Summary
Background
Among interleukin-6 inhibitors suggested for use in COVID-19, there are few robust evidences for the efficacy of sarilumab. Herein, we evaluated the efficacy and safety of sarilumab in severe COVID-19.
Methods
In this phase 3, open-labeled, randomized clinical trial, conducted at 5 Italian hospitals, adults with severe COVID-19 pneumonia (excluding mechanically ventilated) were randomized 2:1 to receive intravenous sarilumab (400 mg, repeatable after 12 h) plus standard of care (SOC) (arm A) or to continue SOC (arm B). Randomization was web-based. As post-hoc analyses, the participants were stratified according to baseline inflammatory parameters. The primary endpoint was analysed on the modified Intention-To-Treat population, including all the randomized patients who received any study treatment (sarilumab or SOC). It was time to clinical improvement of 2 points on a 7-points ordinal scale, from baseline to day 30. We used Kaplan Meier method and log-rank test to compare the primary outcome between two arms, and Cox regression stratified by clinical center and adjusted for severity of illness, to estimate the hazard ratio (HR). The trial was registered with EudraCT (2020-001390-76).
Findings
Between May 2020 and May 2021, 191 patients were assessed for eligibility, of whom, excluding nine dropouts, 176 were assigned to arm A (121) and B (55). At day 30, no significant differences in the primary endpoint were found (88% [95% CI 81–94] in arm A vs 85% [74–93], HR 1.07 [0.8–1.5] in arm B; log-rank p = 0.50). After stratifying for inflammatory parameters, arm A showed higher probability of improvement than B without statistical significance in the strata with C reactive protein (CRP) < 7 mg/dL (88% [77–96] vs 79% [63–91], HR 1.55 [0.9–2.6]; log-rank p = 0.049) and in the strata with lymphocytes <870/mmc (90% [79–96]) vs (73% [55–89], HR 1.53 [0.9–2.7]; log-rank p = 0.058). Overall, 39/121 (32%) AEs were reported in arm A and 14/55 (23%) in B (p = 0.195), while serious AEs were 22/121 (18%) and 7/55 (11%), respectively (p = 0.244). There were no treatment-related deaths.
Interpretation
The efficacy of sarilumab in severe COVID-19 was not demonstrated both in the overall and in the stratified for severity analysis population. Exploratory analyses suggested that subsets of patients with lower CRP values or lower lymphocyte counts might have had benefit with sarilumab treatment, but this finding would require replication in other studies. The relatively low rate of concomitant corticosteroid use, could partially explain our results.
Funding
This study was supported by INMI “Lazzaro Spallanzani” Ricerca Corrente Linea 1 on emerging and reemerging infections, funded by Italian Ministry of Health.
Keywords: Interleukin-6 receptor inhibitors, Sarilumab, SARS-CoV-2 infection, Severe COVID-19 pneumonia, Randomized clinical trial
Research in context.
Evidence before this study
We searched PubMed from Jan, 2020, for English-language articles on phase III randomized clinical trials and meta-analyses regarding the use of sarilumab in the treatment of severe COVID-19. We searched using the terms “COVID-19”, “SARS-CoV-2 infection”, “sarilumab”, “interleukin-6 inhibitors”, “phase III”, “randomized”. This search revealed robust evidences for the efficacy of tocilizumab in the treatment of severe COVID-19, but few published phase III randomized clinical trials for sarilumab with discordant results.
Added value of this study
International guidelines suggest the alternative use of tocilizumab and sarilumab for the treatment of severe COVID-19, even though there are few clear evidences on sarilumab. Two largest trials on IL-6 inhibitors reported their efficacy in patients with COVID-19, mainly when administered in combination with dexamethasone; the REMAP-CAP trial included some patients treated with sarilumab but the RECOVERY trial was focused only on tocilizumab. Moreover, other Randomized Clinical Trials evaluating the efficacy of sarilumab compared to usual care or placebo, failed to demonstrate the superiority of sarilumab in terms of clinical improvement and mortality. Finally, three meta-analyses showed improved outcomes in case of use of IL-6 antagonists, but these findings appeared more marked and consistent for tocilizumab compared to sarilumab. Herein, we contributed to the available evidence, evaluating the efficacy and safety of sarilumab, combined with usual care, in patients with severe COVID-19 pneumonia, in a randomized design.
Implications of all the available evidence
Despite its high tolerability, the efficacy of sarilumab in our population was not demonstrated, with the exception of participants at an early stage of the disease. Together with other existing evidence, these findings shed light on the need to identify targeted subgroups of patients for maximizing benefit of this treatment and minimizing potential adverse events. Moreover, although their similar mechanism of action, further studies are necessary to strengthen the recommendation of the use of sarilumab as an alternative to tocilizumab in the treatment of severe COVID-19.
Introduction
The novel coronavirus, further classified as SARS-CoV-2,1 initially emerged in the city of Wuhan, China, in December 2019, led to a sharply spreading outbreak of human respiratory disease (COVID-19), becoming a global burden of disease and public health. So far, the WHO has confirmed more than 500 million cases and more than 6 million deaths.2
Different therapeutic management has been proposed for non-hospitalized and hospitalized patients and according to severity of illness. So far, the cornerstones of recommended therapy for severe COVID-19 in hospitalized patients are corticosteroids, remdesivir, baricitinib and tocilizumab.3 Tofacitinib and sarilumab have been proposed as immunomodulatory drugs alternative to baricitinib and tocilizumab, respectively, if not available or not feasible to use.3
The pathogenesis of COVID-19 is characterized by a deleterious hyperinflammatory response, associated with the production of a number of proinflammatory cytokines and chemokines, including interleukin-6 (IL-6) 4,5; in fact, SARS-CoV-2 infection induces a great release of IL-6 from macrophages and from bronchial epithelial cells.6 The identification of elevated IL-6 levels in patients with severe COVID-19 led to the rapid development of clinical trials targeting this cytokine, which have provided some evidence that IL-6 inhibitors use in severe COVID-19 may reduce the duration and/or severity of COVID-19.7, 8, 9, 10, 11, 12, 13, 14, 15
Sarilumab is a human monoclonal antibody, which inhibits the binding of IL-6 to its α receptor and is approved for treatment of adults with rheumatologic diseases. Even though it is considered as an alternative to tocilizumab in the treatment of severe COVID-19,3 there are less robust evidences for its efficacy.16, 17, 18, 19, 20, 21, 22 In the present study, we aimed to evaluate, in a randomized design, the efficacy of sarilumab, combined with usual care, in patients with severe COVID-19 pneumonia.
