Abstract
The objective of this study was to review the methods of prior studies that estimate the association between compliance to osteoporosis pharmacotherapy on fracture risk, and make recommendations to guide future research. We completed a systematic search of MEDLINE to identify all English language nonexperimental studies that examined the impact of adherence to osteoporosis pharmacotherapy on fracture risk. Studies that measured compliance were eligible and those that only examined persistence were excluded. We summarized the methodology of each study and make recommendations for future research. We identified 14 eligible articles: nine cohort and five nested case–control. Length of baseline (lookback) periods ranged between 3 months and 2 years, with nearly all studies (86%) restricting inclusion to treatment-naïve users. A threshold of 80% was most commonly used to define compliance (n = 10), with few studies providing a more thorough analysis through categorical (n = 3) or continuous (n = 1) measures. All nine cohort studies adjusted for age, sex, prior fracture, and at least one other comorbidity or drug; two cohort studies adjusted for a comorbidity score. Two of the five case–control studies clearly controlled for age, sex, drug exposure, event date and length of follow up. One study considered a theoretical sensitivity analysis to account for potential healthy adherer bias, yet all mentioned limitations related to possible residual confounding. We identify great variability in methods of prior studies that evaluate the impact of compliance to osteoporosis pharmacotherapy on fracture risk, and make recommendations to guide future research.
Keywords: bone, fracture, medication adherence, osteoporosis, research design
Introduction
Osteoporosis is a disease characterized by microarchitectural deterioration of bone, leading to increased bone porosity and consequently increased susceptibility to fracture [Anonymous, 1993]. The main treatment options for osteoporosis include bisphosphonates, calcitonin, raloxifene, and teriparatide [MacLean et al. 2008; Qaseem et al. 2008]. Despite effectiveness in fracture prevention, adherence to these therapies remains suboptimal [Kothawala et al. 2007]. Adherence to treatment is defined by the extent to which a patient’s behavior coincides with the prescribed regimen, and is quantified by measures of compliance and persistence [Cadarette and Burden, 2010]. Compliance to osteoporosis pharmacotherapy has most commonly been evaluated by the medication possession ratio (MPR) [Wilkes et al. 2010; Imaz et al. 2009; Rabenda et al. 2009; Siris et al. 2009]. MPR is calculated as the total number of days of medication supplied in the observation period, divided by the total number of days in the observation period. When capped at 1 or 100%, MPR is synonymous with the proportion of days covered (PDC). We recently reviewed methods of quantifying adherence to osteoporosis pharmacotherapy and recommend that PDC become the standard measure of compliance [Cadarette and Burden, 2010]. Although healthcare utilization databases can be used to estimate treatment adherence in large real-world populations [Cadarette and Burden, 2010; Grymonpre et al. 2006], using these data to assess the impact of adherence on clinical outcomes can be challenging [Brookhart et al. 2010; International Society for Pharmacoeconomics and Outcomes Research, 2009]. Methodological issues when measuring persistence (length of continuous therapy after treatment initiation) with osteoporosis pharmacotherapy have been reviewed [Cramer et al. 2007], yet most studies report measures of compliance. The aim of this review is to inform future studies that examine the effects of compliance to osteoporosis treatment on fracture rates. We summarize methods of prior studies and then outline recommendations for future research.
Methods
Systematic search strategy
We completed a comprehensive search of the Ovid MEDLINE and PubMed databases (1950–December 2009) to identify all articles that examined the association between adherence to osteoporosis pharmacotherapy on fracture risk. Relevant studies were identified by search terms and subject headings related to adherence, prescription claims, fracture, osteoporosis, bisphosphonate, raloxifene and database (Table 1). Studies were eligible if they evaluated compliance to osteoporosis pharmacotherapy using health-care utilization data, examined the effect of compliance on fracture risk, were published in English, and used a nonexperimental study design. We excluded clinical trials, meta-analyses, editorials, letters, abstracts, conference proceedings, economic evaluations, simulation studies, and review articles.
Table 1.
Search terms.
| MEDLINE search terms |
|
| PubMed search headings |
| (osteoporosis OR bone density OR bone resorption OR bone loss OR bone fragility OR bisphosphonate OR bisphosphonates OR diphosphonate OR alendronate OR risedronate OR ibandronate OR etidronate OR raloxifene) AND (fracture or fractures) AND (medication persistence OR persistence OR adherence OR medication adherence OR compliance OR medication compliance OR medication possession OR medication possession ratio) AND (cohort OR clinic OR outpatient OR database OR administrative database OR record OR prescription OR clinical practice OR drug prescriptions OR claims data OR claims database) |
Data abstraction
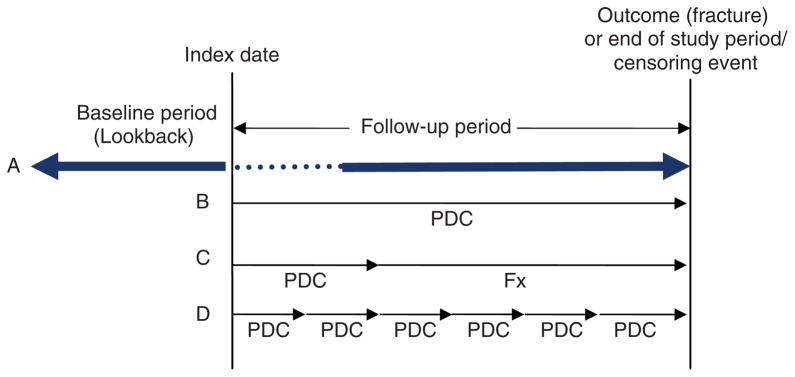
