Abstract
The identification of a stable pool of progenitor/stem cells in the adult pituitary has renewed the interest of identifying mechanisms for maintenance of pituitary cells throughout life. Whereas developmental studies have shown that progenitor expansion is the major source of new differentiated cells during pituitary organogenesis, the contribution of these progenitors for maintenance of the adult tissue is not clear although progenitors were clearly involved in cell expansion following end-organ ablation, notably after adrenalectomy and/or gonadectomy. We have used a genetic trick that eliminates dividing cells by apoptosis in order to assess the contribution of differentiated corticotropes and melanotropes for maintenance of their population in the adult pituitary. The system relies on chromosome instability created by the action of the Cre recombinase on inverted loxP sites. Expression of Cre recombinase in corticotropes and melanotropes led to progressive loss of corticotropes whereas melanotropes were unaffected. Because the Cre transgene is not expressed in progenitors, the data indicate that maintenance of the adult corticotrope pool is primarily due to self-duplication of differentiated cells. In contrast, melanotropes do not divide. Maintenance of corticotropes by self-duplication contrasts with the reported proliferative response of undifferentiated cells observed after adrenalectomy. If corticotrope reentry into cell cycle constitutes a normal mechanism to maintain the adult corticotrope pool, this same mechanism may also be perturbed during corticotrope adenoma development in Cushing’s disease.
The control of cell proliferation is critical during development in order to determine organ size and cell composition of complex tissues. As in most tissues, the developing pituitary contains undifferentiated proliferating cells during every phase of organogenesis. Indeed, fetal pituitary development requires expansion of a pool of progenitor cells that later differentiate (1, 2). Interestingly, the control of progenitor cell proliferation appears to be quite different by comparison to differentiated pituitary cells because pituitary progenitors exit the cell cycle under the action of the Cip/Kip cell cycle inhibitor p57Kip2 whereas differentiated pituitary cells are prevented from entering the cycle by the related p27Kip1 (3, 4).
Analyses of proliferating cells during pituitary development and in adults have largely supported the idea that most pituitary cell expansion is due to division of undifferentiated cells (5). However, division of differentiated cells has also been observed at low frequency (6, 7). Beyond the intrinsic interest of understanding pituitary cell cycle control, the mechanism(s) for maintenance of pituitary cell number either through proliferation of progenitors or by division of differentiated cells has quite different implications in the context of pituitary tumor development.
Significant insight was provided in recent years on the nature of pituitary progenitors, their expansion, and their entry into differentiation (8). In particular, a population of Sox2-positive cells was described in the developing and adult pituitary (9), and their ability to self-renew is consistent with the model that they represent pituitary stem or progenitor cells (10). Sox2-positive cells are maintained around the cleft of the adult pituitary that separates anterior (AL) from intermediate (IL) lobes. These cells have the ability to form self-replicating “pituispheres” in vitro that can be induced to differentiate into each pituitary lineage (9, 10). When they enter differentiation, the cells appear to go through different phases of differentiation that mimic the fetal developmental sequence (11, 12). Recently, pituitary organogenesis and cell differentiation were reconstituted in 3-dimensional cultures using self-organizing embryonic stem cells (13).
The adult Sox2-positive cells form a homotypic cell network in which most Sox2-positive cells maintain direct contacts with other Sox2-positive cells that line the pituitary cleft and, upon stimulation, cells appear to bud from the wedge junction between AL and IL (14). The organization of progenitor cells into a homotypic network is similar to the homotypic networks that have been described for differentiated cells of each pituitary lineage (15, 16).
Different experimental paradigms have supported the idea that acute demands on pituitary function are associated with expansion of pituitary progenitors and their subsequent differentiation into specific lineages. These paradigms have included end-organ ablation such as adrenalectomy (ADX) but also experimental cell ablation produced by various toxins (17). It has been known for a long time that end-organ ablation initially induces proliferation of undifferentiated cells (18–20). Following expansion, these cells undergo differentiation into the appropriate lineages: corticotropes following ADX and gonadotropes following gonadectomy (21). An interesting study showed that combined ADX and gonadectomy result in expansion of a pool of progenitors that may be common for differentiation into corticotropes and/or gonadotropes (21). This common progenitor pool hypothesis is consistent with the model that corticotrope and gonadotrope lineages share a common precursor (1, 22). However, nothing is known about the nature of the pool of progenitors that expand in these conditions or of the signals that are responsible for triggering their proliferation. Further, the mechanism that targets these expanded progenitors toward one or the other lineage also remains elusive.
The analyses of dividing cells, either following experimental manipulation such as ADX or in normal pituitary, have consistently revealed small numbers of mitotic cells that express differentiation markers (21), suggesting that differentiated pituitary cells may divide. The significance of this process remains obscure, however, and, in particular, its importance for tissue development or maintenance is unclear.
In the present work, we exploited a genetic model of replication-dependent apoptosis in order to address the importance of differentiated cell self-duplication in the pituitary. It was observed that cells containing chromosomes with inverted loxP sites would become unstable and undergo apoptosis when they express the Cre recombinase that acts on the loxP sites (23). The ability to target Cre expression in specific cells of the pituitary has allowed us to investigate the role of self-duplication in pituitary corticotropes and melanotropes. This was achieved with a POMC-Cre transgene that directed Cre expression in corticotropes and melanotropes (24). We now provide evidence that self-duplication of corticotropes is a predominant mechanism for corticotrope maintenance in the adult AL and that IL melanotropes do not self-duplicate significantly in the adult. Thus, adult pituitary corticotropes either arise from expansion of progenitors followed by corticotrope differentiation after ADX or from self-duplication for normal tissue maintenance.