Methods
Study design and ethics
In this national, multicenter, open-labeled, phase 3, randomized clinical trial, we enrolled patients from five Italian hospitals in order to assess clinical efficacy and safety of intravenous sarilumab, added to standard of care (SOC), in the treatment of hospitalized adults with severe COVID-19 pneumonia. The study included a follow-up period of 30 days, consisting of regular clinical assessment.
The original protocol (Escape Study version 2.2, May 5, 2020) was approved by the Scientific Committee of the Italian Drug Agency (AIFA) and by the Ethical Committee of the Lazzaro Spallanzani Institute, as National Review Board for COVID-19 pandemic in Italy (approval number 152/2020), and by the ethics committee of the National Institute for Infectious Diseases L. Spallanzani IRCCS and the institutional review boards at each participating hospital. An amendment to the original protocol (Escape Study version 3.0, October 6, 2020) was made in order to extend the duration of recruitment (from 6 to 12 months) and to allow the use of corticosteroids and/or antiviral agents as SOC, according to decision of clinical investigator and current guidelines. It was approved on November 6, 2020 (approval number 205/2020). The study was conducted in accordance with the European Union Clinical Practice Standards, with ICH Good Clinical Practice (GCP) and with the ethical principles expressed in Declaration of Helsinki and its amendments. The clinical study was performed under the regulations of AIFA and of the Italian Ministry of Health. Before entering the study, all patients, gave their written or verbal informed consent (version 3.1. November 13, 2020). All data have been collected anonymously into the Electronic Case Report Forms (eCRF); subjects were identified by numeric codes only, password protected.
The study is registered in the European Union Clinical Trials Register (Eudract Number: 2020-001390-76). The full trial protocol, including legal issues and detailed trial procedures, is available as supplementary material.
Participants
Hospitalized patients aged 18 years or older and with documented SARS-CoV-2 infection, were considered eligible if they had evidence of pulmonary infiltrates by chest imaging and severe or critical clinical condition (oxygen saturation at rest without oxygen supplementation <93%, or ratio of partial pressure of arterial oxygen and fraction of inspired oxygen [PaO2/FiO2] <300 mmHg at rest, in patients requiring oxygen supplementation with Venturi mask or non-invasive ventilation). Participants also had to meet criteria for the evidence of hyperinflammation, defined as at least two among: blood lymphocytes <1000/mm3, ferritin >500 ng/mL, lactate dehydrogenase (LDH) > 300 U/L, D-Dimers >1000 ng/mL, C-reactive protein (CRP) > 3 mg/dL. On the contrary, patients were excluded from the enrollment if they were mechanically ventilated or on extracorporeal membrane oxygenation (ECMO), if they had bowel diverticulitis or perforation, if they had severe hepatic dysfunction or in case of prespecified laboratory abnormalities at baseline. Detailed inclusion and exclusion criteria are listed in the full trial protocol (see supplementary materials).
Randomization and blinding
Randomization was web-based through the use of a permuted-block randomization method to ensure a balanced assignment to each treatment arm. The randomization was stratified by severity of illness (PaO2/FiO2 ≥200 mmHg and PaO2/FiO2 <200 mmHg or the presence of non-invasive ventilation). Eligible participants were randomly assigned in a 2:1 ratio to receive SOC therapy plus sarilumab (treatment group A, experimental arm) or SOC therapy alone (treatment group B, control arm). Participants, their clinicians, people giving the interventions, those assessing outcomes, and those analysing the data were not masked to group assignment and treatment allocation.
Procedures
Sarilumab 400 mg was prepared according to instructions provided in the pharmacy manual and was administered as a single intravenous infusion. Patients could have the infusion stopped for safety-related issues, in which case they did not continue with dosing. A second dose of intravenous sarilumab 400 mg could be administered 12 h later at the discretion of the investigator, if clinical improvement was judged insufficient. Corticosteroids and/or antivirals (mainly remdesivir) could be prescribed as SOC therapy, according to the decision of clinical investigator and current guidelines. Investigator could decide to change or prematurely stop the SOC therapy for safety reasons without the subject being withdrawn from the study. Sarilumab was provided by National Health System and bought by Hospital Pharmacies of each participant site as in routine clinical practice.
Efficacy and safety were assessed daily until day 14 (or until discharge if it occurred before day 14), considering physical examination, vital signs, oxygen supplementation, respiratory status and investigator's report of adverse events (AEs), serious (SAEs) or not. Routine blood tests were performed at days 3, 5, 7, 9 and 15 (or at patient discharge if it occurred before day 14). SARS-CoV-2 PCR on nasopharyngeal swabs was repeated at day 9 and 15. A chest CT scan was performed between day 7 and day 15 to check the evolution of previous radiological findings. Data on secondary infections were also collected. Patients were followed for a total of 30 days with two other visits at days 21 and 30. Patients who were discharged before day 21 or 30 had their follow-up visits conducted by phone call. All the additional tests/procedures required for patient care were provided in accordance with local practice at each site, in order to guaranty best standards of care.
Outcomes
The primary efficacy endpoint was time to clinical improvement, defined as the time from receiving the first dose of drug to an improvement of two or more points (from the status at baseline) on a seven-point category ordinal scale, with numerical values defined as follows: (1) not hospitalized, with resumption of normal activities; (2) not hospitalized, but unable to resume normal activities; (3) hospitalized, not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, requiring noninvasive mechanical ventilation; (6) hospitalized, requiring ECMO, invasive mechanical ventilation, or both; and (7) death. The key secondary efficacy outcomes evaluated the effect of sarilumab on survival. As per protocol, we analyzed the mortality rate within 30 days and the time from treatment initiation to death; additionally, we calculated the proportion of deceased at day 30. As secondary safety outcomes, we evaluated AEs and secondary bacterial or fungal infections. Other secondary outcomes were all prespecified, and details are provided in supplementary materials.
Statistical analysis
Fixing a power of 80% and a two-sided significance level of 5%, assuming that the median time to clinical improvement of participants in the standard-of-care group was estimated as 16 days,23 to observe a reduction in the experimental arm of the endpoint to 10 days (reduction of 37%), 171 patients were needed (according to a design 2:1, 114 in the experimental arm and 57 as control group). Sample size determination was performed using PASS, Version 12.
