evaluate the outcome of COVID-19 in a cohort of patients particularly susceptible to infectious complications and to investigate risk factors that may impact on mortality. Patients were at high risk for severe disease and adverse outcome from COVID-19: a large proportion of patients (70%) received HSCT from alternative donors, 16% had critical infection and required ICU admission. Even more importantly, the median time from HSCT to COVID-19 infection was 268 days, remarkably shorter when compared to other reports (474-790 days) (3, 4, 12, 14). This finding might mirror the adoption of less stringent measures of stewardship among transplant centers after the 1st and 2nd COVID-19 wave. In fact, it should be recognized that one third of the patients had COVID-19 infection diagnosed during the 3rd wave.
Our study demonstrated that COVID-19 infection is a severe complication in allogeneic HSCT recipients with an attributable mortality of 16%. Our results are roughly superimposable to those reported from other recent studies. The CIBMTR reported a COVID-19-related mortality rate of 20% in 184 allogeneic HSCT recipients (14), Piñana et al. analyzed the outcome of COVID-19 in 65 allogeneic HSCT patients and reported a mortality of 18% (3). An EBMT (European Society for Blood and Marrow Transplantation) and GETH (Spanish Group of Hematopoietic Stem Cell Transplantation) prospective study reported an overall mortality rate of 28% among 236 allogeneic HSCT recipients (12). Other smaller series documented similar mortality rates ranging from 20 to 25% (5–7, 9). It is worthwhile recalling that mortality in the general population declined over the 2nd and 3rd waves: this observation should be considered when we consider that only 20% of our patients were diagnosed with COVID-19 during the first wave.
Multivariable analysis showed that age, comorbidities, active disease, and severe/critical infection have been associated with higher mortality, consistent with previous studies (3, 4, 12, 14). Nevertheless, it should be noted that a consistent number of comorbidities and demographic characteristics (i.e. diabetes, BMI, race) potentially influencing the outcome of the patients were not available Similarly, time from HSCT to COVID-19 diagnosis of less than 12 months was a factor significantly associated with fatal outcome. Surprisingly, GVHD at the time of COVID-19 diagnosis did not impact on the outcome of our patients, however it should be underscored that only 5% of the patients had grade II-IV acute GVHD and 10% had moderate/severe chronic GVHD; in addition, data on the cumulative dose of steroids and the different immunosuppressive agents used were not available.
Likewise, mortality was not influenced by the use of immunosuppressive drugs and steroids during the 6 months preceding COVID-19 diagnosis, notwithstanding the number of patients on treatment was extremely high. Whether the administration of two to three doses of vaccine in 20% of the patients might have contributed to dampen the severity of the disease as documented in some studies (18, 19), remains speculative. Regrettably, we do not have data on patient seroconversion and B-cell reconstitution underpinning the effectiveness of vaccination.
ISI did not result as an independent risk factor for poor outcome as shown by Ljungman et al, however several variables included in the ISI (ANC and ALC, GVHD) were poorly represented in our study group. Two studies including a small number of patients reported a favorable outcome of patients receiving PT/Cy as GVHD prophylaxis, since cytokine release syndrome (CRS) associated with haploidentical HSCT and COVID-19 share similar pathophysiology (10, 11). It is well known that a dysregulated, excessive immune response with increased pro-inflammatory cytokines levels during the later phases of COVID-19 infection may lead to multiorgan failure (20). Cy is an alkylating agent largely used in the setting of haploidentical HSCT due to its capacity of depleting rapidly proliferating T-cells while sparing regulatory-T cells (21). According to these observations, it has been postulated a potential effect of Cy in mitigating the COVID-19 infection (22), however in our cohort, we did not find any significant relationship between the type of GVHD prophylaxis and mortality, including PT/Cy in 22% of the cases. ANC and ALC at the time of COVID-19 diagnosis did not result as significant factors for disease severity as shown in other studies (3, 5), however a minority of our patients were neutropenic or had low ALC.