Materials and Methods
Mice
Proopiomelanocortin (POMC)-Cre mice express the Cre transgene under control of the POMC promoter that targets expression in pituitary corticotropes and melanotropes (24, 25). These mice were crossed with mice homozygous for the invloxP allele (23), and resulting litters were humanely destroyed at different ages to assess to number of corticotropes. To further confirm that differentiated corticotropes can divide, we injected wild-type newborn mice with 30 μg/g bromodeoxyuridine (BrdU) (in PBS) ip and euthanized them 1 hour later. Animal experimentation was approved by the IRCM Animal Care and Use Committee, in conformity with regulations of the Canadian Council on Animal Care.
Immunofluorescence
Paraffin-embedded formalin-fixed embryos (embryonic d 15.5 [e15.5]), heads (postnatal d 0 [P0]), or pituitaries (7, 13, 19, 26, 37 wk and 2 y of age) were sectioned and subjected to immunofluorescence with specific antibodies against either Tpit (26), ACTH (CR1096M, Cortex Biochem, Concord, Massachusetts), SF-1 (gift of Dr K. Morohashi), Sox2 (4900S, Cell Signaling Technology, Danvers, Massachusetts) BrdU (G3G4, DSHB), Ki67 (Ab-5; Lab Vision,) or Cre (Novagen, Madison, Wisconsin). Nuclear staining was performed using Hoechst 33258. The total number of cells (Hoechst-positive nuclei) in AL and IL were counted using an in-house developed MATLAB (The MathWorks, Inc, Natick, Massachusetts) script, whereas antibody-stained cells were counted manually. Statistical significance was assessed with Student’s t test.
ADX
In order to ascertain the kinetics of progenitor proliferation following ADX, control mice were subjected to bilateral ADX and provided with 0.9% saline as drinking water. Mice were humanely destroyed at different times after surgery (0, 2, 4, 7, 11, and 14 d), and pituitaries were dissected, fixed in 10% formalin, paraffin embedded, and sectioned in frontal orientation. Thereafter, invloxP/+;POMC-Cre mice were either sham (n = 6) or ADX operated (n = 8), respectively, and maintained 15 days with 0.9% saline before being humanely destroyed. Pituitary tissues were prepared as described above.
Results
Dividing corticotropes
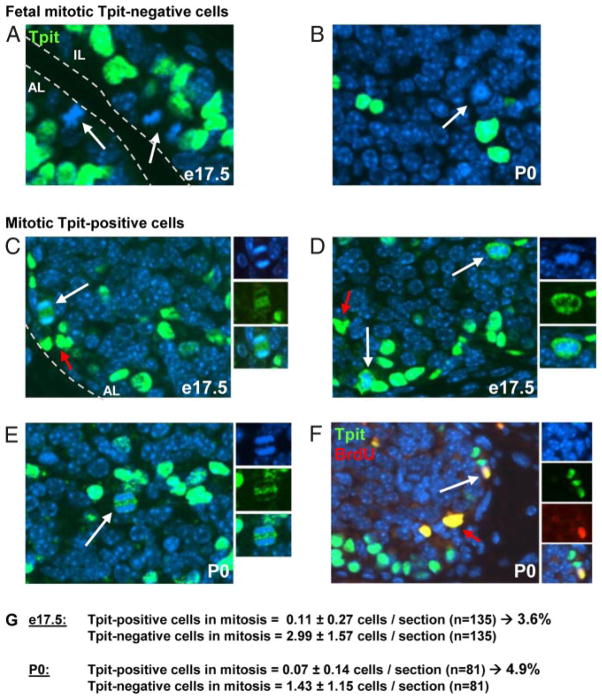
Prior reports had identified dividing differentiated pituitary cells (5–7); however, these are rare occurrences in adult tissues. For example, Taniguchi et al. (5) report 1%–2% BrdU-positive corticotropes at 1 month of age. In order to assess the importance of self-duplication of differentiated pituitary cells, we investigated this possibility at birth when the pituitary undergoes a major cell expansion (24), but after the fetal-expansion phase that primarily involves undifferentiated cells (3). Because the label “self-renewal” is often used to refer to progenitors that divide to produce a copy of themselves with/without differentiation of a daughter cell, we used the term “self-duplication” in the present work to refer to the division of differentiated cells into similar daughter cells. We focused the study on POMC-expressing lineages, AL corticotropes, and IL melanotropes. The earliest marker for terminal differentiation of these lineages is the Tbox transcription factor Tpit that drives their terminal differentiation (22, 26, 27). As expected, the fetal e17.5 and P0 pituitary contains dividing cells with mitotic figures that are predominantly negative for Tpit (Figure 1, A and B); the bulk of these cells line the pituitary cleft (Figure 1A) where Sox2-positive progenitors are found (9). Some Tpit-negative mitotic cells are also observed within the AL (Figure 1B).
Figure 1.
Division of Differentiated Corticotrope Cells. A–E, e17.5 and P0 mouse pituitary sections were subjected to immunofluorescence using Tpit antibody (green) and nuclei counterstained with Hoechst (blue). A and B, Most dividing cells (arrows) are Tpit negative and located around the pituitary lumen (A). Tpit-negative mitotic cells are also observed within the AL (B). C–E, Mitotic Tpit-positive cells (white arrow) are also observed, and Tpit immunoreactivity (green) is excluded from condensed chromatin (blue). Red arrows indicate background autofluorescence by red blood cells. F, Colabeling of P0 pituitary sections for Tpit (green) and BdrU (blue). Tpit and BrdU colabeling indicates recent passage through S phase. G, Count of mitotic Tpit-positive and negative cells in e17.5 and P0 pituitary sections.