Studies were stratified by study design into cohort or nested case–control, and evaluated based on criteria adapted from recent guidelines [Gwadry-Sridhar et al. 2009; International Society for Pharmacoeconomics and Outcomes Research, 2009]. Cohort studies were characterized based on the general study design depicted in Figure 1. We defined index date by the prescription date that defined cohort entry, and baseline period as the period of data collection preceding the index date. The baseline period is also commonly referred to as the ‘lookback’ period by pharmacoepidemiologists. Studies that restricted inclusion to those without an eligible drug claim during the baseline period were identified as having used a new user design [Ray, 2003]. Compliance measurement was classified based on the primary measure of compliance and into studies that used: (1) the entire follow-up period (Figure 1B), (2) an ascertainment period (Figure 1C), or (3) a time-varying measure (Figure 1D). If an analysis used a time-varying measure of compliance, we did not also classify the initial period as an ascertainment period. To help differentiate between studies that capped compliance measurement to 100%, regardless of how the measure was reported in the paper, we defined compliance as PDC when capped to a maximum of 100%, and MPR when no cap was applied [Cadarette and Burden, 2010; Peterson et al. 2007]. We differentiated between studies that considered any fracture during the follow-up period from those that excluded fractures occurring during a predefined ‘treatment-onset’ period (Figure 1A). We also summarized how switching between agents was accounted for, if a minimum number of prescriptions were required for study inclusion, and how potential periods of immeasurable time were accounted for [Gwadry-Sridhar et al. 2009; International Society for Pharmacoeconomics and Outcomes Research, 2009; Suissa, 2008]. Instead of documenting each risk factor included in adjusted analyses, we identified the following four as the minimum set of covariates for adjustment: (1) age, (2) sex, (3) prior fracture and (4) a marker of comorbidity (comorbidity score), such as a comorbidity risk score or number of different medications. Studies that only evaluated females were classified as having adjusted for sex.
Figure 1.
Depiction of a database cohort study design to measure compliance and impact on fracture risk. Index date is identified by the prescription date that defines cohort entry. (A) The baseline (lookback) period is the period of interest prior to the first prescription (index date), and study follow up occurs after the index date and until the outcome or censoring date. Fracture outcomes can be assessed either throughout the entire follow-up period or after a ‘treatment onset’ (dotted line) period. The three primary methods to measure compliance include use of: (B) the entire follow-up period, (C) an ascertainment period, and (D) a time-varying measure. PDC, proportion of days covered (measure of compliance) = total days of drug supplied in the observation period divided by the total days in the observation period and capped at 100%; Fx = specific fracture follow-up period.
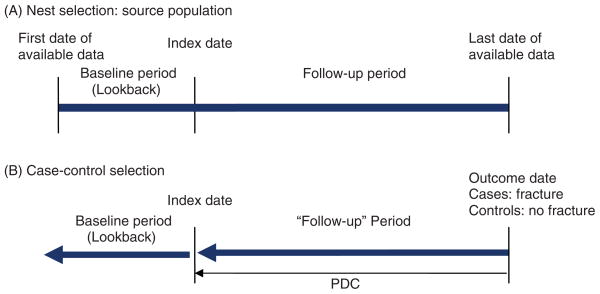
Nested case–control studies were defined as shown in Figure 2. To help standardize terminology in this review, we defined index date as the date of treatment initiation, yet acknowledge that several studies use index date as the ‘outcome’ date. In addition to summarizing similar methods as described under cohort studies, we considered case–control matching criteria. Studies that only evaluated women or one drug class were considered to be matched on sex and treatment, respectively. Given that age is a major risk factor for fracture, if a study matched on age, we deemed matching within 1 year to be appropriate. We also considered matching by date and length of follow up.
Figure 2.
Depiction of a nested case–control study design to measure compliance and impact on fracture risk. Index date is identified by the prescription date that defines cohort entry. (A) The source population (‘nest’) is selected based upon study inclusion and exclusion criteria to identify osteoporosis treatment users. A baseline period prior to drug index date may be used to identify cohort eligibility. (B) Cases are selected as individuals within the nest with the outcome of interest (fracture) and controls as those individuals matched to cases with no fracture. Both cases and controls are followed back until the date of the first prescription (index date), with compliance measured over this period of time (‘follow-up’ period). Covariates are determined from the baseline (lookback) period prior to first treatment and/or during follow up. PDC, proportion of days covered (measure of compliance) = total days of drug supplied in the observation period divided by the total days in the observation period and capped at 100%.
When methods were not clearly defined or were only reported in the results section, we summarized the methods based on the information provided. We focus on methods used in the primary analysis and report sensitivity analyses.
Results
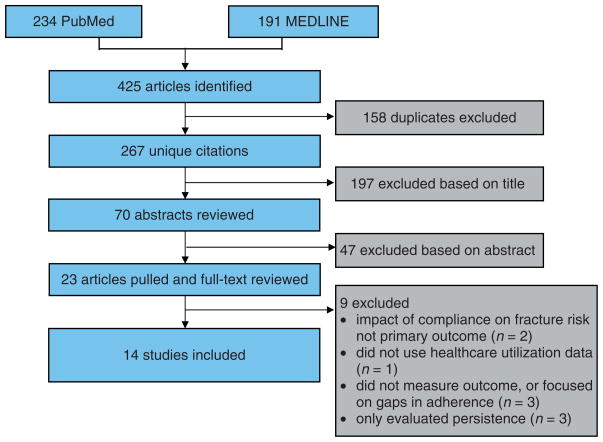
Of 267 unique articles identified, 23 met selection criteria for full-text review (Figure 3). Upon full-text review, nine were excluded: one did not use healthcare utilization data [Adami et al. 2006], one focused on the implications of extended gaps in adherence [Curtis et al. 2008a], two either did not evaluate or simulated fracture risk [Rietbrock et al. 2009; Papaioannou et al. 2003], three only evaluated persistence [Gold et al. 2007; van den Boogaard et al. 2006; McCombs et al. 2004], and two did not measure the effect of patient adherence on fracture risk as the primary outcome [Feldstein et al. 2009; Morin et al. 2007]. The remaining 14 articles were included: nine cohort (Table 2) and five nested case–control studies (Table 3).