Descriptive characteristics were provided using medians and interquartile ranges (IQR) for continuous variables and frequencies and percentages for categorical variables. The efficacy analysis was performed on the modified Intention-To-Treat (mITT) population, defined as all patients who had undergone randomization and received a dose of sarilumab or continued SOC therapy. The efficacy analysis was mainly based on a “time to event” analysis, median time to clinical improvement was estimated by Kaplan Meier method and compared by log-rank test. The baseline of the analysis was treatment initiation. For inter-group comparison, we used chi square or Fisher's exact test for categorical variables, and Wilcoxon or t-test for continuous variables. Shapiro–Wilk test was performed to test normality of distribution of continuous variable before using nonparametric test. Cox regression model stratified by clinical center was employed to estimate the hazard ratio (HR) of the prespecified endpoint. For the primary endpoint, the model was also adjusted for stratification factor in the randomization (PaO2/FiO2 ≥200 mmHg and PaO2/FiO2 <200 mmHg or the presence of non-invasive ventilation). Moreover, two subgroups analysis were performed after stratifying participants by disease severity (PaO2/FiO2 ≥200 mmHg and PaO2/FiO2 <200 mmHg) and by baseline inflammatory parameters (CRP < or ≥7 mg/dL and lymphocytes count < or ≥870/mmc; these cut-offs were selected after calculating the median values in the entire study population). A formal interaction test between strata was also assessed for the primary endpoint. The subgroup analysis was not prespecified in the protocol, but it was added as a post-hoc analysis. Mortality rate was calculated dividing number of deceased over total number of person-years exposed to risk. The safety analysis was performed on the population who received any amount of study medication, according to the treatment that the patients actually received. The number of subjects experiencing any AEs and the number of AEs occurrences during the treatment period, were summarized by treatment arm. In a similar way, we described secondary bacterial or fungal infections occurred over the entire study period.
A statistically significant difference in the variable tested was indicated as p < 0.05 (two-sided). Statistical analysis was performed using STATA 15.1 software.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
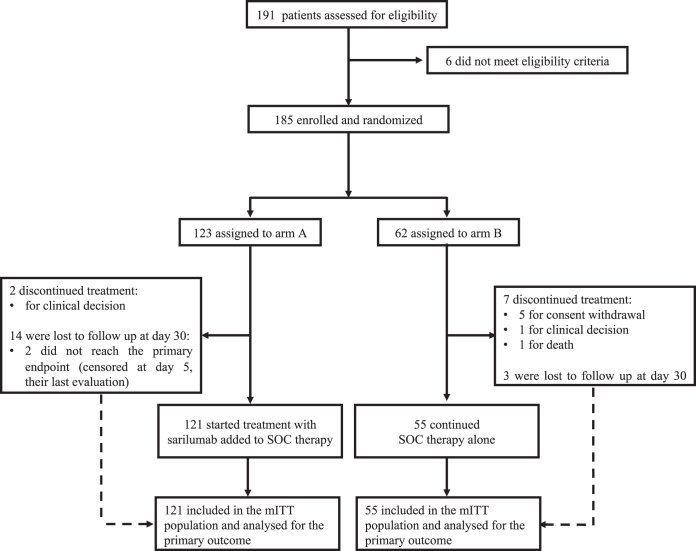
The first patient was screened on May 11, 2020, and the last patient was randomly assigned on May 05, 2021. A total of 191 patients were assessed for eligibility in five Italian hospitals and 185 patients were randomly assigned (123 to the treatment group, arm A, and 62 patients to the SOC group, arm B), 12 before the protocol amendment (8 to arm A, and 4 patients to arm B). Before treatment administration, nine dropouts (two in arm A and seven in arm B) were observed due to consent withdrawal (5/9 in arm B), death (1/9 in arm B) and clinical decision (1/9 in arm B and 2/9 in arm A). The remaining 176 patients (121 in arm A and 55 in arm B) completed the treatment and 17 were lost to follow up after discharge; among these, only two patients (arm A) did not reach the primary endpoint and were censored at their last evaluation (day 5 for both). (Fig. 1). With an overall sample size of 176 participants (55 in the control group and 121 in the treatment group), the study still achieves the fixed power of 80%.
Fig. 1.
CONSORT flow diagram. mITT, modified intention-to-treat, including all the patients who had undergone randomization and received a dose of sarilumab or continued SOC therapy; SOC, standard of care.
Baseline demographic, clinical and laboratory characteristics were similarly distributed between the study arms. In the treatment group (arm A), the median age was 61 years (IQR 53–70), 97 (80%) were men and a total of 45 (37%) comorbidities was reported; in the SOC group the median age was 59 years (52–72), 37 (67%) were men and a total of 19 (35%) comorbidities was described. At baseline, the median PaO2/FiO2 was 212 mmHg (148–280) in arm A and 197 mmHg (160–264) in arm B, and all participants in both groups required supplemental oxygen (at least 4 in the clinical status ordinal scale). During the study period, a similar proportion of patients was treated with corticosteroids (44 [36%] in arm A vs 26 [47%] in arm B, p = 0.170) and remdesivir (22 [18%] in arm A vs 8 [15%] in arm B, p = 0.552) in the two groups. The additional dose of sarilumab was administered to 58 (48%) participants, 12 h after the first dose. Patients’ baseline characteristics are described in Table 1.
Table 1.
Patients’ characteristics at baseline.
| ARM A (treatment group) n = 121 |
ARM B (SOC group) n = 55 |
|
|---|---|---|
| Demographic | ||
| Gender | ||
| Male, N (%) | 97 (80.2%) | 37 (67.3) |
| Female, N (%) | 24 (19.8%) | 18 (32.7) |
| Age, years, median (IQR) | 61 (53–70) | 59 (52–72) |
| Ethnic group | ||
| Caucasian, N (%) | 113 (93.4%) | 52 (94.6%) |
| Not caucasian, N (%) | 8 (6.6%) | 3 (5.5%) |
| Comorbidities | ||
| Chronic cardiac disease, N (%) | 5 (4.1%) | 2 (3.6%) |
| Chronic pulmonary disease, N (%) | 36 (29.8%) | 15 (27.3%) |
| Diabetes, N (%) | 4 (3.3%) | 1 (1.8%) |
| Active malignant neoplasm, N (%) | 0 | 0 |
| Chronic kidney disease, N (%) | 0 | 1 (1.8%) |
| Treatment | ||
| Sarilumab doses | ||
| 1 Dose, N (%) | 63 (52.1%) | – |
| 2 Doses, N (%) | 58 (47.9%) | – |
| Corticosteroids, N (%) | 44 (36.4%) | 26 (47.3%) |
| Remdesivir, N (%) | 22 (18.2%) | 8 (14.6%) |
| Median laboratory parameters (IQR) | ||
| CRP, mg/dl | 7.0 (4.3–12.3) | 6.1 (3.3–9.8) |
| Lymphocytes, X1000/MM3 | 0.85 (0.62–1.24) | 0.89 (0.62–1.23) |
| Ferritin, pg/ml | 975 (493–1485) | 768 (486–1203) |
| D-dimer, ng/ml | 564 (365–919) | 640 (358–994) |
| LDH, UI/L | 356 (272–418) | 334 (271–421) |
| Platelet, x1000/mmc | 229 (170–283) | 232 (176–311) |
| Fibrinogen, median (IQR) | 587 (479–703) | 563 (452–640) |
| Disease severity | ||
| PaO2/FiO2, mmHg, median (IQR) | 212 (148–280) | 197 (160–264) |
| Clinical status on ordinal scalea, median (IQR) | 4 (4–5) | 4 (4–5) |
CRP, C-reactive protein; IQR, interquartile range; LDH, lactate dehydrogenase; n, number of participants; PaO2/FiO2, partial pressure of arterial oxygen/fraction of inspired oxygen.