Interestingly, a low number of Tpit-positive dividing cells are present at both e17.5 (Figure 1, C and D) and P0 (Figure 1, E and F). Tpit immunoreactivity appeared largely excluded from condensed chromatin in these cells whether they are in anaphase (Figure 1, C and E) or in prometaphase/metaphase (Figure 1D). In order to further ascertain the status of Tpit-positive mitotic cells, we performed BrdU incorporation (1 h) in P0 mice and colabeled pituitary sections for BrdU and Tpit. As shown in Figure 1F, double-positive cells for Tpit and BrdU were readily observed, indicating that these cells have recently undergone S phase. Collectively, the data indicate that differentiated Tpit-positive corticotropes can divide and are not postmitotic.
In order to assess the relative contribution of self-duplication relative to division of undifferentiated cells in early pituitary development, we counted the number of Tpit-positive vs negative mitotic cells on sections of e17.5 and P0 pituitaries. Both showed about 4%–5% Tpit-positive mitotic cells, with a slightly greater proportion at P0 compared with e17.5 (Figure 1G). It is noteworthy that all, but one, Tpit-positive mitotic cells were observed in the AL rather than IL. Although these numbers are low, they are very significant when compared with the adult pituitary where it is very difficult to find mitotic cells, let alone Tpit-positive mitotic cells. This is consistent with a very slow turnover of adult pituitary cells.
Ablation of differentiated POMC cells
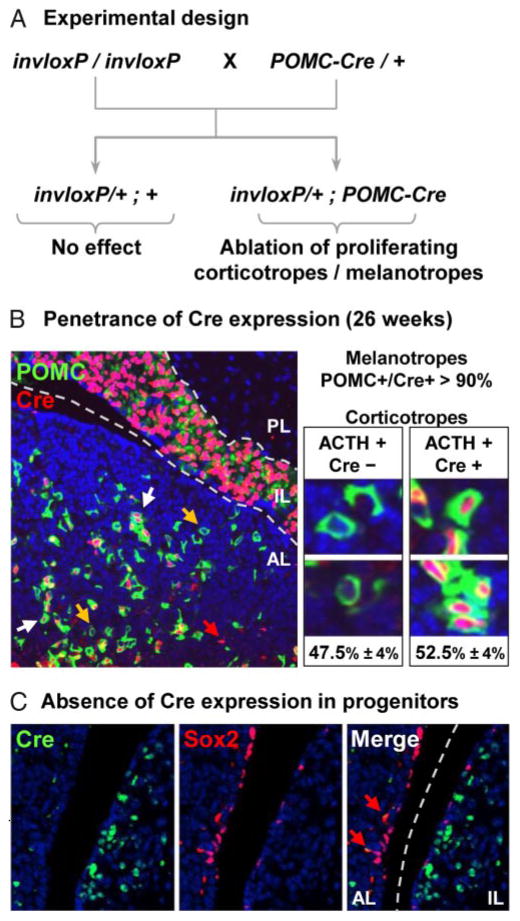
We took advantage of a genetic cell ablation system developed by Grégoire and Kmita (23) to assess the importance of self-duplication of differentiated POMC cells in pituitary tissue maintenance. This system relies on the presence of 2 loxP sites on chromosome 2, one being inserted with reverse orientation with respect to the other one, and on tissue-specific expression of the Cre recombinase. Recombination between these loxP sites (invloxP) leads to chromosome instability and apoptosis exclusively in dividing cells. Chromosome loss was observed during S or G2 phase before the onset of mitosis and may have triggered the apoptotic response (23). We crossed POMC-Cre transgenic mice with mice carrying the chr2 invloxP allele (Figure 2A). POMC-Cre expression relies on the activity of the −480 bp POMC promoter that targets exclusive expression to the 2 differentiated pituitary POMC lineages, with a slight preference for melanotropes (24, 25). This promoter is not active in other cells; in particular, it does not direct expression in hypothalamus consistent with the identification of independent and separate hypothalamus-specific enhancer sequences (28). In agreement with these prior analyses, the POMC-Cre transgene is expressed with high penetrance in IL but less in the AL (Figure 2B) as shown by coimmunofluorescence for Cre and POMC. It is noteworthy that all Cre-positive cells are also stained for POMC. Whereas POMC-Cre penetrance is almost complete in IL, double-positive cells account for 52.5 ± 4% of corticotropes (Figure 2B). This penetrance is similar to values observed previously using the same POMC promoter fragment (24, 25).
Figure 2.
Experimental Design for Assessment of Pituitary Tissue Maintenance. A, Schematic representation of mouse breeding used to generate invloxP/+;POMC-Cre mice. Homozygous female invloxP mice (23) were crossed with heterozygous male POMC-Cre mice. Mice carrying only the invloxP allele(s) are unaffected whereas mice carrying the invloxP allele will have chromosomal instability in cells expressing the POMC-Cre transgene, and these cells will be eliminated by apoptosis if they divide. B, The penetrance of POMC-Cre transgene expression was assessed by staining for POMC (green) and Cre (red) in 26-week-old pituitaries. Whereas most IL melanotropes are Cre positive, AL POMC-positive corticotropes were counted (≥ 100 cells per mouse) and scored for Cre immunoreactivity; we only counted cells with nuclei completely surrounded by POMC-positive cytoplasm. Transgene penetrance is about 53% in AL, and it is noteworthy that no ACTH-/Cre+ cells were detected. Yellow and white arrows indicate Cre-negative and Cre-positive corticotropes, respectively. Isolated red signal is caused by red blood cells autofluorescence (red arrow). C, Coimmunofluorescence staining of adult pituitary for Cre and Sox2 did not show any overlap (red arrow indicates nonspecifically stained red blood cells).