We recognize limitations of our study inherent to the retrospective design. We cannot exclude a possible selection bias in the registration of the patients among such a large number of centers. Our study presents a significant heterogeneity in terms of different variants of concern, namely wild type, delta and omicron variants, each with distinctive transmission rates and infection associated mortality. Information on the treatment of COVID-19 were not available and the management of patients with COVID-19 across centers may differ profoundly, potentially influencing clinical outcome.
Conclusion
Our study on a large number of patients who developed COVID-19 infection following HSCT, shows a high mortality rate compared to the general population. In this respect, it is of upmost relevance to see whether vaccination of patients after HSCT and the availability of pre- and post-exposure prophylactic measures effectively mitigates the severity of the disease in this vulnerable group of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by EPICOVIDEHA was approved by the local ethics committee of the Fondazione Policlinico Universitario Agostino Gemelli - IRCCS, Università Cattolica del Sacro Cuore of Rome, Italy (Study ID: 3226). The patients/participants provided their written informed consent to participate in this study.
A list of EPICOVIDEHA Collaborators members
Juergen Prattes, Malgorzata Mikulska, Gustavo-Adolfo Méndez, Tobias Lahmer, Pavel Jindra, Anna Guidetti, Rita Fazzi, Maria Ilaria Del Principe, Cristina De Ramón, Maria Calbacho, Zlate Stojanoski, Andrés Soto, Alexandra Serris, Irati Ormazabal-Vélez, Ali S. Omrani, Milan Navrátil, Sonia Martín-Pérez, Joyce Marques De Almeida, Sylvain Lamure, Martin Kolditz, Ozren Jaksic, Martin Hoenigl, Carolina Garcia-Vidal, Noemí Fernández, Shaimaa El-Ashwah, Natasha Čolović, Martin Čerňan, Caterina Buquicchio, Valentina Bonuomo, Josip Batinić, Murtadha Al-Khabori, Tatjana Adžić-Vukičević, Juan-Alberto Martín-González, Maria Vittoria Sacchi, María-Josefa Jiménez-Lorenzo, Dominik Wolf, Maria Vehreschild, Raul Cordoba, Ramón García-Sanz, Toni Valković, Miloš Mladenović, Nicole García-Poutón, Ziad Emarah, Julio Dávila-Valls
Author contributions
AB, JS-G, FM, OC and LiP contributed to study design, study supervision, and data interpretation and wrote the paper. AB, JS-G and LiP did the statistical plan. JS-G performed the analysis and AB, JS-G and LiP interpreted the data. All authors recruited participants and collected and interpreted data, contributed to manuscript writing and review of the manuscript, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding Statement
EPICOVIDEHA has received funds from Optics COMMITTM (COVID-19 Unmet Medical Needs and Associated Research Extension) COVID-19 RFP program by GILEAD Science, United States (Project 2020-8223). The funder of the study had no role in study design, data analysis, interpretation, or writing of the report. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Contributor Information
EPICOVIDEHA Consortium:
Juergen Prattes, Malgorzata Mikulska, Gustavo-Adolfo Méndez, Tobias Lahmer, Pavel Jindra, Anna Guidetti, Rita Fazzi, Maria Ilaria Del Principe, Cristina De Ramón, Maria Calbacho, Zlate Stojanoski, Andrés Soto, Alexandra Serris, Irati Ormazabal-Vélez, Ali S. Omrani, Milan Navrátil, Sonia Martín-Pérez, Joyce Marques De Almeida, Sylvain Lamure, Martin Kolditz, Ozren Jaksic, Martin Hoenigl, Carolina Garcia-Vidal, Noemí Fernández, Shaimaa El-Ashwah, Natasha Čolović, Martin Čerňan, Caterina Buquicchio, Valentina Bonuomo, Josip Batinić, Murtadha Al-Khabori, Tatjana Adžić-Vukičević, Juan-Alberto Martín-González, Maria Vittoria Sacchi, María-Josefa Jiménez-Lorenzo, Dominik Wolf, Maria Vehreschild, Raul Cordoba, Ramón García-Sanz, Toni Valković, Miloš Mladenović, Nicole García-Poutón, Ziad Emarah, and Julio Dávila-Valls
Collaborators: EPICOVIDEHA Consortium
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Zhang Y, Xu J, Li H, Cao B. A novel coronavirus (COVID-19) outbreak: A call for action. Chest. (2020) 157:e99–e101. doi: 10.1016/j.chest.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. EBMT COVID-19 guidelines . Available at: https://www.ebmt.org/sites/default/files/2021-06/EBMT%20COVID-19%20guidelines%20v.%2016.03.pdf.