7-point ordinal scale: (1) not hospitalized, with resumption of normal activities; (2) not hospitalized, but unable to resume normal activities; (3) hospitalized, not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, requiring noninvasive mechanical ventilation; (6) hospitalized, requiring ECMO, invasive mechanical ventilation, or both; (7) death.
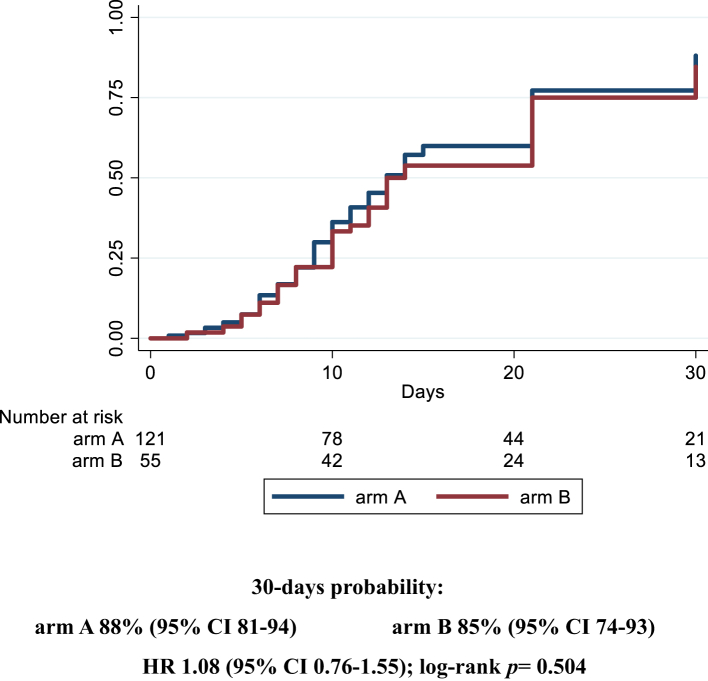
Overall, 97/121 (80.2%) patients and 45/55 (81.8%) patients reached the primary endpoint in arm A and B, respectively, and no significant differences were found between the two groups. Median time to clinical improvement was 13 days (95% CI 11–15) in the experimental arm and 14 (12–21) in the control arm, and 30-days probability of the primary endpoint was 88% (95% CI 81–94) in arm A vs 85% (74–93) in arm B (HR 1.07 [0.8–1.5]; log-rank p = 0.504) (Fig. 2). In a further model adjusting also for the use of additional corticosteroids treatment, we observed similar risk for the two groups (HR arm A vs B 1.05 [0.73–1.50]) (data not depicted). Moreover, we did not observe any statistically significant differences also when considering the number of sarilumab doses administered (84.6% [73.6–92.8] in the SOC group vs 96.2% [88.5–99.3] in case of 1 dose vs 77.6% [65.4–87.9] in case of 2 doses, log-rank p = 0.134; HR SOC vs 1 dose 1.33 [0.90–1.98] and SOC vs 2 doses 0.86 [0.56–1.32]) (data not depicted).
Fig. 2.
Kaplan Meier survival curves estimating the cumulative proportion that experienced the primary endpoint at day 30, in the modified Intention-to-treat population. Cox regression model stratified by clinical center fitted to estimate the hazard ratio (HR) of primary endpoint in arm A vs arm B; 95% confidence intervals (CI) are reported. Primary endpoint: time to clinical improvement of 2 points on a 7-point category ordinal scale. Arm A: sarilumab plus standard of care. Arm B: standard of care.
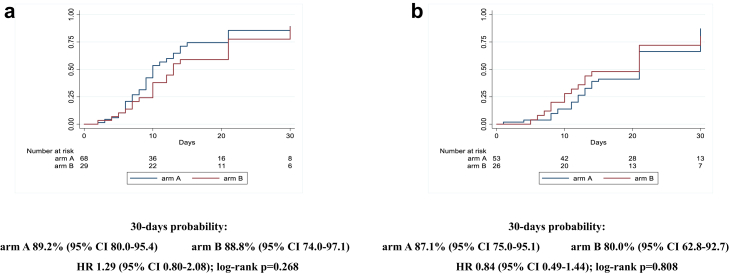
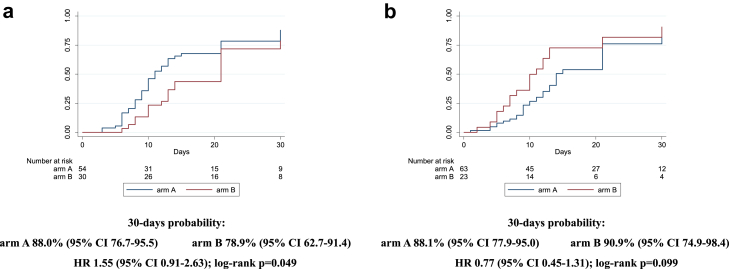
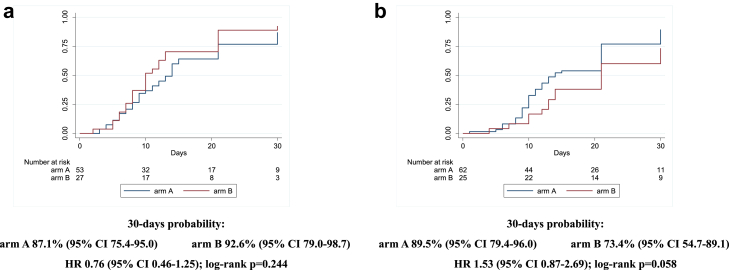
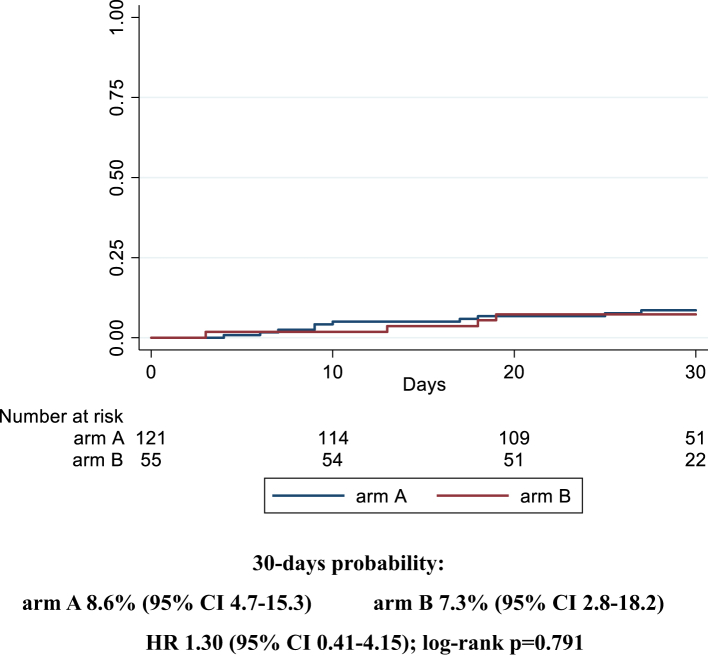
Similarly, after stratifying for disease severity (baseline PaO2/FiO2 ≥ or <200 mmHg), we did not observe any statistically significant difference comparing the two arms (87% [75–95] in arm A vs 80% [63–93] in arm B, HR 0.8 [0.5–1.4]; log-rank p = 0.808, for PaO2/FiO2 <200 mmHg; 89% [80–95] in arm A vs 89% [74–97] in arm B, HR 1.3 [0.8–2.1]; log-rank p = 0.268, for PaO2/FiO2 ≥200 mmHg) (Fig. 3a and b). Interaction test between the two strata was 0.296. Considering the stratification by inflammatory parameters, the probability of improvement resulted similar for the two arms in the strata with CRP ≥7 mg/dL (88% [78–95] vs 91% [75–98] for A vs B, HR 0.8 [0.4–1.3]; log-rank p = 0.099), while, in the strata with CRP <7 mg/dL, a higher probability of improvement was observed in the treatment arm with borderline statistical significance (88% [77–96] vs 79% [63–91] for A vs B, HR 1.6 [0.9–2.6]; log-rank p = 0.049) (Fig. 4a and b). Interaction test between the two strata was 0.090. The last stratification was based on baseline lymphocytes count, < or ≥ the median value (870/mmc). Similarly to the above reported results, the probability of improvement was not significantly different between the two groups when lymphocytes were more than 870/mmc (87% [75–95] vs 93% [79–99] for A vs B, HR 0.8 [0.5–1.3]; log-rank p = 0.244); when lymphocytes were less than 870/mmc, a higher probability of improvement in the sarilumab group (90% [79–96] vs 73% [55–89] for A vs B, HR 1.5 [0.9–2.7]; log-rank p = 0.058) was observed without reaching statistical significance (Fig. 5a and b). Interaction test between the two strata was 