Figure 3.
Study flow diagram for articles identified and reviewed from the OVID MEDLINE and PubMed databases between 1950 and December 2009.
Table 2.
Summary of methodological characteristics of cohort studies.
| [Briesacher et al. 2007] | [Caro et al. 2004] | [Curtis et al. 2008b] | [Gallagher et al. 2008] | [Hoer et al. 2009] | [Huybrechts et al. 2006] | [Penning-van Beest et al. 2008] | [Sheehy et al. 2009] | [Siris et al. 2006] | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | ALD, RSD | ALD, CCT, ETD, RSD, HT | ALD, RSD, IBD | ALD, RSD | ALD, RSD, ETD | ALD, RSD, HT | ALD, RSD | ALD, RSD | ALD, RSD |
| Evaluated drug class separately | NA | No | NA | NA | NA | No | NA | NA | NA |
| Baseline (lookback) period |
1 year | NP | 6 months | 1 year (new user) 0.25–1 year (covariates) | 6 months (new user) 1 year (covariates) | Variable | 1 year | 2 years (new user, prior fx and drug covariates) 1 year (other covariates) | 6 months |
| New users (drug) | Yes (BP) | No | Yes (BP) | Yes (ALD, RSD) | Yes (BP) | No | Yes (All OP drugs) | Yes (weekly BP) | Yes (BP) |
| Allowed switching | Yes | NP | Yes (between BPs; others censored) | NP | Yes | Yes (any) | NP | NP | NP |
| >1 Rx needed | No | No | No | Sub-analysis for 2 + Rxs | No | No | Sub-analysis for 2 + Rxs | No | No |
| Immeasurable time | NP | Assumed 100% compliance |
NP | NP | NP | Assumed 100% compliance |
NP | NP | NP |
| Compliance measure | PDC | MPR | MPR | MPR | MPR | MPR | PDC | MPR | MPR |
| How compliance quantified (1 = primary 2 = secondary) | 0–19%, 20–39%; 40–59%; 60–79%; 80–100% | 1) ≥80% 2) <50%, 50–80%, 80–90%, >90% |
1) ≥80% 2) <50%, 50–80%, ≥80% |
Not Stated: linear regression with 10 subgroups | ≥80% | 1)≥80% 2)<50%, 50–80%, 80–90%, >90% |
1)≥80% 2)<20, 20– 49, 50–69, 70–89, ≥90% |
≥80% | 1) ≥80% 2) Continuous range (0–100%) |
| How compliance measured (1 = primary 2 = secondary) | Time Varying (1 year) | 1) Entire follow-up 2) Ascertainment Period (6/12 months) | 1) Time Varying (90 days) 2) Entire Follow-up |
Entire follow-up | Entire follow-up | Entire follow-up | Time varying (90 days) | Entire follow-up | Entire follow-up |
| Start of fracture identification | Fx after 1 year | Fx after 180 days | Fx after 90 days | Any | Any | Fx after 180 days | Fx after 182 days | Any | Any |
| Length of follow-up to identify fracture risk | Minimum 2 yrs; 60,208 person-years follow-up | 22,753 person-years; mean 2 years | Variable | Mean 2.32 years | Minimum 180 days (82% had 1 + year, 47% 2 + years) | 63,438 person-years; mean of 1.7 years | 22,484 person-years | 1 year | 2 years |
| Minimum confounders for adjustment | |||||||||
| Age | Yes | Yes | Yes | Yesc | Yes | Yes | Yes | Yes | Yes |
| Sex | Yes | Yesa | Yes | Yesc | Yes | Yesa | Yesa | Yes | Yesa |
| Prior fracture | Yes | Yes | Yes | Yesc | Yes | Yes | Yes | Yesb | Yes |
| Comorbidity/drug score |
Yes | No | Yes | Yesc | No | No | No | No | No |
ALD, alendronate; BMD, bone mineral density; BP, bisphosphonates; CCT, calcitonin; ETD, etidronate; Fx, fracture; GCC, glucocorticoids; HT, hormone therapy; IBD, ibandronate; MPR, medication possession ratio; NA, not applicable; NP, data not provided; OP, osteoporosis; PDC, proportion of days covered; RAL, raloxifene; RSD, risedronate; Rx, prescription.
Study only evaluated females.
Study separated cohorts into those with and without a prior fracture (primary and secondary prevention).
Confounder considered –however it is unclear what was included in the final adjusted analysis.
Table 3.
Summary of methodological characteristics of nested case–control studies.