We also assessed putative expression of the POMC-Cre transgene in Sox2-positive pituitary progenitors (9). We observed no overlap between Cre and Sox2 expression in the pituitary (Figure 2C), as best visualized along the cleft that harbors numerous Sox2-positive progenitors.
Depletion of corticotropes in invloxP/+;POMC-Cre pituitaries
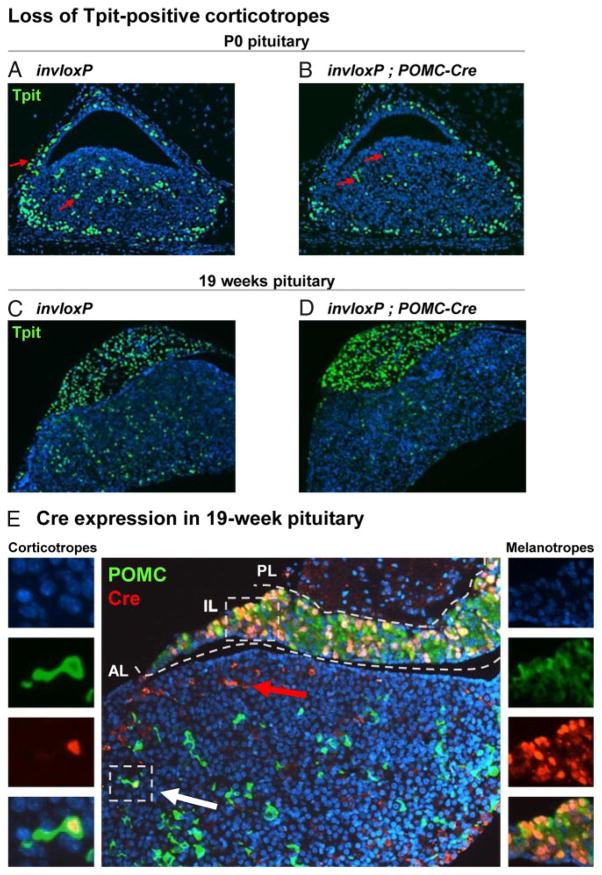
In order to evaluate the relative importance of differentiated POMC cell division for expansion and/or maintenance of corticotropes and melanotropes, we assessed Tpit-positive cells in invloxP/+;POMC-Cre pituitaries compared with invloxP. At birth (P0), there were fewer Tpit-positive cells in invloxP/+; POMC-Cre mice compared with in-vloxP pituitaries from the same litter (Figure 3, B compared with A) or to wild-type pituitaries (data not shown). Throughout these studies, there was no difference between wild-type and invloxP/+ mice: therefore, invloxP/+ littermates were used as control. By 19 weeks of age, the depletion of Tpit-positive cells was much more substantial in the AL of POMC-Cre transgene-carrying mice (Figure 3D) compared with invloxP pituitaries (Figure 3C). However, little difference was observed for IL melanotropes, suggesting that these cells are not sensitive to invloxP-dependent ablation. We verified that the POMC-Cre transgene is indeed still expressed in adult corticotropes and melanotropes, using coimmunofluorescence for POMC and Cre on sections of 19-week-old invloxP/+;POMC-Cre pituitaries. Very few double-positive cells were observed in the AL (Figure 3E), consistent with the interpretation that such cells will be lost by apoptosis if they ever undergo duplication. In contrast, most IL melanotropes coexpressed Cre and POMC (Figure 3E). The survival of Cre-expressing melanotropes suggests that these cells are protected from loxP-mediated apoptosis and/or rarely undergo cell division (23). Consistent with this latter hypothesis, mitotic melanotrope cells are extremely rare (a single mitotic melanotrope was observed in 216 separate sections!). Thus the data suggest that maintenance of AL corticotropes is quite dependent on self-duplication whereas IL melanotropes either 1) do not self-duplicate, 2) are renewed through replication of progenitors that then undergo differentiation, or 3) are maintained throughout adult life without renewal.
Figure 3.
Depletion of Corticotropes in invloxP/+;POMC-Cre Pituitaries. A–D, Sagittal pituitary sections stained for Tpit (green) and nuclear Hoechst (blue) reveal slight corticotrope loss in invloxP/+;POMC-Cre P0 pituitaries (B) compared with control invloxP littermates (A). A more severe depletion of corticotropes is observed at 19 weeks of age (D compared with C). Red arrows indicate background autofluorescence of red blood cells. E, POMC (green) and Cre (red) immunofluorescence staining of 19-week-old invloxP/+;POMC-Cre pituitaries revealed rare occurrence of Cre-positive corticotropes, in striking contrast to the expected 53% Cre penetrance (Figure 2B). On the contrary, IL melanotropes are mostly double positive for POMC and Cre (right insets).