0.050. Finally, no effect of sarilumab on survival was found. Comparing the two arms, A vs B, mortality rate (IR 0.3 [95% CI 1.6–5.7] vs IR 0.3 [1.0–7.0] per 100 person-years follow up [PYFU]), and death probability (9% [5–15] vs 7% [3–18] HR 1.30 [0.4–4.2]; log-rank p = 0.791) were not significantly different (Fig. 6). Including only 159 patients observed until day 30, the proportion of deceased at day 30 was not different between the two groups (10/107 [9.3%] in arm A vs 4/52 [7.7%] in arm B, p = 1.000). The available analyses of the remaining secondary outcomes are listed as supplementary materials.
Fig. 3.
Kaplan Meier survival curves estimating the cumulative proportion that experienced the primary endpoint at day 30, considering patients with baseline PaO2/FiO2 ≥ 200 mmHg (3a) and patients with baseline PaO2/FiO2<200 mmHg (3b). Cox regression model stratified by clinical center fitted to estimate the hazard ratio (HR) of primary endpoint in arm A vs arm B; 95% confidence intervals (CI) are reported. Primary endpoint: time to clinical improvement of 2 points on a 7-point category ordinal scale. Arm A: Sarilumab plus standard of care. Arm B: standard of care.
Fig. 4.
Kaplan Meier survival curves estimating the cumulative proportion who experienced the primary endpoint at day 30, considering patients with baseline C reactive protein <7 mg/dL (4a) and ≥7 mg/dL (4b). Cox regression model stratified by clinical center fitted to estimate the hazard ratio (HR) of primary endpoint in arm A vs arm B; 95% confidence intervals (CI) are reported. Primary endpoint: time to clinical improvement of 2 points on a 7-point category ordinal scale. Arm A: Sarilumab plus standard of care. Arm B: standard of care.
Fig. 5.
Kaplan Meier survival curves estimating the cumulative proportion who experienced the primary endpoint at day 30, considering patients with baselinelymphocytes count ≥870/mmc (5a) and <870/mmc (5b). Cox regression model stratified by clinical center fitted to estimate the hazard ratio (HR) of primary endpoint in arm A vs arm B; 95% confidence intervals (CI) are reported. Primary endpoint: time to clinical improvement of 2 points on a 7-point category ordinal scale. Arm A: Sarilumab plus standard of care. Arm B: standard of care.
Fig. 6.
Kaplan Meier survival curves estimating the cumulative proportion who died considering patients observed until day 30. Cox regression model stratified by clinical center fitted to estimate the hazard ratio (HR) of death in arm A vs arm B; 95% confidence intervals (CI) are reported. Primary endpoint: time to clinical improvement of 2 points on a 7-point category ordinal scale. Arm A: Sarilumab plus standard of care. Arm B: standard of care.
A total of 38 AEs and 29 SAEs occurred in 27 (19 [15.7%] in arm A vs 8 [14.5%] in arm B, p = 0.994) and 28 (22 [18.2%] in arm A vs 6 [10.9%] in arm B, p = 0.221) participants, respectively. Only 2/38 AEs (liver toxicity) and 1/29 SAEs (secondary infection) reported in the treatment group, were considered related to sarilumab and have improved during the study period. Worsening of respiratory condition was the most commonly observed SAE (17/29 in arm A and 5/29 in arm B, p = 0.357). Serious secondary infections were mainly bacterial, except for two cases of candidemia, one in each treatment group. A total of 29 secondary bacterial infections were reported and six patients experienced more than one serious infection (3 [3%] in arm A and 3 [5%] in arm B, p = 0.313). Considering the number of sarilumab doses administered, a higher proportion of infections were observed among patients receiving the additional dose (11/58 [19%] vs 3/63 [5%], p = 0.015). (Table 2).
Table 2.