| [Blouin et al. 2008] | [Cotte et al. 2008] | [Meijer et al. 2008] | [Rabenda et al. 2008] | [Weycker et al. 2007] | |
|---|---|---|---|---|---|
| Treatment | ALD, RSD | ALD, RSD, ETD, RAL, SR | ALD, RSD, ETD | ALD | ALD, RSD, CCT, HT, RAL |
| Evaluated drug class separately | NA | No | NA | NA | No |
| Baseline (lookback) period | 2 years | NP | 1 year | 3 months | 6 months |
| Period of covariate identificationa | 1) Baseline: 1 year for all study covariates (prior fx 2 years) 2) Follow-up: comorbidities |
1) Baseline 2) Follow-up: comorbidities, comedications and supplements in year prior to fx |
1) Baseline 2) Follow-up: Use of non-OP comedications |
Baseline only | 1) Baseline 2) Follow-up: Age at fx date |
| New users (drug) | Yes (all OP drugs) | Yes (all OP drugs) | Yes (any BP) | Yes (Any BP or RAL) | Yes (all OP drugs) |
| Allowed switching | No censored switchers | Yes | NP | No excluded switchers | No excluded switchers |
| >1 Rx needed | No | No | 1 + in two years before outcome | No | No |
| Cases –start of fracture identification | Fx at least 1 year after initiation | Fx 90 days after initiation | Fx 3 months after initiation | First fx after initiation | Fx 90 days after initiation |
| Matching of controls | |||||
| Age | Yes (+/−1yr) | Yes | No | Yes | Yes (+/−3 yr) |
| Sex | Yes | Yes | Yes | Yes | Yes |
| Treatment | Yes | No | Yes | Yes | Yes |
| Index Date | Unclear | Yes | Unclear | Yes | Yes |
| Duration of Follow-up | Yes | Yes | Yes | Yes | Yes |
| Minimum confounders for adjustment in model | |||||
| Age | No (matched) | No (matched) | Yes | No (matched) | Yes |
| Prior fracture | Yes | Yes | Yes | No | Yes |
| Comorbidity Score | Yesb | No | No | No | No |
| Immeasurable time | Assumed 100% compliance | NP | NP | NP | Assumed 100% compliance |
| Compliance measure | PDC | MPR | PDC | PDC | MPR |
| How compliance quantified (1 = Primary 2 = Secondary) | 1) ≥80% 2) <50% vs. ≥ 50%, <90% vs. ≥ 90% |
1) <20%, 20–<40%, 40–<60%, 60–<80%, ≥ 80% 2) Used ROCs to generate threshold |
≥ 80% | 1) 1 Year PDC ≥ 80% 2) Continuous 0–100% |
<30%, 30–69%, 70–89%, ≥ 90% |
ALD, alendronate; BP, bisphosphonates; CCT, calcitonin; ETD, etidronate; Fx, fracture; GCC, glucocorticoids; HT, hormone therapy; IBD, ibandronate; MPR, medication possession ratio; NA, not applicable; NP, data not provided; OP, osteoporosis; PDC, proportion of days covered; RAL, raloxifene, RSD, risedronate; Rx, prescription; SR, strontium ranelate; Unclear, not explicitly stated in the manuscript, however plausible based on the text.
Covariates identified based on 1) ‘baseline’ – data collected during pretreatment index (lookback) period; or 2) ‘follow-up’ – data collected after treatment index date or as time varying.
Confounder considered – however, it is unclear what was included in the final adjusted analysis.
Baseline (lookback) period, restriction to new users and drug exposure
Baseline periods used to identify prior use for exclusion and covariates for adjustment ranged from 3 months to 2 years; three studies (21%) did not provide the length of the baseline period or used variable lengths depending on date of database entry (Table 4). Two studies did not restrict inclusion to new users: both were cohort studies and adjusted for prior osteoporosis drug use as a covariate in their analysis [Blouin et al. 2008; Huybrechts et al. 2006]. Ten articles (71%) examined bisphosphonates exclusively and the other four also considered treatments such as calcitonin, hormone therapy, raloxifene, and strontium ranelate. None of the four studies that examined multiple therapies considered drug classes separately [Cotte et al. 2008; Weycker et al. 2007; Huybrechts et al. 2006; Caro et al. 2004].
Table 4.
Summary of methodological considerations by study design and overall.
| Cohort (n = 9) | Case–control (n = 5) | All studies (n = 14) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Drug exposure | |||
| Bisphosphonate only | 7 (78) | 3 (60) | 10 (71) |
| Bisphosphonate or other osteoporosis drug | 2 (22) | 2 (40) | 4 (29) |
| Baseline prior to index date to define covariates and prior use | |||
| ≤6 months | 3 (33) | 2 (40) | 5 (36) |
| 1 year | 3 (33) | 1 (20) | 4 (29) |
| 2 years | 1 (11) | 1 (20) | 1 (7) |
| variable/not provided | 2 (22) | 1 (20) | 3 (21) |
| New user design | 7 (78) | 5 (100) | 12 (86) |
| Bisphosphonates | 6 (67) | 1 (20) | 7 (50) |
| All osteoporosis drugs | 1 (11) | 4 (80) | 5 (36) |
| Need >1 prescription for inclusion | 2 (22) | 1 (20) | 3 (21) |
| Accounted for switching | 4 (44) | 4 (80) | 8 (57) |
| Allowed between bisphosphonates | 3 (33) | 0 | 3 (21) |
| Allowed any switching | 1 (11) | 1 (20) | 2 (14) |
| Excluded switchers | 0 | 2 (40) | 2 (14) |
| Censored switchers | 0 | 1 (20) | 1 (7) |
| Accounted for immeasurable time | |||
| Assumed 100% compliance | 2 (22) | 2 (40) | 4 (29) |
| Follow-up time for fracture identification began | |||
| 90 days | 1 (11) | 3 (60) | 4 (29) |
| 6 months | 3 (33) | 0 | 3 (21) |
| 1 year | 1 (11) | 1 (20) | 2 (14) |
| Any | 4 (44) | 1 (20) | 5 (36) |
| Compliance measurement method | |||
| Ascertainment period | 0 | ||
| Entire follow-up period | 6 (67) | ||
| Time varying compliance | 3 (33) | ||
| Primary method of quantifying compliance | |||
| ≥80% | 7 (78) | 3 (43) | 10 (71) |
| >2 Categorical groups | 1 (11) | 2 (40) | 3 (21) |
| Continuous (linear regression) | 1 (11) | 0 | 1 (7) |
| Minimum confounders for adjustment in model | |||
| Age | 9 (100) | 2 (40)a | 11 (79) |
| Prior fracture | 9 (100) | 4 (80) | 13 (93) |
| Sex | 9 (100) | 5 (100)b | 14 (100) |
| Comorbidity score | 2 (22) | 0 | 2 (14) |
| Case–control matching | |||
| Age | 4 (80) | ||
| +/−1 year | 1 (20) | ||
| +/−3 years | 1 (20) | ||
| No specification | 2 (40) | ||
| Drug class | 4 (80) | ||
| Sex | 5 (100)b | ||
| Date | 3 (60)c | ||
| Follow up | 5 (100) | ||
| All five criteria | 2 (40) | ||
Index date defined as date of treatment initiation.