We assessed the relative importance of self-duplication for maintenance of corticotropes by quantifying the number of Tpit-positive cells at different ages of development in invloxP/+;POMC-Cre pituitaries by comparison to in-vloxP/+ controls. For AL corticotropes, the data showed a modest loss of Tpit-positive cells at e15.5, suggesting that the bulk of fetal corticotrope accumulation depends on expansion of progenitors followed by differentiation. Nonetheless, self-duplication of differentiated cells is partly responsible for expansion of the fetal corticotrope pool. This mechanism contributes to about 10% of corticotropes in the invloxP/+;POMC-Cre e15.5 pituitary and increases to 20% at P0 (Figure 4A). At 37 weeks of age, progressive loss of corticotropes cumulated to more than 50% corticotrope depletion (Figure 4A), which corresponds to the proportion of total Cre-expressing corticotrope cells (Figure 2B). At this time point, no Cre-positive cells were detected in the AL (data not shown). The depletion of corticotropes did not progress further at 2 years of age (Figure 4A). It is striking that this depletion has not been compensated by recruitment of progenitors: indeed, invloxP/+;POMC-Cre pituitaries do not show increased proportion of Ki67-positive cells (data not shown). Thus, slow depletion of the AL corticotrope pool does not trigger a cell proliferation response as observed for other pituitary cells following acute cell loss (14) or following acute stimulation of corticotropes triggered by ADX (21). This is indeed supported by the unaltered abundance of Sox2-positive progenitors in invloxP/+; POMC-Cre pituitaries (Figure 4, C and D).
Figure 4.
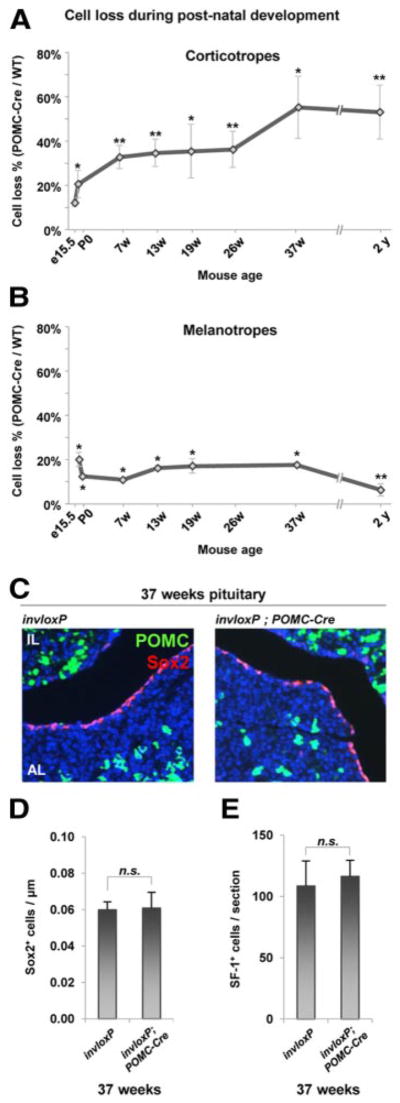
Self-Duplication Is the Major Mechanism for Adult Tissue Maintenance of Corticotropes, but Not Melanotropes. A, The proportion (%) of corticotropes in invloxP/+;POMC-Cre mice compared to control littermates was counted on pituitary sections at different ages of development after staining for Tpit and nuclei (Hoechst). Corticotrope depletion increased slowly and steadily with age, ranging from 12% at e15.5 to 55% at 37 weeks. B, Melanotrope cell number was also assessed on these sections. After a loss of melanotropes observed in early development, no further loss was observed throughout adult life. C, The distribution and number of Sox2-positive cells (in particular along the cleft) do not change in 37-week-old invloxP/+;POMC-Cre pituitaries compared with invloxP. D, Quantitation of Sox2-positive cells along the cleft of pituitaries described above (P = .886). E, Gonadotropes (steroidogenic factor 1 [SF-1]-positive), which were proposed to share a common precursor with corticotropes, are not affected (P = .325) by POMC-Cre expression at 37 weeks of age. Data are presented as means ± SEM (n = 3–7); * and ** indicate P ≤ .05 and P ≤ .01, respectively. n.s., not significant; WT, wild type.
Strikingly and consistent with the immunofluorescence data (Figure 3), quantitation of Tpit-positive IL melanotropes showed a loss of 10%–20% cells at e15.5 and P0, but no further loss till 37 weeks of age (Figure 4B). At 2 years of age, the invloxP/+;POMC-Cre IL had only 6% less melanotropes than control mice (Figure 4B). Because adult IL melanotropes maintain Cre expression (Figure 3E), this indicates that IL melanotropes do not self-duplicate. Maintenance of the melanotrope pool may thus rely principally on division of progenitors followed by their differentiation into melanotropes or, alternatively, these cells are not turning over in adult life.
In order to ensure that expression of the POMC-Cre transgene did not have pleiotropic effects in the pituitary AL, we assessed the number of steroidogenic factor 1-positive gonadotropes in invloxP and invloxP/+;POMC-Cre pituitaries and found this number to be unaltered in Cre-expressing pituitaries (Figure 4E). Because corticotropes and gonadotropes have been proposed to share a common precursor (22), this result is also consistent with exclusive expression of the POMC transgene in differentiated corticotropes and not in their precursors.