Secondary bacterial and fungal infections.
| ARM A (treatment group) n = 121 |
ARM B (SOC group) n = 55 |
P-value | |
|---|---|---|---|
| Bacterial infections | |||
| Pneumonia, N (%) | 6 (5.0%) | 1 (1.8%) | 0.323 |
| Blood stream infections, N (%) | 5 (4.1%) | 4 (7.3%) | 0.381 |
| Urinary tract infection, N (%) | 4 (3.3%) | 1 (1.8%) | 0.582 |
| SSTIS, N (%) | 1 (0.8%) | 0 | 0.499 |
| MDR infection, N (%) | 4 (28.6%) | 3 (75.0%) | 0.093 |
| Fungal infections | |||
| Candidemia, N (%) | 1 (0.8%) | 1 (1.8%) | 0.565 |
| >1 infection, N (%) | 3 (2.5%) | 3 (5.4%) | 0.313 |
| Presence of at least 1 secondary infection according to sarilumab dosesa | |||
| One dose, N (%) | 3/63 (4.8%) | – | |
| Two doses, N (%) | 11/58 (19.0%) | – |
MDR, multi drug resistant pathogens; n, number of participants; SSTIs, skin and soft tissue infections.
Number of infections occurred in one sarilumab dose group vs two sarilumab doses group, p = 0.015.
Discussion
In this randomized, multicenter, open-labeled clinical trial, enrolling patients with severe COVID-19 pneumonia who were receiving the local standard of care, there was no demonstrated efficacy of adding sarilumab (400 mg administered intravenously on day 1, possibly repeated after 12 h), also after stratifying participants according to severity disease. Post-hoc analyses suggested that subsets of patients with CRP <7 mg/dL and lymphocyte count <870/mmc at baseline, might have had some benefits with sarilumab treatment. Furthermore, all the secondary endpoints analyzed did not differ between the two arms, including survival up to 30 days.
Our results seemed to be in contrast with previous studies reporting some remarkable successes of IL-6 inhibitors in patients with COVID-19. Two of the largest trials on IL-6 inhibitors11,13 demonstrated the efficacy of such therapies. The RECOVERY trial,13 focusing only on the assessment of tocilizumab, included not critically ill patients, with hypoxia and systemic inflammation, 2094 receiving usual care and 2022 receiving tocilizumab. The primary outcome was 28-day mortality and tocilizumab use improved both survival and other clinical outcomes. The Immune Modulation Therapy domain of the REMAP-CAP trial11 included 2274 critically ill participants receiving organ support, with 972 participants assigned to tocilizumab, 485 to sarilumab, 378 to anakinra, and 418 to control. Tocilizumab and sarilumab were both effective, when compared with control, and likely to be equivalent in improving survival and reducing duration of organ support. Nonetheless, in both trials, the clinical benefit of IL- 6 blockade was clear when administered in combination with dexamethasone, largely used in both study populations. Moreover, the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group published a prospective meta-analysis of 27 randomized clinical trials assessing the efficacy of IL-6 antagonists compared with SOC or placebo, among patients hospitalized for COVID-19.5 A lower 28-day all-cause mortality and improved clinical outcomes in patients treated with IL-6 antagonists were observed. Collectively, these data supported the use of blocking IL-6 in patients with COVID-19, but the authors clearly declared that associations with improved outcomes appeared more marked among patients receiving corticosteroids and for tocilizumab compared to sarilumab. Similarly, another meta-analysis found mortality reduction for both tocilizumab and sarilumab in combination with corticosteroids, but suggested an increase in the risk of death if used alone.15 In addition, a network meta-analysis of 18 Randomized Clinical Trials14 reported a similar lower mortality for tocilizumab and sarilumab, among hospitalized patients with severe or critical COVID-19, receiving corticosteroids. However, data on sarilumab came only from two trials, the overmentioned REMAP-CAP trial11 and a recently published randomized clinical trial,17 which included only a minority of patients (roughly 30%) receiving concomitant corticosteroids. Considering the general results of this study,17 treatment with sarilumab did not lead to significant improvement in clinical status or mortality. Even though these two anti-IL6 inhibitors share an identical mechanism of action, it has been hypothesized that they may have a different effect on patients with COVID-19, due to the higher binding affinity of sarilumab to the IL-6 receptor24,25 and its possible trapping in the blood in those patients with increased IL-6 receptor concentrations due to the COVID-19 cytokine cascade, potentially leading to decreased penetration of sarilumab in the lungs, the key site of hyperinflammation.19
In line with our findings, evidences supporting the treatment with sarilumab from other Randomized Clinical Trials evaluating its efficacy compared to usual care or placebo,16,18,20,21 were not so certain. Indeed, each of these trials failed to demonstrate sarilumab superiority in terms of clinical improvement and mortality among patients with severe or critical COVID-19. In the SARICOR study,20 only a trend toward better outcomes was observed for the early use of sarilumab, compared to SOC, among patients requiring oxygen support and with features of systemic inflammation, 90% of whom was on corticosteroids therapy. However, important differences in the design of all these studies need to be considered in drawing conclusions about their implications for clinical practice, and their clinical and methodological heterogeneity might limit the extent to which resulted estimates can lead to relevant conclusions.26,27 Moreover, although not published, the press release from the manufacturer of sarilumab strongly suggests no benefit (and possible arm) when used as monotherapy.22
Therefore, studies on sarilumab had the main limitation to have been generally performed earlier in the pandemic, when COVID-19 treatment was not standardized, with low rates of concomitant corticosteroids use. Similarly, in our study, in which adjunctive steroid treatment was not defined by the protocol but was prescribed on the judgment of the clinical investigator, less than half of the participants received such agents at the randomization, equally distributed between the two treatment arms, and this could be one of the explanations for the lack of efficacy of the study drug. Interestingly, and partially supporting this hypothesis, some benefits were shown in participants with low CRP values, in whom IL-6 suppression alone might be sufficient to control the inflammatory burden, also in the absence of the anti-inflammatory effect mediated by corticosteroids, highlighting the importance of the timing of the intervention. A subgroup analysis, considering only patients receiving corticosteroids, was not performed for the relatively small sample size.
Apparently, an inverse correlation with lymphocyte count was found, but inflammatory biomarkers’ trajectories in SARS-CoV-2 infection are not overlapped, and CRP elevation may be delayed compared to the appearance of lymphopenia.28,29
Encouragingly, no new safety signals for sarilumab was identified in terms of AEs, serious and not, and secondary infections. Of note, higher rates of secondary infections were observed among participants receiving two doses of sarilumab, probably as a consequence of the disease severity and the patients’ complexity. This evidence was consistent with previous clinical trial data for sarilumab16,19 and needs to be taken into account in case of corticosteroids concomitant administration.