Does not consider matching.
Studies only evaluated females.
Three clearly matched on date: it is unclear whether the other two case–control studies matched on date.
Compliance measurement
Five studies capped compliance at 100% and thus clearly measured PDC. Half (n = 7) of the articles did not discuss switching between agents. Of the eight articles that mention switching, all four cohort studies permitted switching between eligible agents (three examined only bisphosphonates), two case–control studies excluded switchers, one case–control study censored on switch date and the other case–control study permitted switching between the many agents considered. No study used only an ascertainment period, yet one considered various ascertainment periods within a sensitivity analysis [Caro et al. 2004]. Four studies (29%) reported on immeasurable time, each assumed perfect compliance during hospitalization.
Six of the nine cohort studies examined compliance over the entire follow-up period. The other three cohort studies used a time-varying measure of compliance: two considered 90-day intervals [Curtis et al. 2008b; Penning-van Beest et al. 2008], and one used periods of 1 year [Briesacher et al. 2007]. Ten studies (71%) quantified compliance as a dichotomy of ≥80%, three (21%) used various categorical groups in their primary analysis, and one used linear regression across the entire range of PDC values (Table 4). A single study utilized receiver operating characteristic curves to determine the threshold within their analysis [Cotte et al. 2008].
Control for confounders and matching
Most studies (n = 9, 64%) excluded fractures occurring during the initial period after the index date, with periods ranging from 90 days to 1 year after treatment initiation. All nine cohort studies controlled for age, sex and prior fracture. Of these, one study separated the analysis into primary and secondary prevention cohorts [Sheehy et al. 2009]. All of the cohort studies additionally adjusted for at least one comorbidity or drug, yet only two (22%) adjusted for comorbidity score. Two studies considered the number of medications taken, however it is not clear whether or not this covariate was included in the final adjusted model that examined the association between compliance and fracture risk [Blouin et al. 2008; Gallagher et al. 2008]. The five case–control studies were restricted to women and matched on the length of follow up. Four also matched on age and three clearly matched on index date. Only one study considered a theoretical sensitivity analysis to account for potential healthy adherer bias [Blouin et al. 2008], yet all mentioned limitations related to possible residual confounding.
Discussion
Pharmacoepidemiologic methods are advancing with guidelines regarding good pharmacoepidemiologic practice [Schneeweiss, 2009; Stürmer et al. 2008] and adherence research [Gwadry-Sridhar et al. 2009; Cramer et al. 2007; Peterson et al. 2007] only recently published. We report wide variability in the methods of prior studies that examine the relationship between osteoporosis treatment compliance and fracture risk. To help guide future research, we summarize our recommendations in the following paragraphs.
At minimum, we suggest that the ideal cohort study will: (1) use a new user design, (2) have a minimum 1-year baseline period to exclude prior drug exposure, (3) evaluate each drug class separately, and (4) adjust for fracture risk factors. We also recommend that studies consider restricting inclusion to individuals with at least two prescriptions filled, include switchers within the same drug class and drug efficacy, document the potential for immeasurable time bias, consider a period of drug onset prior to fracture identification, and provide a rationale for how compliance is quantified. Nested case–control studies require similar methodological considerations when selecting the source population that defines the nest. We recommend that case–control studies match cases to controls on: sex, drug class/efficacy, index date and length of follow up. If a case–control study matches on age, we recommend matching within 1 year of age. Case–control studies should additionally control for baseline risk for fracture, such as age, prior fracture and comorbidity, in their analysis.
Baseline (lookback) period and restriction to new users
The new user design is essential when studying drug effects [Schneeweiss, 2009; Ray, 2003]. The most conservative method is to restrict inclusion to those with no prior osteoporosis treatment. However, when studying bisphosphonates, the most commonly prescribed agents, excluding those with prior bisphosphonate use and adjusting for use of other osteoporosis medications may be appropriate. Nonetheless, given that patients largely switch between agents due to adverse drug events or ineffectiveness of the first-line drug therapy, excluding any history of osteoporosis pharmacotherapy other than hormone therapy may be most prudent. All but the first two studies [Huybrechts et al. 2006; Caro et al. 2004] employed a new user design, suggesting that quality is improving over time.
The length of baseline period used to identify prior drug use is important because many patients who stop osteoporosis pharmacotherapy will reinitiate treatment after an extended gap [Roughead et al. 2009; Brookhart et al. 2007a; Melo et al. 2006]. It is estimated that 30% of patients who discontinue osteoporosis pharmacotherapy, defined by a gap of 60 days or more, will reinitiate treatment within 6 months of discontinuation, and 40% will reinitiate osteoporosis treatment within 1 year of discontinuation [Brookhart et al. 2007a]. We therefore recommend a minimum of 1-year baseline period to define incident users.