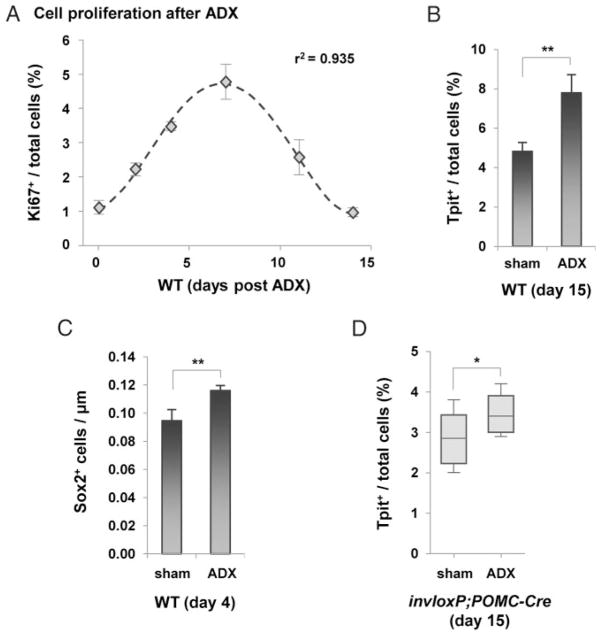
Previous studies indicated that the expansion of corticotropes observed after ADX depends mostly on expansion of progenitors followed by their differentiation (18 – 21). In order to contrast these observations with the self-duplication maintenance mechanism that we identified above (Figures 2– 4), we performed ADX in adult mice. Following ADX, we observed an increase of dividing Ki67-positive cells in the AL with a maximum increment observed at 7 days post-ADX and a return to baseline after 14 days (Figure 5A) in agreement with previous work (21). This increase in mitotic index results in an increase of AL Tpit-positive cells at 15 days post-ADX (Figure 5B and Ref. 29). As expected, ADX led to an increase 4 days after surgery of the Sox2-positive cells lining the cleft on the AL (but not IL) side of the lumen (Figure 5C). These dividing progenitors are presumably the major source of new Tpit-positive cells observed at day 15 (Figure 5B).
Figure 5.
Assessment of Progenitor and Corticotrope Expansion after ADX. A, Progenitor cell proliferation in pituitary following ADX was assessed using Ki67 immunostaining and found to follow a time course that is similar to that observed by Nolan et al. (21). The number of proliferating cells peaks at day 7 and is back to baseline after 14 days. B, Increase of Tpit-positive cells in ADX mice 15 days after surgery compared with sham-operated controls. C, Quantification of Sox2-positive cells along the pituitary cleft 4 days after surgery in sham and ADX mice (10 wk old). Note that the abundance of Sox2-positive cells is higher in these mice compared with the 37-week-old mice of Figure 4D: this reflects an age-dependent depletion of Sox2-positive cells. D, Proportion (%) of Tpit-positive corticotropes in AL 15 days after ADX compared with sham-operated mice. Despite a 40% corticotrope cell loss in the 26-week-old invloxP/+;POMC-Cre mice used for these experiments, the significant increase of corticotropes is consistent with expansion of progenitors followed by differentiation rather than self-duplication of corticotropes. Data are presented as means ± SEM (n = 3–7). * and ** indicate P ≤ .05 and P ≤ .01, respectively.
Sham surgery and ADX were performed on 26-week-old invloxP/+; POMC-Cre mice that had lost about 40% of their AL Tpit-positive corticotropes. Tpit-positive cells were counted 15 days after surgery (Figure 5D), ie, after ADX-induced proliferation (Figure 5A). A significant increase in Tpit-positive cells was observed in ADX invloxP/+; POMC-Cre pituitaries compared with sham controls (Figure 5D).
These data are consistent with previous reports on ADX showing the appearance of new corticotropes by division of progenitors followed by differentiation into corticotropes. They do not support a major role of corticotrope self-duplication following ADX although this mechanism is not completely excluded. It thus appears that maintenance of adult pituitary corticotropes relies primarily on self-duplication of differentiated cells whereas the acute response to ADX triggers progenitor expansion.
Discussion
The organs of complex organisms are composed of multiple cell types and the balance between cells of each lineage is critical for proper function, both in terms of tissue organization and cell number. This balance is established during development and is maintained throughout adult life. In adult tissues, cells need to be replaced as they become nonfunctional and are eliminated. In some tissues, such as gut and skin epithelia, this cell renewal is very frequent and can be measured, and the source of new cells is known because the relative contribution of differentiated cell division compared with expansion of progenitors and subsequent differentiation could be assessed experimentally. For tissues such as skeletal muscles, pools of reserve progenitors, the satellite cells, are recruited for tissue repair after injury. However, some tissues have very slow rates of cell renewal, and this has hindered the assessment of mechanisms for tissue maintenance and renewal. The pituitary and pancreas are such tissues. And in both cases, arguments have been presented throughout the years in support of the importance of progenitor expansion relative to differentiated cell-dependent mechanisms of tissue maintenance. In the adult pancreas, β-cell renewal and maintenance relies extensively on self-duplication of differentiated β-cells (30). Very recent work supported a similar mechanism in the heart (31).
In order to address this question in the pituitary gland, we have taken advantage of a powerful genetic trick that allowed measurement of very low-frequency cell renewal. Thus, the exquisite sensitivity of the invloxP system allowed us to assess the importance of differentiated POMC cell self-duplication for maintenance of pituitary corticotropes and melanotropes in normal fetal and adult life, as well as in an experimental paradigm of tissue stimulation, ADX. The present report showed that whereas early fetal development of the pituitary relies primarily on expansion of undifferentiated progenitors, late fetal and early postnatal organogenesis also involves some self-duplication of differentiated corticotropes and melanotropes (Figure 6). In contrast, maintenance of the adult pool of corticotropes is essentially dependent on self-duplication of these cells whereas the related POMC-expressing melanotropes do not use this mechanism. It is noteworthy, however, that expansion of corticotropes following ADX depends primarily on expansion of Sox2-positive progenitors and subsequent differentiation (Figure 5) rather than to self-duplication of existing corticotropes (Figure 6).
Figure 6.