Our study had several strengths including the randomized, controlled and multicenter design, the stratification based on disease severity, the homogeneous target population of patients with severe COVID-19 and the well-balanced baseline characteristics and cotreatments administered in the two arms. However, some study limitations must be addressed. It was a non-blinded trial, which has the potential for ascertainment bias; besides, awareness of the intervention assignment could affect management in the control group. The recruitment phase was extended over a long period, which could potentially affect background care during the trial, particularly if considering different clinical sites; nonetheless, the main co-treatment choices (corticosteroids and remdesivir) were well balanced between the two arms. Another limitation was that the 400 mg dose may be sub-therapeutic, as suggested in previous studies,16,17 and the second dose has been left to investigator's decision, increasing the risk of heterogeneous choices. Besides, data on sarilumab concentrations were not provided. However, our post-hoc analysis did not demonstrate any superiority of 2 doses of sarilumab. Furthermore, the unequal distribution of males and females between the two groups, despite the web-based randomization, and the predominance of male patients in the treatment arm, may have partially biased the results, considering that men are described as being at a higher risk of severe COVID-19. Nevertheless, the difference between the two arms only showed a trend toward significance (p = 0.063). Finally, it should be noted that the effect estimate used for the power calculation was large, and thus the study may have been underpowered to identify small changes in outcomes. However, given the larger body of research, including other clinical trials and meta-analyses, it is unlikely that conclusions would have been different with a larger sample size.
Taking together all these data, it is clear that the use of corticosteroids is crucial in severe and critical COVID-19 and may provide greater advantage than the specific drug given individually. Indeed, the use of IL-6 inhibitors without adjunctive steroids is not effective (and potentially harmful); as a proof, in the studies showing some benefits, IL-6 inhibitors have been used in combination with other agents. Moreover, despite several certainties regarding its safety, evidences on the efficacy of sarilumab are still not so robust as for tocilizumab and comparative clinical trials are lacking, therefore the alternative use, as suggested by guidelines,2 of these two drugs should be better considered. Our results, while suggesting a lack of efficacy of sarilumab in patients with severe COVID-19, highlight the need to identify targeted subgroups of patients with COVID-19 who are likely to benefit from this treatment, the optimal timing for sarilumab administration and its optimal dosage to maximize the efficacy.
Contributors
Conceptualization: IM, RG, AM, PL, MC, MMP, AA. Methodology and formal analysis: PL. Investigation and resources: IM, RG, FVS, AM, CC, ET, FB, AV, NM, CP, SC, VM, MC, SM, TB, GM, EM, MI, SO. Data curation: PL, SL, MF, AB, MMP. Writing – original draft: IM. Writing - review and editing: RG, AM, PL, AC, AA. Project administration: IM, PL, SL, MMP, AA. Supervision: RM, ML, MF, MA, LS, RC, EN, ADM, FP, AC, AA. Funding acquisition: EG, FV, AA. All authors contributed to the final version of the manuscript, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
IM and PL have directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
The raw data generated and/or analyzed within the present study are available in our institutional repository (rawdata.inmi.it), subject to registration. In the event of a malfunction of the application, the request can be sent directly by e-mail to the Library (biblioteca@inmi.it). No charge for granting access to data is required.
Declaration of interests
Andrea Antinori has served as a paid consultant to Astra-Zeneca, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Merck Sharp and Dohme, Roche, Theratotecnologies and ViiV Healthcare and received research institutional grants from Gilead Sciences, Janssen-Cilag and ViiV Healthcare, payment or honoraria from Gilead Science and ViiV Healthcare and support for attending meetings and/or travel from ViiV Healthcare and AbbVie. Marta Camici received institutional grant, support for attending meetings and/or travel and speakers' honoraria from Gilead Sciences. Stefania Cicalini reports consulting fees paid to self from ViiV Healthcare, Jansen-Cilag, Merck Sharp and Dohme and Gilead Sciences and payment or honoraria from ViiV Healthcare, Jansen-Cilag, Merck Sharp and Dohme and Gilead Sciences. Roberta Gagliardini reports payments to their institution from Gilead Sciences, speakers' honoraria/educational activities for ViiV Healthcare, Merck Sharp and Dohme and Gilead Sciences, support for attending meetings and/or travel from ViiV Healthcare and advisor for Theratechnologies, Janssen-Cilag and Gilead Sciences. Enrico Girardi reports grants from Gilead Sciences and Mylan and fees for lectures and expertise from Gilead Sciences. Marco Iannetta received research institutional grant from Gilead Sciences and personal honoraria from Biogen Italia, AIM Educational and MICOM srl. Ilaria Mastrorosa received institutional research grant and support for attending meetings and/or travel from Gilead Sciences. Annalisa Mondi received speakers' honoraria from Gilead Sciences and ViiV Healthcare and participated in advisory boards sponsored by ViiV Healthcare. Emanuele Nicastri reports grants from Sobi and Roche, payment or honoraria from Eli Lilly, Gilead Sciences, Roche and Sobi, patents planned from Dompè and advisor for Eli Lilly and Roche. Carmela Pinnetti received personal fee for a case presentation and travel grant from Gilead Sciences and participated in an advisory board for Janssen-Cilag. Alessandra Vergori received institutional grant from Gilead Sciences, speakers’ honoraria/educational activities from Merck Sharp and Dohme, ViiV Healthcare and Janssen-Cilag and advisor for Janssen-Cilag. The other co-authors declare no conflicts of interests for this work.
Acknowledgements
This work was supported by INMI “Lazzaro Spallanzani” Ricerca Corrente Linea 1 on emerging and reemerging infections, funded by Italian Ministry of Health.
The authors gratefully acknowledge nurse staff, all the study participants and all members of the ESCAPE Study Group.
ESCAPE Study Group
Chiara Agrati, Massimo Andreoni, Andrea Antinori, Francesca Bai, Alessia Beccacece, Filippo Barreca, Maria Paola Bertuccio, Teresa Bini, Evangelo Boumis, Marta Camici, Roberto Cauda, Carlotta Cerva, Stefania Cicalini, Antonella Cingolani, Antonella D’Arminio Monforte, Angela D’Urso, Margherita De Masi, Federico De Zottis, Cosmo Del Borgo, Francesco Di Gennaro, Arianna Emiliozzi, Massimo Fantoni, Laura Fondaco, Marisa Fusto, Roberta Gagliardini, Francesca Giovannenze, Elisabetta Grilli, Marco Iannetta, Daniele Iodice, Miriam Lichtner, Patrizia Lorenzini, Gaetano Maffongelli, Erminia Masone, Barbara Massa, Ilaria Mastrorosa, Valentina Mazzotta, Paola Mencarini, Eugenia Milozzi, Annalisa Mondi, Silvia Mosti, Rita Murri, Marcantonio Negri, Emanuele Nicastri, Gian Piero Oliva, Giovanna Onnelli, Sandrine Ottou, Pier Giorgio Pace, Fabrizio Palmieri, Jessica Paulicelli, Carmela Pinnetti, Maria Maddalena Plazzi, Loredana Sarmati, Francesco Vladimiro Segala, Chiara Sorace, Eleonora Taddei, Alessandra Vergori, Pietro Vitale
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101895.