Switching between drugs and minimum drug exposure
Most patients switch between osteoporosis drugs due to adverse drug events [Papaioannou et al. 2007]. Switching between agents may be permitted provided drug efficacy is interchangeable. Little evidence suggests that alendronate is more effective than risedronate, and thus switching between these agents may be appropriate [Curtis et al. 2009; Cadarette et al. 2008; MacLean et al. 2008]. However, due to the varying mechanisms of action between therapies, one should take caution when evaluating individuals who switch between different therapy groups (e.g. bisphosphonate to raloxifene) [MacLean et al. 2008; Peterson et al. 2007]. Another option observed is to allow switching but control for the starting treatment regimen [Huybrechts et al. 2006]. In addition, it may be prudent to restrict inclusion to those with a minimum of two drug dispensings. A single treatment dispensing could indicate no drug exposure, be attributed to unmeasured factors such as an adverse side effect that resulted in immediate drug discontinuation, or be a marker of competing illness or unmeasured frailty. Alternatively, filling more than a single prescription could be a marker of unmeasured healthy behaviors [Brookhart et al. 2007b]. Given that there is little potential fracture reduction within the first 3–6 months of exposure [Harrington et al. 2004; Pols et al. 1999], including only patients with a minimum of two drug dispensings may better reflect the effects of drug compliance on fracture risk versus unmeasured factors attributed to only a single prescription.
Immeasurable time
In many healthcare utilization databases, drugs dispensed in hospital or long-term care may be covered by different drug plans and thus these data are not available for analysis. Periods of incomplete drug information is labeled ‘immeasurable time’, and may have an impact on estimated compliance [Suissa, 2008]. For example, one may falsely associate poor compliance due to immeasurable time during hospitalizations with increased fracture rates. All studies that adjusted for immeasurable time assumed that patients were provided with and fully compliant to all medications during their hospital stay, and that previous supplies were continued upon discharge. This method is the most conservative approach because if patients did not receive therapy during hospitalization, then the association between better compliance and fracture reduction would be underestimated, particularly given that patients hospitalized may be the most frail and likely to fracture. Another strategy may be to assume no treatment during hospitalization and consider full coverage in a sensitivity analysis. The best method to adjust for potential immeasurable time is database specific and will depend on the extent of missing data. In addition to periods of missing drug data during hospitalization, some databases may be susceptible to immeasurable time if it is difficult to capture when drug coverage eligibility changes, such as eligibility for Medicaid. Although not all healthcare utilization databases are susceptible to immeasurable time, it is important to discuss potential immeasurable time and clarify when it is not relevant.
Drug exposure and method of compliance measurement
Each drug class used to treat osteoporosis (bisphosphonates, calcitonin, hormone therapy, raloxifene, strontium ranelate, teriparatide), has a unique mechanism of action on bone, require different dosing regimens, and differ in fracture reduction efficacy [MacLean et al. 2008; O’Donnell et al. 2006; Cranney et al. 2002]. When considering the impact of compliance to osteoporosis treatment on fracture risk, it is thus important to consider differences in drug action and efficacy. Pharmacology is also important when considering how to examine the impact of compliance to osteoporosis medication on fracture risk. Osteoporosis medication largely works by improving bone mineral density (BMD) and thus strengthening bone to withstand minimal trauma. Some agents, such as raloxifene, impact BMD by reducing bone loss during drug exposure. The bisphosphonates not only reduce bone loss during drug exposure, but they increase bone mass and persist in bone with a long half-life. Given that bisphosphonates persist in bone, a threshold effect may be relevant [Geusens, 2009]. However, most studies measured compliance over the entire follow-up period and defined compliance as a dichotomous variable (≥80%), and none defined this dichotomy based upon pharmacological or clinical evidence [Andrade et al. 2006]. In one case–control analysis, it was determined that optimal fracture prediction was observed at a PDC threshold of 68% rather than the standard use of 80% [Cotte et al. 2008]. Further, three studies utilized time-varying compliance [Curtis et al. 2008b; Penning-van Beest et al. 2008; Briesacher et al. 2007] and one documented that a nontime-dependent analysis overestimated the effect of compliance on fracture risk [Curtis et al. 2008b]. More research is important to help guide the use of appropriate cut points and this is currently being reviewed by ISPOR [International Society for Pharmacoeconomics and Outcomes Research, 2009].
Fracture identification after a treatment onset period
Requiring a 3–6 month treatment-onset period prior to fracture identification permits time for bisphosphonates to increase BMD and strengthen bone [Harrington et al. 2004; Pols et al. 1999]. In addition, a treatment onset period may provide strength in ability to identify incident fractures distinct from follow up for prevalent fractures, particularly if fracture rates are identified based only on diagnostic codes. Another strategy to improve the validity of incident fracture rates may be to require diagnostic and procedural codes within a defined time period [Ray et al. 1992].
Confounders
When studying the effect of any exposure on fracture risk, it is important to control for potential confounders. An independent risk factor for osteoporotic fracture is a confounder when its prevalence is imbalanced between drug compliance groups under comparison. By failing to control for a confounding factor, the confounder’s effect on fracture risk is falsely attributed to drug compliance. Major risk factors for fracture include age over 65, sex, low BMD, low body weight, prior fracture, frailty/falls risk, and comorbidities or concomitant medications related to fracture risk. Calcium and vitamin D supplementation and weight-bearing exercise may also impact BMD and fracture risk.
All cohort studies controlled for each of age, sex, and prior fracture, and at least one other comorbidity or drug. However, BMD, body weight, lifestyle factors and measures of frailty are typically not available in healthcare utilization databases, and were not included in the studies reviewed. Prior work identifies that better compliance to placebo reduces mortality [Granger et al. 2005] and hip fracture risk [Curtis et al. 2008b]. It is therefore plausible that potential residual confounding exists when studying adherence to osteoporosis treatment on fracture risk. However, recent data suggest that there may be less room for healthy adherer effects to bias results in a homogeneous cohort of frail seniors taking osteoporosis medication to reduce fracture risk [Cadarette et al. 2010]. More research is needed to clarify the potential for healthy adherer bias to inflate the association between better compliance to osteoporosis pharmacotherapy and fracture risk reduction. Each of the 14 studies reviewed appropriately discussed limitations due to possible residual confounding, with more recent studies emphasizing the potential for healthy adherer bias. Theoretical sensitivity analyses that consider the extent of unmeasured confounding needed to explain results are encouraged [Schneeweiss, 2006], and was completed in one study [Blouin et al. 2008].