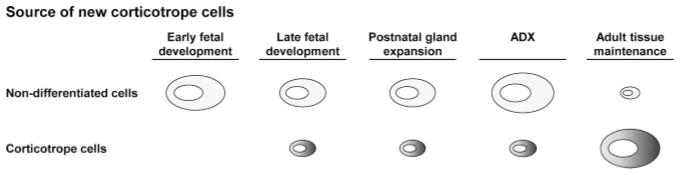
Source of New Corticotropes in Development and in Adult. Schematic representation of the predominant source of new corticotropes in early and late fetal, as well as in postnatal pituitary development. In contrast to development when the bulk of new corticotropes originate from non-differentiated precursor/progenitors, the present work showed that maintenance of adult pituitary corticotropes predominately comes from self-duplication of differentiated corticotropes. Corticotrope expansion following ADX largely depends on expansion of undifferentiated precursors and their subsequent differentiation.
Collectively, these data indicate that maintenance of POMC cells is very different in AL and IL, with self-duplication of differentiated corticotropes representing the major mechanism for maintenance of this pool in adult tissues. Interestingly, the slow depletion of about 55% of corticotropes observed over 8.5 months in inv-loxP/+;POMC-Cre mice is similar to the penetrance of the POMC-Cre transgene (Figure 2B): this may be taken to indicate that the lifespan of corticotropes does not extend beyond 6 – 8 months and hence that all corticotropes would have been lost by 8 months if the POMC-Cre transgene was fully penetrant. This is also consistent with the absence of further corticotrope loss up to 2 years of age. The slow depletion of corticotropes did not trigger expansion of progenitors (Figure 4, C and D) and replacement of the depleted cells as observed after ADX (Figure 5). However, it cannot be excluded that adult pituitary progenitors contribute to the maintenance of the adult corticotrope pool, and this is likely in view of lineage-tracing experiments that relied on expression of Nestin promoter constructs in a subset of pituitary progenitors (32). This interpretation is supported by the very rare occurrence of Cre-positive corticotropes in adult invloxP/+;POMC-Cre transgenic pituitaries (Figure 3E). In view of the incomplete penetrance of the POMC-Cre transgene in corticotropes, it is not possible to compute a half-life for differentiated corticotropes but the data clearly support the conclusion that self-duplication of corticotropes is the major mechanism for maintenance of the adult corticotrope pool.
It is quite intriguing that the slow depletion at more than 50% AL corticotropes did not trigger their replacement by drawing from the pool of Sox2-positive progenitors lining the cleft. Whereas signal from end organs may trigger expansion of progenitor, the slow depletion of corticotropes may be associated with slow compensatory rearrangement of the corticotrope homotypic cell network (15, 33, 34) such that an equilibrium between corticotrope and progenitor networks is not disturbed. In these conditions, the remaining corticotropes may suffice to maintain homeostasis.
The observation that the adult pituitary does not contain a significant number of Cre-positive corticotropes (in contrast to melanotropes) suggests that all corticotropes have ability for self-duplication and therefore suggests that adult corticotropes are mostly in a quiescent or G0 state. These data do not support a model in which a fraction of corticotropes would have the competence for self-duplication and be responsible for tissue maintenance. The control of cell cycle reentry in adult corticotropes thus appears to be tightly controlled, and we have shown the importance of Cip/Kip cell cycle inhibitor p27Kip1 for this control (3). The p27Kip1-dependent cell cycle reentry of adult differentiated pituitary cells contrasts sharply with the role of the related p57Kip2 in cell cycle exit of pituitary progenitors (3). The differential importance of these 2 Cip/Kip cell cycle inhibitors in control of cell cycle in progenitor vs differentiated cells is likely reflected in mechanisms of tumor development involving either undifferentiated or differentiated pituitary cells. It is tantalizing to speculate that these different mechanisms may be involved in pituitary adenomas containing either hormone-negative nonfunctioning adenomas by comparison with adenomas involving differentiated cells such as corticotrope adenomas that cause Cushing’s disease (35). It is noteworthy that, in the mouse, the pituitary is particularly sensitive to loss-of-function mutations affecting cell cycle control genes such as p27Kip1, Rb, Cdk4, etc., (36 – 41). This sensitivity involves both the IL melanotropes as well as AL cells, consistent with the model that all pituitary differentiated cells may be prevented from reentering the cell cycle through p27Kip1-dependent mechanisms (3).
It is noteworthy, however, that IL melanotropes are slightly more sensitive to loss of function of these cell cycle regulators compared with AL cells, and hence the melanotrope fate has unique features that account for this. The apparently rare turnover of melanotropes compared with self-duplication of corticotropes may represent other features unique to each lineage. We have recently identified the transcription factor Pax7 for its unique pioneer role in selection of the melanotrope cell fate (42), and it is thus possible that the unique features of melanotrope and corticotrope cell cycle reentry are controlled by Pax7.
In summary, the present work has revealed very different mechanisms for adult maintenance of the 2 POMC lineages. In particular, adult corticotropes are primarily maintained by self-duplication rather than being dependent on progenitors.
Acknowledgments
We thank Tabasum Abdul-Rasul for her expert secretarial assistance; Manon Laprise for mouse adrenalectomies; and Dominic Filion for the MATLAB nuclei-counting script (all affiliated with Institut de recherches cliniques de Montréal, Quebec, Canada).
This work was supported by grants from the Canadian Institutes for Health Research (CIHR) (to J.D. and M.K.), and from the Canadian Cancer Society (to J.D.). D.L. was supported by a scholarship from Université de Montréal.
Abbreviations
- ADX
adrenalectomy
- AL
anterior lobe
- BrdU
bromodeoxyuridine
- e17.5
embryonic day 17.5
- IL
intermediate lobe
- POMC
proopiomelanocortin
- P0
postnatal d 0 (birth)
Footnotes
Disclosure Summary: The authors do not have any conflict of interests to declare.