Contributor Information
Ilaria Mastrorosa, Email: ilaria.mastrorosa@inmi.it.
ESCAPE study group:
Chiara Agrati, Massimo Andreoni, Andrea Antinori, Francesca Bai, Alessia Beccacece, Filippo Barreca, Maria Paola Bertuccio, Teresa Bini, Evangelo Boumis, Marta Camici, Roberto Cauda, Carlotta Cerva, Stefania Cicalini, Antonella Cingolani, Antonella D'Arminio Monforte, Angela D'Urso, Margherita De Masi, Federico De Zottis, Cosmo Del Borgo, Francesco Di Gennaro, Arianna Emiliozzi, Massimo Fantoni, Laura Fondaco, Marisa Fusto, Roberta Gagliardini, Francesca Giovannenze, Elisabetta Grilli, Marco Iannetta, Daniele Iodice, Miriam Lichtner, Patrizia Lorenzini, Gaetano Maffongelli, Erminia Masone, Barbara Massa, Ilaria Mastrorosa, Valentina Mazzotta, Paola Mencarini, Eugenia Milozzi, Annalisa Mondi, Silvia Mosti, Rita Murri, Marcantonio Negri, Emanuele Nicastri, Gian Piero Oliva, Giovanna Onnelli, Sandrine Ottou, Pier Giorgio Pace, Fabrizio Palmieri, Jessica Paulicelli, Carmela Pinnetti, Maria Maddalena Plazzi, Loredana Sarmati, Francesco Vladimiro Segala, Chiara Sorace, Eleonora Taddei, Alessandra Vergori, and Pietro Vitale
Appendix A. Supplementary data
References
- 1.https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china
- 2.https://covid19.who.int
- 3.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed 23 August 2022. [PubMed]
- 4.Del Valle D.M., Kim-Schulze S.H.H. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar-Hari M., Vale C.L., Godolphin P.J., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan S., Shafiei M.S., Longoria C., Schoggins J.W., Savani R.C., Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife. 2021;10:1–26. doi: 10.7554/eLife.68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas I.O., Diaz G., Gottlieb R.L., et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med [Internet] 2021;47(11):1258–1270. doi: 10.1007/s00134-021-06507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The REMAP-CAP Investigators, Derde L.P.G. Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19 the REMAP-CAP COVID-19 immune modulation therapy domain randomized clinical trial. medRxiv [Internet] 2021 https://www.medrxiv.org/content/10.1101/2021.06.18.21259133v2%0Ahttps://www.medrxiv.org/content/10.1101/2021.06.18.21259133v2.abstract 2021.06.18.21259133. Available from: [Google Scholar]
- 12.Investigators T.R.C., Gordon A.C. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godolphin P.J., Fisher D.J., Berry L.R., et al. Association between tocilizumab, sarilumab and all-cause mortality at 28 days in hospitalized patients with COVID-19: a network meta-analysis. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0270668. e0270668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeraatkar D., Cusano E., Martínez J.P.D., et al. Use of tocilizumab and sarilumab alone or in combination with corticosteroids for Covid-19: systematic review and network meta-analysis. BMJ Med. 2022;1(1) doi: 10.1136/bmjmed-2021-000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lescure F.X., Honda H., Fowler R.A., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivapalasingam S., Lederer D.J., Bhore R., et al. Efficacy and safety of sarilumab in hospitalized patients with COVID-19: a randomized clinical trial. Clin Infect Dis. 2022;75(1):e380–e388. doi: 10.1093/cid/ciac153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancho-López A., Caballero-Bermejo A.F., Ruiz-Antorán B., et al. Efficacy and safety of sarilumab in patients with COVID19 pneumonia: a randomized, phase III clinical trial (SARTRE study) Infect Dis Ther. 2021;10(4):2735–2748. doi: 10.1007/s40121-021-00543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariette X., Hermine O., Tharaux P.L., et al. Sarilumab in adults hospitalised with moderate-to-severe COVID-19 pneumonia (CORIMUNO-SARI-1): an open-label randomised controlled trial. Lancet Rheumatol. 2022;4(1):e24–e32. doi: 10.1016/S2665-9913(21)00315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchante N., Cárcel S., Carlos Garrido-Gracia J., et al. Early use of sarilumab in patients hospitalized with COVID-19 pneumonia and features of systemic inflammation: the SARICOR randomized clinical trial. Antimicrob Agents Chemother. 2022;66(2) doi: 10.1128/aac.02107-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branch-Elliman W., Ferguson R., Doros G., et al. Subcutaneous sarilumab for the treatment of hospitalized patients with moderate to severe COVID19 disease: a pragmatic, embedded randomized clinical trial. PLoS One [Internet] 2022;17(2):1–15. doi: 10.1371/journal.pone.0263591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regeneron Pharmaceuticals I . Sanofi and Regeneron press release; 2020. Regeneron and sanofi provide update on Kevzara® (sarilumab) phase 3 U.S. Trial in COVID-19 patients. [Google Scholar]
- 23.Cao B., Wang Y., Wen D., et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafique A., Martin J., Blome M., Huang T., Ouyang A., Papadopoulos N. AB0037 Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human il-6 receptor (il-6r) alpha. Ann Rheum Dis. 2013;72(Suppl 3):A797.1. [Google Scholar]
- 25.Xu C., Rafique A., Potocky T., et al. Differential binding of sarilumab and tocilizumab to IL-6Rα and effects of receptor occupancy on clinical parameters. J Clin Pharmacol. 2021;61(5):714–724. doi: 10.1002/jcph.1795. [DOI] [PubMed] [Google Scholar]
- 26.Imrey P.B. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):2020–2022. doi: 10.1001/jamanetworkopen.2019.19325. [DOI] [PubMed] [Google Scholar]
- 27.Murthy S., Lee T.C. IL-6 blockade for COVID-19: a global scientific call to arms. Lancet Respir Med. 2021;9(5):438–440. doi: 10.1016/S2213-2600(21)00127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowring M.G., Wang Z., Xu Y., et al. Outcome-stratified analysis of biomarker trajectories for patients infected with severe acute respiratory syndrome Coronavirus 2. Am J Epidemiol. 2021;190(10):2094–2106. doi: 10.1093/aje/kwab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. eBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.