- 3. Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol (2020) 9:21. doi: 10.1186/s40164-020-00177-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah GL, DeWolf S, Lee YJ, Tamari R, Dahi PB, Lavery JA, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest (2020) 130(12):6656–67. doi: 10.1172/JCI141777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varma A, Kosuri S, Ustun C, Ibrahim U, Moreira J, Bishop MR, et al. COVID-19 infection in hematopoietic cell transplantation: Age, time from transplant and steroids matter. Leukemia (2020) 34(10):2809–12. doi: 10.1038/s41375-020-01019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coll E, Fernàndez-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: The Spanish experience. Am J Transplant (2021) 21:1825–37. doi: 10.1111/ajt.16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mushtaq MU, Shahzad M, Chaudhary SG, Luder M, Ahmed N, Abdelhakim H, et al. Impact of SARS-CoV-2 in hematopoietic stem cell transplantation and chimeric antigen receptor T cell therapy recipients. Transplant Cell Ther J (2021) 27(9):796.e1–7. doi: 10.1016/j.jtct.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, et al. COVID-19 in hematopoietic cell transplant recipients. Bone Marrow Transplant (2021) 56:952–5. doi: 10.1038/s41409-020-01084-x [DOI] [PubMed] [Google Scholar]
- 9. Camargo JF, Mendoza MA, Lin R, Moroz IV, Anderson AD, Morris MI, et al. Clinical presentation and outcomes of COVID-19 following hematopoietic cell transplantation and cellular therapy. Wiley Periodicals (2021) 23(4):e13625. doi: 10.1111/tid.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abid MB, Hamadani M, Szabo A, Hari PN, Graham MB, Frank MO, et al. Severity of cytokine release syndrome and its association with infections after T cell-replete haploidentical related donor transplantation. Biol Blood Marrow Transplant (2020) 26:1670–8. [DOI] [PubMed] [Google Scholar]
- 11. Abid MB, Mughal M, Abid MA. Coronavirus disease 2019 (COVID-19) and immune-engaging cancer treatment. JAMA Oncol (2020) 6:1529–30. [DOI] [PubMed] [Google Scholar]
- 12. Ljungman P, de la Cámara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia (2021) 35(10):2885–94. doi: 10.1038/s41409-021-01302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Hematol (2020) 7(10):e737–45. doi: 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol (2021) 8:e185–93. doi: 10.1016/S2352-3026(20)30429-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological malignancies: a European hematology association survey (EPICOVIDEHA). J Hematol Oncol (2021) 14(1):168. doi: 10.1186/s13045-021-01177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salmanton-García J, Busca A, Cornely OA, Corradini P, Hoenigl M, Klimko N, et al. EPICOVIDEHA: A ready to use platform for epidemiological studies in hematological patients with COVID-19. Hemasphere (2021) 5(7):e612. doi: 10.1097/HS9.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA (2020) 323:1239. [DOI] [PubMed] [Google Scholar]
- 18. Abid MB, Rubin M, Ledeboer N, Szabo A, Longo W, Mohan M, et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell (2022) 40(4):340–2. doi: 10.1016/j.ccell.2022.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maillard A, Redjoul R, Klemencie M, Labussière Wallet H, Le Bourgeois A, D'Aveni M, et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. (2022) 139(1):134–7. doi: 10.1182/blood.2021014232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Channappanavar R, Perlman S. Human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol (2017) 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luznik I, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol (2012) 39:683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Revannasiddaiah S, Kumar Devadas S, Palassery R, Kumar Pant N, Maka VV. A potential role for cyclophosphamide in the mitigation of acute respiratory distress syndrome among patients with SARS-CoV-2. Med Hypotheses (2020) 144:109850. doi: 10.1016/j.mehy.2020.109850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