References
- 1.Drouin J. Pituitary development. In: Melmed S, editor. The Pituitary. New York, NY: Elsevier-Academic Press; 2010. pp. 3–19. [Google Scholar]
- 2.Davis SW, Mortensen AH, Camper SA. Birthdating studies reshape models for pituitary gland cell specification. Dev Biol. 2011;352:215–227. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29:1895–1908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roussel-Gervais A, Bilodeau S, Vallette S, et al. Cooperation between cyclin E and p27(Kip1) in pituitary tumorigenesis. Mol Endocrinol. 2010;24:1835–1545. doi: 10.1210/me.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of rat anterior pituitary cells. Anat Embryol (Berl) 2002;206:67–72. doi: 10.1007/s00429-002-0271-8. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Kominami R, Yasutaka S, Kawarai Y. Proliferation and differentiation of pituitary corticotrophs during the fetal and postnatal period: a quantitative immunocytochemical study. Anat Embryol (Berl) 2000;201:229–234. doi: 10.1007/s004290050313. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi Y, Kominami R, Yasutaka S, Shinohara H. Mitoses of existing corticotrophs contribute to their proliferation in the rat pituitary during the late fetal period. Anat Embryol (Berl) 2001;203:89–93. doi: 10.1007/s004290000140. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoti K. Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. Eur J Neurosci. 2010;32:2053–2062. doi: 10.1111/j.1460-9568.2010.07524.x. [DOI] [PubMed] [Google Scholar]
- 9.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146:3985–3998. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Lavandeira M, Quereda V, Flores I, et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS ONE. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida S, Kato T, Yako H, et al. Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol. 2011;23:933–943. doi: 10.1111/j.1365-2826.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suga H, Kadoshima T, Minaguchi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 14.Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21:801–813. doi: 10.1089/scd.2011.0496. [DOI] [PubMed] [Google Scholar]
- 15.Budry L, Lafont C, El Yandouzi T, et al. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA. 2011;108:12515–12520. doi: 10.1073/pnas.1105929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23:261–269. doi: 10.1016/j.tem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Transgenic mice with inducible dwarfism. Nature. 1989;339:538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- 18.Gulyás M, Pusztai L, Rappay G, Makara GB. Pituitary corticotrophs proliferate temporarily after adrenalectomy. Histochemistry. 1991;96:185–189. doi: 10.1007/BF00315991. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi Y, Tamatani R, Yasutaka S, Kawarai Y. Proliferation of pituitary corticotrophs following adrenalectomy as revealed by immunohistochemistry combined with bromodeoxyuridine-labeling. Histochem Cell Biol. 1995;103:127–130. doi: 10.1007/BF01454009. [DOI] [PubMed] [Google Scholar]
- 20.Subburaju S, Aguilera G. Vasopressin mediates mitogenic responses to adrenalectomy in the rat anterior pituitary. Endocrinology. 2007;148:3102–3110. doi: 10.1210/en.2007-0103. [DOI] [PubMed] [Google Scholar]
- 21.Nolan LA, Levy A. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol. 2006;18:655–661. doi: 10.1111/j.1365-2826.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 22.Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17:738–747. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grégoire D, Kmita M. Recombination between inverted loxP sites is cytotoxic for proliferating cells and provides a simple tool for conditional cell ablation. Proc Natl Acad Sci USA. 2008;105:14492–14496. doi: 10.1073/pnas.0807484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavoie PL, Budry L, Balsalobre A, Drouin J. Developmental Dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Mol Endocrinol. 2008;22:1647–1657. doi: 10.1210/me.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlais D, Couture C, Sylvain-Drolet G, Drouin J. A pituitary-specific enhancer of the POMC gene with preferential activity in corticotrope cells. Mol Endocrinol. 2011;25:348–359. doi: 10.1210/me.2010-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamolet B, Pulichino AM, Lamonerie T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 27.Pulichino AM, Vallette-Kasic S, Couture C, et al. Human and mouse Tpit gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17:711–716. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza FS, Santangelo AM, Bumaschny V, et al. Identification of neuronal enhancers of the proopiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol Cell Biol. 2005;25:3076–3086. doi: 10.1128/MCB.25.8.3076-3086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNicol AM, Carbajo-Perez E. Aspects of anterior pituitary growth, with special reference to corticotrophs. Pituitary. 1999;1:257–268. doi: 10.1023/a:1009950308561. [DOI] [PubMed] [Google Scholar]
- 30.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 31.Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gleiberman AS, Michurina T, Encinas JM, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waite E, Lafont C, Carmignac D, et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology. 2010;151:234–243. doi: 10.1210/en.2009-0539. [DOI] [PubMed] [Google Scholar]
- 34.Jelinic P, Stehle JC, Shaw P. The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation. PLoS Biol. 2006;4:e355. doi: 10.1371/journal.pbio.0040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouin J, Bilodeau S, Vallette S. Of old and new diseases: genetics of pituitary ACTH excess (Cushing) and deficiency. Clin Genet. 2007;72:175–182. doi: 10.1111/j.1399-0004.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 36.Fero ML, Rivkin M, Tasch M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 37.Kiyokawa H, Kineman RD, Manova-Todorova KO, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 39.Chien WM, Rabin S, Macias E, et al. Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc Natl Acad Sci USA. 2006;103:4122–4127. doi: 10.1073/pnas.0509514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 41.Franklin DS, Godfrey VL, Lee H, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budry L, Balsalobre A, Gauthier Y, et al. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev. 2012;26:2299–2310. doi: 10.1101/gad.200436.112. [DOI] [PMC free article] [PubMed] [Google Scholar]