Future research directions
Future research is warranted to investigate when, or if, treatment with bisphosphonates may be discontinued without impacting fracture risk. Most papers included in this review considered the impact of compliance within 1–2 years; however, these therapies persist in bone for extended periods after treatment discontinuation [Geusens, 2009; Watts et al. 2008; Rodan et al. 2004]. Perhaps the best, most-compelling evidence comes from the Fracture Intervention Trial Long-term Extension (FLEX) study. FLEX identified that the majority of women who discontinued treatment with alendronate after 5 years of therapy had no significant increase in morphometric vertebral fracture risk compared to those who continued treatment for up to 5 years post-treatment [Black et al. 2006]. Recent post-hoc subgroup analyses, however, suggest that the effect depends on vertebral fracture history and BMD after 5 years of treatment: women with no vertebral fracture after 5 years of alendronate treatment, yet BMD T-score ≤ −2.5 were found to have lower nonvertebral fracture risk (relative risk = 0.50, 95% confidence interval = 0.26–0.96) after continuation of alendronate for an additional 5 years [Schwartz et al. 2010]. Nonetheless, there was little evidence of nonvertebral fracture reduction among women with higher BMD levels or with prior vertebral fracture, and little difference in morphometric vertebral fracture risk was identified after an additional 5 years of alendronate treatment. Further research is important to clarify these findings. Evidence from a real-world cohort study found that the increased risk of hip fracture following discontinuation of bisphosphonates was attenuated among women with higher compliance (e.g. PDC ≥ 80% at 2 years), as well as with longer duration of treatment persistence before treatment discontinuation [Curtis et al. 2008a]. It is not clear, however, how long a patient must persist with therapy or how compliant they need to be before a physician-directed drug holiday may be permitted. The next greatest challenge may thus not be how to quantify compliance and the impact of compliance on fracture risk in general, but rather to determine if, when, how long and among which patients a physician-directed drug holiday may be appropriate.
Conclusion
In summary, we have identified great variability in methods of prior studies and make several recommendations to inform future analyses. As pharmacoepidemiologic methods evolve and we gain a better understanding of the pharmacological effects of different osteoporosis medications, our ability to estimate the effects of compliance to osteoporosis pharmacotherapy on fracture risk and identify if, or when, a physician-directed drug holiday may be appropriate, will improve.
Acknowledgments
Funding
This research was supported by the Canadian Institutes of Health Research (CIHR, CPO-94434). Dr Cadarette holds a CIHR New Investigator Award in the Area of Aging and Osteoporosis (MSH-95364) and Milica Nikitovic receives support from the Toronto Health Economics and Technology Assessment (THETA) collaborative.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Milica Nikitovic, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada.
Daniel H. Solomon, Divisions of Rheumatology, Immunology, and Allergy, Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Suzanne M. Cadarette, Email: s.cadarette@utoronto.ca, Leslie L. Dan Pharmacy Building, University of Toronto, 144 College Street, Toronto, ON, Canada.
References
- Adami S, Isaia G, Luisetto G, Minisola S, Sinigaglia L, Gentilella R, et al. Fracture incidence characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res. 2006;21:1565–1570. doi: 10.1359/jbmr.060715. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- Anonymous, editor. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- Blouin J, Dragomir A, Moride Y, Ste-Marie LG, Fernandes JC, Perreault S. Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol. 2008;66:117–127. doi: 10.1111/j.1365-2125.2008.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesacher BA, Andrade SE, Yood RA, Kahler KH. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882–887. doi: 10.1016/j.bone.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007a;120:251–256. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank WS, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health care services: an investigation of the healthy user effect. Am J Epidemiol. 2007b;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Patrick AR, Shrank WH, Dormuth CR. Validating studies of adherence through the use of control outcomes and exposures. Am J Hypertens. 2010;23:110. doi: 10.1038/ajh.2009.232. [DOI] [PubMed] [Google Scholar]
- Cadarette SM, Burden AM. Measuring and improving adherence to osteoporosis pharmacotherapy. Curr Opin Rheumatol. 2010;22:397–403. doi: 10.1097/BOR.0b013e32833ac7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadarette SM, Katz JN, Brookhart MA, Stürmer T, Stedman MR, Solomon DH. Relative effectiveness of osteoporosis drugs for preventing non-vertebral fracture. Ann Intern Med. 2008;148:637–646. doi: 10.7326/0003-4819-148-9-200805060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadarette SM, Solomon DH, Katz JN, Patrick AR, Brookhart MA. Adherence to osteoporosis drugs and fracture prevention: no evidence of healthy adherer bias in a frail cohort of seniors. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1309-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–1008. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- Cotte FE, Mercier F, De Pouvourville G. Relationship between compliance and persistence with osteoporosis medications and fracture risk in primary health care in France: a retrospective case-control analysis. Clin Ther. 2008;30:2410–2422. doi: 10.1016/j.clinthera.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Silverman SL, Gold DT. Methodological considerations in using claims databases to evaluate persistence with bisphosphonates for osteoporosis. Curr Med Res Opin. 2007;23:2369–2377. doi: 10.1185/030079907X226311. [DOI] [PubMed] [Google Scholar]
- Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, et al. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Rev. 2002;23:570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008a;19:1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008b;23:1435–1441. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JR, Westfall AO, Cheng H, Saag KG, Delzell E. RisedronatE and ALendronate Intervention over Three Years (REALITY): minimal differences in fracture risk reduction. Osteoporos Int. 2009;20:973–978. doi: 10.1007/s00198-008-0772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AC, Weycker D, Nichols GA, Oster G, Rosales G, Boardman DL, et al. Effectiveness of bisphosphonate therapy in a community setting. Bone. 2009;44:153–159. doi: 10.1016/j.bone.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23(10):1569–1575. doi: 10.1359/jbmr.080510. [DOI] [PubMed] [Google Scholar]
- Geusens P. Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep. 2009;7:12–17. doi: 10.1007/s11914-009-0003-6. [DOI] [PubMed] [Google Scholar]
- Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F. A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin. 2007;23:585–594. doi: 10.1185/030079906X167615. [DOI] [PubMed] [Google Scholar]
- Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44:471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
- Gwadry-Sridhar FH, Manias E, Zhang Y, Roy A, Yu-Isenberg K, Hughes DA, et al. A framework for planning and critiquing medication compliance and persistence research using prospective study designs. Clin Ther. 2009;31:421–435. doi: 10.1016/j.clinthera.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Harrington J, St-Marie LG, Brandi M, Civitelli R, Fardellone P, Grauer A, et al. Risedronate rapidly reduces the risk for nonvertebral fracture in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- Hoer A, Seidlitz C, Gothe H, Schiffhorst G, Olson M, Hadji P, et al. Influence on persistence and adherence with oral bisphosphonates on fracture rates in osteoporosis. Patient Preference Adherence. 2009;3:25–30. [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2009 doi: 10.1007/s00198-009-1134-4. in press. [DOI] [PubMed] [Google Scholar]
- International Society for Pharmacoeconomics and Outcomes Research. ISPOR medication compliance and persistence Special Interest Group (SIG) analysis standards. 2009. [accessed December 2009]. http://wwwispororg/sigs/SIGProject-list12-08pdf.
- Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271–287. doi: 10.1016/j.maturitas.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Meijer WM, Penning-van Beest FJA, Olson M, Herings RM. Relationship between duration of compliant bisphosphonate use and the risk of osteoporotic fractures. Curr Med Res Opin. 2008;24:3217–3222. doi: 10.1185/03007990802470241. [DOI] [PubMed] [Google Scholar]
- Melo M, Qiu F, Sykora K, Juurlink D, Laupacis A, Mamdani M. Persistence with bisphosphonate therapy in older people. J Am Geriatr Soc. 2006;54:1015–1016. doi: 10.1111/j.1532-5415.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Morin S, Rahme E, Behlouli H, Tenenhouse A, Goltzman D, Pilote L. Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporos Int. 2007;18:1625–1632. doi: 10.1007/s00198-007-0421-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY. Strontium ranelate for preventing and treating postmenopausal osteoporosis. Cochrane Database Systematic Rev. 2006;4:CD005326. doi: 10.1002/14651858.CD005326.pub2. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Ioannidis G, Adachi JD, Sebaldt RJ, Ferko N, Puglia M, et al. Adherence to bisphosphonates and hormone replacement therapy in a tertiary care setting of patients in the CANDOO database. Osteoporos Int. 2003;14:808–813. doi: 10.1007/s00198-003-1431-2. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging. 2007;24:37–55. doi: 10.2165/00002512-200724010-00003. [DOI] [PubMed] [Google Scholar]
- Penning-van Beest FJA, Erkens JA, Olson M, Herings RMC. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2008;19:511–517. doi: 10.1007/s00198-007-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Internal Med. 2008;149:404–415. [PubMed] [Google Scholar]
- Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10:2303–2315. doi: 10.1517/14656560903140533. [DOI] [PubMed] [Google Scholar]
- Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- Rietbrock S, Olson M, van Staa TP. The potential effects on fracture outcomes of improvements in persistence and compliance with bisphosphonates. Q J Med. 2009;102:35–42. doi: 10.1093/qjmed/hcn130. [DOI] [PubMed] [Google Scholar]
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004;20:1291–1300. doi: 10.1185/030079904125004475. [DOI] [PubMed] [Google Scholar]
- Roughead EE, Ramsay E, Priess K, Barratt J, Ryan P, Gilbert AL. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18:69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S. On guidelines for comparative effectiveness research using nonrandomized studies in secondary data sources. Value Health. 2009;12:1041. doi: 10.1111/j.1524-4733.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX trial. J Bone Miner Res. 2010;25:976–982. doi: 10.1002/jbmr.11. [DOI] [PubMed] [Google Scholar]
- Sheehy O, Kindundu C, Barbeau M, LeLorier J. Adherence to weekly oral bisphosphonate therapy: cost of wasted drugs and fractures. Osteoporos Int. 2009;20:1583–1594. doi: 10.1007/s00198-008-0829-2. [DOI] [PubMed] [Google Scholar]
- Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- Siris ES, Selby PL, Saag KG, Borgström F, Herings RMC, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Stürmer T, Rothman KJ, Avorn J. Pharmacoepidemiology and “in silico” drug evaluation: is there common ground? J Clin Epidemiol. 2008;61:205–206. doi: 10.1016/j.jclinepi.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168:329–335. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- van den Boogaard CHA, Breekveldt-Postma NS, Borggreve SE, Goettsch WG, Herings RMC. Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin. 2006;22:1757–1764. doi: 10.1185/030079906X132370. [DOI] [PubMed] [Google Scholar]
- Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19:365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271–277. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- Wilkes MM, Navickis RJ, Chan WW, Lewiecki EM. Bisphosphonates and osteoporotic fractures: a cross-design synthesis of results among compliant/persistent postmenopausal women in clinical practice versus randomized controlled trials. Osteoporos Int. 2010;21:679–688. doi: 10.1007/s00198-009-0991-1. [DOI] [PubMed] [Google Scholar]