Liao et al. 10.1073/pnas.0611519104. |

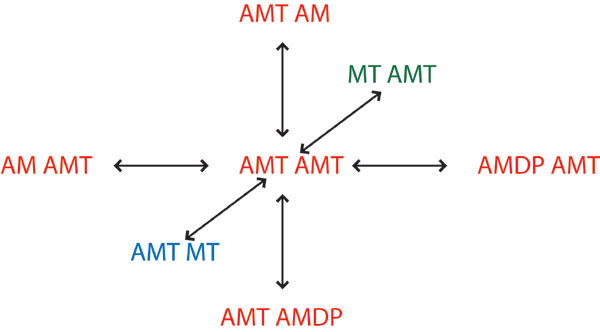
Fig. 5. Six possible transitions of a double-headed myosin state. In this example, the original state is AMT-AMT, where both heads bind to actin with ATP in their individual catalytic sites. Either head can experience ATP hydrolysis and the state transits to either AMT-AMDP or AMDP-AMT. Either head can also release ATP and becomes either AMT-AM or AM-AMT. Another possibility is that either head can detach from the actin to reach state AMT-MT or MT-AMT.

Fig. 6. The kinetic scheme for a double-headed myosin expanded from Fig. 5. The whole kinetic network contains 64 states and 384 possible transitions.
Table 1. Rates for the kinetics of single-headed myosin
Forward rates | Backward rates | |
M↔MT | 1.9x105 M-1s-1(2) | 4.0x10-7 s-1 |
MT↔MDP | 660.6 s-1(2) | 89.4 s-1(2) |
MDP↔MD | 0.1 s-1(2) | 0.1 M-1s-1(2) |
MD↔MD* | 0.1 s-1 | 0.1 s-1 |
MD*↔M | 1.2 s-1(3) | 4.79x106 M-1s-1(3) |
AM↔AMT | 2.4x106 M-1s-1 | 0.03 s-1 |
AMT↔AMDP | 1.0 s-1 | 0.03 s-1 |
AMDP↔AMD | 250 s-1(3) | 9.0 M-1s-1 |
AMD↔AMD* | 100 s-1 | 10.0 s-1 |
AMD*↔AM | 13 s-1(3) | 1.28x107 M-1s-1(3) |
M↔AM | 7.3x107 M-1s-1(3) | 0.166 s-1(2) |
MT↔AMT | 8.0x107 M-1s-1 | 990 s-1 |
MDP↔AMDP | 4.7x106 M-1s-1(3) | 14.3 s-1(2) |
MD↔AMD | 1.4x107 M-1s-1 | 1.6 s-1 |
MD*↔AMD* | 2.2x108 M-1s-1 | 2.0 s-1 |